Abstract
Background
The autoimmune profile of Chronic Urticaria (CU) patients is an increasing topic of interest. Associated diseases suggest shared pathogenic pathways, and they may provide important knowledge on specific targets for future treatment models. In this study we examined the prevalence and risk of comorbidities in CU.
Methods
The Danish National Patient Registry was used to identify all CU patients from 1994 to 2015. Five of 5 specialized dermatological units in Denmark were covered. Analyses were conducted as a nested case control study and a matched cohort study. CSU patients were matched 1:10 on age and sex to an otherwise random group of people from the background population.
Results
A total of 12,185 CU patients were identified, with an overweight of female cases (69% versus 32%). There was an overrepresentation of mast cell mediated diseases including mastocytosis and anaphylaxis, as well as atopic diseases including type 1 allergies and atopic dermatitis. The prevalence of rheumatoid arthritis, systemic lupus erythematosus, thyroiditis and vitiligo was also increased, as was the prevalence of depression. CU patients who did not have any of the co-morbidities at the time of their CU diagnosis had an increased risk of developing both mast cell mediated diseases, atopic diseases, and autoimmune diseases excluding thyroiditis and diabetes.
Conclusion
The autoimmune profile of the comorbidities of CU was demonstrated with an evident risk of developing rheumatoid arthritis. CU patients were also at increased risk of either having or achieving depression. Mast cell related diseases seemed to be overrepresented, although registry data within this disease category are questionable and similar to symptoms of CU to the untrained eye. Thus, CU patients constitute a multimorbid group of patients, which must be recognized among treating physicians.
Keywords: Chronic urticaria, Demographics, Prevalence, Incident risk, Comorbidities
Abbreviations: AD, Atopic Dermatitis; ANA, Anti Nuclear Antibodies; CSU, Chronic spontaneous urticaria; CU, Chronic urticaria; DCRR, Dnish Civil Registration Registry; DNPR, Danish National Patient Registry; HR, Hazard Ratio; ICD, International Classification of Diseases; Ig, Immunglobulin; OR, Odds ration; RECORD, Reporting of Studies Conducted using observational Routinely Collected Health Data; SLE, Systemic Lupus Erythematosus
Introduction
Chronic urticaria (CU) is defined by the presence of hives and itch for 6 weeks or longer.1 Most cases are observed at ages between 20 to 40 years. The prevalence of CU in the general population is 0.5–1% and the average duration of CU is 3–5 years.2 First-line treatment for CU is non-sedating antihistamines in standard doses. However, when the disease control is inadequate, dose escalation of antihistamines to up-to fourfold dosage is recommended. If symptoms persist, add-on therapy with omalizumab (anti-IgE) every fourth week is recommended.1 Previous studies show some efficacy of Cyclosporine A suggesting an active immunological mechanism as part of the pathogenesis.
Recent data suggest that CU is not only a disease of the mast cells, it also is a systemic3,4 autoimmune disease.5 Furthermore, it has been shown that CU patients also have an increased risk of having other autoimmune conditions, which may be associated to urticaria or increase the patient's susceptibility to CU,6 although the pathogenic mechanisms of CU have not been fully understood. The patients may have autoreactive IgG molecules that cross-link IgE or the IgE receptor FcεRI on the surface of the mast cell causing these to degranulate, yet some patients do not have any known cause of the disease. Emerging evidence suggests that the presence of auto-IgE-antibodies may have a pivotal role in the pathogenesis of CU.7
CU influences the health of patients in other ways and is associated with a range of comorbidities. In the Scandinavian AWARE-study, a follow up-study of 158 patients with CU refractory to antihistamine treatment from Denmark, Norway, and Sweden, an increased prevalence of atopic diseases including atopic dermatitis, asthma, and rhino-conjunctivitis was demonstrated.8 Furthermore, a high prevalence of thyroid disease, hypertension, and obesity was observed. Two large registry-studies from Korea and Taiwan demonstrated the same pattern of comorbidities but also an increased prevalence of drug allergies, rheumatic- and inflammatory diseases and cancers as well as psychiatric diseases. Mental disorders and emotional distress including anxiety, depression, and somatoform disorders are the most common comorbidities in CU patients.9,10 A recent systematic review of the literature on autoimmune comorbidities to CU showed that the most common autoimmune comorbidities were autoimmune thyroid diseases and vitiligo (>2% of chronic spontaneous urticaria (CSU) patients), and that the most common circulating auto-antibodies were anti-thyroid antibodies and anti-nuclear antibodies (ANA) (>15% of CSU patients).5,7 In addition, another meta-analysis demonstrated that in Systemic Lupus Erythematosus (SLE) an urticarial rash is common, ranging from 0-21.9% for CSU and 0.4–27.1% for an urticarial rash. On the other hand, data for the prevalence of SLE in CSU patients are lacking and further studies are needed.11
A few studies have examined the prevalence of cardiovascular diseases but not found any increased risk of these in CU patients compared to the general population, although this study did not take severity into account.12 However, hypertension is associated with prolonged duration of CU.13
Taken together, CU seemingly is associated to a specific pattern of comorbidities including autoimmune diseases, atopic diseases, and psychiatric diseases, whereas the pattern is not clear with regards to malignancies and cardiovascular diseases. In this population-based study, comorbidities of 12,185 Danish CU patients were investigated. All dermatological units in Denmark were covered during the period 1994 until 2015.
Patients and methods
Design
The Danish National Patient Registry (DNPR) was used to identify all patients with a diagnosis of urticaria from January 1, 1994 until December 31, 2015. Comorbidities were investigated from a comparative cross sectional perspective as well as from a historical matched cohort perspective, the latter including mortality. The study was approved by the Danish Data Protection Agency and adhered to the RECORD statement (Reporting of studies Conducted using Observational Routinely collected health data).14
The Danish Civil Registration Registry
In Denmark all citizens are given a unique civil registration number at birth. The number is stored in the Danish Civil Registration Registry (DCRR) along with data including date of birth, sex, civil and vital status, and date of death and/or emigration. All information is updated daily on citizens residing in Denmark. The civil registration number is used in all registries administered by the Danish National Health Authorities, and linkage of data can be performed unambiguously.
The Danish National Patient Registry
The DNPR was established in 1977 to monitor activities of hospital and health services in Denmark. The registry contains information on all hospital admissions from 1977 until today, and all outpatient and emergency contacts since 1994. Diagnoses are recorded in accordance to two international disease classification systems; ICD-8 until 1994 and ICD 10 from 1995 until today.
Study population
In this study, patients with a first-time diagnosis of CU (Table 1), diagnosed at a dermatology unit from January 1, 1994 until December 31, 2015, were included. The date of the diagnosis will be referred to as the index date. All patients were matched with 10 age and sex matched controls, which had never been registered with a diagnosis of urticaria. Age was matched within 6 weeks in order to get enough controls. Controls were given the same index date as their case. To increase the validity of the urticaria diagnoses only patients diagnosed in specialized dermatological and allergology departments (5 in total in Denmark) were included along with their matched controls. First time contacts of comorbidity diagnoses of interest were registered during the complete investigation period. All ICD-10 and ICD-8 codes of comorbidity diagnoses (outcomes) are listed in Table 1.
Table 1.
ICD 8 and ICD10 codes of index diagnoses and outcome diagnoses. ICD 8 was used from 1977 until 1993 and ICD from 1994 until today
| Co-morbidities diagnose codes. | ||
|---|---|---|
| Diagnosis | ICD-10 | ICD-8 |
| Urticaria | DL50, DL563, DL282A, DO268H. | |
| Mastocytosis | DQ82, DD47, DC943, DC962 | 75722 |
| Anaphylaxis | DT780, DT782, DT788A, DT634F, DT886, DT805, DD841A | 99949, 70809 |
| Rhino-Conjunctivitis | DJ30, DH101 | 50709, 36003 |
| Thyroditis | DE00-DE07 | 240–246 |
| Depression | DF30-DF39 | 790 |
| Psycosis | DF20-DF29 | 296–299 |
| Osteoporosis | DM80-DM82, DM858A, DM895 | 723 |
| Atopic Dermatitis | DL20 | 69100 |
| Reumatoid Arthritis | DM05-DM14 | 710–718 |
| Lupus Erythematosus | DL93, DM32 | 734, 695 |
| Vitiligo | DL80 | 709 |
| Peptic Ulcer | DK25-DK28 | 532–534 |
| Diabetes Mellitus | DE100-DE101, DE106, DE108-DE109, DE110-DE111, DE116, DE118-DE119, DE120-DE121, DE126, DE128-DE129, DE130-DE131, DE136, DE138-DE139, DE140-DE141, DE146, DE148-DE149 | 24900, 24906, 24907, 24909, 25000, 25006, 25007, 25009 |
Statistics
At the index date, baseline comorbidities in CU patients compared to those of the control population were assessed using a logistic regression model. Results were reported as Odds Ratios (ORs). Time-to-event analysis using Cox proportional hazards regression were performed. Patients and controls were followed from the index date until the date of outcome, the end of follow up, emigration, loss to follow up or death of the index patient. Patients, in which the outcome diagnosis had occurred before the date of CU were excluded along with their controls in the stratified comorbidity analyses. Results were reported as Hazard Ratio's (HRs). In a sub analysis diagnoses within the first year from the index date were excluded (HRadj). The assumption of proportional hazards was verified using log-log plots. Median survival was estimated in a Kaplan-Meier model. All analyses were performed using IBM SPSS statistics, version 22. Comparisons were considered significant when p-values were ≤0.05.
Results
Baseline demographics and characteristics
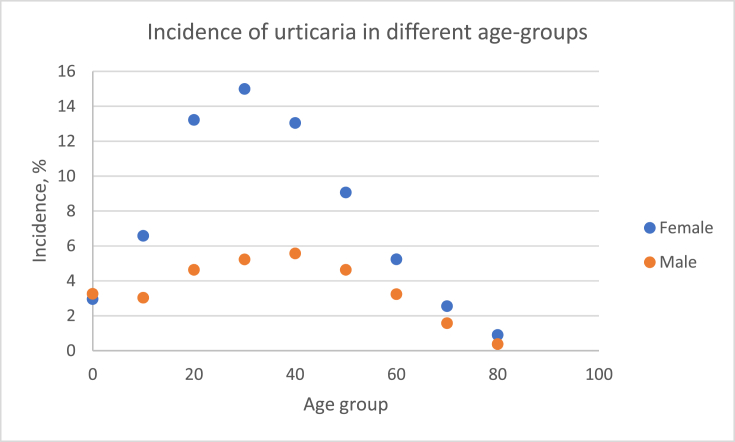
A total of 116,192 patients were found to have one of the inclusion diagnoses registered in the DNPR. Of these 12,185 patients had been diagnosed at a specialized dermatology and allergology center and were included along with their controls. The mean age at the time of inclusion was 38.4 years among the patients diagnosed at a specialized department, while the mean age at inclusion was 38.8 years in the matched cohort. The proportion of female patients was higher than male patients in both groups, 68.5% vs. 31.5% in the urticaria group and 67.1% vs. 32.9% in the matched cohort (Table 2) (That it is not an exact match 1:10 is due to the large number of patients made it impossible to match all 1:10. However there was no significant difference). The peak incidence of CU in both sexes appears between 20-40 years of age (Fig. 1).
Table 2.
Baseline demographics and total number of patients in with each outcome
| Urticaria, n (%) |
Healthy controls |
|
| 12185 |
104007 |
|
| sex | ||
| female | 8352 (68.5) | 69807 (67.1) |
| male |
3833 (31.5) |
34200 (32.9) |
| Age at inclusion, mean |
38,4 years |
38,8 years |
| Inclusion Diagnoses | ||
| Urticaria (L50) | 10500 (86.2) | |
| Urticaria papulosa (L282) | 81 (0.7) | |
| Urticaria Solaris (L563) | 128 (1.1) | |
| Diseases related to pregnancy (O268) |
185 (1.5) |
|
| Comorbidities N (%) |
Total | By diagnosis | After diagnosis | 1 year after diagnosis | Total |
|---|---|---|---|---|---|
| Mastocytosis | 192 (1.6) | 20 (0.2) | 172 (1.4) | 23 (0.2) | 7 (0.0) |
| Anaphylaxis | 73 (0.6) | 33 (0.3) | 40 (0.3) | 21 (0.2) | 72 (0.1) |
| Rhinoconjunctivitis | 351 (2.9) | 276 (2.3) | 75 (0.6) | 0 | 672 (0.6) |
| Atopic dermatitis | 302 (2.5) | 95 (0.8) | 207 (1.7) | 30 (0.2) | 256 (0.2) |
| Thyroditis | 40 (0.3) | 11 (0.1) | 29 (0.2) | 22 (0.2) | 153 (0.1) |
| Rheumatoid Arthritis | 221 (1.8) | 103 (0.8) | 118 (1.0) | 98 (0.8) | 793 (0.8) |
| Lupus Erythematosus | 40 (0.3) | 20 (0.2) | 20 (0.2) | 0 | 69 (0.1) |
| Vitiligo | 7 (0.1) | 7 (0.1) | 0 | 0 | 0 |
| Depression | 535 (4.4) | 292 (2.4) | 243 (2.0) | 217 (1.8) | 3058 (2.9) |
| Psychosis | 121 (1.0) | 69 (0.6) | 52 (0.4) | 48 (0.4) | 836 (0.8) |
| Osteoporosis | 358 (2.9) | 105 (0.9) | 253 (2.1) | 226 (1.9) | 1731 (1.7) |
| Peptic ulcer | 127 (0.1) | 52 (0.4) | 75 (0.6) | 68 (0.6) | 732 (0.7) |
| Diabetes Mellitus | 276 (2.3) | 123 (1.0) | 153 (1.3) | 135 (1.1) | 1812 (1.7) |
Fig. 1.
The age dependent Incidence of Chronic Urticaria in both sexes
Study period comorbidities
The most common comorbidities in the CU group, when counting at the time of diagnosis and during follow up were depressions (4.4%), rhino-conjunctivitis (2.9%), osteoporosis (2.9%), atopic dermatitis (2.5%), and diabetes mellitus (2.3%), while the least common were peptic ulcers (0.1%), psychosis (1.0%), anaphylaxis (0.6%), thyroiditis (0.3%), SLE (0.3%), and vitiligo (0.1%) (Table 2). At the index date previous or present diagnoses of mastocytosis (OR = 171 (95% CI: 27–7086)), anaphylaxis (OR = 7.63 (4.62–12.54)), rhino-conjunctivitis (OR = 5.43 (4.65–6.33)), vitiligo OR = 5.43 ((1.78–15.35)), SLE (OR = 4.72 (2.36–7.4)), and atopic dermatitis OR = 4.69 (3.61–6.06) were significantly higher in the CU population.
No increased OR was seen in patents with CU for thyroiditis, psychosis, peptic ulcer, and diabetes mellitus. Yet when adjusting for sex there was a significantly higher OR of thyroiditis and vitiligo among women than men, OR = 1.45 (2.79–6.72) and OR = 9.75 (2.8–35.15), respectively (Table 3).
Table 3.
Prevalence and Risk of outcomes in chronic urticaria patients
| Comorbidity | OR (95% CI) | OR sex (95% CI) | HR (95% Cl) | HR adj | HR adj stratified sex |
|---|---|---|---|---|---|
| Mastocytosis | 171.0 (27.3–7086.0) | 214.0 (94.6–484.7) | 22.2 (8.6–57.3) | – | |
| Female | 83.7 (11.9–363.0) | 12.4 (3.9–39.2) | |||
| Male | – | 71.1 (8.9–568) | |||
| Anaphylaxsia | 7.6 (4.6–12.5) | 9.02 (5.7–14.3) | 4.8 (2.7–8.4) | – | |
| Female | 5.7 (3.0–10.5) | 4.4 (2.3–8.6) | |||
| Male | 13.9 (5.6–36.5) | 5.8 (2.1–16.3) | |||
| Hay fever | 5.4 (4.7–6.3) | 2.2 (1.6–3.0) | .. | ||
| Female | 5.8 (4.9–7.0) | .. | |||
| Male | 4.6 (3.4–6.1) | .. | |||
| Thyroiditis | 1.3 (0.7–2.6 | 2.0 (1.2–3.3) | 1.4 (0.8–2.6) | ||
| Female | 1.4 (0.8–5.6) | ||||
| Male | 1.5 (2.8–6.7) | <0,001 | |||
| Depression | 1.5 (1.3–1.7) | 1.9 (1.3–2.8) | 1.3 (1.1–1.6) | ||
| Female | 1.4 (1.2–1.6) | 1.2 (1.0–15) | |||
| Male | 1.7 (1.3–2.2) | 1.6 (1.2–2.2) | |||
| Psychosis | 1.1 (0.8–1.4) | 1.3 (0.4–4.2) | 1.4 (1.0–1.9) | ||
| Female | 1.1 (0.8–1.6) | 1.8 (1.2–2.7) | |||
| Male | 1.0 (0.6–1.5) | 0.9 (0.5–1.6) | |||
| Osteoporosis | 1.6 (1.3–1.9) | 1.6 (1.4–1.9) | 1.6 (1.4–1.9) | ||
| Female | 1.5 (1.2–1.9) | 1.5 (1.3–1.8) | |||
| Male | 1.9 (0.9–3.6) | 2.6 (1.7–3.7) | |||
| Atopic dermatitis | 4.7 (3.6–6.1) | 19.2 (14.8–25.0) | 3.1 (2.0–4.8) | ||
| Female | 4.6 (3.4–6.3) | 3.0 (1.7–5.0) | |||
| Male | 4.8 (2.8–7.9) | 3.5 (1.5–7.9) | |||
| Rheumatoid arthritis | 2.2 (1.7–2.7) | 2.0 (1.6–2.5) | 1.8 (1.4–2.3) | ||
| Female | 2.3 (1.8–2.9) | 1.9 (1.4–2.6) | |||
| Male | 1.8 (1.1–2.9) | 1.5 (0.9–2.6) | |||
| Lupus erythematosus | 4.7 (2.4–7.4) | 4.0 (2.1–7.7) | 1.8 (1.4–2.3) | ||
| Female | 4.7 (2.6–8.3) | 1.9 (1.4–2.6) | |||
| Male | . | 1.5 (0.9–2.6) | |||
| Vitiligo | 5.4 (1.8–15.4) | .. | .. | ||
| Female | 9.8 (2.8–35.2) | ||||
| Male | .. | .. | |||
| Peptic ulcer | 1.3 (1.0–1.7) | 1.4 (1.0–1.8) | 1.4 (1.1–1.9) | ||
| Female | 1.4 (1.0–2.0) | 1.7 (1.2–2.3) | |||
| Male | 1.1 (0.6–1.9) | 1.0 (0.6–1.8) | |||
| Diabetes mellitus | 1.1 (0.9–1.4) | 1.3 (1.1–1.6) | 1.3 (1.1–1.6) | ||
| Female | 1.1 (0.8–1.4) | 1.3 (1.0–1.7) | |||
| Male | 1.2 (0.9–1.7) | 1.3 (1.0–1.7) |
Risk of comorbidities after diagnosis of CU
We found an increased risk for atopic dermatitis, HR = 3.09 (2.0–4.8), mastocytosis, HR = 22.21 (8.6–57.3), anaphylaxis, HR = 4.8 (2.7–8.4), allergic rhino conjunctivitis, HR = 1.4 (0.75–2.55) depression, HR = 1.32 (1.13–1.55), and rheumatoid arthritis, HR = 1.8 (1.4–2.3) (Table 2). However, no increased risk was found for psychosis, SLE, and vitiligo. Excluding patients who had the comorbidity, for which the risk was calculated, diagnosed within the first year after diagnosis of CU along with their matched controls, showed that the highest risk was found for mastocytosis, HRadj = 22.21 (8.6–57.3), anaphylaxis, HRadj = 4.8 (2.7–8.4), vitiligo, HRadj = 4.22 (2.1–8.4), and atopic dermatitis, HRadj = 3.09 (2.0–4.8). There was no significantly increased risk for thyroiditis and psychosis, although the risk of psychosis was almost significantly increased, HRadj = 1.38 (0.99–1.93) (Table 3).
The greatest change in risk when adjusting for diagnosis of the comorbidity in question within the first year was seen for mastocytosis, atopic dermatitis, and anaphylaxis, in which the risk decreased.
Survival
A total of 7 patients with their matched controls were excluded from the survival analysis as their end date appeared before the index date. The CU group had a slightly decreased mortality rate compared to controls. A total of 8,444 (8.12%) of the matched controls died in the follow up period of the study, while 693 (5.7%) urticaria patients died. The mean survival was 7,161 days (7,144–7,179) in the control group and 7,418 days (7,374–7,461) in the CU group, HR = 0.638 (p < 0.001).
Discussion
In this register based study; comorbidities and mortality in a cohort of Danish CU patients, were addressed. All tertiary center cases during a 21-year period were included.
More women than men were diagnosed with CU, similar to observations in other studies.3,4,15 It may reflect a gender dependent distribution of CU, but as our data are based on hospital registries, results may also reflect the fact that men are less likely to seek medical attention than women.16 Consequently, we suggest that male cases of CU may need further information on the disease and on its treatments.
We found an increased survival of CU patients compared to controls. If a patient suffers from a malignant disease, especially in the young or middleaged patient, the treating doctor may underestimate the significance of CU, and the patient may be referred to the general practitioner for treatment of this where the diagnosis is not registered. This would lead to underrepresentation of deaths in the CU group.
There was a significantly higher risk of either having or achieving mastocytosis and anaphylaxis in the CSU group. Mastocytosis is a proliferative mast cell disease, and the symptoms are mediated by histamine, and they may be indistinguishable from urticaria.17 It is also a rare disease, with discrete, if any, symptoms at all, and the overrepresentation is probably due to misdiagnosis at their first hospital visit. This hypothesis is further supported by the adjusted HR, which decreased dramatically, when diagnoses of mastocytosis within the first year from the index date were excluded. Most likely, CU-patients will not receive their proper diagnosis before they see a trained dermatologist and become examined with blood tests and biopsies to reveal their true and final diagnosis.
Anaphylaxis is a symptom of mastocytosis, rather than of CU,17 and the overrepresentation may be explained by either overrepresentation of mastocytosis or an overrepresentation of allergic diseases in the CU population. Many CU patients are convinced that their symptoms are due to allergies. Hence, a number of contacts in an ER setting are with a tentative diagnosis of anaphylaxis. About 5% of all CU patients suffer from a subtype, which only cause angioedema. As one of the standard criteria of anaphylactic shock are oedema of the oral mucosa, this is yet another explanation to the increased prevalence of anaphylaxis observed in our CSU population.
The atopic diseases are also strongly overrepresented among CU patients. In case of sensitization and rhino-conjunctivitis, the symptoms of rhino-conjunctivitis may be intensified due to an increased potential of action and activation of the mast cells, and an increased level of specific IgE.18,19 Atopic dermatitis is neither a mast cell driven nor an IgE dependent disease. In atopic dermatitis patients IgE mediated allergies are overrepresented, and AD patients diagnosed with and IgE mediated skin reaction as CU, rather than type I allergy, may lead to an overrepresentation of prior atopic dermatitis in our CU population, as suggested in the study by Bieber et al..20 Furthermore, atopic dermatitis patients have a chronic inflammatory reaction in the skin, in which mast cells are also overrepresented which consequently leads to urticaria symptoms.21 This result is consistent with previous studies both in Scandinavia and the rest of the world.3,4,8
Our data suggest a higher prevalence and risk of autoimmune diseases, including rheumatoid arthritis, vitiligo, and thyroiditis among CU patients. However, they are very differently represented. Rheumatoid arthritis is numerically much higher represented than SLE, thyroiditis, and vitiligo. It could be speculated if the increased presence of rheumatoid arthritis in CU patients is a reflection of an increased inflammatory status overall, destabilizing mast cells and thereby causing urticaria, whereas in lupus, vitiligo, and thyroiditis the pathogenesis is mainly caused by specific auto-antibodies of IgG and IgE type. Our findings are consistent to those of other studies and the autoimmune profile of CU patients need to be acknowledged.
At the index date, there was a higher prevalence of depressions in the CU group. The prevalence of prior diagnoses of psychoses were comparable to that of the background population, yet the risk, especially for females, tended to increase over time. As in atopic dermatitis and psoriasis vulgaris, having chronic skin diseases is a pivotal factor when it comes to the mental health and quality of life of these patients.22 In psoriasis and atopic dermatitis it has been speculated whether the chronic inflammation itself may cause some of the symptoms,23 however, social isolation, altered self-perception, and lack of sleep due to itch are the probable and most likely reasons,22 which may also be the case in CU. The prevalence and future risk of depression was indeed increased, which may reflect that many CU patients have had their diagnosis for years before being referred to a hospital setting. Psychological health, itch, and sleep loss are therefore important parameters in the consultation with CU patients, and perhaps the association between CU on one hand and depression on the other could be prevented, if the guidelines of the EAACI/GA2LEN/EDF/WAO are adhered to when treating AD patients.
We found an increased risk of osteoporosis and diabetes mellitus. Both diseases are increased in patients treated with glucocorticoids, and unfortunately this is still used despite recommendations from EAACI/GA2LEN/EDF/WAO.1,8,24 Yet in mastocytosis, a purely mast cell driven disease, an increased risk of osteoporosis is also observed due to mast cell mediators,17 and this may also be the case in urticaria, yet observational studies are needed to determine if this is the case.
The strength of this study is the nationwide coverage of the Danish registries. Furthermore, we were able to distinguish if the diagnosis had been made at a dermatology- or specialized allergology clinic, which should raise the diagnostic specificity. On the other hand, data are only representable to tertiary center cases of CU, and milder cases, or even some severe cases, were not covered in this study.
In conclusion, CU patients do have an increased burden of co-morbidities especially within the groups of mast cell mediated diseases and atopic diseases as well as in the autoimmune group of diseases. Numerically, the autoimmune diseases are less frequent than the others. The CU patients also have a higher prevalence and risk of developing depression. Thus, CU patients are a multimorbid group of patients, a fact that must be recognized among clinicians treating this group of patients.
Conflict of interest
None to declare for MNG, SFT, LK.
CV Declares: grants from Novartis, grants from Abb-vie, outside the submitted work.
Funding
The work was funded by an unrestricted grant from Novartis.
Ethics approval
The project has been approved by the Danish Data Protection Agency (approval No. 1-16-02-132-16).
Author contributions
Conceptualization and Idea: MNG, LK, SFT, CV.
Calculation and statistics: MNG, LK, SFT and CV.
Writing of manuscript: MNG, LK, SFT and CV.
Footnotes
Full list of author information is available at the end of the article
References
- 1.Zuberbier T., Aberer W., Asero R. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. The 2017 revision and update. Allergy. 2018 Jul;73(7):1393–1414. doi: 10.1111/all.13397. [DOI] [PubMed] [Google Scholar]
- 2.Maurer M., Weller K., Bindslev-Jensen C. Unmet clinical needs in chronic spontaneous urticaria. A GA(2)LEN task force report. Allergy. 2011;66(3):317–330. doi: 10.1111/j.1398-9995.2010.02496.x. [DOI] [PubMed] [Google Scholar]
- 3.Chu C.Y., Cho Y.T., Jiang J.H., Lin E.I., Tang C.H. Epidemiology and comorbidities of patients with chronic urticaria in Taiwan: a nationwide population-based study. J Dermatol Sci. 2017;88(2):192–198. doi: 10.1016/j.jdermsci.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 4.Kim B.R., Yang S., Choi J.W., Choi C.W., Youn S.W. Epidemiology and comorbidities of patients with chronic urticaria in Korea: a nationwide population-based study. J Dermatol. 2018;45(1):10–16. doi: 10.1111/1346-8138.14075. [DOI] [PubMed] [Google Scholar]
- 5.Kolkhir P., Borzova E., Grattan C. Autoimmune comorbidity in chronic spontaneous urticaria: a systematic review. Autoimmun Rev. 2017;16(12):1196–1208. doi: 10.1016/j.autrev.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Kim Y.S., Han K., Lee J.H. Increased risk of chronic spontaneous urticaria in patients with autoimmune thyroid diseases: a nationwide, population-based study. Allergy Asthma Immunol Res. 2017;9(4):373–377. doi: 10.4168/aair.2017.9.4.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kolkhir P., Metz M., Altrichter S., Maurer M. Comorbidity of chronic spontaneous urticaria and autoimmune thyroid diseases: a systematic review. Allergy. 2017;72(10):1440–1460. doi: 10.1111/all.13182. [DOI] [PubMed] [Google Scholar]
- 8.Thomsen S.F., Pritzier E.C., Anderson C.D. Chronic urticaria in the real-life clinical practice setting in Sweden, Norway and Denmark: baseline results from the non-interventional multicentre AWARE study. J Eur Acad Dermatol Venereol. 2017;31(6):1048–1055. doi: 10.1111/jdv.14210. [DOI] [PubMed] [Google Scholar]
- 9.Barbosa F., Freitas J., Barbosa A. Chronic idiopathic urticaria and anxiety symptoms. J Health Psychol. 2011;16(7):1038–1047. doi: 10.1177/1359105311398682. [DOI] [PubMed] [Google Scholar]
- 10.Staubach P., Dechene M., Metz M. High prevalence of mental disorders and emotional distress in patients with chronic spontaneous urticaria. Acta Derm Venereol. 2011;91(5):557–561. doi: 10.2340/00015555-1109. [DOI] [PubMed] [Google Scholar]
- 11.Kolkhir P., Pogorelov D., Olisova O., Maurer M. Comorbidity and pathogenic links of chronic spontaneous urticaria and systemic lupus erythematosus--a systematic review. Clin Exp Allergy. 2016;46(2):275–287. doi: 10.1111/cea.12673. [DOI] [PubMed] [Google Scholar]
- 12.Egeberg A., Kofoed K., Gislason G.H., Vestergaard C., Thyssen J.P. Cardiovascular risk is not increased in patients with chronic urticaria: a retrospective population-based cohort study. Acta Derm Venereol. 2017;97(2):261–262. doi: 10.2340/00015555-2516. [DOI] [PubMed] [Google Scholar]
- 13.Nebiolo F., Bergia R., Bommarito L. Effect of arterial hypertension on chronic urticaria duration. Ann Allergy Asthma Immunol. 2009;103(5):407–410. doi: 10.1016/S1081-1206(10)60360-2. [DOI] [PubMed] [Google Scholar]
- 14.Chen M.Y., Langan S., Benchimol E.I. Routinely collected electronic health data and STI research: RECORD extension to the STROBE guidelines. Sex Transm Infect. 2016;92(1):2–3. doi: 10.1136/sextrans-2015-052360. [DOI] [PubMed] [Google Scholar]
- 15.Lapi F., Cassano N., Pegoraro V. Epidemiology of chronic spontaneous urticaria: results from a nationwide, population-based study in Italy. Br J Dermatol. 2016;174(5):996–1004. doi: 10.1111/bjd.14470. [DOI] [PubMed] [Google Scholar]
- 16.Galdas P.M., Cheater F., Marshall P. Men and health help-seeking behaviour: literature review. J Adv Nurs. 2005;49(6):616–623. doi: 10.1111/j.1365-2648.2004.03331.x. [DOI] [PubMed] [Google Scholar]
- 17.Brockow K. Epidemiology, prognosis, and risk factors in mastocytosis. Immunol Allergy Clin N AM. 2014;34(2):283–295. doi: 10.1016/j.iac.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Christensen C.U., Vestergaard C., Hoffmann H.J. Activation markers CD63 and CD203c are upregulated in chronic urticaria. Ann Dermatol. 2013;25(4):522–523. doi: 10.5021/ad.2013.25.4.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Puxeddu I., Pratesi F., Ribatti D., Migliorini P. Mediators of inflammation and angiogenesis in chronic spontaneous urticaria: are they potential biomarkers of the disease? Mediat Inflamm. 2017;2017:4123694. doi: 10.1155/2017/4123694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bieber T. Atopic dermatitis. N Engl J Med. 2008;358(14):1483–1494. doi: 10.1056/NEJMra074081. [DOI] [PubMed] [Google Scholar]
- 21.Kawakami T., Ando T., Kimura M., Wilson B.S., Kawakami Y. Mast cells in atopic dermatitis. Curr Opin Immunol. 2009;21(6):666–678. doi: 10.1016/j.coi.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dalgard F.J., Gieler U., Tomas-Aragones L. The psychological burden of skin diseases: a cross-sectional multicenter study among dermatological out-patients in 13 European countries. J Investig Dermatol. 2015;135(4):984–991. doi: 10.1038/jid.2014.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farzanfar D., Dowlati Y., French L.E., Lowes M.A., Alavi A. Inflammation: a contributor to depressive comorbidity in inflammatory skin disease. Skin Pharmacol Physiol. 2018;31(5):246–251. doi: 10.1159/000490002. [DOI] [PubMed] [Google Scholar]
- 24.Maurer M., Houghton K., Costa C. Differences in chronic spontaneous urticaria between Europe and Central/South America: results of the multi-center real world AWARE study. World Allergy Organ J. 2018;11(1):32. doi: 10.1186/s40413-018-0216-1. [DOI] [PMC free article] [PubMed] [Google Scholar]