Abstract
Purpose:
Evaluate tumor and ablation zone morphology and densitometry, related to recurrence in participants with stage IA non-small cell lung cancer undergoing radiofrequency ablation in a prospective, multicenter trial.
Materials and methods:
Forty-five participants (median age 76; 25 women, 20 men) from 16 sites were followed for 2 years (December 2006 to November 2010) with CT densitometry. Imaging findings before and after ablation were recorded, including maximum CT attenuation [Hounsfield units (HU)] at precontrast, 45, 90, 180, and 300 seconds post contrast.
Results:
Every 1cm increase in largest axial diameter of the ablation zone at three-month follow-up compared to the index tumor, reduced odds of two-year recurrence by 52% (p= 0.02). A 1cm difference performed best (sensitivity 0.56, specificity 0.93, positive likelihood ratio 8).
CT densitometry precontrast and at 45 seconds showed significantly different enhancement patterns when comparing pretreated lung cancer (delta=+61.2 HU), tumor recurrence (delta=+ 57 HU), and treated tumor/ablation zone (delta=+16.9 HU), (p<0.0001). Densitometry from 45–300 seconds was also different among pretreated tumor (delta=−6.8 HU), recurrence (delta=−11.2 HU), and treated tumor (delta= +12.1 HU), (p=0.01). Untreated and residual tumor demonstrated washout, whereas treated tumor demonstrated increased attenuation.
Conclusion:
An ablation zone ≥1 cm larger than initial tumor on 3-month follow-up imaging is recommended to decrease odds of recurrence. CT densitometry can delineate tumor versus treatment zone.
Introduction
Lung cancer is the leading cancer killer in the United States with an estimated 154,050 deaths from lung cancer in 2018 (1). While surgical resection remains the standard of care, only 20–30% of diagnosed patients are candidates for resection due to advanced stage at initial diagnosis or cardiopulmonary dysfunction (2–4). For high-risk patients, who are deemed surgically inoperable or who refuse surgery, stereotactic body radiotherapy (SBRT) and thermal ablation therapies are well-established treatment alternatives (5–8).
Radiofrequency ablation (RFA) has been used for over a decade in the treatment of lung tumors and offers a minimally invasive treatment option (9). Several studies have shown a correlation between initial tumor size and local recurrence; however, there is little data available on the optimal ablation zone size (7, 10). The International Working Group on Image-Guided Tumor Ablation notes that data is currently lacking to support definitive recommendations regarding ablation margins, although several other studies support an ablative margin of 5–10 mm (11). Ideally, treatment should allow for adequate coverage of the malignant lesion, while also preserving healthy surrounding lung parenchyma.
No standardized imaging protocol for post-ablation follow-up exists; however, most centers will follow patients with conventional contrast-enhanced CT to assess recurrence. Multiphase CT scanning of pulmonary nodules, specifically enhancement patterns of malignant neoplasms versus granulomas and benign neoplasms, was described by Swensen et al. Using a cutoff of 15 Hounsfield Units (HU) to assess enhancement, they noted that malignant neoplasms enhanced significantly more than granulomas and benign neoplasms (12). The use of multiphasic imaging examinations to evaluate suspicious lesions, such as what has been done with breast and prostate cancer patients, has elucidated a better understanding of lesion microcirculation and tumor angiogenesis (13–18). It is hypothesized that, like other malignancies, non-small cell lung cancer (NSCLC) would demonstrate imaging findings of rapid enhancement and plateau or washout, secondary to tumor angiogenesis.
This prospective study, evaluated a target population with stage IA NSCLC treated with RFA and followed at predetermined intervals with dedicated CT densitometry protocols. Imaging characteristics, including ablation zone morphology and densitometry, were assessed as they relate to tumor recurrence.
Materials and Methods
Patient Sample:
This Health Insurance Portability and Accountability Act-compliant study required that each recruiting center obtain institutional review board (IRB) approval. Before participating, each participant provided written informed consent. Data from the 50 study participants included in this study was previously reported. Data were used from a prospective, multicenter group trial funded through the Alliance for Clinical Trials in Oncology (Alliance) National Cancer Institute grant. The prior manuscript focused upon time to local recurrence, overall survival, and complications (7). The current manuscript comments on imaging features that are predictive of recurrence and discusses optimizing early detection of recurrent disease.
Enrollment required that study participants have biopsy-proven NSCLC with a maximum tumor diameter ≤3cm and T1N0M0 staging. All suspicious mediastinal lymph nodes (those measuring >1cm on short-axis or with increased FDG avidity) were biopsied. Criteria for ablation included that the tumor be accessible via a percutaneous route and that the tumor was non-contiguous with vital structures. Additionally, study participants were required to have an Eastern Cooperative Oncology Group/Zubrod performance status between 0 and 2. All study participants were evaluated by an American College of Surgeons Oncology Group-approved thoracic surgeon and were deemed to be high-risk for pulmonary resection, based on previously described criteria (7).
A total of 54 study participants from 16 US sites were enrolled, of these, 49 study participants (22 Men, 27 Women; median age, 76 years; range, 69–81 years) met eligibility requirements and underwent a standardized protocol. These study participants were followed for 2 years, from December 2006 to November 2010. Of those 49 patients, 45 participants had two years of imaging data, and were therefore included in this study. The median tumor size in the group, measured as the largest three-dimensional diameter, was 2.0 cm (range, 0.8 cm- 3.0 cm) (quartiles, 1.7 cm-2.4 cm); 3 patients had tumors ≤ 1.0 cm, 23 patients’ tumors measured between >1.0 - ≤ 2.0 cm, 19 patients had tumors >2.0 cm.
Radiofrequency Ablation Technique:
The Medtronic Cool-tip cluster electrode (Covidien, Boulder, CO) of variable lengths and fixed active tip exposure (2.5 cm) and Cosman Coagulator (Covidien, Boulder, CO) were used. Electrodes were placed within the target lesion using CT or CT fluoroscopic guidance, prior to each activation of the RF generator. At least one RF treatment was done with the maximal allowable current (approximately 1100–2000 mA). Maximal intratumoral temperatures were recorded using a paired temperature probe; the internal temperature of the tumor reached at minimum 60° C. Prior to enrolling patients, all treating physicians were required to have performed at least 25 static or dynamic image-guided thoracic procedures and at least 10 lung RFA procedures (at least 1 with the Covidien system).
Image Acquisition and Analysis:
Archived thin-data from the American College of Radiology Imaging Network (ACRIN) database of study participants’ pretreatment and post treatment follow-up multiphase CT scans were evaluated. Volumetric CT of the chest was performed with the parameters in Table I. Noncontrast and CT densitometry imaging was performed at standard settings at all institutions to minimize variability; the study protocol did not require that the same scanner be used across participants.
Table I.
CT Acquisition Parameters
| Noncontrast CT | CT Densitometry (0, 45, 90, 180, and 300 seconds) | |
|---|---|---|
| kV | ≥ 120 | ≥ 120 |
| mA | ≥ 200 | ≥ 220 |
| Collimation | ≤ 3mm | ≤ 1.5 mm |
| Pitch | ≤ 2 | ≤ 2 |
| Slice Thickness | ≤ 3mm | ≤ 1.5 mm |
| Reconstruction Interval | 30–60% overlap | 30–60% overlap |
| Breathing Instructions | Maximal inspiration | Maximal inspiration |
| Algorithm | Bone | Standard |
| Contrast | None | Omnipaque or equivalent 350 injected at 2 cc/sec IV <100 lbs: 100 cc; 100–200 lbs: 125 cc; >200 lbs : 150 cc |
CT densitometry was performed on all study participants prior to treatment, and follow-up CTs were obtained at 3 months, 6 months, 9 months, 18 months, and 24 months post-procedure. CT images were obtained precontrast, at 45 seconds, 90 seconds, 180 seconds and 300 seconds post contrast, and evaluated for the highest possible Hounsfield Unit (HU) measurement at the treatment site drawn with region of interest (ROI) curves. The ROI curve was placed on the most hyperdense and/or hyperenhancing portion of the tumor and ablation zone, to obtain the maximum HU. This CT protocol was repeated at all interval follow-up studies. Swensen et al.’s technique was used as a guideline to assess lesion and treatment zone enhancement over time (Figure 1) (12). The readers, consisting of two radiologists with between 3 and 26 years of experience, were blinded to information about recurrence or retreatment. Both radiologists double read every study; in cases of inter-observer disagreement, the case was discussed to determine consensus. All the follow up CTs were evaluated for treatment zone size and maximum attenuation at 3 months, 6 months, 9 months, 12 months, 18 months and 24 months (Figure 2). Comparisons were made between three groups: (a) the pretreatment biopsy proven lung cancer, (b) any tumor recurrence, and (c) treated tumor/ablation zone by imaging findings. Measurements of the pretreated tumor and ablation zone size were based on largest axial dimension. Tumor recurrence was determined by the following: growth of the index lesion 1.25 times any dimension on follow-up CT after the 3 month baseline post ablation CT (as determined by lung windows), any mass lesion measuring greater than 9mm in the treatment field that enhances 15 HU or more after contrast injection, or focal hypermetabolic appearance of the peripheral ablation zone on 6-month post-ablation PET/CT compared to background activity. The aforementioned imaging findings were considered suspicious for recurrence; tumor recurrence was confirmed by biopsy.
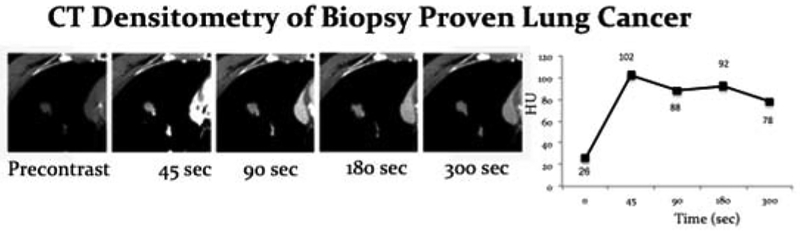
Figure 1:
CT densitometry of the index lesion in a patient with biopsy proven lung cancer. Graphed out HUs at each of the different time points show enhancement at 45 seconds with plateau to slight decrease in contrast in enhancement from 45 seconds to 300 seconds. This pattern of enhancement was seen consistently in the biopsy proven lung cancers.
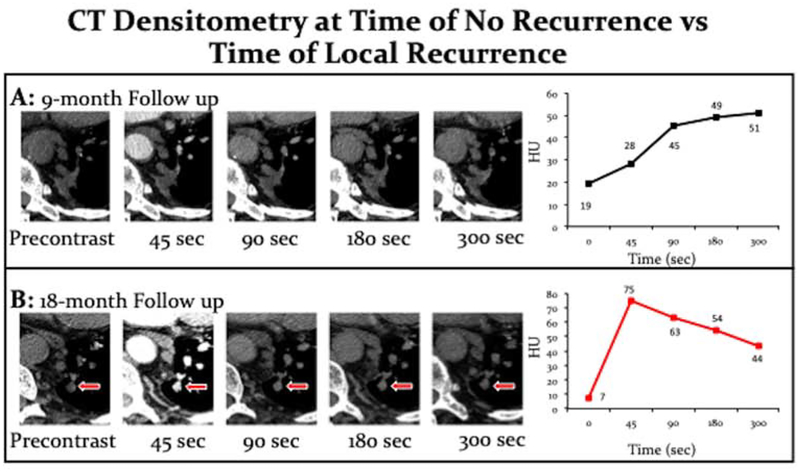
Figure 2:
Comparison of the same patient at their 9-month follow-up (A) versus the 18-month follow up (B). A) The CT densitometry at 9 months demonstrates progressive enhancement overtime consistent with treated tumor/ ablation zone. B) The CT densitometry at 18 month follow up shows rapid enhancement at 45 seconds with wash out from 45 seconds to 300 seconds consistent with recurrent tumor.
In addition, CT scans performed before treatment, and at 3-month and 6-month follow up studies were evaluated for shape (round, ovoid, bilobar, irregular), size (maximum diameter), borders (smooth, speculated, lobulated), attenuation (maximum HU of the ROI), and presence of cavitation in the tumor and subsequent ablation zone. Pretreatment imaging was also evaluated for distance to heat sink (any vessel or airway 3mm in diameter or greater) and distance to the pleura. The procedural CT fluoroscopy images were evaluated to determine the distance between the electrode tip and the chest wall.
Statistical Analysis:
All analyses were conducted using SAS Software 9.4 (SAS Inc. Cary, NC). The relationship between 2-year local recurrence and delta between original maximum tumor size and maximum 3-month post ablation scar size was modeled using generalized linear modeling (GLM) assuming a binary distribution and logit link with PROC GLIMMIX and competing risks hazard regression using the Fine and Gray (FG HR) approach with PROC PHREG. Other competing predictors of recurrence were also examined using FG HR. Size was examined between pre-ablation tumor index size and 3-month post ablation scar in those study participants with and without 2-year local recurrence using generalized mixed modeling with sandwich estimation, where sizes were nested within participants using PROC GLIMMIX assuming a normal distribution. CT densitometry over time between those with and without 2-year local recurrence was examined using generalized estimating equations with sandwich estimation, where Hounsfield Unit (HU) observations were nested within participants. Because ablation is used for local tumor control and because the explicit goal of this study was to evaluate imaging findings that were markers of recurrence, models examined death as a competing risk and censored on the absence of local recurrence. When applicable, a sensitivity analysis was run examining any recurrence and death. Alpha was established a priori at the .05 level and all interval estimates were calculated for 95% confidence. Multiple comparisons were adjusted using the Tukey method where appropriate (19).
Results
Ablation Zone versus Initial Tumor Size:
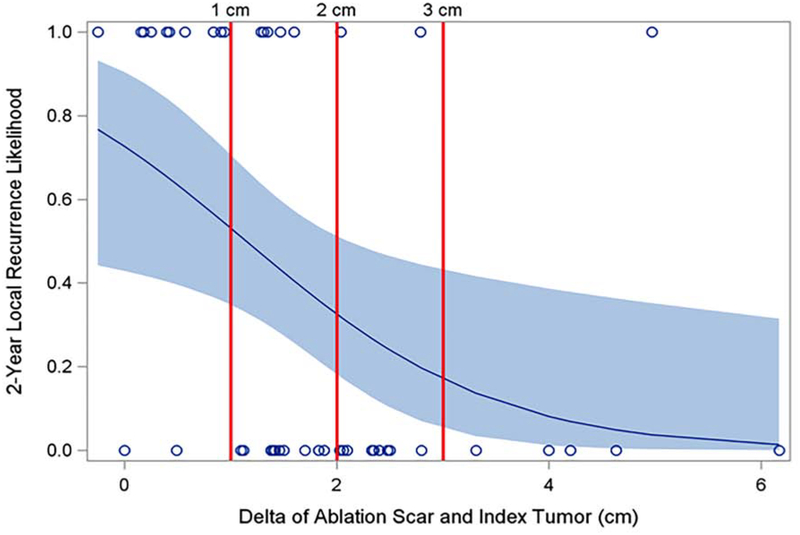
For every 1 cm increase in the difference (delta) between the maximum diameters of the ablation zone at 3 months compared to the index tumor size, the subdistribution hazard of 2-year local recurrence decreased by 52% (HR: 0.476, 95% CI [.256%, .887%], p= 0.0194). the odds of 2-year local recurrence, not controlling for death before local recurrence, was 57% (OR: 0.427, 95% CI [.205%, .886%]) (p= 0.0234); this result was similar for any recurrence or death, p= 0.0077. A 1 cm difference achieved high specificity (sensitivity 0.56, specificity 0.93, positive likelihood ratio 8), while a 3 cm difference achieved high sensitivity (sensitivity 0.94, specificity 0.19, positive likelihood ratio 1.16) (Figure 3), though these estimates exclude those who died before local recurrence in the 2-year window. When the difference between the maximum diameter of the 3-month post ablation scar and the maximum diameter of the index tumor size was less than 1 cm, the subdistribution hazard of 2-year local recurrence was 6.5 (95% CI [2.6, 16.0]) greater compared with when the difference was over 1cm.
Figure 3:
The association between the risk of recurrence at 2-years and the size difference (delta) between the maximum diameter of the 3-month ablation zone and the maximum diameter of the initial tumor, was evaluated. For every 1 cm the ablation zone diameter was larger than the index tumor diameter, the odds of recurrence decreased by 57% (p=0.0234) and risk of 2-year local recurrence decreased 59.3%. A delta of 1 cm had the highest specificity and positive likelihood ratio (sensitivity 0.56, specificity 0.93, positive likelihood ratio 8), while 3 cm was the most sensitive (sensitivity 0.94, specificity 0.19, positive likelihood ratio 1.16).
In addition, a statistically significant relationship in size was observed between maximum ablation zone diameter and maximum index tumor diameter, between those with and without 2-year recurrence, (interaction effect) p= 0.0065, though these estimates exclude those who died before local recurrence in the 2-year window. Of note, no difference was observed in maximum index tumor size between those who eventually had recurrence (2.4cm [2.1,2.7]) and those who did not (2.1cm [1.8,2.3]), p=0.1183. However, those who had recurrence had a significantly smaller ablation zone size (3.6 cm [2.9,4.2]) compared with those who did not have recurrence (4.3 cm [3.9, 4.8]), p=0.0334. These results were mirrored when comparing any recurrence or death versus no recurrence or death, p=0.004.
Additional variables were evaluated as they related to tumor recurrence, including: participant age (p= 0.9482), performance status (p= 0.2535), tumor histology (p= 0.9914), tumor size (p= 0.2054), ablation parameters (peak power (p= 0.4728), peak current (p= 0.6103), maximum temperature (p =0.2927), treatment time (p= 0.1818)), tumor shape (round, ovoid, bilobed, irregular, p= 0.7976), cavitation (p= 0.0634), tumor proximity to airways and vessels (≥3 mm; p= 0.6591), and the distance of the electrode tip to the pleura (p=0.8861). However, none of these variables were statistically predictive of local recurrence.
CT Densitometry:
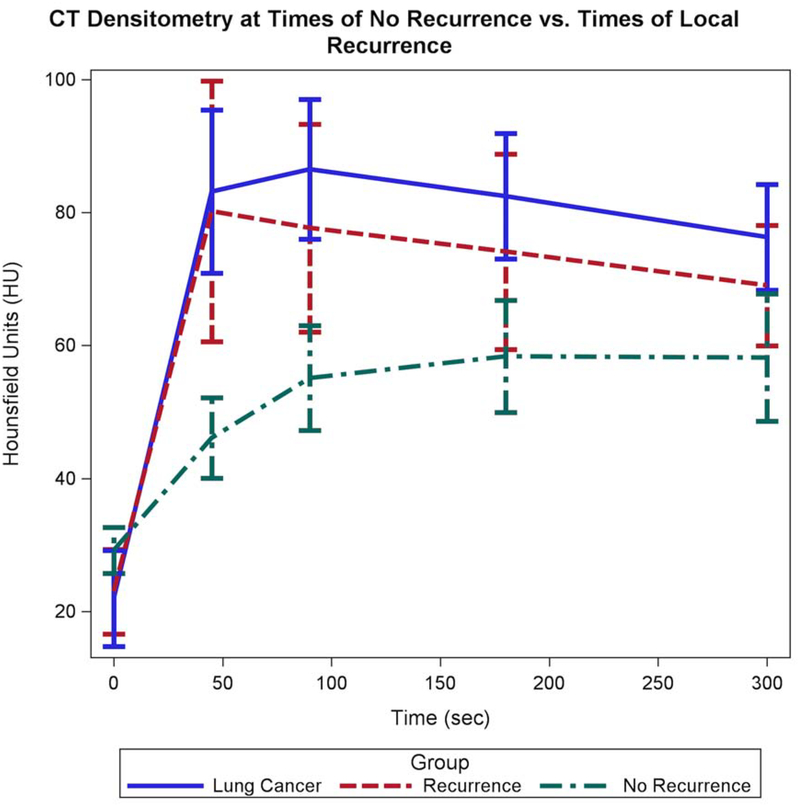
The increase in Hounsfield units value between precontrast (0 seconds) and 45 seconds is dramatically lower for participants without recurrence (delta=+16.9 HU, 95% CI [9.4, 24.4]) relative to those participants with recurrence/untreated tumor (delta=+57.2 HU, 95% CI [27.4, 87.0]) and the pretreatment tumor densitometry measurement (delta= +61.2 HU, 95% CI [41.5, 80.8]), p<0.0001. The mean attenuation at 45 seconds for non-recurrence was 46.1 HU [40.2, 52.9], which was significantly lower than both recurrence 80.2 HU [62.5, 102.9] and pretreatment tumor 83.1 HU [71.4, 96.8], both p<0.001 (Figure 4).
Figure 4:
Graph shows the aggregate data from the CT Densitometry at pretreatment, times of recurrence, and times of no recurrence. There are pronounced similarities between the pretreatment CT Densitometry and times of recurrence. These lines show rapid enhancement at 45 seconds and plateau to slight wash out from 45 seconds to 300 seconds. At times of no recurrence, the ablation zone shows progressive enhancement over time consistent with the enhancement of scar tissue.
The change in attenuation between 45 and 300 seconds is different between baseline pretreatment tumor and recurrence/untreated tumor versus non-recurrence. In non-recurrence or treated tumor, the density (HU) between 45 and 300 seconds increases significantly (delta= +12.1 HU, 95% CI [1.8, 22.4], p=0.0129), in keeping with persistent and increasing enhancement over time. Conversely, the attenuation (HU) of the baseline pretreatment tumor and the recurrence/untreated tumor decreases between 45 and 300 seconds (delta = −6.8 HU, 95% CI [−24.4, 10.6] and −11.2 HU, 95% CI [−31.2, 8.9], respectively), in keeping with washout. The decrease in attenuation between these two time points was not statistically significant (p=0.8164 and p=0.5380, respectively) (Figure 4). A sensitivity analysis considering death as local recurrence mirrored these results.
Discussion
Compared to surgical and SBRT techniques, there are advantages to thermal ablation modalities. Key among them is preservation of lung function and thus functional state, especially in patients with emphysema and low pulmonary reserve (3, 7). A single institution, retrospective study discovered that 37% of patients with anatomically resectable tumors were deemed nonsurgical candidates secondary to poor lung function(20). Many patients with lung cancer have concomitant pulmonary disease and dysfunction, thus an alternative treatment modality for nonsurgical patients that preserves functioning parenchyma is critical. Based upon histopathology data, authors have suggested that the safety zone, or margin of apparently healthy tissue at the periphery of the tumor included in ablation, measures anywhere between 4.1mm and 1cm (21, 22). Evaluation of imaging findings, have yielded disparate recommendations; authors have recommended a ground-glass region of opacification around the index lesion measuring anywhere from 4.5mm to an ablation zone twice the diameter of the index lesion (23, 24).
This study demonstrated a similar trend to that purported by the histopathology studies; when the difference between the ablation zone diameter and index lesion was less than 1 cm, there was a 6.5 times greater risk of local recurrence at 2-years compared to those participants with a greater than 1 cm difference. Additionally, for every centimeter that the ablation zone was larger than the index tumor, the subdistribution hazard of 2-year local recurrence decreased 52%.
Several studies have described the utility of CT densitometry in differentiating pulmonary malignancies from benign nodules (12, 25, 26). CT densitometry allows evaluation of enhancement over time; changes in Hounsfield Units, likely correlate with structurally abnormal and unevenly organized vascular networks caused by tumor angiogenesis (18). This study demonstrated that in tumor (pre-treated tumor and recurrent disease), there was rapid enhancement 45 seconds post contrast, with a decrease to a plateau of enhancement between 45 seconds to 300 seconds. This appearance of ‘washout’ is a characteristic imaging finding of tumor angiogenesis (15). The curve kinetics in this study are similar to kinetic curves in breast and prostate cancer seen using dynamic contrast enhanced MRI (13). In the current study, the treated tumors/ablation zones showed persistent increase in contrast enhancement over time. This is consistent with imaging findings of other scars, such as those caused by myocardial infarction (27, 28).
Post contrast enhancement at 45 seconds was significantly different between local recurrence and treated tumor/ablation zone. Treated tumors/ablation zones demonstrated an average enhancement of 17 HU, whereas recurrent tumor had a mean enhancement of 55 HU, and baseline, pre-treated tumor measured 57 HU. These findings are consistent with studies reporting increased enhancement in malignant nodules compared to benign pulmonary nodules (12, 26). These findings support the current practice of post contrast imaging to follow up patients after ablation. CT densitometry could be a helpful imaging biomarker of lung cancer recurrence after tumor ablation techniques and in the initial diagnosis of lung malignancy.
Over the past decade, radionomics has emerged as a means to extract sub-visual, quantitative imaging features that can be predictive of oncologic response to treatment (29). Several studies have shown that radionomic features can be used to distinguish lung adenocarcinomas from benign lung nodules and to identify prognostic features, including tumor heterogeneity, shape, and phenotype (30–32). Radionomic analysis utilizes sophisticated image analysis tools that allow quantitate features to be extracted using high-throughput computing from medical images (33). In spite of its potential use in diagnosing lung cancers, radionomics was not readily available at the time of the study’s data accrual and is also not currently the standard of care in academic chest radiology departments. This study offers a more utilitarian way to analyze predictors of recurrence and is not reliant on particular computing software but rather the visual and cognitive interpretation of the diagnostic radiologist.
A limitation of this study was the small study size and the distribution of patients across numerous treatment sites. Each site performed their own CT scans; differences between scanners likely created variability in absolute attenuation; although the authors believe the relative changes in enhancement and wash-out are maintained across scanners. In addition each institution had to have a CT scanner capable of performing the protocol as written for the trial. The data included in this study were obtained over ten years ago; during this time different ablation modalities have emerged in the treatment of lung tumors. In particular, MWA has largely supplanted RFA for the treatment of lung tumors due to its larger ablation zone sizes (34, 35). A limitation in the use of CT densitometry to detect recurrence is that it is not feasible in patients with severe renal dysfunction. The added radiation dose conferred by CT densitometry is also a consideration; however, in an older population with a pre-existing malignancy, the benefit of optimized surveillance was felt to outweigh the potential of radiation-induced cancer, which often presents decades after exposure (36–38).
With the introduction of CT lung cancer screening and the aging population worldwide, the number of lung ablations will likely increase. Validation of successful tumor treatment is important and CT densitometry is a readily available technique that should be considered as an adjunct imaging modality for detection of tumor recurrence.
Supplementary Material
Funding:
Alliance for Clinical Trials in Oncology (National Cancer Institute grant U10CA180821)
Footnotes
Conflicts of Interest:
Damian E Dupuy, MD, FACR :
Consultant : Boston Scientific, Perseon Medical
Royalties: Springer Verlag, UpToDate
Hiran C Fernando MBBS, FRCS
Consultant: Merck
Medical Monitor for Study: Galil
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Erica S. Alexander, Hospital of the University of Pennsylvania, Department of Radiology.
Lillian Xiong, Massachusetts General Hospital, Department of Radiology.
Grayson L. Baird, The Warren Alpert Medical School of Brown University, Department of Diagnostic Imaging.
Hiran Fernando, Inova Schar Cancer Institute, Fairfax, VA; Department of Surgery.
Damian E. Dupuy, Cape Cod Hospital, Hyannis, MA.
References
- 1.Cancer Of The Lung And Bronchus - SEER Stat Fact Sheets. 2018 [Internet]. 2018. Available from: https://seer.cancer.gov/statfacts/html/lungb.html.
- 2.Venuta F, Diso D, Onorati I, Anile M, Mantovani S, Rendina EA. Lung cancer in elderly patients. J Thorac Dis. 2016;8(Suppl 11):S908–S14. doi: 10.21037/jtd.2016.05.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mazzone P. Preoperative evaluation of the lung resection candidate. Cleveland Clinic journal of medicine. 2012;79 Electronic Suppl 1:eS17–22. Epub 2012/05/25. doi: 10.3949/ccjm.79.s2.04. [DOI] [PubMed] [Google Scholar]
- 4.British Thoracic S, Society of Cardiothoracic Surgeons of Great B, Ireland Working P. BTS guidelines: guidelines on the selection of patients with lung cancer for surgery. Thorax. 2001;56(2):89–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baumann P, Nyman J, Hoyer M, Wennberg B, Gagliardi G, Lax I, et al. Outcome in a prospective phase II trial of medically inoperable stage I non-small-cell lung cancer patients treated with stereotactic body radiotherapy. J Clin Oncol. 2009;27(20):3290–6. doi: 10.1200/JCO.2008.21.5681. [DOI] [PubMed] [Google Scholar]
- 6.Timmerman R, Paulus R, Galvin J, Michalski J, Straube W, Bradley J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303(11):1070–6. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dupuy DE, Fernando HC, Hillman S, Ng T, Tan AD, Sharma A, et al. Radiofrequency ablation of stage IA non-small cell lung cancer in medically inoperable patients: Results from the American College of Surgeons Oncology Group Z4033 (Alliance) trial. Cancer. 2015;121(19):3491–8. doi: 10.1002/cncr.29507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Healey TT, March BT, Baird G, Dupuy DE. Microwave Ablation for Lung Neoplasms: A Retrospective Analysis of Long-Term Results. J Vasc Interv Radiol. 2017;28(2):206–11. doi: 10.1016/j.jvir.2016.10.030. [DOI] [PubMed] [Google Scholar]
- 9.Dupuy DE, Mayo-Smith WW, Abbott GF, DiPetrillo T. Clinical applications of radio-frequency tumor ablation in the thorax. Radiographics : a review publication of the Radiological Society of North America, Inc 2002;22 Spec No:S259–69. Epub 2002/10/12. doi: 10.1148/radiographics.22.suppl_1.g02oc03s259. [DOI] [PubMed] [Google Scholar]
- 10.de Baere T, Auperin A, Deschamps F, Chevallier P, Gaubert Y, Boige V, et al. Radiofrequency ablation is a valid treatment option for lung metastases: experience in 566 patients with 1037 metastases. Ann Oncol. 2015;26(5):987–91. doi: 10.1093/annonc/mdv037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmed M, Solbiati L, Brace CL, Breen DJ, Callstrom MR, Charboneau JW, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria--a 10-year update. J Vasc Interv Radiol. 2014;25(11):1691–705 e4. doi: 10.1016/j.jvir.2014.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swensen SJ, Viggiano RW, Midthun DE, Muller NL, Sherrick A, Yamashita K, et al. Lung nodule enhancement at CT: multicenter study. Radiology. 2000;214(1):73–80. doi: 10.1148/radiology.214.1.r00ja1473. [DOI] [PubMed] [Google Scholar]
- 13.Kuhl CK, Mielcareck P, Klaschik S, Leutner C, Wardelmann E, Gieseke J, et al. Dynamic breast MR imaging: are signal intensity time course data useful for differential diagnosis of enhancing lesions? Radiology. 1999;211(1):101–10. doi: 10.1148/radiology.211.1.r99ap38101. [DOI] [PubMed] [Google Scholar]
- 14.Kaiser WA, Zeitler E. MR imaging of the breast: fast imaging sequences with and without Gd-DTPA. Preliminary observations. Radiology 1989;170(3 Pt 1):681–6. doi: 10.1148/radiology.170.3.2916021. [DOI] [PubMed] [Google Scholar]
- 15.Knopp MV, Weiss E, Sinn HP, Mattern J, Junkermann H, Radeleff J, et al. Pathophysiologic basis of contrast enhancement in breast tumors. J Magn Reson Imaging. 1999;10(3):260–6. [DOI] [PubMed] [Google Scholar]
- 16.Wang X, Li K, Sun H, Zhao J, Zhang Z, Zheng L, et al. Tumoral angiogenesis in both adrenal adenomas and nonadenomas: a promising computed tomography biomarker for diagnosis. Onco Targets Ther. 2016;9:1823–30. doi: 10.2147/OTT.S93868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao P, Shi C, Zhao L, Zhou Q, Luo L. Differential diagnosis of prostate cancer and noncancerous tissue in the peripheral zone and central gland using the quantitative parameters of DCE-MRI: A meta-analysis. Medicine (Baltimore). 2016;95(52):e5715. doi: 10.1097/MD.0000000000005715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagy JA, Chang SH, Dvorak AM, Dvorak HF. Why are tumour blood vessels abnormal and why is it important to know? Br J Cancer. 2009;100(6):865–9. doi: 10.1038/sj.bjc.6604929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tukey JW. Comparing individual means in the analysis of variance. Biometrics. 1949;5(2):99–114. [PubMed] [Google Scholar]
- 20.Baser S, Shannon VR, Eapen GA, Jimenez CA, Onn A, Keus L, et al. Pulmonary dysfunction as a major cause of inoperability among patients with non-small-cell lung cancer. Clinical lung cancer. 2006;7(5):344–9. doi: 10.3816/CLC.2006.n.017. [DOI] [PubMed] [Google Scholar]
- 21.Abtin FG, Eradat J, Gutierrez AJ, Lee C, Fishbein MC, Suh RD. Radiofrequency ablation of lung tumors: imaging features of the postablation zone. Radiographics. 2012;32(4):947–69. doi: 10.1148/rg.324105181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamamoto A, Nakamura K, Matsuoka T, Toyoshima M, Okuma T, Oyama Y, et al. Radiofrequency ablation in a porcine lung model: correlation between CT and histopathologic findings. AJR Am J Roentgenol. 2005;185(5):1299–306. doi: 10.2214/AJR.04.0968. [DOI] [PubMed] [Google Scholar]
- 23.de Baere T, Palussiere J, Auperin A, Hakime A, Abdel-Rehim M, Kind M, et al. Midterm local efficacy and survival after radiofrequency ablation of lung tumors with minimum follow-up of 1 year: prospective evaluation. Radiology. 2006;240(2):587–96. doi: 10.1148/radiol.2402050807. [DOI] [PubMed] [Google Scholar]
- 24.Anderson EM, Lees WR, Gillams AR. Early indicators of treatment success after percutaneous radiofrequency of pulmonary tumors. Cardiovasc Intervent Radiol. 2009;32(3):478–83. doi: 10.1007/s00270-008-9482-6. [DOI] [PubMed] [Google Scholar]
- 25.Suh RD, Wallace AB, Sheehan RE, Heinze SB, Goldin JG. Unresectable pulmonary malignancies: CT-guided percutaneous radiofrequency ablation--preliminary results. Radiology. 2003;229(3):821–9. doi: 10.1148/radiol.2293021756. [DOI] [PubMed] [Google Scholar]
- 26.Ribeiro SM, Ruiz RL, Yoo HH, Cataneo DC, Cataneo AJ. Proposal to utilize simplified Swensen protocol in diagnosis of isolated pulmonary nodule. Acta Radiol. 2013;54(7):757–64. doi: 10.1177/0284185113481695. [DOI] [PubMed] [Google Scholar]
- 27.Doltra A, Amundsen BH, Gebker R, Fleck E, Kelle S. Emerging concepts for myocardial late gadolinium enhancement MRI. Curr Cardiol Rev. 2013;9(3):185–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peters DC, Wylie JV, Hauser TH, Kissinger KV, Botnar RM, Essebag V, et al. Detection of pulmonary vein and left atrial scar after catheter ablation with three-dimensional navigator-gated delayed enhancement MR imaging: initial experience. Radiology. 2007;243(3):690–5. doi: 10.1148/radiol.2433060417. [DOI] [PubMed] [Google Scholar]
- 29.Thawani R, McLane M, Beig N, Ghose S, Prasanna P, Velcheti V, et al. Radiomics and radiogenomics in lung cancer: A review for the clinician. Lung Cancer. 2018;115:34–41. doi: 10.1016/j.lungcan.2017.10.015. [DOI] [PubMed] [Google Scholar]
- 30.Beig N, Khorrami M, Alilou M, Prasanna P, Braman N, Orooji M, et al. Perinodular and Intranodular Radiomic Features on Lung CT Images Distinguish Adenocarcinomas from Granulomas. Radiology. 2019;290(3):783–92. doi: 10.1148/radiol.2018180910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aerts HJ, Velazquez ER, Leijenaar RT, Parmar C, Grossmann P, Carvalho S, et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun. 2014;5:4006. doi: 10.1038/ncomms5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grove O, Berglund AE, Schabath MB, Aerts HJ, Dekker A, Wang H, et al. Quantitative computed tomographic descriptors associate tumor shape complexity and intratumor heterogeneity with prognosis in lung adenocarcinoma. PLoS One. 2015;10(3):e0118261. doi: 10.1371/journal.pone.0118261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lambin P, Leijenaar RTH, Deist TM, Peerlings J, de Jong EEC, van Timmeren J, et al. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol. 2017;14(12):749–62. doi: 10.1038/nrclinonc.2017.141. [DOI] [PubMed] [Google Scholar]
- 34.Yang X, Ye X, Zheng A, Huang G, Ni X, Wang J, et al. Percutaneous microwave ablation of stage I medically inoperable non-small cell lung cancer: clinical evaluation of 47 cases. J Surg Oncol. 2014;110(6):758–63. doi: 10.1002/jso.23701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolf FJ, Grand DJ, Machan JT, Dipetrillo TA, Mayo-Smith WW, Dupuy DE. Microwave ablation of lung malignancies: effectiveness, CT findings, and safety in 50 patients. Radiology. 2008;247(3):871–9. doi: 10.1148/radiol.2473070996. [DOI] [PubMed] [Google Scholar]
- 36.Ron E, Modan B, Boice JD Jr., Alfandary E, Stovall M, Chetrit A, et al. Tumors of the brain and nervous system after radiotherapy in childhood. N Engl J Med. 1988;319(16):1033–9. doi: 10.1056/NEJM198810203191601. [DOI] [PubMed] [Google Scholar]
- 37.Ozasa K, Grant EJ, Kodama K. Japanese Legacy Cohorts: The Life Span Study Atomic Bomb Survivor Cohort and Survivors’ Offspring. J Epidemiol. 2018;28(4):162–9. doi: 10.2188/jea.JE20170321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Preston DL, Kusumi S, Tomonaga M, Izumi S, Ron E, Kuramoto A, et al. Cancer incidence in atomic bomb survivors. Part III. Leukemia, lymphoma and multiple myeloma, 1950–1987. Radiat Res. 1994;137(2 Suppl):S68–97. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.