Abstract
Decellularized tendon xenografts offer a promising alternative for reconstruction by using ubiquitously available material. This study compares static and centrifugal seeding of avian tendon scaffolds with NIH 3T3 fibroblasts. Incorporation of viable cells was achievable with both techniques, represented by DNA content. Proliferation rate and viability assay showed neither damage by centrifugal force nor superiority of the technique. Cell proliferation after 10 days of culture demonstrated that the scaffold did not hinder 3-D culturing. Confocal laser microscopy revealed structural details as formation of focal adhesions, to provide deeper insight into the process of cell attachment and growth in xenografts.
Keywords: Tendon scaffold, Cell seeding efficiency, Tissue engineering, Confocal laser microscopy, Cell adhesion
1. Introduction
Thirty-two million tendon and ligament injuries each year are placing an enormous burden on the US health care system.1 The goals of treatment in these cases are to restore normal joint motion and function, reduce pain, and prevent further degeneration. However, this treatment is still addressed with limited options. Primary repair may result in poor functional outcome, autografts may cause severe donor site morbidity, and allografts may elicit inflammatory responses caused by remnant cell content.2 Therefore, the emerging field of tissue engineering has sought to find a solution for ligament and tendon replacement. Recent authors have defined the ideal replacement scaffold as biodegradable and porous, which supports cell ingrowth and remodeling of the material with formation of ligamentous/tendinous tissue, resulting in a construct of sufficient strength to withstand the demanding biomechanical strain after implantation.3
Tendon xenografts offer a promising alternative for tendon and ligament reconstruction. These constructs combine the advantage of ubiquitously available graft material with reduced immunogenicity due to decellularization.4 Optimizing these decellularized constructs entails preservation of the construct's signal molecules as well as incorporation of external cells that allow graft remodeling and promote intrinsic tendon healing.5 Pipetting a highly concentrated cell suspension onto a porous graft is adduced as the standard seeding technique in our study and it will be referred to as Static Pipetting. However, benefits of cell seeding supported by an external force, like centrifugation, have also been shown.6, 7, 8
The seeding of tendon scaffolds remains a crucial step in tissue engineering due to their specific architecture: low porosity hinders deep cell infiltration whereas high porosity causes problems in the cell attachment process. It is a commonly observed issue that the cells form flat sheets on the scaffold's surface during the seeding process which may cause an obstruction of the pores.9,10
In order to assess a quantification of the seeding results and efficiency biochemical assays, such as cell viability and cell content, are a commonly used tool.10 The analysis of the efficiency of the seeding process is mostly represented by an absolute number of cells3,11 or DNA content.9 In contrast, the comparison to the native tissue to be replaced is not common and the relation of the observed values lacks an important dimension. Instead, the result of the repopulation of scaffolds is compared to the initially used cell amount for the seeding process.7
Cristino et al. proposed that understanding the early phase of cell seeding in scaffolds is essential to success in developing new devices to enhance tendon/ligament healing.12 Currently, the examination of cell-biomaterial-interactions, as well as cell growth and differentiation remain a challenge for tissue engineering research. Confocal laser microscopy in combination with immunofluorescent staining is a technique that can directly assess ultrastructural details on the cellular level. This technique has proven its potential in examining cell shape and behavior as well as interaction with synthetic scaffold material.13,14
The purpose of our study was to evaluate the efficiency of cell seeding and increase knowledge of colonization mechanism of cells into decellularized natural tendon xenografts that have the potential to be used for reconstruction. We hypothesize that natural tendon xenografts can be successfully seeded with both static and centrifugal cell seeding techniques, facilitating in vitro 3-D culture to achieve a potent tendon and ligament xenograft comparable to native tendon tissue.
2. Materials & methods
2.1. Xenograft preparation
Fresh chicken feet from 56-day-old Leghorn chickens were provided at no cost by Wayne Farms (Dobson, NC). The long digit flexor digitorum profundus (FDP) tendon was harvested (Fig. 1: Steps of scaffold preparation) and immediately decellularized using an established protocol previously described.4 This procedure achieves a reduction of the cellular material by exposing the tendons to peracetic acid, digesting by trypsin and using the osmotic power of de-ionized water to burst the remnant cells. After lyophilization, the decellularized xenografts were stored at −80 °C. Prior to the seeding experiments, the xenografts were thawed individually, the dimensions were measured and cut into uniform specimens (30 mm × 3 mm x 1 mm) discarding non optimal end parts. Care was taken to maintain sterile conditions throughout the thawing and preparation of the tendons.
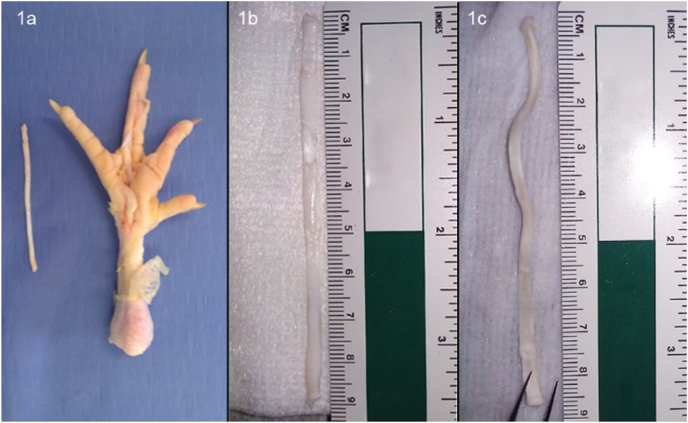
Fig. 1.
Steps of scaffold preparation. 1a: Chicken foot with excised FDP tendon. 1b: Isolated FDP tendon prior to decellularization. 1c: Freeze-dried tendon scaffold.
2.2. Nih 3t3 fibroblast culture
This cell type was chosen due to its widely accepted use in tissue engineering literature.15,16 The cells were purchased from Cell Biolabs Inc. (San Diego, CA), as a stable GFP expressing cell line. The fluorescent feature allowed a histological differentiation between cell remnants of the original chicken tissue and seeded, viable cells.17,18 The cells were cultured at 37 °C and 5% CO2 in vented cap T75 cell culture flasks (Corning, Corning, NY), containing 15 ml of culture medium (Dulbecco's modified Eagle Medium + 10% Newborn Calf Serum + 1% Penicillin/Streptomycin +1% L-Glutamin). The media were changed every three days. Every passage was harvested at approx. 80% confluence via trypsin detachment and subcultured. Only cells of passage two to nine were used for seeding.
2.3. Seeding techniques
2.3.1. Static pipetting
Depending on the literature, the incubation time and/or cell density of a typical static pipetting procedure may vary.19 In our case, 1 × 106 cells in 1 ml medium were pipetted onto the exposed surface of the scaffold in a six well plate. After 30 min of incubation time, the sample was flipped, and the opposite surface was seeded with the same technique and cell number.
2.3.2. Centrifugal seeding
The principles of centrifugal seeding have been documented previously by Godbey and Roh.6,7 We modified Roh's centrifugal seeding method according to our needs in tendon tissue engineering: 2 ml microtubes were filled with the 2 ml (2 × 106 cells) cell suspension and the graft was submerged. One group of centrifugal seeding underwent 10 min of centrifugation en bloc (1 × 10 min). In the other group, the cell pellet which formed on the bottom of the tube, was resuspended every 2 min (5 × 2 minutes). After completing the centrifugation in an Eppendorf 5427 R (Eppendorf, Hamburg, Germany) at 2500 rpm, all samples were transferred including the cell suspension into a six well-plate for incubation and culture.
2.4. Biochemical assays
2.4.1. DNA/proliferation assay
After 24 h and 10 days of culturing, the DNA content was quantified with spectrophotometric analysis. In order to reduce error - due to loosely attached cell debris - the samples were rinsed prior to testing three times with sterile DPBS (Gibco, Carlsbad, CA). The specimen was cut into pieces of approx. 3 mm in length and end pieces were discarded. This step eliminates spuriously high readings from cells attached to the transected scaffold ends.20 Representative 3 mm pieces (1 center, 1 towards each end) were minced, placed in a sterile microtube and weighed prior to DNA testing.
The DNA microtubes were incubated with proteinase for 6 h in a heat-block (Benchmark Digital Dry Bath BSH 1001) at 56 °C in order to achieve proteolytic tissue digestion. The DNA content was extracted using a DNeasy kit (DNeasyTM, Qiagen, Valencia, CA). The absorbance at 260 nm wavelength was measured with a Nanodrop spectrophotometer (Nanodrop 1000, Thermo Fisher Scientific, Wilmington, DE) and the obtained DNA concentrations were normalized to the initial weight of the sample. In addition, the cell content of all samples was calculated by comparison to a standard curve of ascending concentrations of NIH 3T3 fibroblasts.
2.4.2. MTS/viability assay
Six 3 mm pieces of each graft were used for the MTS viability assay.10,20 Under aseptic conditions, each piece was measured, weighed, and then immediately placed in a 96-well plate containing 200 μl sterile PBS per well. The 96-well plate was heated during the preparations to 37 °C to facilitate optimal conditions for the seeded graft pieces. After completing the measurements, 40 μl of MTS reagent (CellTiter 96 AQueous One Solution, Promega, Madison, WI) was added to each well. PBS served as a negative control. Due to no possible tissue lysis we assumed that the reagent will dominantly interact with cells on the scaffold surface. Therefore, all measurements were normalized to the surface area of the graft piece (area of an elliptical cylinder).10
2.5. Histology
Graft samples undergoing histology were harvested and rinsed as mentioned above. The samples were placed in sterile 15 ml conical tubes (BD Biosciences, Bedford, MA) containing 5 ml of paraformaldehyde solution (4%. Alfa Aesar, Ward Hill, MA). All tissue processing steps were protected from light to maintain a strong GFP signal. After 4 h tissue fixation the samples were transferred to fresh conical tubes with 5 ml of 5% Sucrose (Sigma-Aldrich, St. Louis, MO) for 6 h and subsequently, for 24 h in 20% Sucrose at 4 °C.21 The processed grafts were cut into six pieces of 4 mm length. In order to achieve a representative sample, all pieces of one graft were embedded vertically in one 15 × 15 × 5mm cryomold (Thermo Fisher Scientific, Wilmington, DE) with Tissue-Tek O.C.T. freezing medium (Tissue-Tek, Torrance, CA). The samples were flash frozen in pre-chilled isopentane (Thermo Fisher Scientific, Wilmington, DE) and stored at −80 °C. 10 μm sections were obtained and placed on Leica Surgipath X-tra Adhesive Clipped Corner Slides and air dried protected from light for 12 h. The slides were stained either with H&E or according to the instructions of the Millipore Focal Adhesion Kit 100 (EMD Millipore, Merck KGaA, Darmstadt, Germany) with Alexa Fluor 647 (Invitrogen, Carlsbad, CA) as secondary antibody in a 1:300 dilution. Actin microfilaments were stained with immunofluorescent antibodies (Phalloidin-TRITC, Millipore, 1:100) as well as vinculin in the focal adhesion complex (Anti-Vinculin, Millipore, 1:300). All histological slides were mounted with DAPI containing mounting medium (ProLong Antifade Gold with DAPI, Invitrogen, Carlsbad, CA). The images were taken with a Zeiss LSM510 confocal laser microscope and an Olympus VS110 fluorescent microscope.
2.6. Statistical analysis
The obtained results were compared to native tendon tissue and unseeded control constructs. One-way-ANOVA with Bonferroni post-hoc tests and Student's t-tests were used for statistical analysis with p < 0.05.
3. Results
3.1. DNA/proliferation assay
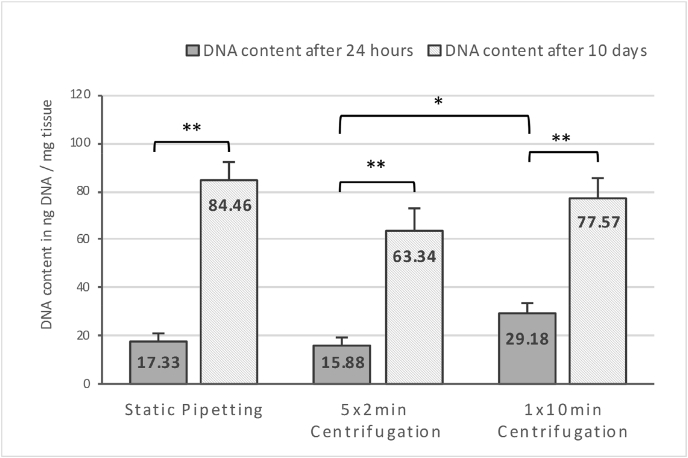
Successful decellularization represented by a mean DNA reduction of 92% was observed (409.87 ± 31.13 ng DNA/mg tissue native tendon vs. 34.21 ± 3.74 ng DNA/mg tissue decellularized construct; n = 8 vs. n = 7; p < 0.05). Compared to an unseeded construct, all groups showed a significant amount of DNA after 24 h of incubation (n = 10; p < 0.05; values in Fig. 2 - DNA content of xenografts after 24 h and 10 days of culturing already zeroed to unseeded constructs).
Fig. 2.
DNA content of xenografts after 24 h (n=10) and 10 days of culturing (n = 16). Values already zeroed to unseeded constructs; Measured in ng DNA/mg tissue. The asterisk marks the significant difference between 1 × 10min and 5 × 2min centrifugation after 24 h of culturing (p < 0.05). Two asterisks mark the significant increase of DNA content after 10 days of culturing in all groups (p < 0.05).
Centrifugal seeding showed no significant difference to static pipetting. Though, resuspending the cell pellet in the microcentrifuge tube every 2 min significantly decreased the DNA concentration at 24 h compared to 10 min of centrifugation without resuspension (15.88 ± 2.94 vs. 29.18 ± 3.92 ng DNA/mg tissue; n = 10; p < 0.05).
The 24-h efficiency calculation was based on the 2 × 106 fibroblasts used for the initial seeding. This cell content calculation at 24 h post seeding yielded efficiency rates of 7.97% (770 cells/mg tissue) for static pipetting, 6.58% (710 cells/mg tissue) for 5 × 2 minutes centrifuging, and 13.31% (1300 cells/mg tissue) for 1 × 10 min centrifuging.
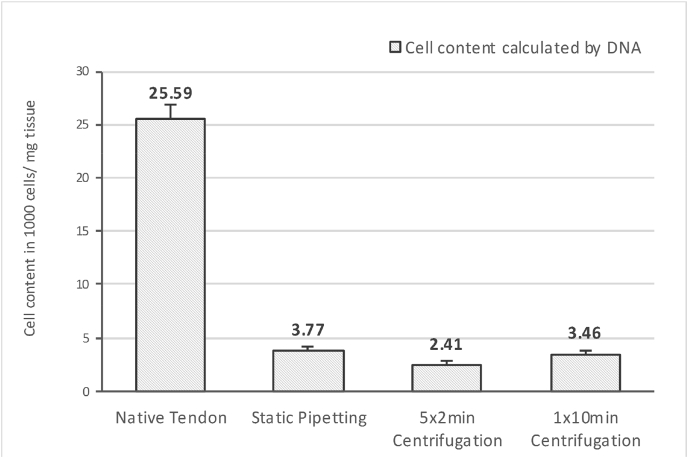
After 10 days of 3-D-culture, all groups of seeded xenografts showed a highly significant increase in DNA (n = 16; p < 0.05) (Fig. 2). During the second evaluation of the seeding success after 10 days of 3-D-culture, the obtained DNA measurements were compared to the cell content of native tendon tissue (1.83 ± 0.14 × 104 cells/mg tissue), as it represents the benchmark for which tissue engineering should aim. The final outcome of seeding and culturing resulted in a cell content of 14.72% of native tendon tissue with static pipetting, 9.42% with 5 × 2 minutes centrifugation, and 13.52% with 1 × 10 min centrifugation (Fig. 3 – Final outcome of seeding and culturing calculated by DNA content after 10 days of 3-D-culture).
Fig. 3.
Final outcome of seeding and culturing. Displayed values show content of cells in 10³ cells/mg tissue. Calculated by DNA content of xenografts after 10 days of 3-D-culture compared to a standard curve of NIH 3T3 fibroblasts in known concentrations; corrected by the inter-species-comparison-factor of 1.4 (Mus musculus vs. Gallus gallus) and compared to the cell content of native avian FDP tendons.
In this calculation we used a corrective factor for an accurate comparison between the species of mouse (NIH 3T3 fibroblast) and chicken (native tendon) [corrective factor = 1.4; because of genome size Mus musculus (2.8 MBp)/Gallus gallus (2.0 MBp)]. Despite no significant inter-group differences (p > 0.05), static pipetting demonstrated the greatest increase from 24 h to 10 days of incubation (Table 1 – Increase of DNA content from 24 h to 10 days of culturing).
Table 1.
Increase of DNA content from 24 h to 10 days of culturing. (Comparison of the different seeding methods, mean values of the measurements of DNA content after 24 h and 10 days of 3-D-cell culture).
| Seeding Method | Increase of DNA content from 24 h–10d |
|---|---|
| Static Pipetting | 4.87-fold |
| 5 × 2min Centrifugation | 3.99-fold |
| 1 × 10min Centrifugation | 2.66-fold |
3.2. MTS/viability assay
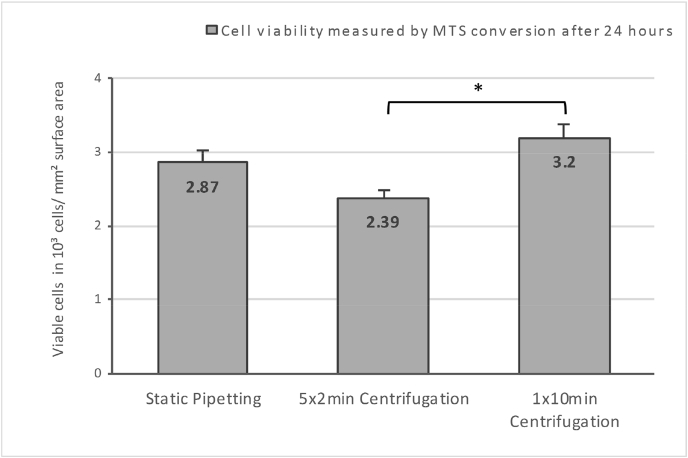
The MTS assay 24 h after seeding confirmed that the DNA results were based on viable cells. Centrifugation did not decrease the number of viable cells compared to static pipetting. However, the 5 × 2 minute group yielded inferior results when compared to 1 × 10 min group (measured in viable cells per mm2 surface area; n = 4; p < 0.05; Fig. 4 - Cell viability 24 h after seeding).
Fig. 4.
Cell viability 24 h after seeding. Measured by MTS conversion and displayed in viable cells per mm2 surface area; n = 4; p < 0.05. The asterisk marks the significant difference between 1 × 10min and 5 × 2min centrifugation.
3.3. Histology
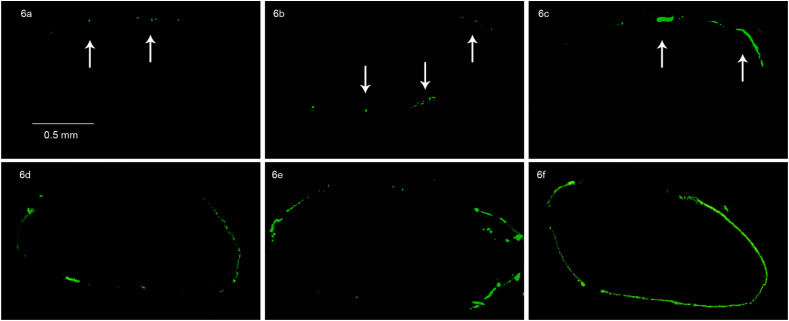
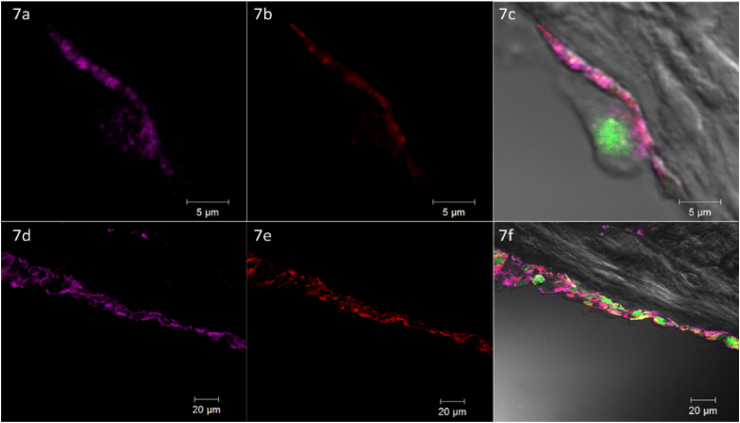
Histology with H&E and DAPI verified successful decellularization through lack of organized nuclei signals (Fig. 5 - Histological comparison of native tendon tissue with a decellularized scaffold) and was consistent with the significant reduction of DNA content observed above. The seeding of the decellularized constructs resulted in a mono- or bilayer of cells (DAPI and GFP positive) on the outer surface of the tendon grafts. This was associated with only sparse evidence of scaffold infiltration by seeding or cell migration after culturing (Fig. 6 - Transversal sections of avian FDP xenografts: GFP signal of seeded cells). Immunofluorescent staining of representative sections of all groups were positive for actin and vinculin, indicative of focal adhesion to the scaffold after 24 h of incubation and 10 days of proliferation (Fig. 7 - Confocal laser microscopy: Xenograft seeded by Static Pipetting after 10 days of culturing).
Fig. 5.
Histological comparison of native tendon tissue with a decellularized scaffold. 5a: Native tendon tissue overview, DAPI staining, 2x magnification, 5b: Decellularized scaffold overview, DAPI staining, 2x magnification, 5c: Decellularized scaffold details, H&E staining, 20x magnification.
Fig. 6.
Transversal sections of avian FDP xenografts: GFP signal of seeded cells. a: Static Pipetting, 24 h incubation. b: Static Pipetting, 10 days of culturing. c: 5 × 2min Centrifugal Seeding, 24 h of incubation. d: 5 × 2min Centrifugal Seeding, 10 days of culturing. e: 1 × 10min Centrifugal Seeding, 24 h of incubation. f: 1 × 10min Centrifugal Seeding, 10 days of culturing.
Fig. 7.
Confocal laser microscopy: Xenograft seeded by Static Pipetting after 10 days of culturing. Legend: Vinculin stain = pink, Actin stain = red, GFP = green. a, b, c: Single Cell @ 63x magnification. d, e, f: Line of Cells on Graft Surface @ 20x magnification, Images of Vinculin (a&d), Actin (b&e) and Merge of DIC and Fluorescent Images (c&f). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
In tendon and ligament tissue engineering, a graft is composed of a biomechanically strong scaffold, preloaded with cells to be carried into the defect, and contains signal molecules to promote intrinsic healing.1,22 Our concept focuses on a naturally derived xenograft from an avian FDP tendon scaffold, which provides biomechanical strength, biocompatibility, and porous architecture.4 The goal of this study was to examine the seeding challenge and 3-D culture of these naturally derived xenografts. The DNA analysis after 24 h revealed that an incorporation of cells into the xenografts is achievable with standard seeding techniques. The viability assay showed that centrifugal force does not damage the cells, although there was no evidence that it offered improvement in seeding efficiency compared to static seeding. The trend after 24 h of incubation implicates that 10 min of centrifuging en bloc increases the initial load of cells compared to static pipetting, represented by a higher DNA content, which was, however, not significant. This trend supports Godbey's and Roh's findings about centrifugal seeding in PGA scaffolds6,7 and makes centrifugal seeding in naturally derived xenografts a possible alternative to static pipetting. But resuspension of the cell pellet during the centrifugation process should be avoided due to significantly lower initial cell load. Further investigation is needed to understand the underlying reason of these attachment issues such as whether the shear stress applied by the fluid flow during resuspension results in rinsing off the cells from the scaffold.
The additional DNA analysis after 10 days of 3-D culture showed significant cell proliferation in all groups. This demonstrated that the graft material did not hinder in vitro 3-D culturing despite the preceding aggressive chemical treatment. According to MTS testing, cells remain viable after centrifugation. Simultaneously the centrifugal seeding groups show lower amplification rates at 10 days culturing than the static pipetting group. This suggests that the centrifuged cells are alive but may be adversely affected, resulting in a lower amplification potential. Further long-term and in-vivo studies are necessary to examine the effects of seeding techniques on cell replication and proliferation in xenografts in detail.
Preliminary analysis of the supernatant after scaffold seeding in previous experiments suggested a high number of non-attached cells. Therefore, we aimed for a deeper insight into the seeding efficiency in this study. Twenty-four hours after the initial loading of the scaffolds with 2 × 106 NIH 3T3 fibroblasts 13.31% (in maximum, 1 × 10min centrifugation) of these cells were detectable in the graft material by DNA analysis. After 10 days of 3-D culturing the cell content of the xenografts was compared to native tendon tissue as the highest addressable standard resulting in 14.72% (in maximum) of the cell content. These two different comparisons at separate times during the seeding process show obvious need for improvement.
The avian FDP scaffold poses a seeding challenge due to its high extracellular matrix density and variable porosity. But it has the advantage of being derived from the same tissue to be replaced. Hypothetically, this results in a construct of physiologic tendon architecture and morphological patterns which will be recognized by exogenous cells and used for adhesion, to the extent that chemical decellularization would not affect them.
If we compare this theory with the measured efficiency, this study outlines a major issue: despite the quantitative augmentation of cell content reflected in biochemical assays, there must be limitations to the attachment process of cells during the seeding of naturally derived graft material. Studying these seeding issues lies beyond the focus of classic biochemical assays, so further tools are needed. In addition, the histological analysis reveals one major limitation of our study: the primary seeding success is confirmed by tracing the seeded GFP positive cells in sections of the grafts; seeding dense natural scaffolds results mainly in a mono- or bilayer of cells on the scaffold surface but does not achieve the goal of homogeneously distributing cells, equivalent to native tendon tissue. This is frequently observed in in-vitro studies.9,23 The efficiency rates and the sparse evidence of cell penetration and migration give rise to the question how initial attachment interaction works and which factors influence the forming of cell-scaffold adhesion.
In response to this question, our current study has proven that confocal laser microscopy is useful in representative sections of naturally derived xenografts after seeding and culturing. The analyzed cells have shown the formation of focal adhesions as an indicator of integral cell-scaffold interaction.
The limited scope of our study does not allow quantification of the focal adhesion nor a standardized comparison between the seeding techniques. But further studies may use confocal laser microscopy to examine the ultra-structure of both material and cell as well as the circumstances facilitating permanent cell adherence. These findings might be of special interest in tissue engineering because tendon grafts frequently undergo a procedure of further preconditioning like the application of cyclic strain in a bioreactor prior to implantation.15,16 This procedure crucially depends on adhesion. Otherwise the beneficial effect of the applied external force would not be transduced to the cells. Further investigation of scaffolds with confocal microscopy might reveal information about the morphological patterns of adhesion on a molecular level, providing valuable insight into the future design of scaffolds. Furthermore, confocal microscopy addresses the structural details based on proteins and might be matched to additional imaging sources such as Scanning Electron Microscopy, adding a possible morphological dimension.
Previous studies have pointed out that the understanding of cells in their microenvironment will have a huge impact on tissue engineering.12 Chemical, mechanical and structural properties including surface features and the 3-D structure of these cell niches affect cellular functioning. The knowledge about how cells interact with other cells and the scaffold's biomaterial during the process of seeding and in-vitro culture will be crucial for graft optimization.
In addition to the study of the mechanism of cell adhesion the common issue of sparse cell loading of porous graft material must be assessed. The superior goal is to achieve seeding results closer to the native tendon tissue. In general, there are several control variables which may be modified during the seeding process: the pore size of the scaffold, the cell density of the seeding fluid, and the external force to support the scaffold infiltration.
The next step considering the presented naturally derived tendon xenograft would be the determination of the optimal relation between cell density and pore size for our naturally derived tendon graft material:
The crucial factor is that a larger pore size of the scaffold may increase the infiltration of cells on one side but may decrease the scaffold's biomechanical properties on the other side. The argument of compromising the biomechanical stability should be considered carefully as the scaffolds presented properties close to native tendon tissue.4 This is an important fact considering that the constructs are facing the high demands of biomechanics in tendon and ligament reconstruction. Similarly, to maintain the scaffold's stability, we refrained from the use of more invasive techniques for cell seeding, like scoring the construct's surface20 or the injection of a cell suspension with a syringe.
A high cell density is primarily linked to a higher cell loading of scaffolds.9,24 However, this relation does not have a positive outcome if it is exaggerated. Using extremely dense cell suspensions results in the clotting of cells, lowering the efficiency of the seeding and hindering scaffold infiltration by the occlusion of the external pores.9,24
Moreover, the actual literature brings forward arguments for the use of external force in order to further optimize the seeding process of porous graft materials. However, there is not an individual technique proving itself to be superior. The synopsis rather indicates that transforming the seeding to a more dynamical process by the additional use of an external force like rotation (in spinner flasks f.e.),9 centrifugation,6,7 orbital shaking9,25 or a bioreactor system using perfusion and or vacuum9 can be beneficial. In this study we showed that the use of centrifugation was not of superior impact on the results of seeding naturally derived tendon xenografts in comparison to static pipetting of a cell suspension. Further studies are needed to explore which kind of external force may contribute to more homogeneous cell distribution, deeper scaffold infiltration and higher cell loading of our scaffolds.
5. Conclusions
In conclusion, our study showed that avian FDP xenografts yield a specific morphology that allows seeding with standard techniques. The microenvironment of the scaffold facilitates significant cell proliferation in 3-D culturing. But despite this quantitative improvement of the cell content, the suboptimal efficiency and cell distribution points to the need to elucidate the cell seeding of porous scaffolds in its structural details. Immunofluorescent staining and confocal laser microscopy may reveal information about the seeding process on the level of cell-scaffold interaction, such as the formation of focal adhesions. This tool may provide insight into the process of cell attachment and growth in future seeding studies of natural decellularized scaffolds.
Efficient seeding techniques for natural decellularized tendon xenografts are vital for the development of an “off-the-shelf” alternative that meets the increasing demand for tendon and ligament reconstructions.
Conflict of interest – statement
(according to the ICMJE form).
The authors declare that neither them nor their institutions received at any time payment or services from a third party for any aspect of the submitted work.
The whole work was supplied by internal funding of the Department of Orthopaedic Surgery, Wake Forest School of Medicine.
In addition, there are no other relationships or activities that readers could perceive to have influenced, or that give the appearance of potentially influencing, what is written in the submitted work.
Disclosures
The presented study was financed by internal, departmental funding. The authors declare that there is no conflict of interest regarding the publication of this manuscript.
CRediT authorship contribution statement
Simon Thönnes: Conceptualization, Methodology, Formal analysis, Investigation, Data curation. Peter Shelton: Formal analysis, Investigation, Data curation. Daniel N. Bracey: Conceptualization, Methodology, Writing - review & editing. Mark Van Dyke: Conceptualization, Resources, Supervision. Patrick Whitlock: Conceptualization, Methodology, Resources. Thomas L. Smith: Resources, Validation, Supervision, Project administration, Funding acquisition. Arash Moghaddam: Writing - review & editing, Project administration, Supervision. Christopher Tuohy: Conceptualization, Resources, Project administration, Funding acquisition.
Acknowledgements
The authors thank Eileen Martin and Jiaozhong Cai for their technical assistance in conducting the experiments and Bailey Fearing, PhD, for her outstanding scientific support.
Contributor Information
Simon Thönnes, Email: simon.thoennes@ukdd.de.
Christopher Tuohy, Email: ctuohy@wakehealth.edu.
References
- 1.Butler D.L., Juncosa-Melvin N., Boivin G.P. Functional tissue engineering for tendon repair: a multidisciplinary strategy using mesenchymal stem cells, bioscaffolds, and mechanical stimulation. J Orthop Res. Jan 2008;26(1):1–9. doi: 10.1002/jor.20456. [DOI] [PubMed] [Google Scholar]
- 2.Poehling G.G., Curl W.W., Lee C.A. Analysis of outcomes of anterior cruciate ligament repair with 5-year follow-up: allograft versus autograft. Arthroscopy. Jul 2005;21(7):774–785. doi: 10.1016/j.arthro.2005.04.112. [DOI] [PubMed] [Google Scholar]
- 3.Subramony S.D., Su A., Yeager K., Lu H.H. Combined effects of chemical priming and mechanical stimulation on mesenchymal stem cell differentiation on nanofiber scaffolds. J Biomech. 6/27/2014;47(9):2189–2196. doi: 10.1016/j.jbiomech.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whitlock P.W., Smith T.L., Poehling G.G., Shilt J.S., Van Dyke M. A naturally derived, cytocompatible, and architecturally optimized scaffold for tendon and ligament regeneration. Biomaterials. Oct 2007;28(29):4321–4329. doi: 10.1016/j.biomaterials.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 5.James R., Kesturu G., Balian G., Chhabra A.B. Tendon: biology, biomechanics, repair, growth factors, and evolving treatment options. J Hand Surg. Jan 2008;33(1):102–112. doi: 10.1016/j.jhsa.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 6.Godbey W.T., Hindy S.B., Sherman M.E., Atala A. A novel use of centrifugal force for cell seeding into porous scaffolds. Biomaterials. Jun 2004;25(14):2799–2805. doi: 10.1016/j.biomaterials.2003.09.056. [DOI] [PubMed] [Google Scholar]
- 7.Roh J.D., Nelson G.N., Udelsman B.V. Centrifugal seeding increases seeding efficiency and cellular distribution of bone marrow stromal cells in porous biodegradable scaffolds. Tissue Eng. Nov 2007;13(11):2743–2749. doi: 10.1089/ten.2007.0171. [DOI] [PubMed] [Google Scholar]
- 8.Way L., Scutt N., Scutt A. Cytocentrifugation: a convenient and efficient method for seeding tendon-derived cells into monolayer cultures or 3-D tissue engineering scaffolds. Cytotechnology. Dec 2011;63(6):567–579. doi: 10.1007/s10616-011-9391-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griffon D.J., Abulencia J.P., Ragetly G.R., Fredericks L.P., Chaieb S. A comparative study of seeding techniques and three-dimensional matrices for mesenchymal cell attachment. J Tissue Eng Regen Med. Mar 2011;5(3):169–179. doi: 10.1002/term.302. [DOI] [PubMed] [Google Scholar]
- 10.Raghavan S.S., Woon C.Y., Kraus A., Megerle K., Pham H., Chang J. Optimization of human tendon tissue engineering: synergistic effects of growth factors for use in tendon scaffold repopulation. Plast Reconstr Surg. Feb 2012;129(2):479–489. doi: 10.1097/PRS.0b013e31823aeb94. [DOI] [PubMed] [Google Scholar]
- 11.Liu H., Fan H., Wang Y., Toh S.L., Goh J.C. The interaction between a combined knitted silk scaffold and microporous silk sponge with human mesenchymal stem cells for ligament tissue engineering. Biomaterials. Feb 2008;29(6):662–674. doi: 10.1016/j.biomaterials.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 12.Cristino S., Grassi F., Toneguzzi S. Analysis of mesenchymal stem cells grown on a three-dimensional HYAFF 11-based prototype ligament scaffold. J Biomed Mater Res A. Jun 1 2005;73(3):275–283. doi: 10.1002/jbm.a.30261. [DOI] [PubMed] [Google Scholar]
- 13.Ryoo S.R., Kim Y.K., Kim M.H., Min D.H. Behaviors of NIH-3T3 fibroblasts on graphene/carbon nanotubes: proliferation, focal adhesion, and gene transfection studies. ACS nano. Nov 23 2010;4(11):6587–6598. doi: 10.1021/nn1018279. [DOI] [PubMed] [Google Scholar]
- 14.Farooque T.M., Camp C.H., Jr., Tison C.K., Kumar G., Parekh S.H., Simon C.G., Jr. Measuring stem cell dimensionality in tissue scaffolds. Biomaterials. 3/2014;35(9):2558–2567. doi: 10.1016/j.biomaterials.2013.12.092. [DOI] [PubMed] [Google Scholar]
- 15.Henshaw D.R., Attia E., Bhargava M., Hannafin J.A. Canine ACL fibroblast integrin expression and cell alignment in response to cyclic tensile strain in three-dimensional collagen gels. J Orthop Res. Mar 2006;24(3):481–490. doi: 10.1002/jor.20050. [DOI] [PubMed] [Google Scholar]
- 16.Webb K., Hitchcock R.W., Smeal R.M., Li W., Gray S.D., Tresco P.A. Cyclic strain increases fibroblast proliferation, matrix accumulation, and elastic modulus of fibroblast-seeded polyurethane constructs. J Biomech. 2006;39(6):1136–1144. doi: 10.1016/j.jbiomech.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 17.Ni M., Lui P.P., Rui Y.F. Tendon-derived stem cells (TDSCs) promote tendon repair in a rat patellar tendon window defect model. J Orthop Res. Apr 2012;30(4):613–619. doi: 10.1002/jor.21559. [DOI] [PubMed] [Google Scholar]
- 18.West A.R., Zaman N., Cole D.J. Development and characterization of a 3D multicell microtissue culture model of airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. Jan 1 2013;304(1):L4–L16. doi: 10.1152/ajplung.00168.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lomas A.J., Webb W.R., Han J. Poly (3-hydroxybutyrate-co-3-hydroxyhexanoate)/collagen hybrid scaffolds for tissue engineering applications. Tissue Eng C Methods. Aug 2013;19(8):577–585. doi: 10.1089/ten.tec.2012.0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woon C.Y., Farnebo S., Schmitt T. Human flexor tendon tissue engineering: revitalization of biostatic allograft scaffolds. Tissue Eng A. July 24, 2012;18(23-24) doi: 10.1089/ten.TEA.2012.0152. [DOI] [PubMed] [Google Scholar]
- 21.Liadaki K., Luth E.S., Kunkell L.M. Co-detection of GFP and dystrophin in skeletal muscle tissue sections. BioTechniques. Jun 2007;42(6):699–700. doi: 10.2144/000112494. [DOI] [PubMed] [Google Scholar]
- 22.Longo U.G., Lamberti A., Petrillo S., Maffulli N., Denaro V. Scaffolds in tendon tissue engineering. Stem Cell Int. 2012;2012:517165. doi: 10.1155/2012/517165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kryger G.S., Chong A.K., Costa M., Pham H., Bates S.J., Chang J. A comparison of tenocytes and mesenchymal stem cells for use in flexor tendon tissue engineering. J Hand Surg. May-Jun 2007;32(5):597–605. doi: 10.1016/j.jhsa.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 24.Dar A., Shachar M., Leor J., Cohen S. Optimization of cardiac cell seeding and distribution in 3D porous alginate scaffolds. Biotechnol Bioeng. 2002;80(3):305–312. doi: 10.1002/bit.10372. Nov 5. [DOI] [PubMed] [Google Scholar]
- 25.Fortier G.M., Gauvin R., Proulx M., Vallee M., Fradette J. Dynamic culture induces a cell type-dependent response impacting on the thickness of engineered connective tissues. J Tissue Eng Regen Med. Apr 2013;7(4):292–301. doi: 10.1002/term.522. [DOI] [PubMed] [Google Scholar]