1. Introduction
Cardiovascular disease and its complications such as myocardial infarction in particular, but also cerebrovascular disease with resulting stroke or peripheral arterial disease with acute limb ischemia or amputation are the leading causes of morbidity and mortality in the high-income countries world-wide [1]. In Europe, cardiovascular disease causes almost 4 million deaths per year, which accounts for almost 50% of all deaths [2]. Importantly, 30–40% of all patients who die from cardiovascular disease are younger than 75 years. Primary and secondary prevention aims at the efficient treatment of the modifiable risk factors, i.e. hypercholesteremia, diabetes mellitus, obesity and the fully established metabolic syndrome, arterial hypertension and smoking. Besides drug and interventional treatment, a healthy diet and an active lifestyle with at least modest regular exercise help reduce or even avoid cardiovascular complications. (See Table 1).
Table 1.
Studies with digital health interventions.
| First and last author | Study acronym | Number of participants (total/intervention vs control) | Primary endpoints | Successful (yes/no) | Type of DHI | doi |
|---|---|---|---|---|---|---|
| Widmer&Lerman | n/a | 80/40 vs. 40 | physical/biochemical metrics; behavorial characteristics | yes | online and smartphone | https://doi.org/10.1016/j.ahj.2017.02.016 |
| Anand&Beyene | n/a | 343/174 vs. 169 | MI (myocardial infarction) risk score | no | emails and text messages | https://doi.org/10.1001/jamacardio.2016.1035 |
| Martin&Blaha | mActive | 48/16 vs. 32 | change in steps/day | yes | text messages | https://doi.org/10.1161/JAHA.115.002239 |
| Glynn&Murphy | SMART MOVE | 90/41 vs. 37 | change in steps/day | yes | smartphone app | https://doi.org/10.3399/bjgp14X680461 |
| Torbjørnsen&Ribu | n/a | 101/51 vs. 50 | acceptability | yes | smartphone app | https://doi.org/10.2196/mhealth.8824 |
| Dallinga&Baart de la Faille-Deutekom | n/a | 3772/ 1976 vs. 1796 | running physical activity | yes | smartphone app | https://doi.org/10.1186/s12889-015–2165-8 |
| Litman&Robinson | n/a | 726/464 vs. 262 | physical activity (self-report) | yes | smartphone app | https://doi.org/10.2196/jmir.4142 |
| Turner-McGrievy&Tate | n/a | 85/48 vs. 37 | activity levels; dietary intake; weight loss | yes | smartphone app | https://doi.org/10.1136/amiajnl-2012–001510 |
| Patel&Hilbert | STEP UP | 602/451 vs.151 | change in steps/day | yes | wearable; gamification | https://doi.org/10.1001/jamainternmed.2019.3505 |
| Bennet&Miranda | Track | 351/176 vs. 175 | weight change | yes | smartphone app; smart scale; telephone calls | https://doi.org/10.1016/j.amepre.2018.07.005 |
| Block&Block | Alive-PD study | 339/163 vs. 176 | fasting glucose; HbA1c; body weight | yes | behavioural intervention via web app; internet; mobile phone; automated calls | https://doi.org/10.2196/jmir.4897 |
| Castro Sweet&Prewitt | n/a | 501 | body weight; glucose/HbA1c; lipid profile; well being | yes | web/mobile information and tracking combined with human coaching | https://doi.org/10.1177/0898264316688791 |
| Alonso-Domínguez& Recio-Rodríguez | EMID Study | 204/102 vs. 102 | adherence to Mediterranean diet | yes | smartphone app; workshop; exercise | https://doi.org/10.3390/nu11010162 |
| Frias&Osterberg | n/a | 109/80 vs. 29 | change of systolic blood pressure | yes | Digital medicine offerings (digital medicine, wearable sensor patch and mobile device app) | https://doi.org/10.2196/jmir.7833 |
| Morawski&Choudhry | MedISAFE-BP | 411/209 vs. 202 | medication adherence; change of systolic blood pressure | yes | Smartphone app (Medisafe app) | https://doi.org/10.1001/jamainternmed.2018.0447 |
| Johnston&Varenhorst | SUPPORT study | 174/91 vs. 83 | medication adherence | yes | smartphone app | https://doi.org/10.1016/j.ahj.2016.05.005 |
| Zhang&Wang | SBCHDP (smartphone-based coronary heart disease prevention) programme | 80/40 vs. 40 | perceived stress; behavioural risk factors | no (but positive tendency) | smartphone app (Care4Heart) | https://doi.org/10.1186/s12955-017–0623-y |
| Polizzi&Tolsma | Quit Smart | 97 (no control group, compared with published data) | smoking cessation | no | audiotape (accompanied by discount vouchers for nicotine replacement therapy, group sessions and a self-help manual) | https://doi.org/10.7812/tpp/03–048 |
| Brendryen&Kraft | Happy ending | 290/ 144 vs. 146 | abstinence from smoking | yes | internet and cell phone | https://doi.org/10.2196/jmir.1005 |
| Webb Hooper & Robinson | n/a | 140/ 70 vs. 70 | feasibility and process variables, including intervention evaluations, readiness to quit | yes | DVD | https://doi.org/10.1093/ntr/ntu079 |
| Burford & Hendrie | n/a | 160/ 80 vs. 80 | quit attempts at 6-month follow-up (self-reported and biochemically validated through testing for carbon monoxide (CO)) | yes | face aging software | https://doi.org/10.2196/jmir.2337 |
| Zeng & Bricker | n/a | 98 | descriptive analysis of user characteristics and utilization of a app for smoking cessation | – | smartphone app | https://doi.org/10.1089/tmj.2014.0232 |
| Heffner & Bricker | n/a | 76 | explorative analysis of most-used app features; prospective associations between feature usage and quitting | – | smartphone app | https://doi.org/10.3109/00952990.2014.977486 |
| Buller & Zimmerman | n/a | 102/ 51 vs. 51 | self-reported usability of REQ-Mobile and quitting behavior | no | smartphone app; text messaging | https://doi.org/10.1089/tmj.2013.0169 |
| BinDhim & Trevena | SSC App | 684/ 342 vs. 342 | smoking abstinence | yes | interactive smoking cessation decision-aid application | https://doi.org/10.1136/bmjopen-2017–017105 |
| Westmaas & Abroms | n/a | 1070/ 535 vs. 535 | abstinence from smoking | yes | https://doi.org/10.1136/tobaccocontrol-2016–053056 | |
| Lewis & Lyons | n/a | 35/ 19 intervention vs. 16 on waitlist (secondary mixed-method analysis) | descriptive analysis of social support patterns using a mobile app for PA | – | Jawbone Up24 activity monitor and Apple iPad Mini; Social support features in the UP app included comments and likes | https://doi.org/10.2196/12496 |
| Tong & Laranjo | n/a | 55 (secondary mixed-methods feasibility study) | descriptive analysis of users’ perspectives regarding mobile social networking interventions to promote physical activity | – | physical activity tracker and a wireless scale integrated with a social networking mobile app | https://doi.org/10.2196/11439 |
| Vandelanotte & Alley | n/a | 243/ 122 vs. 121 | increase in physical activity | yes | 8 modules of theory-based, personally tailored physical activity advice and action planning. Participants were randomized to receive the same intervention either with or without Fitbit tracker integration. | https://doi.org/10.2196/11321 |
| Devi & Singh | n/a | 94/ 48 vs. 46 | change in steps/day | yes | step count via accelerometer | https://doi.org/10.2196/jmir.3340 |
| Harris & Cook | PACE-UPPACE-Lift | PACE-UP: 236 (postal) vs. 231 (nurse support) vs. 214 (control) PACE-Lift: 108 vs. 117 (control) |
change in steps/day | yes | step count via pedometer +/- nurse counselling +/- postal counselling | https://doi.org/10.1371/journal.pmed.1002526 |
Cardiovascular diseases and all modifiable cardiovascular risk factors are chronic conditions and hence demand permanent treatment and care. Furthermore, their incidence is growing in association with a demographically aging population [3]. Projected office visits will increase in the years to come [3]. But in contrast, contacts between patients and physicians are steadily decreasing due to restricted personnel and infrastructural resources, leaving only a few minutes for a single consultation of primary care physicians in most European countries [4]. Although the need drastically increases, the number of trained specialists may not rise adequately and hence aggravate the mismatch of medical experts and the ever-growing patient population [3].
Added to this is a lack of patients’ adherence, that, combined with the missing health care infrastructure, significantly restricts patient outcomes despite definitive medical guidelines and steadily improving treatment possibilities.
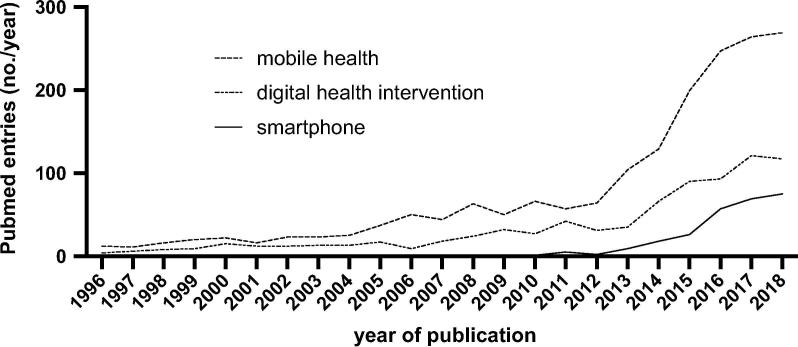
Novel digital interventions and their associated strategies have been tested in a variety of diseases and feature a recently growing number of scientific studies and evidence (Fig. 1). Especially in the period between a widely received review and statement of the American Heart Association in 2015 [5] and now, numerous interventions with a focus on newly available technologies has been tested. Wearables and smartwatches in particular have been probably most recognizably used for the detection of heart arrythmia and are discussed to change the diagnostics of arrythmias sustainably [6], [7], [8], [9]. Studies like the Apple Heart Study promise a higher rate of accurate disease detection than possible under common circumstances, however the significance of these fine-spun diagnostic algorithms and their pathological relevance need to be addressed independently [10]. Image transmission for remote consultations accelerates medical care over long distances and basically ensures availability even in rural areas [11]. Through the wide spread of personal computers and mobile phones with internet connection within the industrialized countries, digital interventions experience their current success story. According to the Dutch market research company Newzoo, the top 5 countries with an estimated smartphone penetration of about 80% (i.e. percentage of the population that owns and uses a smartphone) are the United Kingdom, Netherlands, Sweden, Germany and the United States of America in 2018 [12]. Data by our own group show, that among German patients with peripheral artery disease the mobile phone usage is about 40–60% even in the older patient population of 60 years and above, although we found a decrease in usage with increasing age
Fig. 1.
Number of PubMed-listed publications per year for Mobile Health & cardiovascular, Digital Health Intervention & cardiovascular and Smartphone & cardiovascular.
[13]. This still leaves approximately half of these patients sufficiently equipped for digital interventions at baseline, not considering patients who might acquire a device in order to optimize their treatment.
Digital devices promise a much greater empowerment of patients independent of structural needs and may even improve compliance and adherence to medical treatment regimen and a recommended lifestyle.
In this article, we will review the current approaches and possibilities of next generation patient care in the treatment of atherosclerotic disease and its modifiable risk factors.
2. Methods
A selective scientific literature review from published peer-reviewed work was performed, using the search terms digital health hintervention (DHI), eHealth, mobile health (mHealth), smartphone, phone, messaging, web or internet in combination with cardiovascular disease, vascular disease, cardiovascular risk factors, physical activity, metabolic syndrome, hypertension, diabetes mellitus, lipids, cholesterol, weight loss, obesity, adherence, smoking or smoking cessation. Searches were performed in PubMed, Google Scholar, Science Direct and Scopus. Results were filtered for adequate matches by the authors for adequate matches.
2.1. Digital interventions and physical results
Sufficient physical activity – i.e. at least 150 min of moderate exercise per week – has been identified as beneficial in many ways to promote health and has a central role in the secondary prevention for patients with cardiovascular disease [14]. On the contrary, a sedentary lifestyle is one of the leading risk factors for global mortality, but most adolescents and adults still do not meet the requirements of the current guidelines [15], [16]. Physical inactivity is on the rise not only in Europe or the USA, but affects general health globally in terms of cardiovascular and other non-communicable diseases [17]. Self-monitoring aims at the (at best permanent) modification of behavior [18].
One group performed a digital health intervention during cardiac rehabilitation after acute coronary syndrome. The intervention comprised an online website and a smartphone app, both with an exercise, dietary and weight diary including educational information during the course of a 12-week cardiac rehabilitation. After 90 and 180 days, patients with the digital intervention had a greater persisting weight loss (-5.1 vs −0.8 kg), but also less rehospitalization or visits in an emergency department [19]. A negative result despite a similar approach was in another study, who used text messages and emails to motivate patients to lower their estimated risk for a myocardial infarction. Subjects in both groups did not differ significantly at 12 months concerning their risk score or relevant outcome parameters like blood pressure, HbA1c, and waist-to-hip-ratios [20]. One possible explanation for the diverging results is a potential selection bias, due to the above-average motivation of all eligible participants and the high willingness to receive information about improving their activity and dietary lifestyle at baseline. Further, the mails and messages were addressed too impersonal in terms of the concerns of the study participants and also delivered at random intervals independent of the individual’s interest or need. In another trial, physical activity of subjects who attended a cardiovascular disease prevention program, was tracked with a specific smartphone app (Fitbug Orb). The participants received messages anytime they fall behind the aimed daily number of steps, what resulted in a significant increase in daily activity [21]. Finally, in a primary care setting, the simple use of an activity tracking app improved daily exercise [22].
Over the last years, several tools have been developed to support physical activity aiming for behavior change towards a more active lifestyle. Self-monitoring tools show both growth and a high user acceptance for the management of chronic diseases [23], [24], [25].
Through the fast advancements in the mobile phone sector, app-based mobile health (mHealth) technologies are perfectly suited to serve as a medium to deliver interventional strategies to support an improved health behavior. The increased availability of self-monitoring devices gave the opportunity to use these digital interventions as support for behavior change to implement a more active lifestyle on a large scale. Several studies focusing on self-monitoring using mHealth technologies are found to be associated with higher exercise levels, lower BMI, weight loss, and also healthier eating [26], [27], [28], but the overall effects seem to be modest over a longer period of time [29], [30], [31].
2.2. Novel aids for overweight and metabolic syndrome
Overweight and the metabolic syndrome are a global epidemic and closely related to cardiovascular morbidity [32]. Their treatment and prevention are essential aims in order to reduce cardiovascular disease, and also very suitable to be addressed by studies using innovative, digital strategies.
The TRACK trial combined a coaching/counseling system with self-monitoring including mobile phone app and e.g. a wifi-connected scale in the weight loss program of 351 obese adults. The intervention group had and even sustained a greater weight loss after 6 and 12 months. Further, subjects in the intervention group with a higher commitment to the program yielded better results than those with less [33].
Overweight and diabetes are closely related to the metabolic syndrome, especially in the case of type 2 diabetes. In order to address a digital solution for diabetes prevention and weight loss, tailored, algorithm-based mail, phone and web interventions (so called “fully automated behavioral intervention systems, FABIS”) were tested in obese, in average 55 years old (pre-)diabetics [34]. The program used a weekly, personalized phone contact for the first 6 months, and a biweekly rhythm for the next 6 months, combined with midweek phone calls and email reminders. It was compared with standard care and a delayed start of intervention after 6 months. The patients with FABIS had significantly improved their glycemic control, body weight and lipid profiles after 6 months compared with the standard care control subjects [5]. Another study coalesced online tutorials, personalized human coaching and digital tracking tools in order to reduce the risk of diabetes in 501 participants with prediabetes and/or metabolic syndrome [35]. After a total of 12 months, the patients had lost 7.5% of their body weight and reduced their Hb1Ac for 0.14%. Unfortunately, the study lacked a proper control group, which weakens the significance of the findings, but still contributes to the ongoing debate.
Motivational stimuli are essential for all self-dependent elements in medical interventions. For the individual subjects in a cohort, this may vary from sole self-improvement to head-on-head competition. The suitable type of those stimuli or even the combination of it are necessary to make the digital, self-controlled intervention work. A trial in healthy subjects of the Danish healthcare systems used a web-/mobile-app based tool and tested the benefits of a digital intervention in terms of weight lost, body fat and lipid profiles. Whereas the overall result revealed the difficulty of sustaining motivation to adhere to such tools, the in-detail analysis again showed (mild) improvements of waist circumference, body fat percentage and weight.
The EMID study (Effectiveness of A Multifactorial Intervention in Increasing Adherence to the Mediterranean Diet) [36] investigated the adherence to the Mediterranean diet which has been proven to benefit or even prevent atherosclerotic disease [37], [38], [39], [40], [41]. In that randomized, controlled trial, all subjects received detailed counseling on the diet, and quality and amount of cardioprotective physical exercise. The intervention group (IG) additionally received a smartphone app for 3 months. After 3 months, IG had a better adherence to the Mediterranean diet. This effect was by trend persistent until the second follow-up after a total of 12 months. However, lipid or glycemic parameters did not significantly change [36].
2.3. Adherence to therapeutic regimen
The counseling for a healthier dietary regimen is one potential target for digital interventions, but to increase the adherence to medication and/or regular outpatient visits is another that deeply matters in the treatment of cardiovascular disease and its complications.
A huge challenge to surveil medical adherence is the assessment of actual intake of pills in everyday routine. This issue has been elegantly addressed by Frias et al., who put sensor leaded placebos in the pill box of patients with hypertension and type 2 diabetes. The sensor, once ingested, provided a feedback signal to a wearable sensor patch and allowed an estimation of therapy compliance or inertia. The data were reviewed by patients, treating physicians and investigators and adapted if necessary. As a consequence, the patients featured a significantly improved blood pressure, lower LDL cholesterol and better HbA1c [42].
Nonadherence to medical treatment may account for half of the patients with uncontrolled arterial hypertension. One group used the Medisafe app, a smartphone app that works with reminder alerts, regular adherence reports and even peer support, and evaluated self-reported adherence and the impact on blood pressure. The Morisky Medication Adherence Scale showed a significant improvement in patients with the Medisafe app, however, both groups showed a decrease of systolic blood pressure of 10 mmHg after 12 weeks without significant difference between groups, pointing to a lack of extra value of the easy-to-use app system [43]. The SUPPORT study (A study to evaluate the use of mobile-phone based patient support in patients diagnosed with MI) used a web-based application to track drug adherence with an e-diary and also provide information after myocardial infarction. Whereas drug adherence was expectedly better in the app group, the authors also presented significantly higher effects for LDL lowering and patient satisfaction, but only numerical, non-significant differences in exercise or smoking cessation [44].
Another pillar of today's state-of-the-art treatment is the so called shared decision-making, which is based on partnership-based and equal decision-making by all relevant actors in the disease process [45]. Instead of simple data transmission, the implementation of shared decision-making elements additionally promotes knowledge about disease and treatment concepts and improves medication adherence, disease awareness, and self-management of chronic diseases. Through the process of shared decision-making, patients can take responsibility for their own health, which is called patient empowerment. The combination between digital interventions and the increase in patient empowerment might be promising.
In the 4-week long Smartphone-Based Coronary Heart Disease Prevention (SBCHDP) program, subjects were either briefed and reminded by the Care4Heart app about coronary heart disease (CHD), or recommended website on CHD topics as control. Of note, most of the participants did not have established CHD, thus the primary outcomes were knowledge, perceived stress and behavioral risk factors. After the 4-week period, the intervention group with the Care4Heart app had significantly better CHD awareness and behavior as measured by lower cholesterol levels [46].
2.4. Smart interventions for smoking cessation
In 2004, a study was published, in which they had provided smokers with health instructions in group sessions, discount vouchers for nicotine replacement patches, education materials and interestingly audiotapes for hypnosis and relaxation [47]. This multimodal QuitSmart package was associated with significantly more smoking cessation than the standard care group. The authors found that nicotine patch and daily exercise majorly contribute to the success, but although the audiotape was an interesting, self-empowering component of it, it was omitted in the final analysis [47].
A first real digital cell (and not smart-, but rather dumb-) phone intervention study was published 2008 [48]. The Happy Ending (HE) program took 1 year and was delivered via Internet and cell phone, with some hundred contacts per subject in the course of the program. All components were already fully automated. During the trial period, patients with HE more frequently stopped smoking or were planning and preparing to do. However, the authors found that beyond 1 month (OR 3.46 for abstinence in HE group compared with control), a growing number of patients in both groups relapsed, the overall significant differences between HE and control persisted until 6 months (OR 2.59) and retained a clear trend until 12 months (OR 1.66, p = 0.07) [48].
Another media approach with a specifically, culturally-tailored DVD instead of a standard film yielded better quit rates in African-Americans at the follow-up after 1 month [49].
In Australia, an artificially changed photo was shown to half of the participants of another study, that simulated the aging process in a digital photo if or if not the person will quit smoking. All participants received advice for smoking cessation. The group with subjects who had seen their photoaging had a significantly higher rate of successful cessation when compared with standard care. Of note, in both groups half of the participants who indicated having stopped smoking were still tested positive with a CO breath analyzer, but that did not change the overall results [50].
Due to the wide acceptance and distribution of devices, a new generation of studies finally used smartphone apps to aid smoking cessation. Zeng et al. defined lower education, heavy smoking (greater than10 cigarettes per day for at least the past 30 days) and depression as relevant predictors for patients not to use a smartphone app [51]. The same group examined which parts of an app appeal to subjects and stated, that the users of their app SmartQuit mainly used features that are classically summarized as cognitive behavioral therapy (i.e. tracking, sharing, progress), with only 2 features (viewing a “quit plan” and practice of “letting urges pass”) being significantly associated with quitting [52]. Buller et al. tested a smartphone app vs. text messaging in a group of young (18–30 years old) smokers to achieve abstinence in 2014. The efficacy of text messaging for smoking cessation has been confirmed in a variety of studies before. Both interventions were finally used in about 60% of participants for 30 days, which was the end of the period of intervention. Whereas text messaging was slightly superior for the initial cessation – mainly due to the simple usage compared with the smartphone app- , the absolute abstinence until 12 weeks after start of the study lasted longer in the app group [53]. One of the biggest trials in the field with 684 participants tested a multi-functional app including information, motivational messages, diary and additional benefits vs an information-only version of the app. The subjects with a fully functional app showed a 1-month abstinence of 28.5% (vs 16.9%). However, the great effect at first steadily declined to a 6-months abstinence of 10.2% vs 4.8% in the control group [54].
These findings underline how important single, user-friendly elements of smartphone apps are to finally enable patient empowerment, but also how difficult persistence of non-smoking and adherence to the chosen therapeutic modality will be in real world settings.
A highly patient-centered, very efficient approach was used in another study [55]. Patients received either a variety of tailored, i.e. personalized emails with information and motivation on how to quit smoking and persist a smoke-free life, few still tailored emails or general emails. Only patients with frequent, individual messages showed a significantly higher rate of smoking cessation, whereas both other groups fell behind in an equal measure, pointing out the relevance of individual, but also frequent and constant reminders.
2.5. Impact of web-based communities
The implementation of behavior change theories in apps for physical activity is a relatively new phenomenon [56], [57]. Although, extended research in this field is still missing, so called social support has been identified as a major engagement tool and was shown to be associated to sustained behavior change [58], [59]. A common way to integrate social support into apps is via web-based social networking. This is mainly attached to setting up a personal profile, that can share personal activity and connect to other users, that includes functionalities like assessment through “likes” and comments of other users. Web-based solutions, in contrast to face-to face intervention, offers the benefits of time and cost savings, and include a wide reachability, immediate feedback from the peer group and if wanted also anonymity. Web-Based computer-tailored physical activity interventions were already shown to significantly increased intervention effectiveness [60], [61]. Nevertheless, it has been suggested that the support provided by web-based social networking platforms may mimic the support achieved through face-to- face interventions.
Two major forms of web-based social networking platforms exist: the implementation into already existing platforms (e.g. Twitter or Facebook) and the direct implementation into commercial or researcher-derived health apps.
Results regarding the effectiveness of social network interventions showed an increase in physical activity, but the generalizability is limited due to the heterogeneity of the analyzed studies [62], [63]. In summary, interventions implemented or delivered via web-based social networks were found to have the capacity to modify health-promoting behavior. This might result in a heightened effectiveness in their capacity to reach large audiences and sustain high levels of engagement.
2.6. Current barriers, gaps and future possibilities
The efficacy of digital interventions is significantly influenced by the single person’s engagement with e.g. a specific app [64]. Major limiting factor is the adherence in terms of long-term engagement: only a minority use health and activity apps for more than 6 months, the vast majority rapidly loses interest and finally stops using the apps [65], [66]. This concerns not only healthy subjects who aim for a healthier lifestyle, but importantly also secondary prevention where mainly long-term behavioral changes towards a more active lifestyle are associated with health benefits [67], [68]. Strategies that improve user engagement linked to these technologies may include elements of gamification [29], [69] and devices deeply intertwined with everyday life like smartphones, wearables, or smart homes with fridges or entertainment systems [70]that deliver instant feedback of good or harmful behavior.
The major limitations of scientific studies with digital health interventions are mainly a) the limited study time of mostly only a few weeks or months, b) endpoints with surrogate parameters or self-reports rather than major cardiovascular events like myocardial infarction or re-hospitalization, and finally c) the possibility of a selection bias, because only subjects with the necessary interest and also technical requirements if demanded were included in the majority of studies.
Scientific studies on health psychology including health behavior models, behavioral change techniques, and motivational interviewing and coaching are not worked up systematically [71], [72], hence digital coaching which is based on these data is currently limited. Finally, a relevant impact on medical health care infrastructure and especially its relief from overload still need to be addressed. It will require almost complete independence from human resources like e.g. counselors, which may be possible with the latest, smartphone or web-based alone interventions.
These limitations must not cloud the great possibilities of innovative digital interventions. From the studies in this review one may clearly deduce that well composed digital tools like apps with balanced general, but also personal, individual user interaction have a significant impact on primary and secondary prevention at least for a limited time. From our current point of view, digitization has already changed our healthcare system and patient care sustainably and will most likely become even more prominent in the near future.
Declaration of Competing Interest
The authors have nothing to declare.
References
- 1.Mahmood S.S., Levy D., Vasan R.S., Wang T.J. The Framingham Heart Study and the epidemiology of cardiovascular disease: a historical perspective. Lancet. 2014;383:999–1008. doi: 10.1016/S0140-6736(13)61752-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.N. Townsend, L. Wilson, P. Bhatnagar, K. Wickramasinghe, M. Rayner, M. Nichols, Cardiovascular disease in Europe: epidemiological update 2016., Eur Heart J. 37 (2016) 3232–3245. doi:10.1093/eurheartj/ehw334 [DOI] [PubMed]
- 3.Dall T.M., Gallo P.D., Chakrabarti R., West T., Semilla A.P., Storm M.V. The care span: an aging population and growing disease burden will require a large and specialized health care workforce by 2025. Health Aff. 2013;32:2013–2020. doi: 10.1377/hlthaff.2013.0714. [DOI] [PubMed] [Google Scholar]
- 4.Irving G., Neves A.L., Dambha-Miller H., Oishi A., Tagashira H., Verho A., Holden J. International variations in primary care physician consultation time: a systematic review of 67 countries. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2017-017902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burke L.E., Ma J., Azar K.M.J., Bennett G.G., Peterson E.D., Zheng Y., Riley W., Stephens J., Shah S.H., Suffoletto B., Turan T.N., Spring B., Steinberger J., Quinn C.C. Current Science on Consumer Use of Mobile Health for Cardiovascular Disease Prevention: A Scientific Statement from the American Heart Association. 2015 doi: 10.1161/CIR.0000000000000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tison G.H., Sanchez J.M., Ballinger B., Singh A., Olgin J.E., Pletcher M.J., Vittinghoff E., Lee E.S., Fan S.M., Gladstone R.A., Mikell C., Sohoni N., Hsieh J., Marcus G.M. Passive Detection of Atrial Fibrillation Using a Commercially Available Smartwatch. JAMA Cardiol. 2018;3:409. doi: 10.1001/jamacardio.2018.0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Samol A., Bischoff K., Luani B., Pascut D., Wiemer M., Kaese S. Recording of Bipolar Multichannel ECGs by a Smartwatch: Modern ECG Diagnostic 100 Years after Einthoven. Sensors. 2019;19:2894. doi: 10.3390/s19132894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.L. Krivoshei, S. Weber, T. Burkard, A. Maseli, N. Brasier, M. Kühne, D. Conen, T. Huebner, A. Seeck, J. Eckstein, Smart detection of atrial fibrillation, Europace 19 (2016) euw125. doi:10.1093/europace/euw125 [DOI] [PMC free article] [PubMed]
- 9.Koshy A.N., Sajeev J.K., Nerlekar N., Brown A.J., Rajakariar K., Zureik M., Wong M.C., Roberts L., Street M., Cooke J., Teh A.W. Smart watches for heart rate assessment in atrial arrhythmias. Int J Cardiol. 2018;266:124–127. doi: 10.1016/j.ijcard.2018.02.073. [DOI] [PubMed] [Google Scholar]
- 10.Turakhia M.P., Desai M., Hedlin H., Rajmane A., Talati N., Ferris T., Desai S., Nag D., Patel M., Kowey P., Rumsfeld J.S., Russo A.M., Hills M.T., Granger C.B., Mahaffey K.W., Perez M.V. Rationale and design of a large-scale, app-based study to identify cardiac arrhythmias using a smartwatch: The Apple Heart Study. Am Heart J. 2019;207:66–75. doi: 10.1016/j.ahj.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peng W., Yuan S., Holtz B.E. Exploring the Challenges and Opportunities of Health Mobile Apps for Individuals with Type 2 Diabetes Living in Rural Communities. Telemed e-Health. 2016;22:733–738. doi: 10.1089/tmj.2015.0180. [DOI] [PubMed] [Google Scholar]
- 12.Newzoo, Global Mobile Market Report, Https://Newzoo.Com/Insights/Rankings/Top-Countries-by-Smartphone-Penetration-and-Users/. (2018).
- 13.Lortz J., Simanovski J., Kuether T., Kreitschmann-andermahr I., Ullrich G., Steinmetz M., Rammos C., Jánosi R.A., Moebus S., Rassaf T., Paldán K. Needs and requirements of patients with peripheral arterial disease – a patient-centred approach to designing mobile interventions Table of Contents. JMIR Prepr. X. 2019 doi: 10.2196/15669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeong S.-W., Kim S.-H., Kang S.-H., Kim H.-J., Yoon C.-H., Youn T.-J., Chae I.-H. Mortality reduction with physical activity in patients with and without cardiovascular disease. Eur. Heart J. 2019:1–9. doi: 10.1093/eurheartj/ehz564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamer M., O’Donovan G., Murphy M. Physical Inactivity and the Economic and Health Burdens Due to Cardiovascular Disease: Exercise as Medicine. Adv. Exp. Med. Biol. 2017;999:3–18. doi: 10.1007/978-981-10-4307-9_1. [DOI] [PubMed] [Google Scholar]
- 16.Young D.R., Hivert M.-F., Alhassan S., Camhi S.M., Ferguson J.F., Katzmarzyk P.T., Lewis C.E., Owen N., Perry C.K., Siddique J., Yong C.M. Physical Activity Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Functional Genomics and Translational Biology; and Stroke Council, Sedentary Behavior and Cardiovascular Morbidity and Mortality: A Science Advisory From the American Heart Association. Circulation. 2016;134:e262–e279. doi: 10.1161/CIR.0000000000000440. [DOI] [PubMed] [Google Scholar]
- 17.GBD Mortality and Causes of Death Collaborators, Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet (London, England) 2015;388(2016):1459–1544. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Michie S., Richardson M., Johnston M., Abraham C., Francis J., Hardeman W., Eccles M.P., Cane J., Wood C.E. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med. 2013;46:81–95. doi: 10.1007/s12160-013-9486-6. [DOI] [PubMed] [Google Scholar]
- 19.Widmer R.J., Allison T.G., Lennon R., Lopez-Jimenez F., Lerman L.O., Lerman A. Digital health intervention during cardiac rehabilitation: a randomized controlled trial. Am Heart J. 2017;188:65–72. doi: 10.1016/j.ahj.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 20.Anand S.S., Samaan Z., Middleton C., Irvine J., Desai D., Schulze K.M., Sothiratnam S., Hussain F., Shah B.R., Pare G., Beyene J., Lear S.A. South Asian Heart Risk Assessment Investigators, A Digital Health Intervention to Lower Cardiovascular Risk: A Randomized Clinical Trial. JAMA Cardiol. 2016;1:601–606. doi: 10.1001/jamacardio.2016.1035. [DOI] [PubMed] [Google Scholar]
- 21.Martin S.S., Feldman D.I., Blumenthal R.S., Jones S.R., Post W.S., McKibben R.A., Michos E.D., Ndumele C.E., Ratchford E.V., Coresh J., Blaha M.J. mActive: A Randomized Clinical Trial of an Automated mHealth Intervention for Physical Activity Promotion. J Am Heart Assoc. 2015;4:1–9. doi: 10.1161/JAHA.115.002239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.L.G. Glynn, P.S. Hayes, M. Casey, F. Glynn, A. Alvarez-Iglesias, J. Newell, G. OLaighin, D. Heaney, M. O’Donnell, A.W. Murphy, Effectiveness of a smartphone application to promote physical activity in primary care: the SMART MOVE randomised controlled trial., Br J Gen Pract. 64 (2014) e384-91. doi:10.3399/bjgp14X680461 [DOI] [PMC free article] [PubMed]
- 23.Anderson K., Burford O., Emmerton L. Mobile Health Apps to Facilitate Self-Care: A Qualitative Study of User Experiences. PLoS One. 2016;11:e0156164. doi: 10.1371/journal.pone.0156164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burkow T.M., Vognild L.K., Johnsen E., Bratvold A., Risberg M.J. Promoting exercise training and physical activity in daily life: a feasibility study of a virtual group intervention for behaviour change in COPD. BMC Med Inform Decis Mak. 2018;18:136. doi: 10.1186/s12911-018-0721-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Torbjørnsen A., Småstuen M.C., Jenum A.K., Årsand E., Ribu L. Acceptability of an mHealth App Intervention for Persons With Type 2 Diabetes and its Associations With Initial Self-Management: Randomized Controlled Trial. JMIR MHealth UHealth. 2018;6:e125. doi: 10.2196/mhealth.8824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.J.M. Dallinga, M. Mennes, L. Alpay, H. Bijwaard, M. Baart de la Faille-Deutekom, App use, physical activity and healthy lifestyle: a cross sectional study. BMC Public Health. 15 (2015) 833. doi:10.1186/s12889-015-2165-8 [DOI] [PMC free article] [PubMed]
- 27.Litman L., Rosen Z., Spierer D., Weinberger-Litman S., Goldschein A., Robinson J. Mobile Exercise Apps and Increased Leisure Time Exercise Activity: A Moderated Mediation Analysis of the Role of Self-Efficacy and Barriers. J Med Internet Res. 2015;17:e195. doi: 10.2196/jmir.4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turner-McGrievy G.M., Beets M.W., Moore J.B., Kaczynski A.T., Barr-Anderson D.J., Tate D.F. Comparison of traditional versus mobile app self-monitoring of physical activity and dietary intake among overweight adults participating in an mHealth weight loss program. J Am Med Inform Assoc. 2013;20:513–518. doi: 10.1136/amiajnl-2012-001510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel M.S., Small D.S., Harrison J.D., Fortunato M.P., Oon A.L., Rareshide C.A.L., Reh G., Szwartz G., Guszcza J., Steier D., Kalra P., Hilbert V. Effectiveness of Behaviorally Designed Gamification Interventions With Social Incentives for Increasing Physical Activity Among Overweight and Obese Adults Across the United States: The STEP UP Randomized Clinical Trial. JAMA Intern. Med. 2019:1–9. doi: 10.1001/jamainternmed.2019.3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Romeo A., Edney S., Plotnikoff R., Curtis R., Ryan J., Sanders I., Crozier A., Maher C. Can Smartphone Apps Increase Physical Activity? Systematic Review and Meta-Analysis. J. Med. Internet Res. 2019;21:e12053. doi: 10.2196/12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.D. Baretta, P. Bondaronek, A. Direito, P. Steca, Implementation of the goal-setting components in popular physical activity apps: Review and content analysis., Digit Heal. 5 (n.d.) 2055207619862706. doi:10.1177/2055207619862706 [DOI] [PMC free article] [PubMed]
- 32.Cercato C., Fonseca F.A. Cardiovascular risk and obesity. Diabetol Metab Syndr. 2019;11:74. doi: 10.1186/s13098-019-0468-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bennett G.G., Steinberg D., Askew S., Levine E., Foley P., Batch B.C., Svetkey L.P., Bosworth H.B., Puleo E.M., Brewer A., DeVries A., Miranda H. Effectiveness of an App and Provider Counseling for Obesity Treatment in Primary Care. Am J Prev Med. 2018;55:777–786. doi: 10.1016/j.amepre.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Block G., Azar K.M.J., Romanelli R.J., Block T.J., Hopkins D., Carpenter H.A., Dolginsky M.S., Hudes M.L., Palaniappan L.P., Block C.H. Diabetes Prevention and Weight Loss with a Fully Automated Behavioral Intervention by Email, Web, and Mobile Phone: A Randomized Controlled Trial Among Persons with Prediabetes. J Med Internet Res. 2015;17:e240. doi: 10.2196/jmir.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Castro Sweet C.M., Chiguluri V., Gumpina R., Abbott P., Madero E.N., Payne M., Happe L., Matanich R., Renda A., Prewitt T. Outcomes of a Digital Health Program With Human Coaching for Diabetes Risk Reduction in a Medicare Population. J Aging Health. 2018;30:692–710. doi: 10.1177/0898264316688791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alonso-Domínguez R., García-Ortiz L., Patino-Alonso M.C., Sánchez-Aguadero N., Gómez-Marcos M.A., Recio-Rodríguez J.I. Effectiveness of a multifactorial intervention in increasing adherence to the mediterranean diet among patients with diabetes mellitus type 2: a controlled and randomized study (EMID study) Nutrients. 2019;11 doi: 10.3390/nu11010162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shai I., Spence J.D., Schwarzfuchs D., Henkin Y., Parraga G., Rudich A., Fenster A., Mallett C., Liel-Cohen N., Tirosh A., Bolotin A., Thiery J., Fiedler G.M., Blüher M., Stumvoll M., Stampfer M.J. Dietary Intervention to Reverse Carotid Atherosclerosis. Circulation. 2010;121:1200–1208. doi: 10.1161/CIRCULATIONAHA.109.879254. [DOI] [PubMed] [Google Scholar]
- 38.de Lorgeril M., Salen P. Mediterranean diet in secondary prevention of CHD. Public Health Nutr. 2011;14:2333–2337. doi: 10.1017/S136898001100259X. [DOI] [PubMed] [Google Scholar]
- 39.Camargo A., Delgado-Lista J., Garcia-Rios A., Cruz-Teno C., Yubero-Serrano E.M., Perez-Martinez P., Gutierrez-Mariscal F.M., Lora-Aguilar P., Rodriguez-Cantalejo F., Fuentes-Jimenez F., Tinahones F.J., Malagon M.M., Perez-Jimenez F., Lopez-Miranda J. Expression of proinflammatory, proatherogenic genes is reduced by the Mediterranean diet in elderly people. Br J Nutr. 2012;108:500–508. doi: 10.1017/S0007114511005812. [DOI] [PubMed] [Google Scholar]
- 40.Sleiman D., Al-Badri M.R., Azar S.T. Effect of Mediterranean Diet in Diabetes Control and Cardiovascular Risk Modification: A Systematic Review. Front Public Heal. 2015;3:1–8. doi: 10.3389/fpubh.2015.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruiz-Canela M., Estruch R., Corella D., Salas-Salvadó J., Martínez-González M.A. Association of Mediterranean diet with peripheral artery disease: the PREDIMED randomized trial. JAMA - J Am Med Assoc. 2014;311:415–417. doi: 10.1001/jama.2013.280618. [DOI] [PubMed] [Google Scholar]
- 42.Frias J., Virdi N., Raja P., Kim Y., Savage G., Osterberg L. Effectiveness of Digital Medicines to Improve Clinical Outcomes in Patients with Uncontrolled Hypertension and Type 2 Diabetes: Prospective, Open-Label, Cluster-Randomized Pilot Clinical Trial. J Med Internet Res. 2017;19:e246. doi: 10.2196/jmir.7833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morawski K., Ghazinouri R., Krumme A., Lauffenburger J.C., Lu Z., Durfee E., Oley L., Lee J., Mohta N., Haff N., Juusola J.L., Choudhry N.K. Association of a smartphone application with medication adherence and blood pressure control: the MedISAFE-BP randomized clinical trial. JAMA Intern Med. 2018;178:802–809. doi: 10.1001/jamainternmed.2018.0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnston N., Bodegard J., Jerström S., Åkesson J., Brorsson H., Alfredsson J., Albertsson P.A., Karlsson J.-E., Varenhorst C. Effects of interactive patient smartphone support app on drug adherence and lifestyle changes in myocardial infarction patients: a randomized study. Am Heart J. 2016;178:85–94. doi: 10.1016/j.ahj.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 45.Kambhampati S., Ashvetiya T., Stone N.J., Blumenthal R.S., Martin S.S. Shared Decision-Making and Patient Empowerment in Preventive Cardiology. Curr Cardiol Rep. 2016;18:49. doi: 10.1007/s11886-016-0729-6. [DOI] [PubMed] [Google Scholar]
- 46.Zhang H., Jiang Y., Nguyen H.D., Poo D.C.C., Wang W. The effect of a smartphone-based coronary heart disease prevention (SBCHDP) programme on awareness and knowledge of CHD, stress, and cardiac-related lifestyle behaviours among the working population in Singapore: a pilot randomised controlled trial. Health Qual Life Outcomes. 2017;15:49. doi: 10.1186/s12955-017-0623-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Polizzi K.M., Roblin D.W., Mims A.D., Harris D., Tolsma D.D. Factors Associated with Smoking Cessation Among Quit Smart(TM) Participants. Perm J. 2004;8:28–33. doi: 10.7812/tpp/04.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.H. Brendryen, F. Drozd, P. Kraft, A digital smoking cessation program delivered through internet and cell phone without nicotine replacement (happy ending): randomized controlled trial. J Med Internet Res 10 (2008) e51. doi:10.2196/jmir.1005 [DOI] [PMC free article] [PubMed]
- 49.M. Webb Hooper, E.A. Baker, R.G. Robinson, Efficacy of a DVD-based smoking cessation intervention for African Americans., Nicotine Tob Res. 16 (2014) 1327–35. doi:10.1093/ntr/ntu079 [DOI] [PMC free article] [PubMed]
- 50.Burford O., Jiwa M., Carter O., Parsons R., Hendrie D. Internet-based photoaging within australian pharmacies to promote smoking cessation: Randomized controlled trial. J Med Internet Res. 2013;15:1–13. doi: 10.2196/jmir.2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zeng E.Y., Vilardaga R., Heffner J.L., Mull K.E., Bricker J.B. Predictors of Utilization of a Novel Smoking Cessation Smartphone App. Telemed e-Health. 2015;21:998–1004. doi: 10.1089/tmj.2014.0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heffner J.L., Vilardaga R., Mercer L.D., Kientz J.A., Bricker J.B. Feature-level analysis of a novel smartphone application for smoking cessation. Am J Drug Alcohol Abuse. 2015;41:68–73. doi: 10.3109/00952990.2014.977486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Buller D.B., Borland R., Bettinghaus E.P., Shane J.H., Zimmerman D.E. Randomized trial of a smartphone mobile application compared to text messaging to support smoking cessation. Telemed e-Health. 2014;20:206–214. doi: 10.1089/tmj.2013.0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.BinDhim N.F., McGeechan K., Trevena L. Smartphone Smoking Cessation Application (SSC App) trial: a multicountry double-blind automated randomised controlled trial of a smoking cessation decision-aid “app”. BMJ Open. 2018;8:e017105. doi: 10.1136/bmjopen-2017-017105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Westmaas J.L., Bontemps-Jones J., Hendricks P.S., Kim J., Abroms L.C. Randomised controlled trial of stand-alone tailored emails for smoking cessation. Tob Control. 2018;27:136–146. doi: 10.1136/tobaccocontrol-2016-053056. [DOI] [PubMed] [Google Scholar]
- 56.P. Bondaronek, G. Alkhaldi, A. Slee, F.L. Hamilton, E. Murray, Quality of Publicly Available Physical Activity Apps: Review and Content Analysis., JMIR MHealth UHealth. 6 (2018) e53. doi:10.2196/mhealth.9069 [DOI] [PMC free article] [PubMed]
- 57.Conroy D.E., Yang C.-H., Maher J.P. Behavior change techniques in top-ranked mobile apps for physical activity. Am J Prev Med. 2014;46:649–652. doi: 10.1016/j.amepre.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 58.Lewis Z.H., Swartz M.C., Martinez E., Lyons E.J. Social Support Patterns of Middle-Aged and Older Adults Within a Physical Activity App: Secondary Mixed Method Analysis. JMIR Aging. 2019;2:e12496. doi: 10.2196/12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.H.L. Tong, E. Coiera, L. Laranjo, Using a Mobile Social Networking App to Promote Physical Activity: A Qualitative Study of Users’ Perspectives., J Med Internet Res. 20 (2018) e11439. doi:10.2196/11439 [DOI] [PMC free article] [PubMed]
- 60.Vandelanotte C., Duncan M.J., Maher C.A., Schoeppe S., Rebar A.L., Power D.A., Short C.E., Doran C.M., Hayman M.J., Alley S.J. The Effectiveness of a Web-Based Computer-Tailored Physical Activity Intervention Using Fitbit Activity Trackers: Randomized Trial. J Med Internet Res. 2018;20:e11321. doi: 10.2196/11321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Devi R., Powell J., Singh S. A web-based program improves physical activity outcomes in a primary care angina population: randomized controlled trial. J Med Internet Res. 2014;16:e186. doi: 10.2196/jmir.3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jahangiry L., Farhangi M.A., Shab-Bidar S., Rezaei F., Pashaei T. Web-based physical activity interventions: a systematic review and meta-analysis of randomized controlled trials. Public Health. 2017;152:36–46. doi: 10.1016/j.puhe.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 63.Harris T., Kerry S.M., Limb E.S., Furness C., Wahlich C., Victor C.R., Iliffe S., Whincup P.H., Ussher M., Ekelund U., Fox-Rushby J., Ibison J., DeWilde S., McKay C., Cook D.G. Physical activity levels in adults and older adults 3–4 years after pedometer-based walking interventions: Long-term follow-up of participants from two randomised controlled trials in UK primary care. PLoS Med. 2018;15:e1002526. doi: 10.1371/journal.pmed.1002526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.M. Elliott, F. Eck, E. Khmelev, A. Derlyatka, O. Fomenko, Physical Activity Behavior Change Driven by Engagement With an Incentive-Based App: Evaluating the Impact of Sweatcoin. JMIR MHealth UHealth. 7 (2019) e12445. doi:10.2196/12445 [DOI] [PMC free article] [PubMed]
- 65.Grady A., Yoong S., Sutherland R., Lee H., Nathan N., Wolfenden L. Improving the public health impact of eHealth and mHealth interventions. Aust NZ J Public Health. 2018;42:118–119. doi: 10.1111/1753-6405.12771. [DOI] [PubMed] [Google Scholar]
- 66.Jee H. Review of researches on smartphone applications for physical activity promotion in healthy adults. J Exerc Rehabil. 2017;13:3–11. doi: 10.12965/jer.1732928.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lear S.A., Hu W., Rangarajan S., Gasevic D., Leong D., Iqbal R., Casanova A., Swaminathan S., Anjana R.M., Kumar R., Rosengren A., Wei L., Yang W., Chuangshi W., Huaxing L., Nair S., Diaz R., Swidon H., Gupta R., Mohammadifard N., Lopez-Jaramillo P., Oguz A., Zatonska K., Seron P., Avezum A., Poirier P., Teo K., Yusuf S. The effect of physical activity on mortality and cardiovascular disease in 130 000 people from 17 high-income, middle-income, and low-income countries: the PURE study. Lancet (London, England) 2017;390:2643–2654. doi: 10.1016/S0140-6736(17)31634-3. [DOI] [PubMed] [Google Scholar]
- 68.A. Kononova, L. Li, K. Kamp, M. Bowen, R. V Rikard, S. Cotten, W. Peng, The Use of Wearable Activity Trackers Among Older Adults: Focus Group Study of Tracker Perceptions, Motivators, and Barriers in the Maintenance Stage of Behavior Change., JMIR MHealth UHealth. 7 (2019) e9832. doi:10.2196/mhealth.9832 [DOI] [PMC free article] [PubMed]
- 69.M.N. Kamel Boulos, S. Gammon, M.C. Dixon, S.M. MacRury, M.J. Fergusson, F. Miranda Rodrigues, T. Mourinho Baptista, S.P. Yang, Digital Games for Type 1 and Type 2 Diabetes: Underpinning Theory With Three Illustrative Examples, JMIR Serious Games 3 (2015) e3. doi:10.2196/games.3930. [DOI] [PMC free article] [PubMed]
- 70.Bennett J., Rokas O., Chen L. Healthcare in the Smart Home: A Study of Past Present and Future. Sustainability. 2017;9:840. [Google Scholar]
- 71.Naslund J.A., Aschbrenner K.A., Kim S.J., McHugo G.J., Unützer J., Bartels S.J., Marsch L.A. Health behavior models for informing digital technology interventions for individuals with mental illness. Psychiatr Rehabil J. 2017;40:325–335. doi: 10.1037/prj0000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.O’Connor S., Hanlon P., O’Donnell C.A., Garcia S., Glanville J., Mair F.S. Barriers and facilitators to patient and public engagement and recruitment to digital health interventions: protocol of a systematic review of qualitative studies. BMJ Open. 2016;6:e010895. doi: 10.1136/bmjopen-2015-010895. [DOI] [PMC free article] [PubMed] [Google Scholar]