Abstract
Fragment QRS (fQRS) complex is a myocardial conduction abnormality that indicates myocardial scar. It is defined as additional notches in the QRS complex. Though initially fQRS was defined in the setting of normal QRS duration (<120 m s), later it has been expanded to include conditions with wide QRS complexes as in bundle branch block, ventricular ectopy and paced rhythm, when more than 2 notches are present. It is an important, yet often overlooked marker of mortality and arrhythmic events in many cardiac diseases. The significance of fQRS lies in the fact that it just requires a surface ECG for its recording and the value of information about the condition of the heart it dispenses based on the clinical setting. We review the role of fQRS in predicting adverse cardiac events in various conditions.
Keywords: Fragmented QRS, fQRS, Myocardial scar, Cardiac arrhythmias, Adverse cardiac events, Heart failure, Acute myocardial infarction, Cardiomyopathy
1. Introduction
Appearance of additional spikes in the QRS complexes has gained interest in recent years. These are different from the standard rSR′ pattern seen in right bundle branch block (RBBB) and the notched R waves seen in left bundle branch block (LBBB) [1]. Fragmented QRS (fQRS) was initially defined as additional spikes in the QRS complex in the absence of bundle branch block [2]. But later the definition was widened to include additional notches over and above the pre-existent pattern even in wide QRS due to bundle branch block, paced rhythm or ventricular ectopy. This is termed fragmented wide QRS (f-wQRS) in contrast with fragmented narrow QRS (QRS duration <120 m s). fQRS in a paced rhythm has been designated f-pQRS [3].
Over the past few years, literature on fQRS has evolved, with studies in a wide variety of cardiac conditions ranging from coronary artery disease (CAD), cardiomyopathies, valvular heart disease, aortic dissection, pulmonary embolism, congenital heart disease and cardiac channelopathies. Studies of fQRS in various primarily non-cardiac conditions like obstructive sleep apnea, renal disease, cirrhosis of the liver, radiotherapy in breast cancer, autoimmune disorders and beta thalassemia have also been published. This review is an attempt to compile clinically relevant information from the available literature, focusing primarily on cardiac conditions.
2. Definition
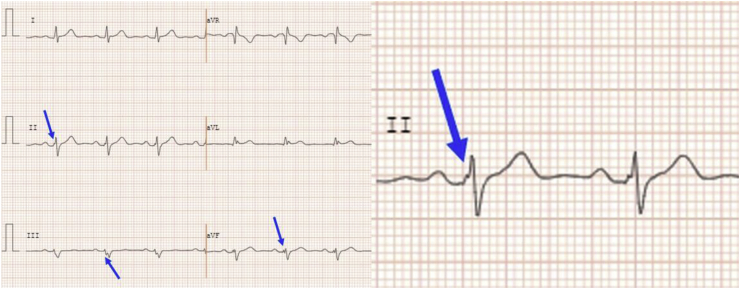
fQRS can be defined as the presence of additional R′ waves or a notch in the nadir of the R or S wave (fragmentation) in two contiguous leads corresponding to a coronary territory in a routine 12-lead ECG (0.5–150 Hz) [4]. Fragmented wide QRS (f-wQRS) is defined as two or more notches in the R or S wave, in two contiguous leads corresponding to a coronary territory (anterior, lateral or inferior) (Fig. 1). The notches should be separated by at least 40 m s.
Fig. 1.
Fragmented QRS in inferior leads.
3. Inter and intra-observer variability
Inter and intra observer variability of fQRS has been studied by Vandenberk B et al. [5]. Hundred ECGs with fQRS were evaluated by two experienced and 3 novel observers. Fleiss and Cohen’s Kappa was calculated among subgroups. There was a significant inter-observer variability with a Kappa of 0.651. Experienced observers had a better agreement with a Kappa of 0.823. Inter-observer variability was much higher in paced-rhythm (f-pQRS) compared with normal rhythm, with Kappa 0.493 vs 0.664 (p < 0.001). Intra-observer variability had a Kappa between 0.736 and 0.880. So visual assessment variability will depend on experience as well as the underlying rhythm.
4. Automation of detection
Conventionally fQRS assessment is done by visual inspection of the 12 lead ECG by trained operators. Automation of detection by computer-based algorithms have been attempted [6]. Out of 40 ECGs randomly selected from a database, 31 were opined to be suitable for analysis by two experienced cardiologists. They demonstrated a sensitivity of 0.897 and specificity 0.899 for the detection of f-QRS by their algorithm. Authors went on to suggest that automation will speed up the detection and reduce human error as well as allow implementation of hospital-based remote monitoring. This could also be an option in implantable cardioverter defibrillator devices (ICD) in future.
5. Coronary artery disease
5.1. Prognostic significance of inferior vs. anterior lead fQRS
Though the correlation of fQRS with various adverse cardiac events has been widely studied, a little was reported comparing the outcomes of fQRS on inferior vs anterior leads. Eyuboglu et al. have studied the severity of CAD in patients with inferior and anterior lead fQRS. Anterior lead fQRS was associated with higher incidence of multivessel disease (p = 0.007) and greater severity of CAD, as indicated by a higher median SYNTAX score (p = 0.047) [7]. Terho HK et al., evaluated the prognostic significance of fQRS on inferior, anterior and lateral leads. fQRS was common in inferior leads. Mere presence of fQRS without an established cardiac disease didn’t predict an adverse cardiac outcome. Lateral lead fQRS had the least incidence among the three but carried a higher risk of all-cause mortality (p = 0.001) [8].
5.2. fQRS: a marker of myocardial scar
The significance of fQRS was initially studied and compared with Q wave in patients undergoing nuclear stress test [2]. The sensitivity, specificity, and the negative predictive value for myocardial scar as were 36.3%, 99.2%, and 70.8%, respectively, for the Q wave alone; 85.6%, 89%, and 92.7%, respectively, for the fQRS; and 91.4%, 89%, and 94.2%, respectively, for the Q wave and/or fQRS. Therefore, fQRS on a 12-lead ECG is a marker of a prior MI, with a substantially higher sensitivity and negative predictive value compared with the Q wave.
Several studies have evaluated the significance of fQRS in conditions like ST elevation [9] and non ST elevation myocardial infarction [10], stable angina pectoris [11], relationship with percutaneous coronary intervention (PCI) [12], cardiac rehabilitation [13], collateral circulation [14], slow flow [15], coronary ectasia [16] left ventricular thrombus [17] and aneurysm [18].
5.3. ST elevation myocardial infarction (STEMI)
fQRS can be used as a tool for risk stratification in STEMI. Tanriverdi et al. studied the association of fQRS with in-hospital mortality rate in STEMI patients. Patients (n = 248) with fQRS on ECG taken within 48 h had higher incidence of in-hospital mortality (p = 0.002). Greater the number of leads with fQRS, higher was the in-hospital mortality rate (p = 0.023) [19]. The prognostic value was better if fQRS was combined with distorted QRS to predict in-hospital mortality rate (p < 0.001) [19].
fQRS seen on admission is often indicative of previous myocardial scar and is associated with increased morbidity and mortality. The relationship between fQRS before and after primary PCI has been assessed by Kocaman SA et al. [20]. Patients with fQRS on admission had higher levels of cardiac troponin, longer pain to balloon time, higher Killip score, higher QRS duration and more frequent Q waves, compared with those without fQRS (P ranging from 0.004 to <0.001). They usually had wider jeopardized myocardium (p < 0.001), in the left anterior descending coronary artery territory. Absence of fQRS on admission predicted more ST resolution, better myocardial reperfusion and reduction in QRS duration.
Many studies on the usefulness of fQRS in predicting the outcomes of STEMI after PCI were published. Akgul et al. [21] published a prospective study comparing the one year outcomes of patients who underwent PCI for STEMI with fQRS and without fQRS. The STEMI with fQRS group had higher all-cause mortality (p < 0.001). fQRS is shown to be a significant independent predictor of one year all-cause mortality (p = 0.001) [21]. STEMI patients with fQRS had higher incidence of in-hospital mortality (p = 0.009) and contrast induced nephropathy (p = 0.029) after undergoing PCI [22]. In a retrospective study, patients with fQRS accompanying STEMI experienced higher rates of cardiovascular mortality (p = 0.028) as well as all - cause mortality (p = 0.022). In addition, it was also established that presence of fQRS was associated with lower left ventricular ejection fraction (LVEF) and higher risk of heart failure (p < 0.001) [23]. Higher cardiac biomarker levels, lower STEMI resolution rate and lower LVEF after PCI were noted among patients with fQRS (p < 0.01) [24]. Kanjanahattakij N., et al. conducted a meta-analysis of six studies that compared mortality rates among STEMI with fQRS and without fQRS groups who underwent PCI, which showed that the presence of fQRS increased the mortality up to 3 times compared to the absence of fQRS [25]. The combined use of fQRS and neutrophil:lymphocyte ratio (NLR) for predicting the in-hospital mortality rate of STEMI patients after PCI was suggested by Tanriverdi et al. It was ratified that NLR ≥5.47 had higher incidence of fQRS (p = 0.001). NLR above the cut off value and fQRS had significant association with higher in-hospital mortality rate (p < 0.001) [26].
fQRS as a manifestation of local conduction abnormalities in the ventricular myocardium and scar tissue has been established time and again. However, its association with atrial conduction abnormalities like atrial fibrillation (AF) in the setting of STEMI is the subject of interest currently. 171 STEMI patients who underwent PCI were studied for new onset AF after revascularization. It was discerned that fQRS with STEMI is associated with higher rates of new onset AF (p = 0.001) [27]. More number of studies with larger sample size are required to vouch for the association.
The success of a cardiac rehabilitation (CR) programme has traditionally been gauged by the quality of life, incidence of reinfarction and mortality rate. Bulut et al. studied 160 patients admitted with STEMI and fQRS who were divided into two groups based on their participation in exercise based CR programme. It was affirmed that the exercisers group had lesser rate of persistent fQRS after CR marking the electrical stabilisation of infarcted myocardium (p = 0.034) [13]. This study might open up newer territories in the assessment of a successful CR programme based on the persistence or disappearance of fQRS on serial ECGs.
5.4. Non-ST elevation myocardial infarction (NSTEMI)
fQRS is conducive in identifying the involved vessel in NSTEMI. In a retrospective study of 183 patients admitted for NSTEMI, fQRS in respective leads had 77.1% sensitivity and 71.5% specificity in identifying the culprit vessel [10]. The usefulness of fQRS in differentiating NSTEMI from unstable angina (UA) was studied by Lang D et al. Incidence of fQRS was higher among the NSTEMI group than the UA group (p = 0.047) [28]. Adverse cardiac events like recurrent angina, recurrent MI, heart failure etc., were higher among the NSTEMI with fQRS group (p = 0.028) [29]. In a study by Bozbeyoglu E et al., out of 433 NSTEMI patients were divided into fQRS (85) and non-fQRS groups (348). In-hospital, 30 – day and 12 month mortality rates of two groups were compared. It was found that there was no significant difference in the in-hospital and 30-day mortality rates but 12 month mortality rate was higher in the fQRS group, 15.2% as compared to 5.4% in non-fQRS group (p = 0.006) [30].
5.5. Collateral circulation in chronic total occlusion (CTO)
The value of fQRS in determining poor collateral coronary circulation in patients with chronic stable angina was studied by Bonakdar H et al. Seventy nine patients with stable angina were evaluated by single photon emission computed tomography (SPECT) for myocardial scar. Coronary angiogram was also performed for CTO of coronary vessels besides understanding the status of collateralisation. fQRS was more frequent in patients with poor collaterals (p < 0.001). SPECT showed significantly higher summed stress score and summed rest score in the patients with poor collateralisation and hence in the positive fQRS group (p < 0.001) [14].
5.6. Coronary slow flow (CSF)
Slow flow of blood through the distal coronary branches in the absence of occlusion is thought to be due to endothelial dysfunction, atherosclerosis, microvascular vasomotor dysfunction and increased platelet aggregability. This can be associated with angina, myocardial ischemia, acute myocardial infarction (AMI). In a study by Yilmaz et al., 60 patients with CSF and 44 patients with normal coronaries were studied. The incidence of fQRS was shown to be higher among the CSF group as compared to the patients with normal coronaries (p = 0.005) [31]. In another study by Cakmak et al., 165 patients were studied, of which 112 patients showed CSF and the rest were controls. It was established that incidence of CSF was higher in the fQRS group than non-fQRS group (p < 0.001). Multivariate analysis showed that fQRS is a reliable marker of CSF (p = 0.03) [15].
5.7. Left ventricular thrombus
Left ventricular thrombus formation is a known complication of AMI and is associated with poor post-PCI outcomes in patients with AMI. The risk factors for left ventricular thrombus formation in the setting of AMI include large infarct size, anterior wall myocardial infarction, apical wall motion abnormality and low ejection fraction. fQRS has been suggested as an ECG marker for left ventricular thrombus formation. In a prospective study of 148 patients admitted for AMI, 53.1% of the patients with left ventricular thrombus had fQRS in leads V4–V6 (p < 0.001) and 75% of these patients had unsuccessful PCI (p = 0.002) [17]. This study projected the significance of fQRS in predicting the risk of left ventricular thrombus in patients with AMI.
5.8. Left ventricular aneurysm
Left ventricular aneurysm is an important long term complication of myocardial infarction and occurs in 3.5–9.4% of cases. fQRS in left sided leads in the absence of LBBB has been associated with left ventricular aneurysm [18]. ECG recordings of patients with left ventricular aneurysm were compared with those of patients without left ventricular aneurysm (with and without CAD). The sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of fQRS in predicting left ventricular aneurysm were 50%, 94.6%, 83.3% and 79.2% respectively.
6. Dilated cardiomyopathy (DCM)
DCM is associated with ECG changes that mark left atrial enlargement, LBBB and sometimes left ventricular hypertrophy. Studies showed that fQRS in non-ischemic DCM was found to be associated with left ventricular dyssynchrony. Tigen K et al., established that the maximal difference in time to attain peak systolic velocity (PSV) between any two myocardial segments and maximal difference between PSV and mean systolic velocity of all segments were significantly high in fQRS group (p = 0.001 and p = 0.003 respectively). This was suggestive of significant intraventricular dyssynchrony in fQRS positive patients with narrow QRS and sinus rhythm [32]. Another prospective study by Zhao L et al., observed that fQRS in idiopathic DCM patients with narrow QRS complex was confederated with left ventricular dyssynchrony as indicated by the time lapse between peak anteroseptal wall and posterior wall strain >130 msec or longitudinal strain delay index >25% (p < 0.001). On follow up after two years, the left ventricular dyssynchrony worsened in the fQRS group (p < 0.05) [33].
7. Role of fQRS in heart failure
The value of fQRS in predicting the mortality of patients with heart failure has been established in numerous studies. A recent meta-analysis that included 5180 patients with heart failure with reduced ejection fraction (HFrEF) established the association of baseline fQRS with increased mortality (risk ratio = 1.49, 95% confidence interval: 1.19–1.86, p = 0.001) [34]. In another study, fQRS in ≥3 leads was identified as an independent predictor of mortality in patients with HFrEF [35]. However, conflicting results were published by two studies vis-à-vis, the value of fQRS in the setting of heart failure with preserved ejection fraction (HFpEF). In a prospective study that included 239 patients admitted with left ventricular diastolic dysfunction, fQRS group had higher incidence of heart failure, higher levels of BNP and hs-TNT than non-fQRS group (p < 0.001, p = 0.001 and p = 0.007 respectively) [36]. Contrarily, in a retrospective study that included 100 patients with asymptomatic left ventricular diastolic dysfunction, no difference was noted in the proportion of patients with fQRS among those who developed HFpEF and those who remained asymptomatic on follow up (p = 0.78) [37].
Currently, cardiac resynchronisation therapy (CRT) is limited to the patients with wide QRS complex (>149 msec) and LBBB pattern. However, various studies demonstrated that 27–52% of the patients with HFrEF have narrow QRS complex (<150 msec) [38,39]. Studies have also shown that 20–50% of the patients with HFrEF and narrow QRS complex have left ventricular dyssynchrony [40,41]. These results demonstrates that a significant number of patients with left ventricular dyssynchrony may be deprived of CRT, as per the current guidelines. On the flip side, echocardiography based studies on application of CRT in HFrEF patients with narrow QRS complex have failed to demonstrate any benefit with CRT in the patient outcome [42,43]. As it was discussed in the previous section, fQRS is a marker of left ventricular dyssynchrony in HFrEF patients with narrow QRS complex on the ECG. This association can be considered in future studies on expanding the scope of CRT to these patients.
Response to CRT is being assessed traditionally by NYHA functional class, 6 min walk test, quality of life questionnaires, left ventricular volumes and LVEF [44]. Resolution of fQRS was ratified as a marker of response to CRT after a follow up of 6 months, in a prospective study. 58% of the patients showed >14% reduction in LV end systolic volume (LVESV) and number of leads with fQRS decreased from 4.4 ± 1.8 to 1.7 ± 1.6 in patients who responded to CRT (p = 0.001 and p < 0.001 respectively) [45]. If further studies show similar results, fQRS resolution might assume a key role in the assessment of CRT response.
8. fQRS in left ventricular noncompaction cardiomyopathy
Left ventricular noncompaction cardiomyopathy (NCM) is a rare genetic disorder characterised by multiple trabeculations in the left ventricular myocardium that arises due to noncompaction of embryonic mesh-like myocardial fibre network [46,47]. NCM results in various complications like left ventricular systolic dysfunction, ventricular arrhythmias and systemic emboli [46]. Murphy RT et al., studied 45 patients admitted for NCM, of which, 91% showed abnormal ECG. LBBB, pathological Q waves, poor R wave progression, ST segment variations and T wave inversion were the abnormalities noted [46]. fQRS was noted in 48% of the patients with NCM in a study by Ning XH et al. It was also demonstrated that fQRS group had higher mortality rate when compared with the non-fQRS group of NCM patients (p = 0.005). Narrow fQRS was noted as an independent predictor of all-cause-mortality in these patients (Hazard ratio, HR = 5.33 and p = 0.045) [48]. In another study, fQRS in the setting of NCM was established as an independent predictor of arrhythmias and cardiovascular mortality (HR = 3.850, 95% CI 1.062 to 9.947, p = 0.002 and HR = 2.719, 95% CI 1.494 to 9.262, p = 0.005 respectively) [49]. These studies assert the usefulness of fQRS in stratifying the risk of mortality and arrhythmic events in NCM patients.
9. Takotsubo cardiomyopathy
In a study of 33 Takotsubo cardiomyopathy (TTC) patients, the incidence of J wave and/or fQRS on ECG was 29% and these patients were categorised as group A. The rest were categorised as group B. On comparing the LVEF of group A with that of group B, it was noted that the group A had lower values. It was also identified that the group A had a higher summed defect score of single-photon emission computed tomography and creatine kinase MB isoenzyme (CKMB). The J wave was a significant marker of sudden cardiac death and ventricular arrhythmias in these patients (p = 0.026) [50]. However, there are no direct studies on correlation of fQRS with adverse cardiac events in the setting of TTC.
10. Hypertrophic cardiomyopathy
Various studies demonstrated the role of fQRS in predicting ventricular arrhythmias in the setting of HCM. In a prospective study of 167 HCM patients, ventricular arrhythmias and sudden cardiac death were considered as the major arrhythmic events. The study established that fQRS is significantly associated with ventricular arrhythmias and major arrhythmic events (unadjusted HR = 6.17, 95% CI 2.46–15.49, p < 0.001 and unadjusted HR 5.12, 95% CI 1.38–19.01, p = 0.014 respectively). fQRS was also identified to be an independent predictor of VA and major arrhythmic events in HCM (adjusted HR 6.28, 95% CI 2.49–15.84, p < 0.001 and adjusted HR 6.04, 95% CI 1.49–24.39, p = 0.011 respectively) [51].
As mentioned in the previous sections, fQRS is a marker of myocardial scar. Ratheendran AC et al. compared ECG abnormalities with Gadolinium enhancement on cardiac MRI (CMR) to predict myocardial scar in patients with HCM. Out of 39 HCM patients, 23 demonstrated fQRS on ECG (63.89%). When all the patients were subjected to Gadolinium enhanced CMR, fQRS group showed higher incidence of late Gadolinium enhancement, that indicated myocardial scar, when compared with non-fQRS group (fQRS group - 84.61%, non-fQRS group - 10%, p < 0.001). Sensitivity, specificity, positive predictive value and negative predictive value of fQRS in predicting myocardial scar were 84.6, 90.0, 95.6 and 69.2% respectively [52].
11. Brugada syndrome
Association of fQRS with Brugada syndrome [53] and its role in predicting adverse events in these patients have been explained in various studies. In a study of 115 BS patients, fQRS was noted in 43% of them. Thirteen patients suffered VF of which, 11 patients (85%) demonstrated fQRS. 50% of the patients who suffered syncope and 34% of the asymptomatic patients also showed fQRS (p value of all adverse events <0.01) [54]. In a meta-analysis by Meng L et al., it was found that unadjusted RR of VF in the presence of fQRS in Brugada syndrome is 4.23 (95% CI 1.68 to 10.61, p = 0.002) based on five studies by Refs. [[54], [55], [56], [57], [58]]. Adjusted HR of sudden cardiac death in Brugada syndrome was found to be 3.61 (95% CI 2.11 to 6.18, p < 0.00001) as demonstrated in three studies [[59], [60], [61], [62]].
12. Arrhythmogenic right ventricular dysplasia (ARVD)
Role of fQRS in foreboding the prognosis of ARVD was described in a few studies. fQRS was noted in 59% of 78 patients studied prospectively by Canpolat U et al., During 38 ± 14 months follow up, 50% of the total patients suffered adverse cardiac events in the form of sudden cardiac death or ventricular arrhythmias. fQRS was significantly associated with arrhythmic events (p < 0.001). Number of leads with fQRS was higher in the fQRS group with greater risk of arrhythmias (fQRS group - 5.08 ± 2.5 vs non-fQRS group - 1.14 ± 1.7 and p < 0.001) [63]. In another study that included 30 ARVD patients, surface ECG abnormalities were compared with corresponding abnormalities on endocardial and epicardial electroanatomic mapping. Twenty five (83%) patients had fQRS in two or more contiguous leads. Endocardial very low bipolar voltage area was larger in fQRS group than in non-fQRS group (median 19 cm2 vs median 5 cm2; p = 0.02). Epicardial late potential percentage (median 24% vs median 8%; p = 0.002) was also significantly larger in fQRS group. These results established fQRS on surface ECG as an indicator of larger voltage substrate abnormalities on electroanatomic mapping in the setting of ARVD [64].
13. Idiopathic ventricular fibrillation
Patients with idiopathic ventricular fibrillation (IVF) commonly succumb to sudden cardiac death. A few suffer ventricular fibrillation (VF) following physical or mental exertion. Studies showed that J point elevation and fQRS on resting ECG are risk indicators of IVF. In a retrospective study of 171 patients who survived cardiac arrest due to VF or syncope due to self-terminating VF. They were divided into three groups based on the presence of fQRS and J wave (group 1), J wave alone (group 2) and normal ECG (group 3). It was established that the incidence of syncope, cardiac arrest and VF episodes, as recorded by implantable cardioverter defibrillator (ICD) or pacemaker, was highest in group 3 followed by group 2 and group 1 (13.4 ± 5.6/year - group 1, 10.8 ± 3.9/year - group 2 and 9.8 ± 4.2/year - group 3) (HR = 3.2; 95% CI, 1.1–7.9; p = 0.01) [65].
14. Conclusion
The value of fQRS in cardiology is much higher than what is being understood currently. The application of fQRS in various clinical settings has been expanding as new studies kept demonstrating its significance. Our article attempted to summarise various studies available on the significance of fQRS in a few important clinical scenarios in the field of cardiology. However, its role has been demonstrated in many non-cardiac diseases, the discussion of which is beyond the scope of this article. Clinical practitioners should heed to fQRS on ECG and investigate the patient for an underlying cardiac disease depending on the clinical context.
Declaration of competing interest
We have no conflicts of interest to declare regarding our manuscript: “Fragmented QRS – Its Significance”.
Acknowledgement
We sincerely acknowledge the advice given by Dr. Mithilesh K. Das, Professor of Clinical Medicine, Indiana University School of Medicine and the Krannert Institute of Cardiology for revising the manuscript.
Footnotes
Peer review under responsibility of Indian Heart Rhythm Society.
References
- 1.Take Y., Morita H., Fragmented Q.R.S. What is the meaning? Indian Pacing Electrophysiol J. 2012 Sep;12(5):213–225. doi: 10.1016/s0972-6292(16)30544-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Das M.K., Khan B., Jacob S., Kumar A., Mahenthiran J. Significance of a fragmented QRS complex versus a Q wave in patients with coronary artery disease. Circulation. 2006;113:2495–2501. doi: 10.1161/CIRCULATIONAHA.105.595892. [DOI] [PubMed] [Google Scholar]
- 3.Das M.K., Suradi H., Maskoun W., Michael M.A., Shen C., Peng J., Dandamudi G., Mahenthiran J. Fragmented wide QRS on a 12-lead ECG: a sign of myocardial scar and poor prognosis. Circ Arrhythm Electrophysiol. 2008 Oct;1(4):258–268. doi: 10.1161/CIRCEP.107.763284. [DOI] [PubMed] [Google Scholar]
- 4.Jain R., Singh R., Yamini S., Das M.K. Fragmented ECG as a risk marker in cardiovascular diseases. Curr Cardiol Rev. 2014 Aug;10(3):277–286. doi: 10.2174/1573403X10666140514103451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vandenberk B., Robyns T., Goovaerts G., Claeys M., Helsen F., Van Soest S., Garweg C., Ector J., Van Huffel S., Willems R. Inter- and intra-observer variability of visual fragmented QRS scoring in ischemic and non-ischemic cardiomyopathy. J Electrocardiol. 2018 May – Jun;51(3):549–554. doi: 10.1016/j.jelectrocard.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Maheshwari S., Acharyya A., Puddu P.E., Mazomenos E.B., Leekha G., Maharatna K., Schiariti M. An automated algorithm for online detection of fragmented QRS and identification of its various morphologies. J R Soc Interface. 2013 Oct 16;10(89):20130761. doi: 10.1098/rsif.2013.0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eyuboglu M., Kucuk U., Senarslan O., Akdeniz B. Comparison of the presence of fragmented QRS complexes in the inferior versus the anterior leads for predicting coronary artery disease severity. Rev Port Cardiol. 2017 Feb;36(2):89–93. doi: 10.1016/j.repc.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Terho H.K., Tikkanen J.T., Junttila J.M., Anttonen O., Kenttä T.V., Aro A.L., Kerola T., Rissanen H.A., Reunanen A., Huikuri H.V. Prevalence and prognostic significance of fragmented QRScomplex in middle-aged subjects with and without clinical or electrocardiographic evidence of cardiac disease. Am J Cardiol. 2014 Jul 1;114(1):141–147. doi: 10.1016/j.amjcard.2014.03.066. [DOI] [PubMed] [Google Scholar]
- 9.Cetin M., Kocaman S.A., Kiris T., Erdogan T., Canga A., Durakoglugil M.E., Ciçek Y., Dogan S., Satiroglu O. Absence and resolution of fragmented QRS predict reversible myocardial ischemia with higher probability of ST segment resolution in patients with ST segment elevation myocardial infarction. Korean Circ J. 2012 Oct;42(10):674–683. doi: 10.4070/kcj.2012.42.10.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo R., Zhang J., Li Y., Xu Y., Tang K., Li W. Prognostic significance of fragmented QRS in patients with non-ST elevation myocardial infarction: results of a 1-year, single-center follow-up. Herz. 2012 Nov;37(7):789–795. doi: 10.1007/s00059-012-3603-3. [DOI] [PubMed] [Google Scholar]
- 11.Cetin M., Kocaman S.A., Canga A., Durakoglugil M.E., Erdogan T., Satiroglu O., Kiris T., Ugurlu Y., Cicek Y., Bostan M. The independent relationship between systemic inflammation and fragmented QRS complexes in patients with stable angina pectoris. Kardiol Pol. 2012;70(7):668–675. [PubMed] [Google Scholar]
- 12.Zhao Q., Zhang R., Hou J., Yu B. Relationship between fragmented QRS and NT-proBNP in patients with ST elevation myocardial infarction who underwent primary percutaneous coronary intervention. Acta Cardiol Sin. 2018 Jan;34(1):13–22. doi: 10.6515/ACS.201801_34(1).20170903A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bulut M., Deniz Acar R., Ergün S., Geçmen Ç., Akçakoyun M. Cardiac rehabilitation improves the QRS fragmentation in patients with ST elevatıon myocardial infarction. J Cardiovasc Thorac Res. 2015;7(3):96–100. doi: 10.15171/jcvtr.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonakdar H., Moladoust H., Kheirkhah J., Abbaspour E., Assadian Rad M., Salari A., Barzigar A., Shad B. Significance of a fragmented QRS complex in patients with chronic total occlusion of coronary artery without prior myocardial infarction. Anatol J Cardiol. 2016 Feb;16(2):106–112. doi: 10.5152/akd.2015.5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cakmak H.A., Aslan S., Gul M., Kalkan A.K., Ozturk D., Celik O., Tasbulak O., Satilmisoglu M.H. Assessment of the relationship between a narrow fragmentedQRS complex and coronary slow flow. Cardiol J. 2015;22(4):428–436. doi: 10.5603/CJ.a2015.0007. [DOI] [PubMed] [Google Scholar]
- 16.Sen F., Yılmaz S., Kuyumcu M.S., Ozeke O., Balcı M.M., Aydoğdu S. The presence of fragmented QRS on 12-lead electrocardiography in patients with coronary artery ectasia. Korean Circ J. 2014 Sep;44(5):307–311. doi: 10.4070/kcj.2014.44.5.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baysal E., Burak C., Yaylak B., Altıntaş B., Öztürk Ö., Çiftçi H., Altındağ R., Söner S. Relationship between fragmented QRS complexes in leads V4-V6 and left ventricular apical thrombus formation in patients presenting with first acute anterior myocardial infarction. Turk Kardiyol Dernegi Arsivi. 2017 Apr;45(3):219–226. doi: 10.5543/tkda.2017.03753. [DOI] [PubMed] [Google Scholar]
- 18.Reddy C.V., Cheriparambill K., Saul B., Makan M., Kassotis J., Kumar A., Das M.K. Fragmented left sided QRS in absence of bundle branch block: sign of left ventricular aneurysm. Ann Noninvasive Electrocardiol. 2006 Apr;11(2):132–138. doi: 10.1111/j.1542-474X.2006.00094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanriverdi Z., Colluoglu T., Unal B., Dursun H., Kaya D. The prognostic value of the combined use of QRS distortion and fragmented QRS in patients with acute STEMI undergoing primary percutaneous coronary intervention. J Electrocardiol. 2018 Mar - Apr;51(2):210–217. doi: 10.1016/j.jelectrocard.2017.09.014. [DOI] [PubMed] [Google Scholar]
- 20.Kocaman S.A., Çetin M., Kırış T., Erdoğan T., Çanga A., Durakoğlugil E., Şatıroğlu Ö., Şahinarslan A., Çiçek Y., Sahin İ., Bostan M. The importance of fragmented QRS complexes in prediction of myocardial infarction and reperfusion parameters in patients undergoing primary percutaneous coronary intervention. Turk Kardiyol Dernegi Arsivi. 2012 Apr;40(3):213–222. doi: 10.5543/tkda.2012.36937. [DOI] [PubMed] [Google Scholar]
- 21.Akgul O., Uyarel H., Pusuroglu H., Surgit O., Turen S., Erturk M., Ayhan E., Bulut U., Baycan O.F., Demir A.R., Uslu N. Predictive value of a fragmented QRS complex in patients undergoing primary angioplasty for ST elevation myocardial infarction. Ann Noninvasive Electrocardiol. 2015 May;20(3):263–272. doi: 10.1111/anec.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurtul A., Duran M. Fragmented QRS complex predicts contrast-induced nephropathy and in-hospital mortality after primary percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction. Clin Cardiol. 2017 Apr;40(4):235–242. doi: 10.1002/clc.22651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uslu N., Gul M., Cakmak H.A., Atam A., Pusuroglu H., Satilmisoglu H., Akkaya E., Aksu H.U., Kalkan A.K., Surgit O., Erturk M., Aksu H., Eksik A. The assessment of relationship between fragmented QRScomplex and left ventricular wall motion score index in patients with ST elevation myocardial infarction who underwent primary percutaneous coronary intervention. Ann Noninvasive Electrocardiol. 2015 Mar;20(2):148–157. doi: 10.1111/anec.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duan W., Ma X., Cui L. Association between fragmented QRS complexes and imperfect ST-segment resolution in patients with ST-elevation myocardial infarction after primary percutaneous coronary intervention. Zhonghua Xinxueguanbing Zazhi. 2014 May;42(5):400–405. [PubMed] [Google Scholar]
- 25.Kanjanahattakij N., Rattanawong P., Riangwiwat T., Prasitlumkum N., Limpruttidham N., Chongsathidkiet P., Vutthikraivit W., Crossey E. Fragmented QRS and mortality in patients undergoing percutaneous intervention for ST-elevation myocardial infarction: systematic review and meta-analysis. Ann Noninvasive Electrocardiol. 2018 Nov;23(6) doi: 10.1111/anec.12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanriverdi Z., Colluoglu T., Dursun H., Kaya D. The Relationship between neutrophil-to-lymphocyte ratio and fragmented QRS in acute STEMI patients treated with primary PCI. J Electrocardiol. 2017 Nov - Dec;50(6):876–883. doi: 10.1016/j.jelectrocard.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 27.Yesin M., Kalçık M., Çağdaş M., Karabağ Y., Rencüzoğulları İ., Gürsoy M., Efe S.Ç., Karakoyun S. Fragmented QRS may predict new onset atrial fibrillation in patients with ST-segment elevation myocardial infarction. J Electrocardiol. 2018 Jan - Feb;51(1):27–32. doi: 10.1016/j.jelectrocard.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 28.Liang D., Zhang J., Lin L., Zong W. The difference on features of fragmented QRS complex and influences on mortality in patients with acute coronary syndrome. Acta Cardiol Sin. 2017 Nov;33(6):588–595. doi: 10.6515/ACS20170810B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li M., Wang X., Mi S.H., Chi Z., Chen Q., Zhao X., Nie S.P. Short-term prognosis of fragmented QRS complex in patients with non-ST elevated acute myocardial infarction. Chin Med J (Engl) 2016 Mar 5;129(5):518–522. doi: 10.4103/0366-6999.176989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bozbeyoğlu E., Yıldırımtürk Ö., Yazıcı S., Ceylan U.S., Erdem A., Kaya A., Dönmez C., Akyüz Ş., Çetin M. Fragmented QRS on admission electrocardiography predicts long-term mortality in patients with non-ST-segment elevation myocardial infarction. Ann Noninvasive Electrocardiol. 2016 Jul;21(4):352–357. doi: 10.1111/anec.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yilmaz H., Gungor B., Kemaloglu T., Sayar N., Erer B., Yilmaz M., Cakmak N., Gurkan U., Oz D., Bolca O. The presence of fragmented QRS on 12-lead ECG in patients with coronary slow flow. Kardiol Pol. 2014;72(1):14–19. doi: 10.5603/KP.2013.0181. [DOI] [PubMed] [Google Scholar]
- 32.Tigen K., Karaahmet T., Gurel E., Cevik C., Nugent K., Pala S., Tanalp A.C., Mutlu B., Basaran Y. The utility of fragmented QRS complexes to predict significant intraventricular dyssynchrony in nonischemic dilated cardiomyopathy patients with a narrow QRS interval. Can J Cardiol. 2009 Sep;25(9):517–522. doi: 10.1016/s0828-282x(09)70137-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao L., Lu J., Cui Z.M., Pavri B.B., Dai M., Qian D.J., Shen W.G., Guo T., Wang R.X. Changes in left ventricular synchrony and systolic function in dilated cardiomyopathy patients with fragmented QRS complexes. Europace. 2015 Nov;17(11):1712–1719. doi: 10.1093/europace/euu408. [DOI] [PubMed] [Google Scholar]
- 34.Kanitsoraphan C., Rattanawong P., Mekraksakit P., Chongsathidkiet P., Riangwiwat T., Kanjanahattakij N., Vutthikraivit W., Klomjit S., Thavaraputta S. Baseline fragmented QRS is associated with increased all-cause mortality in heart failure with reduced ejection fraction: a systematic review and meta-analysis. Ann Noninvasive Electrocardiol. 2019 Mar;24(2) doi: 10.1111/anec.12597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Torigoe K., Tamura A., Kawano Y., Shinozaki K., Kotoku M., Kadota J. The number of leads with fragmented QRS is independently associated with cardiac death or hospitalization for heart failure in patients with prior myocardial infarction. J Cardiol. 2012 Jan;59(1):36–41. doi: 10.1016/j.jjcc.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 36.Onoue Y., Izumiya Y., Hanatani S., Kimura Y., Araki S., Sakamoto K., Yamamoto E., Tsujita K., Tanaka T., Yamamuro M., Kojima S., Kaikita K., Hokimoto S., Ogawa H. Fragmented QRS complex is a diagnostic tool in patients with left ventricular diastolic dysfunction. Heart Vessel. 2016 Apr;31(4):563–567. doi: 10.1007/s00380-015-0651-7. [DOI] [PubMed] [Google Scholar]
- 37.Karagodin I., Strande J., Marong B. Fragmented QRS does not predict onset of heart failure with preserved ejection fraction in patients with diastolic dysfunction. J Investig Med. 2016;64:922. [Google Scholar]
- 38.Bleeker G.B., Schalij M.J., Molhoek S.G., Verwey H.F., Holman E.R., Boersma E., Steendijk P., Van Der Wall E.E., Bax J.J. Relationship between QRS duration and left ventricular dyssynchrony in patients with end-stage heart failure. J Cardiovasc Electrophysiol. 2004 May;15(5):544–549. doi: 10.1046/j.1540-8167.2004.03604.x. [DOI] [PubMed] [Google Scholar]
- 39.Ghio S., Constantin C., Klersy C., Serio A., Fontana A., Campana C., Tavazzi L. Interventricular and intraventricular dyssynchrony are common in heart failure patients, regardless of QRS duration. Eur Heart J. 2004 Apr;25(7):571–578. doi: 10.1016/j.ehj.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 40.Yu C.M., Chan Y.S., Zhang Q., Yip G.W., Chan C.K., Kum L.C., Wu L., Lee A.P., Lam Y.Y., Fung J.W. Benefits of cardiac resynchronization therapy for heart failure patients with narrow QRS complexes and coexisting systolic asynchrony by echocardiography. J Am Coll Cardiol. 2006 Dec 5;48(11):2251–2257. doi: 10.1016/j.jacc.2006.07.054. [DOI] [PubMed] [Google Scholar]
- 41.Dohi K., Suffoletto M.S., Schwartzman D., Ganz L., Pinsky M.R., Gorcsan J., 3rd Utility of echocardiographic radial strain imaging to quantify left ventricular dyssynchrony and predict acute response to cardiac resynchronization therapy. Am J Cardiol. 2005 Jul 1;96(1):112–116. doi: 10.1016/j.amjcard.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 42.Beshai J.F., Grimm R.A., Nagueh S.F., Baker J.H., 2nd, Beau S.L., Greenberg S.M., Pires L.A., Tchou P.J., RethinQ Study Investigators Cardiac-resynchronization therapy in heart failure with narrow QRS complexes. N Engl J Med. 2007 Dec 13;357(24):2461–2471. doi: 10.1056/NEJMoa0706695. [DOI] [PubMed] [Google Scholar]
- 43.Ruschitzka F., Abraham W.T., Singh J.P., Bax J.J., Borer J.S., Brugada J., Dickstein K., Ford I., Gorcsan J., 3rd, Gras D., Krum H., Sogaard P., Holzmeister J., EchoCRT Study Group Cardiac-resynchronization therapy in heart failure with a narrow QRScomplex. N Engl J Med. 2013 Oct 10;369(15):1395–1405. doi: 10.1056/NEJMoa1306687. [DOI] [PubMed] [Google Scholar]
- 44.European Heart Rhythm Association (EHRA), European Society of Cardiology (ESC), Heart Rhythm Society, Heart Failure Society of America (HFSA), American Society of Echocardiography (ASE), American Heart Association (AHA), European Association of Echocardiography (EAE) of ESC, Heart Failure Association of ESC (HFA), Daubert J.C., Saxon L., Adamson P.B., Auricchio A., Berger R.D., Beshai J.F., Breithard O., Brignole M., Cleland J., DeLurgio D.B., Dickstein K., Exner D.V., Gold M., Grimm R.A., Hayes D.L., Israel C., Leclercq C., Linde C., Lindenfeld J., Merkely B., Mont L., Murgatroyd F., Prinzen F., Saba S.F., Shinbane J.S., Singh J., Tang A.S., Vardas P.E., Wilkoff B.L., Zamorano J.L., Anand I., Blomström-Lundqvist C., Boehmer J.P., Calkins H., Cazeau S., Delgado V., Estes N.A., Haines D., Kusumoto F., Leyva P., Ruschitzka F., Stevenson L.W., Torp-Pedersen C.T. 2012 EHRA/HRS expert consensus statement on cardiac resynchronization therapy in heart failure: implant and follow-up recommendations and management. Europace. 2012 Sep;14(9):1236–1286. doi: 10.1093/europace/eus222. [DOI] [PubMed] [Google Scholar]
- 45.Celikyurt U., Karauzum K., Sahin T., Agacdiken A., Vural A., Ural D. Association between resolution of fragmented QRS and response to cardiac resynchronization therapy. Ann Noninvasive Electrocardiol. 2015 Mar;20(2):126–131. doi: 10.1111/anec.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murphy R.T., Thaman R., Blanes J.G., Ward D., Sevdalis E., Papra E., Kiotsekoglou A., Tome M.T., Pellerin D., McKenna W.J., Elliott P.M. Natural history and familial characteristics of isolated left ventricular non-compaction. Eur Heart J. 2005 Jan;26(2):187–192. doi: 10.1093/eurheartj/ehi025. [DOI] [PubMed] [Google Scholar]
- 47.Goud A., Padmanabhan S. A rare form of cardiomyopathy: left ventricular non-compaction cardiomyopathy. J Community Hosp Intern Med Perspect. 2016 Feb 17;6(1):29888. doi: 10.3402/jchimp.v6.29888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ning X.H., Tang M., Chen K.P., Hua W., Chen R.H., Sha J., Liu Z.M., Zhang S. The prognostic significance of fragmented QRS in patients with left ventricular noncompaction cardiomyopathy. Can J Cardiol. 2012 Jul-Aug;28(4):508–514. doi: 10.1016/j.cjca.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 49.Cetin M.S., Ozcan Cetin E.H., Canpolat U., Cay S., Topaloglu S., Temizhan A., Aydogdu S. Usefulness of fragmented QRS complex to predict arrhythmic events and cardiovascular mortality in patients with noncompaction cardiomyopathy. Am J Cardiol. 2016 May 1;117(9):1516–1523. doi: 10.1016/j.amjcard.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 50.Shimizu M., Nishizaki M., Yamawake N., Fujii H., Sakurada H., Isobe M., Hiraoka M. J wave and fragmented QRS formation during the hyperacute phase in Takotsubo cardiomyopathy. Circ J. 2014;78(4):943–949. doi: 10.1253/circj.cj-13-1296. [DOI] [PubMed] [Google Scholar]
- 51.Kang K.W., Janardhan A.H., Jung K.T., Lee H.S., Lee M.H., Hwang H.J. Fragmented QRS as a candidate marker for high-risk assessment in hypertrophic cardiomyopathy. Heart Rhythm. 2014 Aug;11(8):1433–1440. doi: 10.1016/j.hrthm.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 52.Ratheendran A.C., Subramanian M., Bhanu D.K., Prabhu M.A., Kannan R., Natarajan K.U., Saritha Sekhar S., Thachathodiyil R., Harikrishnan M.S., Pai P.G. Fragmented QRS on electrocardiography as a predictor of myocardial scar in patients with hypertrophic cardiomyopathy. Acta Cardiol. 2019 Jan 3:1–5. doi: 10.1080/00015385.2018.1547355. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 53.Mizusawa Y., Wilde A.A. Brugada syndrome. Circ Arrhythm Electrophysiol. 2012 Jun 1;5(3):606–616. doi: 10.1161/CIRCEP.111.964577. [DOI] [PubMed] [Google Scholar]
- 54.Morita H., Kusano K.F., Miura D., Nagase S., Nakamura K., Morita S.T., Ohe T., Zipes D.P., Wu J. Fragmented QRS as a marker of conduction abnormality and a predictor of prognosis of Brugada syndrome. Circulation. 2008 Oct 21;118(17):1697–1704. doi: 10.1161/CIRCULATIONAHA.108.770917. [DOI] [PubMed] [Google Scholar]
- 55.Maury P., Rollin A., Sacher F., Gourraud J.B., Raczka F., Pasquié J.L., Duparc A., Mondoly P., Cardin C., Delay M., Derval N., Chatel S., Bongard V., Sadron M., Denis A., Davy J.M., Hocini M., Jaïs P., Jesel L., Haïssaguerre M., Probst V. Prevalence and prognostic role of various conduction disturbances in patients with the Brugada syndrome. Am J Cardiol. 2013 Nov 1;112(9):1384–1389. doi: 10.1016/j.amjcard.2013.06.033. [DOI] [PubMed] [Google Scholar]
- 56.Tokioka K., Kusano K.F., Morita H., Miura D., Nishii N., Nagase S., Nakamura K., Kohno K., Ito H., Ohe T. Electrocardiographic parameters and fatal arrhythmic events in patients with Brugada syndrome: combination of depolarization and repolarization abnormalities. J Am Coll Cardiol. 2014 May 27;63(20):2131–2138. doi: 10.1016/j.jacc.2014.01.072. [DOI] [PubMed] [Google Scholar]
- 57.Calò L., Giustetto C., Martino A., Sciarra L., Cerrato N., Marziali M., Rauzino J., Carlino G., de Ruvo E., Guerra F., Rebecchi M., Lanzillo C., Anselmino M., Castro A., Turreni F., Penco M., Volpe M., Capucci A., Gaita F. A new electrocardiographic marker of sudden death in Brugada syndrome: the S-wave in lead I. J Am Coll Cardiol. 2016 Mar 29;67(12):1427–1440. doi: 10.1016/j.jacc.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 58.Rivard L., Roux A., Nault I., Champagne J., Roux J.F., Tadros R., Talajic M., Cadrin-Tourigny J., Shohoudi A., Mondésert B., Roy D., Macle L., Andrade J., Dyrda K., Dubuc M., Guerra P.G., Sarrazin J.F., Thibault B., Khairy P. Predictors of ventricular arrhythmias and sudden death in a Québec cohort with Brugada syndrome. Can J Cardiol. 2016 Nov;32(11):1355.e1–1355.e7. doi: 10.1016/j.cjca.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 59.Priori S.G., Gasparini M., Napolitano C., Della Bella P., Ottonelli A.G., Sassone B., Giordano U., Pappone C., Mascioli G., Rossetti G., De Nardis R., Colombo M. Risk stratification in Brugada syndrome: results of the PRELUDE (PRogrammed ELectrical stimUlation preDictive valuE) registry. J Am Coll Cardiol. 2012 Jan 3;59(1):37–45. doi: 10.1016/j.jacc.2011.08.064. [DOI] [PubMed] [Google Scholar]
- 60.Take Y., Morita H., Toh N., Nishii N., Nagase S., Nakamura K., Kusano K.F., Ohe T., Ito H. Identification of high-risk syncope related to ventricular fibrillation in patients with Brugada syndrome. Heart Rhythm. 2012 May;9(5):752–759. doi: 10.1016/j.hrthm.2011.11.045. [DOI] [PubMed] [Google Scholar]
- 61.Apiyasawat S., Sahasthas D., Ngarmukos T., Chandanamattha P., Likittanasombat K. Fragmented QRS as a predictor of appropriate implantable cardioverter-defibrillator therapy. Indian Pacing Electrophysiol J. 2014 Jan;14(1):4–11. doi: 10.1016/s0972-6292(16)30710-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meng L., Letsas K.P., Baranchuk A., Shao Q., Tse G., Zhang N., Zhang Z., Hu D., Li G., Liu T. Meta-analysis of fragmented QRS as an electrocardiographic predictor for arrhythmic events in patients with Brugada syndrome. Front Physiol. 2017 Sep 12;8:678. doi: 10.3389/fphys.2017.00678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Canpolat U., Kabakçi G., Aytemir K., Dural M., Sahiner L., Yorgun H., Sunman H., Bariş Kaya E., Tokgözoğlu L., Oto A. Fragmented QRS complex predicts the arrhythmic events in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. J Cardiovasc Electrophysiol. 2013 Nov;24(11):1260–1266. doi: 10.1111/jce.12202. [DOI] [PubMed] [Google Scholar]
- 64.Tschabrunn C.M., Haqqani H.M., Santangeli P., Zado E.S., Marchlinski F.E. 12-Lead electrocardiogram to localize region of abnormal electroanatomic substrate in arrhythmogenic right ventricular cardiomyopathy. JACC Clin Electrophysiol. 2017 Jul;3(7):654–665. doi: 10.1016/j.jacep.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 65.Wang J., Tang M., Mao K.X., Chu J.M., Hua W., Jia Y.H., Zhao Y.J., Wei W., Chen X.H., Pu J.L., Zhang S. Idiopathic ventricular fibrillation with fragmented QRS complex and J wave in resting electrocardiogram. J Geriatr Cardiol. 2012 Jun;9(2):143–147. doi: 10.3724/SP.J.1263.2011.12121. [DOI] [PMC free article] [PubMed] [Google Scholar]