Abstract
Aim:
Secretory carcinoma (SC) of salivary gland typically harbors ETV6-NTRK3 fusion which can be utilized clinically to assist with diagnosis. Pan-TRK inhibitor therapy has demonstrated drastic responses in patients with NTRK-translocated tumors, including SC. Pan-Trk IHC is emerging as a sensitive and specific tool in detecting NTRK1, NTRK2, and NTRK3 fusions in various cancers. We aimed to establish the specificity and sensitivity of pan-Trk IHC to diagnose SC and to detect ETV6-NTRK3 fusion. A literature review on the utility of pan-Trk IHC was conducted.
Methods and results:
Pan-Trk IHC was performed on 83 salivary gland neoplasms (29 SCs and 54 non-SCs). ETV6-NTRK3 fusion status was established in 25 cases. Using any staining (nuclear or cytoplasmic) as a positive threshold, the sensitivity and specificity of pan-Trk IHC were 90% and 70% in diagnosing SC and 100% and 0% in detecting NTRK3 fusion. When only pan-Trk nuclear staining was considered as positive, the sensitivity and specificity were 69% and 100% in diagnosing SC and 92% and 100% in detecting NTRK3 fusion.
Conclusions:
Nuclear pan-Trk IHC is highly specific for SC diagnosis with a specificity approaching 100%, rendering it a useful and precise diagnostic tool to differentiate SC from its histologic mimickers. On the other hand, any pan-Trk staining (nuclear or cytoplasmic) is highly sensitive for SC, and can serve as an attractive cheap, fast, and accessible screening tool to select patients to undergo confirmative molecular testing for clinical trials using TRK inhibitors.
Keywords: pan-Trk, immunohistochemistry, secretory carcinoma, ETV6-NTRK3 fusion
INTRODUCTION
Secretory carcinoma (SC) of salivary gland, formerly known as mammary analogue secretory carcinoma (MASC), is a distinct salivary gland carcinoma that was first described in 2010 by Skalova et al. and is characterized by ETV6-NTRK3 fusion 1. Histologically, SC is composed of cells with vacuolated or eosinophilic cytoplasm that are commonly arranged in papillocystic, microcystic, or solid architecture, and showing intraluminal dense hyper-eosinophilic material. By immunohistochemistry, this tumor is typically positive for S100, mammaglobin, and GCDFP-15 2, 3. The main differential diagnoses of SC are acinic cell carcinoma (AciCC), (cyst)adenocarcinoma not otherwise specified (NOS), intraductal carcinoma and mucoepidermoid carcinoma 4–7.
Although ETV6-NTRK3 fusion has been reported as the most common molecular event in SC, a subset of tumors was found to have a different fusion partner for ETV6. These tumors were initially reported as ETV-X SC as the fusion partner was unknown 8. Recently, ETV6-RET and ETV6-MAML3 fusion were described in SC 7, 9. Additionally, a recent case series of SC of the skin has reported a novel NFIX-PKN1 translocation 10.
Lately, TRK inhibitors, such as entrectinib and LOXO-101, have been introduced in multiple clinical trials, targeting carcinomas (including SC) or sarcomas harboring NTRK fusions showing promising clinical results 11–16. Therefore, the detection of NTRK3 fusion in salivary gland neoplasms may not only serve as a diagnostic tool but may also have a predictive value to select patients eligible for TRK inhibitor clinical trials.
The gold-standard methods to detect ETV6-NTRK3 fusion are fluorescence in situ hybridization (FISH) and DNA/RNA sequencing 16. Such methods are in general costly and have a turnaround time of one to two weeks. In 2017, Hechtman et al. were the first to publish on the utility of pan-Trk immunohistochemistry (IHC) as an efficient, highly sensitive and specific screening tool for NTRK fusions 17. The authors included all types of tumors with NTRK1, NTRK2, or NTRK3 fusions and reported a high sensitivity (92.5%) and specificity (100%) of the IHC in detecting NTRK fusions. In contrast, a recent study by Hung et al. reported a relatively low sensitivity (64%) and specificity (44%) of pan-Trk IHC in diagnosing secretory carcinoma 18. It appears that pan-Trk IHC is a promising IHC marker but its efficacy in salivary gland neoplasms remains to be validated.
In this study, we aimed to establish the utility of pan-Trk IHC as a diagnostic tool to differentiate SC from its mimickers and as a screening tool in detecting ETV6-NTRK3 fusion in a large cohort of 83 salivary gland neoplasms, including 29 SCs and 25 cases with known ETV6-NTRK3 fusion status.
MATERIAL AND METHODS
Case selection and study cohort
The study was approved by the Institutional Review Board of Memorial Sloan Kettering Cancer Center (MSKCC, New York, NY). Informed consent was not required for this retrospective study. Eighty-three patients with epithelial salivary gland neoplasms who had surgery at MSKCC between 1993 and 2019 with appropriate material for subsequent IHC and molecular studies were included. The histologic slides were reviewed by two head and neck pathologists (NK and BX) to confirm the diagnosis. The study cohort was composed of 29 SC, and a control group of 54 other types of salivary gland neoplasms including 14 AciCC, 7 pleomorphic adenoma, 7 salivary duct carcinoma, 6 mucoepidermoid carcinoma, 6 adenoid cystic carcinoma, 5 myoepithelial carcinoma, 2 polymorphous adenocarcinoma, 1 cribriform adenocarcinoma, 1 adenocarcinoma not otherwise specified (NOS), 1 basal cell adenocarcinoma, 1 carcinoma ex-pleomorphic adenoma, adenocarcinoma NOS, 1 oncocytic cystadenoma, 1 (hyalinizing) clear cell carcinoma, and 1 Warthin tumor (table 1).
Table 1.
Pan-Trk immunopositivity in salivary gland neoplasms.
| N | Pan-Trk positive (any staining) |
Pan-Trk positive (nuclear staining) |
|
|---|---|---|---|
| Secretary carcinoma (SC) | 29 | 26 (90%) | 20 (69%) |
| Non-SC | 54 | 16 (30%) | 0 (0%) |
| Acinic cell carcinoma | 14 | 2 (14%) | 0 (0%) |
| Pleomorphic adenoma | 7 | 5 (71%) | 0 (0%) |
| Salivary duct carcinoma | 7 | 0 (0%) | 0 (0%) |
| Mucoepidermoid carcinoma | 6 | 0 (0%) | 0 (0%) |
| Adenoid cystic carcinoma | 6 | 3 (50%) | 0 (0%) |
| Myoepithelial carcinoma | 5 | 3 (60%) | 0 (0%) |
| Polymorphous adenocarcinoma | 2 | 1 (50%) | 0 (0%) |
| Cribriform adenocarcinoma | 1 | 1 (100%) | 0 (0%) |
| Adenocarcinoma NOS | 1 | 1 (100%) | 0 (0%) |
| Basal cell adenocarcinoma | 1 | 0 (0%) | 0 (0%) |
| Adenocarcinoma NOS ex-pleomorphic adenoma | 1 | 0 (0%) | 0 (0%) |
| Oncocytic cystadenoma | 1 | 0 (0%) | 0 (0%) |
| (Hyalinizing) clear cell carcinoma | 1 | 0 (0%) | 0 (0%) |
| Warthin tumor | 1 | 0 (0%) | 0 (0%) |
NOS: not otherwise specified
Detection of NTRK3-ETV6 fusion
In a subset of 25 cases (23 SC, 1 pleomorphic adenoma, and 1 adenocarcinoma NOS), the ETV6-NTRK3 fusion status (N=23 for ETV6, and N=15 for NTRK3) was assessed using various techniques, including fluorescence in situ hybridization (FISH) for ETV6 (N=18); FISH for NTRK3 (N=8), and MSK-IMPACT targeted exome next generation sequencing platform (N=9).
FISH on interphase nuclei from paraffin-embedded 4-μm sections was performed using custom probes of bacterial artificial chromosomes (BACs) flanking ETV6 or NTRK3 (supplementary table 1). Two hundred successive nuclei were examined for the presence of ETV6 or NTRK3 gene rearrangements/amplifications using a Zeiss fluorescence microscope (Zeiss Axioplan, Oberkochen, Germany), controlled by Isis 5 software (Metasystems, Waltham, MA). A positive FISH score was interpreted when at least 20% of the nuclei showed a break-apart signal. Nuclei with incomplete set of signals were omitted from the score. The MSK-IMPACT™ sequencing assay was a targeted capture massive parallel sequencing that captured somatic genetic alterations and fusions in 410 cancer-related genes as previously described 19, 20.
Pan-Trk IHC
Pan-Trk IHC was performed using monoclonal antibody clone EPR17341 (Abcam, Cambridge, MA) that reacted to a homologous region of Trk A, B, and C near the C terminal. All immunostains were performed using a Leica Bond-3 (Leica, Buffalo Grove, IL) automated stainer platform. The staining pattern, percentage of positive tumor cells, and staining intensity were reviewed and recorded on all cases. The sensitivity and specificity of pan-Trk IHC in detecting SC (diagnosed morphologically) and the ETV6-NTRK3 fusion were calculated. Pan-Trk IHC was considered positive using two criteria: 1) any staining (nuclear or cytoplasmic) within the tumor cells, and 2) any nuclear staining in the tumor cells.
Statistical analysis
All statistical analyses were performed using the SPSS software 24.0 (IBM Corporation, New York, NY, U.S.). The pan-Trk IHC staining pattern, staining intensity, and percentage of positive tumor cells were compared between pan-Trk IHC-positive SCs and pan-Trk IHC-positive non-SC tumors, using Fisher’s exact test and two-tailed student t test respectively. P values less than 0.05 were considered to be statistically significant.
RESULTS
Pan-Trk IHC nuclear staining is highly specific for SC
All SCs in our cohort that were positive for pan-TRK IHC exhibited cytoplasmic and/or nuclear staining for pan-Trk. Membranous and peri-nuclear staining patterns were not noted. Storage period (before 2010 vs. after 2010) did not alter the pan-Trk staining significantly (Fisher’s exact test, p > 0.05). When using any pan-Trk staining (nuclear or cytoplasmic with any staining intensity) as positive, 26 of 29 SCs (90%) and 16 of 54 non-SC (30%) salivary neoplasms were positive, showing a sensitivity of 90% and a specificity of 70% (table 1).
All 16 pan-Trk-positive non-SC tumors showed cytoplasmic but not nuclear staining. The diagnoses of these 16 tumors were as follows: acinic cell carcinoma (2 of 14, 14%), pleomorphic adenoma (5 of 7, 71%), adenoid cystic carcinoma (3 of 6, 50%), myoepithelial carcinoma (3 of 5, 60%), polymorphous adenocarcinoma (1 of 2, 50%), cribriform adenocarcinoma (1 of 1, 100%), and adenocarcinoma NOS (1 of 1, 100%). All the tested salivary duct carcinoma, mucoepidermoid carcinoma, basal cell adenocarcinoma, adenocarcinoma NOS ex-PA, oncocytic cystadenoma, (hyalinizing) clear cell carcinoma, and Warthin tumor were negative for pan-Trk.
None of the 54 tested non-SC tumors showed any nuclear pan-Trk immunopositivity. When immunopositivity was determined as any nuclear staining in tumor cells, 20 of 29 SCs (69%) and 0 (0%) non-SCs were positive for pan-Trk (table 1 and figure 1). Nuclear pan-Trk immunopositivity appears to be a highly specific IHC marker in diagnosing SC with a sensitivity and a specificity of 69% and 100%, respectively.
Figure 1. Pan-Trk immunohistochemistry (IHC) in secretory carcinoma (SC) and acinic cell carcinoma (AciCC).
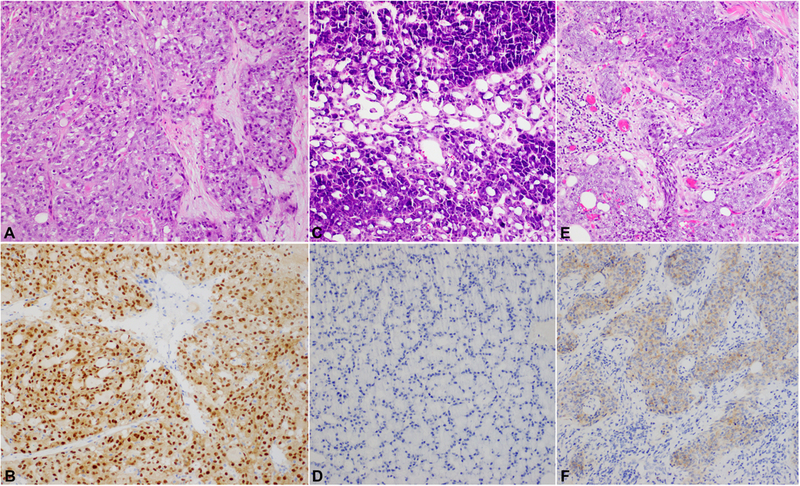
(A/B) A SC that shows solid and microcystic growth pattern. Some microcysts contain hyper-eosinophilic luminal secretion characteristic of SC. The tumor harbors ETV6-NTRK3 fusion and is positive for pan-Trk IHC showing both nuclear and cytoplasmic staining. (C/D) An AciCC that is composed of acinar cells and shows solid and microcystic growth pattern and is entirely negative for pan-Trk (panel D). (E/F) A high grade AciCC showing cytoplasmic pan-Trk immunopositivity (panel F). None of the 14 tested AciCCs shows pan-Trk nuclear staining. Magnification: 400X for all pictures.
The staining details of pan-Trk immunoreactivity in the tumors are shown in table 2. There was a significant difference (p<0.001) between SCs and non-SC tumors in term of staining pattern: the majority of SCs (77%, 20/26) exhibited nuclear staining, whereas only cytoplasmic staining was noted in non-SC tumors. The staining intensity and percentage of positive cells did not differ significantly between SC and non-SC tumors (p>0.05).
Table 2.
Details of pan-Trk immunohistochemistry in positive cases.
| SC (n=26) | Non-SC (n=16) | ||
|---|---|---|---|
| Staining pattern | Cytoplasmic only | 6 (23%) | 16 (100%) |
| Nuclear only | 1 (4%) | 0 (0%) | |
| Cytoplasmic & nuclear | 19 (73%) | 0 (0%) | |
| Staining intensity | Weak | 5 (19%) | 6 (38%) |
| Moderate | 14 (54%) | 8 (50%) | |
| Strong | 7 (27%) | 2 (13%) | |
| Percentage of positive tumor cells (mean ± SEM) | 70%±6% | 68%±6% | |
SEM: standard error of mean.
Correlation of pan-Trk IHC and ETV6-NTRK3 fusion
Among the 23 SCs tested for ETV6 and/or NTRK3 fusion using FISH or MSK-IMPACT next generation sequencing techniques, ETV6 fusion was detected in 19 of 23 SCs (83%); whereas NTRK3 fusion was reported in 13 of 13 (100%).
Four cases were negative for ETV6 translocation by FISH but classified as SC since they exhibited typical histologic features and immunoprofile of SC. Among these four SCs, three showed pan-Trk immunoreactivity: one with 10% weak cytoplasmic, one with 75% weak to moderate cytoplasmic, and one with 70% weak cytoplasmic and nuclear staining. Two non-SC cases were tested negative for ETV6 and NTRK3 fusion. Both cases showed only cytoplasmic pan-TRK immunopositivity.
The positive and negative rate of pan-Trk IHC according to NTRK3 fusion and ETV6 fusion status is stated in table 3. The sensitivity, specificity, positive predictive value, and negative predictive value of pan-Trk IHC in predicting the ETV6-NTRK3 fusion status are listed in table 4. When pan-Trk immunopositivity was defined as nuclear staining only, the IHC showed a high sensitivity (92%) and specificity (100%) in detecting NTRK3 fusion. When using any immunostaining as positive, the specificity decreased to 0%, whereas the sensitivity increased to 100%.
Table 3.
Correlation of pan-Trk immunohistochemistry (IHC) with the diagnosis, NTRK3 fusion status, and ETV6 fusion status.
| Pan-Trk IHC (any staining) | Pan-Trk IHC (nuclear staining) | ||||
|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | ||
| Diagnosis | SC | 26 | 3 | 20 | 9 |
| non-SC | 16 | 38 | 0 | 54 | |
| NTRK3 fusion | Present | 13 | 0 | 12 | 1 |
| Absent | 2 | 0 | 0 | 2 | |
| ETV6 fusion | Present | 19 | 0 | 17 | 2 |
| Absent | 5 | 1 | 1 | 5 | |
SC: Secretory carcinoma
Table 4.
Sensitivity, specificity, positive predictive value, and negative predictive values of pan-Trk immunohistochemistry in predicting SC diagnosis and underlying fusion status.
| Pan-Trk IHC (any staining) | Pan-Trk IHC (nuclear staining) | |||||
|---|---|---|---|---|---|---|
| Diagnosis |
NTRK3 fusion |
ETV6 fusion |
Diagnosis |
NTRK3 fusion |
ETV6 fusion | |
| Sensitivity | 90% | 100% | 100% | 69% | 92% | 89% |
| Specificity | 70% | 0% | 17% | 100% | 100% | 83% |
| Positive predictive value | 62% | 87% | 79% | 100% | 100% | 94% |
| Negative predictive value | 93% | NA | 100% | 86% | 67% | 71% |
The sensitivity and specificity of pan-Trk IHC in predicting ETV6 fusion status was lower compared with predicting NTRK3 fusion status, being 89% and 83% respectively using nuclear staining only threshold, and 100% and 17% using nuclear and/or cytoplasmic staining threshold.
DISCUSSION
NTRK3 gene encodes TrkC protein, a member of tropomysine receptor kinase family that is actively involved in neuronal developments. Fusions involving NTRK3 are oncogenic events in multiple tumor types, e.g. infantile fibrosarcoma, uterine sarcoma, acute myeloid leukemia, pulmonary adenocarcinoma, papillary thyroid carcinoma, sinonasal non-intestinal adenocarcinoma, mammary SC, and salivary SC, through constitutive activation and overexpression of Trk proteins 17, 21–23. In salivary gland, the presence of NTRK3 fusion has only been reported in SC 1, 8, 9. Therefore, the detection of NTRK3 fusion is a useful diagnostic tool in differentiating SC from its mimickers, especially in small biopsy material where the diagnosis can be challenging.
Several recently developed TRK inhibitors have shown promising responses in tumors harboring NTRK3 fusion 11–16. For example, larotrectinib, a highly-potent TRK inhibitor, has demonstrated an overall response rate of 79% in NTRK-translocated tumors, and has been approved by the US Food and Drug Administration (FDA) 16. In these clinical trials, the methods to detect NTRK fusions were FISH or next generation sequencing. These two techniques are relatively time-consuming and costly; they are also not readily available in every pathology laboratory, rendering them less-than-ideal as screening tests for NTRK fusion. 7, 16 Therefore, there is a need to search for an alternative cheaper method, such as IHC, to aid with diagnosis and to support in screening and selecting patients for clinical trials.
Five recent publications 17, 18, 21, 24, 25 and the present study have investigated the utility of pan-Trk IHC in diagnosing specific tumor types and in detecting the underlying fusion. The results are summarized in table 5. All previous studies and the current study used the same EPR17341 (Abcam) antibody clone. While Hechtman et al. 17 and Gatalica et al. 21 studied all tumor types based solely on NTRK fusion status; Rudzinski et al. 24 and Hung et al. 25 focused on mesenchymal tumors; only Hung et al. 18 and our current study assessed the utility of pan-Trk IHC in salivary gland tumors. The two studies by Hung et al. 18, 25 did not include fusion data, but rather explored the utility of pan-Trk IHC in distinguishing tumors that typically harbor NTRK fusion (e.g. infantile fibrosarcoma and SC) from their mimickers. When using a cutoff of any pan-Trk immunostaining (cytoplasmic, membranous, peri-nuclear and/or nuclear), the combined reported sensitivity and specificity are 95% (range: 95–100%) and 95% (range: 70–100%) in detecting NTRK fusion; and 84% (range: 64–90%) and 66% (range: 46–70%) in diagnosing NTRK-rearranged tumors (table 5). These data suggest that any pan-Trk immunopositivity is a relatively sensitive and specific diagnostic marker and a reliable screening tool to detect NTRK fusion status.
Table 5.
Literature review: the sensitivity and specificity pan-Trk IHC in detecting NTRK3 fusion and diagnosing tumors that typically harbor NTRK fusion
| 17 | 24 | 21 | Current study |
Pooled data | |
|---|---|---|---|---|---|
| Pan-Trk IHC any staining | |||||
| NTRK1 or NTRK2-translocated tumorsa | 12/12 (100%) | 15/15 (100%) | 15/17 (88%) | NA | 42/44 (95%) |
| NTRK3-translocated tumors | 8/9 (89%) | 15/16 (94%) | NA | 13/13 (100%) | 36/38 (95%) |
| Control group | 0/20 (0%) | ¾8 (6%) | 166/3942 (4%) | 16/54 (30%) | 185/4064 (5%) |
| Sensitivity for NTRK fusion | 95% | 97% | 75% | 100% | 95% |
| Specificity | 100% | 94% | 96% | 70% | 95% |
| Pan-Trk IHC nuclear staining | |||||
| NTRK1 or NTRK2-translocated tumors | 0/12 (0%) | 0/15 (0%) | 0/17 (0%) | NA | 0/44 (0%) |
| NTRK3-translocated tumors | 4/9 (44%) | 15/16 (94%) | 6/11 (55%) | 12/13 (92%) | 37/49 (76%) |
| Control group | 0/20 (0%) | 0/48 (0%) | NA | 0/54 (0%) | 0/122 (0%) |
| Sensitivity to detecting NTRK3 fusion | 44% | 94% | 55% | 92% | 82% |
| Specificity | 100% | 100% | NA | 100% | 100% |
| 25 | 18 | Current study | Pooled data | |
|---|---|---|---|---|
| Pan-Trk IHC any staining | ||||
| Tumor typically have NTRK1 fusion | 5/5 (100%) | NA | NA | 5/5 (100%) |
| Tumor typically have NTRK3 fusion | 14/15 (93%) | 9/14 (64%) | 26/29 (90%) | 49/58 (84%) |
| Control group | 53/190 (28%) | 39/72 (54%) | 16/54 (30%) | 108/316 (34%) |
| Sensitivity for the diagnosis of tumors that typically harbors NTRK fusion | 95% | 64% | 90% | 86% |
| Specificity | 72% | 46% | 70% | 66% |
| Pan-Trk IHC nuclear staining | ||||
| Tumor typically have NTRK1 fusion | 0/5 (0%) | NA | NA | 0/5 (0%) |
| Tumor typically have NTRK3 fusion | 11/15 (73%) | 9/14 (64%) | 20/29 (69%) | 40/58 (69%) |
| Control group | NA | 6/72 (8%) | 0/54 (0%) | 6/126 (5%) |
| Sensitivity for the diagnosis of tumors that typically harbors NTRK3 fusion | 73% | 64% | 69% | 69% |
| Specificity | NA | 92% | 100% | 95% |
All numbers are expressed as number of positive cases/number of total cases tested (percentage of positive cases).
NA: not available.
Interestingly, all the above-mentioned studies have identified nuclear pan-Trk immunostaining in NTRK3-translocated tumors, whereas NTRK1 or NTRK2-fusion-positive tumors and NTRK-fusion negative tumors had cytoplasmic but no nuclear staining 17, 18, 21, 24, 25. The percentage of NTRK3-translocated tumors that showed nuclear staining for pan-Trk IHC ranged from 44% to 94% 17, 21, 24, and is 76% in the current study. Nuclear staining in non-SC tumors was only identified in the study of Hung et al., where they reported 6 (8%) tumors (5 polymorphous adenocarcinoma and 1 mucoepidermoid carcinoma) showing focal (<10%) nuclear staining for pan-Trk. However, all the other studies including ours 17, 24, 25 did not detect any nuclear immunopositivity in non-SC tumors. The cause of this difference is unclear, as all published studies used the same pan-Trk antibody (EPR17341). Overall, when pan-Trk positivity is defined by any nuclear staining in tumor cells, the sensitivity and specificity of pan-Trk IHC are 82% and 100% in detecting NTRK3 fusion; and 69% and 95% in diagnosing tumors that are typically NTRK3 rearranged. Together, this suggests that pan-Trk nuclear staining is a highly specific diagnostic marker that can be utilized in clinical practice as an adjunct tool in addition to histopathologic features and other IHC markers (e.g. S100 and mammaglobin). The sensitivity and specificity of pan-TRK IHC in detecting ETV6 fusion is lower, which can be explained by the reported fusion of ETV6 with other partners (e.g. RET and MAML3) in SC 7–9. SC harboring fusion other than NTRK3 will not be captured by pan-Trk IHC.
Both Hung et al. 18 and our study have investigated the reliability of pan-Trk IHC in differentiating salivary SC from its mimickers. The rate of pan-Trk IHC cytoplasmic staining is 64% and 90% respectively; and the frequency of pan-Trk nuclear staining is 64% and 69% respectively. The relatively low frequency of immunopositivity can in part be explained by the fact that the fusion partner for ETV6 in salivary SC may not be NTRK3 7–9. The specificity of pan-Trk IHC for a diagnosis of SC in Hung et al. and the present study was 46% and 70% when using any cytoplasmic staining as positive; and 92% and 100% when using any nuclear staining as positive. Clearly, using pan-Trk IHC nuclear staining only markedly improves the specificity of the IHC but compromises its sensitivity in diagnosing SC.
CONCLUSIONS
In summary, in the current study, we provided our experience and the literature review on the utility of pan-Trk IHC in pathology practice. Nuclear pan-Trk IHC positivity is highly specific for NTRK3 fusion and for SC of salivary gland, permitting its use as an adjunct tool in clinical diagnosis. On the other hand, any pan-Trk immunopositivity seems to be highly sensitive for NTRK3 fusion, showing a sensitivity of 100% and a positive predictive value of 87%. Therefore, pan-Trk IHC may be utilized as a screening tool to select patients who can further undergo NTRK3 molecular testing to determine eligibility for clinical trials.
Supplementary Material
Acknowledgments
Source of Funding:
Research reported in this publication was supported in part by the Cancer Center Support Grant of the National Institutes of Health/National Cancer Institute under award number P30CA008748 P50 CA217694 (CRA) and P50 CA140146–01 (CRA). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interest:
The authors have disclosed that they have no significant relationships with, or financial interest in any commercial companies pertaining to this article.
References
- 1.Skalova A, Vanecek T, Sima R et al. Mammary analogue secretory carcinoma of salivary glands, containing the etv6-ntrk3 fusion gene: A hitherto undescribed salivary gland tumor entity. Am J Surg Pathol 2010;34;599–608. [DOI] [PubMed] [Google Scholar]
- 2.Bishop JA, Yonescu R, Batista D, Begum S, Eisele DW, Westra WH. Utility of mammaglobin immunohistochemistry as a proxy marker for the etv6-ntrk3 translocation in the diagnosis of salivary mammary analogue secretory carcinoma. Hum Pathol 2013;44;1982–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skalova A Mammary analogue secretory carcinoma of salivary gland origin: An update and expanded morphologic and immunohistochemical spectrum of recently described entity. Head Neck Pathol 2013;7 Suppl 1;S30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Connor A, Perez-Ordonez B, Shago M, Skalova A, Weinreb I. Mammary analog secretory carcinoma of salivary gland origin with the etv6 gene rearrangement by fish: Expanded morphologic and immunohistochemical spectrum of a recently described entity. Am J Surg Pathol 2012;36;27–34. [DOI] [PubMed] [Google Scholar]
- 5.Chiosea SI, Griffith C, Assaad A, Seethala RR. The profile of acinic cell carcinoma after recognition of mammary analog secretory carcinoma. Am J Surg Pathol 2012;36;343–350. [DOI] [PubMed] [Google Scholar]
- 6.Jung MJ, Song JS, Kim SY et al. Finding and characterizing mammary analogue secretory carcinoma of the salivary gland. Korean J Pathol 2013;47;36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guilmette J, Dias-Santagata D, Nose V, Lennerz JK, Sadow PM. Novel gene fusions in secretory carcinoma of the salivary glands: Enlarging the etv6 family. Hum Pathol 2019;83;50–58. [DOI] [PubMed] [Google Scholar]
- 8.Skalova A, Vanecek T, Simpson RH et al. Mammary analogue secretory carcinoma of salivary glands: Molecular analysis of 25 etv6 gene rearranged tumors with lack of detection of classical etv6-ntrk3 fusion transcript by standard rt-pcr: Report of 4 cases harboring etv6-x gene fusion. Am J Surg Pathol 2016;40;3–13. [DOI] [PubMed] [Google Scholar]
- 9.Skalova A, Vanecek T, Martinek P et al. Molecular profiling of mammary analog secretory carcinoma revealed a subset of tumors harboring a novel etv6-ret translocation: Report of 10 cases. Am J Surg Pathol 2017. [DOI] [PubMed] [Google Scholar]
- 10.Kastnerova L, Luzar B, Goto K et al. Secretory carcinoma of the skin: Report of 6 cases, including a case with a novel nfix-pkn1 translocation. Am J Surg Pathol 2019. [DOI] [PubMed] [Google Scholar]
- 11.Nagasubramanian R, Wei J, Gordon P, Rastatter JC, Cox MC, Pappo A. Infantile fibrosarcoma with ntrk3-etv6 fusion successfully treated with the tropomyosin-related kinase inhibitor loxo-101. Pediatr Blood Cancer 2016;63;1468–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drilon A, Li G, Dogan S et al. What hides behind the masc: Clinical response and acquired resistance to entrectinib after etv6-ntrk3 identification in a mammary analogue secretory carcinoma (masc). Ann Oncol 2016;27;920–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ardini E, Menichincheri M, Banfi P et al. Entrectinib, a pan-trk, ros1, and alk inhibitor with activity in multiple molecularly defined cancer indications. Mol Cancer Ther 2016;15;628–639. [DOI] [PubMed] [Google Scholar]
- 14.Rolfo C, Ruiz R, Giovannetti E et al. Entrectinib: A potent new trk, ros1, and alk inhibitor. Expert Opin Investig Drugs 2015;24;1493–1500. [DOI] [PubMed] [Google Scholar]
- 15.Doebele RC, Davis LE, Vaishnavi A et al. An oncogenic ntrk fusion in a patient with soft-tissue sarcoma with response to the tropomyosin-related kinase inhibitor loxo-101. Cancer Discov 2015;5;1049–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ricciuti B, Genova C, Crino L, Libra M, Leonardi GC. Antitumor activity of larotrectinib in tumors harboring ntrk gene fusions: A short review on the current evidence. OncoTargets and therapy 2019;12;3171–3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hechtman JF, Benayed R, Hyman DM et al. Pan-trk immunohistochemistry is an efficient and reliable screen for the detection of ntrk fusions. Am J Surg Pathol 2017;41;1547–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hung YP, Jo VY, Hornick JL. Immunohistochemistry with a pan-trk antibody distinguishes secretory carcinoma of the salivary gland from acinic cell carcinoma. Histopathology 2019. [DOI] [PubMed] [Google Scholar]
- 19.Cheng DT, Mitchell TN, Zehir A et al. Memorial sloan kettering-integrated mutation profiling of actionable cancer targets (msk-impact): A hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. The Journal of molecular diagnostics 2015;17;251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zehir A, Benayed R, Shah RH et al. Erratum: Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat. Med. 2017;23;1004. [DOI] [PubMed] [Google Scholar]
- 21.Gatalica Z, Xiu J, Swensen J, Vranic S. Molecular characterization of cancers with ntrk gene fusions. Mod Pathol 2018. [DOI] [PubMed] [Google Scholar]
- 22.Hsiao SJ, Zehir A, Sireci AN, Aisner DL. Detection of tumor ntrk gene fusions to identify patients who may benefit from trk inhibitor therapy. The Journal of molecular diagnostics : JMD 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khotskaya YB, Holla VR, Farago AF, Mills Shaw KR, Meric-Bernstam F, Hong DS. Targeting trk family proteins in cancer. Pharmacology & therapeutics 2017;173;58–66. [DOI] [PubMed] [Google Scholar]
- 24.Rudzinski ER, Lockwood CM, Stohr BA et al. Pan-trk immunohistochemistry identifies ntrk rearrangements in pediatric mesenchymal tumors. Am J Surg Pathol 2018;42;927–935. [DOI] [PubMed] [Google Scholar]
- 25.Hung YP, Fletcher CDM, Hornick JL. Evaluation of pan-trk immunohistochemistry in infantile fibrosarcoma, lipofibromatosis-like neural tumour and histological mimics. Histopathology 2018;73;634–644. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


