Abstract
Objective(s):
Cardiovascular disease is a leading cause of morbimortality with over half cardiovascular events occurring in the asymptomatic population by traditional risk stratification. This preliminary study aimed to evaluate systemic plaque vulnerability in patients with prior Coronary Artery Disease (CAD) with a single Whole Body [FDG] PET-CT scan in terms of plaque inflammation and calcifications.
Methods:
Twenty-two patients referred for oncological evaluation and with prior history of advanced CAD or age and gender matched controls without cardiovascular disease, underwent a Whole Body PET-CT scan 90 min after injection of 18F-FDG. A total of 975 transaxial PET images were retrospectively analysed to assess plaque inflammation using a standardized method of analysis with averaged Target-to-Background Ratios (TBRs) at different levels, in the thoracic and abdominal aorta, carotids, LAD, common iliac and femoral arteries, and were correlated with calcium scores from the CT images.
Results:
TBRs from the thoracic aorta were higher in male patients than controls (1.49±0.11, p<0.05) and a gradient was observed (ascending > descending > aortic arch), and were also higher in the carotids in female patients (1.43±0.07) versus controls (p<0.05). A tendency for higher levels of plaque inflammation in the abdominal aorta was noted in all groups, but no significant FDG uptake was found either in the iliac or femoral arteries in any group. Plaque inflammation was also higher in the LAD in males but with large variations. Higher levels of calcifications were noted in the LAD, infra-renal abdominal aorta and common iliac arteries, but without significant correlation with plaque inflammation except sporadic overlapping.
Conclusion:
Patients with advanced CAD are at risk for vulnerable inflamed atheromas in other territories such as the thoracic aorta and carotid arteries, underpinning the systemic nature of the atherosclerotic disease. Coexistence with calcifications is rare, suggesting a different functional status of the plaques and different stages of the disease. Evaluation of subclinical systemic plaque vulnerability in CAD with a Whole Body [FDG] PET-CT scan is feasible and a potentially useful biomarker to assess subclinical vascular risk for risk stratification and treatment optimization, but further studies are needed.
Key Words: PET, Plaque inflammation, Plaque calcifications, Coronary artery disease, Atherosclerosis
Introduction
Atherosclerosis is a chronic systemic inflammatory disease and a leading cause of cardiovascular morbidity and mortality worldwide, with atherosclerotic plaque formation being a long developing process beginning in infancy (1). Endothelial dysfunction, inflammation, lipid and calcium deposition, apoptosis and neo-angiogenesis are among the different molecular mechanisms underlying plaque formation, progression and disruption (2). Although it is difficult to predict the natural evolution of a plaque, it has been demonstrated that plaques with a thin fibrous cap, outward remodelling, high levels of apoptosis and neovascularization and in particular, inflammation, are at higher risk of disruption and subsequent atherothrombosis than plaques without these features (3, 4). Activated macrophages contribute to plaque destabilization by releasing a series of proteolytic enzymes and thrombogenic factors directly linked to the subsequent development of arterial thrombosis (5). The high levels of anaerobic metabolism and energy consumption observed in these macrophages are translated into higher levels of 18F-Fluordeoxyglucose (FDG) uptake on Positron Emission Tomography (PET) images which, in consequence, are an accurate reflection of macrophage activation, inflammation and potential plaque vulnerability (6, 7).
In the clinical arena, it is noteworthy that more than half strokes and myocardial infarcts occur in the low to intermediate risk population assessed by traditional risk stratification (8). In this scenario, the degree of luminal narrowing may no longer be considered the sole determinant for major cardiovascular events, since the majority of them occur with less than 50% stenosis in a critical vessel (9). A special role for the functional status of the atherosclerotic plaque has therefore been proposed, underlining the importance of characterizing and understanding plaque biology for adequate patient evaluation and management (10, 11). Under this approach, new attempts to accurately identify high risk patients are being extensively investigated, with emphasis on different imaging modalities for assessment of plaque vulnerability. Some imaging strategies include the evaluation of carotid intima-media thickness by ultrasound, coronary calcium scores by Computed Tomography (CT) and plaque morphological characterization with Magnetic Resonance Imaging (12). However, current evidence supports the idea that inflammation plays a predominant role in plaque vulnerability and the likelihood of a major adverse cardiovascular event, and that atheromas are not limited to a single vessel or region but are representative of a more widespread disease (10, 12). Therefore, functional plaque imaging with a Whole Body [FDG] PET-CT could potentially play a crucial role to assess this risk and vulnerability non-invasively, and a remarkable body of evidence has been reported with PET to further characterize this phenomenon (13).
The degree of FDG uptake in the atherosclerotic plaque has been positively associated with different cardiovascular risk factors (14) and was also found to be modifiable by anti-inflammatory and lipid lowering therapies (15, 16). High levels of FDG uptake were also extensively reported with PET in the carotids of patients with cerebrovascular disease and stroke (17-19), however, very limited information is available about systemic plaque vulnerability in patients with CAD. This population is particularly at higher risk of atherosclerotic involvement in other critical vascular territories, and every effort to assess this vulnerability needs to be made. The primary objective of this feasibility study was to conduct a preliminary evaluation of systemic plaque vulnerability in different critical vessels in a specific patient population with prior CAD. Secondary objectives were to assess possible correlations between inflamed and calcified atheromas to further evaluate the functional status of the atherosclerotic plaques, and the feasibility of imaging systemic plaque vulnerability with a single Whole Body [FDG] PET-CT scan.
Methods
Study population
Among all patients referred for an [FDG] PET-CT scan to our center for oncological conditions over an eighteen months period, 22 were included in the current study after satisfying the inclusion criteria based on departmental records, and the study was conducted by retrospective observation and analysis. Inclusion criteria consisted of a history of prior CAD for the target population. In addition, age and gender matched subjects without any significant prior history of cardiovascular, peripheral or cerebrovascular disease were included as a reference population. Informed consent was provided by all patients, the study protocol was approved by all pertinent institutional and local Scientific and Ethics Committees and conducted in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and all subsequent revisions. From all patients who underwent an [FDG] PET-CT scan during this period, 11 patients and 11 control subjects meeting the inclusion criteria were identified and were selected for further analysis, with 5 females and 6 males in each group. Mean age was 67±3 years (females) and 70±2 years (males) for both patients and controls. Nine out of eleven patients had prior coronary revascularization (5 patients had a prior coronary artery bypass graft (CABG) and 4 patients had prior percutaneous transluminal coronary angioplasty (PTCA), 1 patient presented severe coronary stenosis by angiography, and 1 patient had a prior history of ischemic heart disease and valvulopathy.
Scanner and imaging protocol
The scanner is a 64 slice time-of-flight PET-CT (GE Discovery 690), equipped with LYSO crystals and 24 detector rings, with an approximate in plane spatial resolution of 3.5 mm and a transaxial field of view of 70 cm for the PET and 50 cm for the CT component. A CT transmission scan was initially performed for localization and attenuation correction purposes. Whole-body PET scans were subsequently acquired in the 3D mode for 20 minutes in different bed positions and in a single acquisition, from the mid-thigh to the base of the skull. Patients fasted for at least 6 hours prior to the scan and were told to refrain from taking caffeine or cold medications 12 hours prior to the study. They were injected with 4 MBq/kg of 18F-FDG (average 283±14.73 MBq), and the scan began 90 minutes after injection of the radiotracer. Average glucose levels were 6±0.36 mmol at the time of injection of the radiotracer.
Image and data analyses
Images were reconstructed using standard procedures by ordered subset expectation maximization algorithms (OSEM), and corrected for attenuation, dead time, randoms and scatters. Images were assessed independently by two investigators (PSR and JR) for consistency and reproducibility, and subsequently analysed on an AW workstation (GE Healthcare). The degree of FDG uptake was evaluated by target-to-background-ratios (TBRs) from the PET images, and the degree of calcification was visually assessed by arterial calcium scores (CSCs) from the non-contrast and co-registered CT images.
a) FDG uptake was semi quantitatively evaluated by target-to-background-ratios (TBRs), which are widely regarded as surrogate markers for plaque inflammation, as previously described (20). A new standardized method of analysis was used by placing regions of interest (ROIs) around the arterial wall on three contiguous transaxial co-registered images at branching and bifurcation points with higher risk of thrombosis for SUVmax calculation (21) and divided by ROIs in the venous mid-lumen for blood pool normalization. This analysis was conducted in 975 transaxial PET images, aided by the CT images for anatomical localization. Target vessels were analysed at specific points and included the carotids at the level of the bifurcation, the ascendinga, transverse and descendingb thoracic aorta (a,b at the level of the aorto-pulmonary window), the mid left anterior descending coronary artery (LAD), the proximal, middle and distal abdominal aorta (at the level of the celiac trunk, renal arteries and above the bifurcation), proximal common iliac arteries (below the aortic bifurcation) and proximal common femoral arteries (pubic symphysis). For venous blood pool normalization, either the jugular vein (for the carotids), superior vena cava (for thoracic aorta and LAD) or inferior vena cava (for abdominal aorta, common iliac and femoral arteries) were selected for analysis. This new standardized approach was used to facilitate the reproducibility of the study. Calculated values represented the average of three contiguous slices at each specific point for each vessel. Vessels were excluded from analyses when extensive regional metastatic disease with high levels of FDG uptake was present. Similarly, when higher than background levels of myocardial glucose uptake were observed, the LAD was not included in the analyses.
b) CSCs were calculated for each vessel based on a visual scale as originally described by Rominger et al (21) and ranging from 0 to 4. The threshold for calcification was considered at >130 Hounsfield units on the non-contrast CT images. When no calcification was present around the arterial wall circumference, a Score 0 was assigned. Similarly, Score 1 corresponded to calcification involving less than 10% of the wall circumference, Score 2 to calcification encompassing between 10-25% of the arterial wall circumference, Score 3 between 25-50% calcification, and Score 4 when calcification was present in more than 50% of the entire wall circumference.
Statistical analyses
For statistical comparison of FDG uptake between arteries in all subject groups, the unpaired Student's t-test was used, and a level of p<0.05 was defined for statistical significance. Descriptive statistics were expressed as mean and standard errors of the mean (S.E.M.) for continuous variables, and as percentage to reflect the degree of calcification by calcium scores. To evaluate the correlations between calcium scores from the CT images and TBRs from the PET images, the Pearson correlation test was performed (SPSS Inc, Chicago, Ill, version 16).
Results
Target-to-background ratios (FDG PET)
The average distribution of TBRs in six major arteries and segments in patients and controls is shown in Figure 1 and Table 1. TBR values were found to be significantly higher in the carotids in female patients vs controls (1.4±0.07 vs 1.1±0.07) and in the thoracic aorta in male patients vs controls (1.5±0.11 vs 1.2±0.05) (p <0.05). These values corresponded to an average SUVmax of 2.5±0.26 for the carotids and 2.5±0.16 for the thoracic aorta, and representative images are shown in Figure 2. Higher baseline levels of FDG uptake were observed in the thoracic aorta in both female patients and controls (1.4±0.07 and 1.5±0.12, respectively), and in the abdominal aorta of all males (1.3±0.10 and 1.4±0.06), but without significant differences between groups. A gradient in FDG uptake was also noted in the thoracic aorta in male patients, being higher in the ascending segment, followed by the descending aorta, and was lower in the aortic arch (1.6±0.14, 1.5±0.29, 1.4±0.10). No gradient was observed in the proximal, middle or distal abdominal aorta in any group. A tendency for higher TBR values was found in the mid LAD in male patients but with large intersubject variations (1.4±0.34), while no FDG uptake was observed in the LAD in any other group. No significant differences in FDG uptake were observed either in the common iliac or common femoral arteries in any group, with TBR ratios close to unity in all cases.
Figure 1.
Vascular distribution of average TBRs. Females (top) and males (bottom). Values from control subjects are represented in green columns and values from patients in blue columns. Bars represent S.E.M, *p <0.05
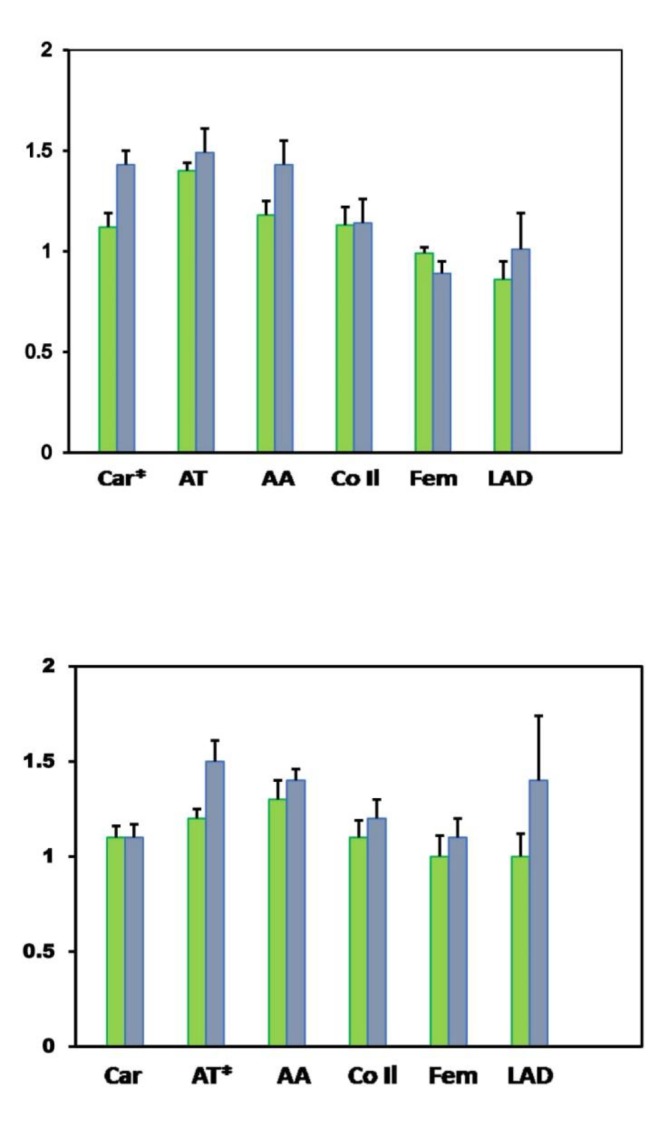
Abbreviations: AA: abdominal aorta; Car: carotid arteries; CoI l: common iliac arteries; Fem: femoral arteries; LAD: left anterior descending coronary artery; TA: thoracic aorta
Table 1.
Average TBR values in different vessels
| Vessel | Control females |
Patient females | Control males |
Patient males |
| Carotid | 1.10±0.07 | †1.40±0.07 | 1.10±0.08 | †1.10±0.07 |
| LAD | 0.90±0.10 | 1.00±0.23 | 1.00±0.12 | 1.40±0.34 |
| Thoracic Aorta | 1.40±0.07 | 1.50±0.12 | 1.20±0.05 | 1.50±0.12 |
| Abdominal Aorta | 1.20±0.04 | 1.40±0.12 | 1.30±0.10 | 1.40±0.06 |
| Common Iliac | 1.10±0.09 | 1.10±0.12 | 1.10±0.08 | 1.20±0.07 |
| Femoral | 1.00±0.03 | 0.90±0.06 | 1.00±0.05 | 1.10±0.10 |
Values represent mean ± S.E.M; † p< 0.05."
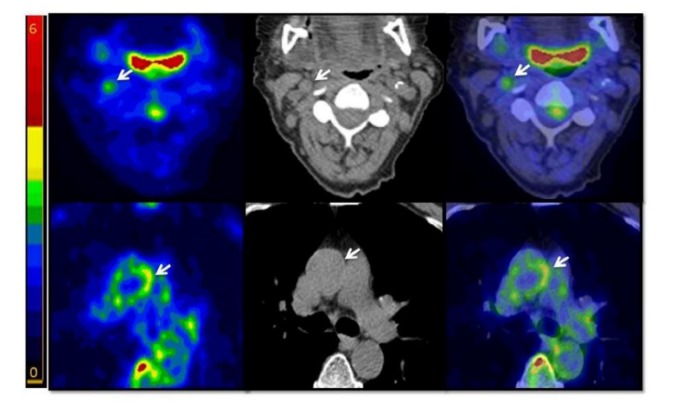
Figure 2.
Representative images showing FDG uptake in atherosclerotic plaques (arrows). Top panel: 71 y.o. female patient with prior PTCA, presenting an FDG-avid non-calcified plaque in the right carotid. Bottom panel: 74 y.o. male patient with prior CABG presenting an FDG-avid non-calcified plaque in the ascending aorta. Left: FDG-PET scan. Centre: non-contrast CT scan. Right: co-registered PET-CT
Calcium scores (CT)
Average percentage CSCs in both the control and patient population are shown in Figure 3. Control subjects only showed minimal or no calcifications in all groups (females, CSC <1 =83%, CSC<2 =17%; males, CSC<1 =100%). In contrast, higher CSCs were observed in all patient groups, with two third of patients presenting scores ≥ 2 (males: CSC<1 =33%, CSC<2 =33%, CSC<3 =16.66%, CSC<4 =16.66%; females: CSC<1 =33%, CSC<2 =67%). These findings were predominantly associated with higher levels of calcium accumulation in the LAD, abdominal aorta and common iliac arteries. When segmental calcification was evaluated in the abdominal aorta, significantly higher CSCs were observed in the lower infra-renal abdominal aorta in male patients when compared with their reference counterparts (2.75 vs 0.75, p<0.001). When comparing TBRs versus CSCs, no significant relationships were found by Pearson’s correlations test. Heavily calcified plaques did not show any significant FDG uptake and overlapping of FDG and calcium in the same plaque was observed in only one patient (1%).
Figure 3.
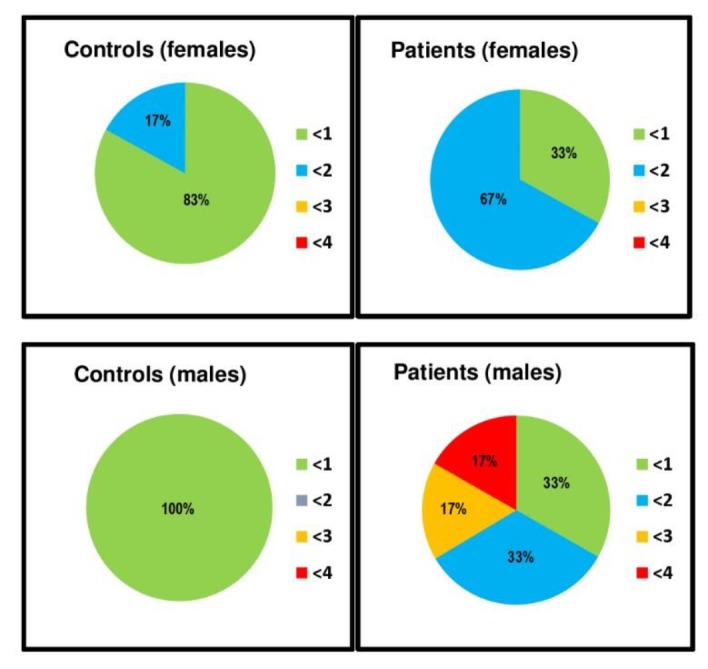
Arterial calcium scores for all vessels. Values are expressed as percentage from total population in each group. Top panel: females group, controls (left) and patients (right). Bottom panel: males group, controls (left) and patients (right)
Discussion
The primary aim of this study was to evaluate plaque vulnerability in major arterial beds in patients with prior CAD with a single Whole Body [FDG] PET-CT scan and in comparison with an age and gender matched reference population. This approach was mainly designed to account for potential cardiovascular risk differences between groups, particularly those associated with age and gender, adding robustness to the analyses of the study by comparison with a baseline population without prior CAD.
Carotid plaque inflammation in association with cerebrovascular disease has been extensively evaluated with [FDG] PET-CT and validated on immunohistochemical grounds, in patients undergoing endarterectomy for severe carotid stenosis. In this context, a direct correlation was reported between TBR and SUVmax values versus the percentage area of macrophages (17, 19) and CD68 macrophage expression (18) as a reflection of macrophage activity. The TBR and SUVmax values reported in this study are within similar range than these previous reports and can be considered as representative of plaque inflammation and vulnerability.
In the present study we identified high FDG-avid plaques in the carotids and in the thoracic aorta in both female and male patients with prior CAD, respectively. Even though carotid plaque inflammation in association with cerebrovascular events has been extensively studied, to our knowledge, only two limited different reports have evaluated plaque inflammation in patients with prior CAD. In one study, the presence of carotid wall inflammation was specifically investigated and was found to be highly prevalent in patients with prior CAD (23). Correlations were also observed in association with age over 65 years, hypertension, smoking, male gender and the metabolic syndrome, though no other vessels were included in this analysis. In another study (14), arterial FDG uptake was evaluated in patients with a diverse history of vascular disease and risk factors, and some preliminary observations in a subgroup of patients with prior CAD were reported with localized imaging protocols. In this patient population, higher levels of FDG uptake and inflammation were reported in the carotids, entire aorta and iliac arteries, and associations with systemic circulatory inflammatory markers such as matrix-metalloproteinases, interleukins and CRP were found in all patients with general vascular disease and risk factors, and a tendency for higher levels of plaque inflammation was observed in those with a prior history of CAD. Our results are in general agreement with these two reports, demonstrating higher levels of FDG uptake in the carotid arteries as reported by Bucerius et al (23), and higher FDG levels in both the carotid arteries and the aorta, as reported by Rudd et al (14) in patients with advanced CAD. In difference to this later study, we did not observe inflamed plaques in the iliac arteries, and the increased aortic uptake was specifically confined to the ascending segment of the thoracic aorta and was not significant in the abdominal aorta at any level. In any case, these results further support the idea of additional vulnerable atherosclerotic involvement in the carotids and thoracic aorta in patients with prior CAD, and the feasibility to image systemic plaque vulnerability with a single Whole Body [FDG] PET-CT scan. Coronary plaque imaging with PET-CT is another potentially emerging area of research, and recent studies reported higher levels of inflammation in culprit coronary lesions associated with acute coronary syndromes versus those with stable disease in patients undergoing percutaneous angioplasty (24). In our study, plaque inflammation was only preliminary examined in the LAD and found to be more prominent in male patients but with large subject variations and without statistical significance.
Another interesting finding in this study was the presence of a gradient in FDG uptake in the thoracic aorta which, to our knowledge, has not been fully described with PET before. FDG uptake was higher in the ascending aorta and lowest in the aortic arch in male patients with prior CAD, whilst no gradient was observed at any level in the abdominal aorta or in women. The closest report has been performed with transesophageal echocardiogram in stroke patients, where a proximal-distal gradient was observed in terms of plaque thickness (25). In this study, the presence of >4 mm atheromas in the ascending aorta were found to be more likely predictive of CAD, while atheromas in the aortic arch and descending aorta were more closely associated with severe carotid stenosis. A single study reported higher FDG uptake in the ascending aorta in association with culprit coronary lesions in acute versus stable coronary syndromes (24), though no other aortic segments were explored. In the present study, a tendency for higher levels of plaque inflammation were noted in the ascending aorta in male patients with prior CAD that further support these preliminary observations, although we could not find an explanation for this finding. Nevertheless, even though the significance of thoracic aortic atherosclerosis in relation to potential cardiovascular events has not been clearly established (26), our results also demonstrate the feasibility to evaluate these observations with functional plaque imaging and a Whole Body [FDG] PET-CT scan. A clear tendency for higher than background levels of FDG uptake with non-overlapping calcifications was also noted in the LAD that needs further investigation.
One of the limitations of this preliminary study is that it is retrospective and with a limited number of patients, and the fact that FDG accumulates in areas where this analysis was conducted, such as the myocardium. At present, various radiotracers are being investigated targeting inflammation in atherosclerotic plaques (27), and these strategies may also prove to be useful to overcome this limitation. In addition, the use of a Whole Body PET-CT scanner in this study was feasible, and the new Total Body PET scanners could also bring additional value for vascular risk stratification protocols in the future (28). In our study, carotid plaque inflammation was primarily observed in female patients, while plaque inflammation in the ascending thoracic aorta was more significant in male patients along with a tendency for higher levels of inflammation in the LAD. To overcome some of the limitations imposed by the number of patients with the target disease and to add robustness to the analysis, the design of the study included comparisons with subjects that did not have prior history of advanced CAD considered as reference population. However, the limited number of patients in each group would not be sufficient to support definitive conclusions about gender differences in terms of risk for subclinical plaque vulnerability despite their statistical significance, and these observations need to be interpreted as preliminary and in the context of this feasibility study, and further evaluated in prospective studies with larger number of patients.
Plaque calcifications were also evaluated in this preliminary report, and significantly higher levels of calcium were observed in the infra-renal abdominal aorta in males, along with a tendency for higher levels in the common iliac arteries and LAD. Abdominal aortic calcification has been associated with age, male gender, diabetes, smoking and ethnicity (29), and a positive relationship between coronary calcifications assessed by CT and risk for cardiac events has been traditionally described (30). However, a recent study identified up to 20% completely occluded vessels with coronary CSCs equal to zero by CT scan (31) and similar findings were reported by others in symptomatic patients with obstructive CAD and absence of calcifications (32).
In terms of correlations between calcification and inflammation, we did not find a direct relationship, in agreement with most of the literature even when different methods of analysis were used. In this study, coexistence of high FDG uptake and calcification was observed in a single plaque in only one patient (<1%), most likely indicating a potential vulnerability in a mixed plaque. This sporadic overlapping of FDG and calcification was previously described as a rare finding (14), and values up to 5-6% were reported when all vascular segments were analysed (33). This lack of correlation is now considered to be representative of different stages in the natural evolution of plaques, with calcification being suggestive of remote and more stable disease and inflammation being the cornerstone of unstable vulnerable plaque (2, 3). These observations are also applicable to our specific patient population with prior CAD, and provide further support for the potential usefulness of functional plaque imaging with [FDG] PET-CT.
Conclusion
Patients with CAD are potentially at risk for vulnerable atherosclerotic plaque involvement in other vessels, and in this preliminary study inflamed plaques were consistently observed in the carotid and ascending thoracic aorta in females and males respectively with sporadic overlapping calcifications. These findings further support the idea that atherosclerosis needs to be evaluated as a systemic disease, and that inflamed and calcified atheromas may represent different stages of the disease in agreement with current perspectives in plaque biology. No inflamed plaques were observed in the abdominal aorta, iliac or femoral arteries, and higher levels of calcifications were noted in the LAD, infra-renal abdominal aorta and common iliac arteries. This preliminary study also provides further evidence that the evaluation of systemic plaque vulnerability in CAD with a single Whole Body [FDG] PET-CT scan is feasible and is a potentially useful biomarker to evaluate systemic subclinical atherosclerosis and vascular risk in addition to traditional risk stratification, but further prospective studies with larger number of patients are still needed.
Acknowledgements
The authors wish to thank Dr Stephen Daniels and Dr Patrick Fielding for statistical and regulatory support, Mr Phillip Facey, Cyclotron and Radiochemistry staff. This work was supported in part by the Welsh Government (WAG-NAW) and the Severnside Alliance for Translational Research. No potential conflicts of interest were disclosed.
References
- 1.Strong JP, Malcom GT, Newman WP, Oalmann MC. Early lesions of atherosclerosis in childhood and youth: natural history and risk factors. J Am Coll Nutr. 1992;11 Suppl:51S–54S. doi: 10.1080/07315724.1992.10737984. [DOI] [PubMed] [Google Scholar]
- 2.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:19–26. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 3.Falk E, Sha P, Fuster V. Coronary plaque disruption. Circulation. 1995;92:657–671. doi: 10.1161/01.cir.92.3.657. [DOI] [PubMed] [Google Scholar]
- 4.Libby P, Theroux P. Pathophysiology of Coronary Artery Disease. Circulation. 2005;28(25):3481–3488. doi: 10.1161/CIRCULATIONAHA.105.537878. [DOI] [PubMed] [Google Scholar]
- 5.Aziz K, Berger K, Claycombe K, Huang R, Patel R, Abela GS. Noninvasive Detection and Localization of Vulnerable Plaque and Arterial Thrombosis. Circulation. 2008;117:2061–2070. doi: 10.1161/CIRCULATIONAHA.106.652313. [DOI] [PubMed] [Google Scholar]
- 6.Ogawa M, Ishino S, Mukai T, Asano D, Teramoto M, Watanabe H, et al. (18)F-FDG accumulation in atherosclerotic plaques: immunohistochemical and PET imaging study. J Nucl Med. 2004;45:1245–1250. [PubMed] [Google Scholar]
- 7.Rudd J, Warburton E, Fryer T. Imaging atherosclerotic plaque inflammation with [18F]-fluordeoxyglucose positron emission tomography. Circulation. 2002;105:2708–2711. doi: 10.1161/01.cir.0000020548.60110.76. [DOI] [PubMed] [Google Scholar]
- 8.Naghavi , Libby P, Falk K, Cassells SW, Litovsky S, Rumberger J, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part I and Part II. Circulation. 2003;108(14):1664–1678. doi: 10.1161/01.CIR.0000087480.94275.97. [DOI] [PubMed] [Google Scholar]
- 9.Ambrose JA, Fuster V. The risk of coronary occlusion is not proportional to the prior severity of coronary stenosis. Heart. 1998;79:3–4. doi: 10.1136/hrt.79.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Falk E, Sillesen H, Muntendam P, Fuster V. The High Risk Plaque Initiative: primary prevention of atherothrombotic events in the asymptomatic population. Curr Atheroscler Rep. 2001;13:359–366. doi: 10.1007/s11883-011-0193-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crea F, Libby P. Acute coronary Syndromes: the way forward from mechanisms to precision treatment. Circulation. 2017;136:1155–1156. doi: 10.1161/CIRCULATIONAHA.117.029870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuster V, Vahl T. The role of non-invasive imaging in promoting cardiovascular health. J Nucl Cardiol. 2010;17:781–790. doi: 10.1007/s12350-010-9264-9. [DOI] [PubMed] [Google Scholar]
- 13.Owe D, Lindsay A, Choudhury R, Fayad Z. Imaging of atherosclerosis. Ann Rev Med. 2011;62:25–40. doi: 10.1146/annurev-med-041709-133809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rudd H, Myers K, Bansilal S, Machac J, Woodward M et al. Relationships among regional arterial inflammation, calcification, risk factors and biomarkers: a prospective Fluorodeoxyglucose Tomography/Computed Tomography imaging study. Circ Cardiovasc Imag. 2009;2:107–115. doi: 10.1161/CIRCIMAGING.108.811752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tahara N, Kai H, Ishibashi M, Nakaura H, Kaida H, Baba K et al. Simvastatin attenuates plaque inflammation: evaluation by fluorodeoxyglucose positron emission tomography. J Am Coll Cardiol. 2006;48(9):1825–1831. doi: 10.1016/j.jacc.2006.03.069. [DOI] [PubMed] [Google Scholar]
- 16.Hacker M. Monitoring anti-inflammatory therapies in patients with atherosclerosis: PET emerges as the method of choice. Eur J Nucl Med Mol Imag. 2012;39:2396–2398. doi: 10.1007/s00259-011-2027-2. [DOI] [PubMed] [Google Scholar]
- 17.Tawakol A, Migrino RQ, Bashian GG, Bedri S, Bermylen D, Cury RC, et al. In vivo 18F-fluordeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am Coll Cardiol. 2006;48:1818–1824. doi: 10.1016/j.jacc.2006.05.076. [DOI] [PubMed] [Google Scholar]
- 18.Graebe M, Pedersen S, Borgwardt L, Hojgaard L, Sillesen H, Kjaer A Molecular pathology in vulnerable carotid plaques: correlation with [18]-fluorodeoxyglucose positron emission tomography (FDG-PET) Eur J Vasc Endovasc Surg. 2009;37:714–721. doi: 10.1016/j.ejvs.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 19.Menezes L, Kotze C, Agu O, Richards T, Brooks J, Goh VJ, et al. Investigating vulnerable atheroma using combined 18F-FDG PET-CT angiography of carotid plaque with immuno-histochemical validation. J Nucl Med. 2011;52:1698–1703. doi: 10.2967/jnumed.111.093724. [DOI] [PubMed] [Google Scholar]
- 20.Rudd J, Myers K, Bansilal S, Machac J, Pinto CA, Tong C, et al. Atherosclerosis Inflammation Imaging with 18F-FDG PET: carotid, iliac and femoral uptake, reproducibility, quantification methods and recommendations. J Nucl Med. 2008;49:871–878. doi: 10.2967/jnumed.107.050294. [DOI] [PubMed] [Google Scholar]
- 21.Warboys C, Amini N, de Luca A, Evans P. The role of blood flow in determining the sites of atherosclerotic plaques. F1000 Medicine Reports. 2011:3–5. doi: 10.3410/M3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rominger A, Saam S, Wolpers S, Cyran CC, Schmidt M, Foerster S et al. FDG PET-CT identifies patients at risk for future cardiovascular events in an otherwise asymptomatic cohort with neoplastic disease. J Nucl Med. 2009;50:1611–1620. doi: 10.2967/jnumed.109.065151. [DOI] [PubMed] [Google Scholar]
- 23.Bucerius J, Duivenvoorden R, Mani V, Moncrieff C, Rudd JH, Calcagno C, et al. Prevalence and risk factors of carotid vessel wall inflammation in coronary artery disease patients. J Am Coll Cardiol Cardiovasc Imag. 2011;4(11):1196–1205. doi: 10.1016/j.jcmg.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rogers I, Nasir K, Figueroa A, Cury RC, Hoffmann U, Vermylen DA, et al. Feasibility of FDG imaging of the coronary arteries Comparison between acute coronary syndrome and stable angina. J Am Coll Cardiol Imag. 2010;3:388–397. doi: 10.1016/j.jcmg.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Sen S, Wu K, McNamara R, Lima J, Piantadosi S, Oppenheimer SM. Distribution, severity and risk factors for aortic atherosclerosis in cerebral ischemia. Cerebrovasc Dis. 2000;10:102–109. doi: 10.1159/000016038. [DOI] [PubMed] [Google Scholar]
- 26.Krozon I, Tunick P. Aortic atherosclerosis disease and stroke. Circulation. 2006;114(1):63–75. doi: 10.1161/CIRCULATIONAHA.105.593418. [DOI] [PubMed] [Google Scholar]
- 27.Bucerius J, Dijkgraaf I, Mottaghy F, Schurgers L. Target identification for the diagnosis and intervention of vulnerable atherosclerotic plaques beyond 18F-fluorodeoxyglucose positron emission tomography imaging: promising tracers on the horizon. Eur J of Nucl Med Mol Imag. 2019;46:251–265. doi: 10.1007/s00259-018-4176-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cherry SR, Jones T, Karp JS, Qi J, Moses WW, Badawi RD. Total Body PET: maximizing sensitivity to create new opportunities for clinical research and patient care. J Nucl Med. 2018;59(1):3–12. doi: 10.2967/jnumed.116.184028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong N, Lopez V, Allison M, Detrano LC, Blumenthal RS, Folson AR. Abdominal carotid calcium and multisite atherosclerosis: The multiethnic study of atherosclerosis. Atherosclerosis. 2011;214:436–441. doi: 10.1016/j.atherosclerosis.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown ER, Kronmal RA, Bluemke DA, Guerci AD, Carr JJ, Goldin J, Detrano R. Coronary calcium coverage score: determination, correlates, and predictive accuracy in the Multi-Ethnic Study of Atherosclerosis. Radiology. 2011;247(3):669–675. doi: 10.1148/radiol.2473071469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gottlieb I, Miller JM, Arbab-Zadeh A, Dewey M, Clouse ME, Sara L et al. The absence of coronary calcification does not exclude obstructive coronary artery disease or the need for revascularization in patients referred for conventional coronary angiography. J Am Coll Cardiol. 2010;55:627–634. doi: 10.1016/j.jacc.2009.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Villines TC, Hulten EA, Shaw LJ, Goyal M, Dunning A, Achenbach S, et al. Prevalence and severity of coronary artery disease and adverse events among symptomatic patients with coronary artery calcification scores of zero undergoing coronary computed tomography angiography: results from the CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter registry. J Am Coll Cardiol. 2011;58(24):2533–2540. doi: 10.1016/j.jacc.2011.10.851. [DOI] [PubMed] [Google Scholar]
- 33.Ben-Haim S, Kupzov E, Tamir A, Israel O. Evaluation of 18F-FDG uptake and arterial wall calcifications using 18F-FDG PET/CT. J Nucl Med. 2004;45:1816–1821. [PubMed] [Google Scholar]