Abstract
Background
Following protein replacement therapy, one-third of severe hemophilia A patients develop antibodies to factor VIII (FVIII), which also hinders the efficacy of gene therapy. Regulatory T cells (Tregs) have a naturally suppressive function to potentially reduce the immune response to FVIII therapy. Furthermore, antigen-specific Tregs are functionally much more potent than polyclonal cells. Adoptive transfer of antigen-specific Tregs can effectively suppress anti-FVIII antibody responses.
Objective
Develop a clinically-feasible protocol to enrich and expand Tregs specific to FVIII for suppressing anti-FVIII immune responses.
Methods
Tregs are isolated from FVIII-sensitized mice, sorted on CD25high markers, and expanded specifically with FVIII, antigen-presenting cells, and IL-2. Subsequently, Tregs are further cultured with anti-CD3/anti-CD28 beads, anti-Crry antibodies, and IL-2 to achieve 10–20 fold expansion. Expanded Tregs are characterized and tested for their suppressive activity in vitro and in vivo.
Results
In vitro FVIII-specific suppressive assays indicate that FVIII-specifically expanded Tregs are more suppressive than non-specifically expanded and naive Tregs. Adoptive transfer of expanded Tregs into HemA mice showed that FVIII-specifically expanded Tregs are significantly more potent in suppressing anti-FVIII immune responses in FVIII plasmid treated HemA mice. Moreover, the FVIII-specific immune tolerance is maintained after a secondary challenge with FVIII plasmid.
Conclusions
Our results demonstrate that the FVIII-specific sensitization and expansion protocol yields more potent Tregs to suppress anti-FVIII antibody responses and induce long term tolerance to FVIII, increasing the potential for adoptive Treg cell therapy to modulate anti-FVIII immune responses.
Keywords: Hemophilia, Regulatory T cells, Foxp3, Factor VIII, Crry
Introduction
Hemophilia A (HemA) is a disorder characterized by a deficiency of the blood coagulation factor VIII (FVIII). Patients are treated acutely or prophylactically by protein replacement therapy to restore coagulation function [1]. However, 25–30% of patients with severe hemophilia develop alloantibodies to FVIII due to the lack of immune tolerance for the protein [2]. These antibodies are referred to as “inhibitors” that neutralize the action of FVIII, causing treatment effectiveness to decline with increasing inhibitor titers.
Immune Tolerance Induction (ITI) is currently used to decrease the inhibitory response to FVIII. The variety of ITI dosing schedules all involve frequent high-dose infusions of FVIII until inhibitor levels drop and therapeutic clotting activity is achieved. This process can take several months to years and is costly and inconvenient. Additionally, successful ITI treatment is largely dependent on initial inhibitor levels and the amount of time since inhibitors first developed, with only ~70% of patients having a favorable outcome [3, 4]. The limited success of ITI underlies the importance of finding alternative treatments to generate immune tolerance to FVIII.
Immunomodulating techniques have been shown to facilitate antigen tolerance and reduce inhibitor titers [5–7] by expanding CD4+CD25+ regulatory T cells (Tregs), a population of immune cells that is crucial to establishing immune tolerance [8–13]. Recently, Tregs have been successfully induced and expanded in vivo, suppressing autoimmune [14] and alloimmune responses [15, 16]. Furthermore, both supplementation of donor grafts with freshly isolated splenic Tregs and in vivo expansion of Tregs can down-regulate graft-versus-host disease lethality to various degrees [17–22]. Additionally, antigen–specific Foxp3-transduced Tregs can control established type-1 diabetes in mice [9]. We have previously demonstrated that adoptive transfer of transgenic CD4+CD25+Foxp3+ Tregs isolated from FVIII-sensitized HemA/Foxp3 double transgenic mice significantly reduced FVIII inhibitor titers in FVIII plasmid-treated recipient HemA mice. We found that the transferred Tregs activated endogenous Tregs in the recipient mice via an infectious tolerance mechanism, leading to long-term tolerance and limited recall responses following a second challenge [8].
Recent advances in understanding how Tregs suppress various immune responses [23–26] provide new opportunities to develop novel strategies to eliminate antibodies and induce long-term immune tolerance to specific antigens. Natural CD4+CD25+Foxp3+ Tregs originate in the thymus during ontogeny as fully differentiated suppressor cells. However, Tregs can also be induced from naive T cells in the periphery [27–29]. Nevertheless, only very small numbers of Tregs can be obtained easily, and their proliferative potential is very low, and their function is mostly antigen nonspecific. These limitations have prompted efforts to identify novel methods to generate antigen-specific Tregs.
Thus, we sought to develop a clinically feasible adoptive Treg cell therapy to modulate FVIII-specific immune responses. The major challenge is difficulty in obtaining sufficient numbers of in vitro expanded Tregs due to the anergic nature of Tregs [30]. It was reported that Tregs can be expanded by co-stimulation of the complement receptor Crry, in addition to CD3/CD28 stimulation and high-dose IL-2 treatment [31]. The expanded cell population displayed suppressive activity in a proteoglycan-induced arthritis model. In this study, we show that the use of Crry stimulation, murine IL-2 (mIL-2), and cell-sorting techniques increased production of suppressive murine Tregs. Furthermore, in order to increase the suppressive effects of Tregs, we developed protocols to enrich and expand FVIII-specific Tregs in the polyclonal Treg population isolated from FVIII-sensitized HemA mice by in vitro stimulation with FVIII and APCs. These FVIII-specifically expanded Tregs show higher suppressive ability in vitro and demonstrate significantly higher efficacy in reducing the anti-FVIII antibody concentration following adoptive transfer into HemA mice.
Materials and Methods
Isolation and expansion of splenic Tregs
CD4+CD25+ Tregs were isolated from murine spleens using magnetic-activated and FACSAria cell sorting (see Supplemental Methods for detailed procedures). To non-specifically expand (NSE) naive Tregs (Table 1), a flat bottom 48-well plate was coated with 0.5mL of 10 μg/mL anti-Crry P3D2 IgG (BD Biosciences) at 37°C for 1-hour minimum. 5×105 CD4+CD25high cells were applied per well suspended in culture medium consisting of 0.5 mL RPMI Medium (Corning) with 2mM L-glutamine, 50μM 2-mercaptoethanol, 100 U/mL penicillin, 100 g/mL streptomycin, and 10% fetal calf serum. Cells were stimulated with 2,000 U/mL IL-2 (Peprotech, Cell Sciences) and anti-CD3/anti-CD28 beads (ThermoFisher Scientific) at a 2:1 ratio. After 3 days incubation, cells were washed and passaged to an anti-Crry coated plate at 1×105 cells per well and treated with IL-2 for an additional 4 days of culture, after which anti-CD3/anti-CD28 beads were removed. Fresh media and IL-2 was added every 2–4 days, as needed.
Table 1.
Definitions of abbreviations used in this article
| Abbreviation | Definition |
|---|---|
| Teff | Effector T-cell, CD4+CD25−Foxp3− |
| Treg | Regulatory T-Cell, CD4+CD25+Foxp3+ |
| Naive Treg | Treg from naïve mice that has not undergone expansion |
| Naive-NSE Treg | Naive Treg that has undergone non-specific expansion |
| F8S Treg | Treg from Factor VIII sensitized mouse that has not undergone expansion |
| F8S-NSE Treg | Treg from Factor VIII sensitized mouse that has undergone non-specific expansion |
| F8S-SE Treg | Treg from Factor VIII sensitized mouse that has undergone Factor VIII specific expansion |
To generate FVIII-sensitized (F8S) Tregs (Table 1), HemA mice were injected with FVIII protein before harvest of their spleens. Mice received intraperitoneal (IP) injections of 2 units FVIII (Bayer Healthcare) in 200 μL of PBS 3 times a week for 2 weeks. Four to six weeks after this sensitization period, non-specific expansion followed the same procedure as for naive NSE Tregs.
For FVIII specific expansion of FVIII-sensitized (F8S-SE) Tregs (Table 1), a flat bottom 48-well plate was coated with anti-Crry IgG as described above. 5×105 CD4+CD25high F8S Tregs were seeded in 0.5 mL culture medium and stimulated with 2,000 U/mL IL-2 and 10 U/mL FVIII and incubated with 1×106 irradiated CD4− splenocytes used as APCs for 3 days. Afterwards, cells were washed and passaged to an anti-Crry coated plate at 1×105 cells per well, stimulated with 2,000 U/mL IL-2 and anti-CD3/anti-CD28 beads at a 2:1 ratio. After 3 days of incubation, cells were passaged to a fresh anti-Crry coated plate at 1×105 cells per well and cultured for 4 days with 2,000 U/mL IL-2, after which anti-CD3/anti-CD28 beads were removed. Fresh media and IL-2 was added every 2–4 days, as needed.
In vitro analysis of Treg suppressive activity
The [3H]-thymidine incorporation suppressive assay was performed in a 96-well round bottom plate. Tregs were co-cultured in triplicate with freshly isolated splenic CD4+CD25− T effector cells (Teffs) (0.8×105 cells/200 μL/well) at ratios of 1:1 and 1:8 (Tregs:Teffs), suspended in 0.2 mL culture medium. Cells were stimulated by the addition of 1.5×105 cells/well CD4− splenocytes used as APCs (irradiated with 3500 rads) and 10U/mL of FVIII. After incubation for 72 hours at 37°C, tritium ([3H]) was added at 1 μCi/well and incubated for an additional 18 hours. Cells were harvested and [3H] activity was measured on a TopCount Scintillation Counter (PerkinElmer). Suppression was calculated as: % suppression = [1 - (c.p.m. (CD4+CD25− T cells with CD4+CD25+ cells)/c.p.m. CD4+CD25− T cells alone)] × 100%. Controls of Teffs without FVIII stimulation and APCs only were included and used to subtract background noise.
In vivo adoptive transfer
Mice were kept according to the guidelines for animal care of the National Institute of Health and Seattle Children’s Research Institute and were housed in a specific pathogen-free vivarium facility. For expansion and adoptive transfer experiments, 8–14 week-old C57BL/6 exon 16 deleted HemA mice were used [8]. CD45.1+ HemA (C57BL/6) mice were used as recipient mice in adoptive transfer experiments [8].
1×106 naive-NSE Tregs, F8S-NSE Tregs, or F8S-SE Tregs re-suspended in 200 μL PBS were injected via tail vein per mouse, while control mice received 200 μL PBS. All mice were injected with 100 μg of the liver specific FVIII plasmid (pBS-HCRHPI-FVIIIA) by hydrodynamic injection (0.1 mL/gram of mouse) [32] 1 day after adoptive transfer of cells. A second injection with 100 μg FVIII plasmid occurred 8 weeks after adoptive transfer of cells.
Anti-FVIII antibody analysis
Peripheral blood was collected from mice by retro-orbital bleeding. Plasma was separated via centrifugation, and hFVIII-specific antibody concentrations (total IgGs) were ascertained by enzyme linked immunosorbent assay (ELISA) and Bethesda assay. For ELISA, 96-well plates were coated with either goat anti-mouse IgG(H+L)-UNLB (Southern Biotech) for control wells or recombinant FVIII (Bayer) for experimental wells and incubated at 4°C overnight. Murine whole IgG (Invitrogen) was added to control wells as IgG standrards and plasma from experimental mice was added to experimental wells. Goat anti-mouse IgG F(ab’)2 HRP (Pierce) was added to all wells and results were analyzed using a VICTOR3 Plate Reader (PerkinElmer). ELISA is very sensitive to evaluate low titers of antibodies. FVIII Inhibitor titers were also measured by Bethesda assay [15, 33]. The inhibitor titers correlated well with the antibody titers measured by ELISA in samples with higher levels of antibody titers.
Flow cytometry analysis of expanded Tregs and adoptively transferred Tregs
Expanded cells were characterized by staining with anti-CD4 Alexa 700 (BD Pharmigen) and anti-CD25 PE-Cy5 (eBioscience) antibodies. Cells were then permeabilized using the Foxp3 Staining Buffer Set (eBioscience) and stained with anti-Foxp3 APC (eBioscience) and anti-CTLA-4 PE (eBioscience) antibodies. Stained cells were analyzed on an LSRII Flow Cytometer (BD Biosciences) and data compiled on FlowJo software (TreeStar).
Peripheral blood lymphocytes from experimental mice were stained for flow cytometry. After lysis of red blood cells with ACK buffer, cells were permeabilized, stained, and analyzed as described above. Detailed descriptions of analysis techniques are provided in Supplemental Methods.
Statistical Analysis
The statistical analysis methods are described in Supplemental Methods. Briefly, repeated measures ANOVA was used to determine statistical significance in experiments involving measurements over an extended time course, while two-tailed student’s T-test or ANOVA was used to evaluate significance of single time point experiments
Data Sharing Statement
For original data, please contact Carol Miao, PhD at carol.miao@seattlechildrens.org
Results
Development of efficient in vitro Treg expansion protocols
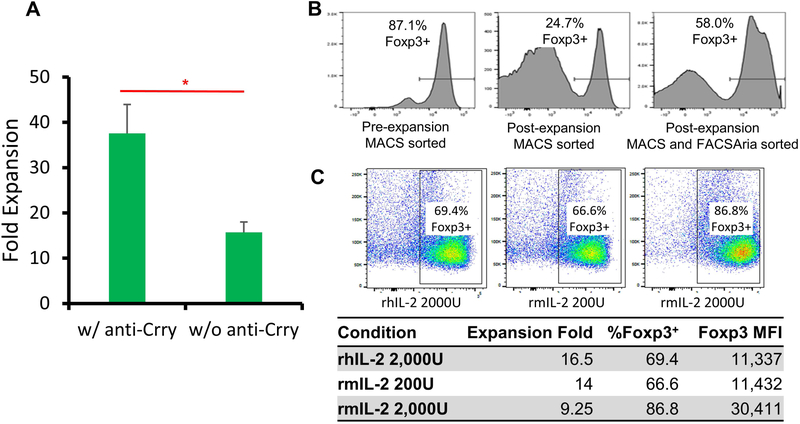
We previously demonstrated that adoptive transfer of FVIIII-sensitized transgenic CD4+Foxp3+ Tregs reduced FVIII antibody titers in FVIII plasmid-treated recipient HemA mice5. To develop a clinically feasible protocol of adoptive Treg cell therapy, we explored three aspects of expanding Tregs isolated from HemA mice. We first explored adding anti-Crry to selectively expand CD4+CD25+ Tregs. Magnetically-isolated Tregs were cultured with plate-bound anti-CD3 (20μg/mL) and high-dose IL-2 (1000U/mL) with or without the presence of plate bound anti-Crry IgG (20μg/mL) for 4 days, followed by culture in IL-2 only for 3 days. After 7 days, Treg expansion with anti-Crry demonstrated 38-fold expansion (Fig. 1A). Without anti-Crry, Tregs had markedly lower levels of proliferation. Based on these results, all subsequent Treg expansions were performed on anti-Crry coated plates.
Figure 1. Non-specific Treg expansion was improved with anti-Crry IgG, cell sorting, and high concentration of murine IL-2.
CD4+CD25+ Tregs were isolated from mouse spleens via magnetic bead separation. Isolated Tregs were cultured in 48 well plates with IL-2, anti-CD3/CD28 beads and plate bound anti-Crry IgG for a total of 7 days. (A) Fold expansion of Tregs expanded in the presence of plate-bound anti-Crry IgG compared to control (n=4). (B) Foxp3+ phenotype of Tregs sorted via FACSAria after magnetic separation compared to Tregs only sorted by magnetic separation. (C) Treg retention of the Foxp3+ phenotype when cultured with murine IL-2 at high concentration was compared to Tregs cultured with human IL-2 or low concentration of murine IL-2. Foxp3+ was gated based on control naive mice. Data is presented as averages from repeated experiments with error bars indicating standard deviation. *p < 0.05. The experiments have been repeated at least once without significant variance between experiments.
Following magnetic separation using autoMACS, 90% of the Treg population expressed Foxp3, the transcription factor that regulates suppressive function of Tregs (Fig. 1B). However, during the 7-day expansion protocol, the residual CD4+CD25+Foxp3− cells expanded robustly and only 27% of the cells maintained Foxp3+ expression, an overall 3.8-fold expansion (Fig. 1B). Thus, following AutoMACS isolation, CD4+CD25+ cells were further sorted for CD25high cells using FACSAria [34]. After 7 days of culture, the CD25high cells expanded by a total of 6.2-fold and 56% of cells maintained Foxp3+ expression (Fig. 1B), resulting in an overall 3.5-fold expansion. Sorting cells using both AutoMACS and FACSAria prior to culture leads to a similar proliferation rate of Foxp3+ cells compared to cells isolated only by MACS but results in a higher purity of CD4+CD25+Foxp3+ cells.
Next, we explored the effect of human IL-2 (hIL-2) versus mIL-2 on Treg expansion. Previous reports showed no difference in the use of hIL-2 compared to mIL-2 for Treg expansion [35]. However, the difference observed in activating T cell proliferation using these two agents in mice [36] led us to investigate the use of mIL-2 during in vitro expansions, as hIL-2 is only 70% homologous to mIL-2. We compared increasing concentrations of mIL-2 and hIL-2 (data using lower concentrations not shown) using our expansion methods described above. We found that mIL-2 significantly improves purity of the expanded cells, resulting in a significant increase in Foxp3+ expression and MFI. 2,000U/mL of hIL-2 resulted in 69% Treg purity after expansion, while 2,000U/mL of mIL-2 produced 83% Treg purity with slightly lower fold expansion (Fig. 1C). With these results, we incorporated the use of anti-Crry, cell sorting on CD25high, and mIL-2 to maximize the Treg purity in a 7-day expansion protocol.
F8S Tregs showed increased suppressive activity and potential to decrease anti-FVIII antibodies
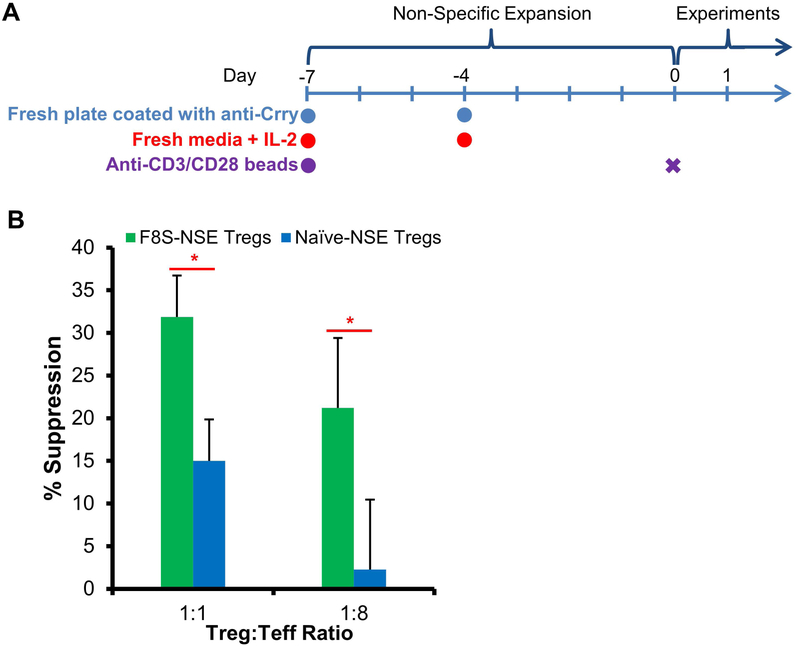
After optimizing our Treg expansion protocol, we then investigated if FVIII-specific Tregs are more potent regulators than nonspecific Tregs. We first generated FVIII sensitized mice by intraperitoneal (IP) injections of 2 units FVIII protein into HemA mice 3 times a week for 2 weeks. We hypothesized that the antigen-specific Tregs were induced in these FVIII sensitized mice. Tregs isolated and sorted from FVIII sensitized mice (F8S Tregs) and non-sensitized mice (naive Tregs) were non-specifically expanded (NSE) using anti-CD3/anti-CD28 beads, anti-Crry, and 2,000U/mL of mIL-2 for 7 days as outlined in Fig. 2A. These F8S-NSE Tregs and naive-NSE Tregs were co-cultured with Teffs in a FVIII specific [3H]-thymidine incorporation suppressive assay. F8S-NSE Treg suppression of Teff proliferation was significantly higher compared to that of naive-NSE Tregs at both 1:1 and 1:8 ratio of Tregs:Teffs (Fig. 2B).
Figure 2. FVIII sensitized Tregs showed higher suppressive function towards FVIII-specific immune responses than naive Tregs in vitro following non-specific expansion.
(A) Timeline of non-specific Treg expansion. HemA mice were first treated with repeated FVIII protein injections to induce generation of anti-FVIII inhibitors. At 4–6 weeks post injection, F8S Tregs were harvested from spleens of the HemA mice with inhibitors. Naive Tregs were harvested from untreated HemA mice. The isolation, purification and non-specific expansion of all Tregs follows the optimized protocol as described in Figure 1 legend. (B) The suppressive activity of F8S-NSE Tregs on F8S Teff proliferation was measured in vitro using the [3H]-Thymidine incorporation assay. F8S CD4+ Teffs were isolated from HemA mice with inhibitors and were cultured with Naive-NSE or F8S-NSE Tregs at 1:1 and 1:8 ratios (Treg:Teff) in the presence of 10U FVIII and APCs. F8S-NSE Treg suppression of Teff proliferation was compared to naive-NSE Treg suppression. Data is presented as averages from repeated experiments with error bars indicating standard deviation. *p < 0.05. The experiments have been repeated three times without significant variance between experiments.
Next, we sought to analyze in vivo suppressive activity (data not shown). Two different experimental groups, injected with either F8S-NSE or naive-NSE Tregs, were used in a preliminary adoptive transfer experiment. Following FVIII plasmid challenge, this adoptive transfer experiment showed a trend of reduced anti-FVIII antibody titers in both experimental groups treated with F8S-NSE Tregs or naive-NSE Tregs as compared to the control group (FVIII plasmid only). Additionally, mice receiving F8S-NSE Tregs had slightly lower anti-FVIII antibody titers after two FVIII plasmid injections compared to mice receiving naive-NSE Tregs.
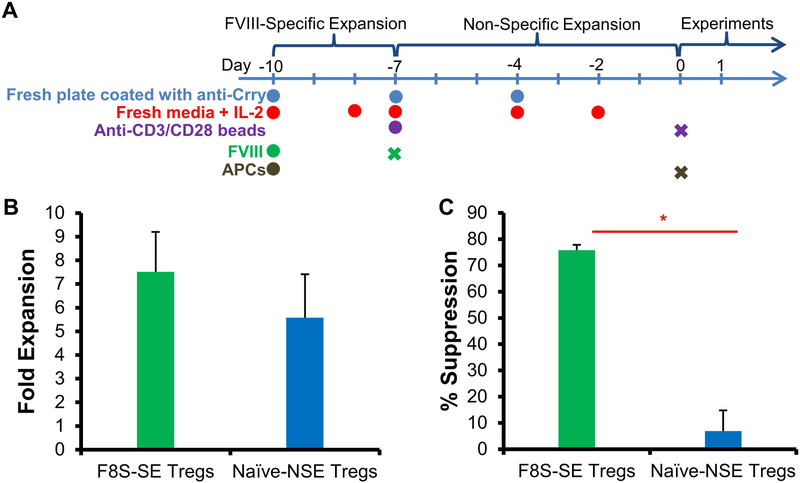
FVIII-specific expansion of Tregs greatly increased suppressive potential of F8S Tregs
To further improve in vivo suppressive activity of F8S Tregs, we developed a FVIII-specific expansion method to increase the antigen-specificity of Tregs prior to adoptive transfer (Fig. 3A). F8S Tregs isolated from FVIII protein sensitized HemA mice were incubated with FVIII protein (10U/mL), APCs (1×106 irradiated CD4− splenocytes), anti-Crry and mIL-2 for 3 days prior to nonspecific expansion by anti-CD3/anti-CD28 beads, anti-Crry, and mIL-2 for 7 days. To assess the suppressive activity of these FVIII-specifically expanded (F8S-SE) Tregs, they were co-cultured with freshly isolated splenic CD4+CD25− Teffs from FVIII sensitized mice at a ratio of 1:2 Tregs:Teffs, and [3H]-thymidine incorporation quantified the suppression of Teff proliferation. The addition of a 3-day FVIII-specific expansion resulted in a dramatic increase in the ability of Tregs to suppress Teff proliferation in vitro (Fig. 3C). F8S-SE Tregs demonstrated 75.8% suppression of Teffs, compared to 6.9% suppression with naive-NSE Tregs. In comparison, the suppressive activities of F8S-SE Tregs and Naïve-NSE Tregs were quite comparable towards CD4+ T cells in a non-specific suppressive assay (Supplemental Figure 1). Tregs expanded with FVIII in conjunction with irradiated APCs have increased specificity for FVIII-inhibiting Teffs. Additionally, there did not appear to be a significant difference in expansion fold between Tregs that underwent FVIII-specific expansion compared to nonspecific expansion (Fig. 3B).
Figure 3. Tregs produced by FVIII specific expansion protocol showed increased FVIII-specific suppressive activity.
F8S Tregs were selectively expanded using APCs and FVIII protein. The proliferation and suppressive effect of F8S-SE Tregs were compared to that of Naive-NSE Tregs. (A) Timeline of the F8S Treg expansion protocol. To increase the F8S Treg populations in the polyclonal Tregs, F8S Tregs were isolated from spleens of HemA mice with anti-FVIII IgG and first cultured for 3 days with FVIII protein and APCs. They were then transferred to a new plate and stimulated with anti-CD3/CD28 beads. Anti-Crry and 2000U/mL rmIL-2 were included in all cultures. (B) Fold expansion of Tregs undergoing FVIII-specific expansion and Tregs undergoing non-specific expansion. (C) F8S-SE Tregs or Naïve-NSE Tregs were cultured with Teff at a 1:2 ratio. Suppression of Teff proliferation was measured using the [3H]-Thymidine incorporation assay. Data is presented as averages from repeated experiments with error bars indicating standard deviation. *p < 0.05. The experiments have been repeated at least once without significant variance between experiments.
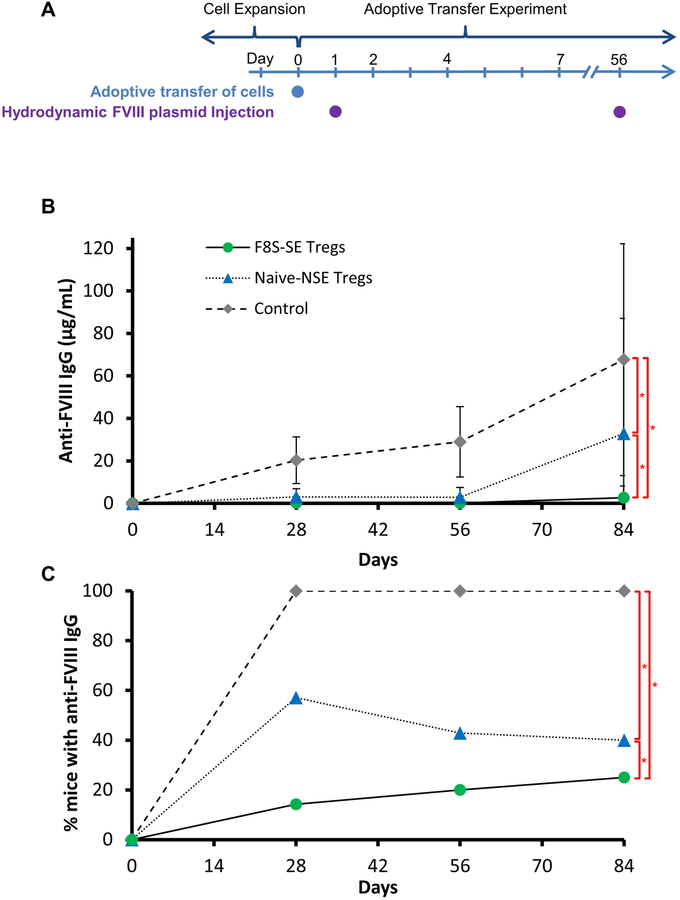
F8S-SE Tregs decreased anti-FVIII antibody concentration in HemA mice
After demonstrating in vitro effectiveness of F8S-SE Tregs in suppressing Teff proliferation, we investigated if F8S-SE Tregs could modulate the anti-FVIII immune response in vivo. 8–10 week old HA/BL6 CD45.1 recipient mice were injected via tail vein with either 1×106 naive-NSE Tregs or F8S-SE Tregs isolated from HA/BL6 CD45.2 donor mice. The following day, recipient and control mice were injected hydrodynamically with 100 μg of FVIII plasmid and then again at week 8 (Figure 4A). The immune response to FVIII was followed over time in all recipient and control mice. ELISA was used, rather than Bethesda assay, to measure total anti-FVIII antibodies due to the low titer inhibitor levels induced in C57/BL6 HemA mice. Control mice that did not receive adoptively transferred cells showed anti-FVIII antibodies starting at week 4 after the first FVIII plasmid injection. These antibody levels increased by week 8, and levels increased even further after the second FVIII plasmid injection performed in week 8. In contrast, F8S-SE Treg treated mice showed no anti-FVIII antibody presence, with the exception of 1 mouse with a very low concentration of antibodies developed after second FVIII plasmid injection (Figure 4B and Supplemental Figure 2). Naive-NSE Treg treated mice also had a reduction in anti-FVIII antibodies, with half showing none throughout the experiment and the rest with low levels through week 12. Only 1 naive-NSE Treg treated mouse developed very high levels of anti-FVIII antibodies following the second FVIII plasmid injection. When compared to controls at each time point, the generation of anti-FVIII antibodies in both F8S-SE Treg and naive-NSE treated mice was dramatically reduced with statistical significance (p<0.05) (Figure 4C and Supplemental Figure 2). Furthermore F8S-SE Treg treated mice produced significantly (p =0.0207) lower titers and a smaller percentage of these mice developed anti-FVIII antibodies, as compared to the naive-NSE Treg treated mice.
Figure 4. Adoptive transfer of expanded Tregs significantly decreased the occurrence of anti-FVIII antibodies in recipient mice.
F8S-SE and Naïve-NSE Tregs were harvested from CD 45.2+ mice and expanded as previously described in Figure 3, then adoptively transferred to HemA/CD45.1+ mice (n=7/group). (A) Following adoptive transfer of cells, recipient mice were subsequently injected with FVIII plasmid at Days 1 and 56. (B) Anti-FVIII antibody concentration in peripheral blood serum was measured via ELISA. Anti-FVIII IgG serum concentrations over 12 weeks is presented as averages from repeated experiments with error bars indicating standard deviation. Individual mouse data is included in supplementary figures (C) The percentage of mice in each group expressing anti-FVIII IgG are compared across groups. The experiments have been repeated at least once without significant variance between experiments. p-values over time were calculated using repeated measures ANOVA, *p < 0.05.
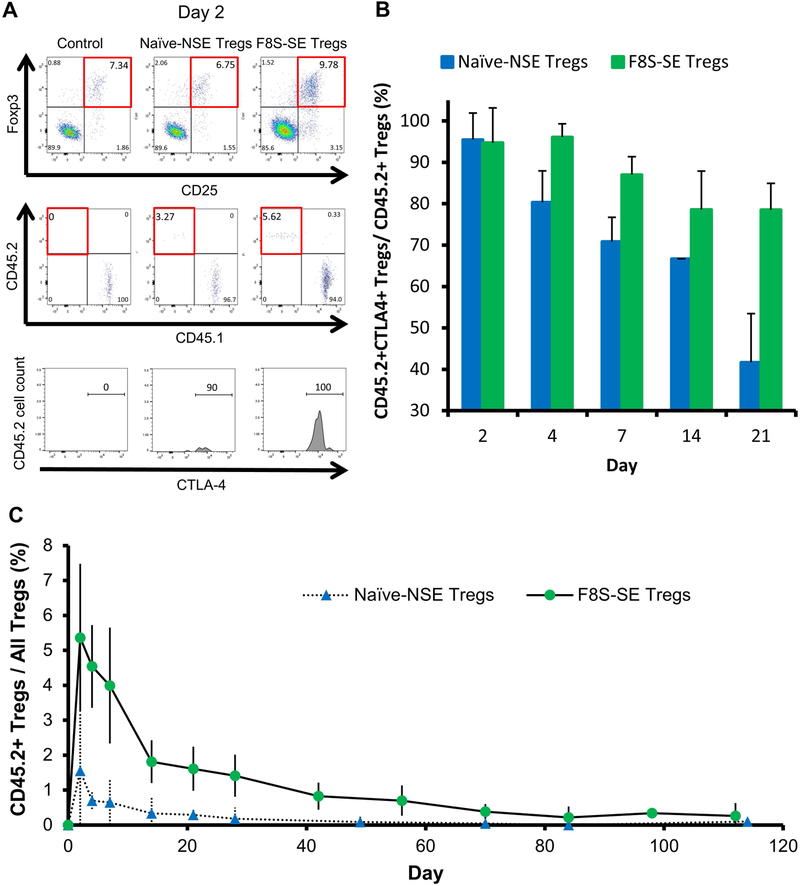
Flow cytometry using CD45.2 as a marker for exogenous donor Tregs showed a predominantly activated state via expression of CTLA-4 in both F8S-SE Tregs and naive-NSE Tregs cells on day 2 post adoptive transfer (Fig. 5A & B). The CTLA-4 activation dropped quickly over time in donor naive-NSE Tregs, but remained over 80% through day 21 in F8S-SE Tregs, probably due to in vivo FVIII stimulation of the F8S-SE Tregs. After day 21, the adoptively transferred cell populations decreased dramatically, making CTLA-4 tracking difficult. Furthermore, there was a higher engraftment percentage of CD45.2+ F8S-SE Tregs compared to naive-NSE Tregs (Fig. 5C). On day 2, the average engraftment for F8S-SE Tregs was 5.5%, compared to 1.75% in naive-NSE Tregs. This is thought to be a result of further in vivo expansion of F8S-SE Tregs stimulated by FVIII expression following hydrodynamic injection of FVIII plasmid.
Figure 5. Flow cytometry shows exogenous Tregs in adoptive transfer experiments have increased activation in F8S-SE Treg treated mice.
Following adoptive transfer of CD45.2+ F8S-SE and Naive-NSE Tregs into CD45.1+ HemA mice (as described in Figure 4), CD45.2+ donor cells were analyzed by flow cytometry. Naive CD45.1+ HemA mice were used as controls. (A) Representative dot plots of CD4+ lymphocytes from peripheral blood. Tregs were sorted via CD25 and Foxp3 expression (top row). CD4+CD25+Foxp3+ gated Tregs were then selected via the CD45.2 marker for exogenous Tregs (middle row). CTLA-4 expression was used to measure percent activation of selected CD45.2+ exogenous Tregs (bottom row). (B) CTLA-4 was measured to characterize the activation state of donor Tregs over the first 21 days of the experiment. Percentage of activated donor Tregs was compared between F8S-SE Treg and Naïve-NSE Treg groups (p=0.009). (C) Percentage of CD45.2+ Tregs over all Tregs was compared between F8S-SE Treg and Naïve-NSE Treg groups (p=0.00986). The experiments have been repeated at least once without significant variance between experiments. p-values over time were calculated using repeated measures ANOVA.
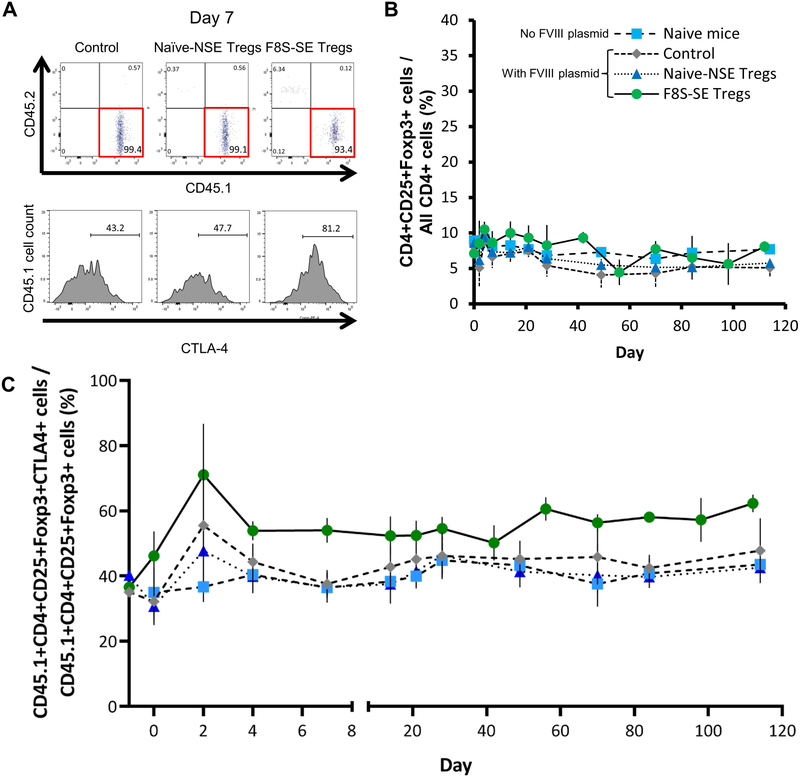
The effect of adoptive transfer therapy on the endogenous Treg population in recipient mice was also analyzed. Flow analysis showed the percentage of Tregs in total CD4+ cells was similar between all experimental groups (Fig. 6A & B). Using CTLA-4 as a marker for Treg activation and CD45.1 as a marker for endogenous Tregs, we saw a statistically significant (p<0.00001) increase of endogenous Treg activation in mice treated with F8S-SE Tregs compared to those treated with naive-NSE Tregs (Fig 6A, 6C). Increased CTLA-4 activation in F8S-SE Treg treated mice lasted for an extended period of time, while no difference in activation was observed between control mice and naive-NSE Treg treated mice. This leads us to believe that F8S-SE Tregs are more potent stimulators of endogenous Tregs than naive-NSE Tregs, thereby decreasing anti-FVIII antibody development.
Figure 6. Flow cytometry of Tregs in adoptive transfer experiments show an increased activation state of endogenous Tregs in F8S-SE Treg treated mice.
Endogenous CD45.1+ Tregs in HemA mice receiving CD45.2+ F8S-SE and Naive-NSE Tregs as described in Figure 4 were analyzed over time. Naïve CD45.1+ HemA mice were used as controls, (A) Representative dot plots of CD4+CD25+Foxp3+ lymphocytes from peripheral blood. Tregs were first gated via CD25 and Foxp3 expression as described in Figure 5. Endogenous CD4+CD25+Foxp3+ Tregs were gated on CD45.1 expression (top row). CTLA-4 expression was measured to compare activation states of CD45.1+ gated endogenous Tregs (bottom row). (B) The percentage of total Tregs relative to total CD4+ cells was compared across all groups. (C) Percentage of activated Tregs endogenous to the recipient mice (CD45.1+) was compared across all groups (p<0.00001). The experiments have been repeated at least once without significant variance between experiments. p-values over time were calculated using repeated measures ANOVA.
Discussion
The critical role of CD4+CD25+Foxp3+ Tregs in the regulation and suppression of autoimmune and alloimmune responses has been demonstrated [37–42]. T cell homeostasis is achieved through a balanced Treg:Teff ratio; therefore, one method of tolerance induction is inducing a balance shift. However, Tregs make up a very small subset of the lymphocyte population and are relatively anergic with low proliferative potential. Our results demonstrate that anti-Crry IgG increases in vitro expansion of Tregs. We also found that mIL-2 greatly enhanced selective expansion of the murine Treg population when compared with human IL-2. Furthermore, we found it imperative to use fluorescence assisted cell sorting in addition to magnetic bead sorting alone to generate purer CD4+CD25+Foxp3+ Tregs as the starting cell population for expansion. Without stringent sorting, CD4+CD25+Foxp3− cells tend to proliferate faster than CD4+CD25+Foxp3+ cells. Even though there were fewer Tregs available for expansion after cell sorting, the final expanded population had higher Treg purity, and thus greater suppressive activity. Our results are consistent with previous literature, where CD25high Tregs have been shown to increase transplantation tolerance in humanized mouse models [12]. With human applications, it may be easier to obtain higher purity of human Tregs with additional markers such as CD127. However, our results emphasize the importance of sorting Tregs with high purity before expansion protocols.
Recent clinical trials showed that adoptive Treg transfer therapy can modulate immune responses in several different diseases [10, 43–45](Treg trial , THRIL trial and TRACT Trial ). Most of these trials used ex vivo expanded polyclonal Tregs. However, it was clearly demonstrated in animal model studies that antigen-specific Tregs are far superior to polyclonal cells to achieve the therapeutic effect [46–49]. Recently, several groups have explored the potential to use T-cell receptors (TCRs) [50–52] or chimeric antigen receptors (CARs) [46, 53, 54] to generate engineered antigen-specific Tregs. However, engineering of T cells can be complicated, and the safety of using these cells still needs to be carefully investigated. Donor-specific expanded Tregs are currently being tested in clinical trials for liver transplantation patients (delta Trial , ARTEMIS Trial ). In this study, we explored a simple and efficient method of enriching FVIII-specific Tregs in a polyclonal population as a first step to establish a safe and translatable adoptive Treg transfer approach to modulate anti-FVIII immune responses.
We previously showed that Tregs isolated from FVIII-immunized HemA/Foxp3 inhibitor mice exerted FVIII-specific immune suppressive activity [8]. In this study, we further demonstrated that Tregs isolated from FVIII-immunized HemA inhibitor mice exerted FVIII-specific suppressive activity, indicating that FVIII-specific Tregs were induced upon antigen stimulation and could provide immunoregulatory effect if a suitable activation mechanism was provided for expansion. It is not surprising that Tregs can be sensitized upon antigen stimulation. For example, functionally active and virus-specific FOXP3+ Tregs were induced in HCV infection [55], and can provide targeted immune regulation in vivo. It was also reported that the induction of Tregs upon vaccination sometimes attenuates the immunity induced by the vaccine [56]. Interestingly, higher frequency of CD4+CD25high Treg cells were detected in some hemophilia patients with FVIII inhibitors [57], suggesting generation of potential FVIII-specific Tregs. We are currently investigating other subimmunogenic or tolerogenic conditions to potentially enhance the generation and isolation of FVIII-specific Tregs in FVIII sensitized HemA mice for in vitro expansion. Additionally, Treg surface activation markers [58] may be used to enhance the identification of antigen-specific Tregs in humans.
To increase the FVIII-specific suppressive activity of isolated Tregs, we tested protocols for sensitizing Tregs to FVIII and FVIII specific expansion of Tregs. While naive Tregs only showed ~5–10% in vitro suppression of Teff proliferation, FVIII sensitization increased this suppression to over 25%. By adding APCs with FVIII protein to the in vitro expansion protocol, we aimed to preferentially increase FVIII-specific Tregs. Following non-specific expansion of F8S Tregs, we sometimes lose some of the FVIII-specific Treg population, as reflected by the reduced suppression of the FVIII-specific responder T cell proliferation and the maintenance and reduction of suppression being variable after each expansion. Thus, we added a FVIII-specific expansion step. The suppressive activity of F8S-SE Tregs consistently and markedly increased to an average of 75% or higher after each expansion (n=4 experiments). These data suggest that FVIII sensitization combined with FVIII-specific expansion selects for and expands the Treg population that has receptors specific for FVIII, thereby increasing the therapeutic potential of the expanded cells to downregulate anti-FVIII immune response.
Next, the in vivo suppressive potential of F8S-SE Tregs and naive-NSE Tregs was tested in HemA mice receiving FVIII plasmid gene therapy via hydrodynamic injection. All control mice generated gradually increasing high-titer anti-FVIII antibodies, while mice receiving adoptively transferred Tregs showed either low or undetectable levels of anti-FVIII antibodies. Approximately half of the naive-NSE Treg treated mice showed undetectable anti-FVIII antibody levels, with only one mouse showing high levels by the end of the experiment. All F8S-SE Treg treated mice, except one with low titers, showed undetectable antibody levels, even after a second round of FVIII plasmid challenge. These results support the hypothesis that sensitized and specifically expanded Tregs with FVIII antigen can facilitate the induction of FVIII tolerance.
The route to tolerance is supported by several key findings. First, analysis of peripheral blood lymphocytes via flow cytometry showed that F8S-SE Tregs were present in larger percentages and more highly activated than naive-NSE Tregs. We hypothesize that this is due to the stimulation by FVIII expression to increase activation and proliferation of adoptively transferred F8S-SE Tregs in the recipient mice. Second, endogenous Tregs showed higher activation in F8S-SE Treg treated mice compared to naive-NSE Treg treated mice. We believe that endogenous FVIII-specific Tregs were induced and activated via infectious tolerance by the transferred F8S-SE Tregs in the presence of FVIII [8]. Taken together, these findings indicate that long-term tolerance may be established by induced endogenous FVIII-specific memory Tregs.
These experiments have important implications for the development of immunomodulation treatments for HemA patients. Currently, HemA patients who develop FVIII inhibitors undergo ITI protocols to reestablish coagulation potential, with an approximately 70% success rate [59]. Furthermore, it was recently reported [60] that high-dose ITI is a risk factor for the evolution from low- to high-titer inhibitors in cohorts of HemA patients and should be avoided as the initial strategy in patients who develop low-titer FVIII inhibitors. By isolating and selectively expanding FVIII sensitized Tregs, the patient’s production of FVIII inhibitors could be reduced or eliminated, forgoing the need for ITI. In addition, the induced tolerance to FVIII would ensure the success of gene therapy treatments currently in development [61, 62] and eliminate the patient’s need for life-long FVIII infusions. We believe that the future of hemophilia treatment will tend toward gene therapy approaches, and thus our mouse model using hydrodynamic delivery of FVIII plasmid, rather than IV injection of FVIII protein, is more relevant for further research in this field. Treg cell therapy has many attractive features compared to other immunomodulating treatments [52, 63, 64]. It is a personalized therapy targeting FVIII-specific immune responses, whereby a transient treatment will achieve the goal of long-lasting suppression of anti-FVIII immune responses in vivo. Compared to ITI protocols currently used for hemophilia patients, adoptive Treg cell therapy has the potential to be a significantly quicker, less costly, and safer method for modulating inhibitory antibody responses. In this study, we have developed a methodology to effectively expand FVIII-specific Tregs and demonstrated that adoptive transfer of expanded antigen-specific Tregs can modulate anti-FVIII antibody responses in HemA mice. Our results have shown that this approach can prevent the formation of anti-FVIII antibodies in mice, and we believe this strategy has potential to translate into clinically feasible protocols to prevent or reduce FVIII inhibitors in HemA patients.
Supplementary Material
Essentials:
Factor VIII (FVIII) inhibitory antibody formation occurs in treatment of hemophilia A patients
Regulatory T cells (Tregs) suppress the immune response of effector T cells.
Treg isolation, sensitization, and expansion were developed to enrich FVIII-specific Tregs.
FVIII-specific Tregs successfully suppressed anti-FVIII immune responses in vitro and in vivo.
Acknowledgements
This work was supported by a pilot project from NIH-NHLBI grant 5 U01 AI101990. We would also like to thank Dr. Chao Lien Liu for helpful initial discussions and suggestions.
Footnotes
Conflict of Interest Disclosures
The authors declare no conflict of interest.
References
- 1.Mannucci PM, Mancuso ME, Santagostino E. How we choose factor VIII to treat hemophilia. Blood. 2012;119:4108–14. [DOI] [PubMed] [Google Scholar]
- 2.Whelan SF, Hofbauer CJ, Horling FM, Allacher P, Wolfsegger MJ, Oldenburg J, et al. Distinct characteristics of antibody responses against factor VIII in healthy individuals and in different cohorts of hemophilia A patients. Blood. 2013;121:1039–48. [DOI] [PubMed] [Google Scholar]
- 3.Meeks SL, Chapman RL, Kempton C, Dunn AL. Late immune tolerance induction in haemophilia A patients. Haemophilia. 2013;19:445–8. [DOI] [PubMed] [Google Scholar]
- 4.Rivard GE, Rothschild C, Toll T, Achilles K. Immune tolerance induction in haemophilia A patients with inhibitors by treatment with recombinant factor VIII: a retrospective non-interventional study. Haemophilia. 2013;19:449–55. [DOI] [PubMed] [Google Scholar]
- 5.Matsui H, Shibata M, Brown B, Labelle A, Hegadorn C, Andrews C, et al. A murine model for induction of long-term immunologic tolerance to factor VIII does not require persistent detectable levels of plasma factor VIII and involves contributions from Foxp3+ T regulatory cells. Blood. 2009;114:677–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miao CH. Advances in Overcoming Immune Responses following Hemophilia Gene Therapy. J Genet Syndr Gene Ther. 2011;S1. [PMC free article] [PubMed] [Google Scholar]
- 7.Scott DW, Pratt KP, Miao CH. Progress toward inducing immunologic tolerance to factor VIII. Blood. 2013;121:4449–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miao CH, Harmeling BR, Ziegler SF, Yen BC, Torgerson T, Chen L, et al. CD4+FOXP3+ regulatory T cells confer long-term regulation of factor VIII-specific immune responses in plasmid-mediated gene therapy-treated hemophilia mice. Blood. 2009;114:4034–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jaeckel E, von Boehmer H, Manns MP. Antigen-specific FoxP3-transduced T-cells can control established type 1 diabetes. Diabetes. 2005;54:306–10. [DOI] [PubMed] [Google Scholar]
- 10.Marek-Trzonkowska N, Mysliwec M, Siebert J, Trzonkowski P. Clinical application of regulatory T cells in type 1 diabetes. Pediatr Diabetes. 2013;14:322–32. [DOI] [PubMed] [Google Scholar]
- 11.Nie H, Zheng Y, Li R, Guo TB, He D, Fang L, et al. Phosphorylation of FOXP3 controls regulatory T cell function and is inhibited by TNF-alpha in rheumatoid arthritis. Nat Med. 2013;19:322–8. [DOI] [PubMed] [Google Scholar]
- 12.Safinia N, Leech J, Hernandez-Fuentes M, Lechler R, Lombardi G. Promoting transplantation tolerance; adoptive regulatory T cell therapy. Clin Exp Immunol. 2013;172:158–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foks AC, Lichtman AH, Kuiper J. Treating atherosclerosis with regulatory T cells. Arterioscler Thromb Vasc Biol. 2015;35:280–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Churlaud G, Jimenez V, Ruberte J, Amadoudji Zin M, Fourcade G, Gottrand G, et al. Sustained stimulation and expansion of Tregs by IL2 control autoimmunity without impairing immune responses to infection, vaccination and cancer. Clin Immunol. 2014;151:114–26. [DOI] [PubMed] [Google Scholar]
- 15.Liu CL, Ye P, Lin J, Djukovic D, Miao CH. Long-term tolerance to factor VIII is achieved by administration of interleukin-2/interleukin-2 monoclonal antibody complexes and low dosages of factor VIII. J Thromb Haemost. 2014;12:921–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu CL, Ye P, Yen BC, Miao CH. In vivo expansion of regulatory T cells with IL-2/IL-2 mAb complexes prevents anti-factor VIII immune responses in hemophilia A mice treated with factor VIII plasmid-mediated gene therapy. Mol Ther. 2011;19:1511–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor PA, Panoskaltsis-Mortari A, Swedin JM, Lucas PJ, Gress RE, Levine BL, et al. L-Selectin(hi) but not the L-selectin(lo) CD4+25+ T-regulatory cells are potent inhibitors of GVHD and BM graft rejection. Blood. 2004;104:3804–12. [DOI] [PubMed] [Google Scholar]
- 18.Rifle G, Herve P. Regulatory (suppressor) T cells in peripheral allograft tolerance and graft-versus-host reaction. Transplantation. 2004;77:S5. [DOI] [PubMed] [Google Scholar]
- 19.Clark FJ, Gregg R, Piper K, Dunnion D, Freeman L, Griffiths M, et al. Chronic graft-versus-host disease is associated with increased numbers of peripheral blood CD4+CD25high regulatory T cells. Blood. 2004;103:2410–6. [DOI] [PubMed] [Google Scholar]
- 20.Singer BD, King LS, D’Alessio FR. Regulatory T cells as immunotherapy. Front Immunol. 2014;5:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matta BM, Reichenbach DK, Zhang X, Mathews L, Koehn BH, Dwyer GK, et al. Peri-alloHCT IL-33 administration expands recipient T-regulatory cells that protect mice against acute GVHD. Blood. 2016;128:427–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolf D, Barreras H, Bader CS, Copsel S, Lightbourn CO, Pfeiffer BJ, et al. Marked in Vivo Donor Regulatory T Cell Expansion via Interleukin-2 and TL1A-Ig Stimulation Ameliorates Graft-versus-Host Disease but Preserves Graft-versus-Leukemia in Recipients after Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2017;23:757–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kitagawa Y, Sakaguchi S. Molecular control of regulatory T cell development and function. Curr Opin Immunol. 2017;49:64–70. [DOI] [PubMed] [Google Scholar]
- 24.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–87. [DOI] [PubMed] [Google Scholar]
- 25.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol. 2008;9:239–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wood KJ, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nat Rev Immunol. 2003;3:199–210. [DOI] [PubMed] [Google Scholar]
- 27.Malek TR, Yu A, Vincek V, Scibelli P, Kong L. CD4 regulatory T cells prevent lethal autoimmunity in IL-2Rbeta-deficient mice. Implications for the nonredundant function of IL-2. Immunity. 2002;17:167–78. [DOI] [PubMed] [Google Scholar]
- 28.Yadav M, Stephan S, Bluestone JA. Peripherally induced tregs - role in immune homeostasis and autoimmunity. Front Immunol. 2013;4:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kanamori M, Nakatsukasa H, Okada M, Lu Q, Yoshimura A. Induced Regulatory T Cells: Their Development, Stability, and Applications. Trends Immunol. 2016;37:803–11. [DOI] [PubMed] [Google Scholar]
- 30.Chai JG, Coe D, Chen D, Simpson E, Dyson J, Scott D. In vitro expansion improves in vivo regulation by CD4+CD25+ regulatory T cells. J Immunol. 2008;180:858–69. [DOI] [PubMed] [Google Scholar]
- 31.Ojeda G, Pini E, Eguiluz C, Montes-Casado M, Broere F, van Eden W, et al. Complement regulatory protein Crry/p65 costimulation expands natural treg cells with enhanced suppressive properties in proteoglycan-induced arthritis. Arthritis Rheum. 2011;63:1562–72. [DOI] [PubMed] [Google Scholar]
- 32.Miao CH, Ye X, Thompson AR. High-level factor VIII gene expression in vivo achieved by nonviral liver-specific gene therapy vectors. Hum Gene Ther. 2003;14:1297–305. [DOI] [PubMed] [Google Scholar]
- 33.Kasper CK, Pool JG. Letter: Measurement of mild factor VIII inhibitors in Bethesda units. Thromb Diath Haemorrh. 1975;34:875–6. [PubMed] [Google Scholar]
- 34.Hoffmann P, Eder R, Kunz-Schughart LA, Andreesen R, Edinger M. Large-scale in vitro expansion of polyclonal human CD4(+)CD25high regulatory T cells. Blood. 2004;104:895–903. [DOI] [PubMed] [Google Scholar]
- 35.Sarkar D, Biswas M, Liao G, Seay HR, Perrin GQ, Markusic DM, et al. Ex Vivo Expanded Autologous Polyclonal Regulatory T Cells Suppress Inhibitor Formation in Hemophilia. Mol Ther Methods Clin Dev. 2014;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lyle MJ, Fu RY, Miao CH. Induction of FVIII tolerance by gene transfer of IL2 and factor VIII plasmids in hemophilia A mice The XXVI Congress of the International Society on Thrombosis and Haemostasis, Berlin, Germany, July 8–13, 2017 2017:PB 1091. [Google Scholar]
- 37.Edinger M, Hoffmann P, Ermann J, Drago K, Fathman CG, Strober S, et al. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med. 2003;9:1144–50. [DOI] [PubMed] [Google Scholar]
- 38.Hoffmann P, Ermann J, Edinger M, Fathman CG, Strober S. Donor-type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J Exp Med. 2002;196:389–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–40. [DOI] [PubMed] [Google Scholar]
- 41.Taylor PA, Lees CJ, Blazar BR. The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft-versus-host disease lethality. Blood. 2002;99:3493–9. [DOI] [PubMed] [Google Scholar]
- 42.Shevach EM. Biological functions of regulatory T cells. Adv Immunol. 2011;112:137–76. [DOI] [PubMed] [Google Scholar]
- 43.Bluestone JA, Buckner JH, Fitch M, Gitelman SE, Gupta S, Hellerstein MK, et al. Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci Transl Med. 2015;7:315ra189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trzonkowski P, Bieniaszewska M, Juscinska J, Dobyszuk A, Krzystyniak A, Marek N, et al. First-in-man clinical results of the treatment of patients with graft versus host disease with human ex vivo expanded CD4+CD25+CD127- T regulatory cells. Clin Immunol. 2009;133:22–6. [DOI] [PubMed] [Google Scholar]
- 45.Martelli MF, Di Ianni M, Ruggeri L, Falzetti F, Carotti A, Terenzi A, et al. HLA-haploidentical transplantation with regulatory and conventional T-cell adoptive immunotherapy prevents acute leukemia relapse. Blood. 2014;124:638–44. [DOI] [PubMed] [Google Scholar]
- 46.Dawson NAJ, Levings MK. Antigen-specific regulatory T cells: are police CARs the answer? Transl Res. 2017;187:53–8. [DOI] [PubMed] [Google Scholar]
- 47.Nishimura E, Sakihama T, Setoguchi R, Tanaka K, Sakaguchi S. Induction of antigen-specific immunologic tolerance by in vivo and in vitro antigen-specific expansion of naturally arising Foxp3+CD25+CD4+ regulatory T cells. Int Immunol. 2004;16:1189–201. [DOI] [PubMed] [Google Scholar]
- 48.Tarbell KV, Petit L, Zuo X, Toy P, Luo X, Mqadmi A, et al. Dendritic cell-expanded, islet-specific CD4+ CD25+ CD62L+ regulatory T cells restore normoglycemia in diabetic NOD mice. J Exp Med. 2007;204:191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsang JY, Tanriver Y, Jiang S, Xue SA, Ratnasothy K, Chen D, et al. Conferring indirect allospecificity on CD4+CD25+ Tregs by TCR gene transfer favors transplantation tolerance in mice. J Clin Invest. 2008;118:3619–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brusko TM, Koya RC, Zhu S, Lee MR, Putnam AL, McClymont SA, et al. Human antigen-specific regulatory T cells generated by T cell receptor gene transfer. PLoS One. 2010;5:e11726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim YC, Zhang AH, Su Y, Rieder SA, Rossi RJ, Ettinger RA, et al. Engineered antigen-specific human regulatory T cells: immunosuppression of FVIII-specific T- and B-cell responses. Blood. 2015;125:1107–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hoeppli RE, MacDonald KG, Levings MK, Cook L. How antigen specificity directs regulatory T-cell function: self, foreign and engineered specificity. HLA. 2016;88:3–13. [DOI] [PubMed] [Google Scholar]
- 53.MacDonald KG, Hoeppli RE, Huang Q, Gillies J, Luciani DS, Orban PC, et al. Alloantigen-specific regulatory T cells generated with a chimeric antigen receptor. J Clin Invest. 2016;126:1413–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yoon J, Schmidt A, Zhang AH, Konigs C, Kim YC, Scott DW. FVIII-specific human chimeric antigen receptor T-regulatory cells suppress T- and B-cell responses to FVIII. Blood. 2017;129:238–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ebinuma H, Nakamoto N, Li Y, Price DA, Gostick E, Levine BL, et al. Identification and in vitro expansion of functional antigen-specific CD25+ FoxP3+ regulatory T cells in hepatitis C virus infection. J Virol. 2008;82:5043–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin PH, Wong WI, Wang YL, Hsieh MP, Lu CW, Liang CY, et al. Vaccine-induced antigen-specific regulatory T cells attenuate the antiviral immunity against acute influenza virus infection. Mucosal Immunol. 2018;11:1239–53. [DOI] [PubMed] [Google Scholar]
- 57.Ding KY, Ji WC, Wu JS, Li T, Sheng YY. Higher frequency of CD4(+)CD25(high) Treg cells in hemophilia patients with factor VIII inhibitor. Genet Mol Res. 2014;13:1774–81. [DOI] [PubMed] [Google Scholar]
- 58.Bacher P, Heinrich F, Stervbo U, Nienen M, Vahldieck M, Iwert C, et al. Regulatory T Cell Specificity Directs Tolerance versus Allergy against Aeroantigens in Humans. Cell. 2016;167:1067–78 e16. [DOI] [PubMed] [Google Scholar]
- 59.Collins P, Baudo F, Knoebl P, Levesque H, Nemes L, Pellegrini F, et al. Immunosuppression for acquired hemophilia A: results from the European Acquired Haemophilia Registry (EACH2). Blood. 2012;120:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mancuso ME, Fischer K, Santagostino E, Oldenburg J, Platokouki H, Konigs C, et al. Risk Factors for the Progression from Low to High Titres in 260 Children with Severe Haemophilia A and Newly Developed Inhibitors. Thromb Haemost. 2017;117:2274–82. [DOI] [PubMed] [Google Scholar]
- 61.Pipe SW. Gene therapy for hemophilia. Pediatr Blood Cancer. 2018;65. [DOI] [PubMed] [Google Scholar]
- 62.Rangarajan S, Walsh L, Lester W, Perry D, Madan B, Laffan M, et al. AAV5-Factor VIII Gene Transfer in Severe Hemophilia A. N Engl J Med. 2017;377:2519–30. [DOI] [PubMed] [Google Scholar]
- 63.Canavan JB, Scotta C, Vossenkamper A, Goldberg R, Elder MJ, Shoval I, et al. Developing in vitro expanded CD45RA+ regulatory T cells as an adoptive cell therapy for Crohn’s disease. Gut. 2016;65:584–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chae WJ, Bothwell ALM. Therapeutic Potential of Gene-Modified Regulatory T Cells: From Bench to Bedside. Front Immunol. 2018;9:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.