Abstract
Inhibitory control is a core executive function (EF) skill, thought to involve cognitive ‘interference suppression’ and motor ‘response inhibition’ sub-processes. A few studies have shown that early bilingualism shapes interference suppression but not response inhibition skills, however current behavioral measures do not fully allow us to disentangle these subcomponents. Lateralized Readiness Potentials (LRPs) are centroparietal event-related potentials (ERPs) that track motor response-preparations between stimulus-presentation and behavioral responses. We examine LRPs elicited during successful inhibitory control on a nonverbal Stroop task, in 6–8 year-old bilingual (n = 44) and monolingual (n = 48) children from comparable socio-economic backgrounds. Relative to monolinguals, bilinguals showed longer and stronger incorrect-response preparations, and a more mature pattern of correct-response preparation (shorter peak-latencies), underlying correct responses on Stroop-interference trials. Neural markers of response-inhibition were comparable between groups and no behavioral differences were found between-groups on the Stroop task. Results suggest group differences in underlying mechanisms of centroparietal motor-response preparation mechanisms in this age group, contrary to what has been shown using behavioral tasks previously. We discuss neural results in the context of speed-accuracy trade-offs. This is the first study to examine neural markers of motor-responses in bilingual children.
Keywords: Inhibitory control, Bilingual children, Lateralized readiness potentials (LRP), Event-related potentials (ERP), Response inhibition, Stroop task
1. Introduction
Inhibition is a core executive function (EF) skill involving the control of one’s attention, behavior, thoughts, and emotions. Inhibition skills, also referred to as inhibitory control, involve two dissociable processes: an earlier “interference suppression” or “interference control” process (used here interchangeably), and a later “response inhibition” process (Booth et al., 2003; Brydges et al., 2012; Bunge et al., 2002; Johnstone et al., 2009; Jongen and Jonkman, 2008; Luk et al., 2010; Vuillier et al., 2016). The earlier interference control process refers to cognitively resisting interference from irrelevant or misleading information, whereas the later response inhibition process refers to stopping or withholding a pre-potent motor response (Vuillier et al., 2016). This distinction may have a reliable neural basis given that underlying neural mechanisms of interference suppression and response inhibition are dissociable (Brydges et al., 2012), with interference suppression areas developing relatively earlier (Bunge et al., 2002; Jongen and Jonkman, 2008).
The inhibitory control skills of bilingual children have come into focus recently within the literature about ‘bilingual advantages’. A number of studies suggest that early exposure to a second language shapes inhibitory control function over the lifespan, leading to certain cognitive advantages (see Bialystok et al., 2009, for review). On the other hand, some studies in adults and older children have reported a lack of bilingual advantages, or advantages on some aspects of EF but not others (Antón et al., 2014; Duñabeitia and Carreiras, 2015; Paap, 2014; Paap and Greenberg, 2013; Paap et al., 2015; Ross and Melinger, 2017), suggesting that early advantages may become muted at stages of peak cognitive ability, and that bilingual advantages should be more carefully qualified. Given the potential interactions between age, early EF development, and bilingualism, efforts to describe neurocognitive mechanisms of specific EF skills control – in both bilingual and monolingual children – are crucial to improving our understanding of how bilingualism may shape cognitive development.
In early inhibitory control skills specifically, bilingualism is thought to shape inhibitory control specifically via interference suppression skills rather than response inhibition skills (Esposito et al., 2013; Martin-Rhee and Bialystok, 2008). For example, Martin-Rhee and Bialystok found that bilingual preschoolers and school-aged children outperformed monolingual controls on tasks that required interference suppression, such as the Simon task, but not on tasks that required verbal or nonverbal response inhibition, such as the Day-Night Stroop. Similarly, Esposito et al. found that bilingual preschoolers outperformed monolingual controls on a modified Stroop task requiring interference suppression but not on a version requiring only response inhibition. Consistent with these findings, Carlson and Meltzoff (2008) showed a broader pattern of bilingual advantages on “conflict” tasks thought to recruit cognitive control, but not on “delay” tasks, thought to recruit response inhibition. Additional studies have reported better performance in bilingual children compared to monolinguals on the Flanker task (Mezzacappa, 2004; Yoshida et al., 2011), classically associated with interference suppression. Overall, therefore, the pattern emerging is that any differences in bilingual children’s inhibitory control skills are driven by interference suppression mechanisms specifically. As an exception, 5-year-old bilinguals outperformed monolinguals on the Go/No-Go task, commonly used to measure response inhibition (Barac et al., 2016).
Vuillier et al. (2016) argue that both interference suppression and response inhibition processes are likely used in all inhibition tasks, and behavioral indicators such as RTs likely correspond to a combination of the two processes. Current behavioral measures of inhibitory control may elicit processes of interference suppression, conflict monitoring, and response inhibition to varying extents depending on their exact design (Kane and Engle, 2003; Tillman and Wiens, 2011). Given that most investigations of bilingual inhibitory control have been behavioral, it is currently difficult to isolate specific mechanisms of inhibition that may be shaped by bilingualism during development. In order to better understand inhibitory control development in bilingual children, we ideally need ways to disentangle interference suppression and response inhibition processes.
One method that has been utilized to exclusively examine response preparation mechanisms of inhibitory control, is by measuring Lateralized Readiness Potentials (LRPs). LRPs are motor-cortex based ERP components indexing motor-response preparation in advance of participants’ motor-responses on a task (Smulders and Miller, 2012; De Jong et al., 1988; Coles, 1989). LRPs can be utilized to shed light on neural mechanisms of the response inhibition process underlying behavioral performance on inhibitory control tasks. For example, when successfully utilizing inhibitory control, it is generally expected that children will inhibit incorrect pre-potent responses before they prepare to make correct responses. This may take different forms on different tasks such as the Stroop, Flanker, Dimensional Change Card Sort, Go/No-Go and other tasks associated with inhibitory control. Since LRPs track motor-preparation, they allow us to track both incorrect and correct response preparations underlying responses on behavioral trials, providing a closer look “behind-the-scenes” of successful response inhibition. By tracking motor-preparation on trials that elicit inhibition (e.g. Incongruent/Stroop trials on a Stroop task), LRPs allow time-locked tracking of the initially expected incorrect response preparation, followed by the correct response preparation, if any. Comparing bilinguals’ and monolinguals’ “behind-the-scenes” response preparations on successful inhibition can therefore shed light on similarities and differences in the motor aspects of their inhibition skills.
Importantly, unlike ERPs which may have visible scalp distributions, LRPs are derived from left and right motor areas specifically. Based on Coles’s (1989) formula for calculating LRPs, LRPs account for the difference between contralateral and ipsilateral activation of motor areas, averaged across left-handed and right-handed responses. That is, LRPs are fundamentally associated with motor-responses, allowing us to disentangle motor-response preparation and inhibition processes from more cognitive aspects of inhibition (e.g. interference suppression), accessible through ERP waveforms studied in bilingual children such as N2 or P3 (e.g. Barac et al., 2016).
LRP analyses have been successfully carried out in young children. One comparison of LRPs indexing response preparation in 5-year-olds and 8-year-olds during a non-verbal Stroop task found that initial incorrect response preparation was similar across the stages of development, whereas correct response preparation was more efficient at older ages (Bryce et al., 2011). While Bryce et al.’s sample included a significant percentage of bilingual children (Bryce, personal communication), their LRPs were not separately analyzed due to the study’s differing focus on the development of inhibitory control mechanisms. Bryce et al. (2011), did however recommend an examination of bilingual children’s LRPs in future work (p.683), which informs the motivations of the current study. The current study extends previous LRP measurement and analysis techniques in children, to understanding bilingual children’s inhibitory control. Specifically, the current study takes a comparative approach, examining similarities and differences in bilingual and monolingual school-aged children’s response preparation and inhibition mechanisms. This can help provide a more complete picture of whether, and how specifically, bilingualism shapes inhibitory control mechanisms, in the context of mixed behavioral findings.
2. The current study
The current study aims to shed light on whether bilingualism shapes neural mechanisms of response inhibition, by comparing electrophysiological markers of response inhibition in bilingual and monolingual children. Specifically, we measured school-aged bilingual and monolingual children’s performance on a nonverbal Stroop task, and extracted LRPs from high-density EEG collected during the task. With this combination of measures, the current study helps disentangle whether, and how, bilingualism may shape response inhibition mechanisms. Since LRPs have not previously been utilized to measure inhibitory control in bilingual children, we hypothesized a few different patterns in the results, and what they would mean. Based on previous studies, we predicted behavioral bilingual advantages on the Stroop task. If bilingual advantages are found, differences in LRPs would suggest that response inhibition mechanisms do partly drive such advantages, contrary to current theories. If behavioral bilingual advantages are absent, differences in LRPs would suggest that bilinguals and monolinguals rely on different cognitive mechanisms to produce the same behavioral results. Lastly, if behavioral bilingual advantages are found, but LRPs do not differ, this would provide more direct neural evidence confirming current theories that bilingual advantages in inhibition are driven by interference control and not response inhibition mechanisms.
3. Methods
3.1. Participants
One hundred and twelve participants (55 females; 57 males; 51 bilinguals; 61 monolinguals) ranging from 6 to 8 years old (M = 6.98 years; SD = 0.57 years) were included in the final analyses. During an initial phone screen, participants were excluded from the study if they had any history of neurological problems or diagnosed attention difficulties or were premature at birth (had a gestational age of less than 37 weeks). Based on the initial phone screen, participants were classified as monolingual if parents reported ≤5 % regular exposure to an L2, and as bilingual if parents reported >20 % exposure to an L2. Specific thresholds for monolingual and bilingual groups were decided based on previous research which suggests that for children to be bilingual they need to receive about 10%–25% exposure to each language (Marchman et al., 2009; Place and Hoff, 2011). Within this range, some consider 20% to be the specific threshold of bilingualism (Gutiérrez-Clellen and Kreiter, 2003), which is consistent with the finding that children are much less likely to make utterances in a second language if they are exposed to it less than 20% of the time (Pearson et al., 1997). Based on our interest in comparing monolinguals and bilinguals, children with between 5% and 20% regular exposure to L2 were excluded from the study after the initial phone screen. Children exposed to South Asian languages (e.g. Hindi, Urdu, Punjabi, Bengali, Gujrati) were also excluded due to the specific exclusion criteria for a separate study utilizing the same participant sample (Nayak et al., 2016). Further, only children who were dominant in English were included in the sample. The final bilingual sample was linguistically heterogeneous, representing a wide range of L2s. Monolinguals and bilinguals were from comparable SES backgrounds, and were of similar ages. Table 1 shows the demographic breakdown of both groups.
Table 1.
Demographic variables by language background.
| Monolinguals | Bilinguals | ||
|---|---|---|---|
| Age (in years) | 6.98 (0.57) | 6.85 (0.62) | |
| Gender (% female) | 47.5 | 51 | |
| Ethnicity (%) | |||
| Caucasian | 77 | 32.7 | |
| African-American | 6.6 | 8.2 | |
| Hispanic/Latino | 1.6 | 10.2 | |
| Asian | 3.3 | 20.4 | |
| Other/Mixed Race | 11.5 | 28.6 | |
| SES | 0.07 (0.74) | −0.06 (0.91) | |
| Parent Occuptational Prestige | 0.14 (0.80) | −0.17 (1.19) | |
| Parent Education | 0.04 (0.98) | −0.04 (1.03) | |
| Income-to-Needs Ratio | 0.04 (0.89) | −0.06 (1.15) | |
| Annual Income ($1000) | 125 (63) | 111 (75) |
Note. SES composite variable and the components of the SES composite are standardized (M = 0, SD = 1).
3.2. Procedures
Children between the ages of 6 and 8 years visited the laboratory with their parent to participate, which lasted between 2 to 2.5 hours. As per Institutional Review Board (IRB) guidelines, informed consent was obtained from the parent, and additional verbal assent was obtained from the child if they were 7 years or older. After consent and assent procedures, children’s inhibition skills were measured using a computerized task, while high-density EEG was recorded. Small prizes and stickers were given to the child after each task, to keep them motivated. EEG data were pre-processed offline, and ERP components of interest were statistically extracted for analyses. All study procedures were IRB approved.
3.3. Background and behavioral measures
3.3.1. Socioeconomic status (SES)
Parents reported on their household’s annual income and composition, highest maternal and paternal level of education attained, and maternal and paternal occupation (as applicable). From these reports, a maternal and paternal occupational prestige variable was coded using the job zone coding scheme from the Occupational Information Network (O*NET, http://www.onetonline.org/help/online/zones), which ranks U.S. census-based occupational categories on a 1–5 scale based on the education, experience, and training required. Parental educational attainment and occupational prestige were computed by averaging across maternal and paternal variables. Further, an income-to-needs ratio variable was computed from household income and composition information, using 2016 U.S. federal poverty guidelines. Parent educational attainment, parent occupational prestige, and income-to-needs ratio were standardized (Z scores with M = 0, SD = 1) and averaged, to create a standardized SES composite variable. For participants who chose not to report annual income data, income-to-needs ratio could not be calculated. For these participants, the SES composite variable was computed by combining the parental education and occupational prestige variables. Similarly, if any one variable comprising SES had missing data for a given participant, the SES composite was computed based on the other two variables. Income data were unavailable for 10 bilinguals and 5 monolinguals, and occupational prestige data were unavailable for 2 bilinguals.
3.3.2. Animal Size Stroop Task (Bryce et al., 2011)
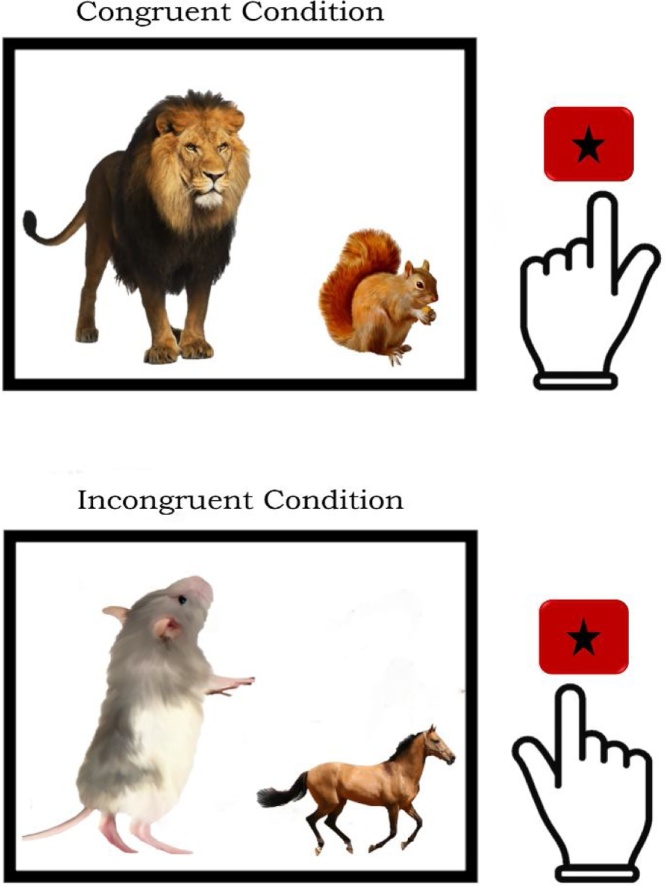
Inhibitory control skills were measured using a modified version of the Animal Size Stroop Task (ASST), a nonverbal Stroop task measuring interference control, adapted from Bryce et al. (2011). On each trial, two animal images of different sizes (big or small) appeared on the screen, on the left and right side. Children were instructed to press the left or right button on a response pad, corresponding to “which animal is bigger in real life” on each trial. On each trial, children made button presses corresponding to the side of the screen that corresponded to the bigger animal “in real life”. Children could press either the left or right button on a response pad, with their left or right index finger respectively. Left button presses (with left index finger) indicated the responses that the animal on the left side of the screen is bigger in real life, and so on. Trials were either congruent, in which the bigger animal on screen is also the bigger animal in real life, or incongruent, in which the smaller animal on screen is bigger in real life. That is, Stroop interference was expected on incongruent trials due to the conflict between animal sizes on screen versus true animal sizes. Fig. 1 provides an example of congruent and incongruent trials along with their correct responses. Each child completed 8 practice trials, followed by 4 blocks of 48 trials, with breaks in between each block to ensure the child was ready for the next set of trials. Each block of 48 trials consisted of an equal amount of congruent and incongruent trials, presented in a predetermined pseudo-random order with half the trials involving left-handed responses for correct responses, and the other half involving right-handed responses. The actual number of left-handed and right-handed responses made by each child depended on their task accuracy. Before each block, children were encouraged to keep their left and right index fingers placed lightly on the left and right response pad. High-density EEG was recorded while the child completed the task.
Fig. 1.
Congruent and Incongruent condition examples from Animal Size Stroop Task, with correct responses. Children are asked to make responses via button press based on “which animal is bigger in real life”. Top image shows congruent condition, where bigger animal on screen is bigger in real life, and bottom image shows incongruent condition, where smaller animal on screen in bigger in real life. Hand images to the right of each example represent the correct button press; in the congruent condition (top), left-handed left-button press is correct, and in the incongruent condition (bottom), right-handed right-button press is correct. Left and right button-presses were always made with the index finger of the corresponding hand.
The task yielded RTs and accuracies on congruent and incongruent (Stroop) trials. Two Stroop effect variables, indexing the extent of Stroop interference experienced, were then computed as the difference between RTs and accuracies in incongruent compared to congruent trials. When calculating RTs, only RTs on correct trials were included. Smaller Stroop effects represented smaller amounts of interference experienced, and therefore better interference control.
4. Neural measures
4.1. EEG recording
EEG data were recorded with the child seated inside an electrically shielded booth, while they completed the ASST. EEG data were recorded using the Clinical Geodesic EEG System 300 (Electrical Geodesics, Inc.), consisting of a 128-channel Hydrocel Geodesic Sensor Net 130, Net Amps 300 Amplifier, E-Prime Professional 2.0 (Psychology Software Tools) with extensions for Net Station, and Net Station 4.5 software. The EEG net was first prepared by soaking in warm saline solution, while the parent’s consent and child’s assent were collected. Before recording began, impedances were measured and electrodes were adjusted till impedances were below a threshold of 80 Ω, an adequate level of impedance for research with children when using a high-impedance EEG acquisition system. To maintain data acquisition quality, after children completed the first 2 blocks of trials on the ASST, impedances were checked again, and electrodes were readjusted or rehydrated where necessary. EEG were recorded to a vertex reference at a 500 Hz sampling rate, with an online high-pass filter of 0.01 Hz applied during recording.
4.1.1. EEG processing and ERP extraction
Offline, EEG data were filtered, applying a bandpass filter of 0.3–30 Hz. Next, data were segmented into epochs of interest, time-locked to stimulus presentation. In order to later allow us to compute LRPs, two categories of ERP segments were created from filtered EEG data: 1) correct left-handed responses on incongruent trials, and 2) correct right-handed responses on incongruent trials.1 Segments were 1200 ms long in total, ranging from 200 ms prior to stimulus presentation (baseline activity) to 1000 ms after stimulus presentation (activity of interest). For each segment, an automatic artifact detection and bad channel replacement paradigm identified channels with excessive artifact (>200 μV) in order to address channels affected by muscle artifact and replaced bad channels via interpolation. Next, the Ocular Artifact Removal tool in NetStation 4.5 was used to excise eyeblink artifacts from the data with a 20 μV/ms blink slope threshold, and the cleaned data were again subjected to artifact rejection and bad channel replacement. Channels that were bad on >15 % of the segments were marked as bad for the entire recording. Segments with >15 bad channels or with remaining eyeblinks (>140 μV differential average) were excluded. Segments were then baseline corrected against the window –200 ms to 0 ms segment window. ERP segment categories were then grand-averaged across participants, and the grand-averaged data was average referenced to the vertex reference electrode VRef (or channel 129). Average referenced data were then baseline corrected again against the –200 ms to 0 ms segment window. Only participants with a minimum of 10 usable segments in incongruent trials were included in computing LRPs from ERP data, and in any statistical analyses. 92 participants (44 bilinguals; 48 monolinguals) were included in the final LRP calculations. Overall, an average of 38 usable segments per participant were retained after artifact rejection (bilinguals = 36 segments; monolinguals = 41 segments), with an overall range of 11–67 usable segments across participants. EEG processing and stimulus-locked ERP extraction were carried out using NetStation 4.5.
4.1.2. LRP calculations and statistical extraction
Based on the aforementioned ERP data extracted from left-handed and right-handed correct responses on incongruent trials, individual and grand-averaged LRP waveforms were computed from the 1200 ms long ERP segments (including baseline) measured at channel 36 and channel 104 (C3 and C4 in the 10–20 international system). Our selection of specific electrodes 36 and 104 to compute LRPs was based on Bryce et al.’s (2011) previous analysis and validation of these electrode sites when computing, visualizing, and statistically analyzing LRPs in children.
Specifically, individual participants’ LRPs were computed by applying the formula: LRP = [(ER – EL) left hand response + (EL – ER) right hand response]/2 (Coles, 1989), where EL and ER represent voltage at left motor and right motor electrode sites respectively. Fig. 2 provides a visual schematic for the electrode positions on the 128-channel net, and the contralateral and ipsilateral activation for left-handed and right-handed responses accounted for by the LRP formula. Individual and grand-averaged LRPs in monolingual and bilingual children were computed and visualized using MATLAB (The MathWorks, Inc.).
Fig. 2.
Visual schematic of LRP calculations from electrical activity elicited by left-handed and right-handed responses, in left and right motor areas. Triangles mark locations of C3 and C4 electrodes on the EGI 128 channel EEG net. Stars represent response buttons on the ASST, and left and right hands represent left-handed and right-handed responses made via button press during the task. As shown in the LRP formula (Coles, 1989), LRPs are computed as a function of contralateral (dashed lines) and ipsilateral activation elicited by left-handed and right-handed task responses via button press. In the current study, only correct responses on incongruent trials of the ASST were included in LRP calculations.
Modeling after Bryce et al.’s (2011) techniques, and based on recommended techniques for LRP analyses, individual LRP data were first smoothed using a 150 ms moving window. In the smoothing process, each data point was recalculated as the mean of all the values occurring 75 ms before and 75 ms after it. Next, the following LRP features of interest were statistically extracted for individual participants:
-
•
Positive-peakamplitudes and latencies: incorrect-response preparation between stimulus-presentation and correct behavioral-response.
-
•
Negative-peakamplitudes and latencies: correct-response preparation between stimulus-presentation and correct behavioral-response.
In addition, the following LRP features were extracted:
-
•
Positive-peak-onsetandpositive-peak-cessationlatencies: latencies corresponding to 75% of positive-peak amplitude on both sides of the peak. Positive-peak-onset therefore occurs before the positive-peak, indicating the “onset” of incorrect-response preparation, whereas positive-peak-cessation occurs after the positive-peak, indicating the “cessation” of incorrect-response preparation.
-
•
Negative-peak-onsetandnegative-peak-cessationlatencies: latencies corresponding to 75% of negative-peak amplitude on both sides of the peak. As above, negative-peak onsets and cessations signal onset and cessations of correct-response preparation, respectively.
-
•
Positive-peak-cessationtonegative-peak-onsettransition: Transitions from incorrect-response preparation to correct-response preparation mark response-inhibition, i.e. inhibition of the incorrect-response preparation in favor of the correct-response preparations.
To acquire the above onset and cessation latencies, smoothed data from individuals were statistically ‘jackknifed’ (or jackknife resampled) in order to improve signal-to-noise ratio, reduce variance, and better localize meaningful values for response-preparation onset and cessation latencies, as has been done by others in the literature, and following Bryce et al.’s technique (2011). In the jackknife resampling technique, at each time point, voltages are recalculated as the mean of (n-1) sub-averages, where each sub-average omits a different participants’ data, or “leaves one out”. Fig. 3 provides an example of jackknife resampling of smoothed LRPs in a sample of n = 5 from the current dataset, for illustrative purposes. Smoothing and jackknife resampling were carried out using MATLAB.
Fig. 3.
Illustrative example of jackknife resampling of smoothed LRP data in n = 5. Smoothed LRPs for each participant are given in red, and jackknifed LRPs for each participant are given in green. At each time point, voltages are recalculated as the mean of (n -1) sub-averages, where each sub-average omits a different participants’ data, or “leaves one out”. Jackknifing is used to capture more meaningful latency values for response-preparation onsets and cessations in individual participants.
Adjustments to jackknifed data were required to capture truer values for each participant’s latencies for peak-onsets and peak-cessation latencies, which were subsequently utilized in statistical analysis and hypothesis testing. These adjustments were made following the procedure outlined by Smulders (2010), in which individual latencies are ‘retrieved’ from jackknifed data, based on the following formula: , where O is the retrieved latency for each participant i, is the mean of jackknifed latencies across all participant, J is the jackknifed latency for each participant i. Any statistical tests pertaining to LRP peak-onset and peak-cessation latencies were carried out using the retrieved latencies O.
5. Analysis plan
Preliminary analyses were conducted on variables of interest to ensure that skew and kurtosis for each variable were within the acceptable −3 to +3 range. Outlier values were winsorized if they fell outside the range of +/−3 SDs from the mean. Participants who had outlying values for all variables were removed from the final analyses. Next, Bivariate Correlations were analyzed in order to understand associations between demographic variables, behavioral variables, and neural (LRP) variables of interest and to choose appropriate covariates.
Behavioral analyses were conducted to understand performance on the ASST. Two-tailed independent samples t-tests were conducted to test whether mean Age and SES were equivalent between groups. Next, in order to investigate any differences between groups on inhibitory control, we conducted two separate independent samples t-tests, or one-way ANCOVAs (as applicable), on Stroop effects in RTs and Stroop effects in Accuracy. Based on preliminary correlational analyses, relevant correlates of the dependent variables were included in the model as covariates.
Based on Coles’ (1989) formula utilized here to compute LRPs, LRPs elicited during correct incongruent (Stroop) trials are expected to show an initial positive component (tracking incorrect motor-response preparation), followed by a negative component (tracking correct motor-response preparation). Within grand-averaged and smoothed LRPs, one sample t-tests were conducted within each available 2 ms time-bin, in order to determine specific latency windows within which LRP amplitudes significantly deflected positively or negatively, i.e. where amplitudes were significantly different from 0 mv in either direction. One-sample t-tests were separately conducted for monolingual and bilingual LRPs.
In order to account for relevant covariates in any statistical models, bivariate correlations were tested between the background (Age, SES) and LRP variables above. Any background variables that correlated with LRP variables of interest were subsequently included as covariates in relevant models. In order to test for group differences on each LRP variable of interest, we conducted two-tailed independent t-tests, or one-way ANCOVAs (as applicable), with language background (monolingual and bilingual) as the between-subjects factor.
6. Results
One participant was removed during preliminary analyses due to all corresponding values being outliers. 3 values were winsorized. Two-tailed independent samples t-tests showed that bilingual and monolingual groups did not differ on Age (t(110) = 1.301, p > .05) or SES (t(95.83) = 0.868, p > .05). Bivariate correlational analyses between demographic variables and behavioral variables of interest showed that smaller Stroop effects in RTs were associated with higher Age (r = −0.25, p = .009) and higher SES (r = −0.21, p = .025). Smaller Stroop effects in Accuracy were also associated with higher SES (r = −0.28, p = .002), but not with Age (r = −0.14, p > .05). Additional correlational analyses between demographic and LRP variables showed that shorter negative-peak latencies were associated with higher Age (r = −0.26, p = .01), but showed no other correlations between demographic and LRP variables. Lastly, correlation analyses between behavioral and neural variables showed that smaller Stoop effects in Accuracy were associated with smaller negative-peak amplitudes (r = 0.21, p = .047) but showed no other correlations between behavioral and LRP variables.
Table 2 shows RTs, accuracies, and Stroop effects in RTs and accuracies, in monolinguals and bilinguals. A one-way ANCOVA with Age and SES included as covariates showed no group difference in Stroop effects in RTs (F(1, 108) = 0.005, p = .946). Similarly, a one-way ANCOVA with SES included as a covariate showed no group difference in Stroop effects in Accuracy (F(1, 109) = 0.272, p = .603).
Table 2.
Behavioral inhibitory control performance and Stroop effects on the Animal Size Stroop Task (ASST) in monolinguals and bilinguals.
| Inhibitory Control |
||||||
|---|---|---|---|---|---|---|
| Congruent |
Incongruent |
Stroop Effects |
||||
| RTs | Accuracy | RTs | Accuracy | RTs | Accuracy | |
| Monolinguals | 1086.58 (31.08) | 0.97(0.00) | 1214.52(35.11) | 0.87(0.02) | 127.94 (11.59) | 0.09 (0.01) |
| Bilinguals | 1092.62 (30.28 | 0.97(0.01) | 1230.07 (38.51) | 0.87(0.02) | 137.45 (18.74) | 0.08 (0.01) |
Note. Performance on congruent and incogruent conditions were derived from the Animal Size Stroop Task. Stroop effects were calculated as the difference between congruent and incongruent conditions. RTs are given in milliseconds, and accuracies are given as a proportion of total trials, between 0 and 1. Standard errors are given in parentheses. N = 112 (Monolingual n = 61; Bilingual n = 51).
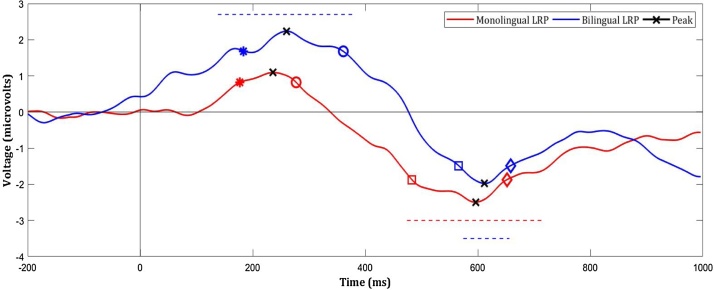
Both language groups showed the expected LRP waveform morphology consisting of an initial positive deflection tracking incorrect response preparation, followed by a secondary negative deflection tracking correct response preparation, although the initial positive component was not significant in monolinguals. Fig. 4 illustrates latencies at which positive and negative amplitudes were significantly different from 0 mv (i.e. significant deflections), based on one-sample t-tests within each 2 ms time bin, following the technique used by Bryce et al. (2011) and others. In bilinguals’ grand averaged LRP, significant positive deflections occurred between 138 ms to 378 ms, and significant negative deflections occurred between 574 ms to 656 ms, relative to stimulus presentation. LRP waveform morphology differed in monolinguals’ grand averaged LRP; significant negative deflections occurred between 474 ms and 714 ms after stimulus presentation, but positive deflections were not significant.
Fig. 4.
Smoothed stimulus-locked LRPs in monolingual and bilingual children, on correct incongruent trials of the Animal Size Stroop Task. LRPs were computed from contralateral and ipsilateral activation in left motor (C3) and right motor (C4) areas, extracted from left-handed and right-handed responses. Stimulus presented at time =0 ms. The early positive component represents incorrect-response preparation, and the later negative component represents correct-response preparation. Incorrect-response preparation-onset (*) and cessation (o), and correct-response preparation-onset (□) and cessation (◊) are marked, and identified as the latency at which the voltage reaches or returns to 75% of its peak amplitude. Peak amplitudes are marked by an ‘X’. Blue dashed lines represent time windows in which response-preparation is significant for bilinguals, and red dashed lines represent the same in monolinguals.
Table 3 shows positive-peak and negative-peak amplitudes and latencies derived from smoothed and grand-averaged LRPs. Two-tailed independent samples t-tests between LRP variables of interests showed that positive-peak amplitudes and latencies, and negative-peak amplitudes were equivalent in bilinguals and monolinguals. However, bilinguals and monolinguals differed on other LRP variables of interest: bilinguals showed later positive-peak-cessation latencies (t(58) = −2.162, p = .035) and later negative-peak-onset latencies (t(90) = −2.263, p = .026) compared to monolinguals. Further, a one-way ANCOVA, with Age included as a covariate in the model based on preliminary correlational analysis, showed earlier negative-peak latencies in bilinguals controlling for Age (F(1,89) = 5.242, p = .02). That is, motor-cortex activation underlying incorrect response-preparations started equivalently but ended later in bilinguals (thereby spanning a longer period compared to monolinguals), and activation associated with correct response preparations started later and peaked earlier in bilinguals (thereby peaking in a shorter amount of time compared to monolinguals).
Table 3.
Positive and negative peak amplitudes and latencies of stimulus-locked Lateralized Readiness Potentials (LRPs) in monolinguals and bilinguals.
| Lateralized Readiness Potentials (LRP) |
||||
|---|---|---|---|---|
| Pos Amp. | Pos Lat. | Neg Amp. | Neg Lat. | |
| Monolinguals | 2.76 (0.56) | 297.33 (15.82) | −5.13 (0.80) | 686.62 (34.64) |
| Bilinguals | 4.05 (0.66) | 280.31 (15.43) | −3.88 (0.70) | 608.31 (20.83) |
Note.Standard error in parentheses. LRP amplitudes (pos.) and latencies (lat.) were derived from accurate incongruent trials of the Animal Size Stroop Task. Raw LRPs were smoothed using a moving average of 150 ms. Amplitudes are given in microvolts and latencies are given in milliseconds from the time of response, with Standard Errors given in parentheses. N = 92 (Monolingual n = 48; Bilingual n = 44).
On the other hand, no group differences were found between positive-peak-onset latencies (t(50) = −0.072, p > .05) or negative-peak-cessation latencies (t(90) = −0.136, p > .05). Additionally, a repeated-measures general linear model showed no significant interactions between language background X transitions (F(1, 90) = .009, p > .05), i.e., bilingual and monolingual showed similarly long transitions from initial positive-peak-cessations (incorrect preparations) to secondary negative-peak-onsets (correct preparation).
7. Discussion
The current study compared 6–8-year-old bilingual and monolingual children’s inhibitory control skills, and underlying neural mechanisms on a nonverbal Stroop task. Consistent with some other examinations of inhibition (Antón et al., 2014; Duñabeitia et al., 2014), the age-matched and SES-matched bilingual and monolingual school-aged children in our study indicated similar abilities to control and respond to stimulus-interference. Extending previous findings showing equivalent inhibitory control skills in the two groups at the behavioral level, our results point to the continued importance of considering age and SES when examining bilingual inhibitory control relative to monolinguals. Although we did not find behavioral differences in inhibition skills, results showed a number of electrophysiological differences in motor-response preparations underlying correct responses on incongruent (Stroop trials).
As expected with the current task and age-group (Bryce et al., 2011), bilinguals showed significant indicators of incorrect-response preparation followed by correct-response preparation. Unexpectedly, monolinguals only showed the later correct-response preparation, with flattened incorrect-response preparation. That is, on average, bilinguals more consistently prepared and inhibited incorrect motor-responses prior to making correct responses on Stroop trials, whereas monolinguals varied on this considerably. Further, bilinguals showed longer positive-peak-cessation latencies, longer negative-peak-onset latencies, and shorter negative-peak latencies, compared to monolinguals. That is, in bilinguals, incorrect-response preparation lasted longer, and transitions between incorrect and correct responses happened later. On the other hand, correct-response preparation lasted for a shorter duration, and peaked earlier in bilinguals.
It should be noted that at the present time it is unclear what the positive and negative peaks represent exactly, given that the more meaningful measures for LRPs are onsets and cessations, as reported in previous LRP studies in children’s incorrect and correct response preparations (e.g., Szűcs et al., 2009; Bryce et al., 2011). However, in our data, shorter negative-peak latencies were also correlated with older ages. Therefore, after ruling out group differences in Age, bilingual children showed more mature correct-response preparation patterns on incongruent (Stroop-interference) trials. Patterns in our data echo Bryce et al.’s findings that correct-response preparations were more efficient in older children, but differ in that negative-peaks (and not negative-peak-onsets) were correlated with Age in our data. It should be noted that age-related changes in the two studies are not directly comparable, since Bryce et al., compared 8 year-olds with 5 year-olds.
In our data, monolinguals’ initial incorrect-response preparations were not significant on average, therefore it is difficult to conclusively interpret group-differences in incorrect-response preparations, and group-similarities in transitions from incorrect to correct response-preparations. However, one possibility is that stronger neural markers of incorrect-response preparations in bilinguals indicate that bilinguals are prioritizing speed over accuracy on the non-verbal Stroop task. If this were the case, bilinguals would be expected to be more susceptible to the pre-potent incorrect response, consistent with our results which show longer, and stronger, incorrect-response preparations in bilinguals. Conversely, if monolinguals were prioritizing accuracy over speed, this may explain their non-significant, flattened incorrect-response preparations. While much more research is required to further understand this potential pattern, here we propose that bilingual children approach inhibitory control differently than monolinguals, particularly in terms of speed-accuracy tradeoffs.
Previous studies have essentially focused on examining cognitive aspects of bilingual inhibitory control skills (and other EF skills) through the tasks commonly utilized. This includes the single neural comparison of bilingual and monolingual inhibitory control in children (Barac et al., 2016). This interpretation would be consistent with previous data suggesting that bilingual preschoolers (3.5–4.5 year olds) show faster RTs than monolinguals on the baseline condition of a card-sort task, but slow down to match monolingual RTs when feedback and rewards are provided for accuracy (Nayak & Tarullo, in press; see also: Tarullo et al., 2018). Here, bilingual preschoolers may be prioritizing speed over accuracy, unless incentivized to do otherwise. This interpretation is further supported by neural markers of error-awareness, which were activated less strongly in bilingual preschoolers in the same study. Future studies should test this emerging idea more systematically.
To our knowledge this is the first study to compare motor function in bilingual and monolingual children. Our results extend Barac et al.’s (2016) important neural examination of inhibitory control skills in monolingual and bilingual children by measuring the motor aspects of inhibitory control – response preparations and inhibition – directly. We studied older school-aged children and utilized a task that elicits robust ERP markers of response preparation and inhibition in children and adults. Taken together with Barac et al.’s results, our results suggest that young bilinguals show differential development of neural mechanisms underlying both cognitive and motor aspects of inhibitory control, i.e. both interference-suppression and response-inhibition processes. Overall, the present results suggest a pressing need to examine neural mechanisms of EF in bilingual children relative to monolinguals, as developmental trajectories may differentiate as early as the preschool years.
Our results contribute to findings pertaining to the burgeoning neuroscientific exploration of differential brain structure and mechanisms in bilingual and monolingual children (Barac et al., 2016; Mohades et al., 2012, 2014, 2015; Nayak & Tarullo, in press), and the robust neuroanatomical and neurofunctional differences between bilingual and monolingual adults (Duncan et al., 2018; see García-Pentón et al., 2016 for review). As indicated in the more recent EEG studies in children noted here, and fMRI work in adults (Grundy et al., 2017; Luk et al., 2010), differential neural mechanisms may underlie similar EF skills and behavioral performance.
Future neuroscientific investigations of bilingual EF development, still extremely sparse, should further examine centroparietal motor areas alongside frontal and prefrontal areas traditionally studied in the context of EF. This could be particularly useful for disentangling cognitive processes elicited by common behavioral tasks for children. A focus on understanding bilingual neural function and development early in life can help us identify how bilingual experiences shape various trajectories of neurocognitive development.
Funding
Funding for this research was provided by grants from the Boston University Clara Mayo Memorial Fellowship, the Princeton University Frank D. Graham Research Fund, and the Princeton University Anonymous Undergraduate Research Assistance Fund, awarded to S.N.
Declaration of Competing Interest
The authors have no conflicts of interest to declare
Acknowledgments
The authors wish to thank G. Luk for extensive comments, and H. Tager-Flusberg and S. Arunachalam for their input on a previous draft of the manuscript, along with feedback from two anonymous reviewers. The authors additionally acknowledge D. Mittone, S. Ortiz, and all the research assistants and families involved with data collection. The authors also acknowledge I. Weber and G. Stangier’s editorial assistance.
Footnotes
Recall that LRPs are computed by taking into account activation during left-handed and right-handed responses, and here the condition of interest is the incongruent (Stroop) condition.
References
- Antón E., Duñabeitia J.A., Estévez A., Hernández J.A., Castillo A., Fuentes L.J. Is there a bilingual advantage in the ANT task? Evidence from children. Front. Psychol. 2014;5(May) doi: 10.3389/fpsyg.2014.00398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barac R., Moreno S., Bialystok E. Behavioral and electrophysiological differences in executive control between monolingual and bilingual children. Child Dev. 2016;87(4):1277–1290. doi: 10.1111/cdev.12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialystok E., Craik F.I., Green D.W., Gollan T.H. Bilingual minds. Psychol. Sci. Public Interest. 2009;10(3):89–129. doi: 10.1177/1529100610387084. [DOI] [PubMed] [Google Scholar]
- Booth J.R., Burman D.D., Meyer J.R., Lei Z., Trommer B.L., Davenport N.D., Li W., Parrish T.B., Gitelman D.R., Mesulam M.M. Neural development of selective attention and response inhibition. Neuroimage. 2003;20(2):737–751. doi: 10.1016/S1053-8119(03)00404-X. [DOI] [PubMed] [Google Scholar]
- Bryce D., Szűcs D., Soltész F., Whitebread D. The development of inhibitory control: an averaged and single-trial Lateralized Readiness Potential study. Neuroimage. 2011;57(3):671–685. doi: 10.1016/j.neuroimage.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Brydges C.R., Clunies-Ross K., Clohessy M., Lo Z.L., Nguyen A., Rousset C., Whitelaw P., Yeap Y.J., Fox A.M. Dissociable components of cognitive control: an event-related potential (ERP) study of response inhibition and interference suppression. PloS one. 2012;7(3) doi: 10.1371/journal.pone.0034482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge S.A., Dudukovic N.M., Thomason M.E., Vaidya C.J., Gabrieli J.D. Immature frontal lobe contributions to cognitive control in children: evidence from fMRI. Neuron. 2002;33(2):301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson S.M., Meltzoff A.N. Bilingual experience and executive functioning in young children. Dev. Sci. 2008;11(2):282–298. doi: 10.1111/j.1467-7687.2008.00675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles M.G. Modern mind-brain reading: psychophysiology, physiology, and cognition. Psychophysiology. 1989;26(3):251–269. doi: 10.1111/j.1469-8986.1989.tb01916.x. [DOI] [PubMed] [Google Scholar]
- De Jong R., Wierda M., Mulder G., Mulder L.J. Use of partial stimulus information in response processing. J. Exp. Psychol. Hum. Percept. Perform. 1988;14(4):682. doi: 10.1037//0096-1523.14.4.682. [DOI] [PubMed] [Google Scholar]
- Duñabeitia J.A., Carreiras M. The bilingual advantage: acta est fabula? Cortex. 2015;73 doi: 10.1016/j.cortex.2015.06.009. [DOI] [PubMed] [Google Scholar]
- Duñabeitia J.A., Hernández J.A., Antón E., Macizo P., Estévez A., Fuentes L.J., Carreiras M. The inhibitory advantage in bilingual children revisited. Exp. Psychol. 2014;61(3):234–251. doi: 10.1027/1618-3169/a000243. [DOI] [PubMed] [Google Scholar]
- Duncan H.D., Nikelski J., Pilon R., Steffener J., Chertkow H., Phillips N.A. Structural brain differences between monolingual and multilingual patients with mild cognitive impairment and Alzheimer disease: evidence for cognitive reserve. Neuropsychologia. 2018;109:270–282. doi: 10.1016/j.neuropsychologia.2017.12.036. [DOI] [PubMed] [Google Scholar]
- Esposito A.G., Baker-Ward L., Mueller S.T. Interference suppression vs. response inhibition: An explanation for the absence of a bilingual advantage in preschoolers’ Stroop task performance. Cognit. Dev. 2013;28(4):354–363. doi: 10.1016/j.cogdev.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Pentón L., Fernández García Y., Costello B., Duñabeitia J.A., Carreiras M. The neuroanatomy of bilingualism: how to turn a hazy view into the full picture. Lang. Cogn. Neurosci. 2016;31(3):303–327. [Google Scholar]
- Grundy J.G., Anderson J.A., Bialystok E. Neural correlates of cognitive processing in monolinguals and bilinguals. Ann. N. Y. Acad. Sci. 2017;1396:183–201. doi: 10.1111/nyas.13333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez–Clellen V.F., Kreiter J. Understanding child bilingual acquisition using parent and teacher reports. Appl. Psycholinguist. 2003;24(2):267–288. [Google Scholar]
- Jongen E.M., Jonkman L.M. The developmental pattern of stimulus and response interference in a color-object Stroop task: an ERP study. BMC neurosci. 2008;9(1):82. doi: 10.1186/1471-2202-9-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone S.J., Barry R.J., Markovska V., Dimoska A., Clarke A.R. Response inhibition and interference control in children with AD/HD: A visual ERP investigation. Int. J. Psychophysiology. 2009;72(2):145–153. doi: 10.1016/j.ijpsycho.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Kane M.J., Engle R.W. Working-memory capacity and the control of attention: the contributions of goal neglect, response competition, and task set to Stroop interference. J. Exp. Psychol: Gen. 2003;132(1):47. doi: 10.1037/0096-3445.132.1.47. [DOI] [PubMed] [Google Scholar]
- Luk G., Anderson J.A., Craik F.I., Grady C., Bialystok E. Distinct neural correlates for two types of inhibition in bilinguals: response inhibition versus interference suppression. Brain Cogn. 2010;74(3):347–357. doi: 10.1016/j.bandc.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Martin-Rhee M.M., Bialystok E. The development of two types of inhibitory control in monolingual and bilingual children. Biling.: Lang. Cogn. 2008;11(1):81–93. [Google Scholar]
- Marchman V.A., Fernald A., Hurtado N. How vocabulary size in two languages relates to efficiency in spoken word recognition by young Spanish–English bilinguals. J. Child Lang. 2010;37(4):817–840. doi: 10.1017/S0305000909990055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezzacappa E. Alerting, orienting, and executive attention: Developmental properties and sociodemographic correlates in an epidemiological sample of young, urban children. Child development. 2004;75(5):1373–1386. doi: 10.1111/j.1467-8624.2004.00746.x. [DOI] [PubMed] [Google Scholar]
- Mohades S.G., Struys E., Van Schuerbeek P., Baeken C., Van De Craen P., Luypaert R. Age of second language acquisition affects nonverbal conflict processing in children: an fMRI study. Brain Behav. 2014;4(5):626–642. doi: 10.1002/brb3.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohades S.G., Struys E., Van Schuerbeek P., Mondt K., Van De Craen P., Luypaert R. DTI reveals structural differences in white matter tracts between bilingual and monolingual children. Brain Res. 2012;1435:72–80. doi: 10.1016/j.brainres.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Mohades S.G., Van Schuerbeek P., Rosseel Y., Van De Craen P., Luypaert R., Baeken C. White-matter development is different in bilingual and monolingual children: a longitudinal DTI study. PLoS One. 2015;10(2):e0117968. doi: 10.1371/journal.pone.0117968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak, S., Tarullo, A.R. (in press). Error-related negativity (ERNs) and ‘hot’ executive function in bilingual and monolingual preschoolers. Bilingualism: Language and Cognition. PsyArxiv. doi:10.31234/osf.io/yzu23.
- Nayak S., Mittone D., Salem H., Tarullo A.R. “The Fruit Attention Task: A novel method for testing incidental word-object mapping”; Poster presented at the Cognitive Neuroscience Society Annual Meeting; 2016. New York, NY. [Google Scholar]
- Paap K.R. The role of componential analysis, categorical hypothesising, replicability and confirmation bias in testing for bilingual advantages in executive functioning. J. Cogn. Psychol. 2014;26(3) [Google Scholar]
- Paap K.R., Greenberg Z.I. There is no coherent evidence for a bilingual advantage in executive processing. Cogn. Psychol. 2013;66(2):232–258. doi: 10.1016/j.cogpsych.2012.12.002. [DOI] [PubMed] [Google Scholar]
- Paap K.R., Johnson H.A., Sawi O. Bilingual advantages in executive functioning either do not exist or are restricted to very specific and undetermined circumstances. Cortex. 2015;69:265–278. doi: 10.1016/j.cortex.2015.04.014. [DOI] [PubMed] [Google Scholar]
- Pearson B.Z., Fernandez S.C., Lewedeg V., Oller D.K. The relation of input factors to lexical learning by bilingual infants. Appl. Psycholinguist. 1997;18(1):41–58. [Google Scholar]
- Place S., Hoff E. Properties of dual language exposure that influence 2-year-olds’ bilingual proficiency. Child Dev. 2011;82(6):1834–1849. doi: 10.1111/j.1467-8624.2011.01660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J., Melinger A. Bilingual advantage, bidialectal advantage or neither? Comparing performance across three tests of executive function in middle childhood. Dev. Sci. 2017;20(4) doi: 10.1111/desc.12405. [DOI] [PubMed] [Google Scholar]
- Smulders F.T. Simplifying jackknifing of ERPs and getting more out of it: retrieving estimates of participants’ latencies. Psychophysiology. 2010;47(2):387–392. doi: 10.1111/j.1469-8986.2009.00934.x. [DOI] [PubMed] [Google Scholar]
- Smulders F.T., Miller J.O. ‘The lateralized readiness potential’. In: Luck S., Kappenman E., editors. The Oxford handbook of event-related potential components. 2012. pp. 209–229. [Google Scholar]
- Szűcs D., Soltész F., Bryce D., Whitebread D. Real-time tracking of motor response activation and response competition in a Stroop task in young children: a lateralized readiness potential study. J. Cogn. Neurosci. 2009;21(11):2195–2206. doi: 10.1162/jocn.2009.21220. [DOI] [PubMed] [Google Scholar]
- Tarullo A.R., Nayak S., John A.M. Performance Effects of Reward-Related Feedback on the Dimensional Change Card Sort Task. J Genet Psychol. 2018;179(4):171–175. doi: 10.1080/00221325.2018.1466264. [DOI] [PubMed] [Google Scholar]
- Tillman C.M., Wiens S. Behavioral and ERP indices of response conflict in Stroop and flanker tasks. Psychophysiology. 2011;48(10):1405–1411. doi: 10.1111/j.1469-8986.2011.01203.x. [DOI] [PubMed] [Google Scholar]
- Vuillier L., Bryce D., Szücs D., Whitebread D. The maturation of interference suppression and response inhibition: ERP analysis of a cued Go/Nogo task. PloS one. 2016;11(11) doi: 10.1371/journal.pone.0165697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H., Tran D.N., Benitez V., Kuwabara M. Inhibition and adjective learning in bilingual and monolingual children. Front. Psychol. 2011;2:210. doi: 10.3389/fpsyg.2011.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]