Abstract
Background
Retinopathy of prematurity (ROP) is a preventable cause of childhood blindness. Without treatment, over 45% of eyes can develop permanent visual loss. Hyperglycaemia has recently been described as a risk factor for the development of retinopathy of prematurity (ROP), a proliferative vascular disease of the retina that primarily affects premature infants. The characteristic neoproliferative growth of blood vessels in the retina is very well under stood with the clinical and experimental experiences with Diabetic retinopathy. The purpose of this study was to evaluate a possible relation between glucose levels in VLBW (Very Low Birth Weight) infants and development of ROP.
Method
All at risk infants of a Neonatal Intensive Care Unit (NICU) of a tertiary care centre in western India were included in the study. The blood sugar values of the neonates were recorded at multiple times during their first week of life. On completion of 31 weeks of gestational age or 04 weeks of birth age, the neonates were subjected to ROP screening as per standard protocols.
Result
A total of 103 neonates were included in the study and were subjected to ROP screening. A total of 32 neonates developed ROP at the end of the study. It was found with statistical significance that the neonates with higher average blood glucose values in the initial period of life had higher incidence of ROP at the time of screening with a Relative Risk of 2.506 (CI = 1.287, 4.882).
Conclusion
A high average blood glucose level in neonates during the first week of life is an indicator for developing ROP at a later date. These neonates should be kept under close follow up in order to facilitate timely detection and prompt intervention.
Keywords: Retinopathy of prematurity, Hyperglycemia, Neonatology, Proliferative retinopathy, Glycaemic status
Introduction
Retinopathy of prematurity (ROP) is a sight-threatening neonatal vasoproliferative condition of the retina during its development, seen in preterm infants. Gilbert et al., in 2008, conducted a study which revealed an estimate that more than 50,000 children are blinded from this condition all across the globe. Other studies suggest that the figure is underestimated.1 There are nearly 15 million preterm births annually, and India tops this list with more than 3.5 million annually.2, 3 ROP is currently a major emerging public health problem affecting the neonatal population. The non-uniform standards of hospital neonatal care, complete absence or delayed screening for ROP and lack of suitably trained ophthalmologists to screen and treat ROP are major factors contributing to increasing blindness and disease burden from ROP along with other morbidities.
Oxygen has been long implicated as the major risk factor for the onset of ROP; however, the search for other risk factors has been ongoing to better understand the pathogenesis of the problem.4, 5 The contribution of hyperglycaemia in the onset of ROP has been studied in lines with other vasoproliferative retinopathies with the existing background knowledge of the pathogenesis of diabetic retinopathy.6, 7, 8
Hyperglycaemia in neonates
The incident population of extremely low-birthweight (ELBW) infants in hospital care with birthweight less than 1000 g is on an increase in neonatal intensive care units as a result of better clinical care and survival.9, 10 Many of these neonates, as a result of glucose intolerance, present with hyperglycaemia, which can be as high as 68% of ELBW neonates within the first few weeks of life. According to the findings of Louik et al.,5 neonates with birthweight less than 1100 g are 18 times more likely to have hyperglycaemia. Conditions such as intravenous fluids, parenteral nutrition, stress and drug treatment, in particular steroids, have been implicated in the development of hyperglycaemia.
Retinopathy of prematurity
Retinopathy of prematurity (ROP) is a neonatal disorder of development of the retinal vascular maturation. Similar to other proliferative retinal disorders, local ischaemia is a major factor in the development of ROP, as seen in diabetic retinopathy, sickle cell retinopathy and so on. The incidence of ROP has an inverse relation with decreasing birthweight and decreasing gestational age.4, 11, 12 With the evident pattern of vasoproliferative retinopathy, ROP results in disorganised growth and lack of maturation of retinal blood vessels, leading to scarring and retinal detachment. Oxygen has been established as a major role player in these cases and is known to cause vascular obliteration of the immature retina.
Proposed mechanism of hyperglycaemia contributing to ROP
Hyperglycaemia has recently been described as an important risk factor for the development of ROP.13 The development and progression of ROP has two phases, namely, a hypoxic preclinical phase and a proliferative clinical phase.14 These nearly overlapping phases arise from the alterations in serum insulin-like growth factor (IGF-1), which is a somatic growth factor, and retinal vascular endothelial growth factor (VEGF), which is a hypoxia-induced vasoproliferative factor.4 The levels of serum IGF-1 fall drastically in cases of premature birth because of premature cessation of supply from the mother in the wake of poor endogenous production.15, 16, 17 Hence, the poor IGF-1 levels hinder the development of retinal vessels, leading to localised ischaemia and a resultant spike in VEGF production which in turn causes the proliferative changes.18, 19 The correlation between IGF-1 and birthweight to the development of ROP has been demonstrated clinically in various studies. The characteristic extensive neoproliferative growth of blood vessels in the retina is very well understood with the clinical and experimental experiences with diabetic retinopathy. The important contribution of hyperglycaemia in the development of proliferative diabetic retinopathy has been studied in adults in great detail.16, 20 Garg et al,6 in 2003, first described the relationship between hyperglycaemia and ROP. Few studies have established significant association between the two conditions.6, 21, 22, 23
In view of the larger number of premature births in India, such a study has become essential. The use of blood sugar levels as an indicator for predicting the onset of ROP will help us plan a prompt screening and management protocol.
ROP diagnosis
The diagnosis of ROP is clinically based on the indirect ophthalmoscopy findings in the neonate. In 1984, the International Committee for Classification of Retinopathy of Prematurity developed an objective diagnostic classification24 for ROP, and the same has since then been further refined.25 It is defined by the parameters of location, stage and extent.
Aim and objective
Aim
The aim of the study is to establish hyperglycaemia as a risk factor for the development of ROP by means of a cohort study.
Materials and methods
Study type: cohort study
The study was a prospective study conducted at two tertiary care hospitals under the care of the same treating physicians. All the neonates who fall in the ‘at-risk’ group admitted to the neonatal intensive care units were part of the cohort. The target population was the clientele population of two hospitals located in Pune city of Maharashtra state in India.
Sample size calculation
Based on the odds ratio (OR) defined in the previous studies, a sample size of 47 cases and 47 controls was reached before the start of the study period.26, 27
Screening of ROP: standard practice
The study centre uses a standardised protocol for ROP screening similar to the All India Institute of Medical Sciences, New Delhi, ROP screening protocol for ‘at-risk infants’.
Defining ‘at-risk neonates’
‘At-risk’ neonates are defined as follows:28
-
•
Neonates with birthweight <1500 g
-
•
Neonates born at ≤32 weeks of gestation
-
•
Selected preterm infants with a birthweight between 1500 and 2000 g or a gestational age of more than 32 weeks with sickness needing cardiorespiratory support, prolonged oxygen therapy and apnoea of prematurity, anaemia needing blood transfusion and neonatal sepsis or believed to be at high risk by their attending paediatrician or neonatologist.
First screening examination was carried out at 31 weeks of gestation or 4 weeks of age, whichever was later.25, 28 The thumb rule followed was as follows: first screening at 1 month of postnatal age in babies born at >26 weeks of gestation age and at 31 weeks of age for others.28
The neonates were fasting for 2 h with instillation of 0.5% tropicamide with 2.5% phenylephrine eye drops according to the AIIMS ROP screening protocol.28 A 28D and 20D condensing lens were used for screening.
Hyperglycaemia
As per the routine protocol of the Neonatal Intensive Care Unit (NICU), the whole blood glucose values were routinely recorded for all the neonates for the first 7 days of life by the finger prick method.
Other risk factors
Other relevant clinical details such as birthweight, period of gestation, preterm complications, intrapartum complications, haemoglobin levels and weight at screening were also noted.
Data collection
The data collection was conducted based on the International Classification of Retinopathy of Prematurity (ICROP)25 diagrams and associated clinical questionnaire.
Outcome measures
The outcome measure of this study was the onset of ROP or complete maturation of the retina, whichever was earlier.
Statistical analysis
The statistical analysis was conducted using IBM SPSS Statistics version 25.0 (2017) and an online tool provided by Dean AG, Sullivan KM and Soe MM (OpenEpi: Open Source Epidemiologic Statistics for Public Health, Version. www.OpenEpi.com, updated 2013/04/06, accessed 2017/11/02).
Results
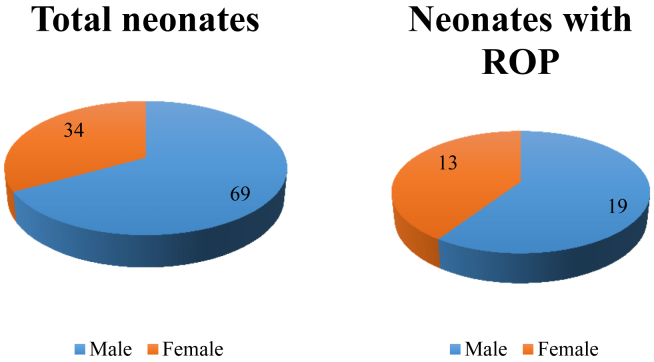
In the given study period, we conducted ROP screening for a total of 103 neonates in the neonatal care units of the two hospitals. The total number of female children was 34 and male children was 69. Among 103 neonates, 32 were found to have ROP at different stages (Fig. 1).
Fig. 1.
Frequency of sex among the study sample. ROP, retinopathy of prematurity.
There was a larger proportion of males among the study sample. The number of neonates with ROP was higher in male sex. However, when adjusted for the sex in the study population, it was found that a higher percentage of female neonates were detected with ROP than their male counterparts (Fig. 1).
Baseline parameters
The baseline parameters of the neonates included in this study are given in Table 1. The mean gestational age at birth was 30.282 weeks (±2.0188) and at the time of screening was 34.846 weeks (±2.0782). The mean duration between the birth and screening was 4 weeks. The neonates had a mean birthweight of 1251 gms (±313.1432), with the maximum being 2028.0 gms and minimum being 530.0 gms. The weight of the neonates during the first screening ranged from 650 gms to 3800 gms, with a mean of 1639.515 gms (±431.5650).
Table 1.
Baseline parameters of the screened population.
| n = 103 | Minimum | Maximum | Mean | Standard deviation |
|---|---|---|---|---|
| Birth age (weeks) | 26.0 | 35.0 | 30.282 | 2.0188 |
| Screened age (weeks) | 31.0 | 43.0 | 34.846 | 2.0782 |
| Birthweight (gms) | 530.0 | 2028.0 | 1251.621 | 313.1432 |
| Screening weight (gms) | 650.0 | 3800.0 | 1639.515 | 431.5650 |
| Oxygen (hrs) | 0.0 | 1344.0 | 62.379 | 142.6051 |
| Weight gain (gms/week) | −63.00 | 460.00 | 90.43 | 64.50 |
| Gmax (mg/dL) | 117.0 | 270.0 | 169.689 | 18.7674 |
| Gmin (mg/dL) | 23.0 | 128.0 | 50.631 | 20.6929 |
| Gavg (mg/dL) | 76.0 | 178.8 | 108.947 | 16.5258 |
Glycaemic state
The minimum, maximum and mean glucose levels of the neonate among the readings recorded in the initial 7 days of life were tabulated separately (Table 1). The mean minimum glucose level of the cohort was 50.631 mg/dL (±20.69), with the lowest level being 23 mg/dL and highest level being 128 mg/dL. The same forms of parameters were drawn for the maximum glucose readings recorded for each child. The mean of Gmax was 169.689 mg/dL (±18.7674), with values ranging from 117 to 270 mg/dL. The mean glucose level of each neonate ranged from 76 mg/dL to 178 mg/dL, with a mean glucose value of 108.947 mg/dL (±16.5258).
Cohort analysis: glycaemic state
The neonates in the study sample were divided into groups according to the average glucose level in their first 7 days of life (Gavg). The group with higher Gavg (mean = 121.66 ± 12.71431 mg/dL) had a higher incidence of ROP (23 of 29 neonates) against the cohort with lower Gavg (mean = 96.475 ± 8.211515 mg/dL), which had only 9 cases of ROP. This difference was found to be statistically significant (chi-square test, p = 0.001779, Table 4).
Table 4.
Chi-square and exact measures of association of ROP with Gmin, Gmax and Gavg.
| Chi-square and exact measures of association | ||||
|---|---|---|---|---|
| Risk factor | Test | Value | p value (1-tailed) | p value (2-tailed) |
| High minimum glucose values | Uncorrected chi-square | 0.8425 | 0.1793 | 0.3587 |
| Mantel–Haenszel chi-square | 0.8343 | 0.1805 | 0.3610 | |
| High maximum glucose values | Uncorrected chi-square | 0.8425 | 0.1793 | 0.3587 |
| Mantel–Haenszel chi-square | 0.8343 | 0.1805 | 0.3610 | |
| High average glucose values | Uncorrected chi-square | 8.496 | 0.001779 | 0.003559 |
| Mantel–Haenszel chi-square | 8.414 | 0.001862 | 0.003724 | |
ROP, retinopathy of prematurity.
The neonates were also divided into two groups according to the minimum glucose (Gmin) value recorded in the first week of life (Table 2). The average value of minimum glucose in the two groups was 65.37 (±20.37249) mg/dL and 36.17 (±4.36) mg/dL. In the first cohort with the higher value, a total of 18 neonates were detected to have ROP against the 14 in the cohort with lower minimum glucose values (chi-square test, p = 0.1793, Table 4).
Table 2.
2 × 2 Table: Gmin versus incidence of ROP.
| Comparison group | Neonates with ROP | Neonates without ROP | Total | Mean minimum glucose (mg/dL) | SD |
|---|---|---|---|---|---|
| Neonates with higher minimum glucose levels (Gmin) | 18 | 33 | 51 | 65.37 | 20.37249 |
| Neonates with lower minimum glucose levels (Gmin) | 14 | 38 | 52 | 36.17 | 4.364389 |
| Total | 32 | 71 | 103 |
ROP, retinopathy of prematurity; SD, standard deviation.
Similarly, when the cohort was grouped using the Gmax (Table 3), it was found that the group with higher Gmax (mean = 182.70 ± 16.041 mg/dL) had a higher incidence of ROP with a total of 18 cases against only 14 cases in the cohort with a lower Gmax (mean = 156.90 ± 10.70212 mg/dL). This difference was found to be not statistically significant as given in Table 4 (chi-square test, p = 0.0.1793).
Table 3.
2 × 2 Table: Gmax versus incidence of ROP.
| Comparison group | Neonates with ROP | Neonates without ROP | Total | Mean maximum glucose (mg/dL) | SD |
|---|---|---|---|---|---|
| Neonates with higher maximum glucose levels (Gmax) | 18 | 33 | 51 | 182.70 | 16.041 |
| Neonates with lower maximum glucose levels (Gmax) | 14 | 38 | 52 | 156.90 | 10.70212 |
| Total | 32 | 71 | 103 |
ROP, retinopathy of prematurity; SD, standard deviation.
Risk of glycaemic status
The cohort, on distribution into two groups according to Gavg, Gmin and Gmax, was analysed for the risk of ROP with different glycaemic states (Table 5). The risk ratio (RR) of the incidence of ROP in the group with higher levels of Gmin was found to be 1.311 (confidence interval [CI] = 0.7326, 2.346). The higher value of Gmax also had a similar RR of 1.311 (CI = 0.7326, 2.346). In both cases, the CI spans on either sides of one, hence statistically non-significant. However, the RR of incidence of ROP in cases with higher values of Gavg was found to be 2.506 (CI = 1.287, 4.882). This value is statistically significant because the RR lies on either sides of 1.
Table 5.
Risk-based estimates of Gmin, Gmax and Gavg for the onset of ROP.
| Point estimates |
Confidence limits |
||
|---|---|---|---|
| Risk factor | Type | Value | Lower, upper |
| High minimum glucose values (Gmin) | Risk in exposed | 35.29% | 23.6, 49.05 |
| Risk in unexposed | 26.92% | 16.67, 40.35 | |
| Overall risk | 31.07% | 22.92, 40.57 | |
| Risk ratio | 1.311 | 0.7326, 2.346 | |
| Risk difference | 8.371% | −9.443, 26.18 | |
| High maximum glucose values (Gmax) | Risk in exposed | 35.29% | 23.6, 49.05 |
| Risk in unexposed | 26.92% | 16.67, 40.35 | |
| Overall risk | 31.07% | 22.92, 40.57 | |
| Risk ratio | 1.311 | 0.7326, 2.346 | |
| Risk difference | 8.371% | −9.443, 26.18 | |
| High average glucose values (Gmax) | Risk in exposed | 44.23% | 31.59, 57.67 |
| Risk in unexposed | 17.65% | 9.344, 30.48 | |
| Overall risk | 31.07% | 22.92, 40.57 | |
| Risk ratio | 2.506 | 1.287, 4.882 | |
| Risk difference | 26.58% | 9.506, 43.66 | |
ROP, retinopathy of prematurity.
Odds ratio (OR): Glycaemic status
Previous studies comparing hyperglycaemia and ROP were case–control studies. To facilitate comparison of the current study with the available literature, OR was required. The OR of a neonate having ROP with higher Gmin was 1.481 (0.6393, 3.429). The similar OR was found for the values of Gmax. In both the cases, the correlation was not significant. However, the OR of a neonate being screened positive for the presence of ROP in cases with higher Gavg was 3.701 (1.498, 9.142).
Discussion
Definition of hyperglycaemia
The definition of hyperglycaemia in neonates varies highly depending on the point of care. Various guidelines and articles have been published to this effect.9, 10, 29 The upper limit values for normal blood glucose levels have been determined by the study and formulated into guidelines, which range from 119 mg/dL to 150 mg/dL. It seems that because of this disparity, various studies looking into the association of hyperglycaemia and ROP have generated their cut-off levels of euglycaemia from their own inherent sample.6, 22, 23, 26, 27, 30, 31
In our study, the level of blood glucose defined as hyperglycaemia is generated from the cohort of neonates being studied. The values were arranged in an ascending order, and the cohort was divided into two groups with high and low glucose levels. This process was followed by the test of statistical significance in the difference of means in these groups, which showed that these two groups were indeed statistically different in terms of their blood sugar values. The values encountered in our study are tabulated in Table 6.
Table 6.
Blood glucose levels in the cohort of neonates (n = 103).
| Quantification of glucose values | Study cohort | Mean value (mg/dL) | SD | Cut-off values used |
|---|---|---|---|---|
| Minimum blood glucose value (Gmin) | High | 65.37 | 20.37249 | >= 43 mg/dL |
| Low | 36.17 | 4.364389 | < 43 mg/dL | |
| Maximum blood glucose value (Gmin) | High | 182.70 | 16.041 | >= 172 mg/dL |
| Low | 156.90 | 10.70212 | < 172 mg/dL | |
| Mean blood glucose value (Gavg) | High | 121.66 | 12.71431 | >=107.5 mg/dL |
| Low | 96.475 | 8.211515 | < 107.5 mg/dL |
ROP, retinopathy of prematurity; SD, standard deviation.
Retinopathy of prematurity (ROP) and hyperglycaemia
Several studies have been conducted to assess the contribution of hyperglycaemia as a risk factor of ROP in various different methodologies.7, 22, 26, 32 These studies were conducted in the form of case–control studies. One of the first studies on the association with hyperglycaemia was undertaken by Garg et al.6 in the year 2003 with a sample of 16 neonates at a single centre. In this study, the author described the effects of glucose levels in terms of the minimum, maximum and average values of the neonate in the first month of life and arrived at the conclusion that there exists a significant relation between incidence of ROP and the maximum as well as the average glucose values of the neonates.6 In our study, we found a statistically significant association between Gavg and the incidence of ROP.
Hence, it can be inferred that the findings of this study is in accordance with the previous studies conducted on this aspect in different regions of the globe. The management pattern of the neonate in the intensive care unit varies according to the local guidelines and policies. However, the relation between hyperglycaemia and ROP is significant across these differences (Table 7). The neonates with a higher average glucose level in their first week of life stand at higher odds of developing ROP compared with those neonates with lower average blood glucose levels (OR = 3.701, CI = 1.498, 9.142). The glycaemic state of the neonate depends of various factors such as prematurity, use of total parenteral nutrition, congenital abnormalities, steroid use and so on.30 However, it can be said with statistical significance that there is a need to screen the neonates with higher average blood glucose values more frequently and earlier to provide an early intervention.
Table 7.
A review of previous studies looking into the relation between hyperglycaemia and ROP compared with the current study (OR).
| Study | Total sample size | Odds ratio | Remarks |
|---|---|---|---|
| Current study | 103 | 3.701 | Correlation with Gavg |
| Carina et al. (2017)12 | 310 | 1.022 | |
| Ahmadpour-Kacho et al. (2014)11 | 75 | 1.03 | Adjusted OR of glucose level |
| Liu et al. (2014)7 | 53 | Not specified | Significant by chi-square test |
| Mohsen et al (2014)22 | 36 | 1.77 | |
| Mohamed et al. (2013)13 | 582 | 1.073 | Adjusted OR of duration |
| Van der Merwe, S K et al (2013) 48 | 160 | Not specified | Casual association |
| Chavez-Valdez et al (2011) 31 | 114 | Not specified | Casual association |
| Bozdag et al (2011) 21 | 167 | 3.26 | Adjusted OR of duration |
| Heimann et al (2007) 30 | 252 | Not specified | |
| Ertl et al (2006) 23 | 201 | Not specified | |
| Garg et al. (2003)10 | 47 | 1.10 |
OR, odds ratio.
Study limitations
ROP being a disease of prematurity, its incidence coexists with other problems of prematurity such as very low birth weight (VLBW), apnoea, metabolic disturbances and so on. Hyperglycaemia in itself might be an outcome of extreme prematurity. These factors confound the exclusivity of hyperglycaemia as a risk factor of ROP. Owing to the small sample size, it is not feasible to achieve statistical significance with adjustment for known confounding factors.
Summary
The findings of this study are summarised as follows:
-
•
A cohort of 103 neonates was screened during the study period.
-
•
Thirty-two neonates were found to have developed ROP during screening.
-
•
A higher average blood glucose value during the first week of life is a predictor for the onset of ROP. These infants are at higher risk of developing ROP. The minimum blood sugar values of the neonate recorded during the initial first week of life are not a significant predictor of the onset of ROP.
Conclusion
Hyperglycaemia in neonates is multifactorial, thus is ROP. Many of these risk factors such as prematurity, LBW/VLBW, intraventricular haemorrhage, sepsis, congenital defects, mechanical ventilation and parenteral nutrition contribute to both ROP and hyperglycaemia. This study failed to gather enough statistical data to independently imply hyperglycaemia as a risk factor for ROP. Analysis of hyperglycaemia as an independent risk factor requires further study, and a standardisation of the definition of hyperglycaemia in neonates is needed.
However, it is imperative to screen neonates with higher average blood glucose levels proactively for ROP because this disease is a blinding condition without timely intervention.
Conflicts of interest
The authors have none to declare.
References
- 1.Blencowe H., Lawn J.E., Vazquez T., Fielder A., Gilbert C. Preterm-associated visual impairment and estimates of retinopathy of prematurity at regional and global levels for 2010. Pediatr Res. 2013;74:35–49. doi: 10.1038/pr.2013.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dogra M.R., Katoch D., Dogra M. An update on retinopathy of prematurity (ROP) Indian J Pediatr. 2017 doi: 10.1007/s12098-017-2404-3. [DOI] [PubMed] [Google Scholar]
- 3.Blencowe H., Cousens S., Oestergaard M.Z. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379(9832):2162–2172. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- 4.Pierce L.M., Raab E.L., Holzman I.R., Ginsburg R.N., Brodie S.E., Stroustrup A. Importance of birth weight as a risk factor for severe retinopathy of prematurity when gestational age is 30 or more weeks. Am J Ophthalmol. 2014;157(6):1227–1230. doi: 10.1016/j.ajo.2014.02.045. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Louik C., Mitchell A.A., Epstein M.F., Shapiro S. Risk factors for neonatal hyperglycemia associated with 10% dextrose infusion. Am J Dis Child. 1985;139(8):783–786. doi: 10.1001/archpedi.1985.02140100045025. http://www.ncbi.nlm.nih.gov/pubmed/4025257 [DOI] [PubMed] [Google Scholar]
- 6.Garg R., Agthe A.G., Donohue P.K., Lehmann C.U. Hyperglycemia and retinopathy of prematurity in very low birth weight infants. J Perinatol. 2003;23(3):186–194. doi: 10.1038/sj.jp.7210879. [DOI] [PubMed] [Google Scholar]
- 7.Ahmadpour-Kacho M., Motlagh A.J., Rasoulinejad S.A. Correlation between hyperglycemia and retinopathy of prematurity. Pediatr Int. 2014;56(5):726–730. doi: 10.1111/ped.12371. [DOI] [PubMed] [Google Scholar]
- 8.Slidsborg C., Jensen L.B., Rasmussen S.C., Fledelius H.C., Greisen G., Cour M de la. Early postnatal hyperglycaemia is a risk factor for treatment-demanding retinopathy of prematurity. Br J Ophthalmol. June 2017 doi: 10.1136/bjophthalmol-2016-309187. bjophthalmol-2016-309187. [DOI] [PubMed] [Google Scholar]
- 9.Hey E. Hyperglycaemia and the very preterm baby. Semin Fetal Neonatal Med. 2005;10(4):377–387. doi: 10.1016/j.siny.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Cowett R.M., Farrag H.M. Selected principles of perinatal–neonatal glucose metabolism. Semin Neonatol. 2004;9(1):37–47. doi: 10.1016/S1084-2756(03)00113-1. [DOI] [PubMed] [Google Scholar]
- 11.Multicenter trial of cryotherapy for retinopathy of prematurity. Preliminary results. Cryotherapy for Retinopathy of Prematurity Cooperative Group. Arch Ophthalmol (Chicago, Ill 1960. 1988;106(4):471–479. doi: 10.1001/archopht.1988.01060130517027. http://www.ncbi.nlm.nih.gov/pubmed/2895630 [DOI] [PubMed] [Google Scholar]
- 12.Eckert G.U., Fortes Filho J.B., Maia M., Procianoy R.S. A predictive score for retinopathy of prematurity in very low birth weight preterm infants∖. Eye. 2012;26∖(3):400–406. doi: 10.1038/eye.2011.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohamed S., Murray J.C., Dagle J.M., Colaizy T. Hyperglycemia as a risk factor for the development of retinopathy of prematurity. BMC Pediatr. 2013;13(1):78. doi: 10.1186/1471-2431-13-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sambasivarao S.V. Algorithms for the prediction of retinopathy of prematurity based upon postnatal weight gain. Clin Perinatol. 2013;18(9):1199–1216. doi: 10.1016/j.clp.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hellstrom A., Engstrom E., Hard A.-L. Postnatal serum insulin-like growth factor I deficiency is associated with retinopathy of prematurity and other complications of premature birth. Pediatrics. 2003;112(5):1016–1020. doi: 10.1542/peds.112.5.1016. [DOI] [PubMed] [Google Scholar]
- 16.Hellstrom A., Perruzzi C., Ju M. Low IGF-I suppresses VEGF-survival signaling in retinal endothelial cells: direct correlation with clinical retinopathy of prematurity. Proc Natl Acad Sci Unit States Am. 2001;98(10):5804–5808. doi: 10.1073/pnas.101113998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Langford K., Nicolaides K., Miell J.P. Maternal and fetal insulin-like growth factors and their binding proteins in the second and third trimesters of human pregnancy. Hum Reprod. 1998;13(5):1389–1393. doi: 10.1093/humrep/13.5.1389. http://www.ncbi.nlm.nih.gov/pubmed/9647578 [DOI] [PubMed] [Google Scholar]
- 18.Pierce E.A., Foley E.D., Smith L.E. Regulation of vascular endothelial growth factor by oxygen in a model of retinopathy of prematurity. Arch Ophthalmol (Chicago, Ill 1960. 1996;114(10):1219–1228. doi: 10.1001/archopht.1996.01100140419009. http://www.ncbi.nlm.nih.gov/pubmed/8859081 [DOI] [PubMed] [Google Scholar]
- 19.Smith L.E.H., Shen W., Perruzzi C. Regulation of vascular endothelial growth factor-dependent retinal neovascularization by insulin-like growth factor-1 receptor. Nat Med. 1999;5(12):1390–1395. doi: 10.1038/70963. [DOI] [PubMed] [Google Scholar]
- 20.Le Y.-Z. VEGF production and signaling in M?ller glia are critical to modulating vascular function and neuronal integrity in diabetic retinopathy and hypoxic retinal vascular diseases. Vis Res. 2017 doi: 10.1016/j.visres.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bozdag S., Oguz S.S., Gokmen T. Serum fructosamine and retinopathy of prematurity. Indian J Pediatr. 2011;78(12):1503–1509. doi: 10.1007/s12098-011-0515-9. [DOI] [PubMed] [Google Scholar]
- 22.Mohsen L., Abou-Alam M., El-Dib M., Labib M., Elsada M., Aly H. A prospective study on hyperglycemia and retinopathy of prematurity. J Perinatol. 2014;34(November 2013):1–5. doi: 10.1038/jp.2014.49. [DOI] [PubMed] [Google Scholar]
- 23.Ertl T., Gyarmati J., Gaál V., Szabó I. Relationship between hyperglycemia and retinopathy of prematurity in very low birth weight infants. Neonatology. 2006;89(1):56–59. doi: 10.1159/000088199. [DOI] [PubMed] [Google Scholar]
- 24.Patz a. The new international classification of retinopathy of prematurity. Arch Ophthalmol. 1984;102(8):1129. doi: 10.1001/archopht.1984.01040030907010. [DOI] [PubMed] [Google Scholar]
- 25.International Committee for the Classification of Retinopathy of Prematurity The international classification of retinopathy of prematurity revisited. Arch Ophthalmol. 2005;123(7):991. doi: 10.1001/archopht.123.7.991. [DOI] [PubMed] [Google Scholar]
- 26.Au S.C.L., Tang S.-M., Rong S.-S., Chen L.-J., Yam J.C.S. Association between hyperglycemia and retinopathy of prematurity: a systemic review and meta-analysis. Sci Rep. 2015;5(1):1–6. doi: 10.1038/srep09091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blanco C.L., Baillargeon J.G., Morrison R.L., Gong A.K. Hyperglycemia in extremely low birth weight infants in a predominantly Hispanic population and related morbidities. J Perinatol. 2006;26(12):737–741. doi: 10.1038/sj.jp.7211594. [DOI] [PubMed] [Google Scholar]
- 28.Jain A. 2010. AIIMS Protocols 2010; pp. 1–13. [Google Scholar]
- 29.Prince R., Hospital A. RPA newborn care protocol book neonatal hyperglycemia Definition : risk factors. RPA Newborn care Protoc B. 2009;1(1):8–10. [Google Scholar]
- 30.Heimann K., Peschgens T., Kwiecien R., Stanzel S., Hoernchen H., Merz U. Are recurrent hyperglycemic episodes and median blood glucose level a prognostic factor for increased morbidity and mortality in premature infants ≤1500 g? J Perinat Med. 2007;35(3):245–248. doi: 10.1515/JPM.2007.057. [DOI] [PubMed] [Google Scholar]
- 31.Chavez-Valdez R., McGowan J., Cannon E., Lehmann C.U. Contribution of early glycemic status in the development of severe retinopathy of prematurity in a cohort of ELBW infants. J Perinatol. 2011;31(12):749–756. doi: 10.1038/jp.2011.19. [DOI] [PubMed] [Google Scholar]
- 32.Nicolaeva G.V., Sidorenko E.I., Iosifovna A.L., Article O. Influence of the blood glucose level on the development of retinopathy of prematurity in extremely premature children. Arq Bras Oftalmol. 2015;78(4):232–235. doi: 10.5935/0004-2749.20150060. [DOI] [PubMed] [Google Scholar]