Abstract
Introduction
Biologics used to treat moderate-to-severe plaque psoriasis may cause injection site reactions (ISRs) characterized by erythema, edema, itch, and sometimes pain. The Federal Adverse Event Reporting System (FAERS) is a repository of spontaneous post-marketing reports of adverse events (AEs) that are reported to the US Food and Drug Administration (FDA). Our objective was to perform a pharmacovigilance analysis of FAERS reports of ISRs associated with the use of subcutaneously administered biologic products approved to treat moderate-to-severe plaque psoriasis.
Methods
The products included in our assessment were adalimumab, etanercept, ixekizumab, secukinumab, and ustekinumab. Reports from the date of US approval for each biologic as treatment for plaque psoriasis through 2 years were included using the search term “injection site.”
Results
The results show that the FAERS database contained reports of ISRs for all of the included biologics during the 2 years following FDA approval. The most common reports on ISRs were on pain, irritation, and erythema for adalimumab; reaction, pain, and erythema for etanercept; erythema, pain, and reaction for ixekizumab; bruising, pain, hemorrhage for secukinumab; and pain, induration, and swelling for ustekinumab. FAERS does not include data on total patient exposure; therefore, ISR rates could not be calculated.
Conclusions
Specific ISRs varied among the biologic therapies assessed. The findings presented could be helpful when patients consider switching therapies due to ISRs.
Funding
Eli Lilly and Company.
Keywords: Adalimumab, Biologics, Etanercept, FAERS, Injection site reactions, Ixekizumab, Psoriasis, Secukinumab, Ustekinumab
Key Summary Points
| The Federal Adverse Event Reporting System (FAERS) is a US Food and Drug Administration (FDA) spontaneous reporting database that contains adverse event (AE) reports, medication error reports, and product quality complaints resulting in AEs that were reported to the FDA. |
| Biologics used to treat moderate-to-severe plaque psoriasis may cause injection site reactions (ISRs). FAERS contained reports of ISRs for all subcutaneously administered biologics of interest (adalimumab; etanercept; ixekizumab; secukinumab; ustekinumab) during the 2 years after US FDA approval as treatment for moderate-to-severe plaque psoriasis. |
| The specific types of ISRs reported to FAERS varied among the biologics, without any obvious pattern by therapeutic target class. The results for individual drugs may be useful when changes in therapy are being considered due to ISRs. |
Introduction
The introduction of biologic agents has revolutionized the treatment of moderate-to-severe plaque psoriasis. Because biologics are high-molecular-weight proteins, the majority are administered via injection subcutaneously, with few being given intravenously. Biologics that are subcutaneously administered for the treatment of moderate-to-severe plaque psoriasis have the potential to cause injection site reactions (ISRs) [1].
ISRs are localized skin reactions that occur at the injection site and are frequently associated with erythema, edema, itch, and/or pain [1, 2]. In clinical trials and some post-marketing settings, ISRs refer to a grouping of multiple ISR-related preferred terms in the Medical Dictionary for Regulatory Activities (MedDRA; https://www.meddra.org/). Although the specific etiology of each type of ISR may not be well defined for each biological agent and could vary depending on the properties and formulation, as well as patient characteristics, an understanding of the ISRs reported for each biologic may be clinically relevant, particularly when patients are switched between therapies due to ISRs.
The Federal Adverse Event Reporting System (FAERS) is a US Food and Drug Administration (FDA) spontaneous reporting database that contains adverse event (AE) reports, medication error reports, and product quality complaints resulting in AEs that were reported to the FDA [3]. In FAERS, AEs and medication errors are coded using terms listed in MedDRA. Post-marketing reports of AEs that are associated with drugs or biologics are voluntarily collected from drug manufacturers and individuals, including healthcare professionals and consumers. FAERS data can be accessed from the FDA website via FAERS data files [4] and a web-based tool (FAERS Public Dashboard) [5] or from commercial sources. FAERS can be an effective tool for detecting adverse drug reactions for acute events known to be associated with drug exposure. In addition, while information on ISRs is available from reports of clinical studies of biologics, real-world experience outside of the restricted populations and relatively uniform self-injection training of subjects in clinical trials may be particularly informative regarding ISR experiences in general clinical practice.
The purpose of this analysis was to characterize, based on the FAERS database, the proportions of specific ISR terms reported for each subcutaneously administered biologic product approved in the US for the treatment of moderate-to-severe plaque psoriasis.
Methods
Data Source
Data were obtained from FAERS [3, 6], which is a source of post-marketing safety surveillance data for drugs in the USA. Anonymized data were collected from a publicly available database and, therefore, this analysis did not require ethics committee approval.
Study Design
This study is a pharmacovigilance analysis of FAERS data. The two inclusion criteria for biologics were: (1) marketed in the USA for treatment of moderate-to-severe plaque psoriasis for at least 2 consecutive years, and (2) subcutaneous administration. For each biologic assessed, 2 years of data beginning at the date of US approval for the treatment of moderate-to-severe plaque psoriasis were examined. The following biologics with their dates of data collection met the criteria for inclusion into this analysis: adalimumab (22 January 2008 to 22 January 2010), etanercept (30 April 2004 to 30 April 2006), ixekizumab (22 March 2016 to 22 March 2018), secukinumab (21 January 2015 to 21 January 2017), and ustekinumab (25 September 2009 to 25 September 2011). FAERS was searched for suspect cases of ISRs, as identified by MedDRA preferred terms containing the words “injection site”. Results were tabulated for each biologic. The distribution of ISRs for each biologic were calculated using the total number of ISRs reported for each biologic as the denominator, with the caveat that it is possible that multiple types of ISRs could be reported for a single injection administered to one individual.
Results
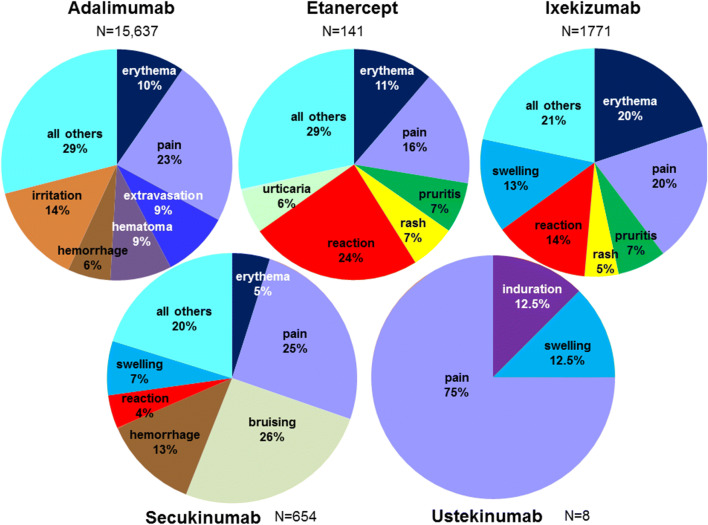
Reports of ISRs in FAERS as listed by MedDRA preferred terms for each indicated biologic are given in Table 1. In FAERS, there were reports of ISRs for adalimumab, etanercept, ixekizumab, secukinumab, and ustekinumab during the first 2 years following their respective US approvals. The total number of ISR-related reports in FAERS for each biologic was 15,637 for adalimumab, 141 for etanercept, 1771 for ixekizumab, 654 for secukinumab, and 8 for ustekinumab. The distributions (%) of the various types of ISRs for each biologic were calculated using the total number of ISRs reported for each biologic as the denominator (Fig. 1). In addition, for each drug, the number of events and the proportion of patients with a given ISR-related AE were calculated (Table 1). The most common types of ISRs reported in FAERS for each biologic were: pain (23.3%), irritation (14.1%), and erythema (9.6%) for adalimumab; reaction (24.1%), pain (16.3%), and erythema (11.4%) for etanercept; erythema (19.9%), pain (19.8%), and reaction (13.6%) for ixekizumab; bruising (25.7%), pain (25.4%), and hemorrhage (12.5%) for secukinumab; and pain (75%), induration (12.5%), and swelling (12.5%) for ustekinumab.
Table 1.
All injection site-related events in the Federal Adverse Event Reporting System database for subcutaneous agents used to treat moderate-to-severe plaque psoriasis
| Preferred term: injection site | Biologica | ||||
|---|---|---|---|---|---|
| Adalimumab (N = 15,637) | Etanercept (N = 141) | Ixekizumab (N = 1771) | Secukinumab (N = 654) | Ustekinumab (N = 8) | |
| n (%) | n (%) | n (%) | n (%) | n (%) | |
| Abscess | 3 (< 0.05) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Anesthesia | 6 (< 0.05) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Atrophy | 0 (0) | 1 (0.7) | 0 (0) | 0 (0) | 0 (0) |
| Bruising | 0 (0) | 1 (0.7) | 49 (2.8) | 168 (25.7) | 0 (0) |
| Cellulitis | 7 (< 0.05) | 0 (0) | 4 (0.2) | 1 (0.2) | 0 (0) |
| Coldness | 1 (< 0.05) | 0 (0) | 0 (0) | 1 (0.2) | 0 (0) |
| Cyst | 0 (0) | 0 (0) | 0 (0) | 2 (0.3) | 0 (0) |
| Dermatitis | 1 (< 0.05) | 0 (0) | 1 (0.1) | 0 (0) | 0 (0) |
| Discharge | 6 (< 0.05) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Discoloration | 75 (0.5) | 1 (0.7) | 9 (0.5) | 9 (1.4) | 0 (0) |
| Discomfort | 39 (0.2) | 1 (0.7) | 8 (0.5) | 5 (0.8) | 0 (0) |
| Dryness | 9 (0.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Eczema | 0 (0) | 0 (0) | 1 (0.1) | 0 (0) | 0 (0) |
| Erosion | 2 (0.01) | 0 (0) | 2 (0.1) | 1 (0.2) | 0 (0) |
| Erythema | 1497 (9.6) | 16 (11.3) | 352 (19.9) | 32 (4.9) | 0 (0) |
| Exfoliation | 9 (0.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Extravasation | 1469 (9.4) | 0 (0) | 0 (0) | 14 (2.1) | 0 (0) |
| Hematoma | 1343 (8.6) | 3 (2.1) | 0 (0) | 0 (0) | 0 (0) |
| Hemorrhage | 942 (6.0) | 8 (5.7) | 25 (1.4) | 82 (12.5) | 0 (0) |
| Hypersensitivity | 6 (< 0.05) | 0 (0) | 3 (0.2) | 1 (0.2) | 0 (0) |
| Hypertrophy | 1 (< 0.05) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Hypoesthesia | 0 (0) | 0 (0) | 1 (0.1) | 2 (0.3) | 0 (0) |
| Induration | 98 (0.6) | 2 (1.4) | 17 (1.0) | 1 (0.2) | 1 (12.5) |
| Infection | 9 (0.1) | 4 (2.8) | 1 (0.1) | 0 (0) | 0 (0) |
| Inflammation | 42 (0.3) | 1 (0.7) | 15 (0.8) | 3 (0.5) | 0 (0) |
| Injury | 5 (< 0.05) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Irritation | 2206 (14.1) | 6 (4.3) | 7 (0.4) | 5 (0.8) | 0 (0) |
| Joint pain | 0 (0) | 0 (0) | 0 (0) | 1 (0.2) | 0 (0) |
| Joint warmth | 0 (0) | 0 (0) | 3 (0.2) | 0 (0) | 0 (0) |
| Laceration | 11 (0.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Macule | 12 (0.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Malabsorption from injection site | 1 (< 0.05) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Mass | 28 (0.2) | 1 (0.7) | 60 (3.4) | 18 (2.8) | 0 (0) |
| Necrosis | 4 (< 0.05) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Nodule | 429 (2.7) | 1 (0.7) | 5 (0.3) | 3 (0.5) | 0 (0) |
| Edema | 9 (0.1) | 1 (0.7) | 5 (0.3) | 2 (0.3) | 0 (0) |
| Pain | 3650 (23.3) | 23 (16.3) | 350 (19.8) | 166 (25.4) | 6 (75) |
| Pallor | 0 (0) | 0 (0) | 1 (0.1) | 0 (0) | 0 (0) |
| Papule | 201 (1.3) | 0 (0) | 2 (0.1) | 0 (0) | 0 (0) |
| Paresthesia | 3 (< 0.05) | 0 (0) | 1 (0.1) | 0 (0) | 0 (0) |
| Pruritus | 840 (5.4) | 10 (7.1) | 123 (6.9) | 20 (3.1) | 0 (0) |
| Pustule | 3 (< 0.05) | 0 (0) | 1 (0.1) | 0 (0) | 0 (0) |
| Rash | 292 (1.9) | 9 (6.4) | 85 (4.8) | 21 (3.2) | 0 (0) |
| Reaction | 850 (5.4) | 34 (24.1) | 241 (13.6) | 28 (4.3) | 0 (0) |
| Recall reaction | 2 (< 0.05) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Scab | 16 (0.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Scar | 13 (0.1) | 0 (0) | 2 (0.1) | 0 (0) | 0 (0) |
| Swelling | 811 (5.2) | 5 (3.5) | 235 (13.3) | 46 (7.0) | 1 (12.5) |
| Ulcer | 1 (< 0.05) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Urticaria | 317 (2.0) | 9 (6.4) | 74 (4.2) | 10 (1.5) | 0 (0) |
| Vesicles | 67 (0.4) | 0 (0) | 6 (0.3) | 7 (1.1) | 0 (0) |
| Warmth | 301 (1.9) | 4 (2.8) | 82 (4.6) | 5 (0.8) | 0 (0) |
FAERS Federal Adverse Event Reporting System, N total number of injection site reaction reports in each category in FAERS, n number of reported events
aSearch dates for each biologic were: adalimumab: 22 January 2008 to 22 January 2010; etanercept: 30 April 2004 to 30 April 2006; ixekizumab: 22 March 2016 to 22 March 2018; secukinumab: 21 January 2015 to 21 January 2017; ustekinumab: 25 September 2009 to 25 September 2011
Fig. 1.
Proportion of injection site-related events in the Federal Adverse Event Reporting System (FAERS) database for each subcutaneous agent used to treat plaque psoriasis. For each drug (adalimumab, etanercept, ixekizumab, secukinumab, and ustekinumab), the most common types of injection site reaction-related events (preferred terms) are shown. Percentages were calculated based on the total number reports of injection site-reactions (N) for each drug
Discussion
In this study we evaluated spontaneous reports to the US FDA FAERS database to obtain information about ISRs occurring with subcutaneously injected biologics indicated for the treatment of plaque psoriasis. Spontaneous reports of ISRs were identified for all subcutaneously administered biologics evaluated in this study (adalimumab, etanercept, ixekizumab, secukinumab, and ustekinumab), with the total number of reports for ustekinumab being very low. In general, the results were consistent with each product’s labeling, although the frequencies of ISRs cannot be determined from the information compiled in this post-marketing database. No conclusions may be drawn from the present dataset regarding the adequacy of rates of reporting of ISRs in FAERS.
This study examined the type of ISRs occurring in a post-marketing setting. Among the most commonly reported ISRs was injection site pain, which was reported in 23% (adalimumab), 16% (etanercept), 20% (ixekizumab), 25% (secukinumab), and 75% (ustekinumab) of all ISRs. Inspection of Fig. 1 suggests that the distribution of ISR types may vary for each drug, with the caveat that it is difficult to draw conclusions for ustekinumab due to the very few reports of ISRs. We did not observe any obvious pattern for the types of ISRs based on classes of biologics (anti-interleukin-17 and anti-tumor necrosis factor [TNF]). This finding could have been affected by the specific biologics used, differences in formulation, or the unknown total patient exposure and reporting rates for each drug. Nonetheless, individual biologics could have characteristic patterns of types of ISRs due to factors such as their unique formulations or their specific effects on the immune system. Future work examining differences in reported ISRs between medications with various mechanisms of action could provide insight into the etiology of ISRs.
FAERS has several limitations, including underreporting and duplicate reporting, inability to verify reports or determine causal association, lack of overall patient exposure data for the calculation of incidence, and lack of information on discontinuation due to ISRs [3, 6]. In the work described here, there was a wide variation in the number of reported ISRs among the various drugs; however, in the absence of information on patient exposure, we could draw no conclusion on the overall incidence of events between drugs. ISR reports may also be confounded by multiple indications for a given drug, as FAERS does not capture specific indication of use. TNF-inhibitors used to treat psoriasis are frequently used in combination with methotrexate to treat rheumatoid as well as psoriatic arthritis. Previous work has shown that combination therapy with methotrexate is associated with fewer ISR reports in the FAERS database than monotherapy [7]. The method of administration is also an important consideration as self-administration could potentially be associated with more ISRs than injections administered by a healthcare professional due to mechanical injuries during self-injection; however, this information is not included in FAERS. Additionally, individual drug formulations may have changed since our study period, which could impact current reports of ISRs; for example, citric acid has been removed from adalimumab to reduce injection site pain [8]. Future studies that will accurately estimate exposure, verify indications of use, and confirm ISR events are needed to make more definitive conclusions about the relative incidence of ISR events.
Finally, FAERS data do not allow for an examination of ISR patterns over time. This is particularly relevant given that the incidence of ISRs associated with products such as adalimumab and ixekizumab do decrease over time. [9, 10].
Conclusions
All of the subcutaneously administered biologics for plaque psoriasis (adalimumab, etanercept, ixekizumab, secukinumab, ustekinumab) that were assessed had reports of ISRs in the FDA FAERS database during the first 2 years following approval for the treatment of plaque psoriasis. The most common specific types of ISRs varied by agent, with no obvious pattern observed between classes of biologics. The findings presented here could be of relevance for patients who consider switching drugs due to ISRs.
Acknowledgements
Funding
This study and the journal’s Rapid Service Fee were supported by funding from Eli Lilly and Company.
Medical Writing Assistance
Medical writing support was provided by Thomas Melby of Syneos Health (Raleigh, NC). This assistance was funded by Eli Lilly and Company.
Authorship
All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Elsie Grace is a full-time employee and minor stock holder of Eli Lilly and Company. Orin Goldblum is a full-time employee and minor stock holder of Eli Lilly and Company. Noah Agada is a full-time employee and minor stock holder of Eli Lilly and Company. Kyoungah See is a full-time employee and minor stock holder of Eli Lilly and Company. Lisa Renda is a full-time employee and minor stock holder of Eli Lilly and Company. Craig Leonardi reports receiving honoraria from AbbVie, Amgen, Boehringer Ingelheim, Dermira, Eli Lilly and Company, Janssen, LEO Pharma, Pfizer, Sandoz, UCB, and Vitae for serving on the advisory board/consultancies; he reports receiving honoraria from AbbVie, Celgene, Novartis, Eli Lilly and Company, UCB, and Sun Pharmaceuticals for speaking engagements; he reports receiving fee-for-service for acting as an investigator for AbbVie, Actavis, Allergan, Amgen, Boehringer Ingelheim, Celgene, Cellceutix, Coherus, Corrona, Dermira, Eli Lilly and Company, Galderma, Glenmark, Janssen, LEO Pharma, Merck, Novartis, Novella, Pfizer, Sandoz, Sienna, Stiefel, and UCB. Alan Menter reports receiving grants and honoraria from Abbott Labs, Amgen, Janssen Biotech, and Sienna (advisory boards, speaker serving investigator, and consulting); he reports receiving grants and honoraria from Boehringer Ingelheim (advisory boards and investigator); he reports receiving grants from Celgene and Merck (investigator); he reports receiving honoraria from Eli Lilly and Company and Novartis (consulting and investigator); and he reports receiving honoraria from UCB (consulting, investigator, and speaker); he is the Editor-in-Chief of this journal.
Compliance with Ethics Guidelines
Anonymized data were collected from a publicly available database and did not require ethics committee approval.
Data Availability
Anonymized data were collected from a publicly available database as described. The FAERS database can be accessed at https://www.fda.gov/drugs/fda-adverse-event-reporting-system-faers/fda-adverse-event-reporting-system-faers-public-dashboard.
Footnotes
Enhanced Digital Features
To view enhanced digital features for this article go to 10.6084/m9.figshare.10070132.
References
- 1.Murdaca G, Spanò F, Puppo F. Selective TNF-alpha inhibitor-induced injection site reactions. Expert Opin Drug Saf. 2013;12:187–193. doi: 10.1517/14740338.2013.755957. [DOI] [PubMed] [Google Scholar]
- 2.Zeltser R, Valle L, Tanck C, Holyst MM, Ritchlin C, Gaspari AA. Clinical, histological, and immunophenotypic characteristics of injection site reactions associated with etanercept: a recombinant tumor necrosis factor alpha receptor: Fc fusion protein. Arch Dermatol. 2001;137:893–899. [PubMed] [Google Scholar]
- 3.United States Food and Drug Administration. Questions and answers on FDA’s Adverse Event Reporting System (FAERS). In: FDA adverse event reporting system (FAERS). 2018. https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/default.htm. Accessed 3 Jan 2019.
- 4.United States Food and Drug Administration. FDA adverse event reporting system (FAERS) quarterly data extract files. https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html. Accessed 7 Jan 2019.
- 5.United States Food and Drug Admionistration. FDA adverse event resporting system (FAERS) public dashboard. https://fis.fda.gov/sense/app/d10be6bb-494e-4cd2-82e4-0135608ddc13/sheet/7a47a261-d58b-4203-a8aa-6d3021737452/state/analysis. Accessed 7 Jan 2019.
- 6.Sakaeda T, Tamon A, Kadoyama K, Okuno Y. Data mining of the public version of the FDA adverse event reporting system. Int J Med Sci. 2013;10:796–803. doi: 10.7150/ijms.6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsui T, Umetsu R, Kato Y, et al. Age-related trends in injection site reaction incidence induced by the tumor necrosis factor-alpha (TNF-alpha) inhibitors etanercept and adalimumab: the Food and Drug Administration adverse event reporting system, 2004–2015. Int J Med Sci. 2017;14:102–109. doi: 10.7150/ijms.17025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.AbbVie. HUMIRA® citrate-free (adalimumab). North Chicago: AbbVie; 2013. https://www.humira.com/citrate-free. Accessed 26 Feb 2019.
- 9.Shear NH, Paul C, Blauvelt A, et al. Safety and tolerability of ixekizumab: integrated analysis of injection-site reactions from 11 clinical trials. J Drugs Dermatol. 2018;17:200–206. [PubMed] [Google Scholar]
- 10.Asahina A, Ohtsuki M, Etoh T, et al. Adalimumab treatment optimization for psoriasis: results of a long-term phase 2/3 Japanese study. J Dermatol. 2015;42:1042–1052. doi: 10.1111/1346-8138.13001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data were collected from a publicly available database as described. The FAERS database can be accessed at https://www.fda.gov/drugs/fda-adverse-event-reporting-system-faers/fda-adverse-event-reporting-system-faers-public-dashboard.