Abstract
Multidrug resistance (MDR) is the resistance of cells toward various drugs commonly used in tumor treatment. The mechanism of drug resistance in oral cancer is not completely understood. Melatonin is an endogenously produced molecule involved in active biological mechanisms including antiproliferation, oncogene expression modulation, antitumor invasion and migration, and anti-inflammatory, antioxidant, and antiangiogenic effects. Despite these functions, the effects of melatonin on vincristine (VCR)-resistant human oral cancer cells remain largely unknown. This study analyzed the role of melatonin in VCR-resistant human oral cancer cells along with the underlying mechanism. We determined that melatonin induced the apoptosis and autophagy of VCR-resistant oral cancer cells; these actions were mediated by AKT, p38, and c-Jun N-terminal kinase (JNK). Melatonin inhibited ATP-binding cassette B1 (ABCB1) and ABCB4 expression in vitro and in vivo. Melatonin reduced the drug resistance and promoted the apoptosis of VCR-resistant oral cancer cells through the upregulation of microRNA-892a (miR-892a) and miR-34b-5p expressions. The expression of miR-892a and miR-34b-5p was related to melatonin-induced apoptosis, but not autophagy. Therefore, melatonin is a potential novel chemotherapeutic agent for VCR-resistant human oral cancer cell lines.
Keywords: melatonin, multi-drug resistance, apoptosis, autophagy, P-glycoprotein, microRNA
Introduction
Oral cancers are highly aggressive malignancies with high mortality rates globally. Approximately 95% of all oral cancers emerge from the squamous epithelium lining the oral cavity.1 Patients with a 5-year survival rate of <50% in the later stages of the disease may be treated with a combination therapy comprising clinical surgery, drug chemotherapy, and radiotherapy. Meta-analyses have reported that chemotherapy is highly beneficial for overall survival in locally advanced oral cancer.2,3 However, many patients respond unfavorably to a combination chemotherapy; thus, drug resistance remains a primary challenge and improving the response and survival of patients with cancer is necessary.4
Multidrug resistance (MDR) is the resistance of cells toward different drugs used in cancer treatments.5 P-glycoprotein (P-gp), MDR-associated protein (MRP1), and the breast cancer resistance protein (BCRP) are widely known for MDR.6 The overexpression of drug efflux transporters may reduce drug influx, leading to MDR phenotypes.7
Low extracellular pH in oral cancer inhibits the effect of clinical chemotherapy drugs, thereby reducing the antitumor effects.8 Oral cancer is highly resistant to various drugs with different structures and cytotoxic action mechanisms. Generally, this cancer recurs within several months of chemotherapy completion. Patients often respond less favorably to drugs on repeating the chemotherapy,9 indicating that oral cancer may be intrinsically chemoresistant. Specific molecules, such as V-ATPases, may play crucial roles in such a phenomenon.10 Ralhan et al.11 observed that P-gp expression increased with increasing dysplasia severity and tumor recurrence, indicating that differential P-gp level may act as a prognostic marker in oral cancer. Moreover, in various cell lines exposed to therapeutic vincristine (VCR; vinca alkaloid antineoplastic drug), MDR1 expression increases P-gp levels, suggesting that P-gp-induced MDR in oral cancer is a critically acquired phenotype caused by the genetic induction of P-gp.6 The accumulation of the genetic alterations of tumor suppressor genes and oncogenes is related to the growth of oral cancer.12 A clear association between P-gp expression and MDR in various tumors is well known;13 however, the mechanism of drug resistance in oral cancer has not been completely studied. Therefore, elucidating the association between the mechanism of carcinogenesis, cancer progression, and MDR development is crucial.
Melatonin (N-acetyl-5-methoxy tryptamine) is synthesized and secreted by the pineal gland and among its many actions regulates the sleep-wake rhythm.14,15 Melatonin also attenuates the oxidative damage caused by doxorubicin.16, 17, 18 It is involved in active biological mechanisms, including antiproliferation,19,20 proapoptotic action,21,22 immunity stimulation,23, 24, 25 oncogene expression modulation,26,27 antitumor invasion and migration,28, 29, 30, 31, 32 uptake glucose transport,33 antiobesogenic and weight-reducing effects,34 antioxidant,35, 36, 37 anti-inflammatory, and antiangiogenic effects.31,32,38 Melatonin enhances cell cytotoxicity and apoptosis in 5-fluorouracil-resistant and cisplatin-resistant colorectal and cervical cancer cells.39, 40, 41 However, the effects of melatonin on VCR-resistant human oral cancer cells have not been studied in detail and clearly understood so far.
The objectives of this work were to understand the effect of melatonin on VCR-resistant human oral cancer cell and discuss its possible mechanisms. Melatonin was observed to be a potential novel chemotherapeutic agent for VCR-resistant human oral cancer cell lines.
Results
Melatonin Inhibited VCR-Resistant Oral Cancer Cell Survival and Proliferation
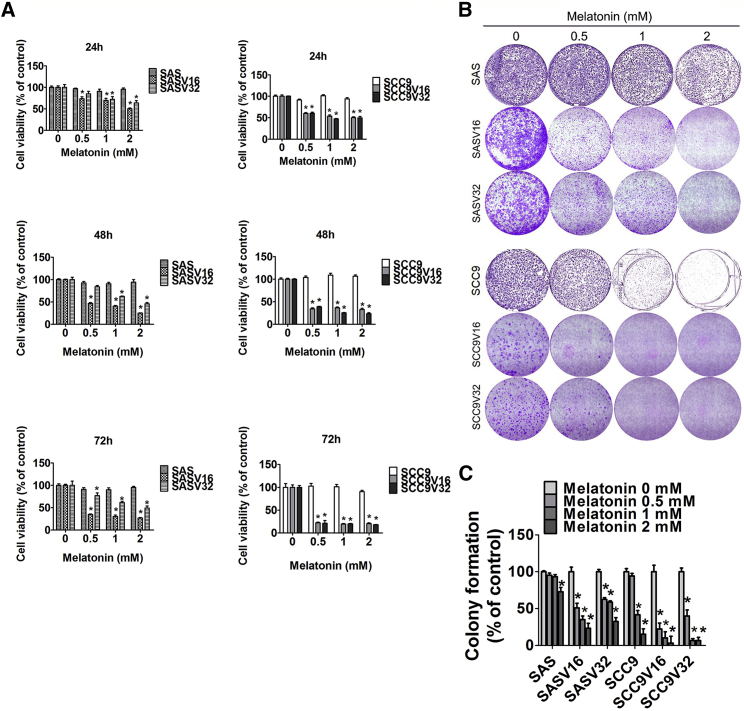
Oral cancer cell lines (SAS and SCC9) and VCR-resistant oral cancer cells (SASV16, SASV32, SCC9V16, and SCC9V32) were treated with varying melatonin concentrations (0.5–2 mM). The 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay revealed that even after a treatment of 72 h with the highest concentration of melatonin, the viability of SAS and SCC9 cell lines remained the same. Notably, four VCR-resistant oral cancer cells were evidently inhibited by the melatonin treatment (Figure 1A). Furthermore, to determine the anti-cell proliferative ability of melatonin for VCR-resistant oral cancer cells, colony-formation data were arranged to investigate the influence of melatonin on VCR-resistant oral cancer cells during a long-term treatment. Figures 1B and 1C illustrate that melatonin (0.5 mM) significantly suppressed the colony-forming ability of four VCR-resistant oral cancer cells. Thus, melatonin inhibited VCR-resistant oral cancer cell survival and proliferation.
Figure 1.
The Influence of Melatonin on Cell Cytotoxicity in VCR-Resistant Oral Cancer Cells
(A) Two oral cancer cells (SAS and SCC9) and four VCR-resistant oral cancer cell lines (SASV16, SASV32, SCC9V16, and SCC9V32) were treated with different concentrations of melatonin and analyzed by MTT assay. (B) Four VCR-resistant oral cancer cells were treated with melatonin for 2 weeks. (C) The formazan crystals were restored by DMSO, and the absorbance was measured. The experiments were repeated at least three times. Values are presented as the mean ± SE of three independent experiments. *p < 0.05, compared with the control group.
Melatonin Induced the Apoptosis of VCR-Resistant Oral Cancer Cell and Reduced Mitochondrial Membrane Potential
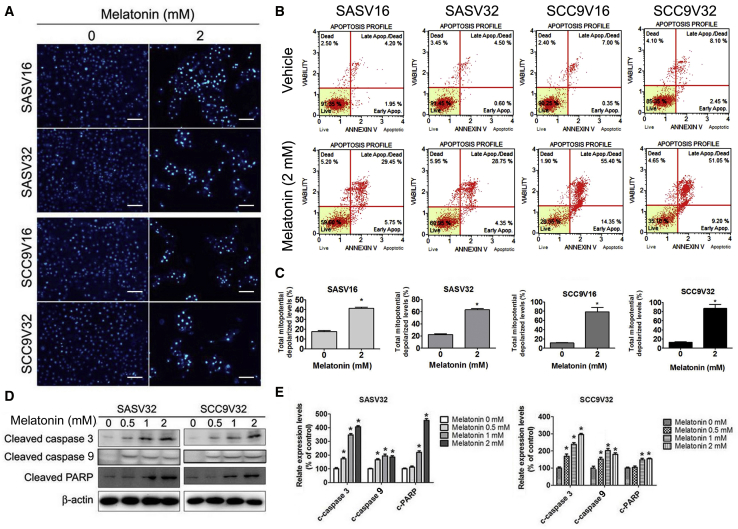
We further investigated the reason for the inhibition of VCR-resistant oral cancer cell survival and proliferation by melatonin. First, apoptosis induction was identified. As presented in Figure 2A, the chromatin condensation of VCR-resistant oral cancer cell lines gradually increased after melatonin treatment. Membrane phosphatidylserine translocation and late apoptosis were observed in melatonin-treated VCR-resistant oral cancer cell lines (Figure 2B). Flow cytometry data indicated that melatonin induced the depolarization of the transmembrane potential (Δψm) in VCR-resistant oral cancer cell lines (Figure 2C). In addition, the expression of activated apoptotic caspase-3, caspase-9, and cleaved poly (ADP-ribose) polymerase (PARP) was significantly upregulated (Figures 2D and 2E). Thus, melatonin induced the apoptosis of VCR-resistant oral cancer cells.
Figure 2.
The Influence of Melatonin on Cell Apoptosis in VCR-Resistant Oral Cancer Cells
(A) Four VCR-resistant oral cancer cells were treated with melatonin (2 mM) for 24 h, and then DAPI staining. (B) Apoptosis cells were measured by Muse Cell Analyzer Assays. (C) Mitochondrial membrane potential was performed by Muse Cell Analyzer Assays. (D) The expression change of cleaved caspase-3, −9, and PARP were analyzed by specific primary antibody, respectively. (E) Bar graphs represent the relative density of each band normalized to β-actin. The experiments were repeated at least three times. Values are presented as the mean ± SE of three independent experiments. *p < 0.05, compared with the control group.
Melatonin-Induced Autophagy in VCR-Resistant Oral Cancer Cells
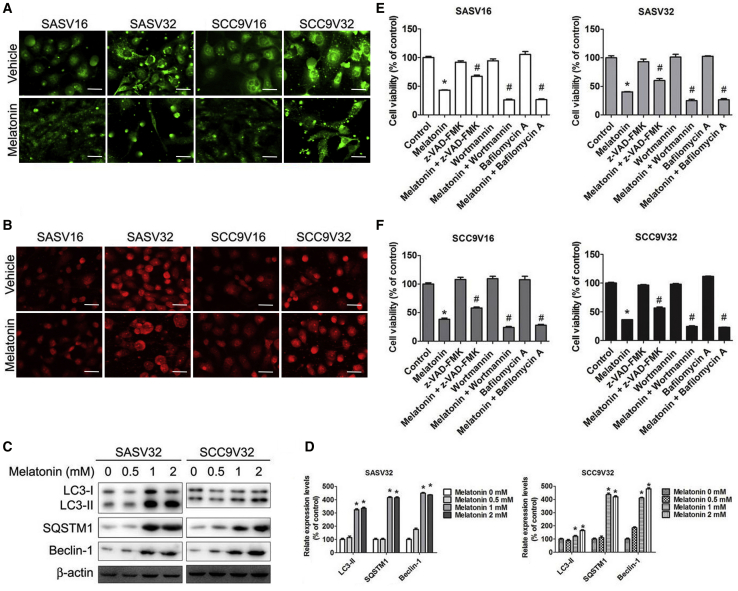
Studies have indicated that autophagy is involved in cell survival and programmed death.42,43 As shown in Figure 3A, LC3-II reflected a melatonin-induced autophagic activity, which was detected using the immunofluorescence and immunoblotting of VCR-resistant oral cancer cell lines (Figures 3C and 3D). The formation of autophagic vacuoles that was detected using specific fluorescent dyes, such as acridine orange (AO), confirmed melatonin-induced autophagy. The melatonin treatment increased acidic vesicular organelle (AVO) formation in VCR-resistant oral cancer cell lines (Figure 3B). Moreover, SQSTM1 and beclin-1 were upregulated in melatonin-treated VCR-resistant oral cancer cell lines (Figures 3C and 3D). To further explain the relationship between melatonin-induced apoptosis and autophagy, we combined the melatonin treatment with one of the pretreatments using z-VAD-FMK, wortmannin, or bafilomycin A. As illustrated in Figures 3E and 3F, living cells were slightly affected after melatonin treatment with wortmannin or bafilomycin A group. Thus, melatonin induced autophagy in VCR-resistant oral cancer cells.
Figure 3.
Melatonin Causes Autophagy in VCR-Resistant Oral Cancer Cells
(A) Four VCR-resistant oral cancer cells were treated with vehicle or melatonin (2 mM) for 24 h, and then cells were stained with Cell Meter Autophagy Assay Kit fluorescent dye. After staining, cells were observed under a fluorescence microscope, which is an indicator of autophagosome formation. (B) After treatment, cells were stained with acridine orange (AO) for acidic vesicular organelles (AVOs) formation, which was examined under a fluorescence microscope. The amount of AVOs (orange-red fluorescence) can be used as a marker of autophagosomes. (C) A representative western blot for expression of LC3-I/II, SQSTM1, and Beclin-1 in cells were treated with increasing concentrations of melatonin. (D) Bar graphs represent the relative density of each band normalized to β-actin. (E) SASV16 and SASV32 oral cancer cells were pretreated with z-VAD-FMK (20 μM), wortmannin (50 μM), or bafilomycin A (1 nM) for 2 h followed by treatment with or without melatonin (2 mM) for 24 h. (F) SCC9V16 and SCC9V32 oral cancer cells were pretreated with z-VAD-FMK (20 μM), wortmannin (50 μM), or bafilomycin A (1 nM) for 2 h followed by treatment with or without melatonin (2 mM) for 24 h. Cell viability was analyzed by MTT assay. The experiments were repeated at least three times. Results are shown as mean ± SEM. *p < 0.05, compared with the control group. #p < 0.05, compared with the only melatonin group.
Melatonin Reduced the Expression of MDR Proteins ABCB1 and ABCB4 in VCR-Resistant Oral Cancer Cells
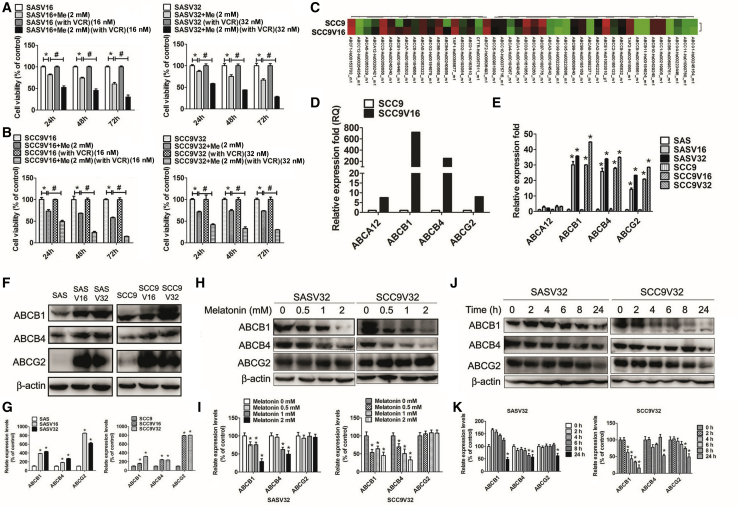
To explain the molecular mechanism, we measured the melatonin-induced inhibition of cell viability in VCR-resistant oral cancer cell-conditioned medium with and without VCR. Although melatonin treatment alone also inhibited cell survival in the conditioned medium without VCR, a more pronounced effect was observed in the conditioned medium with VCR (Figures 4A and 4B). The gene-expression array analysis and real-time PCR data of ATP-binding cassette (ABC) transporter superfamily revealed that the expression of ABCB1, ABCB4, and ABCG2 genes in oral cancer cells and VCR-resistant oral cancer cells was significantly different (Figures 4C–4E). Immunoblot data confirmed that the expression of ABCB1, ABCB4, and ABCG2 significantly increased in VCR-resistant oral cancer cells (Figures 4F and 4G). To further clarify the gene expression of ABCB1, ABCB4, and ABCG2 in melatonin-treated VCR-resistant oral cancer cell lines, we treated cells with melatonin in a conditioned medium with VCR for 24 h. As illustrated in Figures 4H and 4I, ABCB1 and ABCB4 expression decreased in melatonin-treated VCR-resistant oral cancer cell lines. In the conditioned medium without VCR, the gene expression of ABCB1, ABCB4, and ABCG2 gradually decreased within 24 h (Figures 4J and 4K). Thus, we confirmed that the expression of ABCB1, ABCB4, and ABCG2 gradually decreases without VCR. Therefore, melatonin not only suppressed VCR-resistant oral cancer cell line survival but also upregulated the susceptivity of VCR-resistant oral cancer cell line toward VCR, reducing the expression of ABCB1 and ABCB4.
Figure 4.
The Influence of Melatonin on ABCB1 and ABCB4 Expression in VCR-Resistant Oral Cancer Cells
(A and B) (A) SASV16 and SASV32 and (B) SCC9V16 and SCC9V32 oral cancer cells were treated with/without melatonin (2 mM) in conditioned medium (with/without VCR) (16 nM or 32 nM), and then analyzed by MTT assay. (C) Heatmap depiction of ABC transporter superfamily gene differentially expressed between SCC9 cells versus SCC9V16 cells, analyzed by gene-expression array plates. (D) Average expression fold change in ABC transporter superfamily gene expression. (E) Quantified PCR validation of differentially expressed genes between oral cancer cells and VCR-resistant oral cancer cells selected from the microarray analysis. (F) The expression of ABCB1, ABCB4, and ABCG2 between oral cancer cells and VCR-resistant oral cancer cells. (G) Bar graphs represent the relative density of each band normalized to β-actin. (H) The expression of ABCB1, ABCB4, and ABCG2 was treated with melatonin (0.5–2 mM). (I) Bar graphs represent the relative density of each band normalized to β-actin. (J) Cells were incubated with melatonin (2 mM) for the indicated time intervals and the expression levels of ABCB1, ABCB4, and ABCG2 were examined by western blot. (K) Bar graphs represent the relative density of each band normalized to β-actin. All experiments were repeated at least three times. Results are shown as mean ± SEM. *p < 0.05, compared with the control group. #p < 0.05, compared with the only melatonin group.
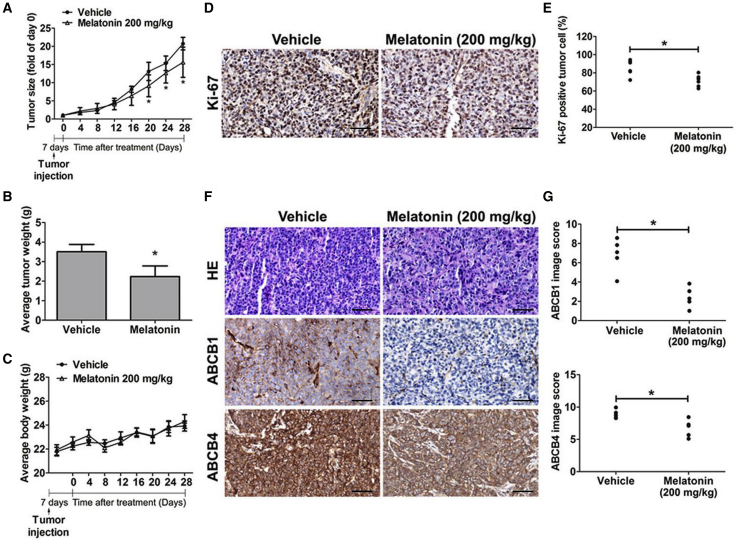
Significant Antitumor Proliferative Effects of Melatonin in a VCR-Resistant Oral Orthotopic Graft Model
To investigate the influence of melatonin on antitumor growth, we investigated antitumor activity of this molecule in vivo. In SASV32 cancer cell models, tumor size progressively increased, as observed in control animals. On day 28, both the average tumor volume and weight in melatonin-treated (200 mg/kg) mice were lower than those in vehicle-treated mice (Figures 5A and 5B). Average body weight was no different (Figure 5C). Cell proliferation detected using the immunohistochemistry (IHC) analysis indicated that the number of Ki67-positive tumor cells decreased in melatonin-treated mice (Figures 5D and 5E). Moreover, ABCB1 and ABCB4 expression decreased in the melatonin-treated tumor specimens (Figures 5F and 5G). Thus, melatonin treatment exhibited an antitumor proliferative effect and inhibited ABCB1 and ABCB4 expression in SASV32 cancer cells in mice.
Figure 5.
The Influence of Melatonin on Anti-Tumor Growth In Vivo
After injection, nude mice were received treatment with melatonin (200 mg/kg). (A–C) The difference changes of (A) tumor size, (B) average tumor weight, or (C) mice body weight are shown. (D) Ki67-positive cells were counted at 200× magnification per tumor section. (E) A quantified value of the Ki67-positive tumor cells expression percentage. (F) Tumor tissues were detected by H&E staining (upper panel) and anti-ABCB1, -ABCB4 IHC staining. Original magnifications: 200×. (G) The ABCB1 (upper panel) and ABCB4 (lower panel) image score is indicated by counting the number of cells. Results are shown as mean ± SEM. *p < 0.05, compared to the vehicle groups.
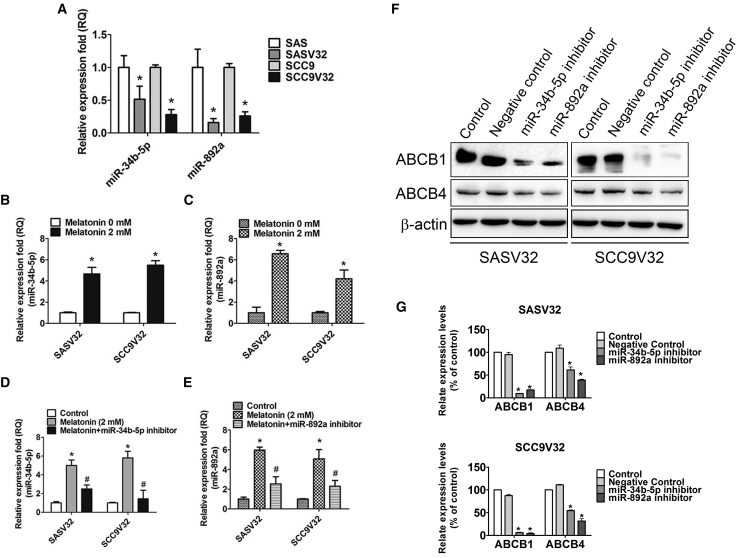
Melatonin Reduced Drug Resistance and Promoted VCR-Resistant Oral Cancer Cell Apoptosis through Induction of miR-34b-5p and miR-892a Expression
Some microRNAs (miRNAs) have been reported to adjust drug-resistance gene expression or induction.44, 45, 46, 47, 48 ABCB1 was the immediate target of miR-34b; miR-34b upregulated apoptosis induction and enhanced the anticancer drug susceptibility of cancer cells.49 CYP1A1 was the goal of miR-892a.50 To clarify the molecular mechanism for the melatonin-induced inhibition of ABCB1 and ABCB4, we analyzed the expression of miRNA genes associated with ABC transporter proteins. miRNA gene expression array and real-time PCR results revealed that the expression level of miR-34b-5p and miR-892a apparently decreased in VCR-resistant oral cancer cells (Figure 6A). miR-34b-5p and miR-892a levels were elevated in melatonin-treated VCR-resistant oral cancer cell lines (Figures 6B and 6C). miR-34b-5p and miR-892a were repressed in melatonin-treated VCR-resistant oral cancer cell lines with specific miRNA inhibitors (Figures 6D and 6E). The expressions of ABCB1 and ABCB4 decreased in miR-34b-5p and miR-892a in melatonin-treated VCR-resistant oral cancer cell lines (Figures 6F and 6G).
Figure 6.
Melatonin Induces miR-34b-5p and miR-892a Expression
(A) miR-34b-5p and miR-892a expression changes between oral cancer cells and VCR-resistant oral cancer cells were confirmed by real-time PCR. (B and C) After incubation with melatonin (2 mM) for 24 h, (B) miR-34b-5p and (C) miR-892a were examined by real-time PCR. (D and E) After transfection with (D) miR-34b-5p inhibitor (1 μM) or (E) miR-892a inhibitor (1 μM) for 24 h, following melatonin treatment for an extra 24 h. The miR-34b-5p and miR-892a expression changes were confirmed by real-time PCR. (F) After transfection with miR-34b-5p inhibitor (1 μM) or miR-892a inhibitor (1 μM) for 24 h, the expression levels of ABCB1 and ABCB4 were examined by western blot. (G) Bar graphs represent the relative density of each band normalized to β-actin. All experiments were repeated at least three times. Results are shown as mean ± SEM. *p < 0.05, compared with the control group. #p < 0.05, compared with the only melatonin group.
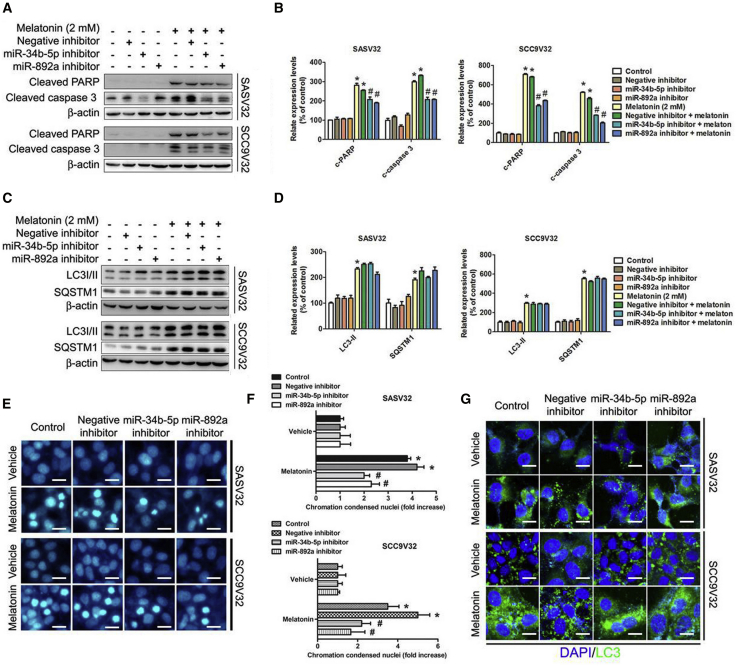
Expression of miR-34b-5p and miR-892a Was Associated with Melatonin-Induced Apoptosis, but Not With Autophagy
To investigate the association between melatonin-induced miR-34b-5p and miR-892a expression, apoptosis, and autophagy, we analyzed the expressions of related proteins. The expression of cleaved PARP and cleaved caspase-3 decreased under the combination treatment of melatonin and miR-34b-5p or miR-892a inhibitors (Figures 7A and 7B). However, autophagosomal markers, LC3-II and SQSTM1, were unaffected by the combination treatment of melatonin and miR-34b-5p or miR-892a inhibitors (Figures 7C and 7D). Moreover, chromatin condensation in nuclei significantly decreased with the combination treatment of melatonin and miR-34b-5p or miR-892a inhibitors (Figures 7E and 7F). The autophagosomal marker LC3-II detected using immunofluorescence indicated that the melatonin-induced autophagic activity was unaffected by the combination treatment of melatonin and miR-34b-5p or miR-892a inhibitors (Figure 7G).
Figure 7.
Relationship of miR-34b-5p and miR-892a with Melatonin-Induced Apoptosis and Autophagy
(A) After transfection with miR-34b-5p inhibitor (1 μM) or miR-892a inhibitor (1 μM) for 24 h, melatonin (2 mM) treatment for an extra 24 h. Protein expression were detected by specific primary antibody, respectively. (B) Bar graphs represent the relative density of each band normalized to β-actin. (C) Cells were transfected with miR-34b-5p inhibitor (1 μM) or miR-892a inhibitor (1 μM) for 24 h, and then melatonin (2 mM) treatment for an extra 24 h. Protein expression was detected by specific primary antibody, respectively. (D) Bar graphs represent the relative density of each band normalized to β-actin. (E) After treatment, cells were fixed and stained with DAPI solution. Nuclear fragmentation and condensation were observed under fluorescence microscope. (F) Bar graphs represent the relative density of nuclear fragmentation and condensation. (G) Results were examined under a confocal microscope. All experiments were repeated at least three times. Results are shown as mean ± SEM. *p < 0.05, compared with the control group. #p < 0.05, compared with the only melatonin (2 mM) group.
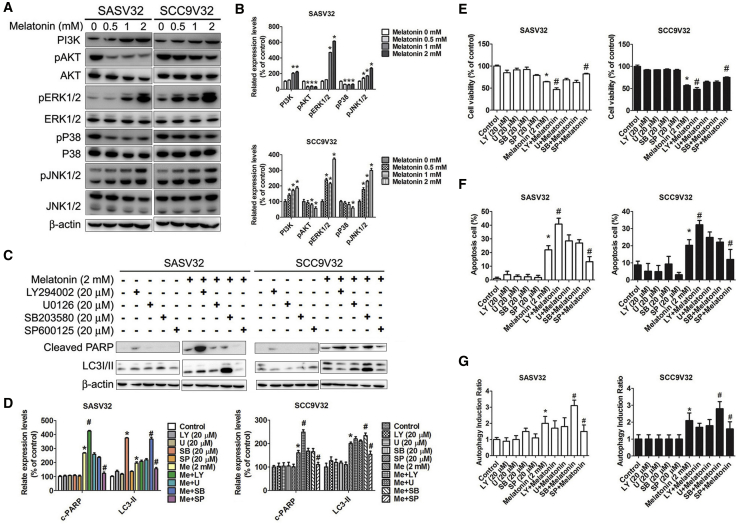
Melatonin-Induced Apoptosis and Autophagy in VCR-Resistant Oral Cancer Cells Were Regulated by AKT, p38, and JNK
The mitogen-activated protein kinase (MAPK) pathway was used to adjust various cell reactions including cell survival, proliferation, differentiation, and death.51 The phosphorylation of phosphatidylinositol 3-kinase (PI3K), ERK1/2, and JNK1/2 was upregulated in melatonin-treated VCR-resistant oral cancer cell lines (Figures 8A and 8B). In addition, the activation of AKT and p38 was significantly inhibited in a concentration-dependent manner. The expression of cleaved PARP increased after combination treatment with melatonin and LY294002 inhibitors, whereas it decreased after combination treatment with melatonin and SP600125 inhibitors. Moreover, LC3-II expression was increased by the combination treatment of melatonin and SB203580 inhibitors, whereas it was reduced by the combination treatment of melatonin and SP600125 inhibitors (Figures 8C and 8D). LY294002 or SP600125 inhibitors in combination with the melatonin treatment influenced cell viability in VCR-resistant oral cancer cell lines (Figure 8E). Apoptotic cells improved with the combination treatment of melatonin and LY294002, a selective PI3K inhibitor (Figures 8F and 8G). In addition, autophagy induction ratios increased with the combination treatment of melatonin and SB203580, which is a specific inhibitor targeting the p38-MAPK pathway. However, both apoptotic cells and the autophagy induction ratio decreased after combination treatment with melatonin and SP600125.
Figure 8.
The Effect of Melatonin-Induced Apoptosis and Autophagy on Activation of AKT and MAPKs in VCR-Resistant Oral Cancer Cells
(A) The expression change of PI3K, AKT, p38, ERK1/2, and JNK1/2 in cells. (B) Bar graphs represent the relative density of each band normalized to β-actin. (C) Cells were pretreated with LY (LY294002), U (U0126), SB (SB203580), or SP (SP600125), and then treated with melatonin for 24 h. Effects of the inhibition of AKT, ERK1/2, p38, and JNK1/2 were assessed by specific primary antibody, respectively. (D) Bar graphs represent the relative density of each band normalized to β-actin. (E) Living cells were detected by MTT assay. (F and G) Apoptosis cells (F) and autophagy induction (G) ratio were detected by Muse Cell Analyzer Assays. All experiments were repeated at least three times. Results are shown as mean ± SEM. *p < 0.05, compared with the control group. #p < 0.05, compared with the only melatonin (2 mM) group.
Discussion
Because patients often respond unfavorably to combination chemotherapies, drug resistance is a major challenge in improving drug response and overall survival among patients with cancer. In this paper, MDR refers to the resistance of cells to numerous drugs used in cancer treatment. The main mechanism causing MDR phenotype is the overexpression of drug efflux transporters. Li et al.52 reported that ethyl lucidenates A, a pure compound of Ganoderma lucidum, inhibits efflux transport and increases the intracellular accumulation of VCR. Hyperin, a flavonoid compound found in Ericaceae, Guttiferae, and Celastraceae, treated with VCR markedly reduces P-gp expression in VCR-resistant colon cells.53 Reducing the P-gp performance upregulates the susceptivity of drug-resistant cancer cell lines to chemotherapeutic drugs and promotes death.
Melatonin is an indoleamine synthesized by the mitochondria and chloroplasts of both animals and plants. Melatonin-protective leukocyte apoptosis depends on the antioxidant action of melatonin, and the protection is melatonin-receptor independent.35 Melatonin treatment significantly reduced both hydrogen peroxide and superoxide anion levels due to free-radical scavenging properties and enhanced leucocyte viability.36 It also was considered a potential tool against oxidative damage and apoptosis.37 The effects of melatonin on MDR human cancer cells remain largely unknown. To date, few studies have discussed the relationship between melatonin and MDR. Fan et al.54 indicated that melatonin increased C/EBP homologous protein (CHOP) expression and improved the cellular cytotoxic effects of doxorubicin, reducing the survival of cancer cells. Melatonin increased the ABCG2/BCRP expression, function, and promotor methylation.55 Melatonin-induced acetylation caused high clofarabine-induced cytotoxicity in drug-resistant cells.56 Melatonin sensitized cervical cancer cells to cisplatin-induced cytotoxicity and apoptosis.40 Melatonin increased the sensitivity of colorectal adenocarcinoma cells toward 5-fluorouracil (FU) treatment.41 Moreover, Xiang et al.18 reported that doxorubicin resistance was driven by the disruption of melatonin signal in breast cancer. Our study determined the effect of melatonin on VCR-resistant oral cancer cells and its mediation mechanism.
SASV16, SASV32, SCC9V16, and SCC9V32 represented cell lines with different degrees of tolerance to VCR concentrations. In our study, melatonin inhibited cell survival and proliferation under low-concentration and long-term treatment (0.5 mM, 72 h). However, melatonin did not alter the viability in SAS and SCC9 cell lines (Figure 1). These results are in accordance with those of studies on the association between melatonin and other cancer cells.28, 29, 30,57 Melatonin caused VCR-resistant oral cancer cell apoptosis and autophagy (Figures 2 and 3). Autophagy is involved in cancer cell survival, maintenance, and death.58,59 Autophagy is defined as a mechanism of programmed cell death (PCD).60 Our study indicated that melatonin-induced autophagy plays a cell-survival role in melatonin-induced VCR-resistant oral cancer cell death.
Melatonin treatment alone also inhibited cell survival in the conditioned medium without VCR (Figures 4A and 4B). ABCB1, ABCB4, and ABCG2 expression decreased gradually for 24 h in the conditioned medium without VCR (Figures 4J and 4K). Therefore, melatonin can inhibit VCR-resistant oral cancer cell line survival. Moreover, melatonin treatment in the conditioned medium with VCR exhibited a more pronounced effect. Thus, melatonin upregulated the susceptivity of VCR-resistant oral cancer cell lines by inhibiting ABCB1 and ABCB4 expression (Figures 4H and 4I). Similarly, an antitumor proliferation effect and the inhibition of ABCB1 and ABCB4 expression were observed in melatonin-treated SASV32 cancer cells in vivo (Figure 5).
miRNAs are potential druggable targets for MDR cancer chemotherapy.61, 62, 63 Some miRNAs have been reported to adjust drug-resistance gene expression or induction.44, 45, 46, 47, 48 ABCB1 was reported as the immediate target of miR-34b; miR-34b upregulation apoptosis induction enhanced the anticancer drugs susceptibility of the cancer cells.49 Moreover, miRNAs exerted deep cellular influences on the adjustment of the cytochrome P450 (CYP) family. CYP1A1 was reported as the goal of miR-892a.50 miR-34b-5p and miR-892a were downregulated in VCR-resistant oral cancer cell lines more than they were downregulated in SAS and SCC9 cell lines (Figure 6). Melatonin-induced miR-34b-5p and miR-892a expression influenced ABCB1 and ABCB4 expression, and these proteins were the direct targets of miR-34b-5p and miR-892a. The expression of cleaved PARP and cleaved caspase-3 decreased on combination treatment with melatonin and miR-34b-5p or miR-892a inhibitors. However, LC3-II and SQSTM1 remained unaffected (Figure 7). These findings indicated that melatonin exhibited the ability to promote apoptosis and increased VCR drug sensitivity by increasing miR-34b-5p and miR-892a expression in VCR-resistant oral cancer cell lines.
In conclusion, the results determined that miR-34b-5p and miR-892a perform a crucial regulating task in the VCR drug resistance of MDR-resistant oral cancer cell lines. In vivo and in vitro findings suggested that melatonin increases miR-34b-5p and miR-892a expression, reduces ABCB1 and ABCB4 expression, promotes apoptosis, and increases drug sensitivity. Melatonin was observed to be a potential novel chemotherapeutic agent for VCR-resistant oral cancer cell lines.
Materials and Methods
Chemicals
Melatonin (purity >99%) was obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). It was dissolved in dimethyl sulfoxide (DMSO) and diluted with culture medium to the aimed concentration on experimental day. The final concentration of DMSO for all treatments was consistently less than 0.1%. Cell culture reagents were obtained from Invitrogen (Carlsbad, CA, USA). The VCR, Coomassie brilliant blue, MTT, 4′,6-diamidino-2-phenylindole (DAPI) dye, protease inhibitor cocktail, phosphatase inhibitor cocktail, and AO were purchased from Sigma-Aldrich (St. Louis, MO, USA). Negative inhibitor (miRNA inhibitor negative control), miRNA-34b-5p inhibitor, and miRNA-892a inhibitor were purchased from Clontech (CA, USA). Antibody against cleaved PARP, cleaved caspase-3, -9, LC3, SQSTM1, Beclin-1, ABCB1, ABCG2, p-AKT, AKT, p-ERK1/2, ERK1/2, p-p38, p38, p-JNK1/2, JNK1/2, and β-actin were purchased from Cell Signaling Technology (Danvers, MA, USA). Antibody against ABCB4 was obtained from MyBioSource (San Diego, CA, USA). Specific inhibitors for AKT inhibitor (LY294002), ERK1/2 (U0126), p38 MAPK (SB203580), JNK (SP600125), wortmannin, bafilomycin A1 (Baf A1), and z-VAD-FMK were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Cell Culture
Oral cancer cell lines (SAS and SCC9) were purchased from American Type Culture Collection. SAS cells were cultured in Dulbecco’s modified Eagle medium (DMEM)/F12 medium supplemented with 10% fetal bovine serum (FBS), 1 mM glutamine, 1% penicillin/streptomycin (10,000 U/mL penicillin and 10 mg/mL streptomycin), 25 mM HEPES (pH 7.4), 1.5 g/L sodium bicarbonate, and 1 mM sodium pyruvate (Sigma, St. Louis, MO, USA). SCC9 cells were cultured in DMEM/F12 medium supplemented with 10% FBS, 0.1 mM non-essential amino acids (NEAA), 1% penicillin/streptomycin, 1 mM glutamine, 1.5 g/L sodium bicarbonate, hydrocrostine (0.4 mg/L), 25 mM HEPES (pH 7.4), and 1 mM sodium pyruvate. Drug-resistant oral cancer cell lines were established as previously described.64 The VCR-resistant subline kept at 16 nM VCR represents SAS/V16 and SCC9/V16. The VCR-resistant subline kept at 32 nM VCR represents SAS/V32 and SCC9/V32.
Cell Cytotoxicity
Cells were seeded into 96-well plates at a density of 0.5 × 105 cells/mL and grown overnight. After melatonin treatment, MTT (5 mg/mL) was treated in conditioned medium followed by incubation in cell culture box (4 h, 37°C). The supernatant was discarded, and DMSO was added to restore the formazan crystals. Finally, data were calculated by measuring the absorbance (595 nm wavelength).
Colony-Formation Assays
As previously described.65 Cell lines were seeded at a concentration of 5 × 103 cells in 6-well cell culture plates in appropriate media. Cells were assigned and incubated, and media contained melatonin at 0.5, 1, and 2 mM. Incubation medium changed every 3 days. After 2 weeks, medium was removed and colonies were fixed with formalin, stained with 0.5% crystal violet, and counted using a stereomicroscope. Colonies of greater than 50 cells were counted.
DAPI Staining
As previously described.65 Cells (1 × 104) were grown in 8-well glass coverslips followed by treatment with melatonin (2 mM) for 24 h. Cells were morphologically fixed for 20 min (4% paraformaldehyde) and then DAPI dye (50 μg/mL) was stained for 20 min. The nuclear morphological changes related to apoptosis were assessed in at least 500 cells. The images were instantly visualized by confocal microscope (Olympus FluoView FV 1200 Confocal Microscope).
Annexin V/PI Double Staining
As previously described,66 cells (1 × 105) were harvested and suspended in 100 μL PBS with 2% BSA after treatment. Cells were then stained with Muse Annexin V & Dead Cell Reagent (100 μL) for 20 min at room temperature in the dark. The signals were analyzed by the Muse Cell Soft V1.4.0.0 Analyzer Assays (Millipore).
Mitochondrial Membrane Potential Assay
As previously described,65 cells were harvested and collected (300 × g, 5 min). Treatment cell precipitates were stained with Muse MitoPotential dye (20 min, 37°C), and then 7-AAD was added for 5 min. The experiment signals were analyzed by the Muse Cell Soft V1.4.0.0 Analyzer Assays (Millipore).
Western Blot Analysis
As previously described.67 Cells were harvested and lysed in radioimmunoprecipitation assay (RIPA) buffer (with protease/phosphatase inhibitor) (Rocho). Equal 20–40 μg of total protein were analyzed by SDS-PAGE, following transfer to 0.22 or 0.45 μm polyvinylidene fluoride (PVDF) membranes (Millipore, Bedford, MA) as previously described.67 Blocking with skim milk (3%–5%) or 0.1% BSA. After appropriate primary antibodies (antibody against cleaved PARP, cleaved caspase-3, -9, LC3, SQSTM1, Beclin-1, ABCB1, ABCG2, p-AKT, AKT, p-ERK1/2, ERK1/2, p-p38, p38, p-JNK1/2, JNK1/2, β-actin, and ABCB4; dilutions ratio 1:1,000) incubation (4°C, overnight), membranes were washed and incubated for 1 h at room temperature with the appropriate secondary antibodies (anti-rabbit, anti-mouse; dilutions ratio 1:5,000) conjugated to horseradish peroxidase (HRP). Membranes were visualized using a chemiluminescence (ECL) detection kit (Millipore). The signals were examined and the relative photographic density was quantitated by ImageQuant LAS 4000 mini (GE Healthcare Life Sciences).
Detection of Autophagosomes and AO
As previously described,65 cells were grown in 8-well glass coverslips followed by treatment with DMSO (vehicle) or indicated concentrations of melatonin for 24 h. Cells were stained with Cell Meter Autophagy Assay Kit (green fluorescence) (AAT Bioquest) or 1 μg/mL AO (30 min, 37°C). Autophagosomes and AO images were observed under confocal microscope (Olympus FluoView FV 1200 Confocal Microscope).
Gene-Expression Analysis
Total cellular RNA and cDNA were prepared with the TaqMan Reverse Transcription Reagents as previously described.55 Quantitative PCR reactions were run by assaying each sample in triplicate using the TaqMan Gene Expression Assays (Applied Biosystems, Carlsbad, CA) or 96-well TaqMan Array plates with StepOne Real-Time PCR System. Levels of mRNA expression were normalized to GAPDH mRNA levels, and differences between samples were analyzed using the delta-delta-Ct (ddCT) method. For quantitative RT-PCR assessment of miRNAs, RNA was extracted from cell lines using Quick-RNA MiniPrep Kit, following the manufacturer’s instruction (Integrated Sciences), and gene expression was assessed using Mir-X miRNA First-Strand Synthesis Kit (Clontech, CA, USA). Normalization was performed using U6 as controls. The following PCR primer sequences were used: has-miR-892a, 5′-CACTGTGTCCTTTCTGCGTAG-3′; has-miR-34b-5p, 5′-d TAGGCAGTGTCATTAGCTGATTG-3′.
In Vivo Anti-Tumor Growth Effects on Xenograft Transplantation
As previously described.68 5-week-old C57BL/6 mice (18–22 g) were used (National Taiwan University Animal Center, Taiwan).68 SASV32 cells injected subcutaneously (s.c.) into mice right flank (2 × 106/PBS). As previously described,69 melatonin was administered 1 h before switching off the room lights. The mice were kept in a pathogen-free environment at the Laboratory Animal Unit (temperature 22°C, humidity 30%∼70%, 5 mice/cage). The control group (5 mice/group) received an equal volume of 0.5% carboxymethyl cellulose vehicle. 7 days after injection, the mice were orally fed melatonin (200 mg/kg) or vehicle three times per week. All animal experiments were conducted in accordance with the institutional animal welfare guidelines of the IACUC of the Changhua Christian Hospital.
Tumor IHC
As previously described,68 slides were deparaffinized, rehydrated, and blocked. The antigen was retrieved in 10 mM pH 6.0 citrate buffers (100°C, 20 min). After primary and secondary antibodies were incubated, and HRP (1 mg/mL)/Fab polymer conjugate (30 min); H&E stain which was used as a light counterstain.
miRNA Inhibitor Transfection
Based on the manufacturer’s protocol, cells were distributed under a concentration of 1 × 104 (96-well) or 5 × 105 cells (6 cm dish) as previously described.70 After 12 h of incubation, negative inhibitor (miRNA inhibitor negative control; 1 μM), miRNA-34b-5p inhibitor (1 μM), or miRNA-892a inhibitor (1 μM) was transfected by Xfect RNA Transfection Reagent (Clontech, CA, USA). After 24 h of transfection, cells were treated with vehicle or melatonin (2 mM) for 24 h. Cell lysates were harvested and protein expressions were determined by western blot analysis.
Statistical Analysis
Statistical analysis was performed by one-way ANOVA. Tukey’s post hoc test was used when more than three groups were analyzed. Student’s t test (Sigma-Stat 2.0, Jandel Scientific, San Rafael, CA, USA) was arranged for comparison among two different groups. In all cases, p value < 0.05 was considered as statistically significant. All results are shown as mean ± SEM. *p < 0.05, compared with the control. #p < 0.05, compared with the only melatonin (2 mM).
Author Contributions
M.J.H., C.W.L., M.K.C., and S.F.Y. conceived and designed the study. M.J.H. and A.W.C. contributed to the carrying out the experiments. M.J.H. and S.C.S. contributed to the data analysis. M.J.H., C.W.L., R.J.R., M.K.C., and S.F.Y. wrote the manuscript. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
This study was supported by grants from National Science Council, Taiwan (MOST 106-2314-B-371-006-MY3; MOST 106-2314-B-371-005-MY3), Changhua Christian Hospital (108-CCH-ICO-145; 108-CCH-IRP-002), and Chung Shan Medical University Hospital, Taiwan (CSH-2016-E-001-Y2).
Contributor Information
Mu-Kuan Chen, Email: 53780@cch.org.tw.
Shun-Fa Yang, Email: ysf@csmu.edu.tw.
References
- 1.Torre L.A., Bray F., Siegel R.L., Ferlay J., Lortet-Tieulent J., Jemal A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Su S.C., Lin C.W., Liu Y.F., Fan W.L., Chen M.K., Yu C.P., Yang W.E., Su C.W., Chuang C.Y., Li W.H. Exome Sequencing of Oral Squamous Cell Carcinoma Reveals Molecular Subgroups and Novel Therapeutic Opportunities. Theranostics. 2017;7:1088–1099. doi: 10.7150/thno.18551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang S.F., Huang H.D., Fan W.L., Jong Y.J., Chen M.K., Huang C.N., Chuang C.Y., Kuo Y.L., Chung W.H., Su S.C. Compositional and functional variations of oral microbiota associated with the mutational changes in oral cancer. Oral Oncol. 2018;77:1–8. doi: 10.1016/j.oraloncology.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Chien M.H., Lin C.W., Cheng C.W., Wen Y.C., Yang S.F. Matrix metalloproteinase-2 as a target for head and neck cancer therapy. Expert Opin. Ther. Targets. 2013;17:203–216. doi: 10.1517/14728222.2013.740012. [DOI] [PubMed] [Google Scholar]
- 5.Granzotto M., Rapozzi V., Decorti G., Giraldi T. Effects of melatonin on doxorubicin cytotoxicity in sensitive and pleiotropically resistant tumor cells. J. Pineal Res. 2001;31:206–213. doi: 10.1034/j.1600-079x.2001.310303.x. [DOI] [PubMed] [Google Scholar]
- 6.Uematsu T., Hasegawa T., Hiraoka B.Y., Komatsu F., Matsuura T., Yamada A.S., Yamaoka M. Multidrug resistance gene 1 expression in salivary gland adenocarcinomas and oral squamous-cell carcinomas. Int. J. Cancer. 2001;92:187–194. doi: 10.1002/1097-0215(200102)9999:9999<::aid-ijc1180>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 7.Nielsen D., Skovsgaard T. P-glycoprotein as multidrug transporter: a critical review of current multidrug resistant cell lines. Biochim. Biophys. Acta. 1992;1139:169–183. doi: 10.1016/0925-4439(92)90131-6. [DOI] [PubMed] [Google Scholar]
- 8.Griffiths J.R. Are cancer cells acidic? Br. J. Cancer. 1991;64:425–427. doi: 10.1038/bjc.1991.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McLeod H.L., Evans W.E. Oral cancer chemotherapy: the promise and the pitfalls. Clin. Cancer Res. 1999;5:2669–2671. [PubMed] [Google Scholar]
- 10.Pérez-Sayáns M., García-García A., Reboiras-López M.D., Gándara-Vila P. Role of V-ATPases in solid tumors: importance of the subunit C (Review) Int. J. Oncol. 2009;34:1513–1520. doi: 10.3892/ijo_00000280. [DOI] [PubMed] [Google Scholar]
- 11.Ralhan R., Narayan M., Salotra P., Shukla N.K., Chauhan S.S. Evaluation of P-glycoprotein expression in human oral oncogenesis: correlation with clinicopathological features. Int. J. Cancer. 1997;72:728–734. doi: 10.1002/(sici)1097-0215(19970904)72:5<728::aid-ijc4>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 12.Fearon E.R., Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 13.Gottesman M.M., Pastan I. The multidrug transporter, a double-edged sword. J. Biol. Chem. 1988;263:12163–12166. [PubMed] [Google Scholar]
- 14.Mayo J.C., Sainz R.M., González Menéndez P., Cepas V., Tan D.X., Reiter R.J. Melatonin and sirtuins: A “not-so unexpected” relationship. J. Pineal Res. 2017;62:e12391. doi: 10.1111/jpi.12391. [DOI] [PubMed] [Google Scholar]
- 15.Reiter R.J., Mayo J.C., Tan D.X., Sainz R.M., Alatorre-Jimenez M., Qin L. Melatonin as an antioxidant: under promises but over delivers. J. Pineal Res. 2016;61:253–278. doi: 10.1111/jpi.12360. [DOI] [PubMed] [Google Scholar]
- 16.Koşar P.A., Nazıroğlu M., Övey I.S., Çiğ B. Synergic Effects of Doxorubicin and Melatonin on Apoptosis and Mitochondrial Oxidative Stress in MCF-7 Breast Cancer Cells: Involvement of TRPV1 Channels. J. Membr. Biol. 2016;249:129–140. doi: 10.1007/s00232-015-9855-0. [DOI] [PubMed] [Google Scholar]
- 17.Fic M., Podhorska-Okolow M., Dziegiel P., Gebarowska E., Wysocka T., Drag-Zalesinska M., Zabel M. Effect of melatonin on cytotoxicity of doxorubicin toward selected cell lines (human keratinocytes, lung cancer cell line A-549, laryngeal cancer cell line Hep-2) In Vivo. 2007;21:513–518. [PubMed] [Google Scholar]
- 18.Xiang S., Dauchy R.T., Hauch A., Mao L., Yuan L., Wren M.A., Belancio V.P., Mondal D., Frasch T., Blask D.E., Hill S.M. Doxorubicin resistance in breast cancer is driven by light at night-induced disruption of the circadian melatonin signal. J. Pineal Res. 2015;59:60–69. doi: 10.1111/jpi.12239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fan C., Pan Y., Yang Y., Di S., Jiang S., Ma Z., Li T., Zhang Z., Li W., Li X. Retraction. J. Pineal Res. 2015;59:321–333. doi: 10.1111/jpi.12261. [DOI] [PubMed] [Google Scholar]
- 20.Shen Y.Q., Guerra-Librero A., Fernandez-Gil B.I., Florido J., García-López S., Martinez-Ruiz L., Mendivil-Perez M., Soto-Mercado V., Acuña-Castroviejo D., Ortega-Arellano H. Combination of melatonin and rapamycin for head and neck cancer therapy: Suppression of AKT/mTOR pathway activation, and activation of mitophagy and apoptosis via mitochondrial function regulation. J. Pineal Res. 2018;64:e12461. doi: 10.1111/jpi.12461. [DOI] [PubMed] [Google Scholar]
- 21.Gatti G., Lucini V., Dugnani S., Calastretti A., Spadoni G., Bedini A., Rivara S., Mor M., Canti G., Scaglione F., Bevilacqua A. Antiproliferative and pro-apoptotic activity of melatonin analogues on melanoma and breast cancer cells. Oncotarget. 2017;8:68338–68353. doi: 10.18632/oncotarget.20124. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Wang J., Guo W., Chen W., Yu W., Tian Y., Fu L., Shi D., Tong B., Xiao X., Huang W., Deng W. Melatonin potentiates the antiproliferative and pro-apoptotic effects of ursolic acid in colon cancer cells by modulating multiple signaling pathways. J. Pineal Res. 2013;54:406–416. doi: 10.1111/jpi.12035. [DOI] [PubMed] [Google Scholar]
- 23.Shi H., Chen Y., Tan D.X., Reiter R.J., Chan Z., He C. Melatonin induces nitric oxide and the potential mechanisms relate to innate immunity against bacterial pathogen infection in Arabidopsis. J. Pineal Res. 2015;59:102–108. doi: 10.1111/jpi.12244. [DOI] [PubMed] [Google Scholar]
- 24.Ahmad R., Haldar C. Melatonin and androgen receptor expression interplay modulates cell-mediated immunity in tropical rodent Funambulus pennanti: an in-vivo and in-vitro study. Scand. J. Immunol. 2010;71:420–430. doi: 10.1111/j.1365-3083.2010.02396.x. [DOI] [PubMed] [Google Scholar]
- 25.Espino J., Pariente J.A., Rodríguez A.B. Oxidative stress and immunosenescence: therapeutic effects of melatonin. Oxid. Med. Cell. Longev. 2012;2012:670294. doi: 10.1155/2012/670294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rao G.N., Ney E., Herbert R.A. Effect of melatonin and linolenic acid on mammary cancer in transgenic mice with c-neu breast cancer oncogene. Breast Cancer Res. Treat. 2000;64:287–296. doi: 10.1023/a:1026552405042. [DOI] [PubMed] [Google Scholar]
- 27.Mediavilla M.D., Güezmez A., Ramos S., Kothari L., Garijo F., Sánchez Barceló E.J. Effects of melatonin on mammary gland lesions in transgenic mice overexpressing N-ras proto-oncogene. J. Pineal Res. 1997;22:86–94. doi: 10.1111/j.1600-079x.1997.tb00308.x. [DOI] [PubMed] [Google Scholar]
- 28.Yeh C.M., Lin C.W., Yang J.S., Yang W.E., Su S.C., Yang S.F. Melatonin inhibits TPA-induced oral cancer cell migration by suppressing matrix metalloproteinase-9 activation through the histone acetylation. Oncotarget. 2016;7:21952–21967. doi: 10.18632/oncotarget.8009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ho H.Y., Lin C.W., Chien M.H., Reiter R.J., Su S.C., Hsieh Y.H., Yang S.F. Melatonin suppresses TPA-induced metastasis by downregulating matrix metalloproteinase-9 expression through JNK/SP-1 signaling in nasopharyngeal carcinoma. J. Pineal Res. 2016;61:479–492. doi: 10.1111/jpi.12365. [DOI] [PubMed] [Google Scholar]
- 30.Lu K.H., Su S.C., Lin C.W., Hsieh Y.H., Lin Y.C., Chien M.H., Reiter R.J., Yang S.F. Melatonin attenuates osteosarcoma cell invasion by suppression of C-C motif chemokine ligand 24 through inhibition of the c-Jun N-terminal kinase pathway. J. Pineal Res. 2018;65:e12507. doi: 10.1111/jpi.12507. [DOI] [PubMed] [Google Scholar]
- 31.Reiter R.J., Rosales-Corral S.A., Tan D.X., Acuna-Castroviejo D., Qin L., Yang S.F., Xu K. Melatonin, a Full Service Anti-Cancer Agent: Inhibition of Initiation, Progression and Metastasis. Int. J. Mol. Sci. 2017;18:E843. doi: 10.3390/ijms18040843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Su S.C., Hsieh M.J., Yang W.E., Chung W.H., Reiter R.J., Yang S.F. Cancer metastasis: Mechanisms of inhibition by melatonin. J. Pineal Res. 2017;62:e12370. doi: 10.1111/jpi.12370. [DOI] [PubMed] [Google Scholar]
- 33.Hevia D., González-Menéndez P., Quiros-González I., Miar A., Rodríguez-García A., Tan D.X., Reiter R.J., Mayo J.C., Sainz R.M. Melatonin uptake through glucose transporters: a new target for melatonin inhibition of cancer. J. Pineal Res. 2015;58:234–250. doi: 10.1111/jpi.12210. [DOI] [PubMed] [Google Scholar]
- 34.Cipolla-Neto J., Amaral F.G., Afeche S.C., Tan D.X., Reiter R.J. Melatonin, energy metabolism, and obesity: a review. J. Pineal Res. 2014;56:371–381. doi: 10.1111/jpi.12137. [DOI] [PubMed] [Google Scholar]
- 35.Espino J., Bejarano I., Paredes S.D., Barriga C., Rodríguez A.B., Pariente J.A. Protective effect of melatonin against human leukocyte apoptosis induced by intracellular calcium overload: relation with its antioxidant actions. J. Pineal Res. 2011;51:195–206. doi: 10.1111/j.1600-079X.2011.00876.x. [DOI] [PubMed] [Google Scholar]
- 36.Espino J., Bejarano I., Paredes S.D., González D., Barriga C., Reiter R.J., Pariente J.A., Rodríguez A.B. Melatonin counteracts alterations in oxidative metabolism and cell viability induced by intracellular calcium overload in human leucocytes: changes with age. Basic Clin. Pharmacol. Toxicol. 2010;107:590–597. doi: 10.1111/j.1742-7843.2010.00546.x. [DOI] [PubMed] [Google Scholar]
- 37.Espino J., Bejarano I., Ortiz A., Lozano G.M., García J.F., Pariente J.A., Rodríguez A.B. Melatonin as a potential tool against oxidative damage and apoptosis in ejaculated human spermatozoa. Fertil. Steril. 2010;94:1915–1917. doi: 10.1016/j.fertnstert.2009.12.082. [DOI] [PubMed] [Google Scholar]
- 38.Calastretti A., Gatti G., Lucini V., Dugnani S., Canti G., Scaglione F., Bevilacqua A. Melatonin Analogue Antiproliferative and Cytotoxic Effects on Human Prostate Cancer Cells. Int. J. Mol. Sci. 2018;19:E1505. doi: 10.3390/ijms19051505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pariente R., Bejarano I., Espino J., Rodríguez A.B., Pariente J.A. Participation of MT3 melatonin receptors in the synergistic effect of melatonin on cytotoxic and apoptotic actions evoked by chemotherapeutics. Cancer Chemother. Pharmacol. 2017;80:985–998. doi: 10.1007/s00280-017-3441-3. [DOI] [PubMed] [Google Scholar]
- 40.Pariente R., Pariente J.A., Rodríguez A.B., Espino J. Melatonin sensitizes human cervical cancer HeLa cells to cisplatin-induced cytotoxicity and apoptosis: effects on oxidative stress and DNA fragmentation. J. Pineal Res. 2016;60:55–64. doi: 10.1111/jpi.12288. [DOI] [PubMed] [Google Scholar]
- 41.Pariente R., Bejarano I., Rodríguez A.B., Pariente J.A., Espino J. Melatonin increases the effect of 5-fluorouracil-based chemotherapy in human colorectal adenocarcinoma cells in vitro. Mol. Cell. Biochem. 2018;440:43–51. doi: 10.1007/s11010-017-3154-2. [DOI] [PubMed] [Google Scholar]
- 42.Yorimitsu T., Klionsky D.J. Autophagy: molecular machinery for self-eating. Cell Death Differ. 2005;12(Suppl 2):1542–1552. doi: 10.1038/sj.cdd.4401765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kroemer G., Levine B. Autophagic cell death: the story of a misnomer. Nat. Rev. Mol. Cell Biol. 2008;9:1004–1010. doi: 10.1038/nrm2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.To K.K., Zhan Z., Litman T., Bates S.E. Regulation of ABCG2 expression at the 3′ untranslated region of its mRNA through modulation of transcript stability and protein translation by a putative microRNA in the S1 colon cancer cell line. Mol. Cell. Biol. 2008;28:5147–5161. doi: 10.1128/MCB.00331-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xia L., Zhang D., Du R., Pan Y., Zhao L., Sun S., Hong L., Liu J., Fan D. miR-15b and miR-16 modulate multidrug resistance by targeting BCL2 in human gastric cancer cells. Int. J. Cancer. 2008;123:372–379. doi: 10.1002/ijc.23501. [DOI] [PubMed] [Google Scholar]
- 46.Mishra P.J., Humeniuk R., Mishra P.J., Longo-Sorbello G.S., Banerjee D., Bertino J.R. A miR-24 microRNA binding-site polymorphism in dihydrofolate reductase gene leads to methotrexate resistance. Proc. Natl. Acad. Sci. USA. 2007;104:13513–13518. doi: 10.1073/pnas.0706217104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kovalchuk O., Filkowski J., Meservy J., Ilnytskyy Y., Tryndyak V.P., Chekhun V.F., Pogribny I.P. Involvement of microRNA-451 in resistance of the MCF-7 breast cancer cells to chemotherapeutic drug doxorubicin. Mol. Cancer Ther. 2008;7:2152–2159. doi: 10.1158/1535-7163.MCT-08-0021. [DOI] [PubMed] [Google Scholar]
- 48.Yang H., Kong W., He L., Zhao J.J., O’Donnell J.D., Wang J., Wenham R.M., Coppola D., Kruk P.A., Nicosia S.V., Cheng J.Q. MicroRNA expression profiling in human ovarian cancer: miR-214 induces cell survival and cisplatin resistance by targeting PTEN. Cancer Res. 2008;68:425–433. doi: 10.1158/0008-5472.CAN-07-2488. [DOI] [PubMed] [Google Scholar]
- 49.Zhou Y., Zhao R.H., Tseng K.F., Li K.P., Lu Z.G., Liu Y., Han K., Gan Z.H., Lin S.C., Hu H.Y., Min D.L. Sirolimus induces apoptosis and reverses multidrug resistance in human osteosarcoma cells in vitro via increasing microRNA-34b expression. Acta Pharmacol. Sin. 2016;37:519–529. doi: 10.1038/aps.2015.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Choi Y.M., An S., Lee E.M., Kim K., Choi S.J., Kim J.S., Jang H.H., An I.S., Bae S. CYP1A1 is a target of miR-892a-mediated post-transcriptional repression. Int. J. Oncol. 2012;41:331–336. doi: 10.3892/ijo.2012.1418. [DOI] [PubMed] [Google Scholar]
- 51.Vilela B., Pagès M., Lumbreras V. Regulation of MAPK signaling and cell death by MAPK phosphatase MKP2. Plant Signal. Behav. 2010;5:1497–1500. doi: 10.4161/psb.5.11.13645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li P., Chen S.Y., Shen S.X., Liu L.X., Xu J.H., Zhang Z.Q. Ethyl lucidenates A reverses P-glycoprotein mediated vincristine resistance in K562/A02 cells. Nat. Prod. Res. 2019;33:732–745. doi: 10.1080/14786419.2017.1402323. [DOI] [PubMed] [Google Scholar]
- 53.Wang L.M., Zhang M.Y., Zhu Q.S., Lu C.F., Bai X. Hyperin Enhances the Sensitivity of HCT8/VCR Colon Cancer Cell Line to Vincristine by Down-Regulating P-Glycoprotein. Clin. Lab. 2018;64:269–275. doi: 10.7754/Clin.Lab.2017.170923. [DOI] [PubMed] [Google Scholar]
- 54.Fan L., Sun G., Ma T., Zhong F., Lei Y., Li X., Wei W. Melatonin reverses tunicamycin-induced endoplasmic reticulum stress in human hepatocellular carcinoma cells and improves cytotoxic response to doxorubicin by increasing CHOP and decreasing survivin. J. Pineal Res. 2013;55:184–194. doi: 10.1111/jpi.12061. [DOI] [PubMed] [Google Scholar]
- 55.Martín V., Sanchez-Sanchez A.M., Herrera F., Gomez-Manzano C., Fueyo J., Alvarez-Vega M.A., Antolín I., Rodriguez C. Melatonin-induced methylation of the ABCG2/BCRP promoter as a novel mechanism to overcome multidrug resistance in brain tumour stem cells. Br. J. Cancer. 2013;108:2005–2012. doi: 10.1038/bjc.2013.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yamanishi M., Narazaki H., Asano T. Melatonin overcomes resistance to clofarabine in two leukemic cell lines by increased expression of deoxycytidine kinase. Exp. Hematol. 2015;43:207–214. doi: 10.1016/j.exphem.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 57.Lin Y.W., Lee L.M., Lee W.J., Chu C.Y., Tan P., Yang Y.C., Chen W.Y., Yang S.F., Hsiao M., Chien M.H. Melatonin inhibits MMP-9 transactivation and renal cell carcinoma metastasis by suppressing Akt-MAPKs pathway and NF-κB DNA-binding activity. J. Pineal Res. 2016;60:277–290. doi: 10.1111/jpi.12308. [DOI] [PubMed] [Google Scholar]
- 58.Paglin S., Hollister T., Delohery T., Hackett N., McMahill M., Sphicas E., Domingo D., Yahalom J. A novel response of cancer cells to radiation involves autophagy and formation of acidic vesicles. Cancer Res. 2001;61:439–444. [PubMed] [Google Scholar]
- 59.Yang Z.J., Chee C.E., Huang S., Sinicrope F.A. The role of autophagy in cancer: therapeutic implications. Mol. Cancer Ther. 2011;10:1533–1541. doi: 10.1158/1535-7163.MCT-11-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tsujimoto Y., Shimizu S. Another way to die: autophagic programmed cell death. Cell Death Differ. 2005;12(Suppl 2):1528–1534. doi: 10.1038/sj.cdd.4401777. [DOI] [PubMed] [Google Scholar]
- 61.Fojo T. Multiple paths to a drug resistance phenotype: mutations, translocations, deletions and amplification of coding genes or promoter regions, epigenetic changes and microRNAs. Drug Resist. Updat. 2007;10:59–67. doi: 10.1016/j.drup.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 62.Blower P.E., Chung J.H., Verducci J.S., Lin S., Park J.K., Dai Z., Liu C.G., Schmittgen T.D., Reinhold W.C., Croce C.M. MicroRNAs modulate the chemosensitivity of tumor cells. Mol. Cancer Ther. 2008;7:1–9. doi: 10.1158/1535-7163.MCT-07-0573. [DOI] [PubMed] [Google Scholar]
- 63.Su S.C., Reiter R.J., Hsiao H.Y., Chung W.H., Yang S.F. Functional Interaction between Melatonin Signaling and Noncoding RNAs. Trends Endocrinol. Metab. 2018;29:435–445. doi: 10.1016/j.tem.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 64.Hsieh M.J., Chen M.K., Yu Y.Y., Sheu G.T., Chiou H.L. Psoralen reverses docetaxel-induced multidrug resistance in A549/D16 human lung cancer cells lines. Phytomedicine. 2014;21:970–977. doi: 10.1016/j.phymed.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 65.Chen J.C., Hsieh M.C., Lin S.H., Lin C.C., Hsi Y.T., Lo Y.S., Chuang Y.C., Hsieh M.J., Chen M.K. Coronarin D induces reactive oxygen species-mediated cell death in human nasopharyngeal cancer cells through inhibition of p38 MAPK and activation of JNK. Oncotarget. 2017;8:108006–108019. doi: 10.18632/oncotarget.22444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hsieh M.J., Chien S.Y., Chou Y.E., Chen C.J., Chen J., Chen M.K. Hispolon from Phellinus linteus possesses mediate caspases activation and induces human nasopharyngeal carcinomas cells apoptosis through ERK1/2, JNK1/2 and p38 MAPK pathway. Phytomedicine. 2016;21:1746–1752. doi: 10.1016/j.phymed.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 67.Hsieh M.J., Lin C.W., Chiou H.L., Yang S.F., Chen M.K. Dehydroandrographolide, an iNOS inhibitor, extracted from Andrographis paniculata (Burm.f.) Nees, induces autophagy in human oral cancer cells. Oncotarget. 2015;6:30831–30849. doi: 10.18632/oncotarget.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen J.C., Hsieh M.J., Chen C.J., Lin J.T., Lo Y.S., Chuang Y.C., Chien S.Y., Chen M.K. Polyphyllin G induce apoptosis and autophagy in human nasopharyngeal cancer cells by modulation of AKT and mitogen-activated protein kinase pathways in vitro and in vivo. Oncotarget. 2016;7:70276–70289. doi: 10.18632/oncotarget.11839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cos S., Sánchez-Barceló E.J. Melatonin and mammary pathological growth. Front. Neuroendocrinol. 2000;21:133–170. doi: 10.1006/frne.1999.0194. [DOI] [PubMed] [Google Scholar]
- 70.Cheng A.W., Wang H., Yang H., Shi L., Katz Y., Theunissen T.W., Rangarajan S., Shivalila C.S., Dadon D.B., Jaenisch R. Multiplexed activation of endogenous genes by CRISPR-on, an RNA-guided transcriptional activator system. Cell Res. 2013;23:1163–1171. doi: 10.1038/cr.2013.122. [DOI] [PMC free article] [PubMed] [Google Scholar]