Abstract
The surgical intervention and adjuvant locally applied measures to reduce the impact of a severe cutaneous hand infection caused by Mycobacterium marinum in a 48-year-old diver, receiving anti-TNFa (anti-tumor necrosis factor αlpha) treatment are presented herein.
Careful surgical intervention- considered controversial so far-and lateral thinking are essential for the outcome of musculoskeletal infections caused by M marinum.
Locally applied disinfection strategies inspired from fields other than medical (e.g. environmental biology) and clearly set surgery goals - aiming at optimizing tissue sampling, antibiotic penetration, decompression while preventing iatrogenic spread are discussed.
Keywords: Anti-TNFa therapy, Mycobacterial musculoskeletal infection, Surgical debridement
Introduction
M. marinum is the species of Mycobacterium causing skin infection following an exposure of cut or abraded skin to organisms present in aquariums, pools, natural water supplies and salt water. Theoretically, it rarely can cause infections involving deeper tissues of the body, such as tendon sheaths, joints, and bones, because compared with other mycobacteria that grow in culture medium at 37 °C this particular species, needs cooler temperatures (30–32 °C for optimal growth).
The incubation period may vary from 3 weeks to 9 months in otherwise healthy individuals [1].
Infections with M marinum have been classified into three clinical categories according to severity and host status [2].
Early diagnosis is of paramount importance -as clinical response to chemotherapy requires months to be attained-and includes histopathology, tissue culture and PCR (Polymerase Chain Reaction). In many instances, especially in patients under anti-TNFa treatment protocol granulomas do not form at all and histopathology may reveal non-specific inflammation. Ziehl-Nielsen stain may or may not reveal acid-fast bacilli. Tissue cultures may have to be repeated and waiting time for a result is not less than ten days.
Body
Two weeks after he punctured the dorsum of his left index finger while working at a ship repair, this 48-year old male diver noticed a painful swelling over the affected area. Five months later, having received oral steroid treatment for arthritis from various physicians he was referred to the orthopaedic department by the rheumatology team of our hospital. The patient was receiving anti TNFa treatment for ankylosing spondylitis over the last 8 years.
At presentation, although his general health was good (normal clinical signs, no temperature and normal laboratory testing except for marginally raised CRP), he was in agonizing pain (Visual Analogue Score VAS = 8) due to extensive swelling of the hand. Subcutaneous abscesses under tension formed in the palm and all over the dorsum of the hand and the fingers, extending in the forearm, that made every movement extremely painful and almost impossible. His sleep was disturbed, he was on paracetamol-tramadol and pregabalin 75 mg three times a day. The distribution of lesions seemed to follow the course of lymphatic vessels across the hand and forearm.
Magnetic Resonance Imaging (MRI) of the left hand revealed multiple subcutaneous collections (abscesses) being extra-synovial and extra-articular (Fig. 1a and b).
Fig. 1.
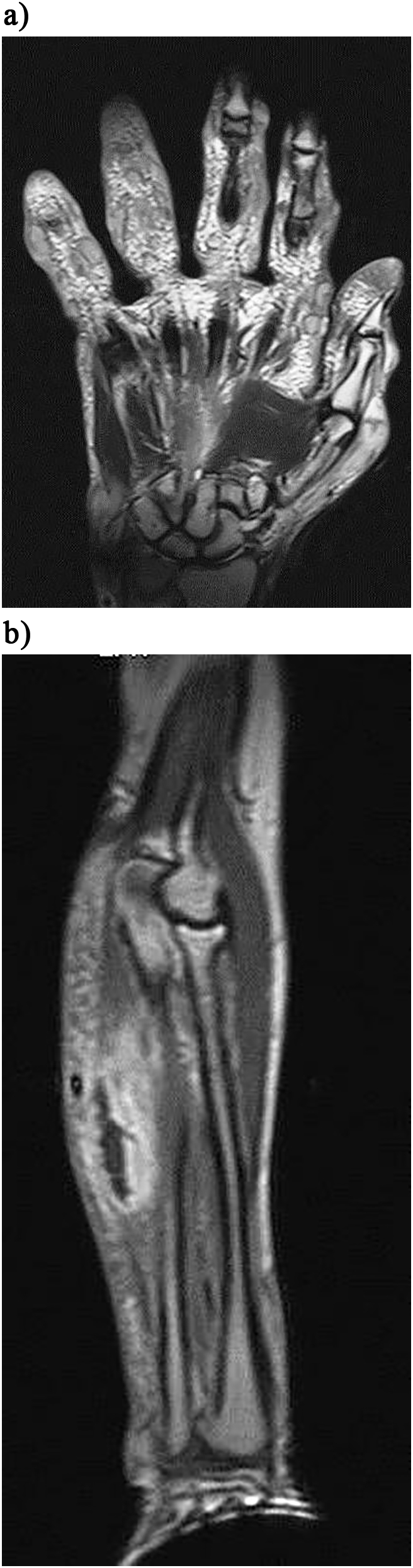
a. MRI scan of the hand showing the distended extra-capsular, extra-synovial abscesses. b. MRI scan of the forearm depicting the forearm similar lesion.
A careful plan was developed aiming at:
-
•
Obtaining adequate tissue samples for cultures and histology.
-
•
Decompressing the distended abscesses with multiple mini incisions (Fig. 2a and b).
-
•
Scrubbing away the purulent content (Fig. 3), thus minimizing the bacteriological load and facilitating antibiotic penetration.
-
•
Employing directly the bactericidal properties of hydrogen peroxide (H2O2) and sodium hypochlorite solutions together with iodine paste.
-
•
Keeping the infection confined within the subcutaneous tissues and prohibiting further iatrogenic inoculation or local extension.
-
•
Repeating minimal invasive surgical intervention as often as needed.
Fig. 2.
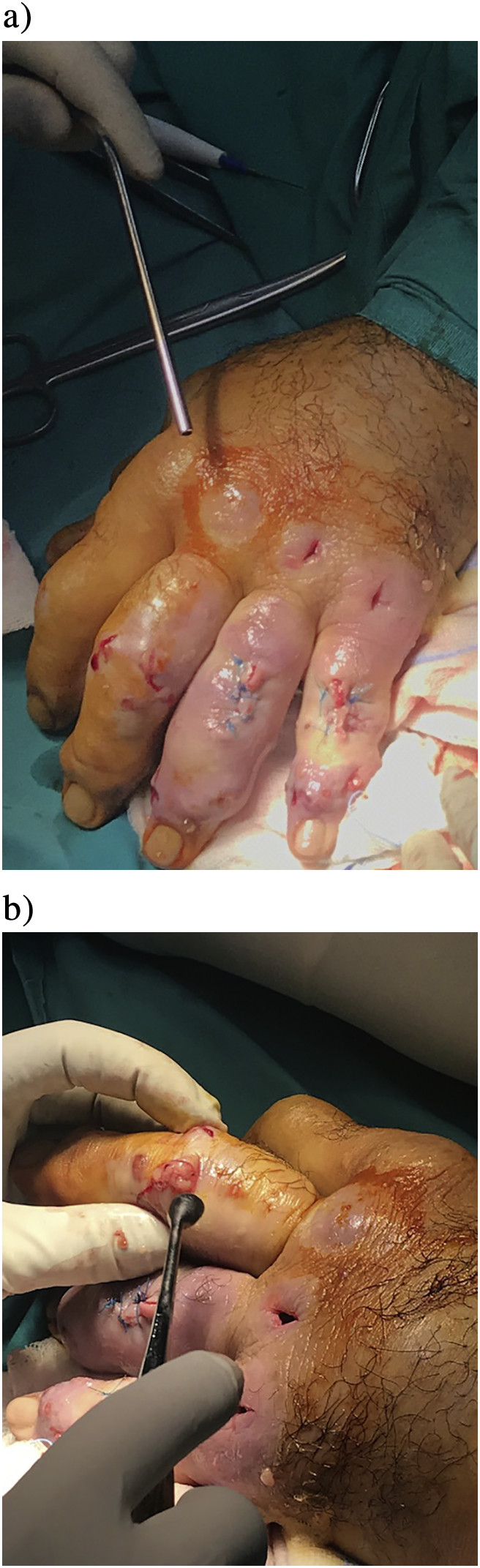
b. The hand at the beginning of the first surgery. b. The hand immediately after the first surgical decompression.
Fig. 3.

Tissue sample of myxomatous texture forming the abscess.
At surgery, the abscesses were debrided by multiple mini incisions, most of which were left to heal by secondary healing, avoiding to spread the infection to deeper tissues.
Having studied current literature on M marinum treatment [2,3,8,11] we devised a custom post-operative wound handling protocol-based on treatments suggested by environmental biologists in order to treat M marinum fish infections-as follows:
Following surgery, every third day the hand was prepped with hydrogen peroxide (30.000 ppm solution) followed by application of dressings immersed in sodium hypochlorite (30 ppm solution) for 10 min. Then it was dried off by sterile dressings and was redressed, every digit separately, and covered by iodine paste and sterile paraffin intertulles. Lastly it was all wrapped in a three-layer natural rayon bandage (velband or similar) and loosely covered by elastic compression bandage, in order to keep it warm and well above 30C.
Anti-TNFa treatment was discontinued and initial administration of three-drug therapy commenced, as suggested in literature [[3], [4], [5]],consisting of Clarithromycin (1 g/day)-Ethambutol (1500 mg/day)-Rifambicin (1200 mg/day), taking into consideration the invasiveness and multi-drug resistance properties of the suspected pathogen; M marinum.
Histology revealed non-specific inflammation of the dermis without granuloma formation -as expected due to anti-TNFa effect- and Ziehl-Nielsen stain for acid-fast bacilli was negative. Following two weeks of incubation in Lowenstein-Jensen medium at 30C, M marinum was isolated and PCR-restriction enzyme analysis of hsp65 confirmed the diagnosis.
Following surgery, the patient immediately improved, the pain was controlled and local lesions withdrew 8 days post-surgery (Fig. 4), primarily those left to heal by secondary healing (without stitching). Recurrence of local symptoms was observed shortly afterwards with newly formed abscesses spreading towards the axilla of the left arm. All new lesions were debrided by repeated surgery and every new incision was left to heal by secondary healing.
Fig. 4.

The hand on the 8th post-operative day.
The patient was thought to have developed Immune Reconstitution Inflammatory Syndrome (IRIS) due to withdrawal of the anti-TNFa, which was further addressed by oral methylprednisolone (16 mg daily) and clinical improvement was immediate.
At three months follow up, infection had completely resolved and the function of the hand, fingers and arm was complete (100%) (Video).
Discussion
All mycobacteria, including M marinum, have extremely resilient waxy cell wall that is highly hydrophobic, they form biofilms thus increasing their resistance to chemotherapy and chemical disinfectants [[6], [7], [8], [9]].
There is increasing evidence that the risk of acquiring non-tuberculous mycobacterial disease is elevated among patients who receive anti–tumor necrosis factor α (TNF-α) therapy [9,10].
In the absence of TNF signaling, bacterial growth is enhanced with various mechanisms: first within macrophages whose microbicidal capacity is significantly but not completely abolished, and then by the death of the infected macrophages that leads to a further enhancement in bacterial replication by eliminating remaining macrophage defense mechanisms. Death of infected macrophages has the potential to increase net bacterial burdens by increasing cell to cell spread [9].
In that respect, the pathogenicity and virulence determinants of M marinum need to be thoroughly and comprehensively investigated and understood in order to achieve effective treatment of the musculoskeletal infections in the immunocompromised patients.
Firstly, we would like to emphasize the need of discussion over surgical intervention. We do believe that a clear distinction between superficial and deep spread is essential, and so is the distinction between quiescent (or self-limiting) and aggressive forms of infection, in order to decide upon surgical debridement. The Bhatty et al. classification [2] recognizes three clinical types of M marinum infection: Type I, is a self-limiting verrucal lesion; Type II refers to the formation of single or multiple subcutaneous granulomas, with or without ulceration; Type III is related to deep infections involving tenosynovium, bursa, bones or joints causing tenosynovitis, septic arthritis or osteomyelitis.
Our experience with this particular case, which we would probably classify as Bhatty Type II, showed that an aggressive lesion- even if subcutaneous- has to be treated with minimal invasive (through multiple mini incisions) and repeated surgeries in order to prevent deeper or further extension. Leaving the incisions to heal by secondary healing lead to a faster outcome in this instance.
Additionally, we would like to point out the paramount importance of a plan which will ensure that iatrogenic spread is avoided. We believe that what Bhatty et al., describe as type III infection, is most likely the consequence of initial misdiagnoses and treatment of the condition with intra-lesional steroid injections that exacerbate the symptoms and spread the infection to tissues like joints and synovium.
Secondly, we observed that local measures deriving from marine biology papers [6,12] are effective in treating M marinum musculoskeletal lesions [2,3]. The wound care protocol we devised lead to immediate improvement of the local symptoms following each dressing change. The patient had one relapse although he was on antibiotics and anti TNFa drug was discontinued. We believe that the multiple surgeries and special wound care protocol helped in controlling the situation locally and had an impact on the excellent functional outcome.
Although phage therapy using the mycobacteriophage D29 proved effective in the treatment of Buruli Ulcer (BU) caused by M. ulcerans in the murine footpad model there is no bacteriophage specific for controlling M. marinum infections [11]. Other therapeutic alternatives such as electrodessication, therapy with X-ray, cryotherapy, local hyperthermic therapy and photodynamic therapy have been recorded and recommended [7].
Conclusion
Clinical data with respect to this pathogen is worth being re-visited, compared and connected at global level in order to produce a successful therapeutic algorithm. It is always useful to gather knowledge from different scientific fields when investigating unresolved problems.
The following is the supplementary data related to this article.
Clinical and functional outcome at 3 months post operatively.
Acknowledgements
We acknowledge the input of Athanasios Tzioufas, Rheumatology Professor at the Kapodistrian University Medical School of Athens and Charalambos Moutsopoulos, emeritus Rheumatology Professor at the Kapodistrian University Medical School of Athens, who kindly put their trust on us to treat their patient.
We also acknowledge the input of Professor N. V Sipsas of Infectious diseases at the Kapodistrian University Medical School of Athens, who worked on the chemotherapy regiment.
References
- 1.Jernigan J.A., Farr B.M. Incubation period and sources of exposure for cutaneous Mycobacterium marinum infection: case report and review of the literature. Clin. Infect. Dis. 2000;31:439–443. doi: 10.1086/313972. [DOI] [PubMed] [Google Scholar]
- 2.Bhatty M.A., Turner D.P.J., Chamberlain S.T. Mycobacterium marinum hand infection: case reports and review of literature. Br. J. Plast. Surg. 2000;53:161–165. doi: 10.1054/bjps.1999.3245. [DOI] [PubMed] [Google Scholar]
- 3.Aubry A., Jarlier V., Escolano S., Truffot-Pernot C., Cambau E. Antibiotic susceptibility pattern of Mycobacterium marinum. Antimicrob. Agents Chemother. 2000;44:3133. doi: 10.1128/aac.44.11.3133-3136.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson M.G., Stout J.E. Twenty-eight cases of Mycobacterium marinum infection: retrospective case series and literature review. Infection. 2015;43:655–662. doi: 10.1007/s15010-015-0776-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramos J.M., García-Sepulcre M.F., Rodríguez J.C., Padilla S., Gutiérrez F. Mycobacterium marinum infection complicated by anti-tumour necrosis factor therapy. J. Med. Microbiol. 2010;59:617–621. doi: 10.1099/jmm.0.017277-0. [DOI] [PubMed] [Google Scholar]
- 6.Chang C.T., Colicino E.G., DiPaola E.J., Al-Hasnawi H.J., Whipps C.M. Evaluating the effectiveness of common disinfectants at preventing the propagation of Mycobacterium spp. isolated from zebrafish. Comp. Biochem. Physiol. Part - C Toxicol. Pharmacol. 2015;178:45–50. doi: 10.1016/j.cbpc.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rallis E., Koumantaki-Mathioudaki E., Frangoulis E., Chatziolou E., Katsambas A. Severe sporotrichoid fish tank granuloma following infliximab therapy. Am. J. Clin. Dermatol. 2007;8:385–388. doi: 10.2165/00128071-200708060-00009. [DOI] [PubMed] [Google Scholar]
- 8.Aubry A., Chosidow O., Caumes E., Robert J., Cambau E. Sixty-three cases of Mycobacterium marinum infection: clinical features, treatment, and antibiotic susceptibility of causative isolates. Arch. Intern. Med. 2002;162:1746–1752. doi: 10.1001/archinte.162.15.1746. [DOI] [PubMed] [Google Scholar]
- 9.Clay H., Volkman H.E., Ramakrishnan L. Tumor necrosis factor signaling mediates resistance to mycobacteria by inhibiting bacterial growth and macrophage death. Immunity. 2008;29:283–294. doi: 10.1016/j.immuni.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liao T.L., Lin C.H., Shen G.H., Chang C.L., Lin C.F., Chen D.Y. Risk for mycobacterial disease among patients with rheumatoid arthritis, Taiwan, 2001–2011. Emerg. Infect. Dis. 2015;21:1387–1395. doi: 10.3201/eid2108.141846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trigo G., Martins T.G., Fraga A.G., Longatto-Filho A., Castro A.G., Azeredo J., Pedrosa J. Phage therapy is effective against infection by Mycobacterium ulcerans in a murine footpad model. PLoS Negl. Trop. Dis. 2013;7 doi: 10.1371/journal.pntd.0002183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mason T. ZEBRAFISH Volume 13, Supplement 1. Mary Ann Liebert, Inc; 2016. Strategies to mitigate a Mycobacterium marinum outbreak in a zebrafish research facility. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clinical and functional outcome at 3 months post operatively.


