Abstract
Background
The aim of this study was to evaluate the expression of p53, p16, Wilms tumor gene (WT1), and Mindbomb E3 Ubiquitin Protein Ligase 1 (MIB-1) index by immunohistochemical (IHC) staining in benign, low-grade, and high-grade serous ovarian tumors.
Methods
Forty-one cases of ovarian serous tumors were included in the study (benign serous tumor [n = 10], low-grade ovarian serous carcinoma [n = 8], and high-grade ovarian serous carcinoma [n = 23]). Expression of p53, p16, WT1, and MIB-1 by IHC was evaluated statistically with the grade of tumor. Semiquantitative scoring system for percentage (0–5) and intensity (1–3) of staining pattern was used to bring about objectivity.
Results
p53, p16, and WT1 showed significantly higher staining scores in ovarian serous carcinoma group than in the benign group (p < 0.05). However, p16 score was not significant in benign versus low-grade tumors. In the carcinoma group, the high-grade serous tumors showed significantly higher staining scores of p53, p16, and WT1 than the low-grade serous tumors (p < 0.05). Papillary serous tumors had comparatively lower p53 and WT1 scores for the same grade of tumor. MIB-1 scores were not significant.
Conclusion
p53, p16, and WT1 are helpful for the subtyping of serous ovarian tumors as low grade and high grade. WT1 is helpful in establishing primary ovarian serous tumors. The combination of moderate-to-high p53 and WT1 scores provides a robust way of confirming high-grade tumors.
Keywords: Immunohistochemistry (IHC), Ovary, Serous carcinoma
Introduction
Ovarian cancer is among the top five cancers affecting Indian women. In a study period from 2001 to 2006, the incidence rates (standardized for age) had a range from 0.9 to 8.4 per 100,000 people.1, 2 The incidence of ovarian cancer in India is lower than the rates in the western world. In spite of this, owing to poor awareness, long relatively asymptomatic latency period, and inadequate diagnostic infrastructure, most women present late in advanced stages of the disease,3 resulting in poor overall survival.
Ovarian carcinomas have conventionally been classified only on the basis of the histology of the primary cell type.4 This is the method that is currently followed in the World Health Organization's classification of ovarian carcinoma.5
Of late, a binary pathway for development of ovarian serous tumors has been proposed based on the grade of tumor. Accordingly, high-grade ovarian serous carcinoma (HOSC) seems to directly arise as cancer from the ovarian surface epithelium or at the fimbrial end of the fallopian tubes or from the epithelium of the cortical inclusion cysts.6, 7 These tumors do not yet have a definitive precursor lesion and are strongly associated with mutations of the p53 tumor suppressor gene.6, 7, 8, 9 Low-grade ovarian serous carcinoma (LOSC), on the other hand, seems to evolve from a benign cystadenoma through the stage of a borderline serous tumor in a sequential step fashion. The LOSC seems to have more mutations in B-RAF and K-RAS genes than in p53 gene than the HOSC.10, 11 Thus, a different set of genes and entirely different pathways seem to be involved in the different grades of ovarian serous carcinoma, and it does not seem to be a simple step-wise gradient as previously thought.
This study aims to objectively evaluate the expression of p53, p16, Wilms tumor gene (WT1) protein antigen, and Mindbomb E3 Ubiquitin Protein Ligase 1 (MIB-1) staining index by immunohistochemistry (IHC) technique in benign, low-grade, and high-grade serous ovarian tumors.
Materials and methods
Forty-one cases of ovarian serous tumors were included in the study. All specimens were fixed in 10% buffered neutral formalin and processed in paraffin wax. The histological type was confirmed by reviewing the hematoxylin/eosin-stained slides. The tumor was graded into either high-grade (HOSC) or low-grade (LOSC) type, using the two-tier system according to the scoring system recommended by Malpica et al.,12 instead of the conventional three-tier system. This system has been shown to have a high interobserver reproducibility and thus was adopted in this study.12, 13
Immunohistochemical methods
Blocks with representative and adequate viable tumor tissue were selected for IHC analysis. For the purpose of immunostaining, 3- to 4-μm-thick serial sections were obtained on poly-l-lysine–coated glass slides and were dried overnight at 37°C. Pretreatment with heat-induced epitome retrieval was carried out using an antigen retrieval system (Decloaking Chamber, Biocare medical) in 0.01 M citrate buffer (pH 6.0). Sections were treated with two drops of 3% hydrogen peroxide for 15–20 min to block the endogenous peroxidases, followed by protein blocking using ultra block of the universal staining kit for 5 min.
Immunostaining for anti-p53 Protein (BP53-12 clone, RTU; BioGenex, Fremont, CA, USA), Monoclonal Mouse anti-human Ki67 antigen (clone MIB-1, RTU, Dako; Agilent Technologies, Santa Clara CA, USA), Monoclonal Mouse anti-human WT1 protein (clone 6F-H2, RTU, Dako; Agilent Technologies, Santa Clara CA, USA), and anti-p16 [INK4] (cloneG175-405, RTU, BioGenex, Fremont, CA, USA) was performed.
Indirect biotin-streptavidin–amplified staining (Super SensitiveTM Link-label IHC Detection system, BioGenex, Fremont, CA, USA) followed by diamino benzidine visualization and hematoxylin counterstaining were performed. With each batch, appropriate positive and negative controls (omitting the primary antibody) were also run. IHC slides were reviewed, and semiquantitative scoring was performed by two pathologists.
For a negative control, the primary antibody was omitted and replaced with immunoglobulin (IgG1; BioGenex, Fremont, CA, USA) at an equivalent concentration.
Immunohistochemical analysis
Two pathologists evaluated the distribution and intensity of immunostaining independently, and the scores were averaged for each case. For assessing the distribution pattern of the immunostain, the cases were scored semiquantitatively as per a scoring system devised by O'Neill et al.7, 9 Briefly, a score of 0–5 was assigned based on the percentage of positive cells with zero score for negative or occasional positive cells and the maximum score of 5 when more than 75% cells were positive. Intensity of immunostain was evaluated in the areas of strongest positivity in all positive cases. They were subjectively assigned a score of 1–3 based on weak, moderate, or strong staining pattern (as compared to reference staining intensity for the particular stain). In case of disagreement, the slide was reevaluated, and the higher score was taken. Finally, an immunohistochemical composite score (ICS) was calculated by multiplying the distribution and intensity scores (possible scores from 0 to 15). Intensity, distribution, and ICS were calculated for the parameters WT1, p16, and p53. For MIB-1, the percentage of positive nuclei was calculated in 100 nuclei count in the best-stained area of the slide (400×).
Statistical analysis
SPSS (IBM SPSS Statistics for window, version 20.0; IBM Crop, Armonk, NY) was used to analyze the data. Briefly, to assess whether there was a significant difference in the various parameters in the three types of ovarian lesions, namely the benign, low grade, and high grade, one-way analysis of variance (ANOVA) was used. The test of homogeneity was carried out, and wherever the homogeneity test failed, instead of one-way ANOVA, non-parametric equivalent Kruskal–Wallis test was used. The pair-wise comparison for significant ANOVA results was carried out by post hoc Bonferroni test. A 5% level of significance was assumed.
The study was approved by the constituted institution ethics committee.
Results
Forty-one cases of ovarian serous tumors were included in the study. There were 10 benign serous tumors, 8 LOSCs, and 23 HOSCs. The mean ages of benign serous tumors were 42 years (range 19–70 years). The mean ages of LOSCs were 50 (range 30–65) years, and the mean ages of HOSCs were 55 (range 40–74) years.
The IHC results for distribution and intensity are given in Table 1, Table 2, respectively. Visual scoring method was carried out as described earlier (Fig. 1). Hematoxylin and eosin (H&E) staining, IHC distribution, and patterns for the various groups are shown in (Fig. 2, Fig. 3, Fig. 4). The mean values of scores for distribution, intensity, and composite score are as per Table 3. The scores were clearly higher in the HOSC group for all the parameters evaluated. There were statistically significant differences in p16, p53, and WT1 in the distribution, intensity, and composite score between the benign and HOSC groups as well as the LOSC and HOSC groups [Table 4]. As a group, the HOSC exhibited a stronger and more diffuse immune staining. Most HOSCs exhibited diffuse p16 positivity with 11 of 23 cases having a distribution score of 5+ (>75% cells positive). In p53 stains, the immunoreactivity was nuclear. In case of p53 positivity, most HOSCs exhibited strong staining with nine of 23 cases having a distribution score of 5+ (>75% cells positive). In case of WT1 staining, most HOSCs exhibited strong staining (52.2%) with 39% of cases showed 4+ or greater staining distribution. MIB-1 scores were zero in benign cases. Only one LOSC showed staining with a 16% count. In HOSCs, 12 cases (52.2%) showed MIB-1 positivity (range of 2%–90%). However, there were no significant differences in p16, p53, and WT1 scores between the benign and LOSC groups.
Table 1.
Immunohistochemistry results with regard to p16, p53, and WT1 staining distribution pattern in benign, low-grade, and high-grade serous ovarian tumors.
| Distribution | Benign (n = 10) |
LOSC (n = 8) |
HOSC (n = 23) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| p16 | p53 | WT1 | p16 | p53 | WT1 | p16 | p53 | WT1 | |
| 0 | 3 (30%) | 9 (90%) | 8 (80%) | 0 | 4 (50%) | 3 (37.5%) | 1 (4.35%) | 3 (13.04%) | 0 |
| 1 | 1 (10%) | 1 (10%) | 2 (20%) | 3 (37.5%) | 2 (25%) | 5 (62.5%) | 1 (4.35%) | 1 (4.35%) | 4 (17.39%) |
| 2 | 5 (50%) | 0 | 0 | 4 (50%) | 2 (25%) | 0 | 0 | 2 (8.70%) | 6 (26.09%) |
| 3 | 1 (10%) | 0 | 0 | 0 | 0 | 0 | 4 (17.39) | 5 (21.74%) | 4 (17.39%) |
| 4 | 0 | 0 | 0 | 1 (12.5%) | 0 | 0 | 6 (26.09%) | 3 (13.04%) | 4 (17.39%) |
| 5 | 0 | 0 | 0 | 0 | 0 | 0 | 11 (47.83%) | 9 (39.13%) | 5 (21.73%) |
Score 0, negative or occasional positive cells; 1+, <10% cells positive; 2+, 10–25% cells positive; 3+, 26–50% cells positive; 4+, 51–75% cells positive; 5+, >75% cells positive. n, number of cases; HOSC, high-grade ovarian serous carcinoma; LOSC, low-grade ovarian serous carcinoma.
Table 2.
Immunohistochemistry results with regard to p16, p53, and WT1 staining intensity in benign, low-grade, and high-grade serous ovarian tumors.
| Intensity | Benign (n = 10) |
LOSC (n = 8) |
HOSC (n = 23) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| p16 | p53 | WT1 | p16 | p53 | WT1 | p16 | p53 | WT1 | |
| Negative (0) | 3 (30%) | 9 (90%) | 8 (80%) | 0 | 4 (50%) | 3 (37.5%) | 1 (4.35%) | 3 (13.04%) | 0 |
| Weak (1+) | 3 (30%) | 1 (10%) | 2 (20%) | 5 (62.5%) | 2 (25%) | 5 (62.5%) | 1 (4.35%) | 1 (4.35%) | 5 (21.74%) |
| Mod (2+) | 4 (40%) | 0 | 0 | 3 (37.5%) | 1 (12.5%) | 0 | 6 (26.09%) | 8 (34.78%) | 6 (26.1%) |
| Strong (3+) | 0 | 0 | 0 | 0 | 1 (12.5%) | 0 | 15 (65.22%) | 11 (47.83%) | 12 (52.2%) |
LOSC, low-grade ovarian serous carcinoma; HOSC, high-grade ovarian serous carcinoma; n, number of cases.
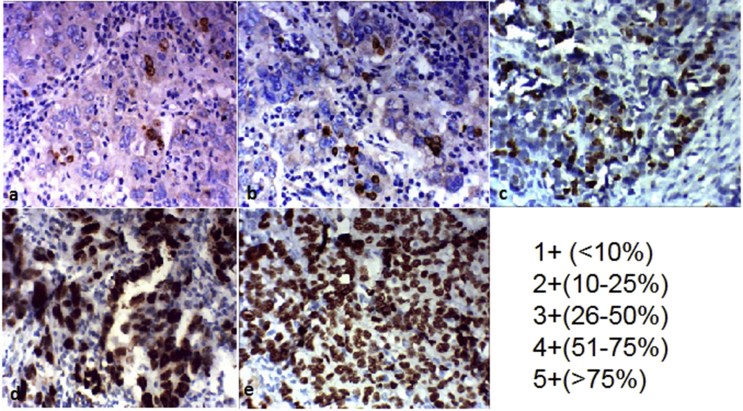
Fig. 1.
Visual scoring system based on distribution pattern and percentage of positive cells (browncolour) on the immunostained slide. (a) Less than 10% positive cells and scored as 1+, (b) 10-25% positive cells and scored as 2+, (c) 26-50% positive cells and scored as 3+, (d) 51-75% positive cells and scored as 4+, (e) More than 75% positive cells and scored as 5+.
Fig. 2.
Staining pattern in benign ovarian neoplasms. (A) Haematoxylin and eosin (H&E) stain at 400 x magnification, (B and C) IHC staining for P16 at 100x and 200x, (D, E & F) Negative IHC staining pattern for p53,WT1(200x) and MIB-1 (400x) respectively.
Fig. 3.
Staining pattern in low-grade serous ovarian neoplasms. (A) Haematoxylin and eosin (H&E) stain at 400 x magnification, (B and C) IHC staining for P16 at 100x and 400x, (D, E & F) IHC staining pattern for p53 (100x),WT1 (200x) and MIB-1 (200x) respectively.
Fig. 4.
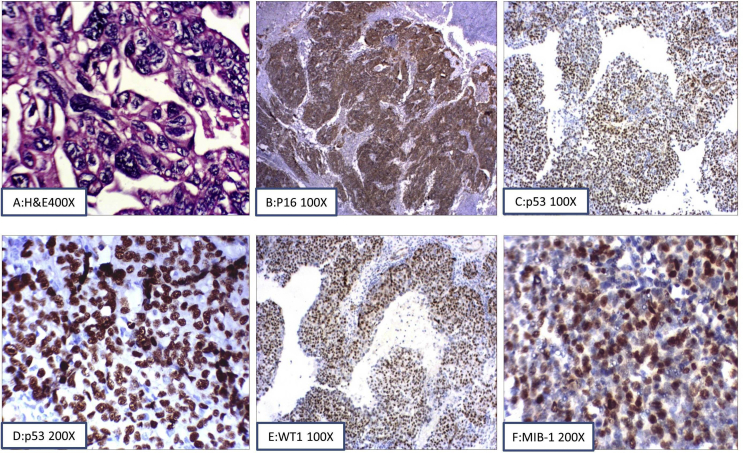
Staining pattern in high-grade serous ovarian neoplasms. (A) Haematoxylin and eosin (H&E) stain at 400 x magnification), (B) Positive IHC staining for P16 at 100x, (C and D) Positive IHC staining pattern for p53 (100x and 200x), (E) Positive IHC staining pattern for WT1 (100x) and (F) Partial positive IHC staining for MIB-1 (200x).
Table 3.
Mean value with standard deviation of various scores in the three groups.
| Parameter | Benign (n = 10) | LOSC (n = 8) | HOSC (n = 23) |
|---|---|---|---|
| p16 intensity | 1.10 ± 0.88 | 1.38 ± 0.52 | 2.52 ± 0.79 |
| p16 distribution | 1.40 ± 1.08 | 1.88 ± 1.00 | 4 ± 1.35 |
| P16 index | 2.20 ± 1.82 | 2.88 ± 2.42 | 10.91 ± 4.62 |
| p53 intensity | 0.10 | 0.88 ± 1.13 | 2.17 ± 1.03 |
| p53 distribution | 0.10 | 0.75 ± 0.98 | 3.35 ± 1.87 |
| P53 index | 0.10 | 1.50 ± 2.36 | 8.96 ± 5.82 |
| WT1 intensity | 0.20 ± 0.42 | 0.63 ± 0.52 | 2.30 ± 0.82 |
| WT1 distribution | 0.20 ± 0.42 | 0.63 ± 0.52 | 3 ± 1.45 |
| WT1 index | 0.20 ± 0.42 | 0.63 ± 0.52 | 7.87 ± 5.17 |
| MIB-1 score | 0.00 | 2.00 | 13.87 ± 24.16 |
LOSC, low-grade ovarian serous carcinoma; HOSC, high-grade ovarian serous carcinoma; n, number of cases.
Table 4.
ANOVA scores between various groups with significance with regard to p16, p53, and WT1 staining scores.
| Parameter | Group | p value | Parameter | Group | p value | Parameter | Group | p value |
|---|---|---|---|---|---|---|---|---|
| p16 distribution | 1 & 2 | 0.456 | P53 distribution | 1 & 2 | 0.088 | WT1 distribution | 1 & 2 | 0.205 |
| 1& 3 | <0.001a | 1& 3 | <0.001a | 1& 3 | <0.001a | |||
| 2 & 3 | 0.001a | 2 & 3 | 0.002a | 2 & 3 | <0.001a | |||
| p16 intensity | 1 & 2 | 0.420 | p53 intensity | 1 & 2 | 0.337 | WT1 intensity | 1 & 2 | 0.437 |
| 1& 3 | <0.001a | 1& 3 | <0.001a | 1& 3 | <0.001a | |||
| 2 & 3 | <0.001a | 2 & 3 | <0.001a | 2 & 3 | <0.001a | |||
| p16 index | 1 & 2 | 0.708 | P53 index | 1 & 2 | 0.205 | WT1 index | 1 & 2 | 0.821 |
| 1& 3 | <0.001a | 1& 3 | <0.001a | 1& 3 | <0.001a | |||
| 2 & 3 | <0.001a | 2 & 3 | <0.001a | 2 & 3 | <0.001a |
Group 1, benign; Group2, low-grade serous ovarian carcinoma (LOSC); Group 3, high-grade serous ovarian carcinoma (HOSC); ANOVA, analysis of variance.
difference is significant.
Discussion
A binary pathway of origin and development between LOSC and HOSC types of ovarian serous carcinoma is being established with many supporting studies.6 The two tumor types also seem to exhibit distinct, clinical, morphological, immunohistochemical, and molecular features. It is important to stress that as per the current evidence, LOSC and HOSC can no longer be considered different grades of the same neoplasm but should be considered as two distinct neoplasms with a different underlying pathogenesis.8, 9, 10 This is important as the basis of the chemotherapy in these tumors is based on the underlying dysfunction in the apoptotic pathway.
Meanwhile, on a more practical front, the dual grading system specifically for serous carcinoma, in which tumors are subdivided into low grade and high grade, has been proposed.12 It has also been shown in studies that this dual grading system is easy to use, more robust, reproducible, and, importantly, is based on underlying molecular differences between LOSC and HOSC.13, 14
The aim of this study was to assess p16, p53, and WT1 staining patterns and MIB-1 expression in a histomorphologically well-characterized series of both LOSC and HOSC in an objective and standardized method that is reproducible. We also included few benign serous tumors because these are often associated with and are thought to be the precursor of many LOSCs.
p16 expression
p16 is a cyclin-dependent kinase-IV inhibitor that mediates its action through inhibitory effect on the cell cycle.15 Various studies have shown that p16 is well expressed in serous ovarian carcinomas and that its expression is generally higher in high-grade tumors.16, 17, 18 However, there was no significant difference in p16 scores between the borderline and LOSC groups.17
Our study has shown similar findings to the previously quoted studies, with regard to p16 staining pattern. The scores, distribution, and intensity were lower in benign tumors and progressively increased in LOSC and HOSC. In spite of some overlap in the extent of staining, HOSCs mostly exhibited diffuse and strong expression of p16 in comparison to LOSCs. The difference in all the p16 parameters were significant between the LOSC and HOSC but not significant between benign and LOSC tumors [Table 4].
p53 expression
In our study, we found p53 staining to be a robust marker of HOSC. Overall, 52% of HOSC showed 4 + scores (39% of the showed 5 + score) in distribution with 48.7% showing strong (3+) staining pattern. Only in four of 23 cases were the scores low. Other studies have shown similar results.7, 19, 20
The difference in all the p53 parameters was significant between the LOSC and HOSC but not significant between benign and LOSC tumors. Interestingly, the three HOSC cases that did not show significant p53 positivity had high p16 and or WT1 scores; probably these had evolved from low-grade tumors or had additional mutations in tumor suppressor pathways other than p53 but morphologically were high-grade lesions. Morphologically high-grade tumors with weak or absent p53 scores may also be due to novel or missense mutations that were not picked up by the antibody used.
WT1 expression
WT1 expression by IHC is observed in approximately 90% of primary ovarian carcinomas and can be used as a diagnostic marker.21 Serous ovarian tumors, in particular, seem to show a higher expression of WT1 than other types of ovarian tumors. Metastatic tumour deposits from unknown primary tumours elsewhere, WT1 is useful to distinguish carcinoma of ovarian origin from carcinoma from other primary sites.22
In our study, all the HOSC tumors stained positive for WT1. Most exhibited strong staining (52.2%) with 39% of cases showed 4 + or greater staining distribution. Five of eight LOSCs were positive, but all had low scores. Interestingly, all four cases of HOSCs which had low scores and three LOSCs with absent staining were papillary adenocarcinomas. The differences in all WT1 parameters were significant between the LOSC and HOSC lesions.
MIB-1 (Ki 67) expression
The immunoexpression of MIB-1 has become a surrogate marker of the proliferative potential of a tumor. Thus, a higher expression correlates with a poor prognosis in several cancers, including ovarian.23 In their study, O'Neill et al. showed that 14% of LOSC and 64% of HOSC had an MIB-1 index of more than 50%. These findings indicate that a morphologically problematic serous carcinoma with a high MIB-1 proliferation index is unlikely to be low grade.7
In our study, MIB-1 scores were zero in benign cases. Only one LOSC case showed staining with a 16% count. In HOSC, 12 cases (52.17%) showed MIB-1 positivity (range of 2%–90%). Only two of 23 cases had an MIB-1 index >50%. We did not find MIB-1 to be a useful marker in our study.
Conclusion
In our study, we have shown that WT1 is a good marker for serous ovarian carcinoma, and in combination with p53 expression patterns, it is a robust way to confirm high-grade tumors. Interestingly, pure papillary neoplasms seem to have comparatively lower p53 and WT1 scores for the same grade of tumor. We have also shown that both the percentage of cells and the staining intensity are of value and have introduced a novel and easy method of creating a scoring index. Our p16 staining pattern is similar to other studies, and we also predict that it is an early event in the development of these neoplasms. We did not find any meaningful use of the MIB-1 staining in our cases.
Conflicts of interest
The authors have none to declare.
References
- 1.National institute of cancer prevention and research. Available from: http://cancerindia.org.in/cp/index.php/know-about-cancer/statistics#cancer-statistics.
- 2.Murthy N.S., Shalini S., Suman G., Pruthvish S., Mathew A. Changing trends in incidence of ovarian cancer – the Indian scenario. Asian Pac J Cancer Prev. 2009;10:1025. [PubMed] [Google Scholar]
- 3.Maheshwari A., Kumar N., Mahantshetty U. Gynecological cancers: a summary of published Indian data. South Asian J Cancer. 2016;5:112–120. doi: 10.4103/2278-330X.187575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seidman J.D., Cho K.R., Ronnett B.M., Kurman R.J. Surface epithelial tumors of the ovary (Chapter 14) In: Kurman R.J., Ellenson L.H., Ronnett B.M., editors. Blaustein's Pathology of the Female Genital Tract. 6th ed. Springer; New York: 2011. pp. 680–772. [Google Scholar]
- 5.Kurman R.J., Carcangiu M.L., Herrington S., Young R.H. vol. 4. IARC; Lyon, France: 2014. (WHO Classification of Tumours of Female Reproductive Organs). [Google Scholar]
- 6.Shih I.M., Kurman R.J. Ovarian tumorigenesis: a proposed model based on morphological and molecular genetic analysis. Am J Pathol. 2004;164:1511–1518. doi: 10.1016/s0002-9440(10)63708-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Neill C.J., Deavers M.T., Malpica A., Foster H., McCluggage W.G. An immunohistochemical comparison between low-grade and high-grade ovarian serous carcinomas: significantly higher expression of p53, MIB1, BCL2, HER-2/neu, and C-KIT in high-grade neoplasms. Am J Surg Pathol. 2005;29(8):1034–1041. [PubMed] [Google Scholar]
- 8.Singer G., Stöhr R., Cope L. Patterns of p53 mutations separate ovarian serous borderline tumors and low and high-grade carcinomas and provide support for a new model of ovarian carcinogenesis: a mutational analysis with immunohistochemical correlation. Am J Surg Pathol. 2005;29(8):218–224. doi: 10.1097/01.pas.0000146025.91953.8d. [DOI] [PubMed] [Google Scholar]
- 9.O'Neill C.J., McBride H.A., Connolly L.E., Deavers M.T., Malpica A., McCluggage W.G. High-grade ovarian serous carcinoma exhibits significantly higher p16 expression than low-grade serous carcinoma and serous borderline tumour. Histopathology. 2007;50:773–779. doi: 10.1111/j.1365-2559.2007.02682.x. [DOI] [PubMed] [Google Scholar]
- 10.Sieben N.L.G., Macropoulos R., Roemen G.M.J.M. In ovarian neoplasms, BRAF, but not KRAS, mutation are restricted to lowgrade serous tumors. J Pathol. 2004;202:336–340. doi: 10.1002/path.1521. [DOI] [PubMed] [Google Scholar]
- 11.Singer G., Kurman R.J., Chang H.W., Cho S.K.R., Shih I.-M. Diverse tumorigenic pathways in ovarian serous carcinoma. Am J Pathol. 2002;160:1223–1228. doi: 10.1016/s0002-9440(10)62549-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malpica A., Deavers M.T., Lu K. Grading ovarian serous carcinoma using a two-tier system. Am J Surg Pathol. 2004;28:496–504. doi: 10.1097/00000478-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Malpica A., Deavers M.T., Tornos C. Interobserver and intraobserver variability of a two-tier system for grading ovarian serous carcinoma. Am J Surg Pathol. 2007;31:1168–1174. doi: 10.1097/PAS.0b013e31803199b0. [DOI] [PubMed] [Google Scholar]
- 14.Ayhan A., Kurman R.J., Vang R., Logani S., Seidman J.D., Shih I.M. Defining the cut-point between low- and high-grade ovarian serous carcinomas: a clinicopathologic and molecular genetic analysis. Am J Surg Pathol. 2009;33(8):1220–1224. doi: 10.1097/PAS.0b013e3181a24354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Milea Anca, George Sophia HL., Matevski Donco. Retinoblastoma pathway deregulatory mechanisms determine clinical outcome in high-grade serous ovarian carcinoma. Mod Pathol. 2014;27:991–1001. doi: 10.1038/modpathol.2013.218. [DOI] [PubMed] [Google Scholar]
- 16.Ferguson D.C., Long D.J., Smith M.C. Comparative analysis of Rb1, P16 and ER as diagnostic, prognostic and potential targets for therapeutic agents in ovarian epithelial tumors: an immunohistochemical study of 130 ovarian carcinomas. J Ovarian Res. 2015;8:34. doi: 10.1186/s13048-015-0163-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Neill C.J., McBride H.A., Connolly L.E., Deavers M.T., Malpica A., McCluggage W.G. High-grade ovarian serous carcinoma exhibits significantly higher p16 expression than low-grade serous carcinoma and serous borderline tumour. Histopathology. 2007;50:773–779. doi: 10.1111/j.1365-2559.2007.02682.x. [DOI] [PubMed] [Google Scholar]
- 18.Shandiz F.H., Kadkhodayan S., Ghaffarzadegan K., Esmaeily H., Torabi S., Khales S.A. The impact of p16 and HER2 expression on survival in patients with ovarian carcinoma. Neoplasma. 2016;63(5):816–821. doi: 10.4149/neo_2016_520. [DOI] [PubMed] [Google Scholar]
- 19.Köbel M., Piskorz A.M., Lee S. Optimized p53 immunohistochemistry is an accurate predictor of TP53 mutation in ovarian carcinoma. J Path Clin Res. 2016;2:247–258. doi: 10.1002/cjp2.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kiyong N.A., Sung Ji-Youn, Kim Hyun-Soo. TP53 mutation status of tubo- ovarian and peritoneal high-grade serous carcinoma with a wild-type p53 immunostaining pattern. Anticancer Res. 2017;37(12):6697–6703. doi: 10.21873/anticanres.12128. [DOI] [PubMed] [Google Scholar]
- 21.Taube Eliane Tabea, Denkert Carsten, Sehouli Jalid. Wilms tumor protein 1 (WT1) — not only a diagnostic but also a prognostic marker in high-grade serous ovarian carcinoma. Gynecol Oncol. 2016;40(3):494–502. doi: 10.1016/j.ygyno.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 22.Liliac L., Carcangiu M.L., Canevari S. The value of PAX8 and WT1 molecules in ovarian cancer diagnosis. Rom J Morphol Embryol. 2013;54(1):17–27. [PubMed] [Google Scholar]
- 23.Mahadevappa A., Krishna S.M., Vimala M.G. Diagnostic and prognostic significance of Ki-67 immunohistochemical expression in surface epithelial ovarian carcinoma. J Clin Diagn Res J Clin Diagn Res. 2017;11(2):EC08–EC12. doi: 10.7860/JCDR/2017/24350.9381. [DOI] [PMC free article] [PubMed] [Google Scholar]