Highlights
-
•
The development of error detection and correction was studied in 6–14 years-old children and adolescents.
-
•
Force and electromyographic activity of button presses were recorded during the performance of a choice-response time task.
-
•
The motor activity had a similar initial part for correct and incorrect responses but was quickly reduced for errors.
-
•
This reduction occurs during error commission and reflects a desperate attempt to inhibit the ongoing error.
-
•
Such error inhibition was present in the youngest children already and was similar across childhood.
Keywords: Performance monitoring, Error detection, Inhibitory control, Electromyographic activity, Cognitive development
Abstract
The maturation of processes involved in performance monitoring, crucial for adaptive behavior, is a core aspect of developmental changes. Monitoring processes are often studied through the analysis of error processing. Previous developmental studies generally focused on post-error slowing and error-related EEG activities. Instead, the present study aims at collecting indicators of error monitoring processes occurring within trials that is, before the erroneous response is produced. Electromyographic (EMG) activity and force produced during responding were registered in 6 to 14-year-olds performing a choice-response task. As already reported in adults, force produced was weaker, EMG bursts were smaller, and motor times (interval between EMG onsets and responses) were longer during errors compared to correct responses. In contrast, the rising part of EMG burst, reflecting the initial motor command, was the same for both response outcomes. This suggests that error inhibition was applied online after the response was triggered but before the actual key was pressed. This error correction was already present in children as young as 6 years old. The effects of reduced EMG and force amplitudes remained stable across childhood. However, the prolonged motor times in young children suggests that they need more time to implement motor inhibition than their older peers.
1. Introduction
The ability to monitor, evaluate and adjust our performance is critical for adaptive behavior. The way we cope with behavioral errors provides valuable insights into performance monitoring processes. Notwithstanding, the developmental trajectory of error monitoring is not clearly understood yet. Previous developmental studies focused mainly on post-error slowing (PES) measured on response times (RTs) and event-related potentials of EEG occurring after error commission. Whereas these markers characterize what happens after an error, research in adults has shown benefits of using indicators of within-trial error-monitoring processes. Derived from EMG activity recorded during responding, these markers capture processes occurring before the execution of the erroneous response is completed (e.g., Allain et al., 2004). These markers are used in the present study to investigate the development of error-monitoring in 6-to-14 year-olds. Before describing them in detail, we will first give a brief overview of the conventional error-processing markers.
PES describes prolonged correct RTs on trials that follow an error as compared to trials following correct responses (Laming, 1979; Rabbitt, 1966). Developmental effects on PES are somewhat inconsistent. Some studies did not show any age-related differences between 7 years old of age and adulthood (Davies et al., 2004a; Ladouceur et al., 2004; van de Laar et al., 2012v; Wiersema et al., 2007) while others have shown that PES decreases with age, from 5 years to adolescence (Fairweather, 1978; Gupta et al., 2009; Schachar et al., 2004; Smulders et al., 2016). In one study PES was not present at all in children aged 7–16 years (Yordanova et al., 2011). Further, the functional meaning of PES is debated. It has classically been interpreted as between-trial engagement of executive control, such as response monitoring and post-error adjustment in decision criteria (Laming, 1979) or increased response inhibition in the following trial (Marco-Pallares et al., 2008; Ridderinkhof et al., 2004). More recently, it has been suggested that PES might be related to processing of novel or unexpected event rather than to executive control (Notebaert et al., 2009; Wessel et al., 2012). While a recent theory of error processing (Wessel, 2018) propose to integrate these interpretations (see: Burns, 1971 for similar proposition), the debate is still open.
The main electrophysiological markers of error processing are the error-related negativity (ERN/Ne) and the error positivity (Pe). ERN/Ne is a negative potential peaking about 50−100 ms after erroneous response (Falkenstein et al., 1991; Gehring et al., 1993). The developmental trajectory of ERN/Ne is again inconsistent. Whereas a few studies evidenced an age-related increase in its amplitude between 7 years old of age and adulthood (Davies et al., 2004b; Ladouceur et al., 2004; Overbye et al., 2019; Wiersema et al., 2007), potentially reflecting the maturation of the neural systems for error monitoring, age effect was only marginally significant in another study with participation of younger children (5–7 years old; Meyer et al., 2012). No effect of age on the ERN/Ne was also obtained in other studies (Eppinger et al., 2009; Grammar et al., 2014; Richardson et al., 2011). ERN/Ne provided valuable information about error monitoring, but there is still a lively debate on processes it indexes: error detection signal (Falkenstein et al., 1991; Gehring et al., 1993), detection of response conflict (Botvinick et al., 2001; Yeung et al., 2004), prediction of error-likelihood (Brown and Braver, 2005), or action monitoring (Bonini et al., 2014; Burle et al., 2008; Vidal et al., 2003). Pe is a positive deflection peaking about 200−500 ms after an error (Falkenstein, Hoorman, Christ, and Hohnstein, 2000) that has been associated with error awareness (Nieuwenhuis et al., 2001; Overbeek et al., 2005; Ficarella et al., 2019). Few studies suggest that Pe amplitude increases between the ages of 3 and 7 (Grammar et al., 2014), many other studies evidenced no further changes from later childhood till adulthood (Davies et al., 2004a, 2004b; Ladouceur et al., 2004; Overbye et al., 2019; Wiersema et al., 2007), suggesting that adults-like conscious error-detection is present already at age of 7.
Given the above inconsistencies in the development of error monitoring, it may be beneficial to study new markers not yet used in the development literature. In the present study, we used indexes that directly highlight the online attempt to interrupt erroneous actions. Forty years ago, Rabbitt (1978) reported that the force produced by skilled typists was reduced on erroneous letters. More recently, extending these results, Allain et al. (2004) recorded electromyographic (EMG) activity of muscles involved in response production in a choice-response time task in adults. The EMG burst of errors was smaller than the EMG burst of correct responses, although the initial part of the EMG burst (as assessed by the rising slope) was the same for both. The rising slope, which indexes the degree of synchrony of the motor unit (Meijers et al., 1976), is, in contrast, modified by factors directly affecting the motor command (e.g. response cues, speed-accuracy trade-off; Possamaï et al., 2002; Spieser et al., 2014). This indicates that the motor command is initially identical for correct and erroneous responses, and hence that the difference appears after the command has been initiated. Moreover, fractioning RT into premotor time (PMT, the interval between stimulus onset and the onset of the EMG burst of correct or incorrect responses) and motor time (MT, the interval between the EMG onset and the response; see Fig. 1D) revealed that MTs of errors were longer than MTs of correct responses. This effect is specific to motor execution, since PMTs (along with RTs) were faster for errors than for correct responses; it hence does not reflect a general slowing (due for example to inattention) that would spread to the motor system. Altogether, these results, replicated several times since then (Allain et al., 2004; Meckler et al., 2011; Rochet et al., 2014; Roger et al., 2014), suggest that erroneous responses are initiated in the same way as correct ones, but that they are later, although unsuccessfully, attempted to be stopped. As being unambiguously linked to motor execution of a response, error-related reduction in force and EMG-burst amplitude (and consequent prolongation of MT duration) provides direct markers of an on-line, within-trial error detection and inhibition even before the actual behavioral response. In the present study force- and EMG-derived markers were used to examine the development of error correction processes in 6 to 14-year-olds performing a choice-response time task.
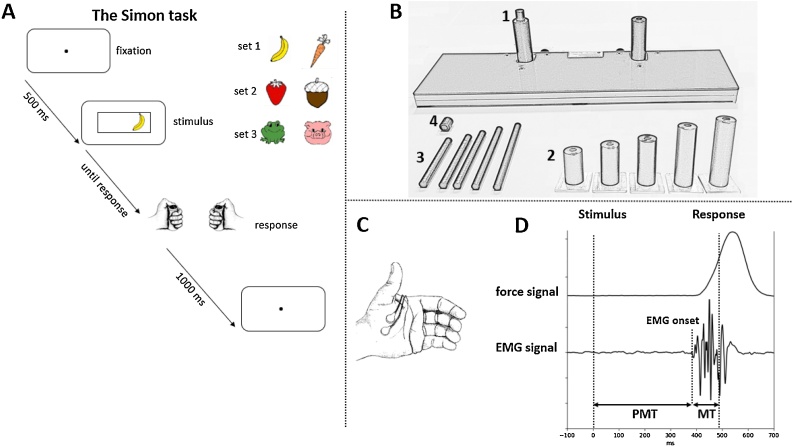
Fig. 1.
Materials and methods used in the experiments. Fig. 1A: the temporal sequence of events and stimulus sets used in the experimental task. Fig. 1B presents the response device: Response buttons were mounted on cylindric handgrips (1B-2), that contained another thin cylinder (1B-3) touching force sensors located in the board (1B-1). The size of the handgrip and the inner thin cylinder were chosen for each child to fit his/her hand size. The top of the thin cylinder was covered by a cap (1B-4) to increase the pressing comfort. Fig. 1C: placement of electrodes for EMG recording. Fig. 1D: typical force and EMG signals recorded in the experiment (vertical dashed lines denote stimulus, EMG onsets and behavioral response; PMT denotes the premotor time and MT the motor time).
2. Method
2.1. Participants
Study participants were 114 children aged from 6 to 14 years old recruited in primary and secondary French schools. Due to the low number of errors (less than 5), 29 participants (equally distributed across age groups) were excluded from the analysis, leaving 85 participants in the final sample. (Eighteen additional children were tested but not included in the initial sample of 114 participants due to very low quality of their EMG signal that had at least 50 % of unclassified EMG bursts, see below for details). Characteristics of the final sample grouped by age (in years) are presented in Table 1. Children provided verbal assent after being explained the procedure and informed written consent was obtained from their legal guardians before the experiment. The study was approved by the head of the regional ethics committee (Comité de Protection des Personnes, CPP, Sud-Est, France). All participants had normal or corrected-to-normal vision, normal color vision and no history of neurological disorders.
Table 1.
Descriptive statistics. Characteristics of the participants.
| Group (age in years) | Number of children | Ratio (girls/ boys) | Mean age (in months) | SD (in months) |
|---|---|---|---|---|
| 6 | 11 | 0.38 | 80.1 | 3.6 |
| 7 | 8 | 1.66 | 92.5 | 3.7 |
| 8 | 13 | 0.63 | 103.9 | 3 |
| 9 | 9 | 0.29 | 117.8 | 5.4 |
| 10 | 9 | 0.80 | 129.4 | 5.3 |
| 11 | 9 | 0.80 | 137.8 | 4 |
| 12 | 7 | 0.75 | 150 | 3.6 |
| 13 | 9 | 1.25 | 164.2 | 4 |
| 14 | 10 | 0.67 | 173.8 | 3.9 |
2.2. Material and apparatus
All children were tested individually in their schools during a single session of about 45 min, which took place in a classroom adapted to the experimental needs (along which EMG recording). The experimental task was controlled by PsychoPy software (Peirce, 2007). Three sets of stimuli were used (see Fig. 1A): cartoonish images of yellow banana and orange carrot (1 st set), brown nut and red strawberry (2nd set), green frog and pink pig (3rd set). All stimuli (3,6° × 3,6°) appeared in a black frame (12,1° × 3,9°) presented in the center of the gray screen located approximately 50 cm in front of the participant. Stimuli appeared with an eccentricity of 3,9° from the central fixation point (0,5°). Responses were collected via a response device designed to be adjustable to children’s hands (Fig. 1B). Response buttons were mounted on cylindric handgrips, that contained another thin cylinder touching force sensors located on the board. Pressure on the thin cylinder was directly transferred to the force sensors that recorded the force of the response button presses. The top of the thin cylinder was covered by a cap to increase the pressing comfort. Before the experiment started, the height of the handgrip and the thin cylinder (one of five handgrips and cylinders possible) were chosen independently to be adjusted to the size of participants’ hands and thumbs. Participants were instructed to keep their thumbs on the response buttons during the entire task and to respond as quickly and as accurately as possible.
2.3. Procedure
Participants performed a child-friendly version of the Simon task (Simon, 1990; Fig. 1A), which took less than 25 min to complete. It consisted of three blocks, each containing a different set of stimuli, which were counterbalanced between the participants. Each trial started with a fixation point displayed for 500 ms. Next, a centrally displayed black frame containing a target-stimulus on its left or right side was presented until a response was given (no response time limit was set) and the next trial started 1 s later. The participants were instructed to press the left or right response button depending on the color of the stimulus independently of its position, with the mapping between the target-color and the response-side counterbalanced between participants. On compatible trials (50 % of all trials), the target was presented on the same side as the required response and on incompatible trials, the target was presented on the side opposite to the required response.
Each block consisted of 100 trials, with short breaks after each 25 trials and longer breaks between blocks. First, the instruction indicating the mapping between target-color and response-side was displayed. Next, two trainings (36 trials in total) were administered to participants to properly learn the task. During the first training, participants received auditory feedback (two contrasting sounds for correct and incorrect response explicitly given before training). The next trial was initiated by the experimenter, who re-explained the task in case a participant committed an error. During the second training, with no feedback, trials started after 1 s independently of the response correctness. After each training, participants received information, displayed on the screen and explained by the experimenter, about the number of errors they committed.
2.4. Electrophysiological recording and processing
Ag/AgCl active flat electrodes (pre-amplified electrodes, Biosemi Inc., Amsterdam, The Netherlands) were used to record EMG activity of the flexor pollicis brevis of both hands (Fig. 1C). The electrodes were placed on the thenar eminence of thumbs with the maximal possible distance apart (1 cm at least). EMG activity was digitized online (sampling rate: 2048 Hz; analog bandwidth limit: –3 dB at 1/5th of the sampling rate) with use of the BioSemi Active-Two system (Biosemi Inc., Amsterdam, The Netherlands). The EMG signal was continuously monitored by a second experimenter (different from the one who runs the experiment and provided instructions) who tracked down the appearance of tonic muscular activity masking small task-related muscular activations. Children were asked to relax their hands whenever the tonic activity appeared increasing during the recording.
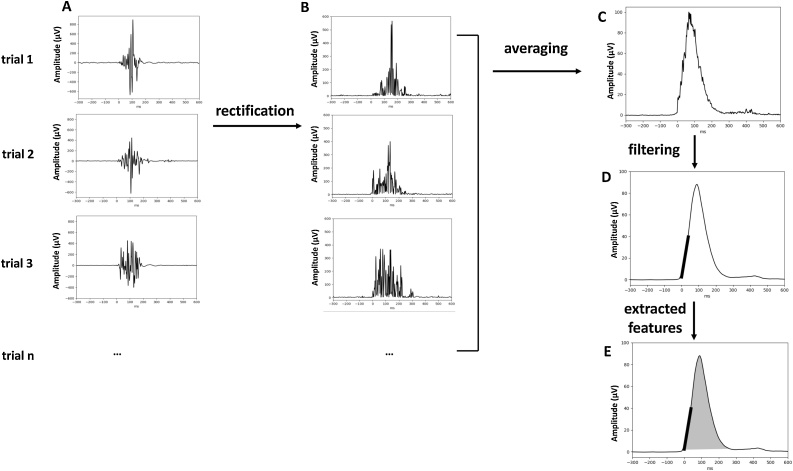
Bipolar montage for left and right hand separately was computed offline and high-pass filtered at 10 Hz to remove slow fluctuations unrelated to EMG activity. To facilitate visual inspection, onsets of force recorded from force sensors and onsets of EMG activity were detected by a home-made custom program written in Python.2 Afterward, force and EMG traces of each participant were inspected visually, and onsets of force and EMG bursts were corrected manually in case of inaccurate detection by the program. Trials in which EMG-burst onsets were undetectable by visual inspection due to low signal-to-noise ratio were excluded from analysis (2 % of all trials in each age group). During this procedure, the person who inspected the traces was unaware of the nature of the trial (compatible vs. incompatible; correct vs. error), nor of the age of the participant the traces corresponded to. Based on this procedure, trials with EMG activity related to correct and incorrect responses were further decomposed into premotor time (PMT; from stimulus onset to the onset of EMG burst) and motor time (MT; from EMG onset to the button press defining the response) as illustrated in Fig. 1D. To calculate the amplitude of the EMG burst, the EMG signal on individual trials (Fig. 2A) was rectified (taking the absolute value of the signal; Fig. 2B), ensuring that the EMG signal does not cancel out during averaging. Next, the rectified signals were averaged time-locked to the burst onset (Fig. 2C) across all trials separately for correct and incorrect responses and filtered at 20 Hz (Fig. 2D). Force was measured from the same trials as EMG bursts. To measure force produced, force traces, consisting of only positive values and having very good signal-to-noise ratio, were averaged time-locked to their onsets across correct and incorrect responses.
Fig. 2.
EMG signal preprocessing. The raw EMG activity on individual trials (panel A) was rectified (panel B), averaged across correct or incorrect trials (panel C) and filtered (panel D). Panel E presents features extracted from EMG signal: surface of the EMG burst (shadowed area) and rising slope of the EMG burst (thick black line).
2.5. Data analysis
The following variables were analyzed: RT, PMT, MT, the force produced, the amplitude of the EMG burst and the leading-edge of the EMG burst. RTs, PMTs and MTs were collected on individual trials and their means were subsequently computed separately for correct responses and errors. The force produced and the amplitude of the EMG burst were quantified on signals that were previously averaged across correct and incorrect trials, time-locked to the force and the EMG burst onset, respectively. The mean force produced and the amplitude of the mean EMG burst were calculated as the surface under the averaged curves of the force and EMG burst, respectively (Fig. 1D). Note that both measures are extracted from different signals (from force and EMG signals) and interpreted as indicating the same underlying mechanism namely, an attempt to stop an error, although at slightly different moments (force onsets are slightly delayed compared to EMG onsets). Finally, the leading-edge of the EMG burst was estimated (Fig. 2E): a linear regression was fitted to the averaged EMG values from the EMG onset to 40 ms after EMG onset. This window was chosen to correspond to the rising part of EMG bursts. Values of the slope of this regression line were used for statistics. The statistical analysis was performed with a linear mixed model with Accuracy as repeated-measures factor (correct vs. error)3 and Age (in months) as a continuous factor. The developmental changes of correct-error differences were additionally evaluated by calculating the ratio (errors divided by correct trials) for all variables, thereby normalizing for age-differences not related to Accuracy. This ratio was subsequently entered as an outcome variable in a linear regression analysis with Age as predictor. Jamovi software (jamovi project, 2018), built on top of R statistical language, was used for statistical analysis.
3. Results
3.1. Errors
Percentages of errors decreased with increasing age (F1,83 = 4.2, p = .04; mean = 4.3 %, SD = 2.4 %, min = 5, max = 39). (This effect was only close to significance in the initial sample of 114 participants, F1,112 = 3.4, p = .07, probably due to ceiling effect). More errors were committed in the incompatible (6.2 %) than in the compatible task condition (2.5 %, F1,83 = 75.4, p < 0.001), with this effect being slightly larger in younger participants (F1,83 = 3.8, p = 0.054).
3.2. Global RT analysis
RTs decreased with increasing age (F1,83 = 109.5, p < 0.001, cf. Fig. 3A4). RTs of errors were shorter than RTs of correct responses (F1,83 = 52.1, p < 0.001), with no interaction between Accuracy and Age (F1,83 = 0.5, n.s.). After normalization for age-related differences in latencies, Age did not modulate the errors/correct RTs ratio (R = 0.05, t1,83 = −0.5, n.s.).
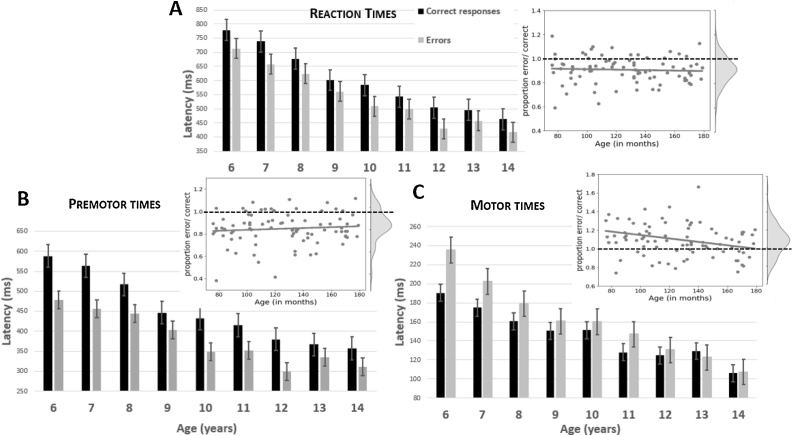
Fig. 3.
Chronometric measures. Reaction times (A), premotor times (B) and motor times (C) presented for categorical age groups separately for correct responses (black bars) and errors (gray bars). Error bars denote the standard error of the mean. Insets present the ratios (latencies of errors divided by latencies of correct trials) and their kernel density estimates. Gray dots represent individual participants, gray line the regression line between Age and ratios. Dotted lines denote the ratio of 1 serving as a reference.
3.3. RT fractioning
PMTs decreased with increasing age (F1,83 = 79.7, p < 0.001). PMTs of errors were shorter than PMTs of correct responses (F1,83 = 97.7, p < 0.001), and this difference decreased with age (Age x Accuracy: F1,83 = 7.3, p = 0.009). However, after normalization, the error/correct PMT ratio was not influenced by Age (R = 0.09, t1,83 = 0.8, n.s.). MTs also decreased with increasing age (F1,83 = 38.4, p < 0.001) but they were longer for errors than for correct responses (F1,83 = 16.4, p < 0.001), with this difference, again, decreasing with age (Age x Accuracy: F1,83 = 12.8, p < 0.001). This effect was still present after normalization (R = 0.3; t1,83 = 2.5, p = 0.02). Fig. 3B and C present the PMTs and MTs, respectively.
3.4. Force and EMG burst
3.4.1. Force
The produced force was smaller for errors than for correct responses (F1,83 = 86.5, p < 0.001). The main effect of Age and the interaction between Age and Accuracy were not significant (F1,83 = 0.01, n.s.; F1,83 = 1.3, n.s., respectively); this absence of interaction survived normalization (R = 0.1; t1,83 = −1.2, n.s.). These results are presented in Fig. 4.
Fig. 4.
Force. Averaged force signal used to press the response buttons (A) and energy of the force (B) presented for categorical age groups separately for correct responses (black lines/bars) and errors (gray lines/bars). Error bars represent the standard error of the mean. Fig. 4C presents the ratio between the force produced during errors with force produced during correct responses and its kernel density estimate. Each grey point represents a participant, the gray line shows the regression between Age and the ratio and the dotted line corresponds to a ratio of 1, serving as a reference.
3.4.2. EMG bursts
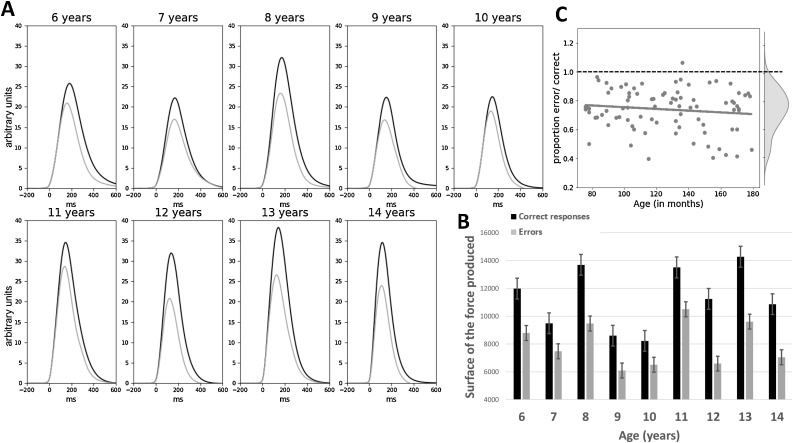
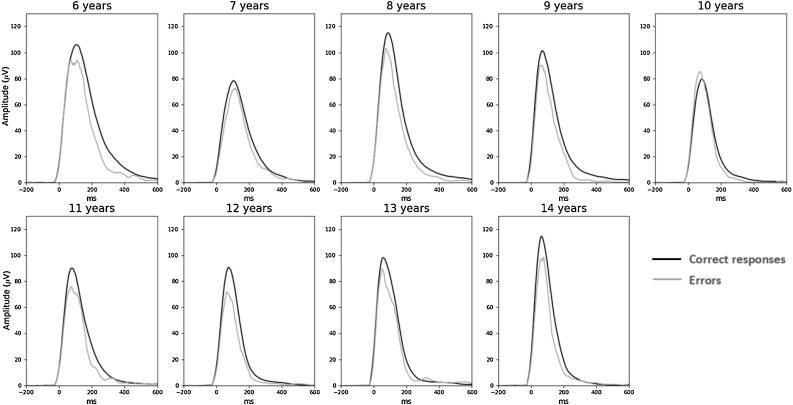
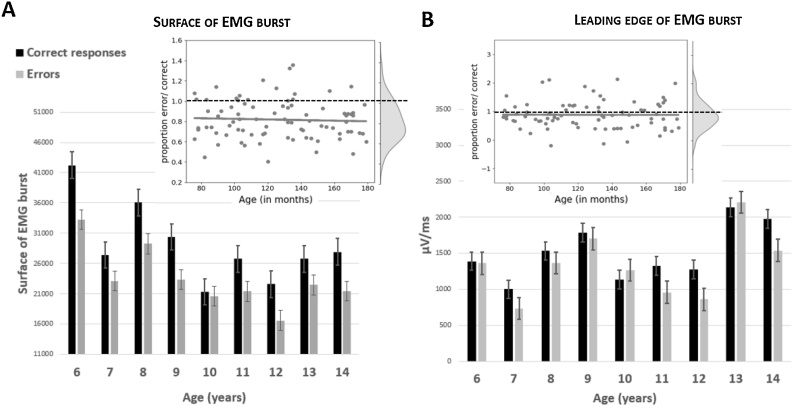
As can be seen on Fig. 5, EMG bursts of errors were smaller compared to EMG bursts of correct responses (F1,83 = 44.3, p = 0.01). The Age effect on EMG amplitudes felt short of significance (Age: F1,83 = 3.8, p = 0.055). The interaction between Accuracy and Age was not significant neither on the raw data (F1,83 = 0.5, n.s.), or nor on ratios (R = 0.05; t1,83 = −0.4, n.s.). The analysis of the rising part of EMG bursts revealed no difference in the leading-edge between EMG bursts related to correct and incorrect responses (F1,83 = 3.0, n.s.) and no main effect of Age (F1,83 = 2.0, n.s.) nor any interaction between Accuracy and Age either the raw data (F1,83 = 0.7, n.s.) or on ratios (R = 0.1; t1,83 = −1.1, n.s., see Fig. 6A for EMG surface and 6B for the leading-edge).
Fig. 5.
EMG. Averaged EMG signal presented for categorical age groups separately for correct (black lines) and incorrect (gray lines) responses.
Fig. 6.
EMG. The surface (A) and leading-edge (B) of EMG bursts presented for categorical age groups separately for correct responses (black bars) and errors (gray bars). Error bars represent the standard error of the mean. The two insets present the ratio between error and correct trials for EMG amplitude (A) and leading-edge (B) and the corresponding kernel density estimates. Gray points denote individual participants, gray lines the regression between ratio and Age and the dotted line indicates the ratio value of 1, representing no effect.
3.5. Compatibility effect on correct trials
It is well established that the number of errors in the Simon task is larger on incompatible than compatible trials. The unequal number of compatible and incompatible trials might then be a confounding factor. Although MTs has repeatedly been reported to be insensitive to compatibility in adults (e.g. Hasbroucq et al., 2009; Spieser et al., 2014), we tested the compatibility effect by contrasting compatible and incompatible trials that resulted in correct responses for each measure of interest, i.e. the force produced, the amplitude of EMG bursts and MT durations (similar analysis on incorrect responses was not possible due to the low number of errors in case of compatible trials). The linear mixed model was used for statistical analysis with one repeated-measurement factor Compatibility (compatible vs. incompatible) and one continuous factor Age (in months). No evidence of Compatibility effect nor interaction between Compatibility and Age was obtained on any of the considered measures (all Compatibility effects: F1,83 ≤ 0.9, n.s.; all interactions: F1,83 ≤ 2.8, n.s.), ruling out a potential bias due to imbalanced number of errors between compatible and incompatible trials.
4. Discussion
Children’s efficiency in performance monitoring and error correction is a decisive factor explaining behavioral adaptation to the environment, hence the importance of understanding its development. Although the use of EEG measures as well as post-error slowing has allowed important progress in this field (for reviews see Ferdinand and Kray, 2014; Tamnes et al., 2013), these markers measure processes after the commission of error. As such, they have concealed the role of error monitoring processes taking place during error commission. In the current study, we used within-trial markers of error monitoring to investigate specifically the development of these processes.
The current findings indicate that even young children try to interrupt the commission of errors, revealing their ability to detect errors and initiate online response inhibition processes. As in adults, errors production in children was associated with reduced EMG amplitudes and reduced force of response button presses that induced prolonged motor times (MTs; intervals between the EMG onset and the response) compared to correct responses. Remarkably, as previously shown in adults, children’s attempts to inhibit errors start during the execution of incorrect responses and does not precede its initiation, as indicated by comparable leading-edge of the EMG bursts of correct and incorrect button presses. It is noteworthy that such within-trial, online inhibitory processes occur before incorrect button presses. Indeed, reducing the force and amplitude of muscle contraction before an incorrect response is finalized indicates some form of advance action inhibition. As such, these findings provide new evidence in favor of a form of proactive control in 6-year-olds (see also Chatham et al., 2009; Gonthier et al., 2019; Lucenet and Blaye, 2014).
Children’s ability to monitor their errors appear to be globally stable across childhood. Age did not modulate the error-related reduction of the force produced nor the EMG amplitude, suggesting that the efficiency of error inhibition was similar for children of all ages. However, such a similar efficacy was achieved at the costs of prolonged MTs in younger children. Indeed, error-related lengthening of MTs, compared to MTs on correct trials, decreased with age suggesting that younger children need more time to implement motor inhibition. In this line, the shortening of time needed to inhibit a response as age increases was observed in the Stop-signal task (Carver et al., 2001; Tillman et al., 2007; van den Wildenberg and van der Molen, 2004; but see Johnstone et al., 2007).
Relatively early development of error correction processes, as evidenced in the current study, is in clear contrast with the well-established long and protracted maturation of cognitive control (Diamond, 2013). This apparent discrepancy may be resolved in the framework of the adaptive orienting theory of error processing (Wessel, 2018). Our within-trial measures potentially reflect the very first stage of adaptive orienting, namely the inhibition of ongoing motor representation. As this inhibition is supposedly automatically triggered, it is consistent with stable error-related reduction of amplitudes of force/EMG bursts from childhood to adulthood. In contrast, control adaptive processes that take place after the commission of error (e.g. task-set reconfiguration, perceptual (re-)tuning, strategic post-error slowing) may require the involvement of processes that continue to mature until adolescence or adulthood. Thus, different control processes might have different developmental trajectories, with error inhibition maturing relatively early, as evidenced in this choice-response time task. An important issue in further studies will be to establish the relationship between EMG and force markers of error inhibition and other electrophysiological (e.g. Ne/ERN and Pe) and behavioral (e.g. PES) markers of error processing and draw an integrative developmental picture of the error monitoring function.
The current study enlightens the importance of indexes derived from force and EMG signals. Recording of EMG and force is relatively easy and allows to determine the onset of the muscular activity on a trial-by-trial basis. This remarkably reduces between-trial jitter and improves the reliability of calculation made on an averaged signal. EMG is easily combined with EEG and both methods can provide complementary information. Moreover, EMG signal might also solve an apparent paradox in the ERN/Ne developmental literature: the ERN/Ne component has been reported as having shorter rather than longer latency in children than in adults (Davies et al., 2004a, 2004b; Wiersema et al., 2007), which is difficult to interpret at the functional level. This result is very likely related to the shortening of MTs as age increases. Indeed, the ERN/Ne is better time-locked to the beginning of motor response (EMG onset) rather than to the mechanical response (Burle et al., 2008). Time-locking the ERN/Ne on the EMG onset should shift it towards shorter latencies, but this shift will be more important for young children due to longer MTs. In line with this hypothesis, no age-related differences in ERN/Ne latency was found when ERN/Ne were locked to the EMG onset (Kim et al., 2007).
Whereas the present study revealed that children can detect errors and to engage inhibition processes in advance of error commission, research in adults suggest that this investigation could be taken one step further. In adults, correct performance is sometimes associated with small EMG activation on the hand corresponding to the incorrect response. These so-called partial errors (Burle et al., 2002) reflect successful inhibition that intervene early enough to prevent errors. The development of this ability in children would be worth considering in future research.
Pointing to a possible shortcoming, the cross-sectional nature of the current study may conceal more subtle age-related changes in error correction processes. Moreover, while EMG is a very valuable method for developmental research, some precautions are needed when interpreting the results. Indeed, besides physiological and functional maturation (which are of direct relevance), electrode placement, and more specifically inter-electrodes distance, is critical. While we tried to keep them as constant as possible, different hand sizes between age groups imply different inter-electrodes distances. Hence, while indexes derived from EMG and force measures are directly interpretable for within participants effects (as used in the present study to investigate the difference between errors and correct responses), they are more difficult to interpret for between-participant factors (e.g. age, gender) and should be taken with caution.
4.1. Conclusions
In conclusion, the analysis of force and EMG activity during the performance of choice-reaction time task is a valuable method that gives direct access to error monitoring processes and can be successfully used in children. The present study indicates that error detection and inhibition processes are present already in children by the age of 6. Although effects on the EMG and force signals do not change across childhood indicating similar control abilities, results on MTs suggests that younger children might need more time to implement motor inhibition.
Declaration of Competing Interest
Authors declare no competing interests.
Acknowledgements
This work was supported by French Agence Nationale de la Recherche (ANR-15-CE28-0008, DOPCONTROL) awarded to A.B and B.B. The authors wish to thank D. Louber and D. Paleressompoulle for designing and setting-up the response device.
Our manuscript meets the guidelines for ethical conduct and report of research. There are no potential or actual conflicts of interest and reported research is unpublished and not under consideration for publication elsewhere.
Footnotes
This home-made custom Python program, which will soon be released with an open-source license, is accessible upon request.
The unbalanced number of trials in both conditions could bias the results. Permutation statistics, applied to control for it, allowed to reject this possibility.
Although Age was processed as a continuous variable in months in statistical analyses, for better clarity of results, graphs present also mean results by age in years.
Contributor Information
Kamila Śmigasiewicz, Email: kamila.smigasiewicz@univ-amu.fr.
Boris Burle, Email: boirs.burle@univ-amu.fr.
References
- Allain S., Carbonnell L., Burle B., Hasbroucq T., Vidal F. On-line executive control: an electromyographic study. Psychophysiology. 2004;41:113–116. doi: 10.1111/j.1469-8986.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Bonini F., Burle B., Liégeois-Chauvel C., Régis J., Chauvel P., Vidal F. Action monitoring and medial frontal cortex: leading role of supplementary motor area. Science. 2014;343:888–891. doi: 10.1126/science.1247412. [DOI] [PubMed] [Google Scholar]
- Botvinick M.M., Braver T.S., Barch D.M., Carter C.S., Cohen J.D. Conflict monitoring and cognitive control. Psychol. Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Brown J.W., Braver T.S. Learned predictions of error likelihood in the anterior cingulate cortex. Science. 2005;307:1118. doi: 10.1126/science.1105783. [DOI] [PubMed] [Google Scholar]
- Burle B., Possamaï C.-A., Vidal F., Bonnet M., Hasbroucq T. Executive control in the Simon effect: an electromyographic study and distributional analysis. Psychol. Res. 2002;66:324–336. doi: 10.1007/s00426-002-0105-6. [DOI] [PubMed] [Google Scholar]
- Burle B., Roger C., Allain S., Vidal F., Hasbroucq T. Error negativity does not reflect conflict: a reappraisal of conflict monitoring and anterior cingulate activity. J. Cogn. Neurosci. 2008;20(9):1637–1655. doi: 10.1162/jocn.2008.20110. [DOI] [PubMed] [Google Scholar]
- Burns J. Error-induced inhibition in a serial reaction time task. J. Exp. Psychol. 1971;90(1):141–148. doi: 10.1037/h0031335. [DOI] [PubMed] [Google Scholar]
- Carver A.C., Livesey D.J., Charles M. Age related changes in inhibitory control as measured by stop signal task performance. Int. J. Neuroscience. 2001;107:43–61. doi: 10.3109/00207450109149756. [DOI] [PubMed] [Google Scholar]
- Chatham C.H., Frank M.J., Munakata Y. Pupillometric and behavioral markers of a developmental shift in the temporal dynamics of cognitive control. Proc. Natl. Acad. Sci. U. S. A. 2009;106(14):5529–5533. doi: 10.1073/pnas.0810002106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P.L., Segalowitz S.J., Gavin W.J. Development of error monitoring event-related potentials in adolescents. In: Dahl R.E., Spear L.P., editors. Vol. 1021. 2004. pp. 324–328. (Adolescent Brain Development: Vulnerabilities and Opportunities). [DOI] [PubMed] [Google Scholar]
- Davies P.L., Segalowitz S.J., Gavin W.J. Development of response monitoring ERPs in 7-to 25-year-olds. Dev. Neuropsychol. 2004;25(3):355–376. doi: 10.1207/s15326942dn2503_6. [DOI] [PubMed] [Google Scholar]
- Diamond A. Executive functions. Annu. Rev. Psychol. 2013;64:135–168. doi: 10.1146/annurev-psych-113011-143750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppinger B., Mock B., Kray J. Developmental differences in learning and error processing: evidence from ERPs. Psychophysiology. 2009;46(5):1043–1053. doi: 10.1111/j.1469-8986.2009.00838.x. [DOI] [PubMed] [Google Scholar]
- Fairweather H. Choice reaction times in children: error and post-error responses, and the repetition effect. J. Exp. Child Psychol. 1978;26:407–418. [Google Scholar]
- Falkenstein M., Hohnsbein J., Hoormann J., Blanke L. Effects of crossmodal divided attention on late ERP components. II. Error processing in choice reaction tasks. Electroencephalogr. Clin. Neurophysiol. 1991;78:447–455. doi: 10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- Falkenstein M., Hoormann J., Christ S., Hohnsbein J. ERP components on reaction errors and their functional significance: a tutorial. Biol. Psychol. 2000;51:87–107. doi: 10.1016/s0301-0511(99)00031-9. [DOI] [PubMed] [Google Scholar]
- Ferdinand N.K., Kray J. Developmental changes in performance monitoring: how electrophysiological data can enhance our understanding of error and feedback processing in childhood and adolescence. Behav. Brain Res. 2014;263:122–132. doi: 10.1016/j.bbr.2014.01.029. [DOI] [PubMed] [Google Scholar]
- Ficarella S., Rochet N., Burle B. Becoming aware of subliminal responses: an EEG/EMG study on partial error detection and correction in humans. Cortex. 2019;120:443–456. doi: 10.1016/j.cortex.2019.07.007. [DOI] [PubMed] [Google Scholar]
- Gehring W.J., Goss B., Coles M.G.H., Meyer D.E., Donchin E. A neural system for error detection and compensation. Psychol. Sci. 1993;4:385–390. [Google Scholar]
- Gonthier C., Zira M., Colé P., Blaye A. Evidencing the developmental shift from reactive to proactive control in early childhood and its relationship to working memory. J. Exp. Child Psychol. 2019;177:1–16. doi: 10.1016/j.jecp.2018.07.001. [DOI] [PubMed] [Google Scholar]
- Grammar J.K., Carrasco M., Gehring W.J., Morrison F.J. Age-related changes in error processing in young children: a school-based investigation. Dev. Cogn. Neurosci. 2014;9:93–105. doi: 10.1016/j.dcn.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R., Kar B.R., Srinivasan N. Development of task-switching and post-error-slowing in children. Behav. Brain Funct. 2009;5(38) doi: 10.1186/1744-9081-5-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasbroucq T., Burle B., Vidal F., Possamai C.-A. Stimulus-hand correspondence and direct response activation: an electromyographic analysis. Psychophysiology. 2009;46(6):1160–1169. doi: 10.1111/j.1469-8986.2009.00865.x. [DOI] [PubMed] [Google Scholar]
- jamovi project . 2018. Jamovi (Version 0.9) [Computer Software]https://www.jamovi.org Retrieved from. [Google Scholar]
- Johnstone S.J., Dimoska A., Smith J.L., Barry R.J., Pleffer C.B., Chiswick D., Clarke A.R. The development of stop-signal and Go/Nogo response inhibition in children 7–12 years: performance and event-related potential indices. Int. J. Psychophysiol. 2007;63(1):25–38. doi: 10.1016/j.ijpsycho.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Kim E.Y., Iwaki N., Imashioya H., Uno H., Fujita T. Error-related negativity in a visual go/no-go task: children vs. adults. Dev. Psychol. 2007;31(2):181–191. doi: 10.1080/87565640701190775. [DOI] [PubMed] [Google Scholar]
- Ladouceur C.D., Dahl R.E., Carter C.S. ERP correlates of action monitoring in adolescence. Ann. N. Y. Acad. Sci. 2004;1021:329–336. doi: 10.1196/annals.1308.040. [DOI] [PubMed] [Google Scholar]
- Laming D. Choice reaction performance following an error. Acta Psychol. 1979;43:199–224. doi: 10.1016/0001-6918(79)90032-5. [DOI] [PubMed] [Google Scholar]
- Lucenet J., Blaye A. Age-related changes in the temporal dynamics of executive control: a study in 5- and 6-year-old children. Front. Psychol. 2014;5:1–11. doi: 10.3389/fpsyg.2014.00831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco-Pallares J., Camara E., Münte T.F., Rodriguez-Fornells A. Neural mechanisms underlying adaptive actions after slips. J. Cogn. Neurosci. 2008;20(9):1595–1610. doi: 10.1162/jocn.2008.20117. [DOI] [PubMed] [Google Scholar]
- Meckler C., Allain S., Carbonnell L., Hasbroucq T., Burle B., Vidal F. Executive control and response expectancy: a Laplatian study. Pychophysiology. 2011;48:303–311. doi: 10.1111/j.1469-8986.2010.01077.x. [DOI] [PubMed] [Google Scholar]
- Meyer A.M., Klein D.N., Torpey D.C., Kujawa A.J., Hayden E.P., Sheikh H.I., Singh S.M., Hajcek G. The additive effects of two dopamine genotypes on the error-related negativity (ERN) in children. Psychophysiology. 2012;49 doi: 10.1111/j.1601-183X.2012.00812.x. 57–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijers L.M.M., Teulings J.L.H.M., Eijkman E.G.J. Model of the electromyographic activity during brief isometric contractions. Biol. Cybern. 1976;25(1):7–16. doi: 10.1007/BF00337044. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S., Ridderinkhof K.R., Blom J., Band G.P.H., Kok A. Error-related brain potentials are differentially related to awareness of response errors: evidence from an anisaccade task. Psychophysiology. 2001;38:752–760. [PubMed] [Google Scholar]
- Notebaert W., Houtman F., Opstal F.V., Gevers W., Fias W., Verguts T. Post-error slowing: an orienting account. Cognition. 2009;111:275–279. doi: 10.1016/j.cognition.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Overbeek T.J.M., Nieuwenhuis S., Ridderinkhof K.R. Dissociable components of error processing: on the functional significance of the Pe vis-a-vis the ERN/Ne. J. Psychophysiol. 2005;19(4):319–329. [Google Scholar]
- Overbye K., Walhovd K.B., Paus T., Fjell A.M., Huster R.J., Tamnes C.H.K. Error processing in the adolescent brain: age-related differences in electrophysiology, behavioral adaptation, and brain morphology. Dev. Cogn. Neurosci. 2019;38:1–10. doi: 10.1016/j.dcn.2019.100665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirce J.W. PsychoPy – psychophysics software in Python. J. Neurosci. Methods. 2007;162(1-2):8–13. doi: 10.1016/j.jneumeth.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Possamaï C.A., Burle B., Osman A., Hasbroucq T. Partial advance information, number of alternatives, and motor processes: an electromyographic study. Acta Psychol. 2002;111:125–139. doi: 10.1016/s0001-6918(02)00019-7. [DOI] [PubMed] [Google Scholar]
- Rabbitt P.M.A. Errors and error correction in choice reaction tasks. J. Exp. Psychol. 1966;71:264–272. doi: 10.1037/h0022853. [DOI] [PubMed] [Google Scholar]
- Rabbitt P.M.A. Detection of errors by skilled typists. Ergonomics. 1978;21(11):945–958. [Google Scholar]
- Richardson C., Anderson M., Reid C.L., Fox A.M. Neural indicators of error processing and intraindividual variability in reaction time in 7 and 9 year-olds. Dev. Psychobiol. 2011;53(3):256–265. doi: 10.1002/dev.20518. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof K.R., van den Wildenberg W.P.M., Segalowitz S.J., Carter C.S. Neurocognitive mechanisms of cognitive control: the role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain Cogn. 2004;50(11):1157–1173. doi: 10.1016/j.bandc.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Rochet N., Spieser L., Casini L., Hasbroucq T., Burle B. Detecting and correcting partial errors: evidences for efficient control without conscious access. Cognit. Affect. Behav. Neurosci. 2014;14(3):970–982. doi: 10.3758/s13415-013-0232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger C., Nunez Castellar E., Pourtois G., Fias W. Changing your mind before it is too late: the electrophysiological correlates of online error correction during response selection. Psychophysiology. 2014;51:746–760. doi: 10.1111/psyp.12224. [DOI] [PubMed] [Google Scholar]
- Schachar R.J., Chen S., Logan G.D., Ornstein T.J., Crosbie J., Ickowicz A., Pakulak A. Evidence for an error monitoring deficit in attention deficit hyperactivity disorder. J. Abnorm. Child Psychol. 2004;32(3):285–293. doi: 10.1023/b:jacp.0000026142.11217.f2. [DOI] [PubMed] [Google Scholar]
- Simon J.R. The effect of an irrelevant directional cue on human information processing. In: Proctor R.W., Reeve T.G., editors. Stimulus-Response Compatibility: An Integrated Perspective. Elsevier; Amsterdam: 1990. pp. 31–38. [Google Scholar]
- Smulders S.F.A., Soetens E., van der Molen M.W. What happens when children encounter an error? Brain Cogn. 2016;104:34–47. doi: 10.1016/j.bandc.2016.02.004. [DOI] [PubMed] [Google Scholar]
- Spieser L., Servant M., Hasbroucq T., Burle B. Beyond decision! Motor contribution to speed-accuracy trade-off in decision-making. Psychon. Bull. Rev. 2014;24(3):950–956. doi: 10.3758/s13423-016-1172-9. [DOI] [PubMed] [Google Scholar]
- Tamnes Ch.K., Walhovd K.B., Torstveit M., Sells V.T., Fjell A.M. Performance monitoring in children and adolescents: a review of developmental changes in the error-related negativity and brain maturation. Dev. Cogn. Neurosci. 2013;6:13. doi: 10.1016/j.dcn.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillman C.M., Thorell L.B., Brocki K.C., Bohlin G. Motor response inhibition and execution in the stop-signal task: development and relation to ADHD behaviors. Child Neuropsychol. 2007;14(1):42–59. doi: 10.1080/09297040701249020. [DOI] [PubMed] [Google Scholar]
- van de Laar M.C., van den Wildenberg W.P., van Boxtel G.J., Huizenga H.M., van der Molen M.W. Lifespan changes in motor activation and inhibition during choice reactions: a Laplacian ERP study. Biol. Psychol. 2012;89:323–334. doi: 10.1016/j.biopsycho.2011.11.005. [DOI] [PubMed] [Google Scholar]
- van den Wildenberg W.P.M., van der Molen M.W. Developmental trends in simple and selective inhibition of compatible and incompatible responses. J. Exp. Child Psychol. 2004;87:201–220. doi: 10.1016/j.jecp.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Vidal G., Burle B., Bonnet M., Grapperon J., Hasbroucq T. Error negativity on correct trials: a reexamination of available data. Biol. Psychol. 2003;64:265–282. doi: 10.1016/s0301-0511(03)00097-8. [DOI] [PubMed] [Google Scholar]
- Wessel J.R., Danielmaier C., Morton J.B., Ullsperger M. Surprise and error: common neuronal architecture for the processing of errors and novelty. J. Neurosci. 2012;32(22):7528–7537. doi: 10.1523/JNEUROSCI.6352-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel J.R. An adaptive orienting theory of error processing. Psychophysiology. 2018;55:1–21. doi: 10.1111/psyp.13041. [DOI] [PubMed] [Google Scholar]
- Wiersema J.R., van der Meere J.J., Roeyers H. Developmental changes in error monitoring: an event-related potential study. Neuropsychologia. 2007;45(8):1649–1657. doi: 10.1016/j.neuropsychologia.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Yeung N., Botvinick M., Cohen J.D. The neural basis of error detection: conflict monitoring and error-related negativity. Psychol. Rev. 2004;111(4):931–959. doi: 10.1037/0033-295x.111.4.939. [DOI] [PubMed] [Google Scholar]
- Yordanova J., Kolev V., Albrecht B., Uebel H., Banaschewski T., Rothenberger A. May posterror performance be a critical factor for behavioral deficits in attention-deficit/hyperactivity disorder? Biol. Psychiatry. 2011;70(3):246–254. doi: 10.1016/j.biopsych.2011.02.026. [DOI] [PubMed] [Google Scholar]