Abstract
Introduction
Skin exposure to ultraviolet radiation (UVR) can cause oxidative stress, particularly in the absence of adequate protective measures or in individuals with a sensitive skin type. Most commonly, protection from UVR entails the use of topical sunscreens. Sunscreens, however, have various limitations. The objective of this study was to evaluate the efficacy and tolerability of an oral food supplement containing a combination of actives with mainly antioxidative properties (vitamins A, C, D3, E, selenium, lycopene, lutein, as well as green tea, polypodium and grape extracts) in the context of photoprotection.
Methods
Photoprotective efficacy was assessed in a 12-week-long, open, prospective and monocentric clinical study with 30 subjects (27 women and 3 men) having a Fitzpatrick skin type I-III and manifesting clinical ageing signs. The study included several visits (14, 28, 56, and 84 days after starting supplement intake), in which photoprotection was evaluated by the measurement of the minimal erythema dose (MED), while the antioxidant capacity of the skin was assessed through ferric reducing antioxidant power (FRAP) and malondialdehyde (MDA) assays. Additionally, several skin parameters (including radiance, elasticity, and moisture) were evaluated. Product evaluation was performed throughout the length of the study by means of a self-assessment questionnaire, and safety was monitored through a self-recording of all observed adverse reactions.
Results
The MED levels increased significantly compared to baseline throughout the study visits, reaching an increase of + 8.1% at T84, p < 0.001. FRAP results also indicated a significant increase in the antioxidant capacity of the skin compared to baseline (+ 22.7% at T84, p < 0.001), while the MDA assay showed a significant decrease in MDA concentration compared to baseline (− 6.4% at T84, p < 0.001) which, in line with the FRAP results, indicated enhanced antioxidative protection of the skin. All assessed skin parameters, including radiance (+ 36.1% at T84, p < 0.001), gross elasticity (+ 13.2% at T84, p < 0.001), net elasticity (+ 28.0% at T84, p < 0.001), and moisture (+ 13.8% at T84, p < 0.001) were also significantly improved. The product was well tolerated as no adverse events were attributed by the investigators to the use of the product. Additionally, the global score obtained from the self-assessment questionnaires provided overwhelmingly positive feedback from the study subjects.
Conclusions
The food supplement evaluated in this study was effective and well-tolerated by the subjects, demonstrating a beneficial effect in terms of photoprotection, enhancing the antioxidative status of the skin and improving general skin condition.
Trial Registration
Retrospectively registered 3rd October 2019, ISRCTN18121679.
Electronic supplementary material
The online version of this article (10.1007/s13555-019-00345-y) contains supplementary material, which is available to authorized users.
Keywords: Antioxidant protection, Clinical study, Oral supplement, Photoprotection, Sunscreen, Ultraviolet radiation
Key Summary Points
| Why Carry Out This Study? |
| Skin exposure to ultraviolet radiation (UVR) can cause oxidative stress, particularly in the absence of adequate protective measures or individuals with a sensitive skin type. Protection from UVR entails the use of topical sunscreens. However, a combined dietary approach to reinforce skin protection could provide a continuous adjunctive measure and could be of great interest. |
| This study aims to assess the efficacy and tolerability of an oral food supplement containing a combination of actives with mainly antioxidative properties. |
| What Was Learned from the Study? |
| The food supplement was effective and well-tolerated, demonstrating a beneficial effect in terms of photoprotection, enhancing the antioxidative status of the skin and improving general skin condition. |
| The outcomes can provide good indications for the protective effects of nutritional interventions in the area of sun protection. |
Introduction
The skin is in continuous contact with the external environment, and because of this, it is daily subjected to several aggressive environmental stressors (e.g. mechanical, chemical, toxic, thermal) as well as to pathogenic microorganisms that affect its physiology. Notably, one of the key environmental agents affecting the skin is solar ultraviolet radiation (UVR) [1]. Excessive UVR exposure has been associated with short-term harmful outcomes such as sunburn (erythema) and photo-immunosuppression [2], but also with long-term consequences such as actinic damage (e.g. actinic keratosis) [3, 4], photo-ageing [5] and skin cancer [6, 7].
The UVR reaching the earth’s surface covers a wide wavelength range (290–400 nm) and is divided into two bands: UVB (290–320 nm) and UVA (320–400 nm). Although UVA has less energy than UVB, it can penetrate deeper into the skin, and its exposure may generate reactive oxygen species (ROS) which can damage DNA through indirect photosensitization reactions [8–11]. Meanwhile, UVB can be absorbed directly by the DNA, forming specific photoproducts such as cyclobutane dimers and 6–4 photoproducts, which cause molecular DNA rearrangements. Since skin irradiation by either UVA or UVB has been shown to modify biological tissues, the International Agency for Research on Cancer (IARC) classified solar UVR as a carcinogen almost three decades ago [12].
Sunscreens are applied topically to protect the skin from solar UVR exposure. It has been shown that the sun protection conferred by these products, measured in sun protection factor (SPF), tends to be overestimated under test conditions compared to real life, as very often the quantity applied in real life is less than the one recommended, and it is difficult to assess whether the product has sufficiently covered the skin area that is going to be exposed [13]. Consequently, a combined dietary approach to reinforce skin protection could provide a continuous adjunctive measure and could be of great interest [14].
Several substances are effective in averting UV-induced skin alterations [15]. Oral agents such as essential micronutrients, vitamins (including vitamins C, D, and D3 — alone or in a mixture), minerals (selenium), polyphenols (including Camellia sinensis, Vitis vinifera L., and Polypodium leucotomos Poir), and carotenoids (including lutein, lycopene and β-carotene), among others, have exhibited photoprotective and anti-photocarcinogenic properties [15, 16]. These constituents display the potential to improve systemic protection not only against UVR but also to the visible and infrared (IR) ranges of sunlight. Skin protection is achieved through different mechanisms that promote antioxidant, anti-inflammatory, and immunomodulatory effects preventing photo-induced carcinogenesis and ageing and improving the appearance and pigmentation of the skin [15].
Vitamins are organic compounds that are involved in skincare and health, displaying antioxidant activity and promoting collagen synthesis, keratinisation, sebum regulation and photoprotection [17]. For instance, vitamin D3, orally administered 1 h after experimental sunburn, has been shown to attenuate the inflammatory response by decreasing pro-inflammatory regulators such as tumour necrosis factor-alpha (TNF-α) and inducible nitric oxide synthase (iNOS) [18–21]. Vitamin C is a well-established antioxidant agent. Skin cells harbour high concentrations of vitamin C [22], to enhance collagen synthesis and provide antioxidant protection against UV-induced photodamage. Vitamin C is also thought to regenerate cutaneous vitamin E from its radical form, and the combination of both vitamins acts synergistically [23]. A combination of vitamins C and E in a double-blind, placebo-controlled study showed that oral treatment reduced sunburn, UV-induced skin damage and cutaneous blood flow when compared to the placebo group [24].
Carotenoids (including lutein, lycopene and β-carotene) are pigments present in a wide variety of vegetables and fruits and humans obtain them through the diet [25]. In vitro and in vivo studies have shown that carotenoids can suppress UVR-mediated reactive oxygen species (ROS) production, and thus prevent photoinactivation of antioxidant proteins, lipid peroxidation, and DNA damage [15, 26]. For instance, it has been demonstrated that when orally supplemented, lutein prompts a significant reduction of the UVA-induced overexpression of several genes [27]. Supplementation with lycopene has been found to decrease ROS production, which in turn protects cellular structures from UVR-induced damage [28]. Regarding β-carotene supplementation, several clinical trials have also shown that it can diminish the intensity of erythema caused by sunlight [29, 30].
Selenium is an essential mineral that increases the activity of various antioxidant enzymes. Some clinical studies propose that selenium supplementation protects the skin against many of the harmful effects of UVB radiation [31].
Polyphenols are plant-derived agents whose photoprotective properties can be linked to their antioxidant, anti-proliferative, anti-carcinogenic and anti-inflammatory properties [32]. For example, green tea polyphenols (GTPs) have been studied extensively in vitro, in vivo and in human studies [33], showing chemo-preventive effects against UVB-induced skin cancer.
Grape seed extract (GSE) is also rich in proanthocyanins. In a 1-year study of Japanese women with chloasma, oral administration of 67 mg of GSE 3 times a day effectively decreased hyperpigmentation, and the extract was shown to be safe and well-tolerated [34].
Finally, Polypodium leucotomos (PL), is a tropical fern plant that has long been used in traditional medicine in Central and South America. PL is rich in polyphenolic compounds, which have been shown to have photoprotective properties inhibiting the UVR-induced generation and release of ROS, which in turn prevents DNA damage while protecting and enhancing the natural antioxidant enzyme systems of the skin [35, 36]. Consequently, PL can be used as an oral adjuvant agent to reduce the adverse effects of UVR exposure by improving the photoprotective capacity of the skin [37].
The goal of this clinical study was to assess the efficacy of a food supplement containing some of these sustances against UV-induced skin damage and oxidative stress.
Methods
Study Design
The present study was an open, prospective and monocentric clinical study, conducted by a contract research organisation (CRO) in San Martino Siccomario (PV) (Italy). The study protocol was approved by the Independent Ethical Committee for Non-Pharmacological Clinical trials of Genova, according to the Italian regulations, on 5 September, 2018 (Record no. E.HU-016-0030.01.005L_2018_2323). Study procedures were carried out following the Declaration of Helsinki, as revised in 2018, and good clinical practice guidelines. Written informed consent was obtained from all patients before enrolment.
Patient Selection
The study enrolled thirty healthy female and male subjects of Caucasian ethnicity, aged between 40 and 65 years with a Fitzpatrick phototype between I and III [38], manifesting clinical ageing signs (chronological-ageing and photo-ageing) and with at least one dark spot on the face. All participants were screened and enrolled under the supervision of a dermatologist, according to protocol inclusion/exclusion criteria.
Sample size was calculated with a two-sided 5% significance level and a power of 80%, considering a 20% variation of the primary endpoints due to both inter-individual human variability and error in the measurement techniques. A sample size of 20 subjects was necessary, given an anticipated dropout rate of 20%.
Subjects were required not to have been recently involved in a similar study, to submit before and after pictures, to use the product during the whole study period, not to vary their daily habits or dietary routine and to use effective contraceptive methods. Additionally, subjects were required to continue using their usual sun protection measures when exposed to the sun.
Exclusion criteria were pregnancy or lactation, allergies or sensitivity to cosmetic products, toiletries, sunscreens, and/or topical drugs, dermatological problems in the test area, positive anamnesis for atopy, use of self-tanning products for at least 1 month before study start or regular use of tanning beds.
Primary and Secondary Endpoints
The primary endpoints concerning photoprotection entailed the assessment of minimal erythema dose (MED) and antioxidant capacity of the skin.
The secondary endpoints confirmed product tolerability and the effect of the product on selected skin parameters related to skin ageing (skin moisturisation, elasticity, radiance and colour of skin dark spot). Furthermore, a subjective evaluation of efficacy and tolerability was performed by the study participants through a self-assessment questionnaire.
Intervention
The study was conducted between October 2018 and January 2019. The starting date of the study was selected to minimize the influence of solar radiation exposure on the parameters evaluated during the study. The investigational product (IP) was an oral food supplement containing VitAox Ultra (Table 1). The intake regime consisted of one capsule per day for 12 weeks, to be taken every morning with plenty of liquid.
Table 1.
Quantitative formula of VitAox Ultra
| Ingredients | Weight/capsule | NRV |
|---|---|---|
| Vitamin C (L-ascorbic acid) | 40 mg | 50% |
| Vitamin E (d-α-tocopherol) | 12 mg | 100% |
| Vitamin D3 (cholecalciferol) | 5 µg | 100% |
| Selenium (sodium selenite) | 41.5 µg | 75.40% |
| Vitamin A (β-carotene) | 800 µg RE | 100% |
| Lycopene | 8 mg | n/a |
| Lutein | 8 mg | n/a |
| Camellia sinensis L. Kuntze extract | 50 mg | n/a |
| Polypodium leucotomos Poir | 480 mg | n/a |
| Vitis Vinifera L. extract | 10.1 mg | n/a |
n/a non-applicable, NRV nutrient reference value [Annex XIII of the Regulation (EU) No. 1169/2011), RE retinol equivalents
Assessment of Minimal Erythema Dose (MED)
A provisional minimal erythema dose (MED) was assessed on the back skin of each study subject up to a week before the primary MED test. The range of UV doses to be applied during the study was established based on the estimated MED (according to laboratory experience, subject SPF testing history, and skin type).
During the primary MED test, six subsites centred around the provisional MED were exposed to incremental UV doses using a 1.15 × geometric progression. The MED was assessed visually, under blind conditions, 20 ± 4 h after UV exposure. MED assessment was carried out in a room with matt neutral wall colour and sufficient illumination conditions (at least 450 lux).
Antioxidant Capacity of the Skin
The antioxidant capacity was determined on the back skin using two standard biochemical methods: ferric reducing antioxidant power (FRAP) and malondialdehyde assay (MDA). Both methods rely on the use of Corneofix® foils (Courage + Khazaka electronic GmbH) to collect different layers of the stratum corneum.
FRAP Assay
The FRAP assay was used as a direct measure of the total reducing power of the skin and as an indirect index of the capability of the skin to resist oxidative damage. The FRAP reaction [39], namely the reduction of the complex TPTZ-Fe(III) into the ferrous form [Fe(II)], was monitored via a colorimetric measurement at 595 nm. The recorded absorbances were compared to an Fe(II) standard curve of known values. The results were directly proportional to the total reducing power of the antioxidant in the reaction mix.
MDA Assay
MDA is a specific biomarker of oxidative stress for lipids, reflecting the state of lipid peroxidation. MDA content was used as an oxidative stress index linked to the lipid peroxide component of skin cells. MDA content was assayed by a colourimetric measurement [40], based on the capability of a chromogen, N methyl 2 phenylindole (NMPI), to react with MDA at 45 °C and acidic pH to produce a stable blue chromophore with an absorption peak between 540 and 590 nm. A calibration curve of growing concentrations of standard MDA was used. The results were expressed as MDA concentration (μM) 4 h and 24 h after UVA exposure. The radiation dose of UVA exposure was 5 J/cm2.
Skin Moisturisation
Measurement of skin moisturisation was performed according to the internationally recognised epidermal capacitance measurement using a Corneometer CM 825 (Courage + Khazaka, electronic GmbH) at five points on the right cheek of each subject [41].
Measurement of Skin Radiance
Skin radiance was measured on the cheek using a spectrophotometer/colourimeter CM 700D (Konica Minolta), by illuminating the skin with diffuse light, generated by the Ulbricht sphere of the colourimeter, which allows the determination of the gloss component by calculating the relationship between directional and diffuse reflection. The method is called 8° gloss value because the sensor measuring the light is 8° to the vertical [42].
Measurement of Colour of Skin Dark Spots (ITA°)
The colour of the skin dark spots was determined through ITA° assessment. ITA° was calculated based on the L* and b* parameter values as follows: ITA° = [arctan(L50)/b*]180/π. The ITA° system classifies the subjects into six skin types, from very light to dark skin (very light > 55° > light > 41° > intermediate > 28° > tan > 10° > brown > − 30° > dark) [43, 44].
Skin Elasticity
Skin elasticity was measured in the same cheek area with a Cutometer® MPA 580 (Courage + Khazaka, electronic GmbH), based on a suction method that mechanically deforms the skin [45]. The device uses negative pressure (450 mbar) so that the skin of the subject is drawn into the aperture of the probe (suction on) for 2 s and released again (suction off). A non-contact optical measuring system determined the penetration depth of the skin. The resistance of the skin to the negative pressure and its ability to return into its original position were displayed as curves (R0 = Uf = penetration depth in mm/time) in real-time during the measurement. Two skin elasticity indices were measured (Fig. 1): (1) R2 (Ua/Uf), gross elasticity or overall elasticity, which represents the ability of re-deformation of the skin to its basal state, and (2) R5 (Ur/Ue), net elasticity which represents the elastic recovery of the skin deformation to its basal state due to its elastic component.
Fig. 1.
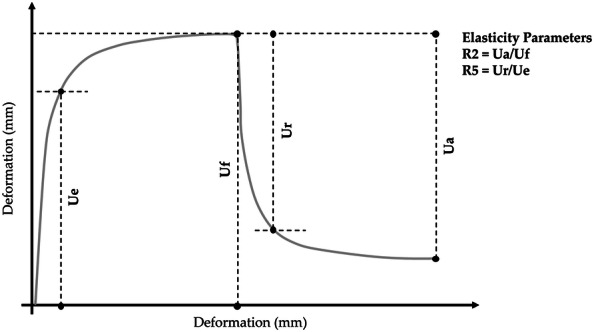
Skin elasticity curve. Graph adapted from the manufacturer’s brochure (https://courage-khazaka.de/images/Downloads/Brochures/Wissenschaftlich/Brochure_Cutometer.pdf)
Subjective Self-Assessment of the Properties of the Product
All study participants evaluated the use of the product by filling out a self-assessment questionnaire starting at week 2 (T14) and during all subsequent study visits [week 4 (T28), 8 (T56) and 12 (T84)]. Subjects were asked several questions to evaluate the ease of use of the product (e.g. size, colour, and taste of the capsules) as well as some skin parameters related to skin ageing (e.g. luminosity, brightness, and flexibility). Participants also had to notify any adverse reactions (see the section below).
Evaluation of Safety and Tolerability of the Product
Subjects were asked to include from the first day of the study, in a personal evaluation form, unpleasant experiences and adverse reactions (AEs) related to the use of product. A dermatologist assessed and recorded all observed AEs and relevant findings to evaluate the safety and tolerability of the product.
Statistical Analyses
Values were expressed as the mean and standard error of the mean (SEM). Intergroup (between treatments) statistical analysis was carried out using multivariate analysis of variance (M-ANOVA) followed by two-way Student’s t-test. A p < 0.05 was considered statistically significant. Statistical analysis output was reported as follows: *p < 0.05 **p < 0.01, and ***p < 0.001.
Results
Subjects
A total of 30 healthy subjects (female and male) were included in the study and completed it. Population characteristics are shown in Table 2. Study subjects attended the clinic at baseline (T0), and after 2 weeks (T14), 4 weeks (T28), 8 weeks (T56) and 12 weeks (T84) of product use. All subjects were included in the safety analysis data set.
Table 2.
Baseline characteristics of subjects
| Units | ||||
|---|---|---|---|---|
| Sex | Male | 2 subjects | 7 | % |
| Female | 28 subjects | 93 | % | |
| Skin phototype (Fitzpatrick*) | I | 3 subjects | 10.0 | % |
| II | 10 subjects | 33.3 | % | |
| III | 17 subjects | 56.7 | % | |
| Age (mean) | 55.6 ± 1.4 | Years | ||
| Minimal erythema dose (MED) | 38.1 | mJ/cm2 | ||
| Skin moisturisation | 37.7 | a.u. | ||
| Skin elasticity (R0 = Uf) | 0.3512 | Ratio | ||
| Skin elasticity (R2 = Ua/Uf) | 0.7137 | Ratio | ||
| Skin elasticity (R5 = Ur/Ue) | 0.2788 | Ratio | ||
| Skin radiance | 8.71 | a.u. | ||
| L values (luminance) | 57.77 | a.u. | ||
| ITA angle | 22.82 | (°) | ||
| MDA (basal) | 1.51 ± 0.01 | µM | ||
| MDA 4 h (after UV exposure) at 0 | 2.62 ± 0.01 | µM | ||
| MDA 24 h (after UV exposure) at 0 | 2.03 ± 0.01 | µM | ||
| FRAP | 160.2 ± 2.3 | Fe(II) µM | ||
*I: always burns on minimal exposure; II: burns easily, tans slightly; III: burns moderately, tans progressively [38]
Minimal Erythema Dose (MED)
A statistically significant increase in the MED threshold compared to baseline (T0) was observed after 14, 28, 56 and 84 days of treatment (Fig. 2). The mean variation in the percentage of MED was + 1.5% at T14 (p < 0.05), + 3.0% at T28 (p < 0.01), + 6.1% at T56 (p < 0.01) and + 8.1% at T84 (p < 0.001), showing a progressive increase of the UV radiation dose necessary to induce erythema.
Fig. 2.
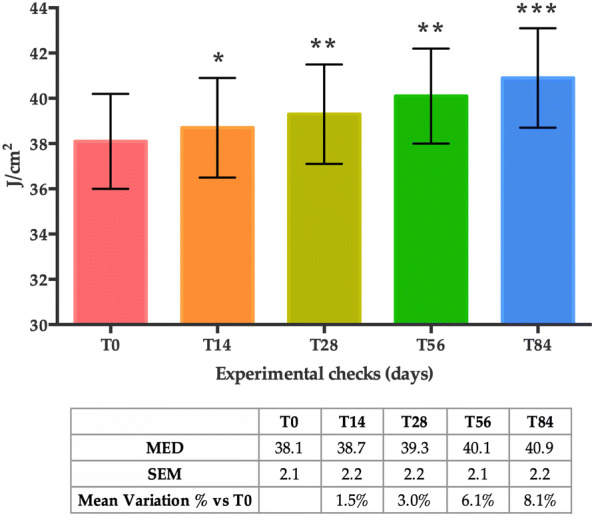
MED score at T0, T14, T28, T56 and T84. Data are reported as the mean value ± SEM in mJ/cm2. Statistical significance was considered at p < 0.05 (*p < 0.05, **p < 0.01, and ***p < 0.001)
The Antioxidant Capacity of the Skin
Ferric Reducing Antioxidant Power (FRAP) Measurement
The mean FRAP values displayed a statistically significant increase after 14, 28, 56 and 84 days of treatment when compared to the baseline (T0) (Fig. 3). The mean variation in the percentage of the FRAP values was + 12.0% at T14, + 13.3% at T28, + 16.5% at T56 and + 22.7% at T84 (all time points, p < 0.001), indicating an increase in the antioxidant capacity of the skin.
Fig. 3.
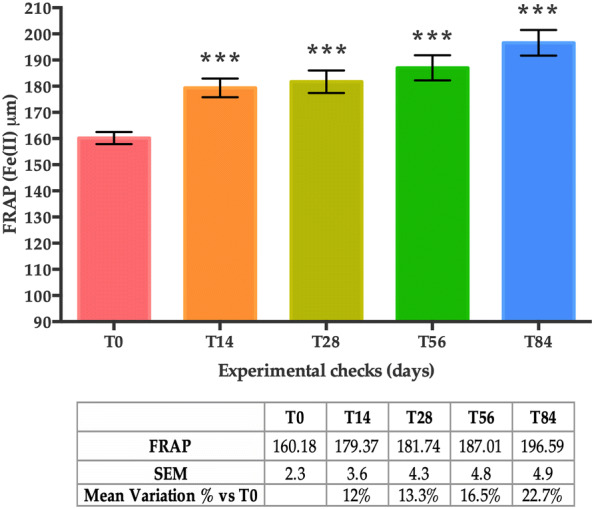
FRAP levels at T0, T14, T28, T56 and T84. Data are presented as the mean of Fe(II) ± SEM in µM. Statistical significance was considered at p < 0.05 (*p < 0.05, **p < 0.01, and ***p < 0.001)
Lipid Peroxidation (LPO) Through Malondialdehyde (MDA) Measurement
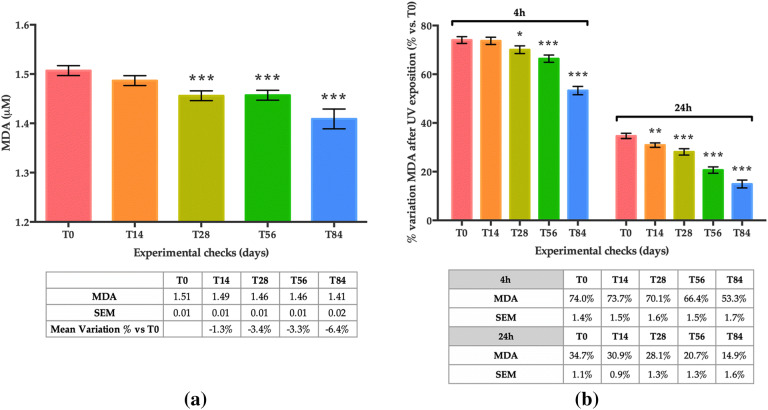
Basal lipid peroxidation (before UVA exposure) as measured by MDA concentration showed a statistically significant decrease at T28 (− 3.4%; p < 0.001), T56 (− 3.3%; p < 0.001), and T84 (− 6.4%; p < 0.001) compared to T0 (Fig. 4a).
Fig. 4.
Lipid-peroxidation (LPO) levels measured by MDA content on the skin cells. a (Basal) — mean concentration of MDA (µM) for the thirty subjects before UVA exposure; b LPO (4 h after UVA exposure) — mean variation in percentage (percentage variation vs baseline (T0) 4 h after UVA exposure) and LPO (24 h) — mean variation in percentage (percentage variation vs baseline (T0) 24 h after UVA exposure). Data are presented as the mean of MDA ± SEM in µM MDA. Statistical significance was considered at p < 0.05 (*p < 0.05, **p < 0.01, and ***p < 0.001)
As expected, in both measurements, 4 h and 24 h after UVA exposure (5 J/cm2), an increase in MDA was observed in all visits (T0 through to T84) compared to measurements before UVA exposure. In Fig. 4b, positive values indicate an increase in MDA concentration expressed as a percentage variation of measurements performed before UVA exposure on the same day.
Nonetheless, compared to previous visits, a smaller increase in MDA concentration was observed at each visit, both 4 h and 24 h after UVA exposure. More specifically, at T0, a 74.0% increase was observed 4 h after UVA exposure, whereas at T84 the increase was 53.3% (p < 0.001) 4 h after UVA irradiation. At 24 h post UVA exposure, a 34.7% increase was recorded at baseline (T0), whereas a 14.9% increase was registered at T84 (p < 0.001). This “reduced rate of increase” is statically significant at T28, T56 and T84 when MDA measurements were performed 4 h after UVA exposure, and at all time points (T14, T28, T56 and T84) when MDA was measured 24 h after irradiation.
Skin Moisturisation
A statistically significant increase in mean skin moisturisation values was achieved after 14 days of treatment when compared to basal conditions (T0). This increase was sustained for the whole of the duration of the study (as measured at T28, T56 and T84) (Fig. 5). Accordingly, the mean variation in the percentage of skin moisturisation compared to T0 was + 9.9% at T14, + 13.5% at T28, + 14.2% at T56 and + 13.8% at T84 (all time points, p < 0.001).
Fig. 5.
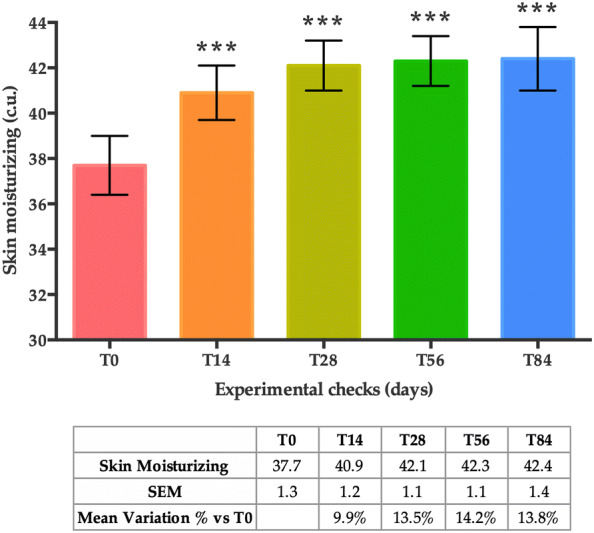
Skin moisturisation at T0, T14, T28, T56 and T84. Data are presented as the mean of analysed skin parameter ± SEM in (c.u.). Statistical significance was considered at p < 0.05 (*p < 0.05, **p < 0.01, and ***p < 0.001)
Skin Radiance
Skin gloss, also referred to as skin radiance, displayed a statistically significant increase after 14, 28, 56 and 84 days of treatment when compared to basal conditions (T0) (Fig. 6). More specifically, the mean variation in the percentage of the skin radiance compared to T0 increased by + 15.2% at T14, + 20.6% at T28, + 30.7% at T56 and + 36.1% at T84 (all time points, p < 0.001).
Fig. 6.
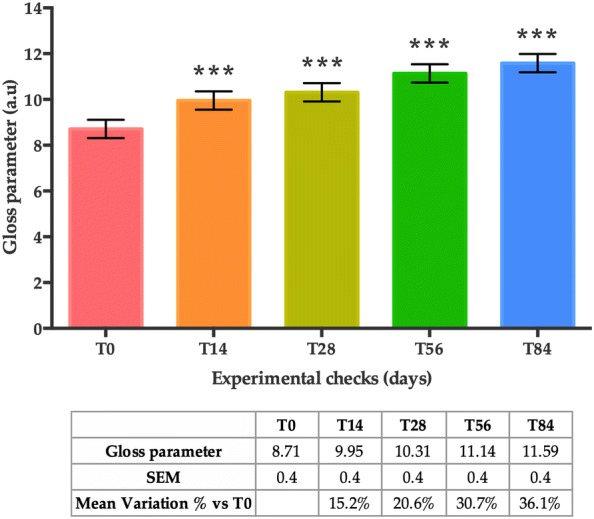
Skin radiance at T0, T14, T28, T56 and T84. Data are presented as the mean of gloss parameter ± SEM in arbitrary units (a.u.). Statistical significance was considered at p < 0.05 (*p < 0.05, **p < 0.01, and ***p < 0.001)
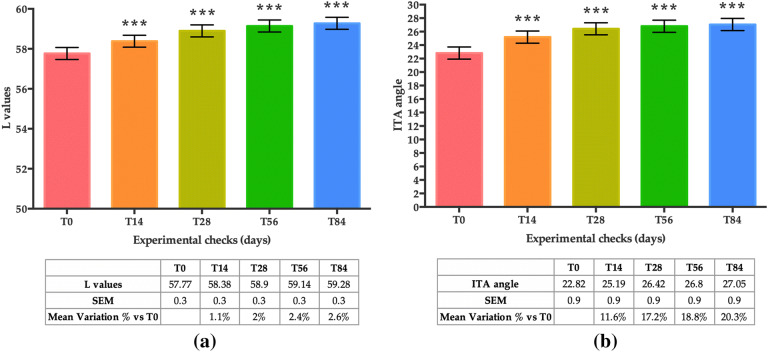
Skin Dark Spots (L and ITA°)
Skin lightness (L), exhibited a statistically significant increase after 14, 28, 56 and 84 days of treatment when compared to basal conditions (T0) (Fig. 7a). More specifically, the mean variation in the percentage of the L values compared to T0 increased by + 1.1% at T14, + 2% at T28, + 2.4% at T56 and + 2.6% at T84 (all time points, p < 0.001).
Fig. 7.
Lightness of the skin. a L values; b ITA° values on an identified brown spot of the subjects. Both graphs show mean values obtained at T0, T14, T28, T56 and T84. Data are represented as the mean of L values ± SEM (in a.u.) and the mean of ITA angle ± SEM in degrees (°), respectively. Statistical significance was considered at p < 0.05 (*p < 0.05, **p < 0.01, and ***p < 0.001)
The mean variation in the percentage of the ITA° (Fig. 7b), measured in the brown spot, displayed an increase of 11.6% at T14, 17.2% at T28, 18.8% at T56, and 20.3% at T84, indicating a lighter skin tone (all time points, p < 0.001).
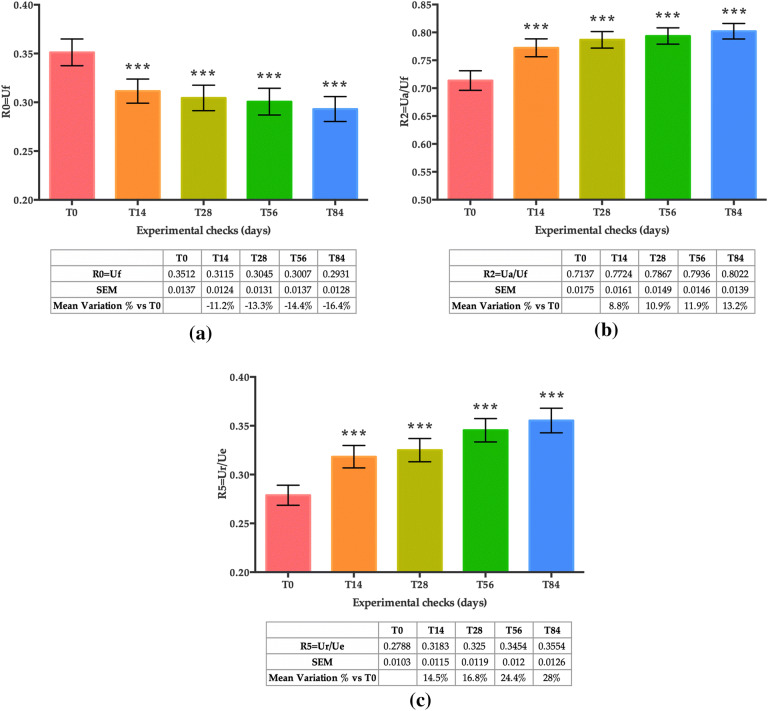
Skin Elasticity
Results showed that the overall skin firmness and elasticity improved in the course of the study (Fig. 8a–c). Skin firmness, measured as R0 (mm/s) with the Cutometer®, significantly increased after 14, 28, 56 and 84 days of treatment compared to basal conditions (T0) (Fig. 8a). The mean skin firmness (R0 = Uf), which is reduced when the skin is firmer, was − 11.2% at T14, − 13.3% at T28, − 14.4% at T56 and − 16.4% at T84 (all time points, p < 0.001).
Fig. 8.
Skin firmness and elasticity measured with the Cutometer®. a R0 parameter — skin firmness; b R2 parameter — gross skin elasticity; c R5 parameter — net skin elasticity. All graphs show mean values obtained at T0, T14, T28, T56 and T84. The data are represented as the mean of Uf ± SEM for R0, Ua/Uf for R2, and Ur/Ue for R5, respectively. Statistical significance was considered at p < 0.05 (*p < 0.05 **p < 0.01, and ***p < 0.001)
Accordingly, both gross elasticity of the skin (Fig. 8b) (R2 parameter, Ua/Uf) and net elasticity (Fig. 8c) (R5 parameter, Ur/Ue) increased after 14, 28, 56 and 84 days of treatment compared to basal conditions (T0). The mean of gross skin elasticity increased by + 8.8% at T14, + 10.9% at T28, + 11.9% at T56 and + 13.2% at T84 (all time points, p < 0.001) and the mean of net skin elasticity increased by + 14.4% at T14, + 16.8% at T28, + 24.4% at T56 and + 28.0% at T84 (all time points, p < 0.001).
Subjective Self-Assessment of the Properties of the Product
The subjective and qualitative evaluation of the efficacy of the product showed that according to study subjects, the use of the product has been beneficial for the skin (Fig. 9: data set available in Table S1). A set of questions was answered by all subjects at all study visits (T14, T28, T56, and T84) using a self-assessment questionnaire form. The score of most of the answers (14/17) continuously improved throughout the length of the study (from T14 to T84).
Fig. 9.
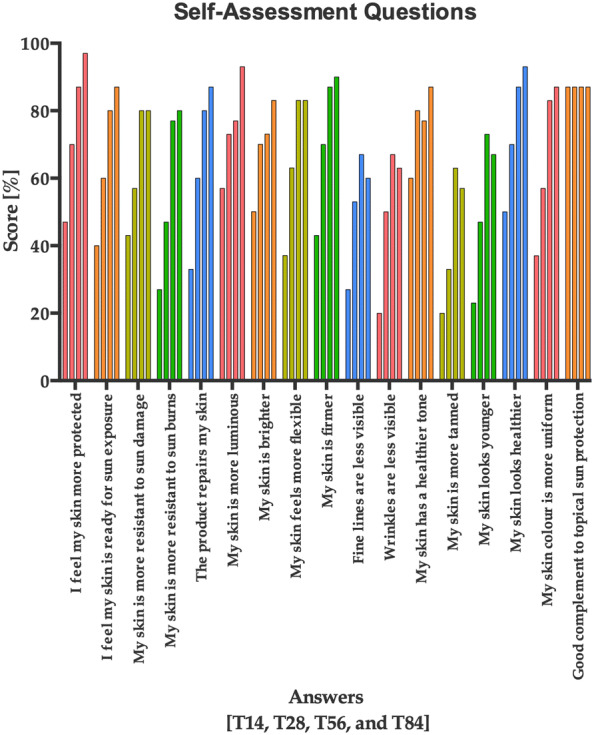
Score in percentage provided by the study subjects on several skin health parameters evaluated throughout the study (T14, T28, T56, and T84). The data are represented as the mean score provided by all the subjects throughout the study length. Each answer has four columns representing the mean of the scores for T14, T28, T56, and T84 (from left to right), respectively
Evaluation of Safety and Tolerability
The study participants reported two adverse events during the length of the study; namely, one reported that the product was difficult to digest, while the second reported slight stomach burns during the last weeks of treatment. However, based on the opinion of the investigators, these adverse events were not clearly attributable to the intake of the product. Therefore, the food supplement was considered well-tolerated.
Discussion
In the quest to provide additional means of increasing sun protection levels for human beings, oral photoprotection may be of significant interest [15, 46].
The study of this food supplement, containing a mix of actives such as carotenoids, vitamins, selenium and phytochemicals, showed that when administered orally for 12 weeks it was capable of increasing the minimal erythema dose (MED), increasing the antioxidant capacity of the skin, and it ameliorated skin parameters related to skin ageing such as skin moisturisation, elasticity, radiance and colour of dark spots.
The MED results showed that oral intake of the product may induce a gradual increase in the amount of UV radiation necessary to induce minimal erythema, and thereby the product may contribute to photoprotection. In comparison with other studies showing significant results for MED after 4 to 12 weeks of supplementation [47, 48] we observed in the present study a modest but statistically significant and progressive impact on MED that was observed as early as after 2 weeks of oral intake.
The FRAP measurement also showed that supplementation with the study product significantly increased the antioxidant capacity of the skin by 12% after 2 weeks (T14) and up to 22.7% after 12 weeks (T84) of product use.
In this study, basal MDA (when no experimental UVA exposure is performed) decreased in consecutive visits, indicating that the process of lipid peroxidation in attenuated. A statistically significant effect has also been observed when looking at MDA levels 4 h and 24 h after experimental UVA exposure. In both cases, the levels of MDA are increased as a result of the UVA exposure, but the per cent increase decreased over consecutive visits, indicating an effect of the food supplement in enhancing the antioxidant capacity of the skin. In comparison with other studies showing significant improvement of hydration, radiance, L*, ITA° and elasticity after 4–25 weeks of oral intake [49, 50], in the present study a statistically significant improvement in these parameters was observed starting after 2 weeks of product use. The skin hydration results show that the food supplement quickly increased skin moisturisation levels, and was able to maintain higher skin hydration during the course of the study. Additionally, skin radiance, L*, ITA° and elasticity continuously improved during the 12 weeks of product use. The product demonstrated a high tolerance since the only two adverse events that were reported by study participants were not serious and not attributable to product intake, based on the opinion of the investigators. Consequently, the treatment was not interrupted by any of the study participants. The methods used to evaluate product tolerability have been previously validated in relevant studies [51–53]. Apart from the two adverse events previously mentioned, all the investigated aspects concerning the product were positively assessed by enrolled subjects.
The design of the study may have certain methodological limitations; for example, most of the subjects in the study were female. Nonetheless, since the molecular, cellular, and tissue-specific events leading to inflammation and photo-ageing are the same among genders, the study outcomes can be extrapolated to the general population. As the product evaluated is a combination of several active ingredients, it is difficult to establish the relevance of each one of the ingredients in the efficacy observed. Even though the present study is an open study (non-randomised study — NRS) with no placebo treatment, outcomes can provide good indications for the protective effects of nutritional interventions in the area of sun protection.
Conclusions
The food supplement evaluated (VitAox Ultra) has been shown to be effective and well-tolerated in increasing minimal erythemal dose (MED), antioxidant capacity of the skin and improving skin parameters related to skin ageing. These positive effects can be explained by an increase in the antioxidant capacity of the skin promoted by the oral intake of this food supplement.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the participants of the study.
Funding
The study and the rapid service fee were funded by Laboratorios ISDIN (Spain).
Medical Writing Assistance
The authors wish to thank Dario Hermida Aponte (SPRIM Health Group) for his contribution in preparing a first draft of the manuscript. This assistance was funded by ISDIN.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published. All authors have participated in the interpretation of the data and in the decision to publish the results.
Disclosures
Sonia Aladrén is an ISDIN employee. Jesús Delgado is an ISDIN employee. Aurora Garre is an ISDIN employee. Corinne Granger, MD is an ISDIN employee. Carles Trullás is an ISDIN employee. Yolanda Gilaberte works at the Dermatology Department of the Hospital Universitario Miguel Servet, Zaragoza, Spain.
Compliance with Ethics Guidelines
The study protocol was approved by the Independent Ethical Committee for Non-Pharmacological Clinical trials of Genova, according to the Italian regulations, on 5 September, 2018 (Record no. E.HU-016-0030.01.005L_2018_2323). Study procedures were carried out following the Declaration of Helsinki, as revised in 2018, and good clinical practice guidelines. Written informed consent was obtained from all patients before enrolment.
Data Availability
The datasets generated during and/or analysed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Footnotes
Enhanced Digital Features
To view enhanced digital features for this article go to 10.6084/m9.figshare.10298486.
References
- 1.Hoel DG, Berwick M, de Gruijl FR, Holick MF. The risks and benefits of sun exposure 2016. Dermatoendocrinol. 2016; 8(1):e1248325. https://linkinghub.elsevier.com/retrieve/pii/S019096221730138X. [DOI] [PMC free article] [PubMed]
- 2.Norval M, Halliday GM. The consequences of UV-induced immunosuppression for human health. Photochem Photobiol. 2011;87:965–977. doi: 10.1111/j.1751-1097.2011.00969.x. [DOI] [PubMed] [Google Scholar]
- 3.Marks R. Nonmelanotic skin cancer and solar keratoses. Int J Dermatol. 1987;26(4):201–205. doi: 10.1111/j.1365-4362.1987.tb00900.x. [DOI] [PubMed] [Google Scholar]
- 4.Bilaç C, Şahin MT, Öztürkcan S. Chronic actinic damage of facial skin. Clin Dermatol. 2014;32(6):752–762. doi: 10.1016/j.clindermatol.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 5.Fisher GJ, Kang S, Varani J, Bata-Csorgo Z, Wan Y, Datta S, et al. Mechanisms of photoaging and chronological skin aging. Arch Dermatol. 2002;138(11):1462–1470. doi: 10.1001/archderm.138.11.1462. [DOI] [PubMed] [Google Scholar]
- 6.Alam M, Ratner D. Cutaneous squamous-cell carcinoma. N Engl J Med. 2001;344(13):975–983. doi: 10.1056/NEJM200103293441306. [DOI] [PubMed] [Google Scholar]
- 7.Morganroth PA, Lim HW, Burnett CT. Ultraviolet radiation and the skin: an in-depth review. Am J Lifestyle Med. 2013;7:168–181. doi: 10.1177/1559827612460499. [DOI] [Google Scholar]
- 8.Adachi H, Murakami Y, Tanaka H, Nakata S. Increase of stratifin triggered by ultraviolet irradiation is possibly related to premature aging of human skin. Exp Dermatol. 2014;23:32–36. doi: 10.1111/exd.12390. [DOI] [PubMed] [Google Scholar]
- 9.Kullavanijaya P, Lim HW. Photoprotection. J Am Acad Dermatol. 2005; 52(6):937–58. https://linkinghub.elsevier.com/retrieve/pii/S0190962204022674. [DOI] [PubMed]
- 10.Mouret S, Baudouin C, Charveron M, Favier A, Cadet J, Douki T. Cyclobutane pyrimidine dimers are predominant DNA lesions in whole human skin exposed to UVA radiation. Proc Natl Acad Sci. 2006;103(37):13765–13770. doi: 10.1073/pnas.0604213103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beard WA, Batra VK, Wilson SH. DNA polymerase structure-based insight on the mutagenic properties of 8-oxoguanine. Vol. 703, Mutation Research - Genetic Toxicology and Environmental Mutagenesis. Elsevier B.V, 2010; pp 18–23. 10.1016/j.mrgentox.2010.07.013. [DOI] [PMC free article] [PubMed]
- 12.Garibyan L, Fisher DE. How sunlight causes melanoma. Curr Oncol Rep. 2010;12:319–326. doi: 10.1007/s11912-010-0119-y. [DOI] [PubMed] [Google Scholar]
- 13.Azurdia RM, Pagliaro JA, Diffey BL, Rhodes LE. Sunscreen application by photosensitive patients is inadequate for protection. Br J Dermatol. 1999;140(2):255–258. doi: 10.1046/j.1365-2133.1999.02658.x. [DOI] [PubMed] [Google Scholar]
- 14.Jansen R, Wang SQ, Burnett M, Osterwalder U, Lim HW. Photoprotection: Part I. Photoprotection by naturally occurring, physical, and systemic agents. J Am Acad Dermatol. 2013;69:853.e1–853.e12. doi: 10.1016/j.jaad.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 15.Parrado C, Philips N, Gilaberte Y, Juarranz A, González S. Oral photoprotection: effective agents and potential candidates. Front Med. 2018;5(June):1–19. doi: 10.3389/fmed.2018.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen AC, Damian DL, Halliday GM. Oral and systemic photoprotection. Photodermatol Photoimmunol Photomed. 2014;30(2–3):102–111. doi: 10.1111/phpp.12100. [DOI] [PubMed] [Google Scholar]
- 17.Shapiro SS, Saliou C. Role of vitamins in skin care. Nutrition. 2001; 17(10):839–44. https://linkinghub.elsevier.com/retrieve/pii/S0899900701006608. [DOI] [PubMed]
- 18.Scott JF, Das LM, Ahsanuddin S, Qiu Y, Binko AM, Traylor ZP, et al. Oral vitamin D rapidly attenuates inflammation from sunburn: an interventional study. J Invest Dermatol. 2017; 137(10):2078–86. https://linkinghub.elsevier.com/retrieve/pii/S0022202X17315580. [DOI] [PMC free article] [PubMed]
- 19.Di Rosa M, Malaguarnera G, De Gregorio C, Palumbo M, Nunnari G, Malaguarnera L. Immuno-modulatory effects of vitamin D3 in human monocyte and macrophages. Cell Immunol. 2012;280(1):36–43. doi: 10.1016/j.cellimm.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 20.Au L, Meisch JP, Das LM, Binko AM, Boxer RS, Wen AM, et al. Suppression of hyperactive immune responses protects against nitrogen mustard injury. J Invest Dermatol. 2015;135(12):2971–2981. doi: 10.1038/jid.2015.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scott JF, Lu KQ. Vitamin D as a therapeutic option for sunburn: clinical and biologic implications. DNA Cell Biol. 2017;36(11):879–882. doi: 10.1089/dna.2017.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pullar JM, Carr AC, Vissers MCM. The roles of vitamin C in skin health. Nutrients. 2017;9(8):866. doi: 10.3390/nu9080866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rizvi S, Raza ST, Ahmed F, Ahmad A, Abbas S, Mahdi F. The role of vitamin E in human health and some diseases. Sultan Qaboos Univ Med J. 2014;14(2):e157–65. http://www.ncbi.nlm.nih.gov/pubmed/24790736. [PMC free article] [PubMed]
- 24.Eberlein-Konig B, Placzek M, Przybilla B. Protective effect against sunburn of combined systemic ascorbic acid (vitamin C) and d-α-tocopherol (vitamin E) J Am Acad Dermatol. 1998;38(1):45–48. doi: 10.1016/S0190-9622(98)70537-7. [DOI] [PubMed] [Google Scholar]
- 25.Bendich A, Olson JA. Biological actions of carotenoids. FASEB J. 1989;3(8):1927–32. https://academic.oup.com/jn/article/119/1/94-95/4738984. [PubMed]
- 26.Stahl W, Sies H. β-Carotene and other carotenoids in protection from sunlight. Am J Clin Nutr. 2012;96(8):1179S–1184S. doi: 10.3945/ajcn.112.034819. [DOI] [PubMed] [Google Scholar]
- 27.Grether-Beck S, Marini A, Jaenicke T, Stahl W, Krutmann J. Molecular evidence that oral supplementation with lycopene or lutein protects human skin against ultraviolet radiation: results from a double-blinded, placebo-controlled, crossover study. Br J Dermatol. 2017;176(5):1231–1240. doi: 10.1111/bjd.15080. [DOI] [PubMed] [Google Scholar]
- 28.Rizwan M, Rodriguez-Blanco I, Harbottle A, Birch-Machin MA, Watson REB, Rhodes LE. Tomato paste rich in lycopene protects against cutaneous photodamage in humans in vivo: a randomized controlled trial. Br J Dermatol. 2011;164(1):154–162. doi: 10.1111/j.1365-2133.2010.10057.x. [DOI] [PubMed] [Google Scholar]
- 29.Gollnick HPM. Systemic beta carotene plus topical UV-sunscreen are an optimal protection against harmful effects of natural UV-sunlight: results of the Berlin-Eilath study. Eur J Dermatol. 1996;6:200–205. [Google Scholar]
- 30.Heinrich U, Gärtner C, Wiebusch M, Eichler O, Sies H, Tronnier H, et al. Supplementation with β-carotene or a similar amount of mixed carotenoids protects humans from UV-induced erythema. J Nutr. 2018;133(1):98–101. doi: 10.1093/jn/133.1.98. [DOI] [PubMed] [Google Scholar]
- 31.Wu X, Cheng J, Wang X. Dietary antioxidants: potential anticancer agents. Nutr Cancer. 2017 doi: 10.1080/01635581.2017.1299872. [DOI] [PubMed] [Google Scholar]
- 32.Matsui MS. The role of topical antioxidants in photoprotection. In: Principles and practice of photoprotection. Cham: Adis; 2016. pp. 361–375.
- 33.Sharma P, Montes de Oca MK, Alkeswani AR, McClees SF, Das T, Elmets CA, et al. Tea polyphenols for the prevention of UVB-induced skin cancer. Photodermatol Photoimmunol Photomed. 2018;34:50–59. doi: 10.1111/phpp.12356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamakoshi J, Sano A, Tokutake S, Saito M, Kikuchi M, Kubota Y, et al. Oral intake of proanthocyanidin-rich extract from grape seeds improves chloasma. Phyther Res. 2004;18(11):895–899. doi: 10.1002/ptr.1537. [DOI] [PubMed] [Google Scholar]
- 35.Gonzalez S, Gilaberte Y, Philips N, Juarranz A. Fernblock, a nutriceutical with photoprotective properties and potential preventive agent for skin photoaging and photoinduced skin cancers. Int J Mol Sci. 2011;12(12):8466–8475. doi: 10.3390/ijms12128466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parrado C, Mascaraque M, Gilaberte Y, Juarranz A, Gonzalez S. Fernblock (Polypodium leucotomos extract): molecular mechanisms and pleiotropic effects in light-related skin conditions, photoaging and skin cancers, a review. Int J Mol Sci. 2016;17(7):1–21. doi: 10.3390/ijms17071026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kohli I, Shafi R, Isedeh P, Griffith JL, Al-Jamal MS, Silpa-archa N, et al. The impact of oral Polypodium leucotomos extract on ultraviolet B response: a human clinical study. J Am Acad Dermatol. 2017 doi: 10.1080/19381980.2016.1248325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124(6):869–871. doi: 10.1001/archderm.1988.01670060015008. [DOI] [PubMed] [Google Scholar]
- 39.Benzie I, Strain J. The ferric reducing ability of plasma as a measure of antioxodant. Anal Biochem. 1996;239(0292):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 40.Erdelmeier I, Gérard-Monnier D, Yadan JC, Chaudière J. Reactions of N-methyl-2-phenylindole with malondialdehyde and 4-hydroxyalkenals. Mechanistic aspects of the colorimetric assay of lipid peroxidation. Chem Res Toxicol. 1998;11(10):1184–1194. doi: 10.1021/tx970180z. [DOI] [PubMed] [Google Scholar]
- 41.Heinrich U, Koop U, Leneveu-Duchemin M, Osterrieder K, Bielfeldt S, Chkarnat C, et al. Multicentre comparison of skin hydration by the capacitive method (Corneometer CM) Int J Cosmet Sci. 2003;25:45–53. doi: 10.1046/j.1467-2494.2003.00172.x. [DOI] [PubMed] [Google Scholar]
- 42.Konica Minolta Sensing Europe BV. The Way Colourimeters See. [Online]. Available from: https://www5.konicaminolta.eu/en/measuring-instruments/learning-centre/colour-measurement/colour/the-way-colourimeters-see.html. Accessed 29 Nov 2019.
- 43.Chaedon A, Cretois I, Hourseau C. Skin colour typology and suntanning pathways. Int J Cosmet Sci. 1991;13(4):191–208. doi: 10.1111/j.1467-2494.1991.tb00561.x. [DOI] [PubMed] [Google Scholar]
- 44.Del Bino S, Bernerd F. Variations in skin colour and the biological consequences of ultraviolet radiation exposure. Br J Dermatol. 2013;169(SUPPL. 3):33–40. doi: 10.1111/bjd.12529. [DOI] [PubMed] [Google Scholar]
- 45.Ohshima H, Kinoshita S, Oyobikawa M, Futagawa M, Takiwaki H, Ishiko A, et al. Use of Cutometer area parameters in evaluating age-related changes in the skin elasticity of the cheek. Ski Res Technol. 2013;19(1):1–5. doi: 10.1111/j.1600-0846.2012.00634.x. [DOI] [PubMed] [Google Scholar]
- 46.D’Orazio J, Jarrett S, Amaro-Ortiz A, Scott T. UV radiation and the skin. Int J Mol Sci. 2013;14:12222–12248. doi: 10.3390/ijms140612222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carrascosa JM, Floriach N, Sala E, Aguilera J. Increase in minimal erythemal dose following oral administration of an antioxidant complex based on a mixture of carotenoids: double-blind placebo-controlled trial. Photodermatol. Photoimmunol Photomed. 2017;33(5):284–286. doi: 10.1111/phpp.12315. [DOI] [PubMed] [Google Scholar]
- 48.Juturu V, Bowman JP, Deshpande J. Overall skin tone and skin-lightening—improving effects with oral supplementation of lutein and zeaxanthin isomers: a double-blind, placebo-controlled clinical trial. Clin Cosmet Invest Dermatol. 2016;9:325–332. doi: 10.2147/CCID.S115519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dumoulin M, Gaudout D, Lemaire B. Clinical effects of an oral supplement rich in antioxidants on skin radiance in women. Clin Cosmet Invest Dermatol. 2016;9:315–324. doi: 10.2147/CCID.S118920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Costa A, Lindmark L, Arruda LH, Assumpçao EC, Ota FS, Pereira Mde O, Langen SS. Clinical, biometric and ultrasound assessment of the effects of daily use of nutraceutical composed by lycopene, acerola extract, grape seed extract and biomarine complex in photoaged human skin. An Bras Dermatol. 2012;87(1):52–61. doi: 10.1590/S0365-05962012000100006. [DOI] [PubMed] [Google Scholar]
- 51.Favrot C, Beal D, Blouin E, Leccia MT, Roussel AM, Rachidi W. Age-dependent protective effect of selenium against UVA irradiation in primary human keratinocytes and the associated DNA repair signature. Oxid Med Cell Longev. 2018;2018:1–9. https://www.hindawi.com/journals/omcl/2018/5895439/. [DOI] [PMC free article] [PubMed]
- 52.Keswick BH, Ertel KD, Visscher MO. Comparison of exaggerated and normal use techniques for assessing the mildness of personal cleansers. J Soc Cosmet Chem. 1992;43(4):187–193. [Google Scholar]
- 53.Edward MJ, Norman FMTR. The controlled use test in a cosmetic product safety substantiation program. Cutan Ocul Toxicol. 1982; 1(2):117–32. http://www.tandfonline.com/doi/full/10.3109/15569528209051517.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are not publicly available but are available from the corresponding author on reasonable request.