Abstract
Background
Inaccuracies in treatment setup during radiation therapy for breast cancers may increase risks to surrounding normal tissue toxicities, i.e. organs at risks (OARs), and compromise disease control. This study was planned to evaluate the dosimetric and isocentric variations and determine setup reproducibility and errors using an online electronic portal imaging (EPI) protocol.
Methods
A total of 360 EPIs in 60 patients receiving breast/chest wall irradiation were evaluated. Cumulative dose–volume histograms (DVHs) were analyzed for mean doses to lung (V20) and heart (V30), setup source to surface distance (SSD) and central lung distance (CLD), and shifts in anterior-posterior (AP), superior-inferior (SI), and medial lateral (ML) directions.
Results
Random errors ranged from 2 to 3 mm for the breast/chest wall (medial and lateral) tangential treatments and 2–2.5 mm for the anterior supraclavicular nodal field. Systematic errors ranged from 3 to 5 mm in the AP direction for the tangential fields and from 2.5 to 5 mm in the SI and ML direction for the anterior supraclavicular nodal field. For right-sided patients, V20 was 0.69–3.96 Gy, maximum lung dose was 40.5 Gy, V30 was 1.4–3 Gy, and maximum heart dose was 50.5 Gy. Similarly, for left-sided patients, the CLD (treatment planning system) was 25 mm–30 mm, CLD (EPIs) was 30–40 mm, V20 was 0.9–5.9 Gy, maximum lung dose was 45 Gy, V30 was 2.4–4.1 Gy, and maximum heart dose was 55 Gy.
Conclusion
Online assessment of patient position with matching of EPIs with digitally reconstructed radiographs (DRRs) is a useful method in evaluation of interfraction reproducibility in breast irradiation.
Keywords: Breast, Setup errors, Irradiation, Portal imaging
Introduction
Patients with breast cancers who have undergone either breast conservation surgery (BCS) or mastectomy will undergo radiation therapy as part of the treatment. Large treatment fields are required to encompass the whole breast (chest) volumes. Any inaccuracy in treatment setup increases the risk of surrounding normal tissue toxicities, i.e. organs at risk (OARs), namely the heart and lung later after radiation treatment. This may also affect disease control. Hence, there is a dire need to generate data about setup reproducibility during treatment of locally advanced breast cancer (LABC) patients in our population. This study is planned to evaluate the dosimetric and isocentric variations and determine setup reproducibility and errors in a cohort of 60 (1 male and 59 females) breast cancer patients treated with mega voltage radiographs using an online electronic portal imaging (EPI) protocol.
Material and methods
This study is a hospital-based cross-sectional observational study that was carried out on approximately 60 patients (1 male and 59 females) with LABC and divided into two groups as Group ‘A’ (30 right-sided) and Group ‘B’ (30 left-sided) breast patients aged between 25 and 75 years (mean age of 50 years) for external beam radiotherapy (EBRT) treated during a period of 1 year between August 2014 and August 2015 at a tertiary care oncology centre. Assuming the standard deviation as 5 mm, we needed a sample of 170 measurements to estimate the true deviation with precision of 1 mm and 99% confidence. A total 360 EPIs (180 right and 180 left sided) were obtained and analyzed in these 60 patients.
Patient inclusion criteria
Pretreatment evaluation including detailed history and physical examination, routine hematological and biochemical investigations, chest radiograph, and mammography to assess local spread and to rule out distant metastasis was carried out in all patients. Contrast-enhanced computed tomography (CECT) chest and Positron emission tomography-computed tomography (PET-CT) scans/bone scans were advised on clinical suspicion of metastases. Clinical staging was completed as per tumor nodal metastases (TNM) staging system. A total of 60 patients (30 right- and 30 left-sided breast [mean age 50 years]) with biopsy-proven breast cancer who had given consent for radiotherapy and were being considered for treatment with external beam radiation therapy (EBRT) as per existing standard protocols for breast cancer treatment were prospectively enrolled in the study protocol approved by the institute's ethics committee.
Treatment scheme/protocol
Sixty patients underwent virtual 3D CT simulation and planned on a treatment planning system (TPS) (Oncentra, version 4.3; Elekta) using two opposed tangential fields (medial and lateral) with 6 MV photons as per international guidelines, and treatment was executed by the existing linear accelerator (Primus Hi; Siemens) to a total dose of 50 Gy in 25 fractions, 5 days in a week for 5–6 weeks. Electron boost to the local site (10–16 Gy/5–8 fractions) was used in patients with BCS. A total 360 EPIs (180 right and 180 left sided) were obtained and analyzed in these 60 patients.
Treatment simulation and treatment planning method
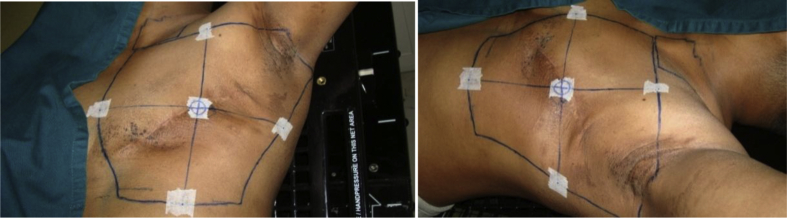
All patients were immobilized on a commercially available inclined breast board in treatment position for virtual CT simulation with the arm on the affected side of the body raised above the head and scanned in supine position with free breathing on a 4 slice CT scanner (Siemens) in the Department of Radio Diagnosis. Immobilization devices (thermoplastic molds) were used for patients with large pendulous breasts. Patients were clinically marked for breast/chest wall field borders and supraclavicular field for nodal irradiation with fiducial radiopaque point-sized (lead) ball markers. Three markers were placed at the central axis of the breast/chest wall field, and CT lasers were matched with this central axis. Two straight lines meeting the superior-inferior (SI) and medial lateral (ML) field borders and joining the central marker were drawn, and four reference markers were placed, one each at the midline of this SI and ML field borders. Distances from upper, lower, and lateral borders with respect to this central marker were noted as SI and ML distance [Fig. 1]. Slice thickness, KVp, and mAs were selected as per the institutional protocol. The whole breast was scanned with a margin of at least 5 cm in cranio caudal direction. This CT image data set of the patient is then imported in Digital Communication in Medicine format to contouring workstation and TPS (Oncentra; Elekta) for contouring structure sets and critical OARs (lung and heart) and planning target volume on each CT slice for all these patients and for three-dimensional (3D) treatment planning. A blanket value of 0.2 was used by TPS to correct isodose plans for lung tissue in homogeneity.
Fig. 1.
Markers and field borders on a patient undergoing irradiation after mastectomy for a left breast cancer.
On TPS, a 3D-approved treatment plan was generated for all patients. Two coplanar conventional tangential fields at two gantry angles (usually for right side between 60 and 240° and for left side between 120 and 300°) were chosen by minimizing the dose to the OARs for treatment on a linear accelerator machine (Primus HI; Siemens) [Fig. 2]. Cumulative dose–volume histograms (DVHs) for mean doses to volume of lung receiving 20 Gy (V20), volume of heart receiving 30 Gy (V30), and linear measurements, namely setup SSD (distance of the perpendicular drawn from isocentre to the upper skin border and named as AP, and SI and ML distances) with respect to central marker and central lung distance (CLD) on isocentric slice were calculated for each case on this 3D reference–approved treatment plan [Fig. 3].
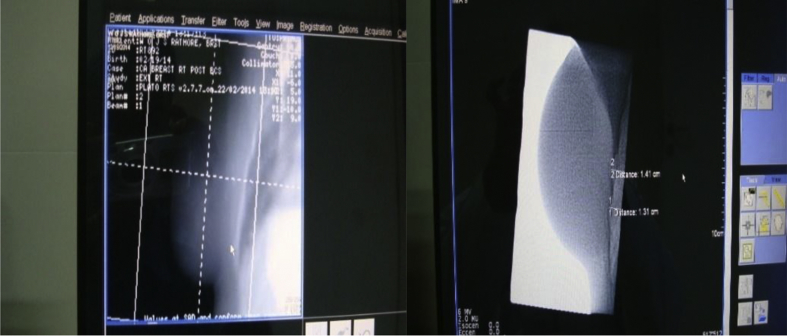
Fig. 2.
Electronic portal images (EPIs) of left-sided chest wall (after mastectomy) irradiation (medial and lateral tangential fields).
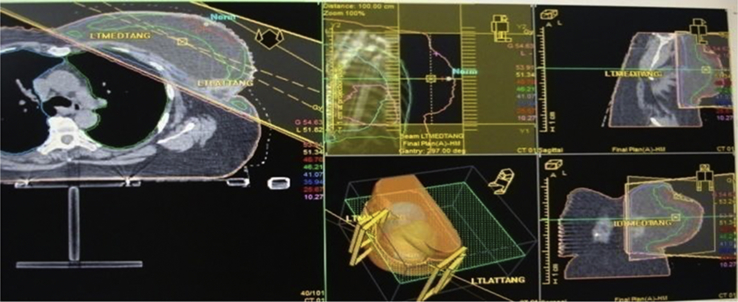
Fig. 3.
3D plan and portal image showing deviations of central lung distance (CLD) of a patient who has undergone conservative breast surgery for a left-sided breast cancer.
This 3D-approved plan along with the reference DRR image generated by the TPS Oncentra was exported to Primeview 3i workstation for EPI with Optivue 500 (amorphous silicon flat panel detector; Siemens), EPI device (EPID) attached to the linear accelerator machine for both medial and lateral tangential fields on the first day and 2 subsequent days thereafter for each patients, and the reference DRR image was superimposed on the EPIs to verify deviations in setup reproducibility in all the three directions (AP, SI, and ML) and deviations in CLD and lung involvement for the tangential fields at two different gantry angles of the coplanar tangential fields. The shifts in superimposed EPIs and the DRR images generated on the TPS 3D treatment plan were evaluated and noted as setup errors, and the dosimetric variations for OARs due to these setup errors were also calculated on TPS Oncentra [Fig. 4].
Fig. 4.
3D reference plan on treatment planning system (Oncentra™).
Definitions of setup deviations
Statistical analysis of the data generated was carried out on a total of 60 patients enrolled in this hospital-based cross-sectional observational dosimetric analysis. Two types of setup deviations were analyzed, one characterized as random deviation was related to the patient (motion while breathing or shift in arm position), and the second systemic deviation was due to the equipment (matching of treatment fields or coordinates). Mean and standard deviations (SDs) were used to characterize these setup deviations. They were calculated in accordance with previously published definitions by Troung et al12 Random deviations [σ] were defined as variations between fractions during a treatment series. This was calculated by estimating the deviations between fractions for each patient, calculating the mean of these readings, plotting the spread (1 SD) around the corresponding mean, followed by calculation of the average of these SDs for the whole group. Systematic deviations [σ] were defined as deviations between the planned position and the average position over the treatment course. These were obtained by estimating the deviations between the plan DRRs and the EPIs and calculating the spread (1 SD) of the individual means of these deviations.
Results
Demographic profile is shown in Table 1.
Table 1.
Demographic profile of patients.
| Age in years | 21–30 | 3 (5%) |
| 31–40 | 6 (10%) | |
| 41–50 | 24 (40%) | |
| 51–60 | 18 (30%) | |
| 61–70 | 6 (10%) | |
| 71–80 | 3 (5%) | |
| Sex | Male (M) | 1 |
| Female (F) | 59 | |
| Surgery (laterality) | After mastectomy (right-sided breast) | 27 (45%) |
| After mastectomy (left-sided breast) | 27 (45%) | |
| BCS (right-sided breast) | 3 (5%) | |
| BCS (left-sided breast) | 3 (5%) | |
| Stage | T1 stage | 3 |
| T2 stage | 30 | |
| T3 stage | 26 | |
| T4 stage | 1 | |
| N0 stage | 6 | |
| N1 stage | 36 | |
| N2 stage | 18 | |
| M1 stage | NIL |
Random and systematic errors
Isocentric variations
The deviation range in setup SSD and displacements in all three directions (AP, SI, and ML) for both sided patients on 3 consecutive treatment days is shown (Table 2).
Table 2.
Deviation range in setup SSD and displacements in three directions for breast cancer patients on 3 consecutive treatment days.
| Patient groups | Nos. of EPIs evaluated on 3 days | Setup SSD (actual on TPS plan) | Setup SSD (measured on EPIs) | Deviation in anterior-posterior (AP) | Deviation in superior-inferior (SI) | Deviation in medial-lateral (ML) |
|---|---|---|---|---|---|---|
| A (30 right sided) | 180 | 20–65 mm | 25–72 mm | 5–7 mm | 2–3 mm | 2–3 mm |
| B (30 left sided) | 180 | 15–70 mm | 20–80 mm | 5–10 mm | 2–3 mm | 2–3 mm |
EPIs, electronic portal images; TPS, treatment planning system.
Analysis of Group ‘A’ patients showed that the shift in setup SSD (shift in AP direction) was from 5 to 7 mm and from 5 to 10 mm for Group ‘B’ patients, and a shift of 2–3 mm was noted for both SI and ML directions. These isocentric deviations for all 60 patients regarding shifts in AP, SI, and ML directions have been statistically analyzed as random and systematic errors in all three directions (Table 3). The random errors ranged from 2 to 3 mm for the breast/chest wall (medial and lateral) tangential treatments and from 2 to 2.5 mm for the anterior supraclavicular nodal treatments. Systematic errors ranged from 3 to 5 mm in the AP direction for the tangential fields and from 2.5 to 5 mm in the SI and ML direction for the anterior supraclavicular nodal field.
Table 3.
Shifts in AP, SI, and ML directions during breast radiotherapy for LABC (random and systematic errors).
| Types of errors | Anterior supraclavicular nodal field |
Medial tangential breast/chest wall field |
Lateral tangential breast/chest wall field |
|||
|---|---|---|---|---|---|---|
| SI | ML | SI | AP | SI | AP | |
| Random errors (mm) | 2.0 | 2.5 | 2.5 | 3.0 | 2.0 | 2.0 |
| Systematic errors (mm) | 2.5 | 5.0 | 2.5 | 3.0 | 2.5 | 5.0 |
SI, superior-inferior; ML, medial-lateral; AP, anterior-posteriorl; LABC, locally advanced breast cancer.
Dosimetric variations
Deviations in CLD and dose variations in OARs due to setup errors: In the analysis of 360 EPIs and DRRs for deviation in CLD and dose variations in OARs due to aforementioned setup errors measured on TPS in all the three directions as per DVHs for tangential breast/chest wall fields of all the 60 patients, the CLD actually measured on TPS was between 20 and 25 mm for Group ‘A’ patients and CLD measured on EPIs was between 25 and 30 mm, showing variation of 5 mm, and the corresponding mean total lung dose V20 was between 0.69 and 3.96 Gy, maximum lung dose was 40.5 Gy, mean heart dose V30 was between 1.4 and 3 Gy, and maximum heart dose measured is 50.5 Gy. Similarly for Group ‘B’, the CLD actually measured on TPS was between 25 and 30 mm, and CLD measured on EPIs was between 30 and 40 mm, showing variation of 10 mm, and the corresponding mean total lung dose V20 was between 0.9 and 5.9 Gy, maximum lung dose was 45 Gy, mean heart dose V30 was between 2.4 and 4.1 Gy, and maximum heart dose measured is 55 Gy. Dosimetric variations are shown in Table 4.
Table 4.
Range of deviation in CLD and mean dose variations in OARs for both sided patients on 3 consecutive treatment days as per dose–volume histograms (DVHs).
| Patient groups | No. of EPIs and DRRs | CLD (actual on TPS plan) | CLD (measured on EPIs) | Mean total lung dose V20 (Gy) | Mean heart dose V30 (Gy) | Max lung dose (Gy) | Max heart dose (Gy) |
|---|---|---|---|---|---|---|---|
| A 30 (right sided) | 180 | 20–25 mm | 25–30 mm | 0.69–3.96 | 1.4–3 | 40.5 | 50.5 |
| B 30 (left sided) | 180 | 25–30 mm | 30–40 mm | 0.9–5.9 | 2.4–4.1 | 45.0 | 55.0 |
CLD, central lung distance; EPIs, electronic portal images; TPS, treatment planning system.
Discussion
Radiation therapy has been used in the treatment of carcinoma breast since early days.1, 2, 3 Recent advances in CT-based and intensity-modulated radiotherapy (IMRT) planning have potential to spare normal tissues, particularly the OARs.4, 5, 6, 7 The use of CT scans for planning of these patients enables us to virtually simulate the gantry angles of the linear accelerator (LA) and select the optimum gantry angles. Combining this with EPID images at the time of treatment delivery further smoothes the workflow in the department and ensures proper positioning and reduction in daily treatment setup errors.8, 9, 10 Thus, implementation of the increasingly complex techniques warrants efforts to evaluate setup verification to ensure accuracy and reproducibility in delivering the planned therapy. However, certain limitations are inherent in the process. Because we used the diagnostic CT available in our hospital, the bore of the CT scanner (65 cm) precludes the imaging of all patients in the treatment position with the shoulders abducted. Hence, we routinely image patients with the ipsilateral arm abducted beyond 90°. This leads to a change in arm position during the treatment of the supraclavicular field, if indicated.
The random and systematic errors in the tangential portion of LABC patients reported in our study are consistent with those in the literature.9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27 Lung and heart doses in these patients are estimated using the International Commission on Radiation Unit and Measurement (ICRU 50 and 62) guidelines.11 Studies have shown that random setup errors in CLD measured by EPIs are due to displacements in three SI, ML, and AP directions for the 3D-planned treatment, and in 90% of all patients, the variation of CLD was less than 10 mm on 3D-simulated plan, and it is the largest setup error noted.12, 13 There is a paucity of information on deviation in doses for OARs due to setup errors.14, 15, 16 Using online EPID imaging, the correction of patient position is improved significantly.17, 18
The doses to the OARs are correlated with toxicity associated with radiation therapy and tolerance tables that have been published for various organs. The tolerance dose TD (Gy)/volume (%) parameters to the lung and heart, i.e. volume receiving 20 Gy (V20) of lung should be ≤30% and volume receiving 30 Gy (V30) of heart should be ≤46% for symptomatic radiation pneumonitis and pericarditis (cardiac failure), respectively. However, the mean and maximum doses to these organs have also been correlated with toxicity. Mean doses of 7–27 Gy to the lung have been found to correlate with a rate of symptomatic pneumonitis between 5–40%, and mean dose to the pericardium (heart) <than 26 Gy has also been found to correlate with rates of pericarditis of less than 15%.27 In another study of estimating heart dose, Kong et al noted for left-sided breast cancer that the mean heart dose varied from <1–8 Gy and maximum dose from 5 to 50 Gy and for right-sided breast cancer that the mean heart dose was always <1 Gy and maximum dose to be < 5 Gy. They have also suggested that CLD is also a measure of TD28, and when CLD variation was ≤5 mm, the formulae by Lorenzen et al showed a mean lung dose increase of no more than 2 Gy with <5% variation at the 20 Gy dose level due to AP movement.29, 30 Our data correlated well with the those of published literature with mean lung dose V20 (Gy) between 0.69–3.96 Gy for right side (5.8%) and 0.9–5.9 Gy for left side (7%) and mean heart doses V30 (Gy) between 1.4–3 Gy for right side and 2.4–4.1 Gy for left side with CLD variation between 5 mm (right sided) and 10 mm (left sided). Hence, CLD is an acceptable surrogate to the total volume of the lung and heart receiving high doses and hence for long-term toxicity associated with radiotherapy to the chest wall and thoracic cavity.
The present study was planned to evaluate dose to OARs due to setup errors during EBRT of breast to generate data for our patient population. This report is among the first in our setup to document the magnitude and directions of setup deviations in LABC for breast cancer in the era of CT-based planning using defined protocols of patient immobilization and EPI.
Conclusion
Online assessment of patient position with matching of EPIs with DRRs is a useful method in evaluation of interfraction reproducibility of tangential fields in breast irradiation, thereby improving on the quality of treatment delivery for our patient population. This simple technique will help to minimize toxicities associated with irradiation of the OARs (heart and lung) as this can be a useful surrogate for the volume of OAR being irradiated. Image-guided radiotherapy protocols including daily imaging and breath-holding techniques as well as the use of advanced techniques of radiation delivery such as IMRT will help to further decrease the long-term toxicities associated with radiotherapy for breast cancers. However, long-term follow-up of these patients is advocated.
Conflicts of interest
The authors have none to declare.
Acknowledgment
This paper is based on Armed forces Medical Research Committee Project No 4495/2014 granted and funded by office of the Directorate General Armed Forces Medical Services and Defence Research Development Organization, Government of India.
References
- 1.Saranath D., Khanna A. Current status of cancer burden: global and Indian scenario. Biomed Res J. 2014;1(1):1–5. [Google Scholar]
- 2.Lichter A.S., Fraas B.A., Yanke B. Treatment techniques in the conservative management of breast cancer. Semin Radiat Oncol. 1992;2:94. doi: 10.1053/SRAO00200094. [DOI] [PubMed] [Google Scholar]
- 3.Das I.J., Cheng E.C., Freedman G., Fowble B. Lung and heart dose volume analyses with CT simulator in radiation treatment of breast cancer. Int J Radiat Oncol Biol Phys. 1998;4(2):11–19. doi: 10.1016/s0360-3016(98)00200-4. [DOI] [PubMed] [Google Scholar]
- 4.Li J.G., Williams S.S., Goffinet D.R., Boyer A.L., Zing Lei. Breast conserving breast radiation therapy using combined electron and IMRT techniques. Radiother Oncol. 2000;56:65–71. doi: 10.1016/s0167-8140(00)00189-4. [DOI] [PubMed] [Google Scholar]
- 5.Herman M.G., Kruse J.J., Hagness C.R. Guide to clinical use of electronic portal imaging. J Appl Clin Med Phys. 2000;1:38–57. doi: 10.1120/jacmp.v1i2.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Creutzberg C.L., Althof V.G., Huizenga H., Visser A.G., Levendag P.C. Quality assurance using portal imaging: the accuracy of patient positioning in irradiation of breast cancer. Int J Radiat Oncol Biol Phys. 1993;25:529–539. doi: 10.1016/0360-3016(93)90077-9. [DOI] [PubMed] [Google Scholar]
- 7.Fein D.A., McGee K.P., Schultheiss T.E., Fowble B.C., Hanks G.E. Intra- and interfractional reproducibility of tangential breast fields:a prospective on-line portal imaging study. Int J Radiat Oncol Biol Phys. 1996:733–740. doi: 10.1016/0360-3016(95)02037-3. [DOI] [PubMed] [Google Scholar]
- 8.Lirette A., Pouliot H., Aubin M., Larochelle M. The role of electronic portal imaging in tangential breast irradiation: a prospective study. Radiother Oncol. 1995;37:241–245. doi: 10.1016/0167-8140(95)01653-8. [DOI] [PubMed] [Google Scholar]
- 9.Valdagni R., Italia C. Early breast cancer irradiation after conservative surgery: quality control by portal localization films. Radiother Oncol. 1991;33:211–213. doi: 10.1016/0167-8140(91)90169-h. [DOI] [PubMed] [Google Scholar]
- 10.Pierce L.J., Butler J.B., Martel M.K. Postmastectomy radiotherapy of the chest wall: dosimetric comparison of common techniques. Int J Radiat Oncol Biol Phys. 2000;52:1220–1230. doi: 10.1016/s0360-3016(01)02760-2. [DOI] [PubMed] [Google Scholar]
- 11.ICRU International Commission on Radiation Units and Measurements, Bethesda 1985(50) and ICRU 62- Supplement of ICRU (50).
- 12.Troung P.T., Berthelet E., Patenaude V. Set up variations in locoregional radiotherapy for breast cancer: an electronic portal imaging study. Br J Radiol. 2005;78:742–745. doi: 10.1259/bjr/11782857. [DOI] [PubMed] [Google Scholar]
- 13.Hurkmans C.W., Remeijer P., Lebesque J.V., Mijnheer B.J. Set up verification using portal imaging review of current clinical practice. Radiother Oncol. 2001;58:105–120. doi: 10.1016/s0167-8140(00)00260-7. [DOI] [PubMed] [Google Scholar]
- 14.van Mourik A., van Kranen S., den Hollander S., Sonke J.J., vanHerk M., van Vliet-Vroegindeweij C. Effects of set up errors and shape changes on breast radiotherapy. Int J Radiat Oncol Biol Phys. 2011;79(5):1557–1564. doi: 10.1016/j.ijrobp.2010.07.032. [DOI] [PubMed] [Google Scholar]
- 15.Prabhakar R., Rath G.K., Julka P.K. Simulation of dose to surrounding normal structures in tangential breast radiotherapy due to set up error. Med Dosim. 2008;33(1):81–85. doi: 10.1016/j.meddos.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 16.Das I.J., Cheng Chee-W., Helen Fosmire, Kase Kenneth R., Fitzgerald Thomas J. Tolerances in set up and dosimetric errors in the radiation treatment of breast cancer. Int J Radiat Oncol Biol Phys. 1993;26(5):883–890. doi: 10.1016/0360-3016(93)90505-p. [DOI] [PubMed] [Google Scholar]
- 17.Krishna M.K., Al-Rahbi Z., Sivakumar S.S., Davis C.A., Ravichandran R., Ghamrawy K.E. Verification of set up errors in external beam radiation therapy using electronic portal imaging. J Med Phys. 2008;33(2):49–53. doi: 10.4103/0971-6203.41192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitine C., Dutreix A., van der Schuren E. Tangential breast irradiation: influence of technique of set-up on transfer errors and reproducibility. Radiother Oncol. 1991;22:308–310. doi: 10.1016/0167-8140(91)90168-g. [DOI] [PubMed] [Google Scholar]
- 19.Thilmann C., Adamietz I.A., Saran F., Mose S., Kostka A., Bottcher H.D. The use of a standardized positioning support cushion during daily routine of breast irradiation. Int J Radiat Oncol Biol Phys. 1998;41:459–463. doi: 10.1016/s0360-3016(98)00022-4. [DOI] [PubMed] [Google Scholar]
- 20.Pouliot T., Lirette A. Verification and correction of setup deviations in tangential breast irradiation using EPID: gain versus workload. Med Phys. 1995;23:1393–1398. doi: 10.1118/1.597722. [DOI] [PubMed] [Google Scholar]
- 21.Dutreix A. When and how can we improve precise in radiotherapy? Radiother Oncol. 1984;2:275–292. doi: 10.1016/s0167-8140(84)80070-5. [DOI] [PubMed] [Google Scholar]
- 22.Van Tienhoven G., Lanson J.H., Cerbeels D., Henkelom S., Mijnheer B.J. Accuracy in tangential breast treatment setup: a portal imaging study. Radiother Oncol. 1991;22:317–322. doi: 10.1016/0167-8140(91)90171-c. [DOI] [PubMed] [Google Scholar]
- 23.Pradier O., Schmidberger H., Weiss E., Bouscayrol H., Daban A., Hess C.F. Accuracy of alignment in breast. Br J Radiol. 1999;72:685–690. doi: 10.1259/bjr.72.859.10624326. [DOI] [PubMed] [Google Scholar]
- 24.Nalder C.A., Bidmead A.M., Mubata C.D., Tait D., Beardmore C. Influence of a vac-fix immobilization device on the accuracy of patient positioning during routine breast radiotherapy. Br J Radiol. 2001;74:249–254. doi: 10.1259/bjr.74.879.740249. [DOI] [PubMed] [Google Scholar]
- 25.Jakobsen A., Iversen P., Gadelberg C., Hansen J.L., Hjem Hansen M. A new system for patient fixation in radiotherapy. Radiother Oncol. 1987;8:145–151. doi: 10.1016/s0167-8140(87)80168-8. [DOI] [PubMed] [Google Scholar]
- 26.Philips Leibel. 2nd ed. 2004. Textbook of Radiation Oncology; pp. 1299–1333. [Google Scholar]
- 27.Marks L.B., Yorke E.D., Jackson A. Use of NTCP models in the clinic. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S10–S19. doi: 10.1016/j.ijrobp.2009.07.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kong F.M., Klein E.E., Bradley J.D. The impact of central lung distance, maximal heart distance, and radiation technique on the volumetric dose of the lung and heart for intact breast radiation. Int J Radiat Oncol Biol Phys. 2002;54:963–971. doi: 10.1016/s0360-3016(02)03741-0. [DOI] [PubMed] [Google Scholar]
- 29.Jones S., Fitzgerald R., Owen R., Ramsay J. Quantifying intra and inter fractional motion in breast radiotherapy. J Med Rad Sci. 2015;62:40–46. doi: 10.1002/jmrs.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lorenzen E.L., Brink C., Taylor C.W., Darby S.C., Ewertz M. Uncertainties in estimating heart doses from 2D-tangential breast cancer radiotherapy. Radiother Oncol. 2016;119:71–76. doi: 10.1016/j.radonc.2016.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]