Abstract
Background
Obesity is associated with decreased brain gray- (GM)/white-matter (WM) volumes in regions. Laparoscopic-sleeve-gastrectomy (LSG) is an effective bariatric surgery associated with neuroplastic changes in patients with obesity at 1-month-post-LSG.
Objective
To investigate whether LSG can induce sustained neuroplastic recovery of brain structural abnormalities, and whether structural changes are accompanied by functional alterations.
Setting
University hospital, longitudinal study.
Methods
Structural MRI/voxel-based morphometry-analysis were employed to assess GM/WM volumes in 30 obese participants at pre-LSG, 1-/3-month-post-LSG. One-way-ANOVA modeled time effects on GM/WM volumes, and then alterations in resting-state-functional-connectivity (RSFC) were assessed.
Results
Significant time effects on GM volumes were in caudate (F=11.20), insula (INS, F=10.11), posterior cingulate cortex (PCC, F=13.32) and inferior frontal gyrus (IFG, F=12.18), and on WM volumes in anterior cingulate cortex (ACC, F=15.70), PCC (F=15.56) and parahippocampus (PHIPP, F=17.96, PFDR<0.05). Post-hoc tests showed significantly increased GM volumes in caudate (mean change ± SEM 0.018±0.005 and P=0.001, 0.031±0.007 and P<0.001), INS (0.027±0.008 and P=0.003, 0.043±0.009 and P<0.001) and PCC (0.008±0.004 and P=0.042, 0.026±0.006 and P<0.001), and increased WM volumes in ACC (0.029±0.006 and P<0.001, 0.041±0.008 and P <0.001), PCC (0.017±0.004 and P<0.001, 0.032±0.006 and P<0.001) and PHIPP (0.031±0.008 and P=0.001, 0.075±0.013 and P<0.001) at 1-/3-month-post-LSG compared to pre-LSG. Significant increases in GM volumes were in caudate (0.013±0.006 and P=0.036), PCC (0.019±0.006 and P=0.006) and IFG (0.019±0.005 and P=0.001), and in WM volumes in ACC (0.012±0.005 and P=0.028), PCC (0.014±0.006 and P=0.017) and PHIPP (0.044±0.014 and P=0.003) at 3-month relative to 1-month-post-LSG. GM volumes in INS and PCC showed a positive correlation at 1-month (r=0.57, P=0.001) and 3-month-post-LSG (r=0.55, P=0.001). GM volume in INS and PCC were positively correlated with RSFC of INS-PCC (r=0.40 and P=0.03, r=0.55 and P=0.001) and PCC-INS (r=0.37 and P=0.046, r=0.57 and P<0.001) at 1-month-post-LSG. GM volume in INS was also positively correlated with RSFC of INS-PCC (r=0.44, P=0.014) and PCC-INS (r=0.38, P=0.037) at 3-month-post-LSG.
Conclusion
LSG induces sustained structural brain changes which might mediate long-term benefits of bariatric surgery in weight-reduction. Associations between regional GM volume and RSFC suggest that LSG-induced structural changes contribute to RSFC changes.
Keywords: obesity, laparoscopic sleeve gastrectomy, functional connectivity, insula, posterior cingulate cortex
Introduction
Obesity is a pervasive public health problem, that causes metabolic diseases, and negatively affects the brain.1 Obesity-related brain structural abnormalities have been reported extensively.2 Using voxel-based morphometry (VBM) analysis, a computational approach that measures neuroanatomic differences in local concentrations of brain tissue,3 our recent study in patients with obesity showed decreased gray- (GM) and white-matter (WM) densities and WM integrity in caudate, inferior frontal gyrus (IFG), insula (INS), hippocampus (HIPP), parahippocampus (PHIPP) and precuneus.4 These regions have all been implicated in processes related to obesity. For instance, the caudate is a region associated with the rewarding component underlying food-intake. The INS is involved in interoceptive sensation and emotional awareness.5 PCC/precuneus plays an important role in self-referential processing involving diverse functions linked with eating behaviors and obesity.6 Finally, PHIPP is critical for memory encoding and retrieval, and IFG/ACC are involved with executive-control. Tuulari et al. also reported that obese presented lower GM densities in cortical areas including the INS, and lower WM densities throughout the brain.7 Obesity-related brain structural changes may have preceded obesity8 and may be detrimental to brain functions including abnormal reward, food-intake control and cognitive-emotional regulation. On the other hand, it has been hypothesized that obesity directly causes deficits in WM/GM structure.9 Long-term caloric excess combined with low energy expenditure result in an energy imbalance that precipitates metabolic disease; in turn, higher levels of circulating free fatty acids may have lipotoxic effects on brain tissue. Still, our understanding of the extent of structural changes in obesity, and whether they recover following weight-loss interventions remains unclear.
Among multiple anti-obesity interventions, laparoscopic-sleeve-gastrectomy (LSG) is currently the primary weight-loss surgery demonstrating long-term efficacy in treating obesity.10 Neuroimaging studies revealed that LSG promoted acute neuroplastic structural recovery (increased GM/WM densities and WM integrity) in patients with obesity at 1-month post-LSG.4 Tuulari and colleagues reported similar findings, where bariatric surgery induced increased GM densities in several brain regions.7 Further, surface-based morphometry analysis, which is a brain morphometric technique used to construct and analyze surfaces that represent structural boundaries within the brain, revealed post-LSG increases in cortical thickness in the INS and anterior cingulate cortex (ACC).11 These suggest a causal link between weight-loss and brain tissue integrity, which might reflect improved brain cognitive performance along with weight-loss post-surgery.9
Obesity- and surgery-related changes to brain structure are often complemented by changes in brain function. Resting-state fMRI (RS-fMRI) studies have indicated abnormal communication between multiple brain regions in patients with obesity.12,13 Obese individuals showed decreased resting-state functional connectivity (RSFC) in regions involved in interoceptive processing (INS) and increased RSFC in regions involved in self-referential processing and cognitive-control (posterior cingulate cortex-PCC).12 Numerous RS-fMRI studies revealed LSG induced profound baseline changes in eating-related neural circuitry including the INS and PCC.13 Studies from our group using seed-based correlation analyses showed enhanced RSFC between the PCC/precuneus and right caudate/left dorsolateral prefrontal cortex (DLPFC) post-LSG and between the HIPP and INS.14,15 A partial reversal of PCC dysfunction and altered brain activity (INS) following body mass index (BMI) reduction have also been reported.14,16 Bariatric surgery also decreased RSFC within the default-mode network (DMN).12,13
Those aforementioned studies provided evidence that bariatric surgery (i.e., LSG) promoted alterations in both brain structures and function along with concomitant weight-loss.4,11,14,15 It remains unclear whether LSG-induced changes in RSFC are associated with structural alterations. In addition, to the best of our knowledge, no study has examined multiple time points post-LSG to investigate if LSG produced sustained changes in brain structures. Here, we employed structural and resting-state MRI to investigate changes in brain GM/WM volumes and RSFC in 30 patients with obesity pre-LSG and at 1-month and 3-month post-LSG. We hypothesized that LSG would induce increased GM/WM volumes in regions implicated in control of food-intake (caudate), interoception (INS), self-referential processing (PCC), executive-control (IFG, ACC) and memory (PHIPP). We also hypothesized that LSG-induced structural changes would be associated with changes in RSFC and with weight-loss.
Materials and Methods
Participants
Fifty-nine morbidly patients with obesity were recruited for LSG at Xijing Gastrointestinal Hospital affiliated with the Fourth Military Medical University in Xi’an, China. To prevent confounds from pre-existing brain lesions, we employed a retrospective questionnaire to exclud subjects who were likely to have had prior brain insults. Patients with psychiatric (including alcoholism) or neurological diseases, previous intestinal surgery/inflammatory intestinal disease/organ dysfunction or taking any current medication that could affect the brain/eating behaviors were excluded. Obese individuals who had a waist circumference (WC) greater than the interior diameter of the scanner were excluded.4,15 Given these criteria, thirteen candidates were disqualified for the magnetic resonance imaging (MRI) scan. Forty-six remaining obese candidates completed the MRI scans at pre-LSG and underwent LSG. The same MRI scans were performed at 1-month and 3-month post-LSG. Sixteen obese subjects who reported having significant weight-loss after surgery (BMI: PreLSG: 38.30±1.56, 1-month: 34.05±1.62) were unable to return for the follow-up MRI assessments due to long distance travel. Thus, thirty patients (age 27.63±1.50 yrs, range 18–47 yrs, 18 females) completed the 3 MRI evaluations (Table 1). The experimental protocol was approved by the Institutional-Review-Board of Xijing Hospital and registered in the Chinese-Clinical-Trial-Registry-Center as: ChiCTR-OOB-15006346 (http://www.chictr.org.cn). The experiments were conducted in accordance with the Declaration of Helsinki. All participants were informed of the nature of the research and provided written informed consent.
Table 1.
Demographic and clinical information of obese subjects.
| Pre-LSG (N=30) | 1-month (N=30) | 3-month (N=30) | ANOVA | Post-hoc tests | ||||
|---|---|---|---|---|---|---|---|---|
| (Mean ± SE) | (Mean ± SE) | (Mean ± SE) | F | P | a | b | c | |
| Weight (Kg) | 114.00 ± 2.98 | 101.54 ± 2.97 | 92.61 ± 2.98 | 181.113 | <0.001 | <0.001 | <0.001 | <0.001 |
| WC (cm) | 121.37 ± 2.31 | 113.85 ± 2.56 | 107.20 ± 2.61 | 65.257 | <0.001 | <0.001 | <0.001 | <0.001 |
| BMI (Kg/m2) | 39.15 ± 0.80 | 34.87 ± 0.82 | 31.79 ± 0.83 | 171.612 | <0.001 | <0.001 | <0.001 | <0.001 |
| YFAS | 5.03 ± 0.56 | 2.97 ± 0.41 | 2.30 ± 0.39 | 8.577 | 0.001 | 0.001 | <0.001 | 0.060 |
| HAMD | 11.97 ± 1.69 | 10.73 ± 1.31 | 8.43±1.20 | 2.887 | 0.072 | 0.422 | 0.034 | 0.069 |
| HAMA | 12.03 ±1.45 | 8.40± 1.10 | 6.60±1.23 | 8.851 | 0.001 | 0.002 | <0.001 | 0.051 |
Abbreviation:
WC, waist circumference; BMI, body mass index; YFAS, Yale Food Addiction Scale; HAMD, Hamilton Depression Rating Scale; HAMA, Hamilton Anxiety Rating Scale; Pre-LSG, obese subjects who received imaging-scanned at pre-LSG;
1-month, obese subjects who received imaging-scanned again at 1-month post-LSG;
3-month, obese subjects who received imaging-scanned again at 3-month post-LSG;
within-group comparison between obese subjects at pre-LSG and 1-month post-LSG (Pre-LSG vs 1-month);
within-group comparison between obese subjects at pre-LSG and 3-month post-LSG (Pre-LSG vs 3-month);
within-group comparison between obese subjects at 1-month and 3-month post-LSG (1-month vs 3-month).
Experimental design
In order to ensure that participants had similar levels of satiety and hunger, all participants underwent 12-h overnight fasting prior to scaning. MRI scans were performed between 9 to 10 A.M. to ensure consistency across assessments and to minimize circadian variability. Subjects completed the Yale-Food-Addiction-Scale (YFAS).17 A designated clinician rated the severity of subjects’ anxiety using the Hamilton-Anxiety-Rating-Scale (HAMA),4,15 and depression using the Hamilton-Depression-Rating-Scale (HAMD).4,15 All these clinical measurements were identically conducted at pre-LSG, 1-month and 3-month post-LSG, and were shown in Table 1. Surgical procedures were performed by the same surgeon on all patients. All clinical measurements were analyzed by IBM SPSS (Statistical Package for Social Sciences, Release 22.0, Chicago: SPSS, IL). One-way within-subject repeated measures ANOVA was employed to assess time effects on clinical measures in patients with obesity at pre-LSG, 1-month and 3-month post-LSG. Paired t-tests were utilized as post-hoc tests where ANOVA indicated significant time effects.
MRI Acquisition
MRI scan was carried out using a 3.0 T GE Signa Excite HD MRI scanner (GE, Milwaukee, WI, USA). First, high-resolution structural images were acquired using T1*-weighted three-dimensional magnetization-prepared rapid acquisition gradient-echo sequences (please see Supplementary Information-SI for detailed parameters settings). Then, a gradient-echo T2*-weighted echo planar imaging sequence was used resting-state (RS) functional images (SI).
Voxel-based morphometry of gray and white matter volume
T1 structural images were analyzed with Matlab 2012a (MathWorks Inc., Natick, MA, USA) using Statistical Parametric Mapping (SPM12, https://www.fil.ion.ucl.ac.uk/spm/) and VBM toolbox (http://dbm.neuro.uni-jena.de/vbm/download/). In the current longitudinal study, preprocessing steps including realignment, bias-correction, tissue classification and spatial normalization were performed for imaging data of the three time points simultaneously (SI).18
Statistical parametric mapping (Region of interest-ROI identification)
SPM12 was employed to perform the voxel-wise analysis on GM/WM brain mappings. One-way ANOVA contrast test (pre- and two post-measurements) was also implemented to assess time effects on GM/WM volumes across the whole brain. Significant GM volumes were identified as ROIs after whole brain false discovery rate (FDR) corrected P<0.05 at the voxel level with a minimum cluster size of 30 (SI).
RSFC analysis
Resting-state images were preprocessed using SPM12 and Data Processing Assistant for Resting-State fMRI (DPARSF, https://sourceforge.net/projects/restingfmri/). Specifically, the first 5 time points were removed to minimize non-equilibrium effects in the fMRI signal, and then slice-timing, realignment-based head movement correction, and spatial normalization (voxel size of 3×3×3 mm3) were performed (SI). After obtaining GM ROIs with significant time effects, a seed region-based (center at the coordinates of the peak value with a 6-mm radius) RSFC analysis was carried out (SI).
Association between behaviors and GM/RSFC
A partial correlation analysis was performed to assess the association between clinical measurements (BMI, YFAS, HAMA, HAMD) and the GM/RSFC ROIs with significant time effects. Bonferroni-correction was applied for multiple-comparisons, and level of significance was set at P<0.003 (0.05/16). In addition, a Pearson’s correlation analysis was carried out between the GM and RSFC to investigate the relationship between structure and RSFC.
Results
Demographic characteristics
There were significant time effects on weight (F(2, 58)=181.113, P<0.001), WC (F(2, 58)=65.257, P<0.001) and BMI (F(2, 58)=171.612, P<0.001) due to significant weight-loss, reduction in WC and BMI in patients with obesity at both 1-month and 3-month post-LSG (P<0.001, Table 1). There were significant time effects on YFAS (F(2, 58)=8.577, P=0.001) and HAMA (F(2, 58)=8.851, P=0.001) due to significant reduction in patients with obesity at 1-month (P=0.001, P=0.002) and 3-month post-LSG (P<0.001) compared to pre-LSG, but not between 1-month and 3-month post-LSG. There were no significant time effects on HAMD, but exploratory post-hoc revealed reductions in HAMD scores at 3-month compared to pre-LSG (P<0.034, Table 1).
Alterations in GM/WM volume
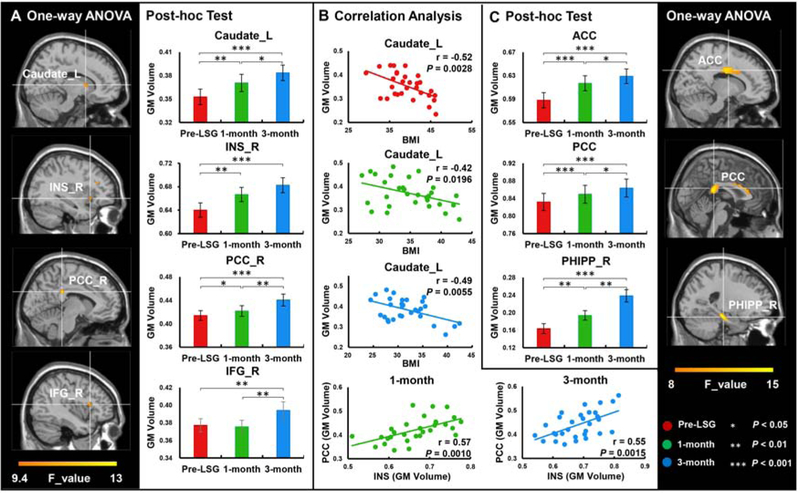
One-way ANOVA showed significant time effects on GM volumes in caudate, INS, PCC and IFG (Fig 1A, Table 2), and on WM volumes in ACC, PCC and PHIPP (PFDR<0.05, Fig 1C, Table 2). Post-hoc tests showed significant increases in GM volumes in caudate and PCC at both 1-month and 3-month post-LSG as compared to pre-LSG, and between 1-month and 3-month post-LSG. There were significant increases in GM volumes in the INS at both 1-month and 3-month post-LSG as compared to pre-LSG, but there was no significant difference between 1-month and 3-month post-LSG. In the IFG the 3-month measure showed significant increases compared to pre-LSG and 1-month post-LSG (Fig 1A). For WM volumes, post-hoc tests showed significant increases over time in ACC, PCC and PHIPP at both 1-month and 3-month post-LSG as compared to pre-LSG, and between 1-month and 3-month post-LSG (Fig 1C).
Figure 1.
One-way ANOVA analysis for GM/WM volumes at pre-LSG and 1-month/3-month post-LSG in patients with obesity (voxel size-corrected, PFDR<0.05). A. There were significant time effects on GM volumes in the caudate, INS, PCC and IFG. LSG significantly increased GM volumes in the caudate, INS and PCC at both 1-month and 3-month post-LSG compared to pre-LSG. LSG significantly increased GM volumes in the caudate, PCC and IFG at 3-month compared to 1-month post-LSG. B. Correlation analysis between BMI and GM volume in the caudate in patients with obesity at pre- and 1-month/3-month post-LSG, and GM volumes in the INS and PCC in patients with obesity at 1-month/3-month post-LSG. C. There were significant time effects on WM volumes in the ACC, PCC and PHIPP. LSG significantly increased WM volumes in the ACC, PCC and PHIPP at both 1-month and 3-month post-LSG compared to pre-LSG and at 3-month compared to 1-month post-LSG. Error bars indicate the standard error.
Abbreviation: INS_R, Right Insula; PCC_R, Right Posterior cingulate cortex; IFG_R, Right Inferior frontal gyrus; ACC, Anterior cingulate cortex; PHIPP_R, Right Parahippocampal gyrus; Pre-LSG, obese subjects who received imaging-scanned at pre-LSG; 1-month, obese subjects who received imaging-scanned again at 1-month post-LSG; 3-month, obese subjects who received imaging-scanned again at 3-month post-LSG.
Table 2.
One-way repeated measures ANOVA analysis on GM/WM volumes in obese patients at pre-LSG and 1-month/3-month post-LSG. (voxel size-corrected, PFDR<0.05).
| Region | Cluster size | MNI | Peak F-value | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Gray matter | |||||
| Caudate_L | 59 | −12 | 6 | 1 | 11.20 |
| INS_R | 35 | 35 | 17 | −8 | 10.11 |
| PCC_R | 71 | 6 | −35 | 31 | 13.32 |
| IFG_R | 63 | 45 | 14 | 13 | 12.18 |
| White matter | |||||
| ACC | 3950 | −15 | −10 | 30 | 15.70 |
| PCC | 623 | 0 | −37 | 15 | 15.56 |
| PHIPP_R | 260 | 23 | −27 | −21 | 17.96 |
Abbreviation: GM, Gray matter; WM, White matter; MNI, Montreal Neurological Institute; INS_R, Right Insula; PCC_R, Right Posterior cingulate cortex; IFG_R, Right Inferior frontal gyrus, opercular part; ACC, Anterior cingulate cortex; PHIPP_R, Right Parahippocampal gyrus.
BMI was negatively correlated with GM volume in caudate at pre-LSG (r(28)=−0.52, P=0.003), and showed a trend at 1-month (r(28)=−0.42, P=0.020) and 3-month post-LSG (r(28)=−0.49, P=0.006, Fig 1B). GM volumes in INS and PCC showed a positive correlation at 1-month (r(28)=0.57, P=0.001) and 3-month post-LSG (r(28)=0.55, P=0.001, Fig 1B).
Alterations in RSFC
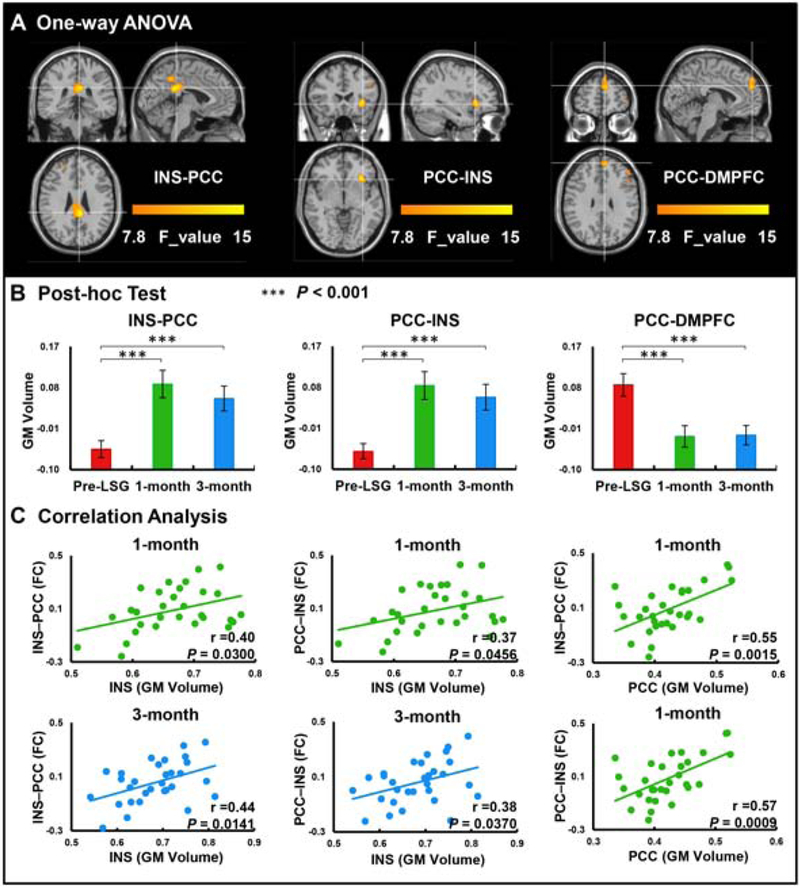
To investigate the relationship between brain structural and functional connectivity post-LSG, an RSFC analysis was carried out with the INS and PCC (extracted from VBM analysis) as seeds. Results showed significant time effects on RSFC between the INS seed and PCC (PFWE<0.05) and between the PCC seed and INS (PFWE<0.05, Fig 2A, Table 3). There were also significant time effects on RSFC between the PCC seed and dorsal medial prefrontal cortex (DMPFC) (PFWE<0.05, Fig 2A, Table 3). Post-hoc tests showed LSG significantly increased RSFC strengths of INS-PCC and PCC-INS, and significantly decreased RSFC strength of PCC-DMPFC at 1-month and 3-month post-LSG as compared to pre-LSG (P<0.001), but there was no significant increase at 3-month compared to 1-month post-LSG (Fig 2B).
Figure 2.
One-way ANOVA analysis of RSFC at pre-LSG and 1-month/3-month post-LSG in patients with obesity (cluster size-corrected, PFWE<0.05). A. There were significant time effects on the RSFC between the INS seed and PCC, between the PCC seed and INS and DMPFC. B. There were significant increases on the RSFC of the INS-PCC and PCC-INS at 1-month and 3-month post-LSG compared to pre-LSG. There were significant decreases on the RSFC of the PCC-DMPFC at 1-month and 3-month post-LSG compared to pre-LSG. C. Correlation analysis between GM volumes in the INS/PCC and RSFC of the INS-PCC/PCC-INS at 1-month and 3-month post-LSG. Error bars indicate the standard error.
Abbreviation: INS, Insula; PCC, Posterior cingulate cortex; DMPFC, Dorsal medial prefrontal cortex; 1-month, obese subjects who received imaging-scanned again at 1-month post-LSG.
Table 3.
One-way repeated measures ANOVA on RSFC in obese patients at pre-LSG and 1-month/3-month post-LSG (cluster size-corrected, PFWE<0.05).
| Seed | Cluster size | MNI | Peak F-value | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| INS-PCC | 238 | 6 | −42 | 24 | 16.85 |
| PCC-INS | 63 | 33 | 21 | −6 | 16.32 |
| PCC-DMPFC | 77 | 9 | 51 | 27 | 13.57 |
Abbreviation: RSFC, resting state functional connectivity; MNI, Montreal Neurological Institute; INS, Insula; PCC, Posterior cingulate cortex; DMPFC, Dorsal medial prefrontal cortex.
GM volume in the INS was positively correlated with RSFC strength between INS and PCC (r(28)=0.40, P=0.030) and between PCC and INS (r(28)=0.37, P=0.046) at 1-month post-LSG (Fig 2C). GM volume in the PCC was also positively correlated with RSFC strength between INS and PCC (r(28)=0.55, P=0.001) and between PCC and INS (r(28)=0.57, P<0.001) at 1-month post-LSG (Fig 2C). GM volume in the INS was also positively correlated with RSFC strength between INS and PCC (r(28)=0.44, P=0.014) and between PCC and INS (r(28)=0.38, P=0.037) at 3-month post-LSG (Fig 2C). Clinical measurements including YFAS, HAMA, and HAMD did not correlate with GM volume and RSFC strength at pre-LSG, 1-/3-month post-LSG.
Discussion
In the present study, we adopted structural MRI with a VBM analysis to investigate LSG-induced changes in GM/WM volumes in patients with obesity at pre-LSG, 1-month and 3-month post-LSG. Results showed significant increases in GM/WM volumes in brain regions associated with the control of food-intake (caudate), interoception (INS), self-referential processing (PCC), executive-function (IFG, ACC) and memory (PHIPP) at both 1-month and 3-month post-LSG as compared to pre-LSG, consistent with sustained structural recovery of the brain following LSG and weight reduction. BMI was negatively correlated with GM volume in caudate pre-LSG and showed a trend at 1-month and 3-month post-LSG such that smaller caudate volumes were associated with larger BMI at all time points. GM volumes in INS and PCC showed positive correlations at 1-month and 3-month post-LSG. Finally, LSG increased RSFC between INS and PCC while it decreased RSFC between PCC and DMPFC.
Alterations in regions associated with control of food-intake
At both 1-month and 3-month post-LSG, GM volumes increased in the caudate, a region associated with control of food-intake. There is increasing evidence that overconsumption of rewarding food can lead to changes in the brain’s reward circuitry that result in compulsive food-intake akin to the phenotype seen with addiction.19 Food reward plays a critical role in overeating and obesity, that has been conceptualize as the addictive dimensional component of obesity.20 Prior structural imaging studies reported that obese subjects had decreased GM densities in the caudate.4 Here we show that in obese individuals the caudate volumes increased post-LSG at both 1-month and 3-month post-LSG, suggesting that LSG may reverse obesity-related neuroanatomical alterations. Similarly, functional alterations associated with obesity also seem to be reversed following LSG. Of particular relevance to the addictive dimensional component of obesity there is evidence that LSG reverses the enhanced reactivity of brain reward regions to hedonic food.21 In addition, BMI was negatively correlated with GM volume in caudate pre-LSG and showed only a trend-level negative association at both 1-month and 3-month post-LSG, which might reflect the relationship between weight-loss and structural recovery in the caudate following LSG. This is consistent with findings of a negative association between BMI and caudate activation in response to rewarding food consumption such that subjects with larger BMIs had decreased caudate activation compared to those with lower BMI.19 Behavioral studies indicate that obese individuals anticipate more reward from food-intake that what they actually experience during its consumption, potentially because of reduced dopamine signaling in striatum (including caudate).22 It is possible that sustained change in the caudate post-LSG might improve its regulation of reward-related eating behaviors.
Alterations in regions associated with self-referential processing and interoception
The INS is a constituent of the salience (SN) ventral network that enables the integration of sensory data with visceral, autonomic, and hedonic “markers,” so that the organism can decide what to do next.23 Patients with obesity show decreased GM volume in INS, which might interfere with perception of internal signals. Impaired awareness of bodily state and satiety may cause obese individuals to consume more food in response to external and internal cues.24 The sustained increases in GM volume in INS post-LSG, might reflect sustained improvement in sensing the interoceptive energy status associated with sustained weight-loss induced by LSG.
The PCC is a midline cortical regions implicated in self-referential and self-consciousness processing and is a prominent integrative hub in the brain that is part of the default-mode network (DMN).6 The DMN plays an important role in self-generated thoughts, including autobiographical memories and future plans.25 This network is most active while processing internal mental status, such as self-referential thinking and during external unfocused attention.25 In addition, PCC plays a critical role in spatial attention, motivation and reward and visual imagery. Previous studies have shown that greater food-cues activation in PCC was found during a fasted versus a sated state,26 which could relate to the role of the PCC in monitoring the external environment and allocating neuronal resources to salient stimuli.
GM volumes in the INS and PCC at 1-month and 3-month post-LSG showed positive correlations with enhanced RSFC between INS and PCC, which might indicate strengthening of the functional connectivitynecessary for linking self-referential processing and interoception. The PCC, which is part of the posterior DMN, exhibits dynamic interactions with other distributed large-scale brain networks including the SN, which includes insula, amydgala and ACC.25 Previous studies found that the interaction among the DMN and SN might be related to low-frequency toggling between introspective states (self-referential states) and extrospective states (interoceptive states), ensuring that the individual is attentive to information from the world around us as well as internal sensations, such as hunger.27 Decreased functional connectivity between the DMN and SN in patients with obesity might reflect an unbalanced regulation between them. Increased connectivity between INS and PCC might lead to enhanced attention to internal states, changes in self-referential processing and greater interoceptive awareness of energy status. On the other hand, hunger and satiety are components of interoceptive signaling that lead to initiation and cessation of food-intake. It is possible that individuals may have greater awareness of bodily signals monitoring feelings of fullness to know when to stop eating post-LSG, while signals of satiety may be more automatic due to the physical restrictions placed on the stomach post-LSG.28
We also observed reduced RSFC between PCC and DMPFC post-LSG. DMPFC plays an important role in central regulation of eating behavior and integration of visceral and reward-based signaling.29 DMPFC is part of the anterior DMN and a prior study demonstrated increased DMN connectivity in overweight/obese individuals; the authors suggested this may be related to an abnormal focus on internal states such as appetite.12 Exercise-related studies also found that DMN activity is higher in obese and overweight individuals compared to controls, and this activity was reduced following chronic exercise. A similar mechanism may underlie post-LSG changes in RSFC between the PCC and DMPFC.
Alterations in regions associated with executive-control
Compared to pre-LSG and 1-month post-LSG, post-hoc tests showed significantly increased GM/WM volumes in the IFG and ACC at 3-month post-LSG, and significantly increased WM volumes in ACC pre-LSG compared to 1-month post-LSG. The IFG is involved with inhibition and attentional control and consistently implicated in inhibitory control in diverse fMRI task paradigms.30 The opercular part of the IFG is part of the ventral frontoparietal network and plays an important role in executive-control, rapidly adapting in order to respond to food stimuli with inhibitory-control. The ACC is implicated in the executive-control of internal/external stimuli, and context-dependent behaviors involving evaluation of salient information and modulation of emotional responses. Previous studies in obese individuals indicated that impaired ACC function might contribute to an imbalance between cognitive/emotional processing and consequentially an increased risk of emotional overeating.12 Sustained increases in GM volumes in IFG and ACC might contribute to improved capability for self-regulation and better control of food-intake at 1-month and 3-month post-LSG.
Alterations in regions associated with memory
Post-LSG there were sustained increases in WM volumes at 1-month and 3-month in PHIPP, which is critical for memory encoding and retrieval. PHIPP is also implicated in food craving, the physiological state of hunger, when motivationally relevant food objects are shown,14 and when tasting a liquid meal. Several neuroimaging studies showed increased PHIPP activation to high-energy visual food cues and decreased PHIPP activation after weight-loss surgery.31 Our previous study also showed that WM densities were lower in PHIPP in obese relative to normal weight individuals. LSG-induced sustained WM volume increases in PHIPP might help restore its function in obesity.
Clinical implications
In the current study, those brain regions showing decreased GM/WM volumes in patients with obesity and sustained increased GM/WM volumes post-LSG are associated with reward processing (caudate), interoception (insula), self-referential processing (PCC), executive-control (IFG/ACC) and memory (PHIPP), suggesting their close relationship with food-intake control. Those surgery-induced brain structural alterations might contribute to the brain activity and functional connectivity changes, resulting in the improvements in eating behaviors. Thus, those brain regions might be used as the targeted areas for clinical treatment such as nonoperative neuromodulation.
Limitations
We identify the following limitations to our study: (1) Due to strict exclusion criteria and difficulty in retaining patients’ post-LSG for follow-up scanning, our sample size is small, which limits the generalizability of our findings. (2) We assessed obese participants at three time points until 3 months post-LSG but longer follow up studies are needed to determine the long-term rate of recovery, their association with functional outcomes, and answer the question of which factors contribute to the improvement of eating behavior and subsequent weight-loss. (3) Super obese subjects were not included because of the limitation of the internal diameter of MRI scanner.
Conclusion
The current study showed that LSG induced sustained structural changes in brain regions involved with control of food-intake, interoception, self-referential processing, executive-control and memory along with strengthening the connectivity between INS and PCC and weakening the connectivity between PCC and DMPFC. These findings suggest that LSG-induced alterations in GM/WM volumes and RSFC between INS and PCC might in part mediate the long-term benefits of LSG in weight reduction.
Supplementary Material
Highlights.
LSG increased brain volumes in fronto-mesolimbic regions at 1-/3-month post-LSG
LSG increased functional connectivity between insula and posterior cingulate cortex
Gray-matter volume in insula/PCC correlated with its functional connectivity
LSG-induced sustained structural brain changes mediate long-term weight reduction
Acknowledgements
This work is supported by the National Natural Science Foundation of China under Grant Nos. 61431013 and 81730016; National Natural Science Foundation of Shaanxi Province under Grant No. 2018JM3007; National Clinical Research Center for Digestive Diseases, Xi’an, China under Grant No. 2015BAI13B07; and support in part from the Intramural Research Program of the United States National Institute on Alcoholism and Alcohol Abuse, Z01AA3009 (PM, NDV, GJW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors have no commercial associations that might be a conflict of interest in relation to this article.
References:
- 1.Poston L, Caleyachetty R, Cnattingius S, et al. Preconceptional and maternal obesity: epidemiology and health consequences. Lancet Diabetes Endocrinol 2016;4(12):1025–1036. [DOI] [PubMed] [Google Scholar]
- 2.Karlsson HK, Tuulari JJ, Hirvonen J, et al. Obesity is associated with white matter atrophy: a combined diffusion tensor imaging and voxel-based morphometric study. Obesity (Silver Spring) 2013;21(12):2530–2537. [DOI] [PubMed] [Google Scholar]
- 3.Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage 2001;14(1 Pt 1):21–36. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y, Ji G, Xu M, et al. Recovery of brain structural abnormalities in morbidly obese patients after bariatric surgery. Int J Obes (Lond) 2016;40(10):1558–1565. [DOI] [PubMed] [Google Scholar]
- 5.Craig AD. Significance of the insula for the evolution of human awareness of feelings from the body. Ann N Y Acad Sci 2011;122572–82. [DOI] [PubMed] [Google Scholar]
- 6.Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain--a meta-analysis of imaging studies on the self. Neuroimage 2006;31(1):440–457. [DOI] [PubMed] [Google Scholar]
- 7.Tuulari JJ, Karlsson HK, Antikainen O, et al. Bariatric Surgery Induces White and Grey Matter Density Recovery in the Morbidly Obese: A Voxel-Based Morphometric Study. Hum Brain Mapp 2016;37(11):3745–3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Opel N, Redlich R, Kaehler C, et al. Prefrontal gray matter volume mediates genetic risks for obesity. Mol Psychiatry 2017;22(5):703–710. [DOI] [PubMed] [Google Scholar]
- 9.O’Brien PD, Hinder LM, Callaghan BC, Feldman EL. Neurological consequences of obesity. Lancet Neurol 2017;16(6):465–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sjostrom L, Narbro K, Sjostrom CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 2007;357(8):741–752. [DOI] [PubMed] [Google Scholar]
- 11.Liu L, Ji G, Li G, et al. Structural changes in brain regions involved in executive-control and self-referential processing after sleeve gastrectomy in obese patients. Brain Imaging Behav 2019;13(3):830–840. [DOI] [PubMed] [Google Scholar]
- 12.Kullmann S, Heni M, Veit R, et al. The obese brain: association of body mass index and insulin sensitivity with resting state network functional connectivity. Hum Brain Mapp 2012;33(5):1052–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Legget KT, Wylie KP, Cornier MA, Melanson EL, Paschall CJ, Tregellas JR. Exercise-related changes in between-network connectivity in overweight/obese adults. Physiol Behav 2016;15860–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li G, Ji G, Hu Y, et al. Bariatric surgery in obese patients reduced resting connectivity of brain regions involved with self-referential processing. Hum Brain Mapp 2018;39(12):4755–4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Ji G, Li G, et al. Ghrelin reductions following bariatric surgery were associated with decreased resting state activity in the hippocampus. Int J Obes (Lond) 2019;43(4):842–851. [DOI] [PubMed] [Google Scholar]
- 16.van Meer F, van der Laan LN, Charbonnier L, Viergever MA, Adan RA, Smeets PA. Developmental differences in the brain response to unhealthy food cues: an fMRI study of children and adults. Am J Clin Nutr 2016;104(6):1515–1522. [DOI] [PubMed] [Google Scholar]
- 17.Gearhardt AN, Corbin WR, Brownell KD. Preliminary validation of the Yale Food Addiction Scale. Appetite 2009;52(2):430–436. [DOI] [PubMed] [Google Scholar]
- 18.Rullmann M, Preusser S, Poppitz S, et al. Gastric-bypass surgery induced widespread neural plasticity of the obese human brain. Neuroimage 2018;172853–863. [DOI] [PubMed] [Google Scholar]
- 19.Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM. Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J Abnorm Psychol 2008;117(4):924–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Volkow ND, Wang GJ, Tomasi D, Baler RD. The addictive dimensionality of obesity. Biol Psychiatry 2013;73(9):811–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scholtz S, Miras AD, Chhina N, et al. Obese patients after gastric bypass surgery have lower brain-hedonic responses to food than after gastric banding. Gut 2014;63(6):891–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang GJ, Volkow ND, Fowler JS. The role of dopamine in motivation for food in humans: implications for obesity. Expert Opin Ther Targets 2002;6(5):601–609. [DOI] [PubMed] [Google Scholar]
- 23.Vianna EP, Naqvi N, Bechara A, Tranel D. Does vivid emotional imagery depend on body signals? Int J Psychophysiol 2009;72(1):46–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frank S, Kullmann S, Veit R. Food related processes in the insular cortex. Front Hum Neurosci 2013;7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andrews-Hanna JR, Smallwood J, Spreng RN. The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Ann N Y Acad Sci 2014;131629–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cornier MA, Salzberg AK, Endly DC, Bessesen DH, Rojas DC, Tregellas JR. The effects of overfeeding on the neuronal response to visual food cues in thin and reduced-obese individuals. Plos One 2009;4(7):e6310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fransson P How default is the default mode of brain function? Further evidence from intrinsic BOLD signal fluctuations. Neuropsychologia 2006;44(14):2836–2845. [DOI] [PubMed] [Google Scholar]
- 28.Lepping RJ, Bruce AS, Francisco A, et al. Resting-state brain connectivity after surgical and behavioral weight loss. Obesity (Silver Spring) 2015;23(7):1422–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tataranni PA, Gautier JF, Chen K, et al. Neuroanatomical correlates of hunger and satiation in humans using positron emission tomography. Proc Natl Acad Sci U S A 1999;96(8):4569–4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hampshire A, Chamberlain SR, Monti MM, Duncan J, Owen AM. The role of the right inferior frontal gyrus: inhibition and attentional control. Neuroimage 2010;50(3):1313–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zoon H, de Bruijn S, Jager G, et al. Altered neural inhibition responses to food cues after Roux-en-Y Gastric Bypass. Biol Psychol 2018;13734–41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.