Abstract
Aims
MicroRNAs play essential roles in tumorigenesis and progression in various cancers including endometrial cancer. Here we assessed the role of miR-135a on proliferation, chemosensitivity, migration and invasion of endometrial cancer cells.
Methods
WST-1 assay was performed to examine the proliferation of HEC-1-B and ISHIKAWA endometrial cancer cells with altered expression of miR-135a, with or without cisplatin treatment. Transwell migration and matrigel invasion assays were used to assess the migration and invasion of endometrial cancer cells. The Caspase-Glo3/7 assay was used to examine the effect of miR-135a on cisplatin-induced apoptosis of endometrial cancer cells. The dual-luciferase reporter assay was conducted to validate the putative binding site.
Results
Upregulation of miR-135a improved the proliferation, and promoted migration and invasion of endometrial cancer cells. Furthermore, miR-135a decreased the sensitivity of HEC-1-B and ISHIKAWA cells after cisplatin treatment. The cisplatin-induced apoptosis in endometrial cancer cells was inhibited by miR-135a by regulation of BAX and Bcl-2 expression. Meanwhile, miR-135a could regulate epithelial to mesenchymal transition (EMT) by altering the expression of E-cadherin, N-cadherin, snail and Vimentin in endometrial cancer cells. Further study showed that the expression levels of PTEN and p-AKT in endometrial cancer cells were changed after aberrant expression of miR-135a.
Conclusion
MiR-135a played important roles in tumorigenesis and disease progression of endometrial cancer by regulating proliferation and chemosensitivy of endometrial cancer cells by targeting AKT signaling pathway. Our study indicates that miR-135a might act as a potential biomarker to predict chemotherapy response and prognosis in endometrial cancer.
Keywords: miR-135a, Endometrial cancer, Apoptosis, AKT
Introduction
Endometrial cancer is one of the most common gynecological malignancy worldwide [1], with estimated 63, 230 new cases and 11, 350 endometrial cancer deaths in 2018. The average age of female diagnosed with endometrial cancer is 60 [2]. Endometrial cancer is traditionally classified to estrogen-dependent endometrioid adenocarcinoma (Type I) and non-estrogen-dependent cancer (Type II) [3]. Type I tumors comprise as much as 75 % of endometrial cancer and are associated with good prognosis. Type II tumors are highly aggressive variant of endometrial cancer and account for most recurrences and cancer death from endometrial cancer. Chemotherapy plays important roles in endometrial cancer treatment [4]. The current NCCN guidelines recommend that patients with poor outcome should receive chemotherapy. The factors of poor prognosis include stage IIIB or stage IIIC disease of any histology and patients with stages IA (with myometrial invasion), IB, II, or IIIA serous or clear cell carcinoma [5]. Cisplatin is one of the most effective cytotoxic platinum-based chemotherapy drug used in a variety of cancers treatment [6]. Cisplatin inhibits proliferation of cancer cells by interfering with the process of cell division and induce programed cell death by damage DNA repair. However, the big challenge of cisplatin-based treatment is the development of drug resistance [7]. Multiple mechanisms have been identified including drug transportation, gene mutation and binding protein inactivation etc [8]. Patients with resistant endometrial cancer have a much shorter median survival time. Therefore, it is important to understand the molecular mechanisms of chemoresistance in the treatment of endometrial cancer and find out more effective therapeutic strategies for endometrial cancer therapy.
MicroRNAs (miRNAs) are a group of endogenous, small non-coding RNA molecules of about 18–25 nucleotides [9]. MiRNAs play important roles in multiple biological processes such as apoptosis, cell proliferation and differentiation by binding to the 3′-untranslated region (3′-UTR) of target mRNAs [10]. Aberrant miRNA expression has been reported in a variety of human malignancies, including endometrial cancer tissues [11]. It has been demonstrated that the expression patterns of miRNAs in cancer tissue are different from those in benign and normal tissues [12]. In endometrial cancer, some miRNAs such as miR-423, miR-103, miR-205, miR-429 and miR-135a are overexpressed and act as oncogenes involved in tumorigenesis, proliferation and cancer progression [11,13]. In contrast, some miRNAs including miR-221, miR-193, miR-30c and miR99b are downregulated in endometrial cancer and act as tumor suppressors involved in repression of invasion and metastasis [10]. In addition, some miRNAs show specific expression profiles in cancer tissues and may be used as biomarkers. For instance, the expression levels of miRNAs are associated with advanced stage and lymph node metastasis in endometrial cancer [14,15]. Recently, some studies have shown that miR-135a promoted migration and invasion of breast cancer cells by directly targeting mRNA and protein of HOXA10 [16]. In Hepatocellular carcinoma (HCC), Huang et al. found that the expression level of miR-135a was significantly increased in HCC tissue, compared with adjacent normal tissue. The elevated miR-135a was also associated with lymphovascular invasion, recurrence and survival rate [17]. Mao et al. reported that miR-135a enhanced invasion and migration of bladd.er cancer cells by activating the Wnt/β-catenin signaling pathway [18].The low expression level of miR-135a in non-small cell lung cancer (NSCLC) tissues was identified by zhou et al. Ectopic expression of miR-135a improved apoptosis and reduced cell proliferation, migration and invasion of NSCLC cells [19]. The expression level of miR135a was significantly upregulated in women with endometriosis in both proliferative and secretory phases by inhibition of HOXA10 [20]. In endometrial adenocarcinoma, there was a 16.6-fold increase in expression level of miR-135a, compared with the matched normal tissue [11]. Interestingly, the level of circulating miR-135a was decreased in women with endometriosis [21]. These findings indicate that miR-135a is an important in tumorigenesis, progression and prognosis in many organs including endometrial cancer.
Here, we investigated the function of miR-135a on proliferation, chemotherapy sensitivity, migration and invasion of endometrial cancer cells. We aimed to identify the role of miR-135a in endometrial cancer.
Materials and methods
Cell lines
Human endometrial cancer cell lines HEC-1B and Ishikawa were purchased from the American Type Culture Collection (ATCC, USA) and cultured in Dulbecco's Modified Eagle's Medium (DMEM) (Gibco, USA). The medium contained 10 % fetal bovine serum (Gibco, USA), 100 units of penicillin/ml and 100 mg of streptomycin/ml (Invitrogen, USA). Human endometrial cancer cells were cultured in a humidified incubator containing 5 % CO2 at 37 °C. Cells in logarithmic growth phase were used in experiments.
Transfection
Human endometrial cancer cells transfections were performed using Lipofectamine 2000 (Invitrogen, USA) according to the manufacturer’s instructions. The negative control was used a scrambled oligonucleotide (Invitrogen, USA). Human endometrial cancer cells were grown in transfection medium for 6 h at 37 °C in a 5 % CO2 atmosphere. Then these cells were cultured in complete medium for further analyses.
qPCR expression analysis
Total RNA from transfected HEC-1B and Ishikawa endometrial cancer cells was extracted using TRIzol reagent (Invitrogen, USA). Then total RNA (1 μg) was used to synthesize the complementary DNA templates using High-Capacity cDNA Reverse Transcription Kit (ThermoFisher Scientific, USA) according to the manufacturer’s instructions. The expression level of miR-135a was measured by real-time quantitative PCR. U6 was used as the endogenous control. The RT-PCR conditions were as follows: 95 °C for 3 min, 36 cycles at 95 °C for 15 s, 60 °C for 55 s, and 72 °C for 30 s, and a dissociation stage.
The primers were synthesized by Sigma (USA) and the sequences were as follow: miR-135a forward: 5′- GCCTCGCTGTTCTCTATGG-3′ and reverse 5′- TGTCCCCGCCGTGCG-3′. All independent experiments were repeated three times in triplicates.
WST-1 assay
The WST-1 assay (Roche, USA) was performed to examine the effect of miR-135a on the proliferation of endometrial cancer cells. Briefly, the transfected endometrial cancer cells were seeded in 96-well plates at density of 1 × 104 cells/well in culture medium and cultured overnight at 37 °C in a humidified incubator. Then the medium was replaced with medium containing different concentration of cisplatin (0, 2, 4 and 6 μM). The endometrial cancer cells were continued to grow at 37 °C in a humidified incubator. At 24, 48, 72, and 96 h, 20 μL of WST-1 solution was added to each well and incubated for 1 h at 37 °C. The absorbance was detected at 490 nm on a microplate reader (BioTek, Germany). All independent experiments were repeated three times in triplicates.
Caspase 3/7 assay
The transfected endometrial cancer cells were cultured in 24-well plates. After 24 -h incubation, cells were treated with different concentrations of cisplatin (Sigma, USA) (0, 2, 4 and 6 μM) in DMEM (Invitrogen, USA) for 48 h. Then the caspase-3/7 activity was measured using Caspase-Glo assay kit (Promega, USA) following the manufacturer’s protocol. Briefly, 100 μl of Caspase-Glo reagent was added to each well and incubated for 2 h in dark at 37 °C. Luminescence of each well was measured using a plate-reading luminometer (ThermoFisher, USA)
Cell migration and invasion assays
The effect of miR-135a on migration and invasion of endometrial cancer cells was examine using transwell assay(BD Bioscience, USA). In brief, the transfected endometrial cancer cells were resuspended in serum free DMEM medium and added into the upper chambers at a density of 3 × 104 cells/well. The lower chambers were filled with 500 μl of DMEM medium supplemented 10 % FBS as chemoattractant. After 18 h incubation, the non-invading cells were removed and the invaded cells were stained, and counted under microscope in five random fields at a magnification of 20 × . The transwell migration assays were the same as the transwell invasion assay except that the top chamber was not coated with matrigel. All independent experiments were repeated three times in triplicates.
Western blot
The total protein was extracted from transfected endometrial cancer cells using RIPA lysis buffer (Abcam, USA). 10 % sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed. 30 μg of protein was loaded and separated at 120 V. Then the proteins were transferred onto PVDF membranes and mounted using 5 % nonfat dried milk in PBS at room temperature for one hour with gentle agitation. Different primary antibodies (Table 1) were incubated with the membranes overnight at 4 °C with gentle agitation. Next, the membranes were washed with phosphate buffered saline (PBS) with tween 20 solution for three times for at least 10 min at room temperature. The membranes then were incubated with secondary antibody at room temperature for 1 h, followed by washing for 10 min each. Membranes were developed in enhanced chemiluminescence reagent (Pierce, USA). GAPDH was used as the loading control. The expression levels of proteins were normalized to GAPDH expression.
Table 1.
The antibodies used in western blot.
| Antibody | Vendor | Dilut. |
|---|---|---|
| BAX | Cell signaling Technology, USA | 1:1000 |
| BCL-2 | Santa cruz Biotechnology, USA | 1:500 |
| E-Cadherin | Cell signaling Technology, USA | 1:500 |
| N-Cadherin | Cell signaling Technology, USA | 1:300 |
| SNAIL | Cell signaling Technology, USA | 1:500 |
| Vimentin | Cell signaling Technology, USA | 1:200 |
| p-AKT | Abcam, USA | 1:1000 |
| PTEN | Cell signaling Technology, USA | 1:500 |
| AKT | Cell signaling Technology, USA | 1:500 |
| GAPDH | Cell signaling Technology, USA | 1:3000 |
Luciferase assay
The potential target of miR-135a was detected using miRDB online database. The fragment of the 3′-UTR of PTEN containing the putative miR-135a binding site was amplified from genomic DNA of human normal cells. Using the In-Fusion Dry-Down PCR Cloning Kit (Clontech, USA) following the manufacturer’s protocol, the amplified fragments were cloned into the Xbal site of the pmirGLO Dual-Luciferase miRNA Target Expression Vector (Promega, USA). Three point mutations were introduced into the seed region of the miR-135a binding sites, which was used as control. HEC-1B cells were seeded in 24-well plates at a concentration of 5 × 104 cells/well overnight. Then the cells were co-transfected with Luc-PTEN and miR-135a using Lipofectamine RNAiMAX following the manufacturer’s protocol. After 48 h, luciferase activities were measured using the Dual-Luciferase Reporter Assay system (Promega, USA). The experiments were performed at least three times in triplicates.
Statistical analysis
SPSS 12.0 software (IBM Corp, USA) was used to calculate the differences. Statistical comparisons were conducted by one-way analysis of variance (ANOVA). P < 0.05 indicated statistical significance.
Results
MiR-135a improved proliferation of endometrial cancer cells and induced cisplatin resistance in dose dependent manner
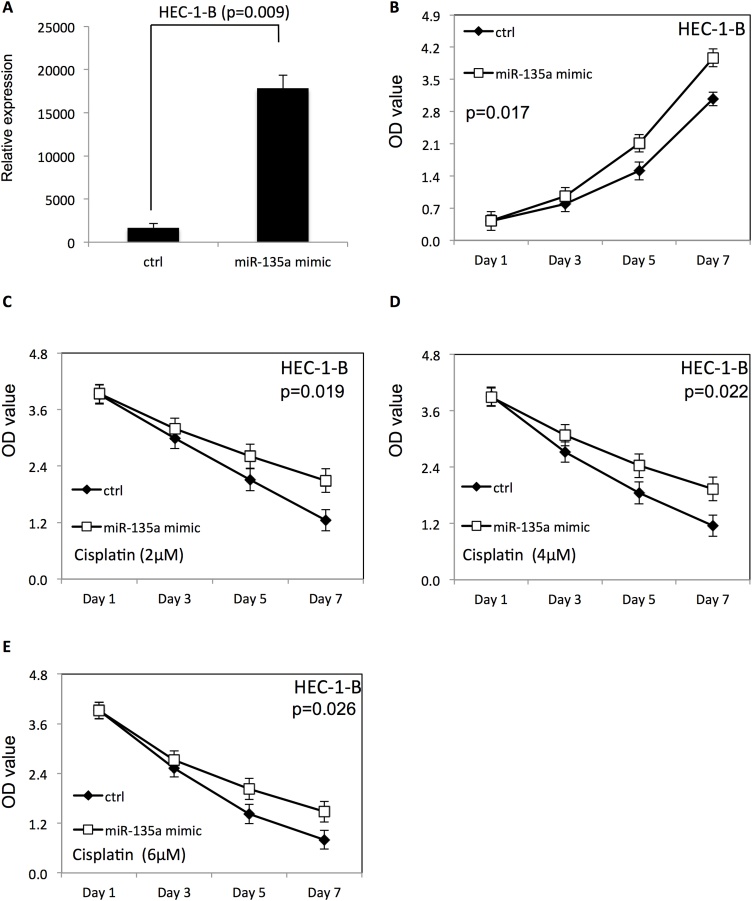
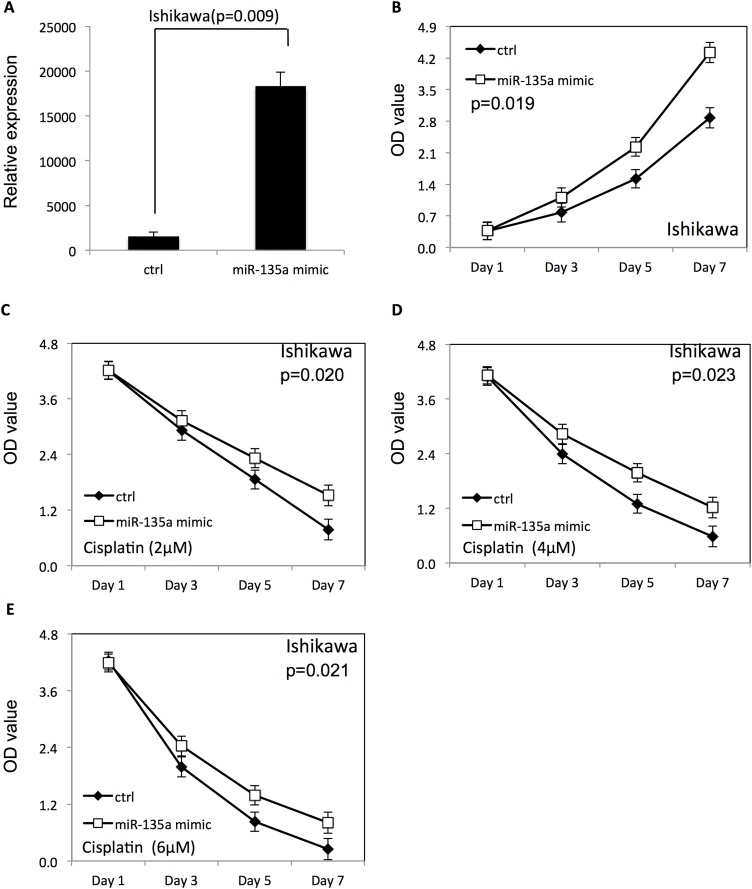
To examine the effect of miR-135a on proliferation of endometrial cancer cells with or without cisplatin treatment, miR-135a mimic or miR-135a inhibitor was transfected to HEC-1-B and ISHIKAWA endometrial cancer cells using Lipofectamine 2000. The proliferation of endometrial cancer cells was examined using WST-1 assay. The scrambled negative control RNAs were used as negative control. As shown in Figs. 1A and 2 A, the miR-135a expression level was significantly increased in both HEC-1-B and ISHIKAWA endometrial cancer cells after transfection of miR-135a mimic. Overexpression of miR-135a promoted proliferation of HEC-1-B (Fig. 1B) and ISHIKAWA cells (Fig. 2B), compared with endometrial cancer cells transfected with negative control RNAs (p < 0.05). Additionally, the survival rate of HEC-1-B (Fig. 1C–E) and ISHIKAWA cells (Fig. 2C–E) was increased after overexpression of miR-135a at presence of cisplatin, compared to the negative control group (p < 0.05).
Fig. 1.
Overexpression of miR-135a improved the proliferation and survival of HEC-1-B cells.
(A) The expression level of miR-135a in HEC-1-B endometrial cancer cells after transfection with miR-135a mimic. (B) The proliferation of HEC-1-B cells after miR-135a overexpression. (C–E) The proliferation of HEC-1-B cells transfected with miR-135a mimic in presence of different doses of cisplatin (2, 4 and 6 μM).
Fig. 2.
Overexpression of miR-135a improved the proliferation and survival of ISHIKAWA cells.
(A) The expression level of miR-135a in ISHIKAWA endometrial cancer cells after transfection with miR-135a mimic. (B) The proliferation of ISHIKAWA cells after miR-135a overexpression. (C–E) The proliferation of ISHIKAWA cells transfected with miR-135a mimic in presence of different doses of cisplatin (20, 40 and 60 μM).
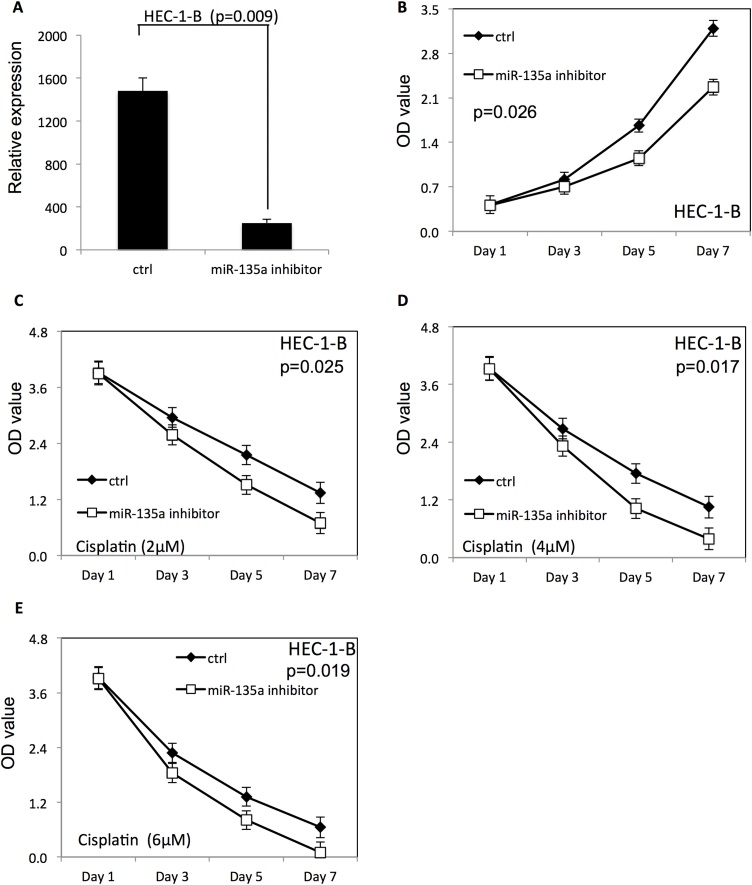
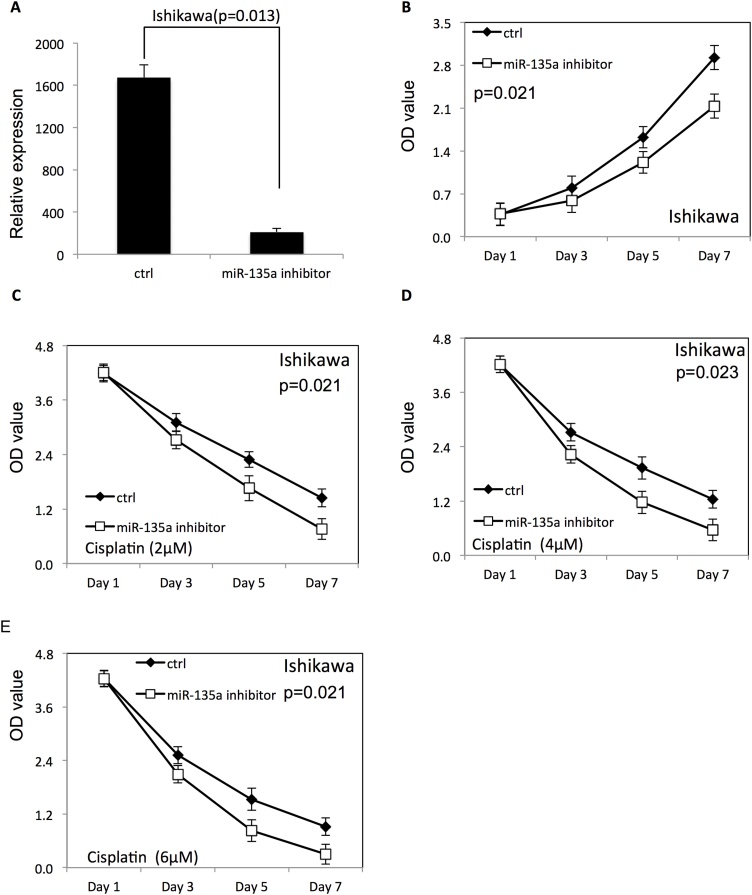
In contrast, the miR-135a expression level was significantly decreased in both HEC-1-B (Fig. 3A) and ISHIKAWA (Fig. 4A) cells after transfection of miR-135a inhibitor. Down-regulation of miR-135a decreased proliferation of HEC-1-B (Fig. 3B) and ISHIKAWA (Fig. 4B) compared with endometrial cancer cells transfected with negative control RNAs. Furthermore, the survival rate of HEC-1-B (Fig. 3C–E) and ISHIKAWA (Fig. 4C–E) was decreased after down-regulation of miR-135a at the presence of cisplatin, compared to the negative control group (P < 0.05).
Fig. 3.
Downregulation of miR-135a decreased the proliferation and survival of HEC-1-B cells.
(A) The expression level of miR-135a in HEC-1-B endometrial cancer cells after transfection with miR-135a inhibitor. (B) The proliferation of HEC-1-B cells after downregulation of miR-135a. (C–E) The proliferation of HEC-1-B cells transfected with miR-135a inhibitor in presence of different doses of cisplatin (2, 4 and 6 μM).
Fig. 4.
Downregulation of miR-135a decreased the proliferation and survival of ISHIKAWA cells.
(A) The expression level of miR-135a in ISHIKAWA endometrial cancer cells after transfection with miR-135a inhibitor. (B) The proliferation of ISHIKAWA cells after downregulation of miR-135a. (C–E) The proliferation of ISHIKAWA cells transfected with miR-135a inhibitor in presence of different doses of cisplatin (20, 40 and 60 μM).
MiR-135a inhibited cisplatin-induced apoptosis of endometrial cancer cells
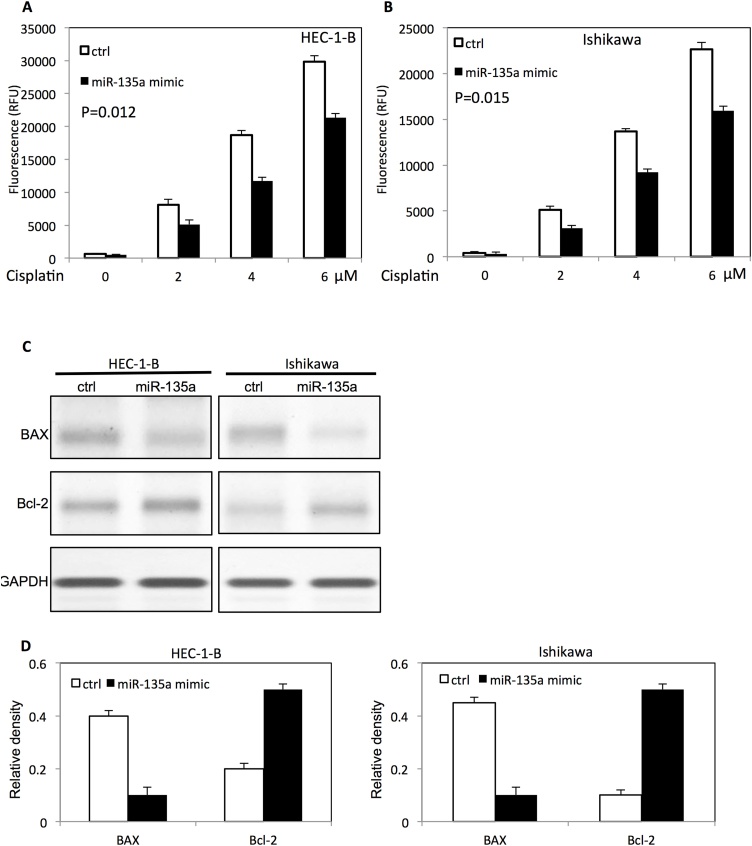
To assess the effect of miR-135a on cisplatin-induced apoptosis, transfected endometrial cancer cells were cultured with different doses of cisplatin. The cisplatin-induced apoptosis was analyzed by caspase 3/7 activity. Up-regulation of miR-135a inhibited cisplatin-induced apoptosis by decreasing the caspase3/7 activities in HEC-1-B cells (Fig. 5A) and ISHIKAWA cells (Fig. 5B). Western blot was used to further evaluate the expression of apoptotic related proteins of transfected endometrial cancer cells at presence of cisplatin. As shown in Fig. 5 C and D, overexpression of miR-135a decreased BAX expression, while the Bcl-2 expression was increased in both HEC-1-B cells and ISHIKAWA cells.
Fig. 5.
MiR-135a inhibits cisplatin-induced apoptosis in endometrial cancer cells in a dose dependent mannter.
(A) The caspase 3/7 activity in HEC-1-B endometrial cancer cells transfected with miR-135a mimic after different doses of cisplatin treatment. (B) The caspase 3/7 activity in ISHIKAWA endometrial cancer cells transfected with miR-135a after different doses of cisplatin treatment. (C) The apoptotic proteins levels in HEC-1-B cells and ISHIKAWA cells after cisplatin treatment. (D) The relative expression level of apoptotic proteins in HEC-1-B cells and ISHIKAWA cells after transfection with miR-135a mimic by densitometric analysis.
MiR-135a altered migration and invasion of endometrial cancer cells
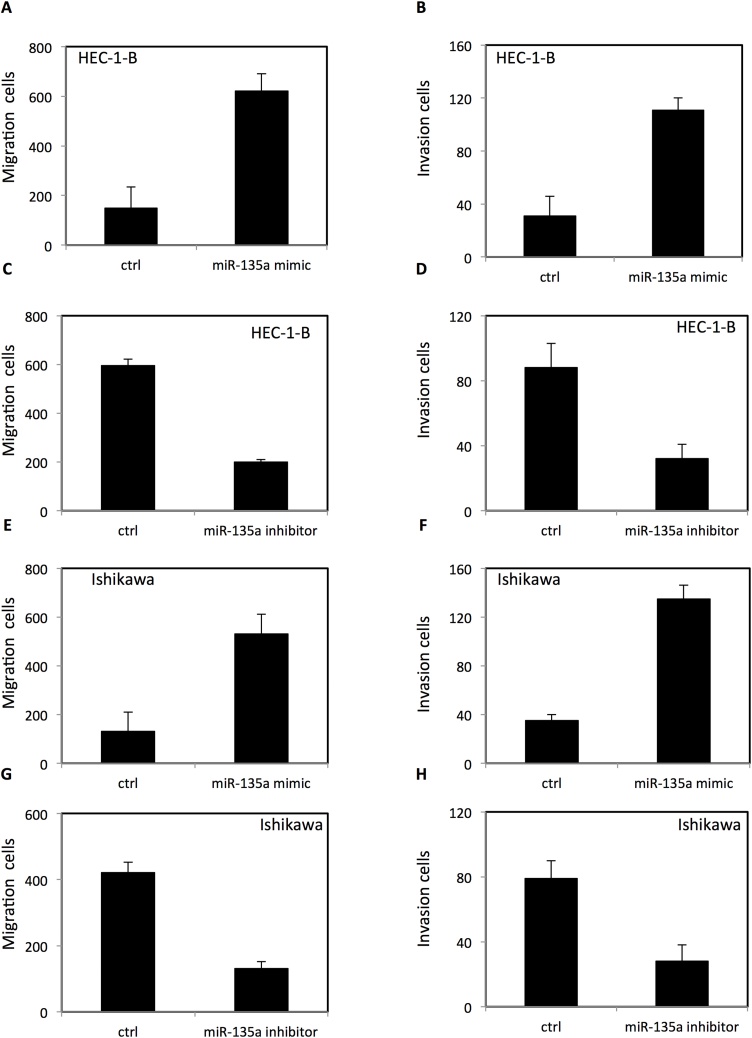
To evaluate the function of miR-135a on migration and invasion of endometrial cancer cells, HEC-1-B and ISHIKAWA endometrial cancer cells were seeded in chambers with or without matrigel. The migration and invasion were examined using transwell migration and invasion assay. Up-regulation of miR-135a enhanced migration and invasion of HEC-1-B cells (Fig. 6A–B) and ISHIKAWA cells (Fig. 6E–F). In contrast, down-regulation of miR-135a decreased the migration and invasion of HEC-1-B cells (Fig. 6C–D) and ISHIKAWA cells (Fig. 6G–H).
Fig. 6.
The effect of miR-135a on migration and invasion of HEC-1-B cells and ISHIKAWA endometrial cancer cells.
(A) Upreguation of miR-135a increased migration of HEC-1-B endometrial cancer cells. (B) Upreguation of miR-135a increased invasion of HEC-1-B cells. (C) Downregulation of miR-135a decreased migration of HEC-1-B endometrial cancer cells. (D) Downregulation of miR-135a decreased invasion of HEC-1-B cells. (E) Upreguation of miR-135a increased migration of ISHIKAWA endometrial cancer cells. (B) Upreguation of miR-135a increased invasion of ISHIKAWA cells. (C) Downregulation of miR-135a decreased migration of ISHIKAWA 7 endometrial cancer cells. (D) Downregulation of miR-135a decreased invasion of ISHIKAWA cells.
MiR-135a altered the expression of EMT markers and activated AKT pathway in endometrial cancer cells
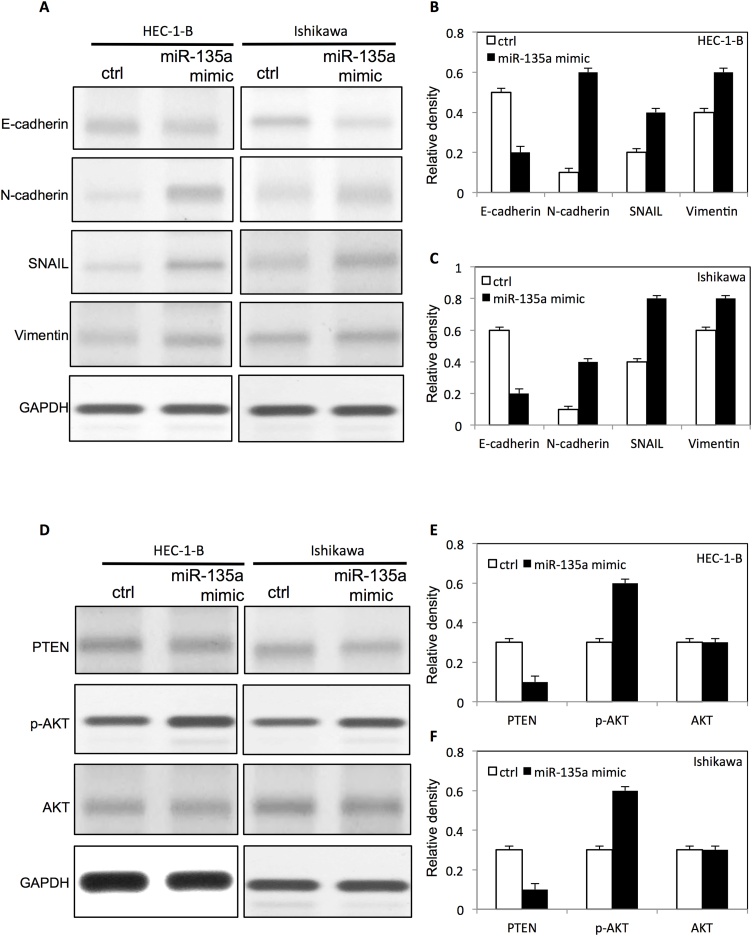
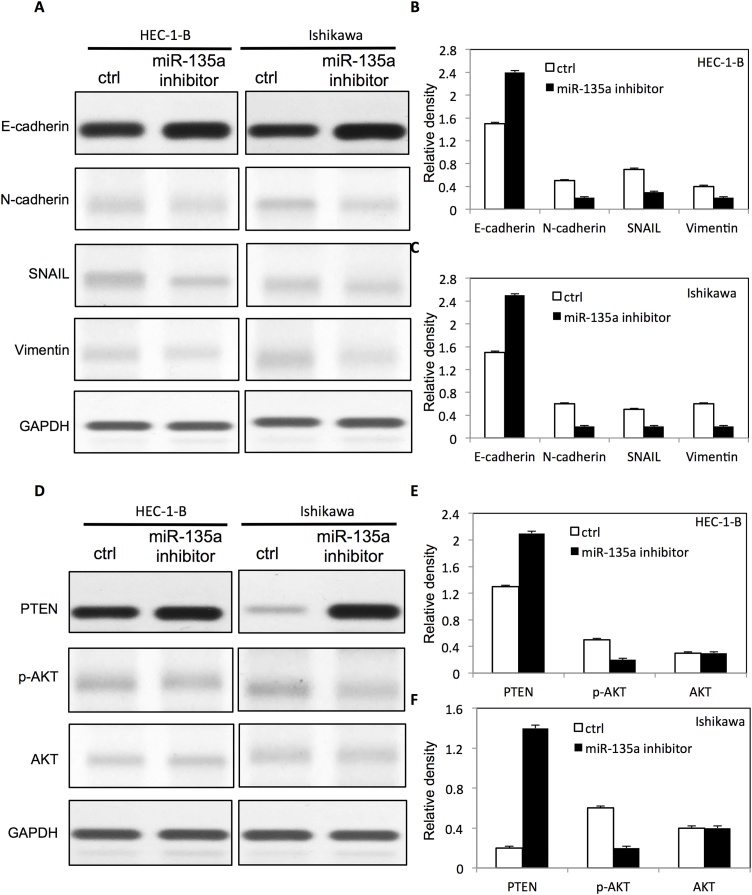
To further examine the role of miR-135a in cell proliferation, migration and invasion in endometrial cancer cells, multiple signaling pathways were analyzed by western blot. We found that the E-cadherin expression was decreased, and the expression level of N-cadherin, snail and Vimentinwere significantly increased in both HEC-1-B cells and ISHIKAWA cells after upregulation of miR-135a (Fig. 7 A–C). However, the down-regulation of miR-135a increased the expression level of E-cadherin, and decreased the expression level of N-cadherin, snail and Vimentin in both HEC-1-B cells and ISHIKAWA endometrial cancer cells (Fig. 8 A–C). Interestingly, we found that miR-135a inhibited the PTEN expression, and increased p-AKT expression in HEC-1-B cells and ISHIKAWA endometrial cancer cells (Fig. 7 D–F). Meanwhile, the down-regulation of miR-135a increased the expression of PTEN, and inhibted the expression of p-AKT in HEC-1-B cells and ISHIKAWA endometrial cancer cells (Fig. 8D–F).
Fig. 7.
MiR-135a altered EMT protein expression and activated AKT expression in endometrial cancer cells.
(A) The expression of EMT proteins in HEC-1-B cells and ISHIKAWA endometrial cancer cells after transfection with miR-135a mimic. The relative expression level of EMT proteins on HEC-1-B cells (B) and ISHIKAWA cells(C) after transfection with miR-135a mimic by densitometric analysis. (D) The expression level of p-AKT, AKT and PTEN in HEC-1-B endometrial cancer cells and ISHIKAWA endometrial cancercells after transfection with miR-135a mimic. The relative expression level of proteins on HEC-1-B cells (E) and ISHIKAWA cells (F) after transfection with miR-135a mimic by densitometric analysis.
Fig. 8.
Downregulation of MiR-135a altered EMT protein expression and inhibited AKT expression in endometrial cancer cells.
(A) The expression of EMT proteins in HEC-1-B cells and ISHIKAWA endometrial cancer cells after transfection with miR-135a inhibitor. The relative expression level of EMT proteins on HEC-1-B cells (B) and ISHIKAWA cells(C) after transfection with miR-135a inhibitor by densitometric analysis. (D) The expression level of p-AKT, AKT and PTEN in HEC-1-B endometrial cancer cells and ISHIKAWA endometrial cancer cells after transfection with miR-135a inhibitor. The relative expression level of proteins on HEC-1-B cells (E) and ISHIKAWA cells (F) after transfection with miR-135a inhibitor by densitometric analysis.
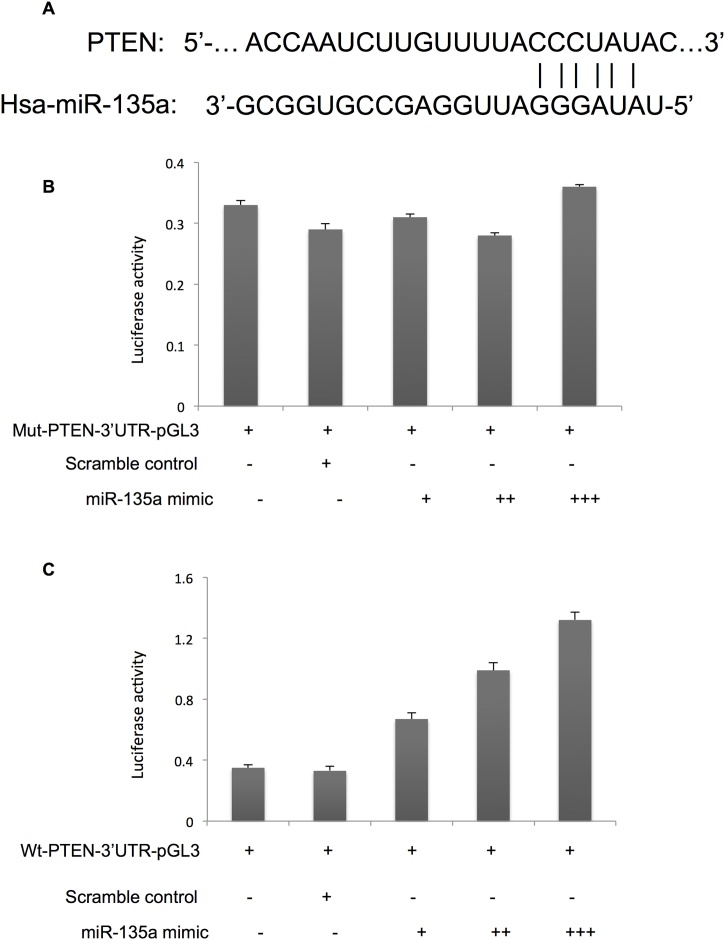
MiR-135a targeted 3′-UTR of PTEN in endometrial cancer cells
We identified the binding sites of miR-135a in 3′-UTR of PTEN using miRNA target prediction tools (Fig. 9A). To validate the putative direct binding site, a dual-luciferase reporter assay was performed using scrambled control and mutated miR-135a binding sites as control. As shown in Fig. 9B, no changes was found in luciferase activity when mu-PTEN-3′-UTR-pGL3 was co-transfected with miR-135a. However, the expression of luciferase of Luc-PTEN was downregulated when wt- PTEN-3′-UTR-pGL3 was co-transfected with miR-135a, compared negative control group (p < 0.05). These results indicated that PTEN was directly regulated by miR-135a in endometrial cancer cells.
Fig. 9.
miR-135a targeted 3′-UTR of PTEN.
A. The potential miR-135a binding site on 3′-UTR of PTEN; B. Luciferase activity of mut-PTEN-3′-UTR-pGL3 co-transfected with miR-135a mimic; C. Luciferase activity of wt-PTEN-3′-UTR-pGL3 co-transfected with miR-135a mimic.
Discussion
Growing evidences have demonstrated that miRNAs can act as either oncogenes or tumor suppressor genes and plays critical roles in tumorigenesis and progression [22]. In the present study, overexpression of miR-135a promoted the proliferation of both HEC-1-B and ISHIKAWA endometrial cancer cells, and down-regulation of miR-135a inhibited proliferation HEC-1-B and ISHIKAWA endometrial cancer cells. These results indicated that miR-135a plays an important role in proliferation of endometrial cancer cells. Cisplatin has been used to treat various cancers as an effective chemotherapy agent for several decades. Cisplatin alone or combination with other chemotherapy agent has significantly improved survival rate in patient with endometrial cancer [23]. However, development of drug resistance is a major problem in endometrial cancer patients with cisplatin-based chemotherapy. Many signaling pathways contribute the development of resistance in cisplatin-based chemotherapy [24]. Many efforts have been made to identify the regulators that affect the sensitivity of cisplatin. Recent studies have shown that some microRNAs, such as miR-92b [25,26], miR-34a [27], miR-98-5p [28], involve cisplatin resistance in difference cancers. In the present study, overexpression of miR-135a increased survival of endometrial cancer cells after cisplatin treatment. And down-regulation of miR-135a decreased survival of endometrial cancer cells after cisplatin treatment. Our results indicated that miR-135a regulated cisplatin resistance of endometrial cancer cells. Further study showed that the expression level of miR-135a was associated with the cisplatin-induced apoptosis in endometrial cancer cells. These findings suggested that miR-135a might affect chemosensitivity of endometrial cancer cells to cisplatin therapy.
Epithelial-mesenchymal transition (EMT) is an important well-described biological process characterized that epithelial cells loss polarity and gain mesenchymal features by multiple signaling pathways. Growing evidence has suggested that aberrant expression of miRNAs regulates EMT program in endometrial cancer. Forced expression of miR-200a inhibited E-cadherin and increased vimentin by repression of FOXA2 expression in endometrial cancer cells [29]. Dong et al. found miR-106b could inhibit TWIST1 expression by directly binding to 3′-untranslated region in endometrial cancer cells [30]. In addition, miR-194 could induce EMT in endometrial cancer stem cells by suppressing the expression of Sox3 [31]. In our study, miR-135a played critical roles in invasion and migration of endometrial cancer cells by regulating expression of EMT-related proteins. These results suggested that miR-135a played essential roles in endometrial cancer progression. Further study indicated that miR-135a changed the expression level of Phosphatase and Tensin homolog (PTEN) and p-AKT in endometrial cancer cells. PTEN is a well-known tumor suppressor and loss of PTEN enhances cancer cell survival, proliferation and decreases apoptosis in various cancers. Loss of PTEN results in activation of PI3K, then initiates protein synthesis and cell cycle. PTEN sequence abnormalities have been detected in up to 55 % of endometrial cancer [32]. MicroRNAs are associated with the expression of PTEN by post-transcriptional regulation. MiR-205 negatively regulates PTEN in early stage endometrial carcinoma and high level of miR-205 indicates poor overall survival in patients with endometrial cancer [33]. Additional studies showed that the upregulation of miR-200a, miR-200b and miR-429 led to suppression of PTEN expression in endometrioid cancer [34]. Our results suggested that miR-135a promoted proliferation of endometrial cancer cells by regulating PTEN.
In summary, miR-135a played important roles in tumorigenesis and disease progression of endometrial cancer by regulating proliferation and chemosensitivy of endometrial cancer cells by targeting AKT signaling pathway. The present study indicated miR-135a could act as a potential biomarker to predict the chemosensitivity and prognosis in endometrial cancer.
Declaration of Competing Interest
No potential conflicts of interest were disclosed.
References
- 1.Cramer D.W. The epidemiology of endometrial and ovarian cancer. Hematol Oncol Clin North Am. 2012;26:1–12. doi: 10.1016/j.hoc.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Nyen T., Moiola C.P., Colas E., Annibali D., Amant F. Modeling endometrial cancer: past, present, and future. Int J Mol Sci. 2018:19. doi: 10.3390/ijms19082348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Felix A.S., Weissfeld J.L., Stone R.A., Bowser R., Chivukula M., Edwards R.P. Factors associated with type I and type II endometrial cancer. Cancer Causes Control. 2010;21:1851–1856. doi: 10.1007/s10552-010-9612-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rauh-Hain J.A., Del Carmen M.G. Treatment for advanced and recurrent endometrial carcinoma: combined modalities. Oncologist. 2010;15:852–861. doi: 10.1634/theoncologist.2010-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koh W.J., Abu-Rustum N.R., Bean S., Bradley K., Campos S.M., Cho K.R. Uterine neoplasms, version 1.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16:170–199. doi: 10.6004/jnccn.2018.0006. [DOI] [PubMed] [Google Scholar]
- 6.Florea A.M., Busselberg D. Cisplatin as an anti-tumor drug: cellular mechanisms of activity, drug resistance and induced side effects. Cancers. 2011;3:1351–1371. doi: 10.3390/cancers3011351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Achkar I.W., Abdulrahman N., Al-Sulaiti H., Joseph J.M., Uddin S., Mraiche F. Cisplatin based therapy: the role of the mitogen activated protein kinase signaling pathway. J Transl Med. 2018;16:96. doi: 10.1186/s12967-018-1471-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brasseur K., Gevry N., Asselin E. Chemoresistance and targeted therapies in ovarian and endometrial cancers. Oncotarget. 2017;8:4008–4042. doi: 10.18632/oncotarget.14021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Felekkis K., Touvana E., Stefanou C., Deltas C. microRNAs: a newly described class of encoded molecules that play a role in health and disease. Hippokratia. 2010;14:236–240. [PMC free article] [PubMed] [Google Scholar]
- 10.Yanokura M., Banno K., Iida M., Irie H., Umene K., Masuda K. MicroRNAS in endometrial cancer: recent advances and potential clinical applications. EXCLI J. 2015;14:190–198. doi: 10.17179/excli2014-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jurcevic S., Olsson B., Klinga-Levan K. MicroRNA expression in human endometrial adenocarcinoma. Cancer Cell Int. 2014;14:88. doi: 10.1186/s12935-014-0088-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ludwig N., Leidinger P., Becker K., Backes C., Fehlmann T., Pallasch C. Distribution of miRNA expression across human tissues. Nucleic Acids Res. 2016;44:3865–3877. doi: 10.1093/nar/gkw116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yanokura M., Banno K., Kobayashi Y., Kisu I., Ueki A., Ono A. MicroRNA and endometrial cancer: roles of small RNAs in human tumors and clinical applications (Review) Oncol Lett. 2010;1:935–940. doi: 10.3892/ol.2010.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vasilatou D., Sioulas V.D., Pappa V., Papageorgiou S.G., Vlahos N.F. The role of miRNAs in endometrial cancer. Epigenomics. 2015;7:951–959. doi: 10.2217/epi.15.41. [DOI] [PubMed] [Google Scholar]
- 15.Li S., Zhang J., Wan X. Role of miRNAs in endometrial cancer. Histol Histopathol. 2015;30:539–548. doi: 10.14670/HH-30.539. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y., Zhang J., Wang H., Zhao J., Xu C., Du Y. miRNA-135a promotes breast cancer cell migration and invasion by targeting HOXA10. BMC Cancer. 2012;12:111. doi: 10.1186/1471-2407-12-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang K.T., Kuo I.Y., Tsai M.C., Wu C.H., Hsu L.W., Chen L.Y. Factor VII-induced MicroRNA-135a inhibits autophagy and is associated with poor prognosis in hepatocellular carcinoma. Mol Ther Nucleic Acids. 2017;9:274–283. doi: 10.1016/j.omtn.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mao X.W., Xiao J.Q., Li Z.Y., Zheng Y.C., Zhang N. Effects of microRNA-135a on the epithelial-mesenchymal transition, migration and invasion of bladder cancer cells by targeting GSK3beta through the Wnt/beta-catenin signaling pathway. Exp Mol Med. 2018;50:e429. doi: 10.1038/emm.2017.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou Y., Li S., Li J., Wang D., Li Q. Effect of microRNA-135a on cell proliferation, migration, invasion, apoptosis and tumor angiogenesis through the IGF-1/PI3K/Akt signaling pathway in non-small cell lung cancer. Cell Physiol Biochem. 2017;42:1431–1446. doi: 10.1159/000479207. [DOI] [PubMed] [Google Scholar]
- 20.Petracco R., Grechukhina O., Popkhadze S., Massasa E., Zhou Y., Taylor H.S. MicroRNA 135 regulates HOXA10 expression in endometriosis. J Clin Endocrinol Metab. 2011;96:E1925–33. doi: 10.1210/jc.2011-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cho S., Mutlu L., Grechukhina O., Taylor H.S. Circulating microRNAs as potential biomarkers for endometriosis. Fertil Steril. 2015;103:1252–1260. doi: 10.1016/j.fertnstert.2015.02.013. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baranwal S. Alahari SK. miRNA control of tumor cell invasion and metastasis. Int J Cancer. 2010;126:1283–1290. doi: 10.1002/ijc.25014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moxley K.M., McMeekin D.S. Endometrial carcinoma: a review of chemotherapy, drug resistance, and the search for new agents. Oncologist. 2010;15:1026–1033. doi: 10.1634/theoncologist.2010-0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen D.W., Pouliot L.M., Hall M.D., Gottesman M.M. Cisplatin resistance: a cellular self-defense mechanism resulting from multiple epigenetic and genetic changes. Pharmacol Rev. 2012;64:706–721. doi: 10.1124/pr.111.005637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y., Li L., Guan Y., Liu X., Meng Q., Guo Q. MiR-92b regulates the cell growth, cisplatin chemosensitivity of A549 non small cell lung cancer cell line and target PTEN. Biochem Biophys Res Commun. 2013;440:604–610. doi: 10.1016/j.bbrc.2013.09.111. [DOI] [PubMed] [Google Scholar]
- 26.Lei L., Huang Y., Gong W. Inhibition of miR-92b suppresses nonsmall cell lung cancer cells growth and motility by targeting RECK. Mol Cell Biochem. 2014;387:171–176. doi: 10.1007/s11010-013-1882-5. [DOI] [PubMed] [Google Scholar]
- 27.Fadejeva I., Olschewski H., Hrzenjak A. MicroRNAs as regulators of cisplatin-resistance in non-small cell lung carcinomas. Oncotarget. 2017;8:115754–115773. doi: 10.18632/oncotarget.22975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y., Bao W., Liu Y., Wang S., Xu S., Li X. miR-98-5p contributes to cisplatin resistance in epithelial ovarian cancer by suppressing miR-152 biogenesis via targeting Dicer1. Cell Death Dis. 2018;9:447. doi: 10.1038/s41419-018-0390-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi W., Wang X., Ruan L., Fu J., Liu F., Qu J. MiR-200a promotes epithelial-mesenchymal transition of endometrial cancer cells by negatively regulating FOXA2 expression. Pharmazie. 2017;72:694–699. doi: 10.1691/ph.2017.7649. [DOI] [PubMed] [Google Scholar]
- 30.Dong P., Kaneuchi M., Watari H., Sudo S., Sakuragi N. MicroRNA-106b modulates epithelial-mesenchymal transition by targeting TWIST1 in invasive endometrial cancer cell lines. Mol Carcinog. 2014;53:349–359. doi: 10.1002/mc.21983. [DOI] [PubMed] [Google Scholar]
- 31.Gong B., Yue Y., Wang R., Zhang Y., Jin Q., Zhou X. Overexpression of microRNA-194 suppresses the epithelial-mesenchymal transition in targeting stem cell transcription factor Sox3 in endometrial carcinoma stem cells. Tumour Biol. 2017;39 doi: 10.1177/1010428317706217. 1010428317706217. [DOI] [PubMed] [Google Scholar]
- 32.Kong D., Suzuki A., Zou T.T., Sakurada A., Kemp L.W., Wakatsuki S. PTEN1 is frequently mutated in primary endometrial carcinomas. Nat Genet. 1997;17:143–144. doi: 10.1038/ng1097-143. [DOI] [PubMed] [Google Scholar]
- 33.Karaayvaz M., Zhang C., Liang S., Shroyer K.R., Ju J. Prognostic significance of miR-205 in endometrial cancer. PLoS One. 2012;7 doi: 10.1371/journal.pone.0035158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoneyama K., Ishibashi O., Kawase R., Kurose K., Takeshita T. miR-200a, miR-200b and miR-429 are onco-miRs that target the PTEN gene in endometrioid endometrial carcinoma. Anticancer Res. 2015;35:1401–1410. [PubMed] [Google Scholar]