Highlights
-
•
At five years old, we start to be able to report that we do not know something.
-
•
Cortical thickness in mOFC linked to childrens' ability to report their ignorance.
-
•
This region was functionally connected to parts of the default-mode network.
-
•
The default-mode network might support the development of metacognitive ability.
Keywords: Metacognition, Brain structure, Functional connectivity
Abstract
Metacognition plays a pivotal role in human development. The ability to realize that we do not know something, or meta-ignorance, emerges after approximately five years of age. We sought for the brain systems that underlie the developmental emergence of this ability in a preschool sample.
Twenty-four children aged between five and six years answered questions under three conditions. In the critical partial knowledge condition, an experimenter first showed two toys to a child, then announced that she would place one of them in a box, out of sight from the child. The experimenter then asked the child whether she knew which toy was in the box.
Children who gave consistently correct answers to this question (n = 9) showed greater cortical thickness in a cluster within left medial orbitofrontal cortex than children who did not (n = 15). Further, seed-based functional connectivity analyses of the brain during resting state revealed that this region is functionally connected to the medial orbitofrontal gyrus, posterior cingulate gyrus and precuneus, and mid- and inferior temporal gyri.
This finding suggests that the default mode network, critically through its prefrontal regions, supports introspective processing. It leads to the emergence of metacognitive monitoring allowing children to explicitly report their own ignorance.
1. Introduction
Metacognition, or the ability to monitor one’s own mental states and processes, is a crucial cognitive function that enables flexible and adaptive behaviour. It has been shown to be a strong predictor of cognitive development and, in particular, school achievements (Schneider, 2008; Williams et al., 2002). “Metacognition” is a broad term, and has been operationalized through a wide variety of behavioural paradigms. These can be explicit, like reporting one’s own memory (Chua et al., 2014), perception (Fleming et al., 2010), or focus of attention (Whitmarsh et al., 2014, 2017), or implicit, like the control of attentional resources (Kentridge and Heywood, 2000), error monitoring (Charles et al., 2013) or allocation of study time (Son and Metcalfe, 2000). Partially different regions within the prefrontal cortex (PFC) have been found to support different aspects of metacognitive monitoring (Dehaene et al., 2017; Fleming and Dolan, 2012).
From a developmental point of view, two core questions remain unanswered. First, what is the specific role of frontal regions and how do they relate to other structures? Second, how does the brain’s monitoring ability develop? Recent brain imaging experiments on adult volunteers (e.g. Baird et al., 2013; Fleming et al., 2010; McCurdy et al., 2013) aimed at answering the first question, whereas behavioural experiments in developing populations aimed at answering the second (e.g. Balcomb and Gerken, 2008; Goupil et al., 2016; Kim et al., 2016; Rohwer et al., 2012; Vo et al., 2014). As a result, these two questions have been largely studied independently of one another. We aimed at bridging these two research approaches to characterize the neural correlates of the emergence metacognitive monitoring in early childhood.
To the best of our knowledge, only one recent study investigated the neural bases of metacognition in a developmental sample. Fandakova et al. (2017) related longitudinal changes in cortical structure with changes in meta-memory ability in the transition from late childhood into adolescence (7- to 12-year-old children). They found that, as in adults, the PFC already plays a crucial role in metacognitive monitoring ability, but whether these same neural networks support the emergence of metacognitive abilities in early childhood remains unknown. Our study attempts to address this question.
In what follows, we first briefly review existing behavioural results on the emergence of metacognition before turning to the task that we employed in this study.
1.1. When do metacognitive abilities develop?
Different aspects of metacognitive monitoring have been shown to develop at different ages. A particularly useful distinction is between implicit metacognition —measured through its effect on behaviour, potentially present from early on— and explicit metacognition, measured through the accuracy of explicit verbal judgements, emerging in the preschool years (Proust, 2013). For example, infants persist in their answers for a longer time after correct than after incorrect choices by 12 months and can regulate their waiting times for a reward in a manner that corresponds to their probability of being correct by 18 months (Goupil and Kouider, 2016). Moreover, Kim et al. (2016) showed that 3- and 4-year olds are able to recognize that they do not have a piece of information by choosing not to inform a third person about it. Crucially however, when Kim et al. explicitly asked the same children whether they themselves had this piece of information, children often (incorrectly) said that they did. It has been suggested that these processes are based on data-driven cues during the learning or performance processes itself (Koriat et al., 2008; Proust, 2013).
Explicit metacognition, on the other hand, refers to our ability to reflect on our cognitive processes and state our (lack of) knowledge. For example, the famous Socratic paradox “I know that I know nothing” is a prototypical explicit metacognitive statement. Classical research has shown that young children tend to equate knowing with seeing for both others and themselves, and develop the ability to distinguish between the two concepts in the preschool years (Pratt and Bryant, 1990; Taylor, 1988; Wimmer et al., 1988).
1.2. How do metacognitive abilities develop?
In order to clarify the neurocognitive mechanisms that subserve the emergence of meta-ignorance in early childhood we sought to identify the brain regions supporting it. We used a task developed by Rohwer et al. (2012) to measure meta-ignorance ability, in which children had to evaluate what they knew: An experimenter placed a toy inside a box either in plain sight or out-of-sight from a child and asked her whether she knew, or did not know, which toy was in the box. The experimenter asked this question in three conditions that differed in terms of the epistemic state of the child. Two of these conditions posed no serious challenge for children as young as 2–3 years old. Children this age could answer correctly in situations in which they had either full informational access, or none at all. However, in the key partial knowledge condition children had seen two possible toys but did not see which of them the experimenter had placed in a box. Here, children under 6 years old had great difficulty recognizing their own ignorance. This effect cannot be easily explained by difficulties with language as, instead, children aged 5 can correctly judge the mental states of others in situations of partial informational access (Pillow et al., 2000; Ruffman, 1996).
We tested children in pre-school behaviourally in this task, and related their performance to cortical thickness and functional connectivity measured on the same day. Given the role of the PFC for metacognitive monitoring in late childhood and adolescents (Fandakova et al., 2017), we expected a relationship between cortical thickness in PFC and task performance.
2. Methods
2.1. Participants
For this study we tested children who were participating in the first wave of an ongoing longitudinal study (Brod et al., 2017). Twenty-four 5- and 6-year olds (mean age (±SD): 5.49 ± 0.4, 14 boys) were included. Children did a meta-ignorance task and a cognitive battery (see below). We tested five additional children but excluded them from analyses due to missing data in one or more of the tasks from the cognitive battery: One child did not do the working memory task, one did not do the cognitive control task, two did not understand or complete the reasoning task, and one did not answer to both repetitions of the partial knowledge.
The HippoKID study aimed at studying the effect of schooling on cognitive development and followed five-year old pre-schoolers longitudinally over two years. Children were recruited through advertisements in kindergartens, newspapers, and Internet forums for parents. Participating children received an honorarium of €10 per hour. All were native German speakers, had no history of psychiatric or neurological disorders or developmental delays (based on parental report), and were born with more than 37 weeks of gestational age. Most children belonged to families with high socioeconomic status.
The testing session lasted approximately 90 min, included cognitive testing and approximately 20 min of magnetic resonance imaging (MRI). To prevent any possible anxiety and excessive movement during MR image acquisition, we let children get accustomed to the scanner by spending time inside a mock scanner that looked and sounded exactly like the real one. Further, an experimenter stood next to the children while they lay on the scanner.
The German Psychological Society (DGPs) approved the study and the children’s parents or legal guardians gave written informed consent. Procedures conformed to the Declaration of Helsinki.
2.2. Behavioural tasks
2.2.1. Meta-ignorance task
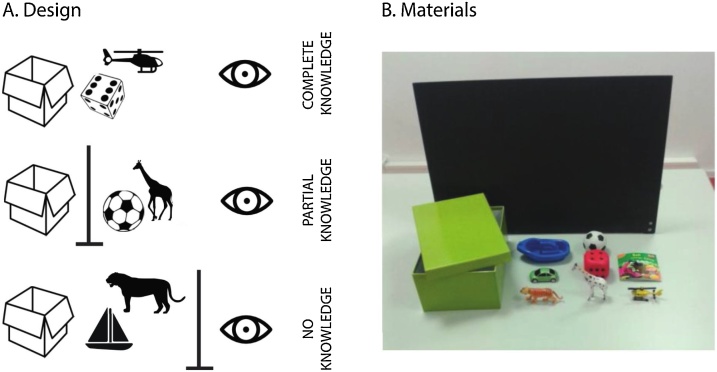
We operationalized explicit metacognition following closely a paradigm developed by Rohwer et al. (2012). The task included three epistemic conditions that differed in terms of how much knowledge a child had about a toy hidden in a box (see Fig. 1A). In all three conditions, the experimenter, sitting across a table from a child, put one of two toys inside a cardboard box, and then asked the child a series of questions about her knowledge. In the complete-knowledge condition, the experimenter first showed two toys to the child and asked her to name them. If she could name them correctly, the experimenter announced that she would place one of the toys in a box with a lid (29 × 18 × 11.5 cm), and did so in plain sight of the child. She then asked the child: “Do you know which toy is in the box, or do you not know?” (In German: “Weißt du, welches Spielzeug in dem Karton ist, oder weißt du es nicht?”). We call this the knowledge question. Depending on the answer, the experimenter asked follow-up questions. If the child said that she knew which toy was in the box, the experimenter asked, first: “O.K., then tell me which toy is in the box”, then the confirmation question: “Do you really know, or are you guessing?” (“Weißt du das wirklich, oder rätst nur?”); and finally, “How do you know that the [toy’s name] is in the box?” If, instead, the child said that she did not know which toy was in the box, the experimenter would ask: “Why don’t you know which toy is inside the box?” The procedure for the other two conditions was identical save for what the experimenter showed to the child. In the no-knowledge condition, the experimenter did not show the two toys to the child, before announcing that she would place one of them inside the box, behind the partition screen. Hence, in the no-knowledge condition, the child had seen neither of the toys. In the crucial partial-knowledge condition, the experimenter showed the two toys to the child and asked her to name them. Before placing one of the toys in the box, the experimenter placed a black partition screen (60 × 39 cm) that occluded the child’s view of the toys and box. Thus, in the partial-knowledge condition, the child knew what two toys the experimenter could have put in the box, but did not know which one.
Fig. 1.
Meta-ignorance task. (A.) Task design. In the complete-knowledge condition, children saw an experimenter put one of two toys in a box. In the partial-knowledge condition, children saw two toys and the experimenter put one of them inside a box, behind an occlusion screen (represented in the figure by the vertical line) and out of sight from the child. In the no-knowledge condition, children saw neither the toys nor the experimenter as she put one of them inside a box. The experimenter asked children the same epistemic questions in all conditions. (B.) Materials. Toys used in the task.
All children completed the three tasks twice in a fixed order: complete-, no-, partial-, partial-, no-, complete-knowledge. For each child, the experimenter randomly drew one of four predetermined sequences of toys and followed it. Eight different toys (see Fig. 1B) were available for the two repetitions of the two different conditions (complete- and partial-knowledge) that required two toys each. One child could not name one of the toys, so the experimenter replaced it with an additional toy available. We coded the responses to each of the questions as correct or incorrect. We recorded a video of the testing sessions for all but three children, due to technical problems.
2.2.2. Cognitive control - hearts and flowers task
We operationalized cognitive control using the “hearts and flowers” task (Davidson et al., 2006). The details have been described elsewhere (Brod et al., 2017). Briefly, the task included three conditions with 20 trials each. On each trial of the congruent condition (first block), a heart was displayed for 1.5 s either on the right or left side of a computer screen and children pressed a key with the corresponding right or left hand, on the same side as the displayed heart. The trial ended 2 s after image onset. In the incongruent condition (second block) a flower appeared on either side of the screen, and children pressed a key on the side opposite to the flower. In the mixed condition (third block), hearts and flowers were interleaved and children pressed a key on the same side of a heart but on the opposite side of a flower. This task requires sustained attention, maintenance of task rules in working memory and, in the mixed condition, inhibitory control and cognitive flexibility. Following recommendations for the age range of our sample, we calculated each child’s accuracy as the rate of correct responses in the mixed condition (Diamond et al., 2007; Diamond and Kirkham, 2005).
2.2.3. Working memory
We used the computerized Working Memory Test Battery for Children Aged Five to Twelve Years (AGTB 5–12; Hasselhorn, 2012; Michalczyk et al., 2013), a German standardized tool assessing working memory according to the multicomponent model by Baddeley (1986). We administered two subtests and used their average score as a measure of working memory ability for each child. Each subtest is a span measure with an adaptive testing procedure and consists of ten trials, grouped into five testing blocks of two trials. The first block starts with a two-item sequence and sequence length is adjusted after each response: If the child recalls the presented trial correctly (or incorrectly), the sequence length of the subsequent trial increases (or decreases) by one item. In the remaining four testing blocks, the sequence length is adjusted more conservatively, following two correct (or incorrect) recalls. The minimum span length is two items. If recall is incorrect for only one of the two trials, the span length remains the same. The span score is based on the mean performance in the last four testing blocks. For each correct response, the child receives a score that corresponds to the span length (i.e., the number of items within the presented sequence). For instance, if the child correctly recalls a five-item sequence, she receives five points. A false response is assigned the span length minus one (e.g., incorrect repetition of a five-item sequence results in four points).
Corsi span: Using a sequential presentation format, this task captures the inner scribe of the sketchpad (e.g. Logie, 1995). A smiley face is displayed sequentially in one of nine white squares (950 ms display time, 50 ms inter-stimulus interval), placed pseudo-randomly on a grey background. At the end of each trial, children touch the squares where the smiley was shown, in the same sequential order.
Colour span backwards: This task captures two aspects of the central executive: coordinative complexity of controlling encoding and recall, and selective focus on relevant information. A sequence of filled coloured circles is presented in the centre of the screen for 2 s each. At recall, children reproduce the sequence in reversed order by touching filled coloured circles arranged in a larger circle.
2.2.4. Reasoning ability
We operationalized reasoning ability using the Culture Fair IQ Test (Cattell, 1950). Briefly, the test consists of ten questions where children see a series of images that follow a logical pattern. Because the pattern is never explicitly given, children have to infer it and choose the correct answer (out of five available) that is consistent with the inferred pattern. We considered the number of correct responses to the task.
2.3. MRI data acquisition
Structural data were acquired using a T1-weighted 3-D magnetization-prepared rapid gradient-echo sequence (repetition time =2500 ms, echo time =2500 ms, sagittal slice orientation, spatial resolution = 1 × 1 × 1 mm). T2*-weighted echo-planar images were acquired using a 3-T Siemens TIM Trio MRI scanner with a 12-channel head coil (transverse slice orientation, interleaved ascending scanning direction), field of view = 216 mm, repetition time =2000 ms, echo time =30 ms, 36 slices, slice thickness = 3 mm, matrix = 72 × 72, voxel size = 3 × 3 × 3 mm, distance factor = 10%, 152 volumes).
2.4. MRI data analysis
2.4.1. Preprocessing MRI data with the computational anatomy toolbox (CAT12)
We estimated cortical thickness using surface-based morphometry as implemented in CAT12 (r1278) (Jena University Hospital, Departments of Psychiatry and Neurology, http://www.neuro.uni-jena.de/cat/) running on SPM12 (Wellcome Department for Imaging Neuroscience, London, United Kingdom; http://www.fil.ion.ucl.ac.uk/spm) and Matlab R2016b (The MathWorks, MA, USA). We used an age-adequate tissue probability map (TPM), generated though the average approach of the Template-o-matic toolbox (Wilke et al., 2008) instead of the default TPM for the segmentation, and default parameters otherwise. The data were then affine-registered to the MNI space and a non-linear deformation was applied. The deformation parameters were calculated with classical registration to the existing DARTEL template in MNI space generated from 555 participants from the IXI Dataset (Ashburner, 2007) (http://brain-development.org/ixi-dataset/). We did not correct the data manually, and a check of sample homogeneity revealed no issues. Surface and thickness were then estimated using projection-based thickness estimation methods (Dahnke et al., 2013). Finally, we applied a smoothing kernel of 15 FWHM and submitted the resulting images to statistical analyses.
2.5. Functional connectivity analyses
2.5.1. Preprocessing
We excluded the first five MR images of the series from the functional analyses to ensure steady-state longitudinal magnetization. We used SPM12 to preprocess the remaining images. We first performed slice timing correction and realignment, followed by coregistration between functional images and the individual anatomical T1 images. We then segmented the anatomical images into white matter, gray matter, and cerebrospinal fluid using the same age-adequate TPM as for the structural analyses. We normalized the resulting functional images to the MNI template and applied spatial smoothing with a 6-mm FWHM to improve signal-to-noise ratio. To reduce physiological high-frequency respiratory and cardiac noise and low-frequency drift, we used the REST toolbox (Song et al., 2011) to bandpass-filter (0.01–1 Hz) and detrend the data. We regressed out the signal from white matter and cerebrospinal fluid as well as the motion parameters. Additionally, we calculated the framewise displacement (FD) according to Power et al. (Power et al., 2012). We excluded from the analyses one child who had an FD above the recommended threshold of 0.6.
2.5.2. Functional connectivity and seed-based functional connectivity
To examine connectivity between brain regions using seed-based functional connectivity (FC) as implemented in the REST toolbox, we calculated voxel-wise temporal (Pearson) correlations between a seed based on the structural results (see below) and the whole brain. We then applied Fischer transformations to the individual FC maps, to obtain z-scores to improve normality; and then submitted the z-score maps to a second-level analysis in SPM12, using movement (FD), age, sex, working memory, cognitive control and reasoning ability as covariates. We identified regions showing consistent levels of FC using a one-sample t-test and performed group comparison using a two-sample t-test. Both analyses were FWE corrected with an additional threshold of p < 0.05 at the voxel level, and cluster size >100 voxels.
3. Results
3.1. Behavioural results
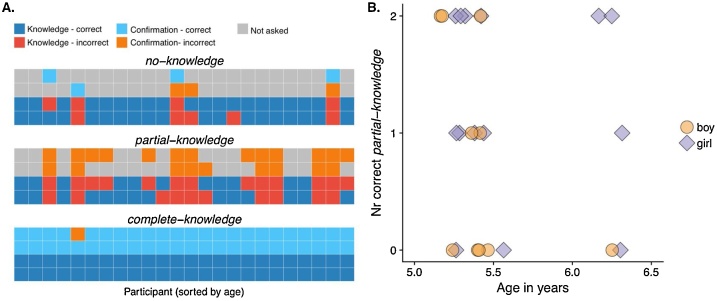
Fig. 2A shows all responses to the knowledge and confirmation questions for each child and each condition. As Rohwer et al. (2012) reported, the complete-knowledge condition posed no challenge for children. Here, all 24 children answered (correctly) in both repetitions of the task that they knew which toy was in the box, but one child answered incorrectly in the confirmation question (i.e., they responded that they guessed the contents of the box, although they had seen the experimenter put the toy in the box). Although the no-knowledge condition was slightly more difficult, most (18 out of 24) children replied correctly to both instances. However, six children incorrectly responded in at least one instance that they knew which of two toys was inside the box, although they had not seen either of the toys. Finally, in the crucial partial-knowledge condition, 15 children responded (incorrectly) in at least one instance that they knew which of the two toys was in the box (and 9 children responded correctly to both instances). The partial-knowledge condition appeared to be the most difficult for children and —in all but one case— those children who responded incorrectly to at least one instance of the no-knowledge condition also responded incorrectly to the partial-knowledge condition.
Fig. 2.
Behavioural results. (A.) All conditions: Responses to each of the six questions (two per condition) for each child, for both the knowledge question and the first follow up (confirmation) question. Each child’s responses are arranged vertically, children are sorted by age (increasing towards the right). The colours code for response accuracy for each of the knowledge and confirmation questions, as it is indicated in the legend. (B.) Partial-knowledge condition: Number of correct responses to the partial knowledge condition as a function of age.
We studied the effect of age on performance in the partial knowledge task (Fig. 2B) using a logistic regression. We found no significant effect of age (χ2(1) = 0.529, p = 0.467. In fact, the evidence favoured the null hypothesis of no effect of age, as estimated by a Bayes Factor with a standard Cauchy prior (BF10 = 0.267).
3.2. Structural correlates of explicit metacognition
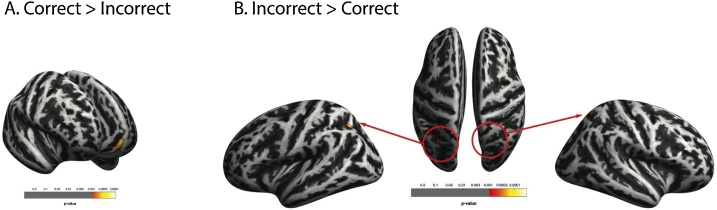
To identify brain structures that support meta-ignorance, we compared cortical thickness in the whole brain between two groups of children: those who responded correctly to both trials of the partial-knowledge condition and those who did not (similar to Wimmer and Perner, 1983). In a multiple regression, we looked for differences between the groups while accounting for age (in months), sex, working memory, cognitive control, and reasoning scores as covariates of no interest. After correction for non-stationary smoothness and expected cluster size, the group comparison revealed a positive effect in a single cluster spanning medial (mOFC, 79%) and superior orbitofrontal cortex (21%) (p = 0.00021, cluster extent k = 61, see Fig. 3A). Further, two small but bilateral clusters showed a negative effect on superior parietal cortex (left: p = 0.00011, k = 30, 87% superior parietal, 13% inferior parietal; right: p = 0.00028, k = 15, 100% superior parietal, see Fig. 3B).
Fig. 3.
Group differences in cortical thickness (correct vs. incorrect responses in the partial-knowledge condition). Accounting for sex, age, working memory, cognitive control, and reasoning, the group that responded correctly showed greater cortical thickness in a region within the left mid- and superior orbitofrontal cortex (A.), and thinner cortex in bilateral superior parietal cortex (B.), as compared to the group with at least one incorrect response.
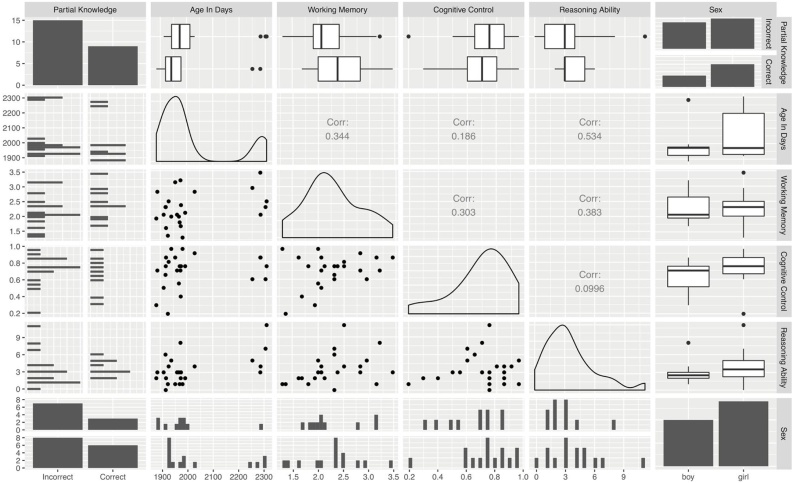
The appropriate way to control for potential effects of the covariates of no interest is, as we did above, to include them in the multiple regression analysis. But this approach is obscure, in that it is not clear what effect, if any, these additional regressors have on the results. To try to reveal these potential effects, we additionally explored potential relationships between performance in the metacognitive task and the other behavioural measures that we considered (see Fig. 4). In a series of tests, we found no differences between groups in working memory ability (t(17.2) = 1.14, p = 0.27, BF10 = 0.60), cognitive control (t(15.62) = -0.51, p = 0.62, BF10 = 0.42) or reasoning ability (t(21.06) = 0.29, p = 0.77, BF10 = 0.39). We also found no differences between the two groups in terms of age in days (t(16.14) = -0.35, p = 0.72, BF10 = 0.4) or sex (X2(1) = 0.04, p = 0.83, BF10 = 0.57). The two groups also did not differ in their tendency to move inside the scanner (measured as mean framewise displacement during resting state; t(16.14) = -0.36, p = 0.73, BF10 = 0.4). Hence, our effects are specific to metacognitive ability and cannot easily be explained by any of the other covariates included in the model.
Fig. 4.
Relationships between all variables considered in the multiple regression model. We isolated the differences related to performance in the partial- knowledge condition whilst controlling for age, sex, working memory ability, cognitive control, and reasoning ability. The figure shows the relationships between all these variables. There was no association between our variable of interest and covariates of no interest, suggesting that the effect is specific to metacognitive performance.
3.3. Seed-based functional connectivity results
Structural analyses revealed that a region in the left mOFC region is involved in the accurate processing of meta-ignorance. To better understand the role of this region, we measured seed-based connectivity during resting state, which infers the functional network between a region of interested given by a specific seed and all voxels in the brain. We calculated whole brain functional connectivity z-maps (based on Pearson’s correlations) for each child using a 10 mm-radius seed centred on (x = −8, y = 53, z = −1) and regressing out FD as a measure of movement (Power et al., 2012). We ran two exploratory analyses on the functional connectivity data. In the first analysis we compared the two groups to determine whether differences in functional connectivity during resting state could explain the behavioural results. In the second analysis we ran a one-sample t-test (across both groups) to identify the network that the frontal region we identified in structural analyses is part of. As in the analysis of brain structure, we used age (in months), sex, working memory, cognitive control, and reasoning ability scores as covariates of no interest in the statistical models.
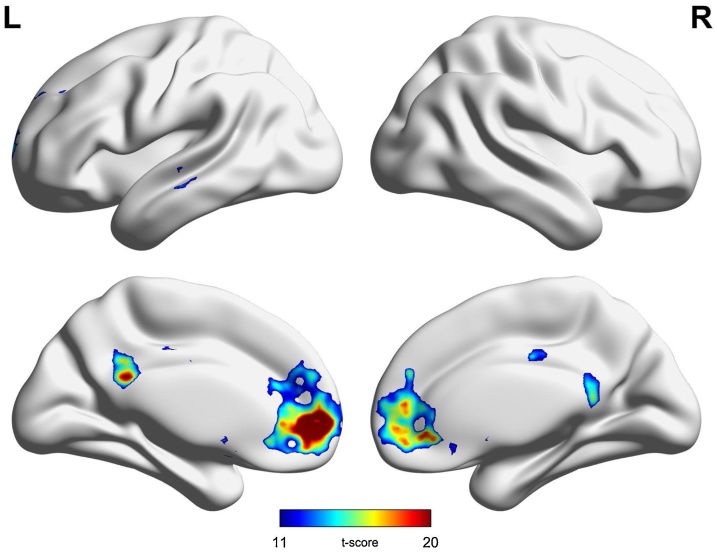
The comparison between groups yielded no significant results (i.e., no cluster of voxels survived our corrections for multiple comparisons consisting of FWE correction, p < 0.05 at the voxel level, and cluster size >100 voxels). Instead, our group analysis showed statistically significant (i.e., consistent) functional connectivity between the prefrontal seed and left medial orbitofrontal gyrus, left posterior cingulate gyrus and right precuneus, and mid- and inferior temporal gyri (see Table 1 and Fig. 5).
Table 1.
Seed-based whole brain voxel functional connectivity results for the whole sample.
| Peak MNI Coordinates |
t-score | Cluster size (voxels) | |||
|---|---|---|---|---|---|
| x | y | z | |||
| Left medial orbitofrontal cortex | −2 | 52 | −6 | 30 | 4676 |
| Left anterior cingulate gyrus | −12 | 48 | 0 | 30 | |
| Left medial frontal gyrus | −10 | 58 | 2 | 28 | |
| Left posterior cingulate gyrus | −4 | −42 | 24 | 21 | 919 |
| −10 | −46 | 30 | 17 | ||
| Right precuneus | 4 | −48 | 14 | 16 | |
| Left middle temporal gyrus | −60 | −20 | −6 | 17 | 189 |
| −56 | −28 | −8 | 13 | ||
| Left inferior temporal gyrus | −52 | −22 | −20 | 13 | |
Fig. 5.
Seed-based functional connectivity. A 10mm-radius seed based on the results from the structural analyses and the whole brain revealed consistent fronto-temporal fronto-cingulate connections for the whole group.
4. Discussion
We investigated the neural mechanisms relating to the emergence of explicit metacognition in preschool children by means of MRI. We concentrated on a “meta-ignorance” task, where children are required to recognize —and explicitly report— that they lack certain information. Previous results had shown that children develop this ability at around 5−6 years of age (Kloo et al., 2017; Rohwer et al., 2012). Our structural analyses revealed that a cortical region within the medial prefrontal cortex was thicker in those children that correctly identified that they did not know something. Our functional resting state analyses, in turn, revealed that this region is connected to the left medial orbitofrontal gyrus, left posterior cingulate gyrus and right precuneus, and mid- and inferior temporal gyri, which are regions belonging to the default-mode network (Deco et al., 2011). These results highlight the neural networks that give rise to the emergence of explicit metacognition in early childhood. In the following sections, we bring our results in the context of the existing literature in both children and adults.
4.1. Behavioural results
In their original study, Rohwer et al. (2012) used a cross-sectional design to test 2–7 year-old children’s performance in the meta-ignorance task. In their sample, the strongest differences in performance in the partial-knowledge condition occurred between 5 and 6 years-old children. In line with these results, we found that under half (9 out of 24) of the children aged 5–6 responded correctly to both repetitions in the partial-knowledge condition, suggesting that this is a critical transition age. However, we did not find a positive relationship between performance and age, but we note that we tested children within a narrower age range than Rohwer et al. Overall, this demonstrates that we successfully implemented the task and allows us to interpret the results of our structural and connectivity analyses.
4.2. Structural results
We considered that a child is proficient in the meta-ignorance task if she could answer correctly to both instances of the partial-knowledge condition (similar to Wimmer and Perner, 1983). Nine out of 24 children could answer correctly to the two instances of this condition. We exploited this fact to build two groups: those that answered correctly to the meta-ignorance task and those who did not. The group of children who answered correctly showed significantly greater estimates of cortical thickness within left orbitofrontal cortex, even when accounting for other higher-order cognitive abilities like working memory, reasoning and cognitive control, as well as sex and age. Further, children who responded correctly to the task showed two small, bilateral clusters in the superior parietal cortex with significantly smaller cortical thickness estimates. Both frontal and parietal regions have been described previously as relevant for performance monitoring and the formation of confidence (for a review, see Chua et al., 2014). Our study adds to previous research by demonstrating that these regions also subserve the emergence of metacognition. Here we discuss the two regions separately.
The cluster in mOFC is consistent with previous studies showing that structural parameters within the prefrontal cortex are associated with metacognitive ability in adults (Bertrand et al., 2018; Cul et al., 2009; Filevich et al., 2015; Fleming et al., 2010; McCurdy et al., 2013; Schnyer et al., 2004). Moreover, the direction of the effect (namely thicker cortex associated with better cognitive performance) is in line with neurodevelopmental trajectory studies showing that thickness increases in children with the age of our sample and peaks only later —at around 8 years of age— over the whole cortex (Raznahan et al., 2011) and in frontal regions specifically (Shaw et al., 2008). In particular, a recent study (Fandakova et al., 2017) on meta-memory found that cortical thickening in the ventromedial PFC predicted metacognitive improvements in 7–12 years-old children.
To the best of our knowledge, we are the first to report a negative relationship between metacognitive ability and structural cortical characteristics in the superior parietal cortex. The contribution of neural activity in primate parietal regions to decision confidence is well established (e.g., Kiani and Shadlen, 2009). In humans, BOLD (blood-oxygen-level dependent) signal levels in parietal cortex vary with reported confidence, In particular, Chua et al. (2014) recently noted that whereas inferior parietal cortex is often associated with high vs. low confidence, superior parietal cortex shows the inverse effect, i.e. higher BOLD signal levels in low confidence trials (Hayes et al., 2011; Kim and Cabeza, 2007, 2009; Moritz et al., 2006). Based on these results, Chua et al. hypothesised distinct roles of superior and inferior parietal cortex in confidence formation.
The negative relationship that we found between superior parietal cortex and meta-ignorance ability is in line with the hypothesis put forward by Chua et al. (2014). However, we should note that a link between functional results and structural characteristics is equivocal and should be re-examined in future studies, possibly by including both functional and structural measures in order to test their links to meta-ignorance ability explicitly.
4.3. How does metacognition develop?
Two main hypotheses have been put forward to explain the emergence of explicit metacognitive ability. The “Simulation theory” proposes that children learn to recognize their own mental states by building on the neural systems that monitor the mental states of others (i.e., theory of mind (ToM) e.g Goldman, 2006; Harris, 1992; Lecce et al., 2015; Lockl and Schneider, 2007). A different notion, stemming from the so called theory-theory suggests instead that children rely on lay theories and rules that are applied to self and other in order to understand mental states (e.g. Gopnik and Wellman, 1994; Perner, 1991) and that, in the way of Bayesian observers, children inform and narrow their priors as they learn to understand the world (Gopnik and Wellman, 2012). Rohwer et al. (2012) interpreted their results of the partial-knowledge condition in line with the latter notion: they argued that children follow cues to answer metacognitive questions concerning their own knowledge: it will suffice that they can produce a plausible answer to a question (regardless of its accuracy) for children to judge that they know the answer to the question, regardless of whether this answer is correct.
Our experiment was not designed to arbitrate between these two theories, and our data alone can therefore not solve the dispute. Instead, here we take a neurocognitive approach: we rely on existent activation-likelihood estimation (ALE) meta-analyses to compare our results to previous literature on ToM. Meta-analyses from studies in healthy young adults reveal ToM consistent activations across different tasks in the medial PFC, but these are mostly located ventrally to the cluster we identified (Schurz et al., 2014 -c.f. Fig. 5-;van Veluw and Chance, 2014. Further, a meta-analysis identified several regions related to domain-general metacognition distinct from those supporting ToM (Vaccaro and Fleming, 2018). In order to better understand the role of the prefrontal region that we identified through structural analyses, we ran exploratory analyses to examine its functional connectivity pattern, which we describe below.
4.4. Functional connectivity results
We used the cluster identified through structural analyses to calculate seed-based FC to explore consistent intrinsic functional connectivity patterns from the seed to the rest of the brain. In this set of exploratory analyses, we did not find any significant differences in functional connectivity between the two groups of children. In the second analysis we found that the seed in left medial orbitofrontal cortex was functionally connected to left medial orbitofrontal gyrus, left posterior cingulate gyrus and precuneus, and mid- and inferior temporal gyri. This connectivity pattern includes three core regions of the default model network (DMN), namely the medial PFC (mPFC), posterior cingulate cortex/precuneus and lateral temporal cortices and is in line with the literature in including the mOFC in the DMN (Raichle, 2015). We note that, with our comparatively small sample size, we cannot make a strong claim about the network function of the mOFC. Nor do we aim to make general inferences about the composition of the DMN in 5−6 year old children. Instead, we take this result to show that our sample shows roughly the same connectivity as has been described in the literature, validating our interpretation, described below.
BOLD activity within DMN regions is typically associated with self-referential thought and introspection (Davey et al., 2016; Northoff et al., 2006; Qin and Northoff, 2011). And a handful of studies that related functional connectivity during resting state to inter-individual differences in metacognitive ability found that the PFC is both a key component of metacognitive monitoring and connected to regions within the DMN (Barttfeld et al., 2013; Soto et al., 2017; Francis et al., 2017). Thus, we speculate that the mOFC may enable accurate metacognitive monitoring thought its role in the self-referential/self-monitoring network.
In light of this interpretation, one important question is whether the DMN in our developmental sample is analogous to that of adults. Overall, the existing literature indicates that an adult-like DMN architecture is already present and relatively stable at one year of age. In particular, the posterior cingulate and retrosplenial cortices, as well as the mPFC appear to be crucial network hubs, already integrated in the newborn brain (Gao et al., 2009). While brain regions active during rest in very young infants overlap only partially with the adult DMN (Fransson et al., 2007; Gao et al., 2009), functional connections (in particular long-distance) develop rapidly during the first year of age (Gao et al., 2009), and continue to develop at a slower pace for several years after that (Fair et al., 2008; Gao et al., 2011; Khan et al., 2018). In short, because the DMN network continues to mature and develop into adolescence, we caution against committing to an unqualified interpretation of these results. But, to the extent that the main features of the network are already in place at the age of the children in our sample, we are justified in interpreting the functional connectivity results in light of self-reflection and DMN. We stress that this interpretation is speculative, as we did not find a direct connection between task performance and functional connectivity. But our results do suggest that the mOFC is already connected with DMN-related regions at a young age. Future studies may directly test this idea.
4.5. Limitations
Several limitations of these results should be mentioned. First, as virtually any empirical study of metacognitive processing, the results cannot a priori be generalized to other metacognitive functions. Just as studies in adults have revealed different neural bases for different metacognitive tasks and suggested some degree of domain-specificity (Baird et al., 2014; Fleming et al., 2014), studies in developmental populations have revealed that different aspects of metacognitive monitoring develop at different developmental stages (Goupil and Kouider, 2016; Goupil et al., 2016; S. Kim et al., 2016). An interesting question for future studies could be whether inter-individual differences between these different aspects are stable over development.
We now consider three potential objections to the experimental paradigm. First, could these results be explained by the parsimonious interpretation that children had a general bias against admitting their own ignorance, or that they did not understand the terms “knowing” and “guessing”? The results of the no-knowledge condition argue against this explanation: If any of these two scenarios were true, those children that answered incorrectly in the partial-knowledge condition should have also answered incorrectly in the no-knowledge condition (i.e. answering that they knew which toy was in the box in both cases). This was only the case for five out of the 15 children in our sample, so this effect alone cannot explain our results. Further, previous literature suggests that children this age understand the difference between (and spontaneously use) the terms “knowing” and “guessing” without ambiguity (e.g., Moore et al., 1989; Shatz et al., 1983). See Rohwer et al. (2012) for a more detailed argument.
Second, our analysis of children’s behaviour in the meta-ignorance task followed Wimmer and Perner (1983) by treating metacognitive ability as binary: a child can either solve the problem, providing consistently correct answers, or she cannot yet. This allowed us to maximize the contrasts in our analysis. Yet, metacognitive ability is not all-or-none and its development does not finish during childhood. Other paradigms that quantify metacognitive ability on a continuous scale offer finer grained information (Geurten et al., 2015; Hembacher and Ghetti, 2014; Paulus et al., 2014; Weil et al., 2013; see also Ghetti et al., 2013for a review). Future studies may consider these paradigms in early developmental samples like ours to draw connections between the developmental trajectory or metacognitive ability and its neural bases.
A final limitation of these results is our relatively small sample size. We nevertheless note that our cortical thickness results are in line with previous studies and consistent internally, with our functional connectivity analyses. All in all, our results go beyond previous literature, and may help constrain and inform future developmental studies.
4.6. Conclusion
Children who answered correctly to a metacognitive task had greater cortical thickness in a cluster within medial prefrontal cortex, compared to children who answered incorrectly to the task. This cluster did not overlap with regions previously identified in adults as supporting theory of mind, a cognitive function thought to be related to —and, under some accounts, be a precursor of— metacognition. Instead, together with our functional connectivity analyses, the complete pattern of results recalls the default-mode network, often associated with self-referential thought and introspection. Our results suggest that children’s metacognitive ability to recognize that they do not know something depends on a mature default-mode network that supports introspective processing.
Declaration of Competing Interest
None.
Acknowledgements
We thank Yana Fandakova for very helpful comments on an earlier version of this manuscript. This work was supported by a Minerva Research Group to YLS from the Max Planck Society. YLS has been funded by the European Union (ERC-2018-StG-PIVOTAL-758898) and a Fellowship from the Jacobs Foundation (JRF 2018–2020). EF and CF are supported by the Volkswagen Foundation (grant number 91620). SK has been funded by two grants from the German Research Foundation (DFG KU 3322/1-1, SFB 936/C7), the European Union (ERC-2016-StG-Self-Control-677804) and a Fellowship from the Jacobs Foundation (JRF 2016-2018). MP has been supported by a Fellowship from the Jacobs Foundation (JRF 2016 1217 12). The design of the study, the collection, analysis, and interpretation of the data, and the writing of the manuscript was the sole responsibility of the authors. We acknowledge support by the German Research Foundation (DFG) and the Open Access Publication Fund of Humboldt-Universität zu Berlin.
Contributor Information
Elisa Filevich, Email: elisa.filevich@bccn-berlin.de.
Caroline Garcia Forlim, Email: c.garcia-forlim@uke.de.
Carmen Fehrman, Email: carmen-fehrmann@gmx.de.
Carina Forster, Email: forsteca@hu-berlin.de.
Markus Paulus, Email: markus.paulus@lmu.de.
Yee Lee Shing, Email: yshing@mpib-berlin.mpg.de.
Simone Kühn, Email: skuehn@uke.de.
References
- Ashburner J. A fast diffeomorphic image registration algorithm. NeuroImage. 2007;38(1):95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Clarendon Press/Oxford University Press; New York, NY, US: 1986. Working Memory. [Google Scholar]
- Baird B., Mrazek M.D., Phillips D.T., Schooler J.W. Domain-specific enhancement of metacognitive ability following meditation training. J. Exp. Psychol. Gen. 2014;143(5):1972–1979. doi: 10.1037/a0036882. [DOI] [PubMed] [Google Scholar]
- Baird B., Smallwood J., Gorgolewski K.J., Margulies D.S. Medial and lateral networks in anterior prefrontal cortex support metacognitive ability for memory and perception. J. Neurosci. 2013;33(42):16657–16665. doi: 10.1523/JNEUROSCI.0786-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balcomb F.K., Gerken L. Three-year-old children can access their own memory to guide responses on a visual matching task. Dev. Sci. 2008;11(5):750–760. doi: 10.1111/j.1467-7687.2008.00725.x. [DOI] [PubMed] [Google Scholar]
- Barttfeld P., Wicker B., McAleer P., Belin P., Cojan Y., Graziano M., Sigman M. Distinct patterns of functional brain connectivity correlate with objective performance and subjective beliefs. Proc. National Acad. Sci. 2013;110(28):11577–11582. doi: 10.1073/pnas.1301353110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand E., Azar M., Rizvi B., Brickman A.M., Huey E.D., Habeck C., Cosentino S. Cortical thickness and metacognition in cognitively diverse older adults. Neuropsychology. 2018;32(6):700–710. doi: 10.1037/neu0000458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brod G., Bunge S.A., Shing Y.L. Does one year of schooling improve children’s cognitive control and alter associated brain activation? Psychol. Sci. 2017;28(7):967–978. doi: 10.1177/0956797617699838. [DOI] [PubMed] [Google Scholar]
- Cattell R.B. Institute for Personality and Ability Testing; 1950. Culture Fair Intelligence Test: Test of" g": Scale 1. [Google Scholar]
- Charles L., Opstal Fvan, Marti S., Dehaene S. Distinct brain mechanisms for conscious versus subliminal error detection. NeuroImage. 2013;73:80–94. doi: 10.1016/j.neuroimage.2013.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua E.F., Pergolizzi D., Weintraub R.R. The cognitive neuroscience of metamemory monitoring: understanding metamemory processes, subjective levels expressed, and metacognitive accuracy. In: Fleming S.M., Frith C.D., editors. The Cognitive Neuroscience of Metacognition. Springer; Berlin Heidelberg: 2014. pp. 267–291.http://link.springer.com/chapter/10.1007/978-3-642-45190-4_12 Retrieved from. [Google Scholar]
- Cul A.D., Dehaene S., Reyes P., Bravo E., Slachevsky A. Causal role of prefrontal cortex in the threshold for access to consciousness. Brain. 2009;132(9):2531–2540. doi: 10.1093/brain/awp111. [DOI] [PubMed] [Google Scholar]
- Dahnke R., Yotter R.A., Gaser C. Cortical thickness and central surface estimation. NeuroImage. 2013;65(Supplement C):336–348. doi: 10.1016/j.neuroimage.2012.09.050. [DOI] [PubMed] [Google Scholar]
- Davey C.G., Pujol J., Harrison B.J. Mapping the self in the brain’s default mode network. NeuroImage. 2016;132:390–397. doi: 10.1016/j.neuroimage.2016.02.022. [DOI] [PubMed] [Google Scholar]
- Davidson M.C., Amso D., Anderson L.C., Diamond A. Development of cognitive control and executive functions from 4 to 13 years: evidence from manipulations of memory, inhibition, and task switching. Neuropsychologia. 2006;44(11):2037–2078. doi: 10.1016/j.neuropsychologia.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deco G., Jirsa V.K., McIntosh A.R. Emerging concepts for the dynamical organization of resting-state activity in the brain. Nat. Rev. Neurosci. 2011;12(1):43–56. doi: 10.1038/nrn2961. [DOI] [PubMed] [Google Scholar]
- Dehaene S., Lau H., Kouider S. What is consciousness, and could machines have it? Science. 2017;358(6362):486–492. doi: 10.1126/science.aan8871. [DOI] [PubMed] [Google Scholar]
- Diamond A., Barnett W.S., Thomas J., Munro S. Preschool program improves cognitive control. Science (New York, N.Y.) 2007;318(5855):1387–1388. doi: 10.1126/science.1151148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A., Kirkham N. Not quite as grown-Up as We like to think: parallels between cognition in childhood and adulthood. Psychol. Sci. 2005;16(4):291–297. doi: 10.1111/j.0956-7976.2005.01530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair D.A., Cohen A.L., Dosenbach N.U.F., Church J.A., Miezin F.M., Barch D.M., Schlaggar B.L. The maturing architecture of the brain’s default network. Proc. National Acad. Sci. 2008;105(10):4028–4032. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fandakova Y., Selmeczy D., Leckey S., Grimm K.J., Wendelken C., Bunge S.A., Ghetti S. Changes in ventromedial prefrontal and insular cortex support the development of metamemory from childhood into adolescence. Proc. National Acad. Sci. 2017;2017:03079. doi: 10.1073/pnas.1703079114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filevich E., Dresler M., Brick T.R., Kühn S. Metacognitive mechanisms underlying lucid dreaming. J. Neurosci. 2015;35(3):1082–1088. doi: 10.1523/JNEUROSCI.3342-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming S.M., Dolan R.J. The neural basis of metacognitive ability. Philos. Trans R. Soc. B: Biol. Sci. 2012;367(1594):1338–1349. doi: 10.1098/rstb.2011.0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming S.M., Ryu J., Golfinos J.G., Blackmon K.E. Domain-specific impairment in metacognitive accuracy following anterior prefrontal lesions. Brain. 2014;221 doi: 10.1093/brain/awu221. awu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming S.M., Weil R.S., Nagy Z., Dolan R.J., Rees G. Relating introspective accuracy to individual differences in brain structure. Science. 2010;329(5998):1541–1543. doi: 10.1126/science.1191883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis M.M., Hummer T.A., Leonhardt B.L., Vohs J.L., Yung M.G., Mehdiyoun N.F., Breier A. Association of medial prefrontal resting state functional connectivity and metacognitive capacity in early phase psychosis. Psychiatry Res. 2017;262:8–14. doi: 10.1016/j.pscychresns.2016.12.014. [DOI] [PubMed] [Google Scholar]
- Fransson P., Skiöld B., Horsch S., Nordell A., Blennow M., Lagercrantz H., Åden U. Resting-state networks in the infant brain. Proc. National Acad. Sci. 2007;104(39):15531–15536. doi: 10.1073/pnas.0704380104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W., Gilmore J.H., Giovanello K.S., Smith J.K., Shen D., Zhu H., Lin W. Temporal and spatial evolution of brain network topology during the first Two years of life. PLOS ONE. 2011;6(9) doi: 10.1371/journal.pone.0025278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W., Zhu H., Giovanello K.S., Smith J.K., Shen D., Gilmore J.H., Lin W. Evidence on the emergence of the brain’s default network from 2-week-old to 2-year-old healthy pediatric subjects. Proc. National Acad. Sci. 2009;106(16):6790–6795. doi: 10.1073/pnas.0811221106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geurten M., Willems S., Meulemans T. Beyond the experience: detection of metamemorial regularities. Conscious. Cogn. 2015;33:16–23. doi: 10.1016/j.concog.2014.11.009. [DOI] [PubMed] [Google Scholar]
- Ghetti S., Hembacher E., Coughlin C.A. Feeling uncertain and acting on It during the preschool years: a metacognitive approach. Child Dev. Perspect. 2013;7(3):160–165. [Google Scholar]
- Goldman A.I. Oxford University Press; 2006. Simulating Minds: The Philosophy, Psychology, and Neuroscience of Mindreading.http://www.oxfordscholarship.com/view/10.1093/0195138929.001.0001/acprof-9780195138924 Retrieved from. [Google Scholar]
- Gopnik A., Wellman H.M. The theory theory. In: Hirschfeld L.A., Gelman S.A., editors. Mapping the Mind: Domain Specificity in Cognition and Culture. Cambridge University Press; New York, NY, US: 1994. pp. 257–293. [Google Scholar]
- Gopnik A., Wellman H.M. Reconstructing constructivism: causal models, bayesian learning mechanisms, and the theory theory. Psychol. Bull. 2012;138(6):1085. doi: 10.1037/a0028044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goupil L., Kouider S. Behavioral and neural indices of metacognitive sensitivity in preverbal infants. Curr. Biol. 2016;26(22):3038–3045. doi: 10.1016/j.cub.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goupil L., Romand-Monnier M., Kouider S. Infants ask for help when they know they don’t know. Proc. National Acad. Sci. 2016;113(13):3492–3496. doi: 10.1073/pnas.1515129113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris P.L. From simulation to folk psychology: the case for development. Mind & Language. 1992;7(1-2):120–144. [Google Scholar]
- Hasselhorn M. Hogrefe; 2012. Arbeitsgedächtnistestbatterie für Kinder Von 5 Bis 12 Jahren: AGTB 5-12. [Google Scholar]
- Hayes S.M., Buchler N., Stokes J., Kragel J., Cabeza R. Neural correlates of confidence during item recognition and source memory retrieval: evidence for both dual-process and strength memory theories. J. Cognit. Neurosci. 2011;23(12):3959–3971. doi: 10.1162/jocn_a_00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hembacher E., Ghetti S. Don’t look at my answer: subjective uncertainty underlies preschoolers’ exclusion of their least accurate memories. Psychol. Sci. 2014;25(9):1768–1776. doi: 10.1177/0956797614542273. [DOI] [PubMed] [Google Scholar]
- Kentridge R.W., Heywood C.A. Metacognition and awareness. Conscious. Cogn. 2000;9(2):308–312. doi: 10.1006/ccog.2000.0448. [DOI] [PubMed] [Google Scholar]
- Khan S., Hashmi J.A., Mamashli F., Michmizos K., Kitzbichler M.G., Bharadwaj H., Kenet T. Maturation trajectories of cortical resting-state networks depend on the mediating frequency band. NeuroImage. 2018;174:57–68. doi: 10.1016/j.neuroimage.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiani R., Shadlen M.N. Representation of confidence associated with a decision by neurons in the parietal cortex. Science. 2009;324(5928):759–764. doi: 10.1126/science.1169405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Cabeza R. Trusting our memories: dissociating the neural correlates of confidence in veridical versus illusory memories. J. Neurosci. 2007;27(45):12190–12197. doi: 10.1523/JNEUROSCI.3408-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Cabeza R. Common and specific brain regions in high- versus low-confidence recognition memory. Brain Res. 2009;1282:103–113. doi: 10.1016/j.brainres.2009.05.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Paulus M., Sodian B., Proust J. Young children’s sensitivity to their own ignorance in informing others. PLOS ONE. 2016;11(3) doi: 10.1371/journal.pone.0152595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloo D., Rohwer M., Perner J. Direct and indirect admission of ignorance by children. J. Exp. Child Psychol. 2017;159:279–295. doi: 10.1016/j.jecp.2017.02.014. [DOI] [PubMed] [Google Scholar]
- Koriat A., Nussinson R., Bless H., Shaked N. Handbook of Metamemory and Memory. Psychology Press; New York, NY, US: 2008. Information-based and experience-based metacognitive judgments: evidence from subjective confidence; pp. 117–135. [Google Scholar]
- Lecce S., Demicheli P., Zocchi S., Palladino P. the origins of children’s metamemory: the role of theory of mind. J. Exp. Child Psychol. 2015;131:56–72. doi: 10.1016/j.jecp.2014.11.005. [DOI] [PubMed] [Google Scholar]
- Lockl K., Schneider W. Knowledge about the mind: links between theory of mind and later metamemory. Child Dev. 2007;78(1):148–167. doi: 10.1111/j.1467-8624.2007.00990.x. [DOI] [PubMed] [Google Scholar]
- Logie R.H. Psychology Press; 1995. Visuo-Spatial Working Memory. [Google Scholar]
- McCurdy L.Y., Maniscalco B., Metcalfe J., Liu K.Y., Lange F., de P., Lau H. Anatomical coupling between distinct metacognitive systems for memory and visual perception. J. Neurosci. 2013;33(5):1897–1906. doi: 10.1523/JNEUROSCI.1890-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalczyk K., Malstädt N., Worgt M., Könen T., Hasselhorn M. Age differences and measurement invariance of working memory in 5-to 12-year-old children. Eur. J. Psychol. Assess. 2013;29(3):220–229. [Google Scholar]
- Moore C., Bryant D., Furrow D. Mental terms and the development of certainty. Child Dev. 1989;60(1):167–171. [Google Scholar]
- Moritz S., Gläscher J., Sommer T., Büchel C., Braus D.F. Neural correlates of memory confidence. NeuroImage. 2006;33(4):1188–1193. doi: 10.1016/j.neuroimage.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Northoff G., Heinzel A., de Greck M., Bermpohl F., Dobrowolny H., Panksepp J. Self-referential processing in our brain--a meta-analysis of imaging studies on the self. NeuroImage. 2006;31(1):440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Paulus M., Tsalas N., Proust J., Sodian B. Metacognitive monitoring of oneself and others: developmental changes during childhood and adolescence. J. Exp. Child Psychol. 2014;122:153–165. doi: 10.1016/j.jecp.2013.12.011. [DOI] [PubMed] [Google Scholar]
- Perner J. The MIT Press; 1991. Understanding the Representational Mind. [Google Scholar]
- Pratt C., Bryant P. Young children understand that looking leads to knowing (so Long as they are looking into a single barrel) Child Dev. 1990;61(4):973–982. [PubMed] [Google Scholar]
- Proust J. OUP Oxford; 2013. The Philosophy of Metacognition: Mental Agency and Self-Awareness. [Google Scholar]
- Pillow B.H., Hill V., Boyce A., Stein C. Understanding inference as a source of knowledge: children’s ability to evaluate the certainty of deduction, perception, and guessing. Dev. Psychol. 2000;36(2):169. doi: 10.1037//0012-1649.36.2.169. [DOI] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin P., Northoff G. How is our self related to midline regions and the default-mode network? NeuroImage. 2011;57(3):1221–1233. doi: 10.1016/j.neuroimage.2011.05.028. [DOI] [PubMed] [Google Scholar]
- Raichle M.E. The brain’s default Mode network. Annu. Rev. Neurosci. 2015;38(1):433–447. doi: 10.1146/annurev-neuro-071013-014030. [DOI] [PubMed] [Google Scholar]
- Raznahan A., Shaw P., Lalonde F., Stockman M., Wallace G.L., Greenstein D., Giedd J.N. How does your cortex grow? J. Neurosci. 2011;31(19):7174–7177. doi: 10.1523/JNEUROSCI.0054-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohwer M., Kloo D., Perner J. Escape from metaignorance: how children develop an understanding of their own lack of knowledge. Child Dev. 2012;83(6):1869–1883. doi: 10.1111/j.1467-8624.2012.01830.x. [DOI] [PubMed] [Google Scholar]
- Ruffman T. Do children understand the mind by means of simulation or a theory? Evidence from their understanding of inference. Mind & Language. 1996;11(4):388–414. [Google Scholar]
- Schneider W. The development of metacognitive knowledge in children and adolescents: Major trends and implications for education. Mind, Brain, and Edu. 2008;2(3):114–121. [Google Scholar]
- Schnyer D.M., Verfaellie M., Alexander M.P., LaFleche G., Nicholls L., Kaszniak A.W. A role for right medial prefrontal cortex in accurate feeling-of-knowing judgments: evidence from patients with lesions to frontal cortex. Neuropsychologia. 2004;42(7):957–966. doi: 10.1016/j.neuropsychologia.2003.11.020. [DOI] [PubMed] [Google Scholar]
- Schurz M., Radua J., Aichhorn M., Richlan F., Perner J. Fractionating theory of mind: a meta-analysis of functional brain imaging studies. Neurosci. Biobehav. Rev. 2014;42:9–34. doi: 10.1016/j.neubiorev.2014.01.009. [DOI] [PubMed] [Google Scholar]
- Shatz M., Wellman H.M., Silber S. The acquisition of mental verbs: a systematic investigation of the first reference to mental state. Cognition. 1983;14(3):301–321. doi: 10.1016/0010-0277(83)90008-2. [DOI] [PubMed] [Google Scholar]
- Shaw P., Kabani N.J., Lerch J.P., Eckstrand K., Lenroot R., Gogtay N., Rapoport J.L. Neurodevelopmental trajectories of the human cerebral cortex. J. Neurosci. 2008;28(14):3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son L.K., Metcalfe J. Metacognitive and control strategies in study-time allocation. J. Exp. Psychol. Learn. Mem. Cogn. 2000;26(1):204–221. doi: 10.1037//0278-7393.26.1.204. [DOI] [PubMed] [Google Scholar]
- Song X.-W., Dong Z.-Y., Long X.-Y., Li S.-F., Zuo X.-N., Zhu C.-Z., Zang Y.-F. REST: a toolkit for resting-State functional magnetic resonance imaging data processing. PLOS ONE. 2011;6(9) doi: 10.1371/journal.pone.0025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto D., Theodoraki M., Paz-Alonso P.M. How the human brain introspects about one’s own episodes of cognitive control. Cortex. 2017;107:110–120. doi: 10.1016/j.cortex.2017.10.016. [DOI] [PubMed] [Google Scholar]
- Taylor M. Conceptual perspective taking: children’s ability to distinguish what they know from what they see. Child Dev. 1988;59(3):703–718. [PubMed] [Google Scholar]
- Vaccaro A.G., Fleming S.M. Thinking about thinking: a coordinate-based meta-analysis of neuroimaging studies of metacognitive judgements. Brain and Neurosci. Adv. 2018;2 doi: 10.1177/2398212818810591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veluw S.J., Chance S.A. Differentiating between self and others: an ALE meta-analysis of fMRI studies of self-recognition and theory of mind. Brain Imaging and Behav. 2014;8(1):24–38. doi: 10.1007/s11682-013-9266-8. [DOI] [PubMed] [Google Scholar]
- Vo V.A., Li R., Kornell N., Pouget A., Cantlon J.F. Young children bet on their numerical skills: metacognition in the numerical domain. Psychol. Sci. 2014;25(9):1712–1721. doi: 10.1177/0956797614538458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmarsh S., Barendregt H., Schoffelen J.-M., Jensen O. Metacognitive awareness of covert somatosensory attention corresponds to contralateral alpha power. NeuroImage. 2014;85(Part 2):803–809. doi: 10.1016/j.neuroimage.2013.07.031. [DOI] [PubMed] [Google Scholar]
- Whitmarsh S., Oostenveld R., Almeida R., Lundqvist D. Metacognition of attention during tactile discrimination. NeuroImage. 2017;147:121–129. doi: 10.1016/j.neuroimage.2016.11.070. [DOI] [PubMed] [Google Scholar]
- Wilke M., Holland S.K., Altaye M., Gaser C. Template-O-matic: a toolbox for creating customized pediatric templates. NeuroImage. 2008;41(3):903–913. doi: 10.1016/j.neuroimage.2008.02.056. [DOI] [PubMed] [Google Scholar]
- Williams W.M., Blythe T., White N., Li J., Gardner H., Sternberg R.J. Practical intelligence for school: developing metacognitive sources of achievement in adolescence. Dev. Rev. 2002;22(2):162–210. [Google Scholar]
- Wimmer H., Hogrefe G.-J., Perner J. Children’s understanding of informational access as source of knowledge. Child Dev. 1988;59(2):386–396. [Google Scholar]
- Wimmer H., Perner J. Beliefs about beliefs: representation and constraining function of wrong beliefs in young children’s understanding of deception. Cognition. 1983;13(1):103–128. doi: 10.1016/0010-0277(83)90004-5. [DOI] [PubMed] [Google Scholar]