Abstract
Background
Evidence regarding the role of non-invasive marker of airway inflammation, fractional exhaled nitric oxide (FeNO) to guide asthma treatment is equivocal. We aimed to evaluate if the use of FeNO to adjust inhaled corticosteroid treatment resulted in reduced daily corticosteroid use and lesser exacerbations.
Methods
100 patients of bronchial asthma in the age group of 12–70 years were randomised to receive inhaled corticosteroids based on either FeNO measurements (n = 50) or as per Global Initiative for Asthma (GINA) guidelines. Follow up was done every 2 months for period of 12 months. Results were compared in terms of mean daily inhaled corticosteroid use and number of exacerbations.
Results
After the follow up period of 12 months, mean daily dose of ICS (SD) required in FeNO group was 267.5 μg (126.29), as opposed to control group in which mean daily dose of steroid was 320.00 μg (138.69). However this observed difference in steroid dose was statistically insignificant (p value = 0.061). The estimated mean (SD) rate of asthma exacerbation experienced in follow up period of 12 months in FeNO group was 0.3 episodes (0.54) per patient per year (95% confidence interval, 0.145–455) and 0.4 episodes (0.61) per patient per year in control group (95% confidence interval, 0.228–572). However this difference in rate of exacerbations between the two study groups was not statistically significant (p = 0.387).
Conclusion
FeNO guided management strategy for asthma did not result in statistically significant reduction in dose of inhaled corticosteroids or number of asthma exacerbations.
Keywords: Exhaled nitric oxide, Bronchial asthma, Inhaled corticosteroids
Introduction
Asthma is a heterogeneous disease characterised by variable symptomatology and expiratory airflow limitation which is associated with chronic airway inflammation.1 Chronic airway inflammation remains the hallmark of bronchial asthma. Diagnostic and monitoring tools for bronchial asthma in current practice include pulmonary function tests (PFT), bronchodilator reversibility assessment and bronchial challenge tests. However, none of these tests are able to directly measure the airway inflammation. In such scenario arises the need of a test which can directly measure the level of airway inflammation and which can be useful in assessing treatment response in a non-invasive manner.2 Inhaled corticosteroids are the mainstay of treatment for bronchial asthma which is aimed at controlling ongoing airway inflammation. But inhaled corticosteroid dose requires close monitoring as lower doses will be ineffective at alleviating airway inflammation as well as symptomatology and higher than required dose will put the patient at risk of adverse effects.3 Recently Fractional Exhaled Nitric Oxide (FeNO) has been gaining attention as a sensitive marker of airway inflammation. Nitric Oxide (NO) is produced in airway epithelium by upregulation of nitric oxide synthase in inflammation. Its role has been studied in airways as a vasodilator, bronchodilator, neurotransmitter and inflammatory mediator.4, 5 Asthma cases have been found to have high levels of FeNO as compared to those having only atopy or the normal controls.5 FeNO levels rise with severity of asthma and its levels reduce in dose dependent manner with inhaled corticosteroid usage.6, 7 Ultimate aim in asthma management consists of alleviation of symptomatology and reduction of exacerbations at lowest possible dose of inhaled corticosteroids.1 Many scientific papers and reports recommend FeNO as an investigative and research tool presently. International studies are available titrating the dose of inhaled steroids based on FeNO levels.8, 9, 10 The switchover from research to clinical practice of FeNO requires more scientific research globally and more so in our country. Meta analysis reviewing the results of various studies showed no improvement in exacerbation rate in the patients adjusting the dose of inhaled steroids as per FeNO levels, in comparison with standard approach.11 However comparing the cumulative doses of inhaled steroids at end of study there were conflicting results with FeNO group having lower cumulative dose in adults and higher in children.11 As there are conflicting results in various available studies and paucity of Indian data using FeNO levels for guiding asthma treatment, we conducted this study comparing the dose adjustment of inhaled steroids as per FeNO levels versus adjustment as per GINA guidelines. We tested the null hypothesis that there will be no difference in mean daily inhaled corticosteroid dose between two groups at the end of study.
Materials and methods
Study size
A single blind randomised controlled trial was conducted in Respiratory outpatient department of a tertiary care hospital. Sample size was calculated to test null hypothesis H0: μ1 − μ2 = 0 against alternate hypothesis H1: μ1 − μ2 ≠ 0 with 5% level of significance and 90% power of study. Sample size calculations were done based on results of randomised control trial conducted by Smith et al.8 It showed mean daily dose of fluticasone to be 370 ± 360 mcg per day for FeNO group comprising of forty-six patients (95% confidence interval, 263–477) as opposed to 641 ± 396 mcg per day for control group consisting of forty-eight patients (95% confidence interval, 526–756).8 Required sample size was calculated to be 41 subjects in each group.
Participants and study plan
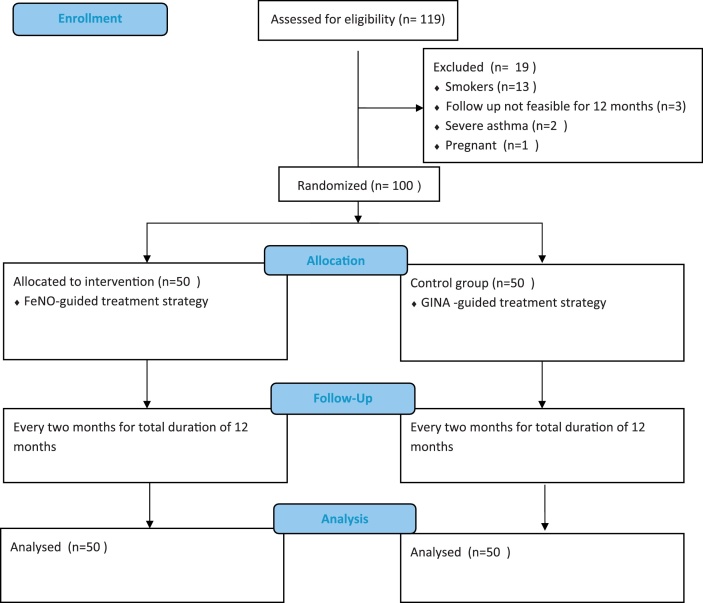
Study plan is elaborated as per consort flow diagram (Fig. 1). A single blind randomised controlled trial was conducted in Respiratory outpatient department of a tertiary care hospital. Ethical clearance was obtained from the institutional ethics committee. Patients were blinded and were unaware of group to which they were assigned. A total of 119 patients in age group of 12–70 years who were diagnosed cases of Bronchial Asthma as per Global Initiative for Asthma (GINA) guidelines were initially recruited in this study. They were analysed for initial 2 months. Patient with history of smoking, severe asthma cases, h/o ≥ 2 exacerbations in preceding year, requiring oral prednisolone for asthma exacerbation in these two months of pre-allocation period and pregnant females were excluded from the study group. Out of 119 participants, 19 were excluded due to above mentioned reasons and 100 were randomised to treatment or control group by simple randomisation method using a online software (http://www.randomization.com/) generated randomisation sequence with equal allocation in two groups with each group comprising of 50 patients. Participants were allocated to either group with the randomisation sequence concealed in sequentially numbered, opaque sealed envelopes. All patients were clinically evaluated with baseline clinical profile, laboratory parameters and dose of inhaled corticosteroid (ICS) at the commencement of the study. Base line characteristics of both the groups are as depicted in Table 1. In group A which was conventional therapy group, the treatment was subsequently tailored based on clinical symptoms and spirometry (FEV1). While Group B patients, the FeNO group, received treatment strategy guided by FeNO measurement. In group A patients, spirometry was performed for each subject as per ATS guidelines. Asthma control test (ACT) score was calculated for each patient.12 Group B patients underwent measurement of FeNO levels with an electrochemical analyser (NO breath FeNO monitor; Bedfont Scientific Limited, Maidstone, United Kingdom) as per ATS/European Respiratory Society recommendation. Thereafter all these parameters were followed once in every two months for a total period of 12 months as part of the standard protocol for outcome comparison in both the groups. Outcome was compared in terms of frequency of exacerbation, and change in requirement of mean daily inhaled corticosteroids (ICS) dose for both groups and FeNO values for group B in addition to all these parameters. Through out the study period Metered Dose Inhaler (MDI) Fluticasone propionate was used as standard ICS in strength of multiples of 125 μg/puff. ICS dose was stepwise upregulated or downregulated by 125 μg/daily based on GINA guidelines in conventional therapy group and FeNO levels in FeNO group.
Fig. 1.
Consort flow diagram depicting study plan.
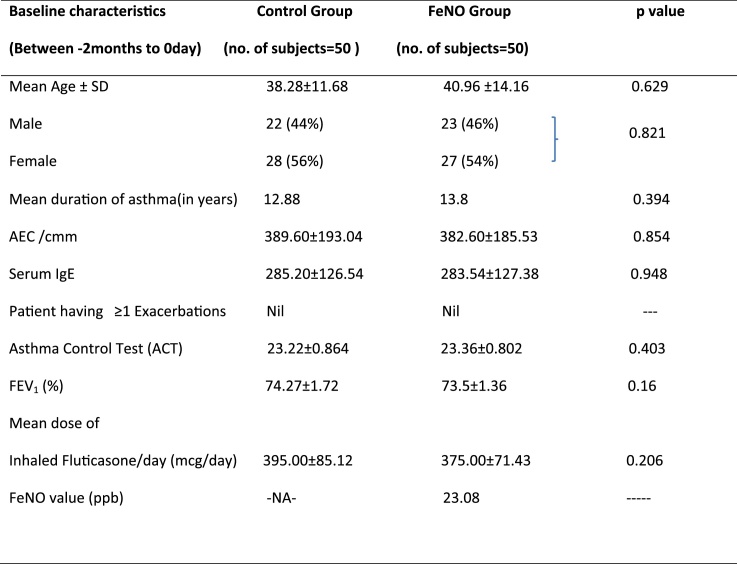
Table 1.
Baseline characteristics of the control group and intervention (FeNO) group.
Definition of abbreviations: AEC = absolute eosinophil count, FEV1 = forced expiratory volume in 1 s, FeNO = fraction of exhaled nitric oxide.
Values presented as mean ± standard deviation (SD).
During the study period, dose adjustment of ICS in Group A (conventional therapy control group) was primarily based on clinical symptoms, signs and spirometry (FEV1). ICS dose adjustment was done as per step up-step down recommendations of GINA guidelines after reviewing the medication technique, compliance and avoidance of risk factors. In group B (FeNO group) ICS dose adjustment was based on FeNO values as per ATS recommendation 2011. Increase in FeNO was considered as significant when it increased by greater than 20% for values over 50 ppb or more than 10 ppb for values lower than 50 ppb from one visit to the next and a reduction of at least 20% in FeNO for values over 50 ppb or more than 10 ppb for values lower than 50 ppb as the cut point to indicate a significant response to anti-inflammatory therapy.13 ICS dose was increased for significant increase in FeNO and decreased for its significant fall.
Measurement of FeNO
FeNO values were measured with an electrochemical analyzer (NO breath FeNO monitor; Bedfont Scientific Limited, Maidstone, United Kingdom) at an expiratory flow rate of 50 ml/s as per ATS/European Respiratory Society recommendation. FeNO values were expressed in parts per billion (ppb).13
Statistical analysis
Statistical analysis was performed using a statistical software package (SPSS Statistics 20.0). Tests for significant difference in means for continuous variables were calculated using Mann–Whitney Test as variables were not following normal distribution. Chi-square test was applied to determine the significance between categorical variables. A ‘p’ value of less than or equal to 0.05 was considered statistically significant.
Results
Subject characteristics
During the study period of twelve months 100 subjects between age group 12–70 years, were analysed. In conventional group 19 (38%) were males and 31 (62%) were female subjects. In FeNO group 16 (32%) were males and 34(68%) were females. The age of the patients in the study group was ranged from 12 to 70 years and the majority of patients were in between 31 to 40 years of age in both the groups. The mean age of group A population was 33.82 ± 12.36 years and the mean age of group B population was 34.34 ± 14.05 years. At 0 month total number of patients having ≥1 exacerbation in both the group A and B were nil. The baseline mean FEV1 in group A and group B was 74.27 ± 1.72% and 73.5 ± 1.36% respectively. Mean total requirement of ICS dose at 0 month in group A and group B was 395 ± 85.12 μg/day and 375 ± 71.43 μg/day respectively. Difference in mean dose of ICS between group A and group B at ‘0’ month was 20mcg/day but the difference was statistically insignificant (p = 0.206). Baseline mean Asthma Control Test (ACT) score in group A and B was 23.22 ± 0.864 and 23.36 ± 0.802 respectively. The difference in baseline ACT of both the groups was again statistically insignificant (p = 0.403). The baseline mean FeNO value in group B was 23.08 ppb. Baseline characteristics of the patients of both groups showed patients with controlled bronchial asthma on medium dose of ICS. The characteristics when analysed at baseline, there were no significant differences observed between the study groups.
Table 2 depict the categorization of ICS (Fluticasone propionate in μg/day) as low (125–250), medium (>250–500) and high (>500–1000) and the number of subjects using ICS as low, medium and high dose. There was significant increase in number of subjects in both study groups requiring low dose ICS from 08 to 26 in group A and from 08 to 32 in group B from month 0 to month 12 and decrease in number of subjects requiring high dose of ICS from 16 subjects to 9 in group A and from 8 to 6 in group B during the study period. Mean dose of ICS required at end of 12 months of follow up was 320 ± 138.69 μg/day in the control group as opposed to 267.5 ± 126.29 μg/day in the FeNO group (Table 3). The difference in distribution of daily ICS dose between both the groups at the end of 12 months failed to achieve statistically significant value (p = 0.061). Minimum, maximum values observed after 12 months were 125 μg and 750 μg in control group comparing with 125 μg and 500 μg in FeNO group. Mean reduction in the daily dose of ICS at the end of 12 months as compared to 0 month was 75 ± 131.22 mcg in the control group and 107.5 ± 121.14 mcg in FeNO group. At the end of study participants in FeNO group had greater reduction in daily dose of ICS as compared to control. However, this difference in reduction of daily dose of ICS between both control and FeNO group was statistically insignificant (p = 0.306).
Table 2.
Number of subjects in control group and intervention (FeNO) group requiring low, medium and high dose inhaled fluticasone (μg/day) at 0 months and 12 months.
| ICS daily dose | Control group (no of subjects) |
FeNO group (no of subjects) |
||
|---|---|---|---|---|
| At 0 month | At 12 month | At 0 month | At 12 month | |
| Low (125–250) | 08 | 26 | 08 | 32 |
| Medium (>250–500) | 26 | 15 | 34 | 12 |
| High (>500–1000) | 16 | 09 | 08 | 06 |
| Total | 50 | 50 | 50 | 50 |
| p valuea | <0.001 | <0.001 | ||
Chi-square test was done to calculate p value.
Table 3.
Comparison of mean dose of inhaled fluticasone ± standard deviation (μg/day) required at 0 month and at 12 months in control group and intervention (FeNO) group.
| Mean fluticasone dose (μg/day) |
p-Value a | ||
|---|---|---|---|
| Control group | FeNO group | ||
| At 0 month | 395 ± 85.12 | 375.00 ± 71.43 | 0.206 |
| At 12 month | 320.00 ± 138.69 | 267.50 ± 126.29 | 0.061 |
Mann–Whitney Test was applied to calculate p value.
After the follow up period of 12 months, in group A 33 patients never had any episode of asthma exacerbation while 17 subjects experienced exacerbation, out of which 14 subjects had single episode of exacerbation and 03 subjects had 2 episodes of asthma exacerbation during the follow up (Table 4). In group B 37 patients never had any episode of asthma exacerbation while 13 subjects experienced exacerbation, out of which 11 subjects had single episode of exacerbation in any period of two monthly follow up during study period and only 02 subjects had more than 2 episodes of asthma exacerbation during the study period (Table 4). Total no of asthma exacerbations in FeNO group was 15 in 13 patients as compared to 20 in 17 patients in control group. The estimated mean(SD) rate of asthma exacerbation experienced in follow up period of 12 months in FeNO group was 0.3 episodes (0.54) per patient per year (95% confidence interval, 0.145–455) and 0.4 episodes (0.61) per patient per year in control group (95% confidence interval, 0.228–572). However this difference in rate of exacerbations between the two study groups was not statistically significant (p = 0.387).
Table 4.
Comparison of number of subjects who experienced exacerbations over the study period in the control group and intervention (FeNO) group.
| No of exacerbations | Control group (no. of subjects) | FeNO group (no. of subjects) | p-Valuea |
|---|---|---|---|
| Nil | 33 | 37 | |
| One or more episode | 17 | 13 | 0.38 |
| Total | 50 | 50 |
Chi-square test was applied to calculate p value.
Discussion
Bronchial asthma being a chronic inflammatory disorder leads to airflow limitation which is assessed by characteristic symptoms and spirometry in current practice. FENO has recently gained attention in regards of a quick, non-invasive and a reliable tool for assessing the airway inflammation. In our study we aimed to assess the efficacy of Fractional exhaled nitric oxide (FENO) in monitoring of bronchial asthma compared to conventional methods, to minimise the number of exacerbations per year and to assess the reduction in inhaled corticosteroid use in patients. In our study overall numbers of exacerbation were less in FENO group with 37 patients never having exacerbation during study period as compared to 33 patients with no exacerbation in conventional group. Moreover absolute no of ≥1 exacerbations were also less in FeNO group. However, this difference was statistically insignificant. Our findings are consistent with those of other studies on FeNO except Syk et al. Shaw et al. estimated mean exacerbation frequency 0.33 per patient per year in FeNO group as compared to 0.42 per patient per year in control group (p = 0.43).9 In the study conducted by Szefler et al. measures of asthma exacerbations like unscheduled visits and hospitalizations did not differ significantly in both the groups (FeNO versus Reference group) during 46 weeks follow up period.10 Honkoop et al. also found lower incidence of asthma exacerbation in FeNO based treatment strategy as compared to asthma symptom based strategy.14 Syk et al. established significant reduction in asthma exacerbation rate (exacerbations/patient/year).15 It was reduced by almost 50% in the FENO-guided group (0.22 [CI, 0.14–0.34] versus 0.41 [CI, 0.29–0.58]; p = .024) as compared to control group. All studies mentioned above have shown some reduction in asthma exacerbation rate when FeNO was used as guiding tool for asthma treatment, although significant reduction in exacerbation rate could have been proven by Syk et al. only.15 It implies FeNO used as monitoring tool can detect airway inflammation better and earlier than clinical symptoms, spirometry combined together. It helps in better targeting the ICS usage according to airway inflammation,leading to fewer exacerbation rate. Slight difference in outcome rates observed in different studies may be due to diverse study population, as well as dissimilar step up/step down protocols.
In our study, both study groups showed clinically and statistically significant reduction in mean ICS daily dose at the end of study when compared with the daily required dose of ICS before the initiation of study protocol with p value <0.001 for both the groups. It has also been observed that there was significant increase in number of subjects in both study groups requiring low dose ICS and decrease in number of subjects requiring high dose of ICS. This is likely due to intense monitoring and enhanced confidence of patient to reduce ICS dose under the supervision of specialist in tertiary care setting. At the completion of study period mean daily dose of ICS required in FeNO group was less than the control group. As stated previously both the groups showed reduction in ICS dose, however reduction of ICS in FeNO group was more than the control group when compared with pre study values. But this reduction in dose of inhaled steroids achieved in FeNO group failed to reach statistically significant values. Our results are in agreement with the previous studies which failed to show significant reduction in ICS dose in FeNO group.9, 11, 16 In the study conducted by Shaw and coworkers final daily dose of ICS in FeNO group was less than that of control group, however total ICS dose over the study period in FeNO group was 11% greater than control group. Calhoun and colleagues failed to demonstrate any reduction in ICS usage with biomarker-based treatment adjustment (exhaled nitric oxide) as compared to symptom-based adjustment.16 Moreover latest meta-analysis by Petsky et al.11 showed that strategy of tailoring asthma medication based on FeNO levels did not exhibit any beneficial effect in inhaled corticosteroid use. This latest meta-analysis results surpass the findings of Smith et al. who demonstrated statistically significant (p value = 0.008) difference of 270 mcg per day in mean daily dose ICS between the two groups.
There is consistent beneficial effect of FeNO guidance on asthma exacerbation rate including the results of our study and the latest meta-analysis report,11 although significant reduction in exacerbation rate could have been demonstrated in a single study conducted by Syk et al.15 However, FeNO group in different studies did not substantially reduce ICS usage and had contradictory results in different studies. In conclusion FeNO guided treatment strategy resulted in less number of exacerbations and lower corticosteroid use, but the difference was statistically insignificant. Larger and longer studies in future are needed to reach clear consensus regarding universal use of FeNO, which at present should be considered for research purpose only. At present sufficient evidence is lacking permitting its regular use in clinical practise.
Conflicts of interest
The authors have none to declare.
Acknowledgements
This paper is based on Armed Forces Medical Research Committee Project No. 4385/2013 granted by the office of the Directorate General Armed Forces Medical Services and Defence Research Development Organization, Government of India.
References
- 1.Global Initiative for Asthma . 2016. Global Strategy for Asthma Management and Prevention. Available from: www.ginasthma.org. [Google Scholar]
- 2.Revathy N., Vinodkumar S., Kadhiravan T., Manju R., Sandhiya S., Adithan C. Clinical utility of fractional exhaled nitric oxide (FeNO) as a biomarker to predict severity of disease and response to inhaled corticosteroid (ICS) in asthma patients. J Clin Diagn Res. 2016;10:FC01–FC06. doi: 10.7860/JCDR/2016/20656.8950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta R., Fonacier L.S. Adverse effects of nonsystemic steroids (inhaled, intranasal, and cutaneous): a review of the literature and suggested monitoring tool. Curr Allergy Asthma Rep. 2016;16:44. doi: 10.1007/s11882-016-0620-y. [DOI] [PubMed] [Google Scholar]
- 4.Guo F.H., Comhair S.A., Zheng S. Molecular mechanisms of increased nitric oxide (NO) in asthma: evidence for transcriptional and post-translational regulation of NO synthesis. J Immunol. 2000;164:5970–5980. doi: 10.4049/jimmunol.164.11.5970. [DOI] [PubMed] [Google Scholar]
- 5.Kharitonov S.A., Yates D., Robbins R.A., Logan-Sinclair R., Shinebourne E.A., Barnes P.J. Increased nitric oxide in exhaled air of asthmatic patients. Lancet. 1994;343:133–135. doi: 10.1016/s0140-6736(94)90931-8. [DOI] [PubMed] [Google Scholar]
- 6.Pijnenburg M.W., Bakker E.M., Hop W.C., De Jongste J.C. Titrating steroids on exhaled nitric oxide in children with asthma: a randomized controlled trial. Am J Respir Crit Care Med. 2005;172:831–836. doi: 10.1164/rccm.200503-458OC. [DOI] [PubMed] [Google Scholar]
- 7.Silkoff P.E., McClean P., Spino M., Erlich L., Slutsky A.S., Zamel N. Dose–response relationship and reproducibility of the fall in exhaled nitric oxide after inhaled beclomethasone dipropionate therapy in asthma patients. Chest. 2001;119:1322–1328. doi: 10.1378/chest.119.5.1322. [DOI] [PubMed] [Google Scholar]
- 8.Smith A.D., Cowan J.O., Brassett K.P., Herbison G.P., Taylor D.R. Use of exhaled nitric oxide measurements to guide treatment in chronic asthma. N Engl J Med. 2005;352:2163–2173. doi: 10.1056/NEJMoa043596. [DOI] [PubMed] [Google Scholar]
- 9.Shaw D.E., Berry M.A., Thomas M. The use of exhaled nitric oxide to guide asthma management: a randomized controlled trial. Am J Respir Crit Care Med. 2007;176:231–237. doi: 10.1164/rccm.200610-1427OC. [DOI] [PubMed] [Google Scholar]
- 10.Szefler S.J., Mitchell H., Sorkness C.A. Management of asthma based on exhaled nitric oxide in addition to guideline-based treatment for inner-city adolescents and young adults: a randomised controlled trial. Lancet. 2008;372:1065–1072. doi: 10.1016/S0140-6736(08)61448-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petsky H.L., Kew K.M., Turner C., Chang A.B. Exhaled nitric oxide levels to guide treatment for adults with asthma. Cochrane Database Syst Rev. 2016;9:CD011440. doi: 10.1002/14651858.CD011440.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nathan R.A., Sorkness C.A., Kosinski M. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113:59–65. doi: 10.1016/j.jaci.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Dweik R.A., Boggs P.B., Erzurum S.C. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184:602–615. doi: 10.1164/rccm.9120-11ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Honkoop P.J., Loijmans R.J., Termeer E.H. Symptom- and fraction of exhaled nitric oxide-driven strategies for asthma control: a cluster-randomized trial in primary care. J Allergy Clin Immunol. 2015;135:682–688. doi: 10.1016/j.jaci.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 15.Syk J., Malinovschi A., Johansson G. Anti-inflammatory treatment of atopic asthma guided by exhaled nitric oxide: a randomized, controlled trial. J Allergy Clin Immunol Pract. 2013;1:639–648. doi: 10.1016/j.jaip.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 16.Calhoun W.J., Ameredes B.T., King T.S. Comparison of physician-, biomarker-, and symptom-based strategies for adjustment of inhaled corticosteroid therapy in adults with asthma. The BASALT Randomized Controlled Trial. JAMA. 2012;308:987–997. doi: 10.1001/2012.jama.10893. [DOI] [PMC free article] [PubMed] [Google Scholar]