Abstract
Congenital blindness modifies the neural basis of language: “visual” cortices respond to linguistic information, and fronto-temporal language networks are less left-lateralized. We tested the hypothesis that this plasticity follows a sensitive period by comparing the neural basis of sentence processing between adult-onset blind (AB, n = 16), congenitally blind (CB, n = 22) and blindfolded sighted adults (n = 18). In Experiment 1, participants made semantic judgments for spoken sentences and, in a control condition, solved math equations. In Experiment 2, participants answered “who did what to whom” yes/no questions for grammatically complex (with syntactic movement) and simpler sentences. In a control condition, participants performed a memory task with non-words. In both experiments, visual cortices of CB and AB but not sighted participants responded more to sentences than control conditions, but the effect was much larger in the CB group. Only the “visual” cortex of CB participants responded to grammatical complexity. Unlike the CB group, the AB group showed no reduction in left-lateralization of fronto-temporal language network, relative to the sighted. These results suggest that congenital blindness modifies the neural basis of language differently from adult-onset blindness, consistent with a developmental sensitive period hypothesis.
Keywords: Sensitive periods, Language development, Adult-onset blindness
1. Introduction
When it comes to neural and cognitive development of language, timing is of the essence. Young children acquire language rapidly and effortlessly, without explicit training (Bonvillian et al., 2012; Gleitman and Wanner, 1982; Petitto et al., 2001a, 2001b; Gleitman and Newport, 1995; Lightbrown and Spada, 1993). By contrast, when language is acquired in adulthood, learning proceeds more slowly and plateaus at lower levels of proficiency (Johnson and Newport, 1989, 1991; Neville et al., 1992; Newport et al., 2001). This pattern is evident in second language learners and individuals born deaf who do not gain access to sign language until later in life (Emmorey et al., 1995; Mayberry et al., 2011; Mayberry and Eichen, 1991; Mayberry and Lock, 2003).
There is also evidence that the neural systems supporting language can be altered early in life (MacSweeney et al., 2008a, 2008b; Mayberry et al., 2011). Delays in language acquisition modify the neural basis of language processing (Neville et al., 1998; Allen et al., 2013; MacSweeney et al., 2008a, 2008b; Mayberry and Kluender, 2018). Individuals with early but not late damage to left hemisphere language networks have language processing abilities in the normal range, and recruit right-hemisphere homologues of left-hemisphere fronto-temporal language regions during language tasks (Dronkers et al., 2004; Rasmussen and Milner, 1977; Zevin et al., 2012; Newport et al., 2017; Kempler et al., 1999; Rosen et al., 2000; Tivarus et al., 2012).
Evidence from studies of blindness also suggests that the language network can be augmented with cortical real-estate in the occipital lobe that is typically occupied by visual perception. Individuals who are blind from birth recruit a network of “visual” areas during sentence processing, lexical retrieval, reading and word production tasks (Hamilton and Pascual-Leone, 1998; Kupers et al., 2007; Sadato et al., 1998; Bedny et al., 2011; Lane et al., 2015, 2017; Röder et al., 2002; Watkins et al., 2012). This recruitment is part of a broader phenomenon, whereby in blindness, regions of the “visual” cortex are recruited by non-visual cognitive functions, including, spatial localization and numerical processing (e.g. Sadato et al., 1998; Collignon et al., 2011; Kanjlia et al., 2016; Röder et al., 2000; Bedny, 2017). Subsets of “visual” cortex are selectively involved in language-processing, rather than other auditory and tactile tasks. For example, different subsets of “visual” cortex respond preferentially to sentences as opposed to math equations and vice versa (Kanjlia et al., 2016; Bedny, 2017; Abboud and Cohen, 2019). These language-responsive “visual” regions are, furthermore, sensitive to high-level linguistic information i.e. semantics and grammar. They respond more to sentences than Jabberwocky, and more to Jabberwocky than lists of unconnected non-words (Bedny et al., 2011; Lane et al., 2015; Röder et al., 2000). Responses are also higher for grammatically complex than simpler sentences (Röder et al., 2002; Lane et al., 2015). Finally, language-responsive “visual” areas are co-lateralized with the fronto-temporal language network, and show higher functional correlations with classical “language” regions even in the absence of a task (i.e. at rest) (Kanjlia et al., 2016; Bedny et al., 2011; Lane et al., 2017; Liu et al., 2007). Some evidence suggests that visual cortex responses to linguistic information are behaviorally relevant: Transcranial Magnetic Stimulation (TMS) to the occipital pole impairs generation of semantically related verbs to heard nouns among individuals born blind (Amedi et al., 2004). Congenitally blind individuals also outperform sighted people on verbal working memory tasks and one recent study finds enhanced sentence-processing performance among congenitally blind individuals (Amedi et al., 2003; Loiotile et al., 2019; Occelli et al., 2017; Pasqualotto et al., 2013). It is not known whether blindness-related changes to the language network are restricted to a developmental sensitive period, like other previously observed modifications to the neural basis of language.
One hypothesis is that blindness during development modifies cortical specialization and enables the incorporation of parts of “visual” cortex into the language system (Bedny et al., 2015). Alternatively, it remains possible that “visual” cortex has a latent ability to respond to linguistic information in all humans, irrespective of developmental visual history, and absence of visual input unmasks this latent ability. These hypotheses make different predictions with respect to how “visual” cortex recruitment for language is affected by the timing of blindness onset. The developmental specialization hypothesis predicts that “visual” cortex responses to language are particular to congenital blindness. By contrast, the unmasking hypothesis predicts that “visual” cortex recruitment for language would also occur in people who lose their vision as adults.
Although the question of how blindness onset affects plasticity for language in particular has not yet been conclusively answered, previous studies find age-of-blindness onset effects on visual cortex involvement in various auditory and tactile tasks (reviewed in Noppeney, 2007; Voss, 2013) (Büchel et al., 1998a, 1998b; Cohen et al., 1999; Veraart et al., 1990; Collignon et al., 2013; Fieger et al., 2006; Voss et al., 2008; Bedny et al., 2010; Jiang et al., 2016). Previous studies have found differences in visual cortex recruitment between late onset and congenital blindness in auditory spatial and pitch processing (Collignon et al., 2013), auditory motion perception (Bedny et al., 2010), monaural and binaural auditory localization tasks (Voss et al., 2008), tactile discrimination (Cohen et al., 1999) and numerical cognition (Kanjlia et al., 2018). With regard to visual cortex plasticity for language in particular, the available evidence is limited and mixed. Like congenitally blind individuals, late-onset blind individuals activate “visual” cortices during Braille reading and some spoken language tasks (Aguirre et al., 2016; Büchel et al., 1998a, 1998b; Burton et al., 2002; Burton, Diamond, et al., 2006; Burton and McLaren, 2006; Büchel et al., 1998a, 1998b; Burton et al., 2006a, 2006b; Burton et al., 2002; Elli et al., 2019). For example, Burton and colleagues reported activity in ‘visual’ cortices of late-onset blind individuals during a semantic judgment task with heard words (Burton and McLaren, 2006.) One study of resting state connectivity found that individuals with retinitis pigmentosa, who did not become totally blind until adulthood, show elevated correlations between inferior frontal language areas and occipital cortices (Sabbah et al., 2016).
On the other hand, there is also some evidence that language-related “visual” cortex plasticity differs between congenitally and late-onset blind individuals. Increases in resting state correlations between fronto-temporal language networks and occipital language areas are much larger in individuals blind from birth (Kanjlia et al., 2018). Several studies find that responses to spoken and written language in visual cortex are more anatomically extensive and robust in congenitally as compared to adult-onset blind individuals (Burton, Snyder, et al., 2006; Burton et al., 2002; Sadato et al., 2002; Bedny et al., 2012; but see Aguirre et al., 2016). One study reported that TMS to occipital cortices impaired Braille reading in people born blind but not in people who lost vision as adults (Cohen et al., 1999).
An outstanding question is whether “visual” cortex activity during language tasks in congenitally and adult-onset blind people reflects similar or different cognitive operations. Prior studies with late-onset blind individuals have compared language tasks to relatively low-level control conditions (e.g. rest or backwards speech). It therefore remains uncertain whether in adult-onset blind individuals, “visual” cortices are selective for linguistic information, as is found in people who are born blind. Do “visual” cortices of adult-onset blind individuals, like those of people who are born blind, respond to higher-order linguistic information, such as syntax?
To address these questions, we compared the neural basis of language in adult-onset blind, congenitally blind and sighted individuals in two experiments. Experiment 1 compared spoken sentence comprehension to an auditory math task. Experiment 2 compared sentences to lists of non-words, and manipulated the grammatical complexity of the sentences using a syntactic movement dependency, while holding lexical semantics constant. These experiments were designed to test two previously identified functional signatures of language-responsive cortical networks. Previous studies find that fronto-temporal language networks respond more to stimuli that are linguistically rich than stimuli that are less linguistically rich, even when the non-linguistic stimuli are more working memory intensive. Larger responses are observed in fronto-temporal networks to sentences than math equations, whereas math equations produce larger responses in parietal and dorsal frontal areas (Cappelletti et al., 2001; Fedorenko et al., 2011; Monti et al., 2009, 2012; Zago et al., 2001). Fronto-temporal language regions also respond more to sentences than matched lists of non-words, which lack both grammatical and lexical semantic information (Fedorenko et al., 2011; Fedorenko and Thompson-Schill, 2014). Neuroimaging studies of linguistic processing in sighted individuals have also repeatedly found higher responses in classic fronto-temporal language regions, such as the Inferior Frontal Gyrus (IFG), to sentences with higher syntactic complexity (Ben-Shachar et al., 2003; Caplan, 2007; Chen et al., 2006; Fedorenko et al., 2012a, 2012b; Grodzinsky and Friederici, 2006; Keller, 2001; Noppeney and Price, 2004). Although domain general fronto-parietal working memory systems also show heightened responses to grammatically complex sentences, the combined signature of higher responses to linguistic stimuli over working memory intensive non-linguistic stimuli as well as sensitivity to grammatical complexity is a signature of fronto-temporal language regions in sighted people (Ben-Shachar et al., 2004; Caplan, 2007; Chen et al., 2006; Fedorenko et al., 2011). Previous studies find that this functional signature is also present in the “visual” cortex of congenitally blind individuals (Bedny et al., 2011; Lane et al., 2015; Röder et al., 2002). The goal of the current study was to ask whether visual cortices of adult-onset blind individuals also show this functional profile.
Working with adult-onset blind individuals also enabled us to ask a second question about the neural basis of language in blindness. In addition to the recruitment of “visual” areas for language, congenital blindness is also associated with reduced left-lateralization of front-temporal language areas themselves (Lane et al., 2015, 2017; Röder et al., 2002). This phenomenon appears to be unrelated to the recruitment of visual cortex for language. Across congenitally blind individuals, the amount of “visual” cortex recruitment for language does not predict the laterality of fronto-temporal language networks. Furthermore, although there is some evidence that recruitment of the “visual” cortex for language processing confers cognitive benefits, reduction in left lateralization appears to have no consequences for behavior (Lane et al., 2015, 2017). In the current study we tested whether the left lateralization of the language network is also reduced in adult-onset blind individuals.
One challenge in answering the question of whether developmental blindness has unique effects on the neural basis of language is determining the relevant cut off point for “late” blindness onset. Previous studies have defined late blindness in various ways, including vision loss starting at 7, 9, 11 and 16 years of age. In the current study, we took a conservative approach - the “late” blind group includes only participants who lost their vision at 17 years of age or later. Therefore, we henceforth refer to this group as “adult-onset blind”.
2. Methods
2.1. Participants
Sixteen adult-onset blind individuals (AB; 5 female, mean age = 56.87, SD age = 10.39, mean years of education = 17.31, SD years of education = 3.11), twenty-two congenitally blind (CB; 16 female, mean age = 46.50, SD age = 17.18, mean years of education = 16.67, SD years of education = 2.26) and eighteen blindfolded sighted controls (S; 9 female, mean age = 46.50, SD age = 15.32, mean years of education = 16.34, SD years of education = 1.37) contributed data to the current study. Data for all but 3 of the congenitally blind and all of the sighted participants have previously been reported (Lane et al., 2015). Adult-onset blind participants were blind for at least 4 years (Mean = 16.06 years SD = 10.58 years, see Table 1 for details). One adult-onset blind participant only contributed data to Experiment 1. This participant did not learn English until 11 years of age, and was therefore excluded from data analyses of Experiment 2, which manipulated syntactic complexity. One additional adult-onset blind participant acquired English at 5 years-of-age, however, as their acquisition was early and their performance was not different from the group, they were included in both experiments. We additionally excluded any scanned participant who did not perform above chance (performed below 55 %) on the sentence condition of either experiment. This resulted in exclusion of 3 congenitally blind participants, not included in the subject count.
Table 1.
Participant demographic information and vision loss history summary for the congenitally blind (CB), adult-onset blind (AB) and sighted (S) groups. Duration of blindness is calculated by subtracting age at time scanned from age when current level of vision was reached for the AB group, and age at time tested for the CB group. Braille reading score was self-reported on a scale of 1-5. For expanded table, see Supplementary Material (Table S4).
| GROUP | SAMPLE SIZE |
AGE | BLINDNESS ONSET | DURATION OF BLINDNESS | SELF-REPORTED BRAILLE READING SCORE | ||
|---|---|---|---|---|---|---|---|
| N | M | F | Mean (years) | Mean (years) | Mean (years) | Mean on a scale of 1–5 | |
| CB | 22 | 6 | 16 | 46.5 (17.8) | -- | 46.5 (17.8) | 4.8 (1.3) |
| AB | 16 | 11 | 5 | 56.9 (10.4) | 33.2 (12.8) | 16.1 (10.6) | 2.5 (0.4) |
| S | 18 | 9 | 9 | 46.5 (15.3) | -- | -- | -- |
For both groups of blind individuals, all causes of blindness were related to pathology of the retina or optic nerve, not brain damage (Table 2). Adult-onset blind participants were fully sighted until 17 years of age or later (vision loss between the ages of 17–70, mean = 33.19, SD = 12.81, Table 1). At the time of the experiment, all blind participants had at most minimal light perception (LP) or no light perception (NLP), and the proportion of participants with light perception did not differ across blind groups (proportion with light perception AB 38 %, CB 45 %). None of the participants suffered from any known cognitive or neurological disabilities. All participants gave written informed consent and were compensated $30 per hour.
Table 2.
Etiology summary for the congenitally blind (CB) and adult-onset blind (AB) groups showing causes of blindness.
| GROUP | BLINDNESS ETIOLOGY | EXP 1 | EXP 2 |
|---|---|---|---|
| CB | TOTAL | 22 | 22 |
| Leber Congenital Amaurosis | 7 | 7 | |
| Retinopathy of Prematurity | 13 | 13 | |
| Detached Optic Nerve | 1 | 1 | |
| Unknown | 1 | 1 | |
| AB | TOTAL | 16 | 15 |
| Trauma | 3 | 3 | |
| Glaucoma and Macular Degeneration | 3 | 3 | |
| Optic Nerve Neuropathy | 1 | 1 | |
| Diabetic Retinopathy | 4 | 4 | |
| Retinitis Pigmentosa | 4 | 3 | |
| Autoimmune | 1 | 1 | |
Since adult onset blind participants were not matched to the sighted and congenitally blind on age, we repeated all behavioral and ROI analyses with age matched subsets for both Experiments 1 and 2. All results stayed the same and are reported in the Supplemental Materials (See Supplemental Fig. S2, Supplemental Results S2.).
2.2. Experimental procedures
Participants were scanned while performing two separate auditory tasks. All stimuli were presented over Sensimetrics MRI compatible earphones (http://www.sens.com/products/model-s14/). Volume was adjusted to a comfortable level for each participant. All participants, blind and sighted, wore a blindfold for the duration of the experiment.
2.2.1. Experiment 1 (Sentences and Equations)
Experiment 1 consisted of a language task and a mathematical control task (Kanjlia et al., 2016; Lane et al., 2015). In the language task, participants judged whether the meanings of two consecutively presented sentences, one presented in active voice and one in passive voice, were the same. For the “same” trials, the relations and roles of the people in the sentences were maintained. On the “different” trials, the roles were reversed (e.g. “The bartender that the mailman knew cut the grass” and “The grass was cut by the mailman that the bartender knew.”).
In the mathematical control task, participants judged whether the value of ‘X’ in two consecutively presented subtraction equations was the same. ‘X’ could occur as either the operand (e.g.: 6 – X = 3) or answer (e.g.: 16 – 13 = X). The equations varied in difficulty level, however, the difficulty manipulation was not analyzed in the present experiment (see Kanjlia et al., 2016 for further details).
There were 48 sentence trials and 96 mathematical trials. Each trial was 14 s long, starting with a 0.25 s tone followed by two sentences/equations of 3.5 s each, separated by a 2.75 s interval. After hearing the second stimulus, participants had 4 s to respond. The experiment included 36 rest blocks that were 16 s long.
2.2.2. Experiment 2 (Sentences and Non-words)
In Experiment 2, participants performed a sentence processing task, and a non-word working memory control task. In the sentence task, participants listened to a sentence, followed by a yes or no question, which required participants to judge who did what to whom. Half of the sentences were more syntactically complex (MOVE) and half were less complex (NONMOVE). The MOVE sentences contained a syntactic movement dependency in the form of an object-extracted relative clause (e.g.: “The accountant [that the corrupt detective in the organized crime division dislikes] advises the Sicilian mob.”). Sentences with movement require listeners to relate distant elements (words and phrases) to each other during the derivation of the sentence’s structure (Chomsky, 1957). The NONMOVE sentences had similar meanings and contained nearly identical words to the MOVE sentences, but did not contain an object extracted relative clause (e.g.: "The corrupt detective in the organized crime division dislikes [that the accountant advises the Sicilian mob.]"). Sentences were yoked across conditions, such that each sentence had both a MOVE and a NONMOVE version. Each participant heard one version of the sentence, counterbalanced across participants.
In the non-word working memory control task, participants heard a long list of non-words (target), followed by a shorter list of non-words (probe) which consisted of non-words from the first list - either in the same order as they were initially presented, or in a different order. Subjects judged whether the non-words in the shorter probe list were in the same order as they had occurred in the initially presented, longer target list.
There were 54 trials each of the MOVE, NONMOVE and NONWORD conditions divided across 6 runs, i.e. 9 in each run. All the trials were 16 s long, consisting of a tone, a 6.7 s sentence/target non-word list, 2.9 s question/probe non-word list, giving participants until the end of the 16 s periods to respond. We matched the sentences and target non-word sequences for number of items (words and nonwords, sentence = 17.9, nonword lists = 17.8; p = 0.3), number of syllables per item (sentence = 1.61, nonword = 1.59; p = 0.3), and mean bigram frequency per item (sentence = 2.34, nonword = 2.35; p = 0.3) (Duyck et al., 2004). For further details, see Lane et al., 2015.
2.3. MRI acquisition and data analysis
MRI structural and functional scans were acquired on a 3 Tesla Phillips MRI. For the structural T1 weighted images, 150 axial slices with 1 mm isotropic voxels were collected, and for the functional BOLD images, 36 axial slices with 2.4 × 2.4 × 3 mm voxels were collected with TR 2 s.
We created cortical surface models for each subject using the Freesurfer pipeline, and used FSL, Freesurfer, HCP workbench and custom software for surface-based analyses. Functional data were motion corrected, high pass filtered (128 s cutoff), resampled to the cortical surface and smoothed with a 6 mm FWHM Gaussian kernel on the cortical surface. Only cortical data, excluding the cerebellum and subcortical structures, was analyzed. BOLD activity as a function of condition was analyzed using a GLM and combined across runs within subjects using fixed-effects analyses. For both experiments, predictors were entered after convolving with a canonical HRF and its first temporal derivative. In Experiment 1, each type of math and language trial was a separate predictor. For Experiment 2, the non-word trials and each kind of sentence trial were separate predictors. We dropped trials where the participants failed to respond by including a regressor of no interest (average drops per run CB = 1.21, AB = 1.32, S = 1.38). We also dropped time-points with excessive (>1.5 mm) motion. Data were combined across participants using random effects analysis. In whole-cortex analysis, we used cluster-wise correction for multiple comparisons as implemented in FSL, using Monte Carlo simulations (n = 5000) across the cortical surface, and thresholding significance at p < 0.05 (Winkler et al., 2014). For within-group results, each permutation switches the condition labels for a subset of the participants, effectively inverting the vertex value signs for those participants. The group map obtained from this permutation is thresholded at p < 0.01 to obtain the largest number of contiguous vertices. A null distribution with the largest cluster size of each permutation is created, and the clusters from the true results that pass the correction lie within an alpha of p < 0.05 of this distribution. For between-group results, group labels are changed in every permutation, and the same procedure is followed.
2.4. ROI analyses
Region of interest (ROI) analyses were used to probe responses to language in the visual cortices of adult-onset blind participants and compare them to those of the congenitally blind and blindfolded sighted participants. A two-step procedure was used to define individual-subject specific functional ROIs (Fedorenko et al., 2010; Saxe et al., 2006). First, visual cortex search-spaces were defined based on a combination of anatomical landmarks, previous literature and orthogonal group-wise contrasts. Next, individual subject orthogonal ROIs were defined within these search-spaces by either using data from one experiment to select task-responsive vertices and extracting data from the other, or performing a leave-one-run out procedure, described in detail below.
To examine responses to sentences relative to math equations (Experiment 1), we first used a leave-one-run-out procedure within an anatomically defined V1 search-space. The V1 search-space was defined in each individual participant based on sulcal and gyral landmarks, according to previously published procedures (Hadjikhani et al., 1998; Van Essen, 2005). Within this search space, we selected the top 20 % most responsive vertices to sentences > equations for each subject. Vertices were selected based on data from all but one run, and PSC was extracted from the left-out run, iteratively over all possible leave-one-out combinations. The results of each leave-one-out procedure were averaged together.
Second, we looked for a sentences > equations effect (Experiment 1) in those visual cortex regions that responded more to sentences than nonwords in the adult-onset blind group as compared to the sighted in Experiment 2. A group-wise searchspace was defined as adult-onset blind > sighted for sentences > nonwords, at a leninent threshold of p < 0.01, uncorrected (AB language responsive visual cortex region, AB LangOccip). This search-space was then truncated anteriorly using the PALS atlas occipital lobe boundary (Van Essen, 2005). Within this search-space, we performed the same leave-one-run out procedure as described above to define individual-subject functional ROIs using the sentences > equations contrast from Experiment 1.
To test for the sentences > nonwords and grammatical complexity effects in Experiment 2 we used three ROIs. First, we examined activity in V1. An anatomical V1 search-space was defined as described above. Within this search-space, orthogonal individual subject functional ROIs were defined for each participant using the sentence > equations contrast from Experiment 1 (top 20 % sentences > equations). No leave-one-run-out procedure was necessary, since Experiment 1 data were used to define ROIs for Experiment 2 analyses.
Second, we examined activity in visual areas that have previously been found to respond to spoken language in those who are congenitally blind (Kanjlia et al., 2016; Kim et al., 2017; Lane et al., 2015). A search space was created using the group-wise data from Experiment 1, defined as the occipital cortex regions that responded to sentences > equations (p < 0.05) in the congenitally blind more than sighted group (CB language responsive occipital cortex region – CB LangOccip). This search-space was then truncated anteriorly using the PALS atlas occipital lobe boundary (Van Essen, 2005). Next, within each search-space, we defined individual-subject-specific functional ROIs by choosing the top 20 % of vertices that showed the sentences > equations effect for that particular subject. Again, no leave-one out procedure was necessary because ROIs were defined based on an independent experiment.
To further probe for the grammatical complexity effect, we also conducted an ROI analysis within the AB language responsive occipital cortex region (AB LangOccip), that was more responsive to sentences than nonwords in the adult-onset blind than sighted group. This analysis was conducted to ensure that the grammatical complexity effect was not missed in the adult-onset blind group by focusing on regions that were more relevant to the congenitally blind group. Individual subject functional ROIs were defined within the AB occipital search-space by taking the top 20 % of sentences > equations responsive vertices contrast from Experiment 1.
All of the above search-spaces and contrasts used were orthogonal with respect to the contrasts of interest. Note however that the AB language responsive visual cortex search-space is specifically looking at parts of the visual cortex that respond to spoken language in the AB group more so than in the sighted, whereas the CB language responsive visual cortex search-space focuses on areas that are more responsive to language in those who are congenitally blind relative to the sighted. These approaches are therefore complementary to each other, ensuring that no effects are missed because of different visual cortex areas recruited for language in these two populations. In practice, these approaches yield similar results, suggesting that similar visual cortex regions become responsive to language in congenitally and adult-onset blind individuals.
A classically language responsive ROI was defined using a similar procedure to the individual subject visual cortex CB occipital functional ROIs. Within the Inferior Frontal Gyrus (IFG) search space from Fedorenko et al., we selected the top 20 % most responsive vertices to Experiment 1 (sentences > equations) in each individual subject, and examined responses to Experiment 2 (Fedorenko et al., 2010). All ROIs were defined in both hemispheres. Previous studies have found reduced left lateralization of language in congenitally blind individuals (Lane et al., 2017; Röder et al., 2000). Whether lateralization is also reduced in adult-onset blindness is not known. To account for potential lateralization differences across congenitally blind, adult-onset blind and sighted participants we conducted analyses in every subject’s language dominant hemisphere (see Lane et al., 2015 for similar analysis). For each participant, we calculated (L–R)/(L + R), where L and R are the sum of positive z-statistics > 2.3 (p < 0.01 uncorrected) in the left and right hemisphere, respectively (Lane et al., 2015, 2017). Laterality was defined based on the entire hemisphere, minus the occipital lobe. This was done to avoid biasing laterality indices based on visual cortex plasticity differences across groups. We used data from Experiment 1 to determine the laterality index and then analyzed results from Experiment 2, and vice versa.
For all of the above ROIs, PSC was calculated as BOLD signal during the predicted peak window (8−14 s for Experiment 1, 6−12 s for Experiment 2) relative to rest ((Signal condition - Signal baseline)/Signal baseline). PSC was averaged across vertices within each ROI.
All ANOVAs performed on PSC values obtained from each ROI are two-way ANOVAs with repeated measures, comparing means between two groups at a time, using condition as a within subject factor, and subject as a random factor for repeated measures. All t-tests reported (paired or unpaired) are two-tailed. Only planned comparisons were performed to test prespecified predictions, and no multiple comparison correction was applied in ROI or behavioral analyses (Perneger, 1998). Since the sighted and most of the congenitally blind data have previously been reported (Lane et al., 2015), comparisons between these two groups are presented for expository purposes only as a reference point to evaluate effects in the adult-onset blind group.
5. Results
5.1. Behavioral results
5.1.1. Experiment 1
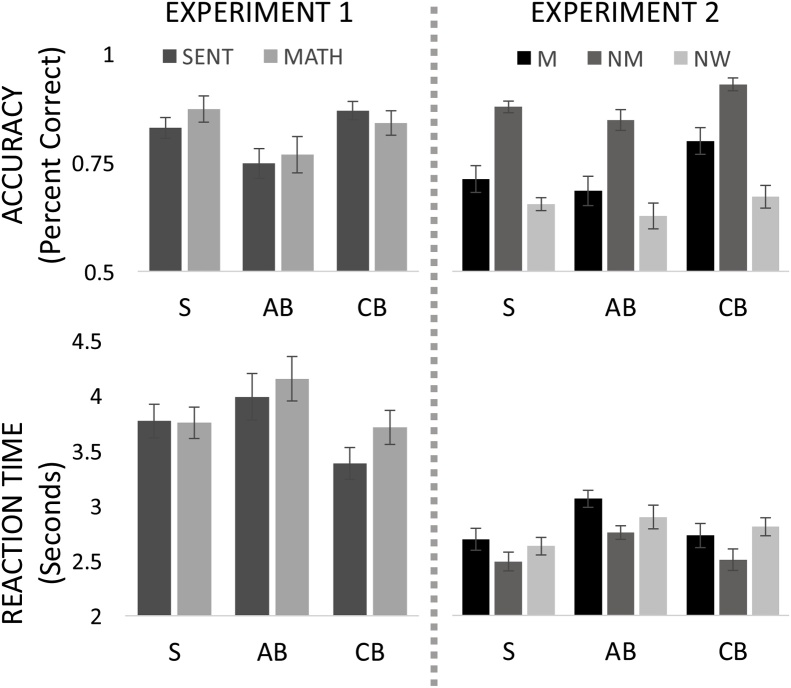
Adult-onset blind participants were as similarly accurate on the language and math conditions (t(15) = 0.26, p = 0.800), and marginally faster on the language relative to math condition (t(15) = 2.18, p = 0.052). There were no significant differences between any of the three groups in their accuracy on the math trails (One way ANOVA effect of group F(2,52) = 2.02, p = 0.143) or the sentence trials (One way ANOVA effect of group F(2,52) = 1.3, p = 0.282). The congenitally blind group was faster on the language trials than both the adult-onset blind (t(15) = 2.24, p = 0.036) and the sighted groups (t(17) = 2.06, p = 0.046), who were not different from each other (t(15) = −0.44, p = 0.662). There was no difference between any of the three groups in their RT on math trials (One way ANOVA effect of group F(2,52) = 2.10 p = 0.133). This pattern of results did not change when the groups were matched for age (Supplementary Material S2).
5.1.2. Experiment 2
Like congenitally blind and sighted participants, adult onset blind participants were less accurate and slower on the MOVE than NONMOVE sentences (Fig. 1, AB: Accuracy t(14) = -6.77, p < 0.001; RT: t(14) = 7.01, p < 0.001 CB: Accuracy t(21) = -5.35, p < 0.001; RT t(21) = 6.06, p < 0.001 S: Accuracy t(17) = -7.69, p < 0.001 RT t(17) = 5.26, p < 0.001). The effect of movement on accuracy and RT in the adult-onset blind was no different from either the congenitally blind or the sighted (group-by-condition ANOVA, group-by-condition interaction: Accuracy CB vs AB: F(1,35) = 1.25, p = 0.272; Accuracy AB vs S: F(1,31) = 0.25, p = 0.619; RT CB vs AB: F(1,35) = 0.95, p = 0.335; RT AB vs S: F(1,31) = 1.84, p = 0.185). The movement effect was also not different when comparing the congenitally blind and sighted groups (group-by-condition ANOVA, group-by-condition interaction: Accuracy CB vs S: F(1,38) = 1.92, p = 0.174; RT CB vs S: F(1,38) = 0.16, p = 0.695).
Fig. 1.
Behavioral performance of Sighted (S), Adult-onset Blind (AB) and Congenitally Blind (CB) on Experiment 1 (Sentence (SENT) and Mathematical equations (MATH)) and Experiment 2 (MOVE (M), NONMOVE (NM) and NONWORD (NW)) conditions. Error bars represent standard error of mean (SEM).
Across sentence types, the adult-onset blind participants were no different in their accuracy from the sighted (group-by-condition ANOVA main effect of group AB vs S F(1,31) = 0.53, p = 0.474) but were significantly less accurate than the congenitally blind (group-by-condition ANOVA main effect of group CB vs AB F(1,35) = 10.77, p = 0.002). Congenitally blind participants were also more accurate than the sighted group (group-by-condition ANOVA main effect of group CB vs S F(1,38) = 6.91, p = 0.012). Congenitally blind participants were more accurate than adult-onset as well as sighted participants even when matched for age (Supplementary Material S2).
In reaction time, the adult-onset blind group was slightly slower at the sentence comprehension task than both the sighted and congenitally blind groups (group-by-condition ANOVA main effect of group AB vs S F(1,31) = 5.18, p = 0.030; CB vs AB F(1,35) = 3.66, p = 0.063), which were not different from each other (group-by-condition ANOVA main effect of group CB vs S F(1,38) = 0.02, p = 0.889). However, these differences in RT were no longer significant when the groups were matched for age.
For the non-word condition, there was no difference between the three groups in accuracy or response time (One way ANOVA effect of group: Accuracy F(2,52) = 0.77, p = 0.467; RT F(2,52) = 1.37, p = 0.264).
5.2. fMRI results
5.2.1. Larger responses to language in “visual” cortex of congenitally than adult-onset blind individuals (Experiment 1)
5.2.1.1. Whole-brain analysis
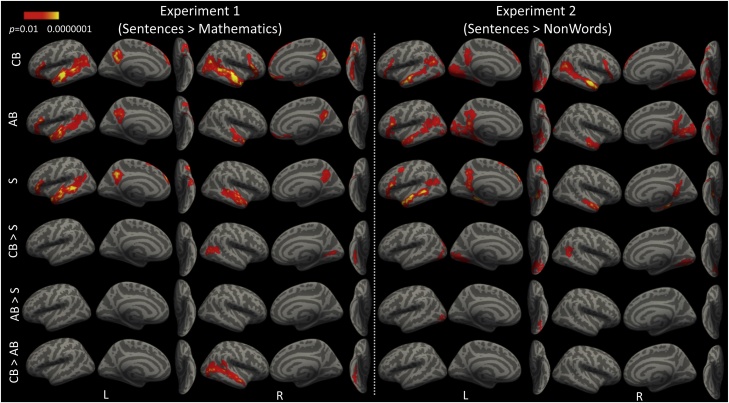
Congenitally blind, but not sighted participants show larger responses to sentences than mathematical equations in lateral occipital and posterior fusiform cortices (p < 0.05, within CB group and CB > S group-by-condition interaction, cluster corrected, Fig. 2). In adult-onset blind participants, occipital responses did not reach significance in this contrast (Fig. 2, AB > S group-by-condition interaction). No regions were more active in sighted compared to congenitally blind or adult-onset blind participants.
Fig. 2.
Whole brain analysis results of all subjects in the left and right hemispheres on the lateral, medial and ventral surface (cluster corrected, p < 0.05) for Experiment 1 (left) and Experiment 2 (right) in the Congenitally Blind (CB, n = 22), Adult Onset Blind (AB, n = 16 (Experiment 1), 15 (Experiment 2)) and Sighted (S, n = 18) groups.
5.2.1.2. ROI analysis
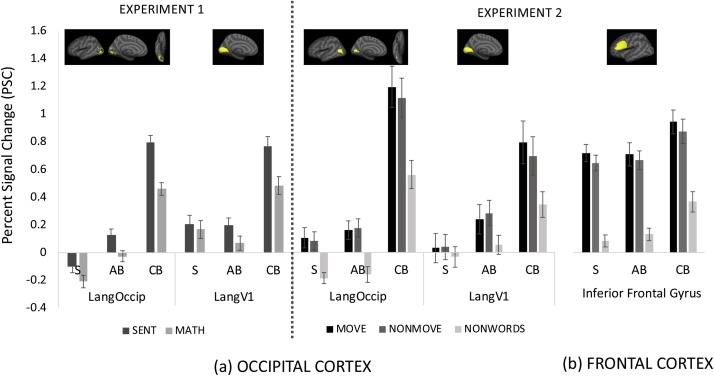
In the ROI analysis, the response to sentences compared to equations was larger in the congenitally blind than the sighted group in both visual cortex ROIs (group-by-condition ANOVA CB vs S group-by-condition interaction LangOccip: F(1,38) = 12.97, p < 0.001, LangV1: F(1,38) = 8.79, p = 0.005). By contrast, the difference between the adult-onset blind group and the sighted group did not reach significance in either secondary visual areas or in V1 (group-by-condition ANOVA AB vs S group-by-condition interaction LangOccip: F(1,32) = 0.53, p = 0.471; LangV1: F(1,32) = 2.30, p = 0.139).
When adult-onset and congenitally blind adults were directly compared to each other, the response to sentences (relative to equations) was smaller in the adult-onset blind group in secondary visual areas (LangOccip: group-by-condition ANOVA CB vs AB group-by-condition interaction F(1,36) = 6.94, p = 0.012) but was not different from congenitally blind individuals in V1 (LangV1: group-by-condition ANOVA CB vs AB main effect of condition F(1,36) = 22.61, p < 0.001; group-by-condition interaction F(1,36) = 2.54, p = 0.119).
In within group t-tests, we found a larger response to sentences than equations in the congenitally blind group in both visual cortex ROIs (LangOccip: t(21) = 6.95, p < 0.001; LangV1: t(21) = 4.09, p < 0.001). In the sighted group, a significant effect was present in the LangOccip ROI (t(17) = 2.74, p = 0.014) but there was no effect for sentences > equations in V1 (t(17) = 0.85, p = 0.407). In adult-onset blind individuals, the effect was present in in both V1 and secondary visual areas (LangV1: t(15) = 2.043, p = 0.028; LangOccip: t(15) = 3.09, p = 0.007) (Fig. 3).
Fig. 3.
Percent Signal Change (PSC) in response to the Sentence and Equation conditions of Experiment 1 and the MOVE, NONMOVE and NONWORD conditions of Experiment 2 in the Sighted (S), Adult-Onset blind (AB) and Congenitally Blind (CB) groups in (a) occipital cortex and (b) frontal cortex ROIs. Error bars represent SEM.
The overal response relative to rest was also larger in congenitally blind as compared to the adult-onset blind individuals. In the LangOccip ROI, the adult-onset blind individuals fell intermediate between sighted and congenitally blind (LangOccip CB vs AB: F(36) = 28.95, p < 0.001; AB vs S: F(32) = 7.05, p = 0.012). In V1, adult-onset blind participants were no different from the sighted (LangV1 CB vs. AB F(1,36) = 10.10, p = 0.003; AB vs S F(32) = 0.30, p = 0.585).
In sum, the “visual” cortices of adult-onset blind participants showed a smaller response to language (i.e. sentences > math) relative to congenitally blind participants. However, in some “visual” regions, responses to language were observed even in the adult-onset blind group.
5.2.2. “Visual” cortex of adult-onset blind individuals responds to spoken sentences more than to lists of non-words, but less so than in congenitally blind adults (Experiment 2)
5.2.2.1. Whole-brain analysis
There were larger responses to sentences than nonwords in the visual cortex of congenitally blind but not sighted participants in lateral occipital cortex bilaterally, retinotopic visual cortices on the medial surface (in the location of V1, V2 and V3) as well as the posterior fusiform on the ventral surface (CB > S group-by-condition interaction, p < 0.05, cluster corrected) (Fig. 2).
The visual cortices of adult-onset blind participants showed a qualitatively similar but weaker response to spoken sentences compared to the congenitally blind group. Larger responses to spoken language than non-words was observed in posterior lateral occipital cortex, within the vicinity of the lateral occipital complex (LO) and V5 (MT/MST). Small patches of activation were also present on the medial surface in pericalcarine and extrastriate cortices (in the regions of V1, V2 and V3). In a group-by-condition interaction analysis, we observed larger visual cortex responses to sentences than nonwords in the adult-onset blind as compared to the sighted in the posterior lateral occipital cortex (AB > S, p < 0.05, cluster corrected). There were no statistically significant differences between the adult-onset blind and the congenitally blind groups in this contrast (Fig. 2). No regions showed larger responses in sighted compared to congenitally blind or adult-onset blind participants.
5.2.2.2. ROI analysis
In the secondary visual cortices (LangOccip ROI), the sentences > non-words effect was larger in the congenitaly blind group than the sighted group (group-by-condition ANOVA CB vs S group-by-condition interaction F(1,38) = 8.28, p = 0.006), larger in the congenitally blind relative to the adult-onset blind group (group-by-condition ANOVA, AB vs. CB, group-by-condition interaction F(1,35) = 5.22, p = 0.028) and not significantly different between sighted and adult onset blind groups (group-by-condition ANOVA AB vs S group-by-condition interaction F(1,31) = 0.26, p = 0.616).
In V1, the response to sentences was larger in the congenitally blind relative to the adult-onset blind group (LangV1 group-by-condition ANOVA CB vs AB main effect of group F(1,35) = 6.17, p = 0.018 group-by-condition interaction F(1,35) = 3.43, p = 0.072), and the adult-onset blind showed a trending difference from the sighted group (group-by-condition ANOVA AB vs S group-by-condition interaction F(1,31) = 3.55, p = 0.068), suggesting an intermediate response profile.
In post-hoc within group comparisons, there was a significant difference between sentences and nonwords in the LangOccip ROI of the congenitally blind group (t(21) = 7.32, p < 0.001), the adult-onset blind group (t(14) = 4.54, p < 0.001) and in the sighted group (t(17) = 4.07, p < 0.001). In V1, there was a significant response to sentences > nonwords in the congenitally blind (t(21) = 5.01, p < 0.001) and adult-onset blind groups (t(14) = 4.29, p < 0.001), but not in the sighted group (t(17) = 1.25, p = 0.226).
In sum, the adult-onset blind group showed higher responses to sentences than non-words in both secondary visual areas and primary visual cortex, but this effect was smaller than what is observed in congenital blindness (Fig. 3).
5.2.3. Sensitivity to syntactic complexity in visual cortex of congenitally blind but not adult-onset blind individuals (Experiment 2)
Both V1 and secondary visual cortices were more sensitive to movement congenitally than adult-onset blind adults (movement-by-group ANOVA AB vs CB, movement-by-group interaction: LangV1: F(1,35) = 6.61, p = 0.015; LangOccip: F(1,35) = 6.25, p = 0.017). By contrast, there were no differences between the adult-onset blind and sighted groups with respect to the movement effect in any visual cortex regions (movement-by-group ANOVA, movement-by-group interaction LangOccip: F(1,31) = 1.18, p = 0.284; LangV1: F (1,31) = 0.43, p = 0.514) (Fig. 3).
The same pattern held when we examined responses to syntactic movement in the region specifically responsive to sentences more than non-words in the adult-onset blind group (AB LangOccip). The adult-onset blind participants were no different from the sighted in their response to syntactic movement (movement-by-group ANOVA, movement-by-group interaction F(1,31) = 0.34, p = 0.559) in this region, and significantly differed from the congenitally blind (movement-by-group ANOVA, movement-by-group interaction F(1,35) = 13.95, p < 0.001).
There was a syntactic movement effect in the language-responsive secondary visual areas and V1 of congenitally blind adults (LangOccip: t(21) = 3.48, p = 0.002, LangV1 t(21) = 3.20, p = 0.004), and no effect of syntactic movement in sighted participants (LangOccip: t(17) = 1.23, p = 0.234, LangV1 t(17) = −0.24, p = 0.813). The adult-onset blind participants patterned like the sighted group on this measure, i.e. there were no effects of syntactic movement in either LangOccip (t(14) = -0.47, p = 0.646) or in V1 (t(14) = −0.91, p = 0.378). Again, in the language-responsive region of the adult-onset blind group (AB LangOccip), there was a significant movement effect in the congenitally blind group (t(21) = 3.74, p = 0.001), but not in the adult-onset blind (t(14) = −1.78, p = 0.101) or the sighted group (t(17) = −1.18, p = 0.253). To ensure that we were not missing a small effect in the adult-onset blind group, we repeated the analysis at smaller ROI sizes (top 10 %, 5 % and top 20 vertices). The adult-onset blind group failed to show a syntactic movement effect in any ROI, regardless of ROI size (all t’s < 1.0, all p’s > 0.1). We additionally repeated all ROI analyses with age-matched subsets of all three groups, and the pattern of results did not change in any ROI.
5.2.4. Relationship of blindness duration and age of blindness onset to visual cortex responses to language (Experiments 1 and 2)
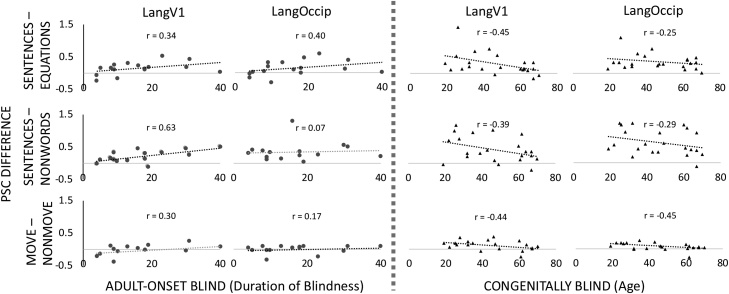
In the adult onset-blind group, there was a tendency for responses to language in the visual cortex to increase with duration of blindness. Blindness duration and age of onset predicted the size of the sentences > non-words effect (Experiment 2) in the adult onset blind group in V1 (LangV1, duration: r = 0.63, t(13) = 2.94, p = 0.011, age of onset: r = −0.54, t(13) = −2.27, p = 0.040). When blindness duration and onset were both entered into a multiple regression, only the effect of blindness duration remained significant in the adult onset-blind group (LangV1, duration t(12) = 2.32, p = 0.037, blindness onset t(12) = −1.01, p = 0.327, adjusted r = 0.41). No other correlations were significant in any ROI, although all effects were in the same direction (p’s > 0.1) (Fig. 4).
Fig. 4.
Effect of blindness duration (in Adult Onset Blind) and age (in Congenitally Blind) on visual cortex responses to language. PSC extracted from functionally defined individual LangV1 and CB LangOccip ROIs for the SENTENCE-NONWORD and MOVE-NONMOVE conditions in Experiment 2, and LangV1 and AB LangOccip ROIs for the SENTENCE-EQUATION conditions of Experiment 1.
By contrast, in the congenitally blind group, the response to language in visual cortex tended to decrease with blindness duration (i.e. age). This correlation was only significant in V1 for Experiment 1 (LangV1 r = −0.45, t(20) = −2.25, p = 0.036), and trending in V1 for Experiment 2 (LangV1 r = −0.39, t(20) = −1.88, p = 0.075), but was in the same direction in all comparisons (p’s > 0.1) (Fig. 4).
The size of the MOVE-NONMOVE effect in both ROIs in the adult-onset blind group was not significantly predicted by duration of blindness (LangOccip: r = 0.17, t(13) = 0.63, p = 0.537; LangV1: r = 0.30, t(13) = 1.15, p = 0.272) or age of blindness onset (LangOccip: r = 0.36, t(13) = 1.40, p = 0.184; LangV1: r = 0.24, t(13) = 0.91, p = 0.379). In the congenitally blind group, the MOVE-NONMOVE effect showed a significant reduction in effect size with age in all visual cortex ROIs (LangOccip: r = −0.45, t(20) = −2.26, p = 0.035; LangV1: r = −0.44, t(20) = −2.17, p = 0.042).
5.2.5. Similar responses to language among sighted, congenitally blind and adult onset blind groups in inferior frontal cortex
The inferior frontal gyrus (IFG) showed a similar response profile in the adult-onset blind group relative to the sighted and congenitally blind groups (Fig. 3). The IFG responded more to sentences than nonwords across groups (Two-by-two AB vs. CB ANOVA main effect of condition (sentences vs. nonwords) F(1,35) = 198.91, p < 0.001, group-by-condition interaction F(1,35) = 0.02, p = 0.877; Two-by-two AB vs. S ANOVA main effect of condition (sentences vs. nonwords) F(1,31) = 262.51, p < 0.001, group-by-condition interaction F(1,31) = 0.33, p = 0.567). There was also a larger response to the MOVE than NONMOVE sentences across groups (IFG AB vs. CB, main effect of condition (MOVE vs. NONMOVE) F(1,35) = 12.18, p = 0.002, group-by-condition interaction F(1,35) = 0.61, p = 0.442; AB vs. S main effect of condition (MOVE vs. NONMOVE) F(1,31) = 13.35, p < 0.001, group-by-condition interaction F(1,31) = 0.84, p = 0.365).
In post-hoc t-tests within the IFG, the sentences > nonwords effect was present in the congenitally blind (t(21) = 9.63, p < 0.001), adult-onset blind (t(14) = 10.98, p < 0.001) as well as the sighted group (t(17) = 11.94, p < 0.001). The syntactic movement effect was also present in all three groups (CB: t(21) = 3.67, p = 0.002; AB: t(14) = 1.79, p = 0.055; S: t(17) = 4.15, p < 0.001).
5.2.6. Reduced left lateralization of fronto-temporal reponses to language in congenitally but not adult-onset blind individuals
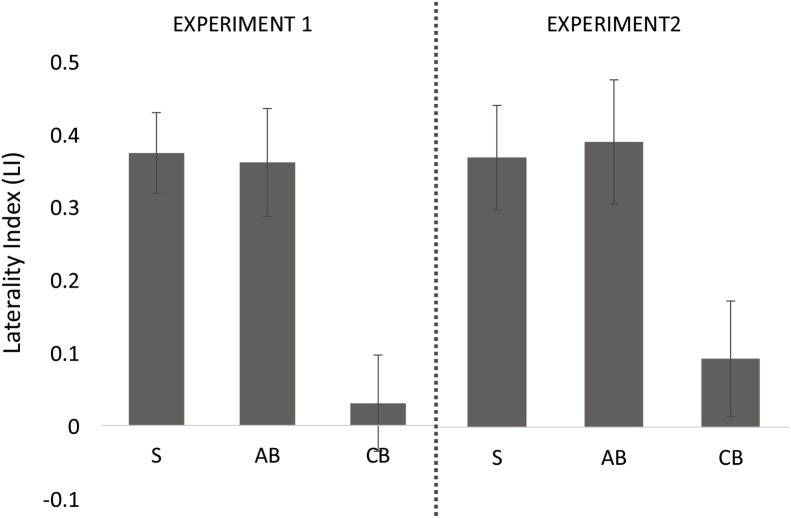
In whole-brain analyses, all three groups showed comparable fronto-temporal responses to sentences > math equations (Experiment 1) and sentences > nonwords (Exeriment 2) (Fig. 2). However, fronto-temporal responses were left-lateralized in the sighted and adult-onset blind groups, but not in the congenitally blind group. We used laterality index analysis to directly test the laterality of fronto-temporal language responses and observed reduced left-lateralization relative to the sighted in the congenitally but not adult-onset blind group (Fig. 5). The response to spoken language in the fronto-temporal language network of the adult-onset blind group were as left-lateralized as in the sighted, both in the sentences > math contrast (t(13) = 0.14, p = 0.889) and the sentences > non-words contrast (t(13) = −0.19, p = 0.846). The adult-onset blind group was significantly more left lateralized than the congenitally blind group (sentences > mathematics t(13) = −3.33, p = 0.002; sentences > nonwords t(13) = −2.56, p = 0.015).
Fig. 5.
Mean laterality index for sighted (S), adult-onset blind (AB) and congenitally blind (CB) participants in response to the sentences > mathematics (Experiment 1) and sentences > nonwords (Experiment 2) condition in the whole brain excluding the occipital cortex. Error bars represent SEM.
Reduction of left lateralization in the congenitally blind group was comprable among CB participants with and without ROP (59 %) as cause of blindness. There was no correlation between Braille reading scores and the laterality index in either group, in Experiment 1 or 2 (all p’s > 0.1). There was also no correlation in either group or experiment between laterality index and duration of blindness (all p’s > 0.1).
6. Discussion
6.1. Evidence for a sensitive period in the neural substrates of language in blindness
Previous studies identified two ways in which the neural basis of language processing is modified in blind individuals. First, parts of the “visual” cortex are incorporated into the language network and become sensitive to the grammatical structure of spoken sentences (Bedny et al., 2011; Lane et al., 2015; Röder et al., 2002). Second, fronto-temporal language areas are less left-lateralized in congenitally blind than sighted individuals (Lane et al., 2017). Here, we report that both of these phenomena are either absent, or present to a much lesser extent in individuals who lose their vision as adults.
Adult-onset blind individuals show left-lateralization of fronto-temporal language areas that is indistinguishable from sighted adults, and greater than congenitally blind participants. “Visual” cortices of adult-onset blind individuals differentiate less between sentences and equations and sentences and lists of non-words than those of congenitally blind individuals, although some difference between these conditions is observed even in individuals who became blind as adults. We observed sensitivity to syntactic movement in “visual” cortex in congenitally blind, but not adult-onset blind individuals. These results are consistent with the hypothesis that blindness has different effects on the neural basis of language during and after a developmental sensitive period, and therefore support the developmental specialization hypothesis.
An important caveat to the sensitive period interpretation of the current findings is that the present evidence is indirect. Non-invasive methods, such as fMRI, do not measure plastic properties of cortex (e.g. spine motility, excitatobry/inhibitory neurotransmiter balance) (Hensch and Fagiolini, 2004; Knudsen, 2004; Wallace and Stein, 2007; Hensch, 2004) Studies with humans also measure the consequences of naturally occuring varation in experience, rather than the experimental manipulation of experience. As a result, the timing of experience is confounded with other interindividual differences. In the present case, people who become blind as adults and those who are born blind differ in multiple ways, including blindness etiology, Braille proficiency and perhaps most significantly, blindness duration. In the current study, the adult-onset blind group was blind for 16 years on average, whereas the congenitally blind group, where duration of blindness is equal to age, was blind for an average of 46 years. We found, however, that blindness duration has either weak or no effects on occipital responses to languge in adult-onset blind individuals, and in congenitally blind people, ‘visual’ cortex responses to language tend to decrease with age (and blindness duration), possibly because BOLD data are generally noisier from older participants (D’Esposito et al., 2003; Huettel et al., 2001). Although the current samples are admittedly small and not designed to test effects of blindness duration, at the very least they suggest that in the current sample effects of age of blindness onset on occipital plasticity are larger than any putative effects of blindness duration or age. Together with prior evidence, the present results therefore offer support for the hypothesis that the capacity of human cortex to specialize for linguistic processes follows a sensitive period. Our findings further suggest that the neural systems supporting language processing are modifiable during development.
The absence of a syntactic movement effect in the “visual” cortex in adult-onset blind adults is particularly intriguing, and consistent with claims that sensitivity to aspects of syntax depends on cortical flexiblity that is inherent to sensitive periods (Friederici, 2017; Lenneberg, 1967; Neville et al., 1992; Ruben, 1999). Studies of delayed first language exposure, as well as second language learning also suggest that the develping brain is especially capable of acquiring grammatical abilties and conversely some aspects of syntax are especially volnurable to delays in langauge exposure (Cormier et al., 2012; Mayberry, 2007; Mayberry and Eichen, 1991; Mayberry and Lock, 2003). For example, several studies suggest that deaf children who do not have access to sign language and do not receive cochlear implants until later in life perform poorly on morpho-syntactic production and comprehension tasks, while performance on vocabulary and lexical semantic tasks is comprable to age matched hearing children (Friedmann and Rusou, 2015; Geren and Snedeker, 2009; Lopez-Higes et al., 2015). Indeed, there is some evidence that processing of syntactic movement per se is affected by delays in language access (Friedmann, 2005; Friedmann and Rusou, 2015). In a neuroimaging study of grammaticality and phonemic judgements, age of acquisition also affected the neurobiology of sign language, with later learners of ASL showing lower BOLD responses to syntactic tasks in left inferior frontal regions (Mayberry et al., 2011). Analogously, speakers who aquire a second language as adults show a reduced ability to acquire some aspects of grammatical structure (e.g. morphosyntax, more syntacticially complex constructions) relative to vocabulary and basic word order (Johnson and Newport, 1989; Newport, 1990; Patkowski, 1980; Weber-Fox and Neville, 1996). A recent large-scale study of second language proficiency in over half a million participants confirmed age-of-acqusition effects on sentence-level syntax in second language learners, although it also suggests that the cortical learning rate itself may not fall off until the late teens (Hartshorne et al., 2018). There is also evidence of different neural signatures for syntax in first and second languages speakers matched for proficiency (Neville et al., 1998; Pakulak and Neville, 2011; Weber-Fox and Neville, 2001).
The present results provide complementary evidence for the hypothesis that acquisition of aspects of grammar depends on the special cortical flexibility afforded by sensitive periods. One interpretation of previous age of acquisition effects in grammar is that they arise uniquely from the maturational timetable characteristic to fronto-temporal language areas (Lenneberg, 1967; Newport and York, 2003). An alternative, non-mutually exclusive possiblity suggested by the present findings is that certain aspects of syntax acquisition depend on critical period plasticity more generally, even outside the fronto-temporal network. It is worth noting that because syntactic movement effects are relatively small in size and the present study is the first to examine them in this population, it will be important to replicate the presently observed group differences in future studies. Furthermore, although we observed neural responses to the syntactic movement manipulation, the underlying cognitive and neural computations that produce these responses are debated, and remain to be understood (Ben-Shachar et al., 2004; Christensen, 2008; Dehaene et al., 2015; Gibson, 1998; Hickok, 1993; Levy, 2008; MacDonald et al., 1992, 1994; McRae et al., 1998; Staub, 2010).
Appart from blindness-onset effects on occipital responses to language, we also find that unlike congenitally blind individuals, adult-onset blind individuals show typical left-lateralization of the frontotemporal language network. Prior developmental neuroimaging studies show that left lateralized language responses emerge as early as 5 years of age, with precursors of left-lateralization being apparent in infants as young as 3 months of age. (Dehaene-lambertz et al., 2002; Holland et al., 2007; Szaflarski et al., 2006; Wood et al., 2004). At the same time, there is evidence that lateralization of language is both variable across people and malleable in childhood (Bishop, 2013). While massive damage to the left hemisphere in adulthood typically leads to severe language processing deficits, damage suffered in childhood to the left or right hemisphere has similarly small effect on ultimate language performance (Dronkers et al., 2004; Max, 2004; Newport et al., 2017; Rasmussen and Milner, 1977; Thal et al., 1991; Zevin et al., 2012). Why congenital blindness in particular affects language lateralization is not known. It has been noted that language lateralization is affected by a variety of heterogenous developmental factors (Flagg et al., 2005; Mayberry et al., 2011; Sommer et al., 2001; Szaflarski et al., 2006). One possibility is that language becomes left lateralized, in part, as a result of competitive interactions with other visuo-spatial functions that dominate the right hemisphere (Leybaert and D’hondt, 2009; Röder et al., 2002). If so, the absence of vision may modify these interactions and reduce left-lateralization. Another possibly is that reduced left-lateralization is related to transient language delays observed in blind infants (Bohannon et al., 1986). Modifying the timing of neural specialization for language could affect lateralization (Bates, 1993; Bishop, 2013; Mayberry et al., 2011). Irrespective of which, if any, of these accounts explains reduced lateralization in congenital blindness, the present results suggest that it is a developmental phenomenon. Together these findings support the claim that the neural basis of language is uniquely malleable during development.
6.2. Visual cortex of adult-onset blind individuls responds to spoken sentences
Although we observed different responses to spoken sentences in visual cortices of congenitally and adult-onset blind individuals, we found that even in adult-onset blind individuals, both V1 and secondary “visual” areas respond to spoken sentences more than to lists of non-words and equations. In some cases, small effects were observed even in the secondary visual areas of the blindfolded sighted group. This is despite the task demands of our control conditions being equal (Experiment 1) or higher (Experiment 2) than the language conditions, evidenced by the behavioral performance. Furthermore, in the adult-onset blind group, there was a tendency for responses to spoken language to increase over the course of many years, with AB individuals who are blind for 30 years showing larger responses to sentences than those who are blind for 20. Thus, age-of-blindness-onset effects coexist with continued plasticity throughout life. One interpretation is that the “visual” cortex retains the capacity for functional reorganization into adulthood, but in adulthood, the rate of learning is much slower (Merabet and Pascual-Leone, 2010). Within this framework, lack of responses to syntactic movement in the visual cortex of adult-onset blind individuals might reflect the fact that the human lifespan is insufficiently long to acquire such sensitivity, given the slower learning rate in adult cortex.
The presence of responses to language in the visual cortex in individuals who become blind as adults is consistent with the observation of increased resting-state connectivity between Broca’s area and the “visual” cortex in this population (Sabbah et al., 2016). Analogously, a recent study found increased resting-state connectivity between parts of the “visual” cortex that are responsive to number, and fronto-parietal number networks, even in adult-onset blind individuals (Kanjlia et al., 2018). This latter study also showed that resting-state increases are significantly smaller in the adult-onset as opposed to the congenitally blind population. Furthermore, like in the current study, sensitivity to task-based cognitive manipulations was reduced or absent in adult-onset blindness - in the case of this prior study, responses to the difficulty of math equations were present only in people blind from birth. One possibility is that acquisition of sensitivity to fine-grained cognitive distinctions (e.g. to syntax and equation difficulty) depends on critical period plasticity in local cortical circuits, whereas resting state connectivity changes and more gross responses to language do not (Hensch, 2005).
Previous studies also suggest that the behavioral relevance of “visual” cortex activity in adult onset and congenital blindness is different. In congenital blindness, transcranial magnetic stimulation (TMS) to the occipital pole induces semantic errors in a verb generation task (Amedi et al., 2004). Congenitally blind adults also show superior performance on some linguistic tasks that recruit the “visual” cortex, e.g. verbal memory (Amedi et al., 2003; Occelli et al., 2017; Pasqualotto et al., 2013). One study also found better sentence processing performance among congenitally blind individuals relative to the sighted (Loiotile et al., 2019). In the current study, congenitally blind individuals responded somewhat faster (Experiment 1) and more accurately (Experiment 2) on sentence trials but not math and non-word control conditions, relative to the sighted as well as adult-onset blind individuals. The present behavioral effect should, however, be interpreted with caution since three blind participants were excluded from the study for poor performance. We found no difference in sentence-processing performance between adult-onset and sighted groups. There is also at present no evidence for behavioral relevance of visual cortex activity in adult-onset blindness. Indeed, as noted in the introduction, one study found that TMS to the occipital pole impairs Braille reading in congenitally blind, but not adult-onset blind individuals (Cohen et al., 1999). It thus remains possible that activity in visual cortices of adult-onset blind individuals is epiphenomenal with respect to behavior. This could be because the degree of involvement of the “visual” cortex is so small in the adult-onset blind population as to be task irrelevant. It also remains possible, however, that cross-modal “visual” cortex activity in adult onset blind individuals has a different cognitive role from that of congenitally blind individuals, and this role has not yet been tested.
Even if responses to language in the visual system of adult-onset blind individuals are not behaviorally relevant, their presence evidences communication between visual and language systems in people who grew up with vision. Similar effects have been observed even in blindfolded sighted adults (e.g. Elli et al., 2019). Communication between visual and language systems occurs in sighted individuals during visual tasks such as describing a visual scene, identifying objects based on verbal labels, or retrieving abstract linguistic representations from visual symbols i.e. reading (Jackendoff, 1987; Landau and Jackendoff, 2013). Parts of the visual cortex are both proximal and anatomically connected to language areas in sighted adults. The visual word form area (VWFA) is one of the key cortical nodes that connects visual and language systems (Bouhali et al., 2014; Yeatman et al., 2014). In sighted individuals, this region develops selectivity for written language, possibly because its anatomical location is ideally suited to connect visual symbols with linguistic content (Dehaene and Dehaene-Lambertz, 2016; Saygin et al., 2016; Osher et al., 2016). A number of anatomical tracts connect language and visual systems and could enable communication. In humans, the inferior fronto-occipital fasciculus (IFOF) contains a set of fibers passing from the occipital lobe to the inferior frontal cortex, the inferior longitudinal fasciculus (ILF) connects the occipital to the anterior and medial temporal lobe, and the vertical occipital fasciculus (VOF) of Wernicke connects the ventral occipitotemporal cortex to the lateral occipito-parietal junction (Ashtari, 2012; Forkel et al., 2014; Yeatman et al., 2013). One possibility is that blindness from birth modifies the functional role of these tracts by changing the way language-related information is used by “visual” cortex (Atilgan et al., 2017; Hasson et al., 2016; Leporé et al., 2010). A recent neurocomputational model was proposed to explain how the functional pattern that is observed in blind individuals might emerge from a common sighted/blind architecture (Tomasello et al., 2019).
6.3. Summary and conclusions
The present results provide evidence for an effect of the age-of-blindness onset on the reorganization of language networks in blindness. Only in congenitally blind individuals do visual cortices respond to syntactic movement, and visual cortex responses to spoken sentences are much larger in congenitally than adult-onset blind individuals. These results are consistent with the idea that in the absence of dominating visual input from the lateral geniculate nucleus, parts of the visual system are incorporated into the language network during language acquisition. The plasticity observed in congenital blindness supports the idea that the neural basis of language, while evolutionarily constrained, nevertheless emerges through a dynamic process that includes competition for the same cortical territory by multiple cognitive functions (Bates, 1993; Johnson et al., 2002; Karmiloff-Smith, 1998). The presence of some high-level language responses even in the visual system of adult-onset blind and blindfolded sighted people suggests that the plasticity observed in congenital blindness is made possible by existing channels of communication between the visual and language systems.
The current results add to prior evidence of different cognitive sensitivity in the visual cortices of congenitally and adult-onset blind individuals (eg: Bedny et al., 2010, 2012; Büchel et al., 1998a, 1998b; Burton et al., 2006a, 2006b; Burton et al., 2002; Cohen et al., 1999; Kanjlia et al., 2018). Together with the present results, these studies support the hypothesis that human cortex has a different capacity for cognitive specialization during childhood, as opposed to adulthood.
Declaration of Competing Interest
The authors declare no conflict of interests.
Acknowledgements
We thank the blind and sighted participants for taking part in this research. We are also grateful to Connor Lane and Dr. Akira Omaki for assistance with the design of the stimuli and task in Experiment 2. This study was supported by grant R01EY027352-02 from the National Institute of Health, United States of America to Dr. Marina Bedny.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2019.100744.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Abboud S., Cohen L. Distinctive Interaction Between Cognitive Networks and the Visual Cortex in Early Blind Individuals. Cereb. Cortex. 2019 doi: 10.1093/cercor/bhz006. [DOI] [PubMed] [Google Scholar]
- Aguirre G.K., Datta R., Benson N.C., Prasad S., Jacobson S.G., Cideciyan A.V., Bridge H., Watkins K.E., Butt O.H. Patterns of individual variation in visual pathway structure and function in the sighted and blind. PLoS ONE. 2016 doi: 10.1371/journal.pone.0164677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen J.S., Emmorey K., Bruss J., Damasio H. Neuroanatomical differences in visual, motor, and language cortices between congenitally deaf signers, hearing signers, and hearing non-signers. Front. Neuroanat. 2013 doi: 10.3389/fnana.2013.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amedi A., Floel A., Knecht S., Zohary E., Cohen L.G. Transcranial magnetic stimulation of the occipital pole interferes with verbal processing in blind subjects. Nat. Neurosci. 2004;7(11):1266–1270. doi: 10.1038/nn1328. [DOI] [PubMed] [Google Scholar]
- Amedi A., Raz N., Pianka P., Malach R., Zohary E. Early “visual” cortex activation correlates with superior verbal memory performance in the blind. Nat. Neurosci. 2003;6(7):758–766. doi: 10.1038/nn1072. [DOI] [PubMed] [Google Scholar]
- Ashtari M. Anatomy and functional role of the inferior longitudinal fasciculus: a search that has just begun. Dev. Med. Child Neurol. 2012;54(1):6–7. doi: 10.1111/j.1469-8749.2011.04122.x. [DOI] [PubMed] [Google Scholar]
- Atilgan H., Collignon O., Hasson U. Structural neuroplasticity of the superior temporal plane in early and late blindness. Brain Lang. 2017 doi: 10.1016/j.bandl.2017.03.008. [DOI] [PubMed] [Google Scholar]
- Bates E. Modularity, domain specificity and the development of language. Discuss. Neurosci. 1993 [Google Scholar]
- Bedny M. Evidence from blindness for a cognitively pluripotent cortex. Trends Cogn. Sci. 2017 doi: 10.1016/j.tics.2017.06.003. [DOI] [PubMed] [Google Scholar]
- Bedny M., Konkle T., Pelphrey K., Saxe R., Pascual-Leone A. Sensitive period for a multimodal response in human visual motion area MT/MST. Curr. Biol. 2010;20(21):1900–1906. doi: 10.1016/j.cub.2010.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedny M., Pascual-Leone A., Dodell-Feder D., Fedorenko E., Saxe R. Language processing in the occipital cortex of congenitally blind adults. Proc. Natl. Acad. Sci. 2011;108(11):4429–4434. doi: 10.1073/pnas.1014818108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedny M., Pascual-Leone A., Dravida S., Saxe R. A sensitive period for language in the visual cortex: distinct patterns of plasticity in congenitally versus late blind adults. Brain Lang. 2012 doi: 10.1016/j.bandl.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedny M., Richardson H., Saxe R. “Visual” cortex responds to spoken language in blind children. J. Neurosci. 2015 doi: 10.1523/JNEUROSCI.0634-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shachar M., Hendler T., Kahn I., Ben-Bashat D., Grodzinsky Y. The neural reality of syntactic transformations: evidence from functional magnetic resonance imaging. Psychol. Sci. 2003 doi: 10.1111/1467-9280.01459. [DOI] [PubMed] [Google Scholar]
- Ben-Shachar M., Palti D., Grodzinsky Y. Neural correlates of syntactic movement: converging evidence from two fMRI experiments. NeuroImage. 2004 doi: 10.1016/j.neuroimage.2003.11.027. [DOI] [PubMed] [Google Scholar]
- Bishop D.V.M. Cerebral asymmetry and language development: cause, correlate or consequence? Why are some children poor at language learning? Science. 2013;340(6138) doi: 10.1126/science.1230531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohannon J.N., Landau B., Gleitman L. Language and experience: evidence from the blind child. Language. 1986 [Google Scholar]
- Bonvillian J.D., Orlansky M.D., Novack L.L., Folven R.J. The Acquisition of Symbolic Skills. 2012. Early sign language acquisition and cognitive development. [Google Scholar]
- Bouhali F., Thiebaut de Schotten M., Pinel P., Poupon C., Mangin J.-F., Dehaene S., Cohen L. Anatomical connections of the visual word form area. J. Neurosci. 2014;34(46):15402–15414. doi: 10.1523/JNEUROSCI.4918-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büchel C., Price C., Frackowiak R.S.J., Friston K. Different activation patterns in the visual cortex of late and congenitally blind subjects. Brain. 1998;121(3):409–419. doi: 10.1093/brain/121.3.409. [DOI] [PubMed] [Google Scholar]
- Büchel C., Price C., Friston K. A multimodal language region in the ventral visual pathway. Nature. 1998 doi: 10.1038/28389. [DOI] [PubMed] [Google Scholar]
- Burton H., Diamond J.B., McDermott K.B. Dissociating cortical regions activated by semantic and phonological tasks: a fMRI study in blind and sighted people. J. Neurophysiol. 2006 doi: 10.1152/jn.00279.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton H., McLaren D.G. Visual cortex activation in late-onset, Braille naive blind individuals: An fMRI study during semantic and phonological tasks with heard words. Neurosci. Lett. 2006 doi: 10.1016/j.neulet.2005.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton H., Snyder A.Z., Conturo T.E., Akbudak E., Ollinger J.M., Raichle M.E. Adaptive changes in early and late blind: a fMRI study of braille reading. J. Neurophysiol. 2002 doi: 10.1152/jn.00285.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton H., Snyder A.Z., Diamond J.B., Raichle M.E. Adaptive changes in early and late blind: a fMRI study of verb generation to heard nouns. J. Neurophysiol. 2006 doi: 10.1152/jn.00129.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan D. Functional neuroimaging studies of syntactic processing in sentence comprehension: a critical selective review. Lang. Linguist. Compass. 2007 [Google Scholar]
- Cappelletti M., Butterworth B., Kopelman M. Spared numerical abilities in a case of semantic dementia. Neuropsychologia. 2001 doi: 10.1016/s0028-3932(01)00035-5. [DOI] [PubMed] [Google Scholar]
- Chen E., West W.C., Waters G., Caplan D. Determinants of BOLD signal correlates of processing object-extracted relative clauses. Cortex. 2006 doi: 10.1016/s0010-9452(08)70397-6. [DOI] [PubMed] [Google Scholar]
- Chomsky N. Mouton; The Hague, The Netherlands: 1957. Syntactic Structures. [Google Scholar]
- Christensen K.R. Interfaces, syntactic movement, and neural activation: a new perspective on the implementation of language in the brain. J. Neurolinguistics. 2008 [Google Scholar]
- Cohen L.G., Weeks R.A., Sadato N., Celnik P., Ishii K., Hallett M. Period of susceptibility for cross-modal plasticity in the blind. Ann. Neurol. 1999;45(4):451–460. doi: 10.1002/1531-8249(199904)45:4<451::aid-ana6>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Collignon O., Dormal G., Albouy G., Vandewalle G., Voss P., Phillips C., Lepore F. Impact of blindness onset on the functional organization and the connectivity of the occipital cortex. Brain. 2013;136(9):2769–2783. doi: 10.1093/brain/awt176. [DOI] [PubMed] [Google Scholar]
- Collignon O., Lassonde M., Voss P., Vandewalle G., Charbonneau G., Lepore F., Albouy G. Functional specialization for auditory-spatial processing in the occipital cortex of congenitally blind humans. Proc. Natl. Acad. Sci. 2011 doi: 10.1073/pnas.1013928108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cormier K., Schembri A., Vinson D., Orfanidou E. First language acquisition differs from second language acquisition in prelingually deaf signers: evidence from sensitivity to grammaticality judgement in British Sign Language. Cognition. 2012 doi: 10.1016/j.cognition.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Esposito M., Deouell L.Y., Gazzaley A. Alterations in the BOLD fMRI signal with ageing and disease: a challenge for neuroimaging. Nat. Rev. Neurosci. 2003 doi: 10.1038/nrn1246. [DOI] [PubMed] [Google Scholar]
- Dehaene-lambertz G., Dehaene S., Hertz-Pannier L. Functional neuroimaging of speech perception. In Infants. Science. 2002;298(December 2002):2013–2015. doi: 10.1126/science.1077066. [DOI] [PubMed] [Google Scholar]
- Dehaene S., Dehaene-Lambertz G. Is the brain prewired for letters? Nat. Neurosci. 2016;19(9):1192–1193. doi: 10.1038/nn.4369. [DOI] [PubMed] [Google Scholar]
- Dehaene S., Meyniel F., Wacongne C., Wang L., Pallier C. The neural representation of sequences: from transition probabilities to algebraic patterns and linguistic trees. Neuron. 2015 doi: 10.1016/j.neuron.2015.09.019. [DOI] [PubMed] [Google Scholar]
- Dronkers N.F., Wilkins D.P., Van Valin R.D., Redfern B.B., Jaeger J.J. Lesion analysis of the brain areas involved in language comprehension. Cognition. 2004 doi: 10.1016/j.cognition.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Duyck W., Desmet T., Verbeke L.P.C., Brysbaert M. WordGen: a tool for word selection and nonword generation in Dutch, English, German, and French. Behav. Res. Methods Instrum. Comput. 2004 doi: 10.3758/bf03195595. [DOI] [PubMed] [Google Scholar]
- Elli G.V., Lane C., Bedny M. 2019. A Double Dissociation in Sensitivity to Verb and Noun Semantics Across Cortical Networks. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmorey K., Bellugi U., Friederici A., Horn P. Effects of age of acquisition on grammatical sensitivity: evidence from on-line and off-line tasks. Appl. Psycholinguist. 1995 [Google Scholar]
- Fedorenko E., Behr M.K., Kanwisher N. Functional specificity for high-level linguistic processing in the human brain. Proc. Natl. Acad. Sci. 2011 doi: 10.1073/pnas.1112937108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E., Hsieh P.-J., Nieto-Castañón A., Whitfield-Gabrieli S., Kanwisher N. New method for fMRI investigations of language: defining ROIs functionally in individual subjects. J. Neurophysiol. 2010 doi: 10.1152/jn.00032.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E., Nieto-Castañon A., Kanwisher N. Lexical and syntactic representations in the brain: an fMRI investigation with multi-voxel pattern analyses. Neuropsychologia. 2012 doi: 10.1016/j.neuropsychologia.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E., Nieto-Castañón A., Kanwisher N. Syntactic processing in the human brain: what we know, what we don’t know, and a suggestion for how to proceed. Brain Lang. 2012 doi: 10.1016/j.bandl.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E., Thompson-Schill S.L. Reworking the language network. Trends Cogn. Sci. 2014 doi: 10.1016/j.tics.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fieger A., Röder B., Teder-Sälejärvi W., Hillyard S.A., Neville H.J. Auditory spatial tuning in late-onset blindness in humans. J. Cogn. Neurosci. 2006 doi: 10.1162/089892906775783697. [DOI] [PubMed] [Google Scholar]
- Flagg E.J., Cardy J.E.O., Roberts W., Roberts T.P.L. Language lateralization development in children with autism: insights from the late field magnetoencephalogram. Neurosci. Lett. 2005 doi: 10.1016/j.neulet.2005.05.037. [DOI] [PubMed] [Google Scholar]
- Forkel S.J., Thiebaut de Schotten M., Kawadler J.M., Dell’Acqua F., Danek A., Catani M. The anatomy of fronto-occipital connections from early blunt dissections to contemporary tractography. Cortex. 2014;56:73–84. doi: 10.1016/j.cortex.2012.09.005. [DOI] [PubMed] [Google Scholar]
- Friederici A.D. Neurobiology of syntax as the core of human language. Biolinguistics. 2017 [Google Scholar]
- Friedmann N. Syntactic movement in orally trained children with hearing impairment. J. Deaf Stud. Deaf Educ. 2005 doi: 10.1093/deafed/enj002. [DOI] [PubMed] [Google Scholar]
- Friedmann N., Rusou D. Critical period for first language: the crucial role of language input during the first year of life. Curr. Opin. Neurobiol. 2015;35(JUNE):27–34. doi: 10.1016/j.conb.2015.06.003. [DOI] [PubMed] [Google Scholar]
- Geren J., Snedeker J. Harvard University; 2009. Syntactic and Lexical Development in Children with Cochlear Implants; pp. 1–35. Unpublished Paper, Retrieved from Retrieved from http://www.wjh.harvard.edu/∼lds/pdfs/Geren Snedeker CI PRELIMINARY.pdf. [Google Scholar]
- Gibson E. Linguistic complexity: locality of syntactic dependencies. Cognition. 1998 doi: 10.1016/s0010-0277(98)00034-1. [DOI] [PubMed] [Google Scholar]
- Gleitman L.R., Newport E.L. The invention of language by children: environmental and biological influences o the acquisition of language. Language. 1995;1995(2nd):1–24. [Google Scholar]
- Gleitman L.R., Wanner E. Language Acquisition: the State of the Art. 1982. Language acquisition: the state of the art. [Google Scholar]
- Grodzinsky Y., Friederici A.D. Neuroimaging of syntax and syntactic processing. Curr. Opin. Neurobiol. 2006 doi: 10.1016/j.conb.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Hadjikhani N., Liu A.K., Dale A.M., Cavanagh P., Tootell R.B.H. Retinotopy and color sensitivity in human visual cortical area V8. Nat. Neurosci. 1998 doi: 10.1038/681. [DOI] [PubMed] [Google Scholar]
- Hamilton R.H., Pascual-Leone A. Cortical plasticity associated with Braille learning. Trends Cogn. Sci. 1998 doi: 10.1016/s1364-6613(98)01172-3. [DOI] [PubMed] [Google Scholar]
- Hartshorne J.K., Tenenbaum J.B., Pinker S. A critical period for second language acquisition: evidence from 2/3 million English speakers. Cognition. 2018 doi: 10.1016/j.cognition.2018.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U., Andric M., Atilgan H., Collignon O. Congenital blindness is associated with large-scale reorganization of anatomical networks. NeuroImage. 2016 doi: 10.1016/j.neuroimage.2015.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch T.K. Critical period regulation. Annu. Rev. Neurosci. 2004 doi: 10.1146/annurev.neuro.27.070203.144327. [DOI] [PubMed] [Google Scholar]
- Hensch T.K. Critical period plasticity in local cortical circuits. Nat. Rev. Neurosci. 2005;6(11):877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- Hensch T.K., Fagiolini M. Excitatory-inhibitory balance and critical period plasticity in developing visual cortex. Prog. Brain Res. 2004;147(SPEC. ISS):115–124. doi: 10.1016/S0079-6123(04)47009-5. [DOI] [PubMed] [Google Scholar]
- Hickok G. Parallel parsing: evidence from reactivation in garden-path sentences. J. Psycholinguist. Res. 1993 [Google Scholar]
- Holland S.K., Vannest J., Mecoli M., Jacola L.M., Tillema J.-M., Karunanayaka P.R., Schmithorst V.J., Yuan W., Plante E., Byars A.W. Functional MRI of language lateralization during development in children. Int. J. Audiol. 2007 doi: 10.1080/14992020701448994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huettel S.A., Singerman J.D., McCarthy G. The effects of aging upon the hemodynamic response measured by functional MRI. NeuroImage. 2001 doi: 10.1006/nimg.2000.0675. [DOI] [PubMed] [Google Scholar]
- Jackendoff R. On beyond Zebra: the relation of linguistic and visual information. Cognition. 1987 doi: 10.1016/0010-0277(87)90026-6. [DOI] [PubMed] [Google Scholar]
- Jiang F., Stecker G.C., Boynton G.M., Fine I. Early Blindness Results in Developmental Plasticity for Auditory Motion Processing within Auditory and Occipital Cortex. Front. Hum. Neurosci. 2016 doi: 10.3389/fnhum.2016.00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J.S., Newport E. Critical period effects in second language learning: the influence of maturational state on the acquisition of english as a second language. Cogn. Psychol. 1989;21:60–99. doi: 10.1016/0010-0285(89)90003-0. [DOI] [PubMed] [Google Scholar]
- Johnson J.S., Newport E.L. Critical period effects on universal properties of language: the status of subjacency in the acquisition of a second language. Cognition. 1991 doi: 10.1016/0010-0277(91)90054-8. [DOI] [PubMed] [Google Scholar]
- Johnson M.H., Halit H., Grice S.J., Karmiloff-Smith A. Neuroimaging of typical and atypical development: a perspective from multiple levels of analysis. Dev. Psychopathol. 2002 doi: 10.1017/s0954579402003073. 10.1017.S0954579402003073. [DOI] [PubMed] [Google Scholar]
- Kanjlia S., Lane C., Feigenson L., Bedny M. Absence of visual experience modifies the neural basis of numerical thinking. Proc. Natl. Acad. Sci. 2016 doi: 10.1073/pnas.1524982113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanjlia S., Pant R., Bedny M. Sensitive period for cognitive repurposing of human visual cortex. Cereb. Cortex. 2018 doi: 10.1093/cercor/bhy280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmiloff-Smith A. Development itself is the key to understanding developmental disorders. Trends Cogn. Sci. 1998 doi: 10.1016/s1364-6613(98)01230-3. [DOI] [PubMed] [Google Scholar]
- Keller T.A. The neural bases of sentence comprehension: a fMRI examination of syntactic and lexical processing. Cereb. Cortex. 2001 doi: 10.1093/cercor/11.3.223. [DOI] [PubMed] [Google Scholar]
- Kempler D., Van Lancker D., Marchman V., Bates E. Idiom comprehension in children and adults with unilateral brain damage. Dev. Neuropsychol. 1999 [Google Scholar]
- Kim J.S., Kanjlia S., Merabet L.B., Bedny M. Development of the visual word form area requires visual experience: evidence from blind braille readers. J. Neurosci. 2017 doi: 10.1523/JNEUROSCI.0997-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen E.I. Sensitive periods in the development of the brain and behavior. J. Cogn. Neurosci. 2004;16(8):1412–1425. doi: 10.1162/0898929042304796. [DOI] [PubMed] [Google Scholar]
- Kupers R., Pappens M., De Noordhout A.M., Schoenen J., Ptito M., Fumal A. rTMS of the occipital cortex abolishes Braille reading and repetition priming in blind subjects. Neurology. 2007 doi: 10.1212/01.wnl.0000255958.60530.11. [DOI] [PubMed] [Google Scholar]
- Landau B., Jackendoff R. Bridges Between Psychology and Linguistics. Psychology Press; 2013. Spatial language and spatial cognition; pp. 157–182. [Google Scholar]
- Lane C., Kanjlia S., Omaki A., Bedny M. “Visual” cortex of congenitally blind adults responds to syntactic movement. J. Neurosci. 2015 doi: 10.1523/JNEUROSCI.1256-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane C., Kanjlia S., Richardson H., Fulton A., Omaki A., Bedny M. Reduced left lateralization of language in congenitally blind individuals. J. Cogn. Neurosci. 2017 doi: 10.1162/jocn_a_01045. [DOI] [PubMed] [Google Scholar]
- Lenneberg E.H. New Directions in the Study of Language. 1967. A biological perspective of language. [Google Scholar]
- Leporé N., Voss P., Lepore F., Chou Y.Y., Fortin M., Gougoux F., Lee A.D., Brun C., Lassonde M., Madsen S.K., Toga A.W., Thompson P.M. Brain structure changes visualized in early- and late-onset blind subjects. NeuroImage. 2010 doi: 10.1016/j.neuroimage.2009.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R. Expectation-based syntactic comprehension. Cognition. 2008;106(3):1126–1177. doi: 10.1016/j.cognition.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Leybaert J., D’hondt M. Neurolinguistic development in deaf children: the effect of early language experience. Int. J. Audiol. 2009 doi: 10.3109/14992020309074622. [DOI] [PubMed] [Google Scholar]
- Lightbrown P., Spada N. How Languages Are Learned. 1993. How languages are learned. [Google Scholar]
- Liu Y., Yu C., Liang M., Li J., Tian L., Zhou Y. Whole brain functional connectivity in the early blind. Brain. 2007 doi: 10.1093/brain/awm121. [DOI] [PubMed] [Google Scholar]
- Loiotile R., Omaki A., Bedny M. Enhanced sentence processing abilities among congenitally blind adults. PsyArXiv. 2019 [Google Scholar]
- Lopez-Higes R., Gallego C., Martin-Aragoneses M.T., Melle N. Morpho-syntactic reading comprehension in children with early and late cochlear implants. J. Deaf Stud. Deaf Educ. 2015;20(2):136–146. doi: 10.1093/deafed/env004. [DOI] [PubMed] [Google Scholar]
- MacDonald M.C., Just M.A., Carpenter P.A. Working memory constraints on the processing of syntactic ambiguity. Cogn. Psychol. 1992 doi: 10.1016/0010-0285(92)90003-k. [DOI] [PubMed] [Google Scholar]
- MacDonald M.C., Pearlmutter N.J., Seidenberg M.S. The lexical nature of syntactic ambiguity resolution. Psychol. Rev. 1994 doi: 10.1037/0033-295x.101.4.676. [DOI] [PubMed] [Google Scholar]
- MacSweeney M., Capek C.M., Campbell R., Woll B. The signing brain: the neurobiology of sign language. Trends Cogn. Sci. 2008 doi: 10.1016/j.tics.2008.07.010. [DOI] [PubMed] [Google Scholar]
- MacSweeney M., Waters D., Brammer M.J., Woll B., Goswami U. Phonological processing in deaf signers and the impact of age of first language acquisition. NeuroImage. 2008 doi: 10.1016/j.neuroimage.2007.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Max J.E. Effect of side of lesion on neuropsychological performance in childhood stroke. J. Int. Neuropsychol. Soc. 2004;10(5):698–708. doi: 10.1017/S1355617704105092. [DOI] [PubMed] [Google Scholar]
- Mayberry R.I. When timing is everything: age of first-language acquisition effects on second-language learning. Appl. Psycholinguist. 2007 [Google Scholar]
- Mayberry R.I., Chen J.K., Witcher P., Klein D. Age of acquisition effects on the functional organization of language in the adult brain. Brain Lang. 2011;119(1):16–29. doi: 10.1016/j.bandl.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Mayberry R.I., Eichen E.B. The long-lasting advantage of learning sign language in childhood: another look at the critical period for language acquisition. J. Mem. Lang. 1991;30(4):486–512. [Google Scholar]
- Mayberry R.I., Kluender R. Rethinking the critical period for language: new insights into an old question from American sign language. Bilingualism. 2018 doi: 10.1017/S1366728917000724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberry R.I., Lock E. Age constraints on first versus second language acquisition: evidence for linguistic plasticity and epigenesis. Brain Lang. 2003;87(3):369–384. doi: 10.1016/s0093-934x(03)00137-8. [DOI] [PubMed] [Google Scholar]
- McRae K., Spivey-Knowlton M.J., Tanenhaus M.K. Modeling the influence of thematic fit (and other constraints) in on-line sentence comprehension. J. Mem. Lang. 1998 [Google Scholar]
- Merabet L.B., Pascual-Leone A. Neural reorganization following sensory loss: the opportunity of change. Nat. Rev. Neurosci. 2010 doi: 10.1038/nrn2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti M.M., Parsons L.M., Osherson D.N. The boundaries of language and thought in deductive inference. Proc. Natl. Acad. Sci. 2009 doi: 10.1073/pnas.0902422106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti M.M., Parsons L.M., Osherson D.N. Thought beyond language: neural dissociation of algebra and natural language. Psychol. Sci. 2012 doi: 10.1177/0956797612437427. [DOI] [PubMed] [Google Scholar]
- Bavelier D., Corina D., Rauschecker D.J., Karni A., Lalwani A., Braun, Clark V., Jezzard P., Turner R. Cerebral organization for language in deaf and hearing subjects: biological constraints and effects of experience. Proc. Natl. Acad. Sci. 1998 doi: 10.1073/pnas.95.3.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville H.J., Mills D.L., Lawson D.S. Fractionating language: different neural subsystems with different sensitive periods. Cereb. Cortex. 1992 doi: 10.1093/cercor/2.3.244. [DOI] [PubMed] [Google Scholar]
- Newport E.L. Maturational constraints on language-learning. Cogn. Sci. 1990;14(1):11–28. [Google Scholar]
- Newport E.L., Bavelier D., Neville H.J. Language, Brain and Cognitive Development. 2001. Critical thinking about critical periods: perspectives on a critical period for language acquisition. [Google Scholar]
- Newport E.L., Landau B., Seydell-Greenwald A., Turkeltaub P.E., Chambers C.E., Dromerick A.W., Carpenter J., Berl M.M., Gaillard W.D. Revisiting Lenneberg’s hypotheses about early developmental plasticity: language organization after left-hemisphere perinatal stroke. Biolinguistics. 2017;11:407–422. https://www.ncbi.nlm.nih.gov/pubmed/30556058 Retrieved from. [PMC free article] [PubMed] [Google Scholar]
- Newport E.L., York N. Language development, critical periods. Encycl. Cognit. Sci. 2003:737–740. [Google Scholar]
- Noppeney U. The effects of visual deprivation on functional and structural organization of the human brain. Neurosci. Biobehav. Rev. 2007 doi: 10.1016/j.neubiorev.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Noppeney U., Price C.J. An fMRI study of syntactic adaptation. J. Cogn. Neurosci. 2004 doi: 10.1162/089892904323057399. [DOI] [PubMed] [Google Scholar]
- Occelli V., Lacey S., Stephens C., Merabet L.B., Sathian K. Enhanced verbal abilities in the congenitally blind. Exp. Brain Res. 2017 doi: 10.1007/s00221-017-4931-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osher D.E., Saxe R.R., Koldewyn K., Gabrieli J.D.E., Kanwisher N., Saygin Z.M. Structural connectivity fingerprints predict cortical selectivity for multiple visual categories across cortex. Cereb. Cortex. 2016 doi: 10.1093/cercor/bhu303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakulak E., Neville H.J. Maturational constraints on the recruitment of early processes for syntactic processing. J. Cogn. Neurosci. 2011 doi: 10.1162/jocn.2010.21586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasqualotto A., Lam J.S.Y., Proulx M.J. Congenital blindness improves semantic and episodic memory. Behav. Brain Res. 2013 doi: 10.1016/j.bbr.2013.02.005. [DOI] [PubMed] [Google Scholar]
- Patkowski M.S. The sensitive period for the acquisition of syntax in a second language. Lang. Learn. 1980;30(2):449–468. [Google Scholar]
- Perneger T.V. What’s wrong with Bonferroni adjustments. BMJ. 1998 doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitto L.A., Holowka S., Sergio L.E., Ostry D. Language rhythms in baby hand movements. Nature. 2001 doi: 10.1038/35092613. [DOI] [PubMed] [Google Scholar]
- Petitto L.A., Katerelos M., Levy B.G., Gauna K., Tétreault K., Ferraro V. Bilingual signed and spoken language acquisition from birth: implications for the mechanisms underlying early bilingual language acquisition. J. Child Lang. 2001 doi: 10.1017/s0305000901004718. [DOI] [PubMed] [Google Scholar]
- Rasmussen T., Milner B. The role of early left-brain injury in determining lateralization of cerebral speech functions. Ann. N. Y. Acad. Sci. 1977 doi: 10.1111/j.1749-6632.1977.tb41921.x. [DOI] [PubMed] [Google Scholar]
- Röder B., Rösler F., Neville H.J. Event-related potentials during auditory language processing in congenitally blind and sighted people. Neuropsychologia. 2000;38(11):1482–1502. doi: 10.1016/s0028-3932(00)00057-9. [DOI] [PubMed] [Google Scholar]
- Röder B., Stock O., Bien S., Neville H., Rösler F. Speech processing activates visual cortex in congenitally blind humans. Eur. J. Neurosci. 2002 doi: 10.1046/j.1460-9568.2002.02147.x. [DOI] [PubMed] [Google Scholar]
- Rosen H.J., Petersen S.E., Linenweber M.R., Snyder A.Z., White D.A., Chapman L., Dromerick A.W., Fiez J.A., Corbetta M. Neural correlates of recovery from aphasia after damage to left inferior frontal cortex. Neurology. 2000 doi: 10.1212/wnl.55.12.1883. [DOI] [PubMed] [Google Scholar]
- Ruben R.J. A time frame of critical/sensitive periods of language development. Indian J. Otolaryngol. Head Neck Surg. 1999;51(3):85–89. doi: 10.1007/BF02996542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbah N., Authié C.N., Sanda N., Mohand-Saïd S., Sahel J.A., Safran A.B., Habas C., Amedi A. Increased functional connectivity between language and visually deprived areas in late and partial blindness. NeuroImage. 2016;136:162–173. doi: 10.1016/j.neuroimage.2016.04.056. [DOI] [PubMed] [Google Scholar]
- Sadato N., Okada T., Honda M., Yonekura Y. Critical period for cross-modal plasticity in blind humans: a functional MRI study. NeuroImage. 2002 doi: 10.1006/nimg.2002.1111. [DOI] [PubMed] [Google Scholar]
- Sadato N., Pascual-Leone A., Grafman J., Deiber M.P., Ibañez V., Hallett M. Neural networks for Braille reading by the blind. Brain. 1998 doi: 10.1093/brain/121.7.1213. [DOI] [PubMed] [Google Scholar]
- Saxe R., Brett M., Kanwisher N. Divide and conquer: a defense of functional localizers. NeuroImage. 2006 doi: 10.1016/j.neuroimage.2005.12.062. [DOI] [PubMed] [Google Scholar]
- Saygin Z.M., Osher D.E., Norton E.S., Youssoufian D.A., Beach S.D., Feather J., Gaab, Gabrieli J.D.E., Kanwisher N. Connectivity precedes function in the development of the visual word form area. Nat. Neurosci. 2016 doi: 10.1038/nn.4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer I.E.C., Ramsey N.F., Kahn R.S. Language lateralization in schizophrenia, an fMRI study. Schizophr. Res. 2001 doi: 10.1016/s0920-9964(00)00180-8. [DOI] [PubMed] [Google Scholar]
- Staub A. Eye movements and processing difficulty in object relative clauses. Cognition. 2010 doi: 10.1016/j.cognition.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Szaflarski J.P., Holland S.K., Schmithorst V.J., Byars A.W. fMRI study of language lateralization in children and adults. Hum. Brain Mapp. 2006 doi: 10.1002/hbm.20177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thal D.J., Marchman V., Stiles J., Aram D., Trauner D., Nass R., Bates E. Early lexical development in children with focal brain injury. Brain Lang. 1991 doi: 10.1016/0093-934x(91)90145-q. [DOI] [PubMed] [Google Scholar]
- Tivarus M.E., Starling S.J., Newport E.L., Langfitt J.T. Homotopic language reorganization in the right hemisphere after early left hemisphere injury. Brain Lang. 2012 doi: 10.1016/j.bandl.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasello R., Garagnani M., Wennekers T., Pulvermüller F. 2019. Recruitment of Visual Cortex for Language Processing in Blind Individuals Is Explained by Hebbian Learning; pp. 1–16. August 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen D.C. A Population-Average, Landmark- and Surface-based (PALS) atlas of human cerebral cortex. NeuroImage. 2005 doi: 10.1016/j.neuroimage.2005.06.058. [DOI] [PubMed] [Google Scholar]
- Veraart C., De Volder A.G., Wanet-Defalque M.C., Bol A., Michel C., Goffinet A.M. Glucose utilization in human visual cortex is abnormally elevated in blindness of early onset but decreased in blindness of late onset. Brain Res. 1990 doi: 10.1016/0006-8993(90)90735-t. [DOI] [PubMed] [Google Scholar]
- Voss P. Sensitive and critical periods in visual sensory deprivation. Front. Psychol. 2013;4(SEP):1–13. doi: 10.3389/fpsyg.2013.00664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss P., Gougoux F., Zatorre R.J., Lassonde M., Lepore F. Differential occipital responses in early- and late-blind individuals during a sound-source discrimination task. NeuroImage. 2008;40(2):746–758. doi: 10.1016/j.neuroimage.2007.12.020. [DOI] [PubMed] [Google Scholar]
- Wallace M.T., Stein B.E. Early experience determines how the senses will interact. J. Neurophysiol. 2007 doi: 10.1152/jn.00497.2006. [DOI] [PubMed] [Google Scholar]
- Watkins K.E., Cowey A., Alexander I., Filippini N., Kennedy J.M., Smith S.M., Ragge N., Bridge H. Language networks in anophthalmia: maintained hierarchy of processing in “visual” cortex. Brain. 2012 doi: 10.1093/brain/aws067. [DOI] [PubMed] [Google Scholar]
- Weber-Fox C.M., Neville H.J. Maturational constraints on functional specializations for language processing: ERP and behavioral evidence in bilingual speakers. J. Cogn. Neurosci. 1996 doi: 10.1162/jocn.1996.8.3.231. [DOI] [PubMed] [Google Scholar]
- Weber-Fox C., Neville H.J. Sensitive periods differentiate processing of open- and closed-class words: an ERP study of bilinguals. J. Speech Lang. Hear. Res. 2001 doi: 10.1044/1092-4388(2001/104). [DOI] [PubMed] [Google Scholar]
- Winkler A.M., Ridgway G.R., Webster M.A., Smith S.M., Nichols T.E. Permutation inference for the general linear model. NeuroImage. 2014 doi: 10.1016/j.neuroimage.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood A.G., Harvey A.S., Wellard R.M., Abbott D.F., Anderson V., Kean M., Saling M.M., Jackson G.D. Language cortex activation in normal children. Neurology. 2004 doi: 10.1212/01.wnl.0000140707.61952.ca. [DOI] [PubMed] [Google Scholar]
- Yeatman J.D., Rauschecker A.M., Wandell B.A. Anatomy of the visual word form area: adjacent cortical circuits and long-range white matter connections. Brain Lang. 2013 doi: 10.1016/j.bandl.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatman J.D., Weiner K.S., Pestilli F., Rokem A., Mezer A., Wandell B.A. The vertical occipital fasciculus: a century of controversy resolved by in vivo measurements. Proc. Natl. Acad. Sci. 2014;111(48):E5214–E5223. doi: 10.1073/pnas.1418503111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zago L., Pesenti M., Mellet E., Crivello F., Mazoyer B., Tzourio-Mazoyer N. Neural correlates of simple and complex mental calculation. NeuroImage. 2001 doi: 10.1006/nimg.2000.0697. [DOI] [PubMed] [Google Scholar]
- Zevin J.D., Datta H., Skipper J.I. Sensitive periods for language and recovery from stroke: conceptual and practical parallels. Dev. Psychobiol. 2012 doi: 10.1002/dev.20626. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.