Abstract
This study investigated the accuracy, drift, and clinical usefulness of a new optical transcutaneous oxygen tension (tcPO2) measuring technique, combined with a conventional electrochemical transcutaneous carbon dioxide (tcPCO2) measurement and reflectance pulse oximetry in the novel transcutaneous OxiVenT™ Sensor. In vitro gas studies were performed to measure accuracy and drift of tcPO2 and tcPCO2. Clinical usefulness for tcPO2 and tcPCO2 monitoring was assessed in neonates. In healthy adult volunteers, measured oxygen saturation values (SpO2) were compared with arterially sampled oxygen saturation values (SaO2) during controlled hypoxemia. In vitro correlation and agreement with gas mixtures of tcPO2 (r = 0.999, bias 3.0 mm Hg, limits of agreement − 6.6 to 4.9 mm Hg) and tcPCO2 (r = 0.999, bias 0.8 mm Hg, limits of agreement − 0.7 to 2.2 mm Hg) were excellent. In vitro drift was negligible for tcPO2 (0.30 (0.63 SD) mm Hg/24 h) and highly acceptable for tcPCO2 (− 2.53 (1.04 SD) mm Hg/12 h). Clinical use in neonates showed good usability and feasibility. SpO2-SaO2 correlation (r = 0.979) and agreement (bias 0.13%, limits of agreement − 3.95 to 4.21%) in healthy adult volunteers were excellent. The investigated combined tcPO2, tcPCO2, and SpO2 sensor with a new oxygen fluorescence quenching technique is clinically usable and provides good overall accuracy and negligible tcPO2 drift. Accurate and low-drift tcPO2 monitoring offers improved measurement validity for long-term monitoring of blood and tissue oxygenation.
Graphical abstract
Keywords: Transcutaneous, tcPO2, tcPCO2, Oxygen, Fluorescence quenching
Introduction
Transcutaneous blood gas monitoring is based on the diffusion of oxygen (O2) and carbon dioxide (CO2) from the blood to the skin surface [1]. Transcutaneous blood gas sensors locally heat the skin to induce vasodilation, resulting in an increase in supplied O2 and clearance of CO2 [2, 3]. The diffusion capacity of the skin is however markedly lower for O2 than for CO2 [4], additionally influenced by the thickness [5, 6] and microcirculatory condition [7] of the skin. As a consequence the measurement of transcutaneous oxygen (tcPO2) [8] requires relatively high sensor temperatures of 43 to 44 °C [9] for tcPO2 to correlate with arterial oxygen tension (PaO2), which due to skin thickness only results in tcPO2 values approaching PaO2 in infants and young children [10–12].
Conventional transcutaneous blood gas sensors are based on the electrochemical techniques introduced by Clark [13] for tcPO2 and Stow-Severinghaus [14] for tcPCO2. For decades the Clark-type electrode has been the only clinically available technique for tcPO2 measurements [15]. It measures oxygen by reduction, lowering the actual and thereby measured oxygen level in the superficial skin [16, 17]. Additionally there is measurement drift over time with both techniques [18], hindering usability due to reduced accuracy, frequent calibrations and membrane changes. These limitations in reliability and usability of tcPO2 measurements [19] have held back a widespread clinical use similar to that of tcPCO2 measurements. However, tcPO2 offers advantages over SpO2 in infants in which blood gas sampling is indicated for the measurement of PaO2, precise PaO2 targeting is required or the oxygen dissociation curve is markedly shifted [20, 21]. In adults the use of tcPO2 is limited to oxygen trend monitoring due to an insuperable underestimation of PaO2 [22]. In addition measurement drift hinders clinical usability. Removing measurement drift as an influence on the measurement by implementing drift-free optical techniques could therefore significantly improve usability of tcPO2 measurements [23]. The recently introduced OxiVenT™ Sensor (SenTec AG, Therwil, Switzerland) combines reflectance pulse oximetry and a conventional electrochemical Stow-Severinghaus-type tcPCO2 measurement with an optical oxygen sensing technique for measuring tcPO2. Fluorescence quenching [24] is the optical technique used for the measurement of oxygen, making it potentially free of drift. The main challenge in the development of this sensor was to combine two optical techniques, fluorescence quenching and pulse oximetry, without mutual interference into a single sensor which also contains an electrochemical Stow-Severinghaus tcPCO2 measurement. In this article we will discuss the technical aspects of implementing fluorescence quenching in a combined sensor, provide the first results on measurement accuracy and evaluate its clinical implications.
Methods
A novel combined transcutaneous sensor
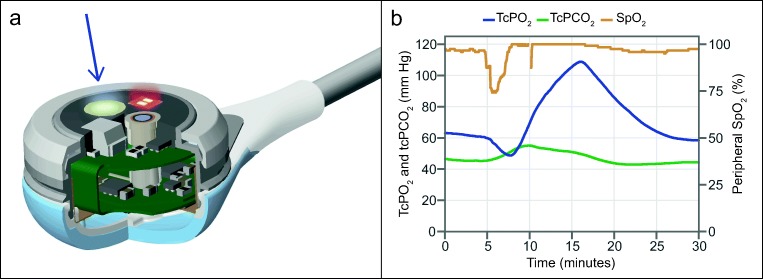
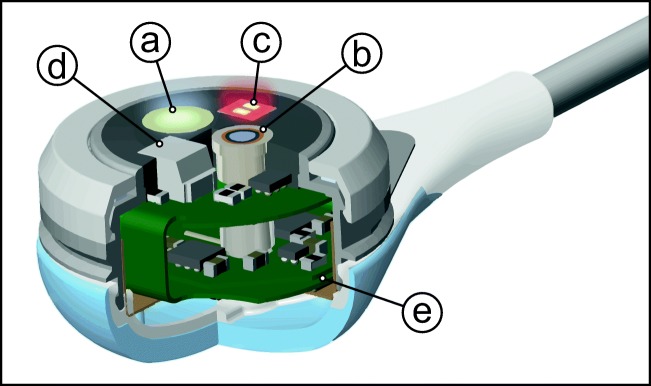
The OxiVenT™ Sensor is the first transcutaneous sensor in which an optical tcPO2 measurement is combined with an electrochemical Stow-Severinghaus-type tcPCO2 measurement and reflective pulse oximetry (Fig. 1). The sensor weighs 2.7 g and has a diameter of 14 mm and a height of 9 mm. All measurements are digitized within the sensor and preprocessed. The principle of an electrolyte-filled diffusion chamber is retained for the tcPCO2 measurement. For measuring oxygen, the sensor contains an oxygen fluorescence quenching dye surface which is back-lit by an excitation light-emitting diode. On the same side of the dye, the excitation light is measured with a wavelength-filtered photodetector. In order to provide parallel optical measurements of tcPO2 and SpO2, the respective light sources emit in an alternating intermittent fashion. The sensor contains dual temperature sensing for accurate heating control. The sensor can be attached to the skin using either an ear clip or adhesive rings, minimizing pressure on the skin.
Fig. 1.
Inside view of the fully digital OxiVenT™ Sensor, showing (a) tcPO2 optical module, (b) tcPCO2 electrochemical module, (c) pulse oximetry light-emitting diode, (d) pulse oximetry photodiode, and (e) flexible circuit board containing the temperature sensors and all electronic components and microprocessor
Measuring principles and technology
TcPO2 measurement and fluorescence quenching
The OxiVenT™ Sensor measures oxygen levels with an optical technique called oxygen fluorescence quenching [24]. This technique relies on the excitation of a dye molecule by the absorption of a photon emitted by a light-emitting diode with a peak wavelength of approximately 500 nm, moving the molecule to a higher energy state. Without the presence of an oxygen molecule, the dye molecule will emit a photon at a lower specific emission wavelength (approximately 650 nm) and return to its base energy state. In the presence of an oxygen molecule, the oxygen will quench the dye and thereby prevent photon emission. In the sensing dye surface of the OxiVenT™ Sensor, fluorescence emission of each dye molecule occurs non-synchronously during a certain time interval. This results in a fluorescence intensity and decay time interval that relates to the amount of oxygen that quenches dye fluorescence. Selectively and intermittently the light intensity at the 650-nm band is measured, out of which the decay curve is reconstructed and the measured oxygen values are inferred. The oxygen diffusion to the dye results in a typical 90% response time of under 150 s. Contrary to a Clark-type electrode which reduces oxygen, influencing the oxygen level measurement itself, the fluorescence quenching technique does not affect oxygen levels.
TcPCO2 measurement
In the OxiVenT™ Sensor, CO2 is measured with a Stow-Severinghaus-type electrode. This technique is used in the majority of currently commercially available transcutaneous sensors and consists of a pH electrode in an electrolyte buffer containing sodium bicarbonate, covered by a gas-permeable membrane. Carbon dioxide diffuses from the skin through the membrane, where it causes a carbonic acid dissociation reaction. This in turn changes the pH of the solution, which is detected by the pH electrode and causes a potential change between the pH electrode and the reference silver/silver chloride electrode. In sensors with an electrochemical tcPO2 (Clark-type) and tcPCO2 measurement the Clark-type electrode and its inherent oxygen consumption influence pH within the diffusion chamber. Without this influence on the tcPCO2 measurement, there is potentially a reduction in measurement drift. Multiple patient factors and sensor temperature influence the speed at which CO2 diffuses from the skin, and thereby the delay in measuring the changes in arterial values transcutaneously. In practice, this delay is usually 20–80 s from changes in ventilation to their effect on transcutaneous measurements [25, 26].
Reflective two-wavelength pulse oximetry
In pulse oximetry, the optically measured ratio between oxygenated and deoxygenated hemoglobin is used to measure oxygen saturation. By sending two light frequencies (660-nm and 880–890-nm wavelengths) through tissue, the light intensity that results after absorption of light by the two forms of hemoglobin can be used to calculate a ratio between the two. Only the pulsatile part of the signal is analyzed as it ideally represents the arterial component of the signal. Using a calibration model, based on measurements in healthy volunteers, for each ratio, this results in a specific oxygen saturation. Although a shift in the oxygen dissociation curve can influence the interpretation of SpO2 values in relation to the actual PaO2, this technique is one of the most used oxygen monitoring techniques. Two variants of the technique are often used; transmission and reflectance pulse oximetry. In transmission pulse oximetry the light emitter and detector are placed opposite to each other on both sides of tissue (e.g. a finger), while in reflectance pulse oximetry the emitter and detector are placed next to each other. This means that in transmission pulse oximetry the light path is linear and a relatively large part of the emitted light reaches the detector. In reflectance pulse oximetry the detected light is the part that is scattered and reflected back from the tissue, resulting in a weaker signal when compared with transmission pulse oximetry. In transcutaneous sensors the arterialization caused by locally heating the skin markedly improves the reflective signal-to-noise ratio [27].
Sensor validation methods
Hardware and software
All studies were performed using OxiVenT™ sensors with software versions 01.09-01.58, connected to a SenTec Digital Monitor (SDM) with software versions 08.00.0-08.01.1 (SenTec Monitoring Board) and 06.00.01-06.01.00 (Multi Parameter Board).
In vitro gas studies for the validation of tcPO2 and tcPCO2
An in vitro validation of the transcutaneous (O2 and CO2) measurements of the OxiVenT™ Sensor was performed with 10 sensors for each parameter in order to determine the accuracy and drift of these measurements. Prior to the protocol, the sensors were allowed to stabilize. Testing methods were in concordance with the FDA Guidance on cutaneous carbon dioxide and oxygen monitors (clause 6.2), as well as IEC 60601-2-23 [28]. Accuracy was tested by cycling through different combinations of gas concentrations of O2 and CO2. Each gas mixture was allowed to stabilize for 10 min, after which a data point was collected for each step. In the tcPCO2 accuracy test, a total of 4 data points for both 3% CO2 and 5% CO2 as well as 8 data points for 10% CO2 were collected. After 4 cycles, an additional measurement of nitrogen with 0% CO2 was performed. A comparable method was used for the tcPO2 accuracy test. This results in 4 data points for both 2% O2 and 10% O2 as well as 8 data points for 20% O2 after 4 cycles. Following these 4 cycles, additional measurements with nitrogen (0% O2) and with 100% O2 were performed. For the drift test, the sensors were exposed to humidified test gas (20% O2/10% CO2) for the duration of the calibration interval (24 h for tcPO2 and 12 h for tcPCO2). The total drift over the calibration interval is given as a percentage of the initial reading. In addition, the drift is given as %/h for the first hour (0–1 h) and last hour (11–12 h/23–24 h) of the calibration interval.
Clinical use of tcPO2 and tcPCO2
At the Neonatal Intensive Care Unit at Erasmus MC – Sophia Children’s Hospital (Rotterdam, the Netherlands), transcutaneous blood gas monitoring in preterm (24–32 weeks GA) and term neonates is performed as standard care. Existing, local, age-specific protocols for sensor temperatures and site times were applied for extreme preterm neonates (< 26 weeks GA: 42 °C, 2 h) and less preterm and term neonates (≥ 26 week GA: 43 °C, 3 h). TcPCO2 was calibrated initially, and when the site time elapsed, tcPO2 was calibrated initially and daily for verification during a tcPCO2 calibration. Several clinical examples were selected to demonstrate the usability and feasibility of transcutaneous blood gas monitoring of tcPO2 and tcPCO2 with the OxiVenT™ Sensor during various clinical events. SpO2 measurements (Masimo SET®, Masimo, Irvine, CA, USA) were recorded simultaneously with averaging over 12 s.
Validation of SpO2 in healthy volunteers
Validation of the OxiVent™ Sensor SpO2 measurements was performed with a clinical study in healthy volunteers at the University of California (San Francisco, USA). Approval from the institutional IRB was obtained for the study protocol. The study was carried out according to the FDA Guidance on the validation of SpO2 accuracy [29] and ISO 80601-2-61 [30]. The healthy volunteers underwent a desaturation protocol consisting of stepwise adjustments of the fraction of inspired oxygen (FiO2), targeting specific arterial oxygen saturation (SaO2) level plateaus. A total of two “runs” per volunteer were performed. Every SpO2 plateau was held for about 30–60 s. Two blood samples were collected during the saturation plateaus. Each run was then ended by several breaths of 100% O2 followed by room air while taking another sample pair of blood samples. The number of plateaus per “run” was adapted to the tolerance of the subjects to the desaturation protocol. SpO2 was measured with several sensors on 5 different application sites; earlobe, forehead, cheek, upper arm, and shoulder blade. SpO2 averaging time was set to 6 s. The sensor temperature was set to 44 °C for most sensors. For increasing statistical variety, several measurements were done at 37 °C and 41 °C.
Statistical analysis
Descriptive statistics (mean/standard deviation or median/range, depending on the distribution of the data) are given for demographic data (age, gender, and BMI). Correlation and Bland-Altman analyses were performed in order to determine Pearson’s correlation coefficient (r), bias (d), and standard deviation (SD). In concordance with the uniformity of data presentation that follows from guideline ISO 80601-2-61:2011, the accuracy root mean square error (Arms) was calculated with limits of agreement that did not take repeated measurements into account (). In this formula, is the SpO2 value for iteration number i, yi is the measured SaO2 value for the iteration number i, n is the number of samples, and d is the bias. The presented limits of agreement and the between-subject variance were calculated according to the methods of repeated measurements as described by Bland and Altman [31].
Results
In vitro accuracy and drift of tcPO2 and tcPCO2
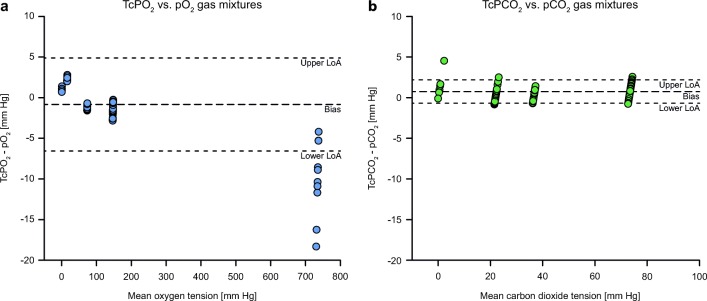
A total of 17 tcPCO2 and 18 tcPO2 data points were collected with each of the 10 sensors. The number of available data points and the correlation and Bland-Altman analyses of the tcPO2 and tcPCO2 data compared with the gas O2 and CO2 partial pressures are shown in Fig. 2 and summarized in Table 1. At oxygen tensions of over 700 mm Hg, agreement of tcPO2 with the reference gas has decreased, underestimating the pO2. Measurement drift over different intervals shows a very small overall O2 drift (Table 2). Drift of tcPCO2 is notably highest during the first hour, tcPO2 drift is not equally affected.
Fig. 2.
Bland-Altman plots of the agreement of in vitro tcPO2 (a) and tcPCO2 (b) measurements with calibration gas mixtures. The accuracy, bias, and limits of agreement (LoA) for each measurement are shown in Table 1.
Table 1.
In vitro accuracy of tcPO2 (0–100%) and tcPCO2 (0–10%) measurements
| Measurement | Data points (n) | Accuracy (mm Hg) | Bias (mm Hg) | Limits of agreement (mm Hg) | r | |
|---|---|---|---|---|---|---|
| Arms | Lower | Upper | ||||
| tcPO2 | 180 | 3.0 (2.9) | − 0.8 | − 6.6 | 4.9 | 0.999 |
| tcPCO2 | 170 | 1.1 (0.7) | 0.8 | − 0.7 | 2.2 | 0.999 |
Values measured with the OxiVenT™ Sensor and compared with calibration gas mixtures
Table 2.
Data on drift of tcPO2 (24-h calibration interval) and tcPCO2 (12-h calibration interval)
| Total drift during calibration interval (12 h/24 h) (%) | Drift during first hour of calibration interval (%/h) | Drift during last hour of calibration interval (%/h) | |
|---|---|---|---|
| tcPO2 | 0.30 (0.63) | 0.14 (0.28) | 0.03 (0.21) |
| tcPCO2 | − 2.53 (1.04) | 0.49 (0.28) | 0.18 (0.09) |
Data is shown as mean (SD)
Clinical use of tcPO2 and tcPCO2
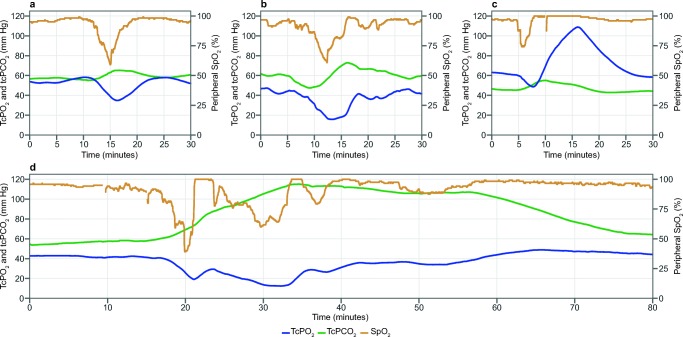
Four examples of clinical events were selected from patient files, are shown in Fig. 3, and include tcPO2 and tcPCO2 data, as well as the SpO2 data obtained from standard of care pulse oximetry. These examples contain both cardiorespiratory patient events and related clinical interventions. A tcPO2 response time of approximately 2 min longer when compared with SpO2 and a consequential dampening effect can be observed.
Fig. 3.
Clinical examples of tcPO2 and tcPCO2 measured in preterm neonates with the OxiVenT™ Sensor during relevant events, supplemented with standard of care peripherally measured transmission pulse oximetry. These examples show the following events: (a) Very preterm neonate, born at a gestational age (GA) of 28 weeks and with a birth weight (BW) of 1200 g. Drop in oxygen saturation to 56% SpO2 due to retention of sputum, following by suctioning, accompanied by a transient rise of tcPCO2 and decrease of tcPO2 down to 35 mm Hg. (b) Extreme preterm neonate, GA 27 weeks, BW 800 g. Capillary blood sampling at an extremity, leading to agitation and crying with a consequential drop in oxygen saturation to 55% and tcPO2 to 16 mm Hg. Noteworthy is the temporary drop in tcPCO2 due to crying, followed by a rise due to a decline in respiratory effort. The patient’s lungs were recruited due to clinical indications of bronchospasms. The FiO2 was increased from 0.21 to 0.40 during this process. (c) Late preterm neonate, GA 36 weeks, BW 2500 g. Short period of bradycardia which was followed by a drop in oxygen saturation. As a clinical intervention, the FiO2 was increased from 0.21 to 0.39 for 4 min, leading to a period of hyperoxia up to 109 mm Hg that was undetected by pulse oximetry. (d) Extreme preterm neonate, GA 24 weeks, BW 700 g. During nursing with patient repositioning multiple episodes of bradycardia down to 50 heart beats per minute, with drops in SpO2 down to 40% and slow recovery. The decline in respiratory effort and slow recovery are reflected by the clear and persistent elevation of CO2 levels
Validation of SpO2 in healthy volunteers
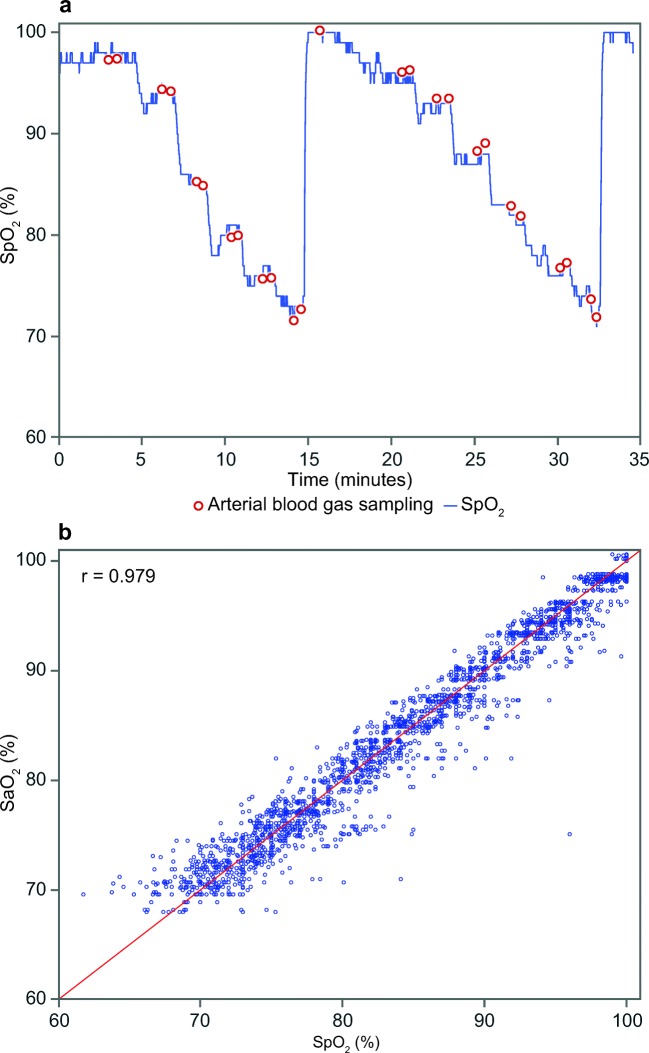
A total of 12 healthy volunteers participated in the study. The study demographics are shown in Table 3. At each step of the test protocol, two blood samples were drawn, of which a single-patient example is shown in Fig. 4(a). This resulted in a total of 2244 SaO2-SpO2 data pairs. The median of all measured SaO2 values is 84.8% (IQR 76.1–93.4%, range 68.0–100.6%). The correlation plot of the SaO2 values with the corresponding SpO2 measurements obtained with the OxiVenT™ Sensor at all five measurement sites is shown in Fig. 4(b). The accuracy and agreement analyses for the separate measuring sites show the narrowest limits of agreement when measuring at the forehead and cheek, with the highest accuracy when measured at the forehead (Table 4).
Table 3.
Volunteers characteristics
| No. of volunteers | 12 |
|---|---|
| Age (years) | 25 (23–34) |
| Male/female | 7/5 |
| Skin type | |
| - Dark | 2 |
| - Medium | 5 |
| - Light | 5 |
Values are listed as median (range), where applicable
Fig. 4.
(a) Single-patient example of the controlled desaturation protocol in which a stepwise decrease in SpO2 is achieved by controlled lowering of the FiO2. Dots indicate the moments when an arterial blood sample was taken during a relative plateau phase. (b )Correlation plot (r = 0.979) of SaO2 in % from all arterial blood samples and SpO2 in % measured with the OxiVenT™ Sensor at the moment of blood sampling (n = 2244)
Table 4.
Agreement between SpO2 and SaO2 at different measurement sites
| Application site | SaO2-SpO2 pairs | Accuracy (%) | Bias (%) | Limits of agreement (%) | r | |
|---|---|---|---|---|---|---|
| Arms | Lower | Upper | ||||
| Earlobe | 451 | 2.44 | 1.16 | − 3.17 | 5.49 | 0.973 |
| Forehead | 526 | 1.35 | − 0.29 | − 2.89 | 2.31 | 0.990 |
| Cheek | 476 | 1.29 | 0.56 | − 1.75 | 2.86 | 0.992 |
| Upper arm | 415 | 2.41 | 0.38 | − 4.39 | 5.15 | 0.971 |
| Shoulder blade | 376 | 2.13 | − 1.34 | − 4.64 | 1.96 | 0.989 |
| Overall | 2244 | 2.09 | 0.13 | − 3.17 | 3.98 | 0.979 |
Pooled SaO2-SpO2 Arms (not corrected for repeated measurements), bias, limits of agreement (corrected for repeated measurements), and Pearson’s correlation coefficient (not corrected for repeated measurements) per application site
Discussion
With these studies, we present data on the OxiVenT™ Sensor, the first combined tcPO2, tcPCO2, and SpO2 transcutaneous sensor incorporating an optical tcPO2 measurement that is designed to eliminate measurement drift. The in vitro results confirm a good tcPO2 accuracy and negligible overall measurement drift. Decreased tcPO2 accuracy and precision can be observed at very high oxygen tensions, together with underestimation of PaO2. This is most likely a consequence of the abundance of oxygen, leading to a short fluorescence decay time in combination with a high intensity. However, these supraphysiological levels are not likely to be clinically relevant. TcPCO2 accuracy and drift are on par with previous sensor generations [32]. TcPCO2 drift is highest during the first hour of measurement, possibly due to equilibration effects. TcPO2 drift does not seem to be equally affected, providing a more consistently accurate measurement from onset. Furthermore, SpO2 shows excellent correlation and agreement with SaO2 values in adult volunteers, particularly when measuring at the forehead or cheek. Although transcutaneous blood gas measurements have retained their place in the clinic after the introduction of pulse oximetry, the technique has remained laborious [33–35]. When measurements are considered to be in disagreement with arterial values, they require training to be able to distinguish technical failure or measurement drift from patient factors influencing the measurement. As a consequence, transcutaneous monitoring is most often used when the required dedicated attention is outweighed by the advantages, such as in neonatal intensive care units or sleep laboratories. The logical innovation in transcutaneous blood gas monitoring is consequently the introduction of drift-free measurement techniques, making transcutaneous monitoring more accurate and easy to use. In the investigated OxiVenT™ Sensor, an optical tcPO2 measurement has been implemented for this purpose. The main patient-related limitation of transcutaneous tcPO2 and tcPCO2 measurements is inaccuracy due to the influence of skin thickness and microcirculatory impairment on the diffusion of blood gases [2, 18, 36]. TcPO2 accuracy is known to suffer more from these influences than tcPCO2 accuracy due to the higher skin diffusion resistance to oxygen [4], leading to wide limits of agreement in clinical studies on tcPO2 [11, 37]. In addition, the traditional electrochemical tcPO2 sensors contained Clark-type electrodes, which consume oxygen as part of the measurement [2, 6]. The implementation of an optical measurement technique for tcPO2 therefore potentially has a greater measurement technique–related impact on accuracy for than it would have for tcPCO2. Clinical measurements of tcPO2 and tcPCO2 in the Neonatal Intensive Care Unit suggest good usability and response to clinical events. The relatively long tcPO2 response time makes it unsuitable for detecting apneic episodes and oxygenation dips. In adults, the inability to measure tcPO2 values that mirror PaO2 values limits the use in the adult population to oxygen trend monitoring. However, the improved reliability of the tcPO2 trend could clinically have a greater impact than improved agreement with blood gas samples. Data on the user preference of using either absolute values or trends is however limited and specific for patient populations. With the new OxiVenT™ Sensor, the potential of optical techniques has been demonstrated. In clinical use, this combined sensor will however still require frequent calibration of the electrochemical tcPCO2 measurement, negating the potential benefit on calibration strain for both patients and personnel. Although this study provides useful information on the technical performance of this new combined sensor, clinical validation is needed to evaluate its impact and limitations.
Conclusion
Our results show the successful integration of a new optical oxygen measuring technique in a non-invasive, combined tcPO2, tcPCO2, and SpO2 sensor. In vitro tcPCO2 measurement performance is unchanged when compared with literature on previous sensor generations. Reflectance pulse oximetry correlates well in a study on healthy volunteers. The new optical tcPO2 measurement is virtually drift-free in vitro. Despite showing good usability in clinical examples, the clinical benefit needs to be proven. Additionally, clinical data is needed to validate this sensor to arterial blood samples in specific patient populations.
Acknowledgments
We would like to thank Prof. Bickler and Prof. Feiner and their team from the University of California, San Francisco, for their expertise on SpO2 measurement validation and permission to publish the data collected in their lab.
Biographies
Willem van Weteringen
, M.D., M.Sc., studied at Erasmus University Medical Center and holds masters in Medicine and Neurosciences. His Ph.D. research focuses on minimal access surgery and non-invasive monitoring.
Tom G. Goos
, M.Sc., studied Biomedical Engineering at Delft University of Technology. His research focuses on patient monitoring and closed-loop control, and he is working towards a Ph.D. at Delft University of Technology.
Tanja van Essen
, B.Sc., is a PhD student at the division of Neonatology. Her research focuses on non-invasive neonatal monitoring modalities such as near-infrared spectroscopy and transcutaneous blood gas measurements.
Christoph Ellenberger
, Ph.D., completed his Ph.D. (2006) at the Laboratory for Solid State Physics at the ETH Zurich and is now working as a Senior Algorithm Developer for medical Applications at the R&D department of SenTec AG.
Josef Hayoz
, Ph.D., holds a Ph.D. from the Physics Institute of the University of Fribourg, Switzerland, and was a post-doctoral fellow at The Pennsylvania State University, USA. He joined SenTec in 2001 and was CEO from 2005 to 2015.
Rogier C.J. de Jonge
, M.D., Ph.D., studied medicine in Amsterdam, followed by a specialization in neonatology and training in epidemiology. In 2013, he finished his Ph.D. study in the long-term outcome of bacterial meningitis in children.
Irwin K.M. Reiss
, M.D., Ph.D., studied medicine at the Medical University Lübeck. After a Habilitation on surfactant therapy, he obtained a doctorate on injury to the developing lung. Since 2011, he is professor of Neonatology.
Peter M. Schumacher
, Ph.D., is an Electronics Engineer with a Ph.D. in Biomedical Engineering from ETH Zurich. Professional experience: 10+ years in academia and 20+ years in industry, of which 10 years at SenTec as Head of R&D.
Funding information
Financial support for the presented studies was provided to Erasmus MC – Sophia Children’s Hospital by SenTec AG.
Compliance with ethical standards
Conflict of interest
The authors J. Hayoz, Ch. Ellenberger, and P.M. Schumacher are employees and shareholders of SenTec AG. J. Hayoz is a board member of SenTec AG.
Research involving human participants and/or animals
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
For the healthy volunteer study, informed consent was obtained from all individual participants; for the other studies, formal consent was not required.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lubbers DW. Theory and development of transcutaneous oxygen pressure measurement. Int Anesthesiol Clin. 1987;25(3):31–65. doi: 10.1097/00004311-198702530-00005. [DOI] [PubMed] [Google Scholar]
- 2.Lubbers DW. Theoretical basis of the transcutaneous blood gas measurements. Crit Care Med. 1981;9(10):721–733. doi: 10.1097/00003246-198110000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Tremper KK, Huxtable RF. Dermal heat transport analysis for transcutaneous O2 measurement. Acta Anaesthesiol Scand Suppl. 1978;68:4–8. doi: 10.1111/j.1399-6576.1978.tb01385.x. [DOI] [PubMed] [Google Scholar]
- 4.Hansen TN, Sonoda Y, McIlroy MB. Transfer of oxygen, nitrogen, and carbon dioxide through normal adult human skin. J Appl Physiol Respir Environ Exerc Physiol. 1980;49(3):438–443. doi: 10.1152/jappl.1980.49.3.438. [DOI] [PubMed] [Google Scholar]
- 5.Jaszczak P, Sejrsen P. Oxygen tension and consumption measured by a tc-PO2 electrode on heated skin before and after epidermal stripping. Acta Anaesthesiol Scand. 1987;31(5):362–369. doi: 10.1111/j.1399-6576.1987.tb02585.x. [DOI] [PubMed] [Google Scholar]
- 6.Falstie-Jensen N, Spaun E, Brochner-Mortensen J, Falstie-Jensen S. The influence of epidermal thickness on transcutaneous oxygen pressure measurements in normal persons. Scand J Clin Lab Invest. 1988;48(6):519–523. doi: 10.3109/00365518809085767. [DOI] [PubMed] [Google Scholar]
- 7.Versmold HT, Linderkamp O, Holzmann M, Strohhacker I, Riegel KP. Limits of tcPO2 monitoring in sick neonates: relation to blood pressure, blood volume, peripheral blood flow and acid base status. Acta Anaesthesiol Scand Suppl. 1978;68:88–90. doi: 10.1111/j.1399-6576.1978.tb01399.x. [DOI] [PubMed] [Google Scholar]
- 8.Huch A, Huch R, Arner B, Rooth G. Continuous transcutaneous oxygen tension measured with a heated electrode. Scand J Clin Lab Invest. 1973;31(3):269–275. doi: 10.3109/00365517309082431. [DOI] [PubMed] [Google Scholar]
- 9.Al-Siaidy W, Hill DW. The importance of an elevated skin temperature in transcutaneous oxygen tension measurement. Birth Defects Orig Artic Ser. 1979;15(4):149–165. [PubMed] [Google Scholar]
- 10.Rooth G. Transcutaneous oxygen tension measurements in newborn infants. Pediatrics. 1975;55(2):232–235. [PubMed] [Google Scholar]
- 11.Lanigan C, Ponte J, Moxham J. Performance of transcutaneous PO2 and PCO2 dual electrodes in adults. Br J Anaesth. 1988;60(6):736–742. doi: 10.1093/bja/60.6.736. [DOI] [PubMed] [Google Scholar]
- 12.Huch R, Lubbers DW, Huch A. Quantitative continuous measurement of partial oxygen pressure on the skin of adults and new-born babies. Pflugers Arch. 1972;337(3):185–198. doi: 10.1007/BF00586844. [DOI] [PubMed] [Google Scholar]
- 13.Clark LC. Monitor and control of blood and tissue O2 tensions. Trans Am Soc Artif Intern Organs. 1956;2:41–48. [Google Scholar]
- 14.Severinghaus JW. A combined transcutaneous PO2-PCO2 electrode with electrochemical HCO3- stabilization. J Appl Physiol Respir Environ Exerc Physiol. 1981;51(4):1027–1032. doi: 10.1152/jappl.1981.51.4.1027. [DOI] [PubMed] [Google Scholar]
- 15.Severinghaus JW, Astrup PB. History of blood gas analysis IV. Leland Clark’s oxygen electrode. J Clin Monit. 1986;2(2):125–139. doi: 10.1007/BF01637680. [DOI] [PubMed] [Google Scholar]
- 16.Eberhard P, Severinghaus JW. Measurement of heated skin O2 diffusion conductance and Po2 sensor induced O2 gradient. Acta Anaesthesiol Scand. 1978;68:1–3. doi: 10.1111/j.1399-6576.1978.tb01384.x. [DOI] [PubMed] [Google Scholar]
- 17.Kimmich HP, Kreuzer F. Model of oxygen transport through the skin as basis for absolute transcutaneous measurement of PaO2. Acta Anaesthesiol Scand Suppl. 1978;68:16–19. doi: 10.1111/j.1399-6576.1978.tb01387.x. [DOI] [PubMed] [Google Scholar]
- 18.Eberhard P, Mindt W, Jann F, Hammacher K. Continuous pO2 monitoring in the neonate by skin electrodes. Med Biol Eng. 1975;13(3):436–442. doi: 10.1007/BF02477116. [DOI] [PubMed] [Google Scholar]
- 19.Rich K. Transcutaneous oxygen measurements: implications for nursing. J Vasc Nurs. 2001;19:55–59. doi: 10.1067/mvn.2001.115782. [DOI] [PubMed] [Google Scholar]
- 20.Quine D, Stenson BJ. Does the monitoring method influence stability of oxygenation in preterm infants? A randomised crossover study of saturation versus transcutaneous monitoring. Arch Dis Child Fetal Neonatal Ed. 2008;93(5):F347–F350. doi: 10.1136/adc.2007.132282. [DOI] [PubMed] [Google Scholar]
- 21.Collins JA, Rudenski A, Gibson J, Howard L, O’Driscoll R. Relating oxygen partial pressure, saturation and content: the haemoglobin-oxygen dissociation curve. Breathe (Sheff) 2015;11(3):194–201. doi: 10.1183/20734735.001415EDU-0014-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tremper KK, Shoemaker WC. Transcutaneous oxygen monitoring of critically ill adults, with and without low flow shock. Crit Care Med. 1981;9(10):706–709. doi: 10.1097/00003246-198110000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Urban M, Fouasson-Chailloux A, Signolet I, Colas Ribas C, Feuilloy M, Abraham P. Comparison of two devices for measuring exercise transcutaneous oxygen pressures in patients with claudication. Vasa. 2015;44(5):355–362. doi: 10.1024/0301-1526/a000454. [DOI] [PubMed] [Google Scholar]
- 24.Opitz N, Lubbers DW. Theory and development of fluorescence-based optochemical oxygen sensors: oxygen optodes. Int Anesthesiol Clin. 1987;25(3):177–197. doi: 10.1097/00004311-198702530-00011. [DOI] [PubMed] [Google Scholar]
- 25.Kesten S, Chapman KR, Rebuck AS. Response characteristics of a dual transcutaneous oxygen/carbon dioxide monitoring system. Chest. 1991;99(5):1211–1215. doi: 10.1378/chest.99.5.1211. [DOI] [PubMed] [Google Scholar]
- 26.Nickerson BG, Patterson C, McCrea R, Monaco F. In vivo response times for a heated skin surface CO2 electrode during rest and exercise. Pediatr Pulmonol. 1986;2(3):135–140. doi: 10.1002/ppul.1950020304. [DOI] [PubMed] [Google Scholar]
- 27.Eberhard P, Gisiger PA, Gardaz JP, Spahn DR. Combining transcutaneous blood gas measurement and pulse oximetry. Anesth Analg. 2002;94(1 Suppl):S76–S80. [PubMed] [Google Scholar]
- 28.IEC 60601-2-23:2011 - Medical electrical equipment - Part 2-23: Particular requirements for the basic safety and essential performance of transcutaneous partial pressure monitoring equipment (2011).
- 29.FDA Pulse Oximeters – Premarket Notification Submissions [510(k)s] – Guidance for Industry and Food and Drug Administration Staff (2013)
- 30.ISO 80601-2-61:2011 Medical Electrical Equipment – Part 2-61 Particular Requirements for basic safety and essential performance of pulse oximeter equipment (2011) [DOI] [PubMed]
- 31.Bland JM, Altman DG. Agreement between methods of measurement with multiple observations per individual. J Biopharm Stat. 2007;17(4):571–582. doi: 10.1080/10543400701329422. [DOI] [PubMed] [Google Scholar]
- 32.Roediger R, Beck-Schimmer B, Theusinger OM, Rusch D, Seifert B, Spahn DR, Schmid ER, Baulig W. The revised digital transcutaneous PCO2/SpO2 ear sensor is a reliable noninvasive monitoring tool in patients after cardiac surgery. J Cardiothorac Vasc Anesth. 2011;25(2):243–249. doi: 10.1053/j.jvca.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 33.Rudiger M, Topfer K, Hammer H, Schmalisch G, Wauer RR. A survey of transcutaneous blood gas monitoring among European neonatal intensive care units. BMC Pediatr. 2005;5:30. doi: 10.1186/1471-2431-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sandberg KL, Brynjarsson H, Hjalmarson O. Transcutaneous blood gas monitoring during neonatal intensive care. Acta Paediatr. 2011;100(5):676–679. doi: 10.1111/j.1651-2227.2011.02164.x. [DOI] [PubMed] [Google Scholar]
- 35.Poets CF, Southall DP. Noninvasive monitoring of oxygenation in infants and children: practical considerations and areas of concern. Pediatrics. 1994;93(5):737–746. [PubMed] [Google Scholar]
- 36.Eickhoff JH, Ishihara S, Jacobsen E. Effect of arterial and venous pressures on transcutaneous oxygen tension. Scand J Clin Lab Invest. 1980;40(8):755–760. doi: 10.3109/00365518009095592. [DOI] [PubMed] [Google Scholar]
- 37.Palmisano BW, Severinghaus JW. Transcutaneous PCO2 and PO2: a multicenter study of accuracy. J Clin Monit. 1990;6(3):189–195. doi: 10.1007/BF02832145. [DOI] [PubMed] [Google Scholar]