Abstract
Background:
Cytomegalovirus (CMV) infection is a serious complication in immunosuppressed patients, specifically transplant recipients. Here, we describe the development and use of an assay to monitor the incidence and treatment of CMV viremia in a Cynomolgus macaque model of bone marrow transplantation (BMT) for tolerance induction. We address the correlation between the course of viremia and immune reconstitution.
Methods:
Twenty-one animals received a nonmyeloablative conditioning regimen. Seven received cyclosporine A (CyA) for 28 days and fourteen received rapamycin. A CMV PCR assay was developed and run twice per week to monitor viremia. Nineteen recipients were CMV seropositive before BMT. Immune reconstitution was monitored through flow cytometry and CMV viremia was tracked via qPCR.
Results:
Recipients developed CMV viremia during the first month post-BMT. Two animals developed uncontrollable CMV disease. CMV reactivation occurred earlier in CyA-treated animals compared to those receiving rapamycin. Post-BMT, T-cell counts remained significantly lower compared to pretransplant levels until CMV reactivation, at which point they increased during the viremic phase and approached pretransplant levels three months post-BMT. Management of CMV required treatment before viremia reached 10,000 copies/mL, otherwise clinical symptoms were observed. High doses of ganciclovir resolved the viremia, which could subsequently be controlled with valganciclovir.
Conclusion:
We developed an assay to monitor CMV in Cynomolgus macaques. CMV reactivation occurred in 100% of seropositive animals in this model. Rapamycin delayed CMV reactivation and ganciclovir treatment was effective at high doses. As in humans, CD8+ T cells proliferated during CMV viremia.
1. Introduction
Cytomegalovirus (CMV) is a β-herpesvirus that affects a high percentage (50-90%) of the human population.1,2 After primary infection, CMV remains latent in immunocompetent individuals. Clinically significant disease can occur during primary infection in seronegative individuals or as reactivation of latent infection in immunocompromised individuals, such as immunosuppressed transplant recipients. CMV is a particularly frequent complication in bone marrow transplant (BMT) recipients, where treatment with myelosuppressive antiviral drugs is undesirable during stem cell engraftment.3 CMV may have additional impact on the outcomes of bone marrow transplant recipients. Cellular immunity through T cell responses is the most important pathway for controlling CMV replication.4–9 Virus-specific memory T cells have been associated with bone marrow (BM) rejection or graft-versus-host disease (GVHD) at the time of CMV infection,10 suggesting that viremia may induce heterologous immunity to the donor or recipient, respectively. Early detection and control of CMV in BMT patients is therefore crucial for optimal clinical outcomes.
Our laboratory has utilized Mauritian origin Cynomolgus macaques (MCM) as a translational model to study the ability to induce transplantation tolerance through long-term mixed hematopoietic chimerism across major histocompatibility complex (MHC) barriers. Our current tolerance induction protocol uses a nonmyeloablative preparative regimen along with a short course (28 days) of cyclosporine A (CyA) or rapamycin monotherapy, BMT and in vitro expanded polyclonal recipient regulatory T cell (Treg) infusions.11 In our MCM model, as in humans, we found that CMV primary infection or reactivation is a significant complication after BMT. We therefore developed a qPCR assay to monitor CMV viremia in our animals.
Here, we report the results of different treatment approaches for the control of CMV infection in MCM, the effect of the use of CyA versus rapamycin on CMV reactivation timing and on the reconstitution of CD4+ and CD8+ T cell numbers and naïve/memory phenotypes in the context of CMV reactivation and control.
2. Materials and Methods
2.1. Animals
Adult Mauritian origin Cynomolgus macaques were used in this study (Charles River Primates, Wilmington, MA; Sanofi-Synthelabo, Bridgewater, NJ; Bioculture Group, Glenmoore, PA). Recipient and donor pairs were selected for ABO compatibility and mismatching of Cynomolgus leukocyte antigens.12 Cytomegalovirus serology was assessed prior to transplant (VRL Laboratories). All animals were negative for B virus, simian T-lymphotropic virus, simian retrovirus, simian immunodeficiency virus, simian varicella virus and malaria. All macaques were housed at the Institute of Comparative Medicine (Columbia University Medical Center, New York, NY). This facility holds a current USDA registration, PHS assurance and is AAALAC accredited. All surgical and experimental procedures were approved by the Columbia University Institutional Animal Care and Use Committee. All postoperative animals were continuously monitored by a team of veterinarians and pediatric surgeons, providing uninterrupted medical care day-and-night.
2.2. Development of Cynomolgus CMV quantitative polymerase chain reaction (qPCR) and CMV monitoring
A quantitative PCR assay was designed to detect a fragment of the DNA polymerase gene of Cynomolgus cytomegalovirus (CMV). Using Primer3 software, ten primer pairs were designed and tested in a gel-based assay. To generate template material for testing, we used the EasyMag extraction platform (Biomerieux, Marcy-l’Étoile, France) to extract DNA from serum and brain of a Cynomolgus macaque with clinical signs of systemic CMV reactivation. From the initial tests, we selected one optimal primer pair, consisting of primers CMV-Forward-GATGGGACCGCTCAAGTTTC, CMV-Reverse-TGACGGTAGCGAGGAGACAA, and CMV-Probe-(Fam) GGTCGATGGGGTTTTGACTCACGA (Tam). To generate a quantified standard for the assay, we cloned a PCR product containing the primer and probe binding sites into the PGem T-easy (Promega, Madison, WI) ligation and vector system. Plasmids were isolated and purified using the PureLink plasmid miniprep kit (ThermoFisher, Waltham, MA). The concentration of the standard was quantified, and then serially diluted. These serial dilutions were used to optimize the qPCR assay using TaqMan Universal PCR Master Mix (ThermoFisher) and to test the sensitivity of the primers and probe. The assay had an efficiency of 95% and a sensitivity of ≥5 copies/4μl of DNA.
For CMV testing, 200μl of serum were extracted on the EasyMag platform and eluted in 40μl of DNA using the Qiagen QIAamp DNA Mini Kit (Qiagen, Hilden, Germany). PCR reactions were run in triplicate using 4μl of DNA along with serially diluted standards and a no-template control on a ViiA7 Real-Time PCR machine (Applied Biosystems, Foster City, CA). Results were analyzed using QuantStudio Real-Time PCR software (Applied Biosystems). Results of the assay were multiplied by 50 to yield DNA copies/mL of serum.
2.3. Cell sorting and flow cytometric analysis
Whole blood was lysed and labeled with a combination of the following monoclonal antibodies: MHC I-PE-Cy7 (G46-2.6, BD Biosciences, San Jose, CA), CD3-PerCP-Cy5.5 (SP34-2, BD Biosciences), CD4-BV510 (L200, BD Horizon, Franklin Lakes, NJ), CD4-APC (L200, BD Pharmingen, Franklin Lakes, NJ), CD8-APC (RPA-T8, BD Biosciences), CD8-BV421 (RPA-T8, BD Biosciences), CD95-PE (DX2, BD Biosciences), CD28-Pacific Blue (CD28.2, BioLegend, San Diego, CA). Samples were acquired using FACSCanto II or LSRFortessa (BD Bioscience). Samples were analyzed using FCS Express (De Novo Software, Glendale, CA). CD4+ and CD8+ T cells were defined as CD20-CD3+CD4+ or CD20-CD3+CD8+ cells, respectively. NK cells were defined as CD20-CD3-CD56+CD8+ cells.13,14 Naïve T cells were defined as CD28+CD95-. Central and effector memory T cells were defined as CD28+CD95+ and CD28-CD95+, respectively.
2.4. Conditioning regimen
Recipients were conditioned with 2.5-3 Gy of total body irradiation (TBI) (Table 1) administered on days −6 and −5, 7 Gy of thymic irradiation (TI) on day −1, T-cell depletion with anti-horse anti-thymocyte globulin (ATG, Pfizer, New York, NY) on days −2, −1 and 0, co-stimulation blockade with anti-CD40L (NHP Reagent Resource, Boston, MA) (day 0, 2, 5, 7, 9 and 12) and a short course of immunosuppression post-BMT (either CyA (Novartis, Basel, Switzerland) or rapamycin (LC Laboratories, Woburn, MA)). BM was administered from a MHC-mismatched donor ± in vitro expanded autologous Tregs. CyA levels were maintained between 200-400 ng/mL and rapamycin levels between 20-30 ng/mL for 28 days and tapered to 0 thereafter.11 Animals treated with CyA were included in Group A if they received Tregs or Group B if they did not, and rapamycin-treated animals that received Tregs were included in Group C or Group D if they did not receive Tregs.
Table 1.
CMV reactivation time point and antiviral treatment
| Animal ID | Immunosuppressive Therapy | TBI (cGy) | Day of Reactivation | Day of CMV >1,000 copies/mL | Day of CMV >10,000 copies/mL | Day of Initiation of Antiviral Treatment | Antiviral Treatment | Cause of Euthanasia |
|---|---|---|---|---|---|---|---|---|
| 90-39 | CyA | 300 | 0 | 0 | 12 | 17 | GCV, VGC | CMV viremia |
| 90-15 | CyA | 300 | 19 | 19 | 23 | 23 | GCV, VGC, Foscarnet | Scientific Endpoint |
| 6C64 | CyA | 300 | 5 | 5 | 19 | 2 | Foscarnet, GCV, VGC | Scientific Endpoint |
| 6C1 | CyA | 300 | 7 | 12 | NA | 7 | GCV | Scientific Endpoint |
| 90-47 | CyA | 300 | NA | NA | NA | NA | NA | CMV viremia |
| 90-7 | CyA | 300 | 1 | 1 | 19 | 10 | GCV, VGC | Scientific Endpoint |
| 90-1 | CyA | 300 | NA | NA | NA | NA | NA | Scientific Endpoint |
| SA196A | Rapamycin | 300 | 8 | 8 | 29 | 30 | GCV | Scientific Endpoint |
| AM538C | Rapamycin | 250 | 26 | 26 | 34 | 34 | GCV | Scientific Endpoint |
| AK746F | Rapamycin | 250 | 26 | 26 | 28 | 29 | GCV | Scientific Endpoint |
| BF418F | Rapamycin | 250 | 6 | NA | NA | 13 | GCV, Foscarnet, Cidofovir | Scientific Endpoint |
| AG531J | Rapamycin | 250 | 16 | 20 | NA | 20 | GCV | Scientific Endpoint |
| BM12B | Rapamycin | 250 | 29 | NA | NA | NA | NA | Scientific Endpoint |
| AF201G | Rapamycin | 250 | 8 | 26 | NA | 19 | GCV | GVHD |
| V59M | Rapamycin | 250 | 10 | NA | NA | 25 | GCV | GVHD |
| AJ606D | Rapamycin | 250 | 29 | 33 | NA | 36 | GCV | Scientific Endpoint |
| AH260F | Rapamycin | 250 | 9 | 9 | 32 | 32 | GCV | Scientific Endpoint |
| AT468G | Rapamycin | 250 | 11 | NA | NA | 17 | GCV, Foscarnet | Renal failure/Infection |
| BY648F | Rapamycin | 250 | 10 | NA | NA | 16 | GCV | Scientific Endpoint |
| AP532B | Rapamycin | 250 | 30 | 49 | NA | 43 | GCV | GVHD |
| F813M | Rapamycin | 250 | 4 | 28 | 36 | 27 | GCV | GVHD |
“Day” represents the number of days after conditioning was completed at which the first CMV positive sample was detected (Day of Reactivation), CMV copies/mL threshold was reached (Day of CMV >1,000 copies/mL or Day of CMV >10,000 copies/mL), or the time point when the antiviral treatment was started (Day of initiation of antiviral treatment). 90-1 (CMV seronegative), 90-47 and BM12B did not receive antiviral treatment. NA, not applicable; CMV, cytomegalovirus; CyA, cyclosporine A; TBI, total body irradiation; GCV, ganciclovir; VGC, valganciclovir.
2.5. Major histocompatibility complex (MHC) genotyping
Comprehensive MHC genotypes were determined by the Genetic Services Unit of the Wisconsin National Primate Research Center at the University of Wisconsin-Madison (http://www.primate.wisc.edu/wprc/services/genetics.html). Genomic DNA isolated from whole blood samples served as templates for PCR with a panel of primers that flank the highly polymorphic peptide binding domains encoded by exon 2 of MHC class I (Mafa-A, -B, -I, -E) and class II (Mafa-DRB, -DQA, -DQB, -DPA and -DPB) loci. These PCR products were generated with Fluidigm Access Arrays that allow all reactions to be multiplexed in a single experiment. After cleanup and pooling, these products were sequenced on an Illumina MiSeq instrument and the resulting sequence reads mapped against a custom database of Mauritian Cynomolgus macaque class I and class II.15
2.6. Statistics
Data were analyzed applying two-tailed paired or unpaired Student’s t-Test using GraphPad Prism 7.05 (San Diego, CA). P values of ≤0.05 were considered statistically significant (*= p ≤ 0.05, **= p ≤ 0.01, ***= p ≤ 0.001, ****= p ≤ 0.0001, NS = Not significant).
3. Results:
3.1. Hematologic effects of the BMT preparative regimen and CMV reactivation postconditioning
Our MCM transplant tolerance induction protocol across MHC barriers included BMT ± infusion of in vitro expanded polyclonal recipient Tregs. In addition, prior to the transplant, the animals received a preparative regimen that included a T cell depleting agent (ATG), TBI and TI in addition to CyA (n=7) or rapamycin (n=14) for a minimum of 28 days (cf., Materials and Methods).11
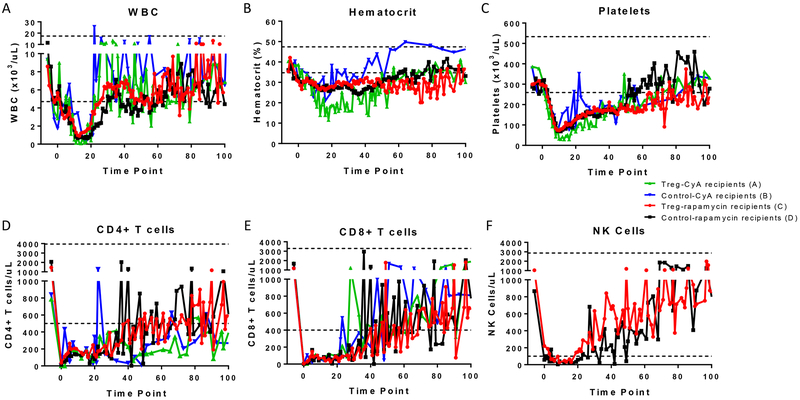
Effects of this preparative regimen included transient leukopenia, anemia, and thrombocytopenia. All hematopoietic cell lineages reached their nadir approximately two weeks post-TBI (Figure 1A, 1B, 1C). In addition, CD4+ T cell, CD8+ T cell and NK cell counts decreased significantly compared to the preconditioning levels (Figure 1D,1E, and 1F respectively) and the recovery of these populations was slower compared to the white blood cell count (WBC), hematocrit and platelets. We compared the effects of the regimen with posttransplant CyA vs rapamycin with and without expanded Tregs on hematologic recovery during the first month post-BMT (Groups A through D). All four groups responded with a similar pattern, with the exception of Treg-treated animals receiving CyA (Group A), where anemia and thrombocytopenia were more pronounced than the other groups (Figure 1A–1F and Table 2).
Figure 1. Hematological effects of the BMT preparative regimen.
Mean of (A) WBC, (B) hematocrit, (C) platelets, (D) CD4+ T cells, (E) CD8+ T cells, (F) and NK cells at different time points after BMT in recipients receiving a nonmyeloablative conditioning regimen. Each colored line represents a different group based on the immunosuppressive therapy (CyA or rapamycin) and the administration of Tregs: Group A [green], CyA and Tregs (n=4); Group B [blue], CyA (n=3); Group C [red], rapamycin and Tregs (n=9); Group D [black], rapamycin (n=5). NK cell data is only available for the rapamycin-treated recipients. Dotted lines represent normal ranges.44
Table 2.
T-test analysis between different groups depending on the administration of Tregs and rapamycin or CyA.
| Groups | WBC | Hematocrit | Platelets |
|---|---|---|---|
| Treg-CyA (A) vs Control-CyA (B) | ns | ** Control-CyA (B) |
** Control-CyA (B) |
| Treg-CyA (A) vs Treg-Rapamycin (C) | ns | *** Treg-Rapamycin (C) |
**** Treg-Rapamycin (C) |
| Treg-CyA (A) vs Control-Rapamycin (D) | * Treg-CyA (A) |
*** Control-Rapamycin (D) |
*** Control-Rapamycin (D) |
| Control-CyA (B) vs Treg-Rapamycin (C) | ns | * Control-CyA (B) |
* Control-CyA (B) |
| Control-CyA (B) vs Control-Rapamycin (D) | ns | * Control-CyA (B) |
* Control-CyA (B) |
| Treg-Rapamycin (C) vs Control-Rapamycin (D) | * Treg-Rapamycin (C) |
ns | ns |
Two-tailed paired Student’s t-Test analysis was performed between each group for the first 30 days of the study (*= p ≤ 0.05, **= p ≤ 0.01, ***= p ≤ 0.001, ****= p ≤ 0.0001, ns = not significant). Recipients were classified as “Group A” (received Tregs and CyA), “Group B” (CyA), “Group C” (Tregs and rapamycin), and “Group D” (rapamycin). WBC, white blood cell count. The group with higher counts is indicated in the case of a significant difference.
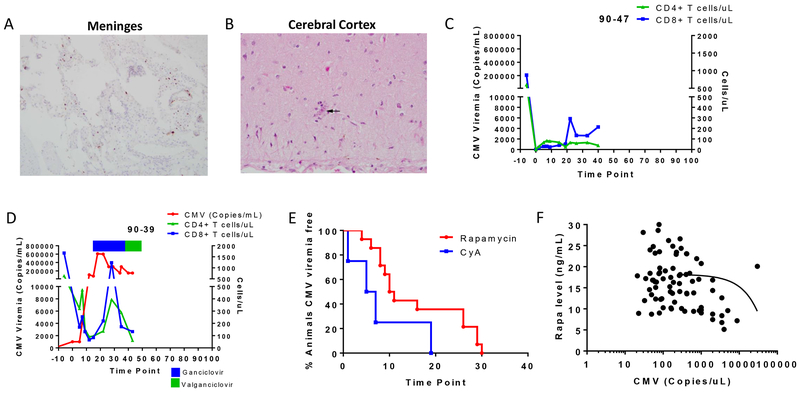
Twenty of 21 animals that underwent conditioning and BMT developed CMV viremia between day 0 and 19 in the case of CyA-treated recipients and between day 4 and 30 if they received rapamycin as posttransplant immunosuppression (Table 1). In some cases, when CMV viremia exceeded >10,000 copies/mL, clinical signs were observed (lethargy, loss of appetite, tremor). Two of the CyA recipients were euthanized due to uncontrollable CMV disease before the qPCR assay was developed and the coordination of antiviral treatment was perfected. Histology from the first animal, 90-47, showed acute meningitis featuring meningeal neutrophilic infiltrates as well as cytomegalic cells (Figure 2A) and the cerebral cortex showed scattered neurons with cytomegalic changes including nuclear and cytoplasmic inclusions, that were positive on CMV immunostaining (Figure 2B), which correlated with the clinical findings. This animal’s CD4 and CD8 counts remained under 400 cells/μL until sacrifice (Figure 2C). Due to these outcomes, our qPCR assay was developed to monitor MCM CMV in the subsequent animals (cf., Materials and Methods) and antiviral treatment was instituted in all animals that developed CMV viremia. The second animal that developed uncontrollable CMV disease (90-39) was seronegative before the transplant conditioning. After the recipient developed a primary infection from the donor, whose serologic testing revealed it converted to CMV+ just prior to donation, CMV copies increased rapidly, reaching >600,000 copies/mL (Figure 2D). The animal had clinical signs of CMV disease that included Bell’s palsy. Ganciclovir (GCV) treatment was started at 10 mg/kg BID IV on day 17, increased to 12.5 mg/kg BID on day 25 and switched later to valganciclovir (VGC) after a poor response to treatment, but the animal did not recover and was euthanized. The lack of successful CMV control was perhaps in part due to the low T cell counts from the conditioning regimen (Figure 2D), the lack of preexisting immunity against CMV and the timing of antiviral treatment.
Figure 2. Effects of CMV viremia and reactivation timing.
Histological findings in the (A) meninges and (B) cerebral cortex of a CMV+ recipient (90-47) that developed uncontrollable CMV disease. C) CD4+ T cell (green) and CD8+ T cell (blue) counts for 90-47. D) CMV level (red), CD4+ T cell (green), and CD8+ T cell (blue) counts of recipient 90-39. Antiviral treatment with ganciclovir (blue bar) and valganciclovir (green bar) is indicated at the top of the graph. E) CMV reactivation time point for recipients receiving rapamycin (red, n=14) or CyA (blue, n=5) as immunosuppressant. F) Correlation between the rapamycin level and the day of CMV reactivation posttransplant.
3.2. Rapamycin delayed the use of antivirals post-BMT compared to CyA.
Since the early CMV reactivation in the animals that received CyA post-BMT had a detrimental effect on bone marrow engraftment due to the toxic effect of CMV and antivirals on donor stem cells as we have previously shown,11 we next sought to evaluate whether an alternate maintenance immunosuppressant would affect the kinetics of CMV reactivation. We chose to replace CyA with rapamycin given that rapamycin has been reported to reduce the risk of CMV infection in organ transplant recipients16 while favoring the proliferation and survival of Tregs that are part of the conditioning regimen being tested in our tolerance induction protocol.17,18 We performed 14 BMTs with rapamycin including Treg-treated animals and controls (no Tregs infused) (Groups C and D). Animals receiving rapamycin had a trend toward delayed CMV reactivation detected through qPCR analysis compared to those receiving CyA (15.86±2.62 (n=14) vs 6.4±3.4 days (n=5) respectively, p=0.0687) (Figure 2E). Therefore, the use of rapamycin instead of CyA permitted a delay in the use of antiviral treatment while potentially providing a more favorable environment for Tregs.
3.3. Lower rapamycin levels correlated with CMV reactivation prior to antiviral treatment
Animals in this study received rapamycin doses targeted at a level of 20-30 ng/mL during the first 30 days, followed by a taper during the ensuing three weeks. In some cases, the rapamycin levels reached lower levels than desired (during the first month post-BMT) and we noted that CMV viremia started to increase when the rapamycin levels dropped to ~10 ng/mL if GCV had not previously been started (Figure 2F). These data support the antiviral activity of rapamycin in MCM and its use as an immunosuppressant post-BMT, permitting a delay in antiviral use.
3.4. T cell counts during CMV viremia and recovery
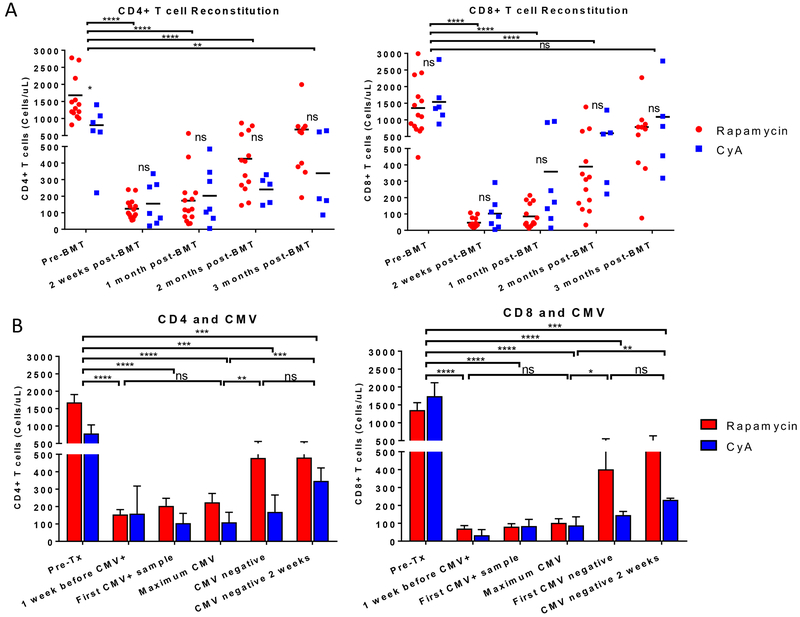
We studied the T cell recovery in blood at different time points (Figure 3A) and their correlation with CMV viremia (Figure 3B). We observed an expected and significant decrease in the T cell counts during the first month post-BMT compared to the pretransplant levels for both the CD4 and the CD8 populations (p<0.0001) due to T cell depleting therapy. The decrease in T cells was similar for the rapamycin and CyA recipients (Figure 3A). T cell counts started to recover during the second month post-BMT, although not yet reaching pre-BMT levels. Although CD8+ T cells reached lower absolute numbers/μL in blood than CD4+ T cells, CD8+ T cell recovery occurred earlier than CD4+ T cell recovery. By the third month post-BMT, CD8 counts were comparable to those pre-BMT. On average, recipients that received rapamycin and CyA recovered their T cell counts at a similar rate, with no significant difference between these two groups posttransplant (Figure 3A).
Figure 3. T cell counts during CMV viremia and recovery.
A) CD4+ and CD8+ T cell counts at different time points post-BMT in rapamycin-treated (red, n=14) and CyA-treated (blue, n=7) recipients. Two-tailed unpaired Student’s t-Test was run between the different time points including the rapamycin and CyA animals within each comparison. Two-tailed unpaired Student’s t-Test was run between the rapamycin and CyA recipients at each time point. B) Mean ± SEM of CD4+ and CD8+ T cell counts in rapamycin-treated (red, n=14) and CyA-treated (blue, n=5) recipients in correlation with the CMV reactivation and clearance time points. Two-tailed unpaired Student’s t-Test was run between the different time points including the rapamycin and CyA animals within each comparison. 90-1 (CMV seronegative) and 90-47 (CMV was not assessed) were excluded.
T cell number analysis was performed at different stages of CMV reactivation to determine if CMV influenced T cell proliferation. Both prior to and at the time of CMV reactivation, T cell levels were significantly lower compared to the pretransplant counts (Figure 3B and Table 1). By the time CMV became undetectable in serum, while animals were on GCV treatment, we observed an increase in the CD4 and CD8 counts. At that point, we kept the animals on GCV because T cell counts were still low. When CD8 counts surpassed 500 cells/μL the GCV treatment was switched to VGC in order to protect from reactivation and T cell numbers remained relatively stable for over two weeks (Figure 3B).
3.5. Expansion of naïve and memory T cells
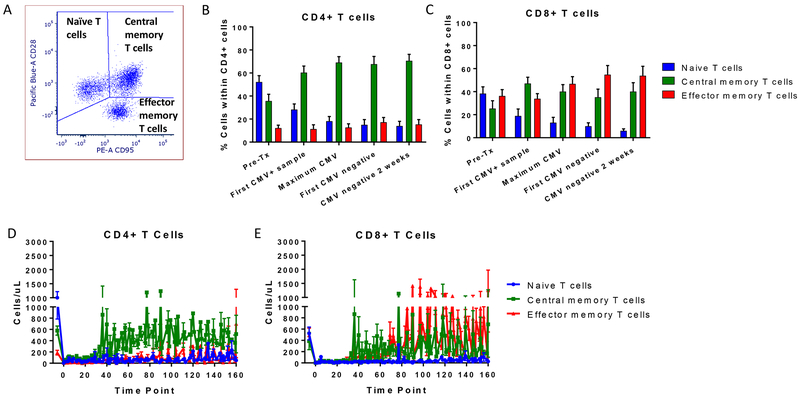
We further studied the T cell reconstitution during CMV reactivation in the rapamycin-treated recipients based on their naïve (CD28+CD95-), central memory (CD28+CD95+) and effector memory (CD28-CD95+) phenotype19 in CD8+ and CD4+ cells (Figure 4A). Prior to conditioning, T cells with naïve phenotype were the predominate subset among both CD4+ and CD8+ T cells. Following conditioning and transplantation, the naïve CD4+ and CD8+ T cell percentages were markedly reduced. In contrast to CD4+ T cells, which shifted toward a central memory phenotype, the percentage of both central and effector memory phenotypes increased among CD8+ cells (Figure 4B and 4C). The trends for absolute counts mirrored the trends for the percentage of naïve and memory cells for both CD4 and CD8 T cells (Figure 4D and 4E, respectively).
Figure 4. Evolution of naïve and memory T cells post-BMT.
A) Schema of the analysis of naïve and memory T cells based on the CD28 and CD95 marker. Mean ± SEM of the percentage of naïve (blue), central memory (green) and effector memory (red) cells within B) CD4+ and C) CD8+ T cells in correlation with the CMV reactivation and clearance time points in recipients receiving rapamycin (n=14). Mean ± SEM of naïve (blue), central memory (green) and effector memory (red) cell counts within D) CD4+ and E) CD8+ T cells over time in recipients receiving rapamycin (n=14).
3.6. CMV clearance and antiviral treatment
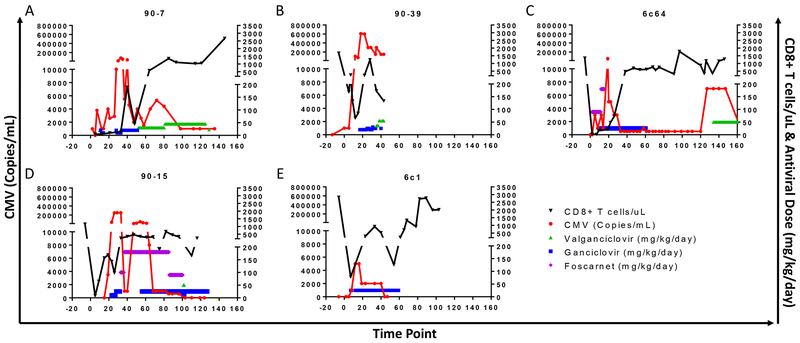
We studied different preemptive treatment approaches for MCM that received this tolerance induction regimen. In one recipient (90-7), GCV at a dose of 12.5 mg/kg BID controlled viremia after initiation at close to 200,000 copies/mL, but CMV reactivated late despite maintenance treatment with VGC that was started to allow oral drug administration (Figure 5A). Here we focused on the CD8+ T cell counts as have been shown to be fundamental for the control of CMV. Viremia decreased to 4,000 copies/mL after CD8+ T cells increased to >350 cells/μL while the animal was maintained on VGC. In a second animal (90-39), antiviral treatment with GCV (starting at 10 mg/kg up to 15 mg/kg BID) was initiated when the viral load exceeded 150,000 copies/mL but was unable to control viremia that eventually peaked at >600,000 copies/mL (Figure 5B). In addition, it has been our experience that foscarnet alone (90 mg/kg) did not prevent CMV reactivation (Figure 5C). Furthermore, in one animal (90-15) the combination of foscarnet at 90 mg/kg BID with GCV at low doses (5 mg/kg BID) intended to limit myelosuppression likewise failed to control CMV reactivation (Figure 5D). Overall, we found that CMV clearance was efficient with GCV at 12.5 mg/kg BID when treatment was instituted before CMV viremia rose above 10,000 copies/mL and maintained at this dose until viremia was undetectable for >2 weeks and CD8+ T cell numbers exceeded 500 copies/mL in the peripheral blood (e.g. Figure 5E).
Figure 5. Antiviral approaches in CyA-treated animals.
CD8+ T cell counts (black), CMV viremia (red), and antiviral treatment (valganciclovir [green], ganciclovir [blue], foscarnet [magenta]) in CyA-treated recipients (A through E, n=5). 90-1 (CMV seronegative) and 90-47 (CMV was not assessed) were excluded.
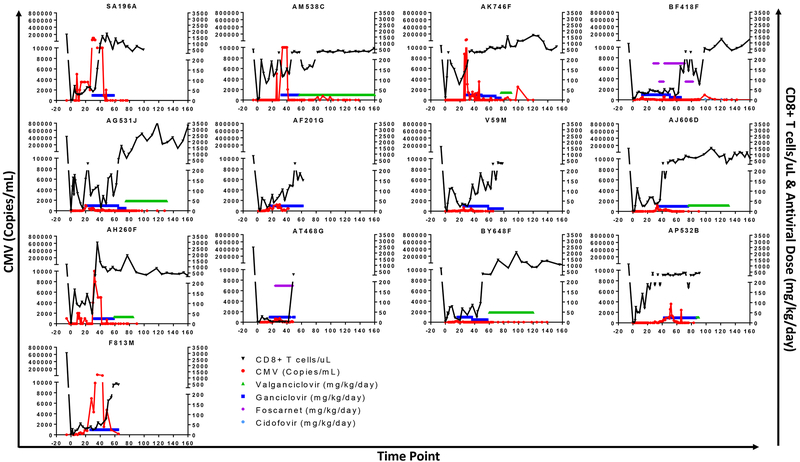
Based on this initial experience, when the rapamycin-treated animals reactivated CMV, GCV treatment was initiated at 12.5 mg/kg BID when viremia reached >1,000 copies/mL. After CMV in serum was tested negative for two consecutive weeks and the CD8+ cells recovered >500 cells/μl, GCV was switched to VGC. CMV was controlled successfully with this strategy, which frequently allowed us to delay antiviral treatment with myelosuppressive drugs during the first few weeks after BMT (Figure 6).
Figure 6. Antiviral treatment in rapamycin-treated animals.
CD8+ T cell counts (black), CMV viremia (red), and antiviral treatment (valganciclovir [green], ganciclovir [blue], foscarnet [magenta], cidofovir [light blue]) in rapamycin-treated recipients (n=13). Recipient BM12B did not receive antiviral treatment.
4. Discussion
Human CMV is a ubiquitous virus, highly prevalent among humans.1,2 CMV remains the most important opportunistic pathogen in immunocompromised individuals such as BMT recipients.20–23 The CMV status of the donor and recipient plays a major role in the development of viremia, with seronegative recipients receiving transplants from seropositive donors having the highest risk of disease. In addition, the intensity of recipient immune system manipulation has a major impact on the development of opportunistic infections. Despite the highly species-specific nature of each CMV strain, NHPs represent an excellent model for the study of CMV as the genomic organization and coding potential closely resembles human CMV, facilitating mechanistic studies of viral pathogenesis and its effect on the host immune response.24–26 The CMV viruses that are endemic in NHPs and humans are evolutionarily related.27 Studies have compared the structure and genetics of CMV virus in Cynomolgus macaques to those isolated from Rhesus macaques and humans. They demonstrated that the Cynomolgus CMV glycoprotein B amino acid sequence is 88% homologous to rhesus and 76% homologous to human glycoprotein B, respectively.24 Of the 262 open reading frames identified in Cynomolgus CMV, 137 are homologous to human CMV.28
Here, we present the outcomes of CMV infection in Cynomolgus macaque recipients that underwent a nonmyeloablative conditioning regimen, BMT with or without in vitro expanded polyclonal recipient Tregs and a short course of immunosuppression monotherapy with either CyA or rapamycin.11 In our study, all seropositive recipients reactivated CMV post-BMT irrespective of the immunosuppressive therapy. Our qPCR assay was able to detect and monitor CMV viremia, which allowed us to initiate treatment only once viremia was detected, thus avoiding the use of myelosuppressive antivirals during the period of stem cell engraftment.
We studied several approaches to control CMV in our MCM transplant tolerance induction protocol. High levels of CMV viremia were difficult to control and became lethal unless promptly treated. Foscarnet as prophylaxis did not effectively inhibit CMV viremia in our model. We found that GCV at 12.5 mg/kg BID was able to control CMV infection if instituted soon after viremia was detected and before the viremia reached 10,000 copies/mL. Lower GCV doses were not sufficient to clear the viremia. Long-term CMV control was maintained when VGC was given once the viremia cleared and CD8 counts were >500 cells/μL. Thus, we show here in our MCM model that CMV can be controlled by high doses of GCV followed by VGC after viremia clears and CD8 T cell counts recover.
Rapamycin is an immunosuppressant with reported antiviral properties that may be effective against CMV.16 We compared the serologic titers and response to treatment in animals that received CyA versus rapamycin. We found that animals receiving rapamycin had delayed CMV reactivation compared to CyA-treated animals. By delaying the CMV reactivation, rapamycin used with our preemptive treatment protocol delayed the use of myelosuppressive antiviral treatment, thus allowing the donor BM additional time to engraft without being affected by the toxic effect of the drugs, representing a promising approach in BMT recipients.29 The beneficial effects on CMV viremia that we observed after changing from CyA to rapamycin must be balanced with the knowledge of the different side effects and toxicities of each drug. While, CyA is associated with hypertension, diabetes, nephrotoxicity, neurotoxicity, liver toxicity, and diarrhea, rapamycin can cause impaired wound healing and angiogenesis, mouth ulcers, thrombocytopenia, peripheral edema, arthralgia, and interstitial pneumonitis. Thus, the appropriate immunosuppression for a particular patient must be chosen after considering how the drugs may affect their overall health status.
Our conditioning protocol, including ATG-based T-cell depletion, uniformly resulted in substantial CD4+ and CD8+ T cell reduction. It was during this lymphopenic period that CMV reactivation occurred. We found that T cell recovery, predominantly by CD8+ T cells, started at the peak of CMV viremia and progressed rapidly until the virus became undetectable in the serum. The expansion occurred mostly in central and effector memory T cells. There are several potential explanations for this pattern of T cell expansion. First, lymphopenia-driven expansion is known to drive the division of T cells that then assume a memory phenotype. However, CMV is also known to drive T cell recovery after T cell depletion. In a clinical study of kidney recipients that received rabbit ATG, when T cell recovery was compared between recipients based on their CMV serostatus, CD8+ T cells were shown to repopulate faster (1-2 months) in those patients that reactivated CMV compared to seronegative recipients (taking up to two years).30 The CD8+ T cells had an effector and memory phenotype, suggesting that memory T cell expansion was driven by CMV replication. CMV reactivation was also reported in a BMT study performed in Rhesus macaques that sought to induce mixed chimerism. Similar to our study, that group also showed that the repopulating CD8+ T cells shifted from a naïve to a central and effector phenotype during CMV infection.31 These findings could suggest that CMV infection promoted T cell expansion that in turn caused immune activation against CMV and potentially the graft due to heterologous immunity. Consistent with this possibility, we previously reported that the only Cynomolgus recipient that remained CMV negative in a prior study of BMT achieved a markedly increased level and of duration chimerism compared to the rest of the group.11 However, in order to rigorously study the effect of CMV on BM engraftment and rejection, we would require a large CMV seronegative control group. Unfortunately, CMV-seronegative MCM are extremely rare making a study of this nature infeasible.
Novel approaches are currently being studied for the prevention and control of CMV to avoid or decrease the requirements for conventional antiviral therapies. Adoptive transfer of donor CMV-specific T cells offers an alternative to restore CMV immunity that would reduce the need for the conventional antiviral treatments and their side effects.32–34 In addition, they provide an alternative in the face of CMV drug-resistance, which is currently a growing problem.35,36 One potential pitfall of this strategy is the possibility of heterologous immunity of anti-CMV T cells and precipitation of rejection by the use of cellular immunotherapy.37 Alternatively, CMV vaccines represent an approach being investigated for CMV prevention.38 Although there are currently no licensed CMV vaccines, there is an increasing interest in this approach and vaccines are under clinical development. CMV-specific CD8+ and CD4+ T cell recovery is associated with protection against CMV.39 Therefore, monitoring the CMV-specific T cell population might be a strategic approach to predict CMV reactivation in BMT recipients and improve the timing for the initiation of antiviral treatment.40,41 The use of tetrameric complexes of HLA molecules loaded with a CMV peptide has been investigated in human BMT recipients to monitor the recovery of CMV-specific CD8+ T cells posttransplant.6 Findings support the need for CMV-specific CD8+ T cells for CMV protection and suggest that graft-origin CMV-specific memory T cells contributed to CMV protection. The development of nonhuman primate tetramers and other assays for the study of these populations in MCM transplant models would facilitate the determination of whether or not CMV represents a barrier to the induction of transplantation tolerance.
Our treatment strategy with GCV starting at the time of viremia reaching 1,000 copies/mL with eventual transition to VGC was successful in controlling CMV in infected animals. This strategy is also adopted in the clinic in BMT recipients, where antiviral treatment is started after CMV detection. Unlike solid organ transplantation, where CMV prophylaxis is an option, the early use of antivirals is avoided in BMT recipients due to the myelosuppressive component of these drugs. We propose that for solid organ transplantation studies in macaques, prophylactic dosing with GCV be administered if T cell depletion is utilized. For BMT studies, we propose a rigorous monitoring protocol for CMV reactivation and initiation of GCV at 12.5 mg/kg BID once viremia exceeds 1,000 copies/mL. Other groups that study BMT in NHPs have used cidofovir prophylaxis during the peritransplant period. They found a significant incidence of CMV reactivation, consistent with our experience that it was unable to suppress viremia completely, and therefore frequent monitoring for viremia is still required.31 Recently, a new CMV prophylaxis approach that lacks the toxic effects of the currently available antiviral drugs, letermovir, has been developed.42,43 Letermovir inhibits CMV replication by binding to components of the terminase complex (UL51, UL56 or both). It is now clinically available and clinical trials showed that prophylaxis with letermovir resulted in a significant lower risk of clinically CMV infection compared to those that received placebo. Further studies in MCM are necessary to establish its potential in this model.
In conclusion, CMV infection remains a challenge for BMT recipients. Viremia and antiviral therapy not only interfere with BM engraftment, hindering successful outcomes, but also cause morbidity and mortality in immunocompromised recipients. Similar results were observed in our Cynomolgus macaque transplant tolerance induction protocol, where recipients developed CMV viremia that progressed to clinical CMV disease if not treated promptly. Additionally, the immune response to CMV may contribute to immune activation, thus preventing the development of chimerism and donor-specific tolearance.11 Newer antivirals without bone marrow toxicity are a promising approach to avoid CMV reactivation and its consequent adverse effects.
Acknowledgments
ATG used in this study was generously provided by Pfizer.
Funding
Funding for these studies was provided by NIH grant R01OD017949, by startup funds from Columbia University Departments of Medicine and Surgery (to MS and RDS), the Banting Foundation (to MS), the Columbia University core award (to RDS), and the Irving Pilot Translational science award for new investigators (to RDS). These studies used the resources of the Herbert Irving Comprehensive Cancer Center Flow Cytometry Shared Resources funded in part through Center Grant P30CA013696 and the Diabetes and Endocrinology Research Center Flow Core Facility funded in part through Center Grant 5P30DK063608.
Abbreviations:
- CMV
Cytomegalovirus
- MCM
Mauritius Cynomolgus macaque
- BMT
Bone marrow transplant
- MHC
Major histocompatibility complex
- NHP
Nonhuman primate
- CyA
Cyclosporine A
- GCV
Ganciclovir
- VGC
Valganciclovir
- GVHD
Graft-versus-host disease
- Treg
Regulatory T cell
- TBI
Total body irradiation
- TI
Thymic irradiation
- ATG
Anti-thymocyte globulin
- qPCR
Quantitative polymerase chain reaction
Footnotes
Disclosure of Conflicts of Interest
Authors do not report any conflict of interest pertaining to the work shown in this manuscript.
References:
- 1.Staras SA, Dollard SC, Radford KW, et al. Seroprevalence of cytomegalovirus infection in the United States, 1988-1994. Clin Infect Dis. 2006;43(9):1143–1151. [DOI] [PubMed] [Google Scholar]
- 2.Xu GJ, Kula T, Xu Q, et al. Viral immunology. Comprehensive serological profiling of human populations using a synthetic human virome. Science. 2015;348(6239):aaa0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azevedo LS, Pierrotti LC, Abdala E, et al. Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo). 2015;70(7):515–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li CR, Greenberg PD, Gilbert MJ, et al. Recovery of HLA-restricted cytomegalovirus (CMV)-specific T-cell responses after allogeneic bone marrow transplant: correlation with CMV disease and effect of ganciclovir prophylaxis. Blood. 1994;83(7):1971–1979. [PubMed] [Google Scholar]
- 5.Cwynarski K, Ainsworth J, Cobbold M, et al. Direct visualization of cytomegalovirus-specific T-cell reconstitution after allogeneic stem cell transplantation. Blood. 2001;97(5):1232–1240. [DOI] [PubMed] [Google Scholar]
- 6.Gratama JW, van Esser JW, Lamers CH, et al. Tetramer-based quantification of cytomegalovirus (CMV)-specific CD8+ T lymphocytes in T-cell-depleted stem cell grafts and after transplantation may identify patients at risk for progressive CMV infection. Blood. 2001;98(5):1358–1364. [DOI] [PubMed] [Google Scholar]
- 7.Singhal S, Shaw JC, Ainsworth J, et al. Direct visualization and quantitation of cytomegalovirus-specific CD8+ cytotoxic T-lymphocytes in liver transplant patients. Transplantation. 2000;69(11):2251–2259. [DOI] [PubMed] [Google Scholar]
- 8.Hassan-Walker AF, Vargas Cuero AL, Mattes FM, et al. CD8+ cytotoxic lymphocyte responses against cytomegalovirus after liver transplantation: correlation with time from transplant to receipt of tacrolimus. J Infect Dis. 2001;183(6):835–843. [DOI] [PubMed] [Google Scholar]
- 9.Jin X, Demoitie MA, Donahoe SM, et al. High frequency of cytomegalovirus-specific cytotoxic T-effector cells in HLA-A*0201-positive subjects during multiple viral coinfections. J Infect Dis. 2000;181(1):165–175. [DOI] [PubMed] [Google Scholar]
- 10.Amir AL, D’Orsogna LJ, Roelen DL, et al. Allo-HLA reactivity of virus-specific memory T cells is common. Blood. 2010;115(15):3146–3157. [DOI] [PubMed] [Google Scholar]
- 11.Duran-Struuck R, Sondermeijer HP, Bühler L, et al. Effect of Ex Vivo-Expanded Recipient Regulatory T Cells on Hematopoietic Chimerism and Kidney Allograft Tolerance Across MHC Barriers in Cynomolgus Macaques. Transplantation. 2017;101(2):274–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Budde ML, Wiseman RW, Karl JA, et al. Characterization of Mauritian cynomolgus macaque major histocompatibility complex class I haplotypes by high-resolution pyrosequencing. Immunogenetics. 2010;62(11–12):773–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vargas-Inchaustegui DA, Demberg T, Robert-Guroff M. A CD8alpha(−) subpopulation of macaque circulatory natural killer cells can mediate both antibody-dependent and antibody-independent cytotoxic activities. Immunology. 2011;134(3):326–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zitsman JS, Alonso-Guallart P, Ovanez C, et al. Distinctive Leukocyte Subpopulations According to Organ Type in Cynomolgus Macaques. Comp Med. 2016;66(4):308–323. [PMC free article] [PubMed] [Google Scholar]
- 15.Karl JA, Bohn PS, Wiseman RW, et al. Major histocompatibility complex class I haplotype diversity in Chinese rhesus macaques. G3 (Bethesda). 2013;3(7):1195–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Demopoulos L, Polinsky M, Steele G, et al. Reduced risk of cytomegalovirus infection in solid organ transplant recipients treated with sirolimus: a pooled analysis of clinical trials. Transplant Proc. 2008;40(5):1407–1410. [DOI] [PubMed] [Google Scholar]
- 17.Sugiyama H, Maeda Y, Nishimori H, et al. Mammalian target of rapamycin inhibitors permit regulatory T cell reconstitution and inhibit experimental chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2014;20(2):183–191. [DOI] [PubMed] [Google Scholar]
- 18.Zhao T, Yang C, Qiu Y, et al. Comparison of regulatory T cells and FoxP3-positive T-cell subsets in the peripheral blood of renal transplant recipients with sirolimus versus cyclosporine: a preliminary study. Transplant Proc. 2013;45(1):148–152. [DOI] [PubMed] [Google Scholar]
- 19.Nadazdin O, Boskovic S, Murakami T, et al. Phenotype, distribution and alloreactive properties of memory T cells from cynomolgus monkeys. Am J Transplant. 2010;10(6):1375–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyers JD, Flournoy N, Thomas ED. Nonbacterial pneumonia after allogeneic marrow transplantation: a review of ten years’ experience. Rev Infect Dis. 1982;4(6):1119–1132. [DOI] [PubMed] [Google Scholar]
- 21.Meyers JD, Flournoy N, Thomas ED. Risk factors for cytomegalovirus infection after human marrow transplantation. J Infect Dis. 1986;153(3):478–488. [DOI] [PubMed] [Google Scholar]
- 22.Ljungman P, Brand R, Hoek J, et al. Donor cytomegalovirus status influences the outcome of allogeneic stem cell transplant: a study by the European group for blood and marrow transplantation. Clin Infect Dis. 2014;59(4):473–481. [DOI] [PubMed] [Google Scholar]
- 23.Teira P, Battiwalla M, Ramanathan M, et al. Early cytomegalovirus reactivation remains associated with increased transplant-related mortality in the current era: a CIBMTR analysis. Blood. 2016;127(20):2427–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ambagala AP, Marsh A, Chan J, et al. Isolation and characterization of cynomolgus macaque (Macaca fascicularis) cytomegalovirus (CyCMV). Virology. 2011;412(1):125–135. [DOI] [PubMed] [Google Scholar]
- 25.Powers C, Früh K. Rhesus CMV: an emerging animal model for human CMV. Med Microbiol Immunol. 2008;197(2):109–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yue Y, Barry PA. Rhesus cytomegalovirus a nonhuman primate model for the study of human cytomegalovirus. Adv Virus Res. 2008;72:207–226. [DOI] [PubMed] [Google Scholar]
- 27.Russell JN, Marsh AK, Willer DO, et al. A novel strain of cynomolgus macaque cytomegalovirus: implications for host-virus co-evolution. BMC Genomics. 2016;17:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marsh AK, Willer DO, Ambagala APN, et al. Genomic Sequencing and Characterization of Cynomolgus Macaque Cytomegalovirus. J Virol. 2011;85(24):12995–13009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piñana JL, Perez-Pitarch A, Guglieri-Lopez B, et al. Sirolimus exposure and the occurrence of cytomegalovirus DNAemia after allogeneic hematopoietic stem cell transplantation. Am J Transplant. 2018;18(12):2885–2894. [DOI] [PubMed] [Google Scholar]
- 30.Havenith SH, Remmerswaal EB, Bemelman FJ, et al. Rapid T cell repopulation after rabbit anti-thymocyte globulin (rATG) treatment is driven mainly by cytomegalovirus. Clin Exp Immunol. 2012;169(3):292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zheng HB, Watkins B, Tkachev V, et al. The Knife’s Edge of Tolerance: Inducing Stable Multilineage Mixed Chimerism but With a Significant Risk of CMV Reactivation and Disease in Rhesus Macaques. Am J Transplant. 2017;17(3):657–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peggs KS, Thomson K, Samuel E, et al. Directly selected cytomegalovirus-reactive donor T cells confer rapid and safe systemic reconstitution of virus-specific immunity following stem cell transplantation. Clin Infect Dis. 2011;52(1):49–57. [DOI] [PubMed] [Google Scholar]
- 33.Blyth E, Clancy L, Simms R, et al. Donor-derived CMV-specific T cells reduce the requirement for CMV-directed pharmacotherapy after allogeneic stem cell transplantation. Blood. 2013;121(18):3745–3758. [DOI] [PubMed] [Google Scholar]
- 34.Walter EA, Greenberg PD, Gilbert MJ, et al. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med. 1995;333(16):1038–1044. [DOI] [PubMed] [Google Scholar]
- 35.Macesic N, Langsford D, Nicholls K, et al. Adoptive T cell immunotherapy for treatment of ganciclovir-resistant cytomegalovirus disease in a renal transplant recipient. Am J Transplant. 2015;15(3):827–832. [DOI] [PubMed] [Google Scholar]
- 36.Holmes-Liew CL, Holmes M, Beagley L, et al. Adoptive T-cell immunotherapy for ganciclovir-resistant CMV disease after lung transplantation. Clin Transl Immunology. 2015;4(3):e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Che JW, Daniels KA, Selin LK, Welsh RM. Heterologous Immunity and Persistent Murine Cytomegalovirus Infection. J Virol. 2017;91(2):e01386–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schleiss MR. Cytomegalovirus vaccines under clinical development. J Virus Erad. 2016;2(4):198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gerna G, Lilleri D, Chiesa A, et al. Virologic and immunologic monitoring of cytomegalovirus to guide preemptive therapy in solid-organ transplantation. Am J Transplant. 2011;11(11):2463–2471. [DOI] [PubMed] [Google Scholar]
- 40.Baldanti F, Lilleri D, Gerna G. Monitoring human cytomegalovirus infection in transplant recipients. J Clin Virol. 2008;41(3):237–241. [DOI] [PubMed] [Google Scholar]
- 41.Legendre C, Pascual M. Improving outcomes for solid-organ transplant recipients at risk from cytomegalovirus infection: late-onset disease and indirect consequences. Clin Infect Dis. 2008;46(5):732–740. [DOI] [PubMed] [Google Scholar]
- 42.Marty FM, Maertens J, Badshah C. Letermovir Prophylaxis for Cytomegalovirus. N Engl J Med. 2018;378(10):963–965. [DOI] [PubMed] [Google Scholar]
- 43.Marty FM, Ljungman P, Chemaly RF, et al. Letermovir Prophylaxis for Cytomegalovirus in Hematopoietic-Cell Transplantation. N Engl J Med. 2017;377(25):2433–2444. [DOI] [PubMed] [Google Scholar]
- 44.Caldwell RG, Marshall P, Fishel J. Method validation and reference range values for a peripheral blood immunophenotyping assay in non-human primates. J Immunotoxicol. 2016;13(1):64–76. [DOI] [PubMed] [Google Scholar]