Abstract
Objective(s):
99mTc-PSMA SPECT/CT is a cost effective alternative for 68Ga-PSMA PET/CT. The aim of this study was to directly compare these two techniques in patients with prostate cancer.
Methods:
28 man with prostate cancer were studied using 99mTc-PSMA SPECT/CT and 68Ga-PSMA PET/CT in a short time period (<60 days). No intervention was done between the studies. Whole body PET/CT was done 60 minutes after IV injection of 2 MBq/Kg of 68Ga-PSMA. 99mTc-PSMA kit (PSMA I+S) was used for SPECT/CT and whole body imaging was performed 4 hours after IV injection of 740 MBq of 99mTc-PSMA. Images were interpreted independently and the results of each imaging were recorded.
Results:
The mean age of the patients was 64.7±9.6 years old and the mean time difference between two sets of images was 16.6±13.5 days. Abnormal uptake was seen in 25 (89.2%) patients by 68Ga-PSMA PET/CT and 20 (71.4%) patients with 99mTc-PSMA SPECT/CT. No patients with positive 99mTc-PSMA SPECT/CT had negative 68Ga-PSMA PET/CT. The mean number of detected lesions was 26.07±27.5 by 68Ga-PSMA PET/CT and 10.52±10.99 by 99mTc-PSMA SPECT/CT (P<0.001). Detection of lymph nodes and bone metastases were not significantly different between two sets of imaging (P>0.05), however 68Ga-PSMA PET/CT were more successful in detection of prostate bed lesions compared to 99mTc-PSMA scan. Interestingly, no patient with PSA level of >2.1 ng/ml had discordant result between two sets of images.
Conclusion:
99mTc-PSMA SPECT/CT is as accurate as 68Ga-PSMA PET/CT in M staging, however 68Ga-PSMA PET/CT detected more lesions compared to 99mTc-PSMA SPECT/CT. Detection rate was not significantly different between two techniques in patients with PSA levels>2.1 ng/ml.
Key Words: Prostate Cancer, Tc-99m-PSMA, Ga-68-PSMA
Introduction
Prostate cancer is the most common cancer diagnosis made in men. Despite its indolent course in majority of patients, it is the third-leading cause of cancer death in men throughout the world (1).
Multiple treatment options are available for patients with prostate cancer according to their risk of disease which is usually determined by Gleason score, PSA level and imaging results (1).
Traditionally imaging evaluation for patients with prostate cancer included ultrasonography, CT, MRI and whole body bone scan. From PET/CT options, considering indolent nature of the disease FDG-PET/CT was not remarkable and 18F-Choline and
68Ga-PSMA showed more sensitivity for detection of recurrence or metastases (2, 3). Radiolabeled prostate specific membrane antigen (PSMA) imaging of prostate cancer has been increasingly used in the last few years with promising results (3). Furthermore, labeling of PSMA with beta and alpha emitters provided a new option in treatment of patients with prostate cancer (4, 5).
PSMA is expressed in different tissues and organs including prostate, kidney, proximal small intestine and salivary glands and more importantly is overexpressed in high risk prostate cancer tissues (6, 7). Its overexpression in prostate cancer gives a promising outcome in imaging and treatment of patients with prostate cancer (7).
Although 68Ga-PSMA has been widely studied, 99mTc-PSMA is introduced as a cost effective alternative either for imaging or for radio-guided surgery (8). Wide availability of SPECT/CT machines facilitated accurate localization of the lesions (9).
However there are limited studies comparing 68Ga-PSMA PET/CT scan with 99mTc-PSMA SPECT/CT in terms of sensitivity, specificity and accuracy (10-13). The aim of this study was performing a head to head comparison of 68Ga-PSMA PET/CT scan with 99mTc-PSMA SPECT/CT in a group of patients with prostate adenocarcinoma who referred either for initial staging, restaging or recurrence evaluation.
Methods
We studied 28 men with prostate carcinoma who referred to our center for PSMA imaging. The indications for imaging were recurrence evaluation or restaging in 24 patients and initial staging in 4 patients. All patients were explained about the procedures and probable use of data for research project and signed a written consent. The study were confirmed by local ethics committee (DSREC-02/2019_20). All patients underwent 68Ga-PSMA PET/CT and 99mTc-PSMA SPECT/CT in a short time interval (<60 days). Whole body PET/CT was done using GE MI-DR( 64 SLICE CT) and patients studied 60 minutes after IV injection of 2 MBq/Kg of 68Ga-PSMA (8 beds and 4 minutes per bed position, CT: 120 Kvp, auto mA (range 30 to 300 mA) with 40 % dose reduction and noise index 16.0.
99mTc-PSMA kit (PSMA I+S) was labeled with 99mTc-pertechnetate and whole body imaging was performed 4 hrs after IV injection of 740 MBq of 99mTc-PSMA (Exposure time per pixel: 480 sec and pallet velocity: 5.0 cm/min, stored in a 1024×256 matrix). SPECT was done with step and shoot protocol, 30 sec/view over 360 degree and stored in a 128×128 matrix.
The CT part of the SPECT/CT was done according to the following protocol: helical, 120kvp, smart mA (50-150 mA) Noise index: 18.4752, slice thickness: 3.75 mm.
Images were reviewed separately and anonymously by two experienced nuclear physicians and were categorized as normal or abnormal. Any non-physiologic uptake was considered as abnormal finding. Any suspicious finding was further evaluated by delayed imaging and post void images. In case of discrepancy between readers, consensus was obtained by consulting with a third expert. Furthermore, the locations of abnormal uptake were fully described in both sets of images with special attention on prostate bed, local or regional lymph nodes and skeletal system.
Statistical analysis:
Statistical analysis was done using SPSS software (Version 16, SPSS Inc. USA). Frequency and cross tables were used for descriptive analysis of the variables. Comparison of quantitative and qualitative variables between two sets of images was done using paired t-test and McNemar test respectively. P<0.05 was considered statistically significant in all comparisons.
Results
We studied 28 men with prostate cancer with mean age of 64.7±9.6 years old and age range of 49 to 90 years old. Histopathologic analysis revealed classic type adenocarcinoma in all patients with evidence of poor differentiation in one patient. The reason for referral was restaging in 16 patients (57.1%), recurrence evaluation in 8 (28.6%) and initial staging in 4 (14.3%) patients. The median Gleason score was 7. Serum PSA level ranged from 0.03 to 438 ng/ml with a mean of 54.8±97.0. All patients underwent 68Ga-PSMA and 99mTc-PSMA whole body imaging. The time difference between two sets of imaging ranged between 2 to 50 days with a mean of 16.6±13.5 days.
68Ga-PSMA scan showed abnormal uptake in 25 patients while 99mTc-PSMA scintigraphy found abnormal uptake in 20 patients. Three patients had negative finding in both sets of images. Five patients had negative 99mTc-PSMA scintigraphy while 68Ga-PSMA scan was abnormal (table 1). All patients with abnormal 99mTc-PSMA scan had abnormal findings on 68Ga-PSMA imaging too. The 68Ga-PSMA scan seems to be more sensitive than 99mTc-PSMA scan in detection of patients with abnormality (P=0.06).
Table 1.
Comparison of overall finding of 99mTc-PSMA scan versus 68Ga-PSMA scan (McNemar test; P=0.06)
|
99m
Tc-PSMA scan
|
T otal | |||
|---|---|---|---|---|
| Negative | Positive | |||
| 68Ga-PSMA scan | Negative | 3 | 0 | 3 |
| Positive | 5 | 20 | 25 | |
| Total | 8 | 20 | 28 | |
Further analysis was done for lesion detection in prostate bed, lymph nodes and skeletal system. Abnormal uptake in prostate bed was seen in 24 patients by 68Ga-PSMA scan which was much higher than 15 patients with abnormal prostate bed uptake in 99mTc-PSMA scan (P=0.004). Table 2 shows abnormal uptake in prostate bed in two sets of images.
Table 2.
Prostate bed lesion in 99mTc-PSMA scan versus 68Ga-PSMA scan. (McNemar test: P=0.004)
|
99m
Tc-PSMA scan
|
Total | |||
|---|---|---|---|---|
| Negative | Positive | |||
| 68Ga-PSMA scan | Negative | 4 | 0 | 4 |
| Positive | 9 | 15 | 24 | |
| Total | 13 | 15 | 28 | |
We found a remarkable agreement between 99mTc-PSMA scan and 68Ga-PSMA scan for detection of lymph node metastasis (Kappa coefficient=0.92). Anyhow one patient had no lymph node metastasis in 99mTc-PSMA scan while 68Ga-PSMA scan detected a lymph node metastasis. Table 3 shows no significant difference between two sets of images in terms of detection of lymph node metastasis (McNemar Test; P=1.0).
Table 3.
Lymph node metastasis in 99mTc-PSMA scan versus 68Ga-PSMA scan (P=1.0)
|
99m
Tc-PSMA scan
|
Total | |||
|---|---|---|---|---|
| Negative | Positive | |||
| 68Ga-PSMA scan | Negative | 16 | 0 | 16 |
| Positive | 1 | 11 | 12 | |
| Total | 17 | 11 | 28 | |
Regarding detection of bone metastasis, complete agreement was noted between two sets of images. All patients with bone metastases was detected by both methods (P=1.0).
Overall 68Ga-PSMA scan detected more abnormal foci compared to 99mTc-PSMA scan (P=0.017). The number of detected lesions ranged from 0 to 65 in 68Ga-PSMA scan while it ranged from 0 to 30 in 99mTc-PSMA scan. Using paired t-test, the mean number of lesions was compared between two set of images. It was significantly higher in 68Ga-PSMA scan (26.07±27.5) compared to 99mTc-PSMA (10.52±10.99) scintigraphy (P<0.001).
Five patients had discordant scan results between 68Ga-PSMA scan and 99mTc-PSMA scan that was further analyzed. PSA level ranged from 0.03 to 2.1 ng/ml in these patients and the mean PSA level was 0.63±0.85 ng/ml which was significantly lower than mean PSA level of 66.5±103.6 ng/ml in patients with concordant results. No patient with PSA >2.1 ng/ml had discordant results. None of the patients with discordant results had more than 1 lesion in 68Ga-PSMA scan. The mean age, mean time interval between two imaging and mean Gleason score was not significantly different between patients with discordant results and those with concordant results (P>0.3).
Defining a PSA threshold of more than 0.5 ng/ml, there was no statistically significant difference between two sets of scans in detection of abnormality. In these patients, concordance was noted in 21 out of 23 patients between the two sets of images while 2 patients had positive 68Ga-PSMA scan and negative 99mTc-PSMA scan (P=0.50). There was no significant difference between the two methods in detection of lymph node metastasis (P=1). Anyhow the number of lesions in 68Ga-PSMA scan was higher compared to 99mTc-PSMA scan, independent of PSA level.
Discussion
Overall we found that 68 Ga-PSMA PET/CT seems to be more sensitive than 99mTc-PSMA SPECT/CT in terms of detection of lesions, however this superiority is not evident in detection of bone metastases.
Bone metastasis is the most common distant metastasis in patients with prostate cancer and bone scan using 99mTc-HDP is usually used for detection of it , however false positive results are common due to HDP uptake in variety of benign boney lesions and the overall specificity is low (14) . Lengana et al observed the superiority of 68Ga-PSMA PET/CT over bone scan in detecting lytic and bone marrow lesions and suggested it as a better alternative to replace the bone scan (15) . In another study, 99mTc-PSMA SPECT/CT imaging found 95 bone metastases in 25 patients which was superior to other imaging modalities such as bone scan and MRI (16) .
Considering equal sensitivity of 68Ga-PSMA PET/CT and 99mTc-PSMA SPECT/CT in our study for detection of bone metastasis, we may expect superior sensitivity of 99mTc-PSMA SPECT/CT over 99mTc-HDP bone scan too, although further studies with direct comparison is needed to confirm the findings.
The use of 68Ga-PSMA PET/CT in initial staging of prostate cancer can make a significant impact on therapy. It was more sensitive and specific in detection of prostate cancer compared to current conventional imaging modalities (17) .
Additionally a study group from Munich showed that even at low PSA levels of 1 to >2 ng/ml, 68Ga-PSMA PET/CT could detect 93% of lesions (18) . A strong association has been shown between increased PSA levels and metastatic detection using 68Ga-PSMA PET/CT in many studies (16,18, 19) . Natarajan et al showed that the detection rate was higher for higher PSA levels using 68Ga-PSMA PET/CT i.e: 86%, 85% and 95% for PSA levels of 2-5, 1-10, and 10 ng/ml respectively (20) . In another study with 99m Tc-PSMA-SPECT-CT, the detection rate according to PSA levels were 30%, 80% and 100% for PSA levels of >1, 4-10, < 10 ng/ml respectively (21) .
Anyhow, joint SNM/EANM guideline on 68Ga-PSMA PET/CT recommends it for localization of the recurrence in patients with PSA levels of 0.1-10 ng/ml (22) . Our study showed that 99mTc-PSMA SPECT/CT has no significant difference with 68Ga -PSMA PET/CT in localization of recurrence in patients with PSA levels of more than 2.1 ng/ml.
Considering limited availability and high cost of 68Ga-PSMA PET/CT, prostate cancer imaging with 99mTc-PSMA SPECT/CT should be considered as a good alternative. We checked the costs of performing 68Ga-PSMA PET/CT and 99mTc-PSMA SPECT/CT in few countries in the middle East and found that 68Ga-PSMA PET/CT is 2.4 to 7.7 times more expensive than 99mTc-PSMA SPECT/CT. Our findings showed that using 99mTc-PSMA SPECT/CT in management of patients with prostate cancer, may have significant impact on health economics of prostate cancer in the developing countries..
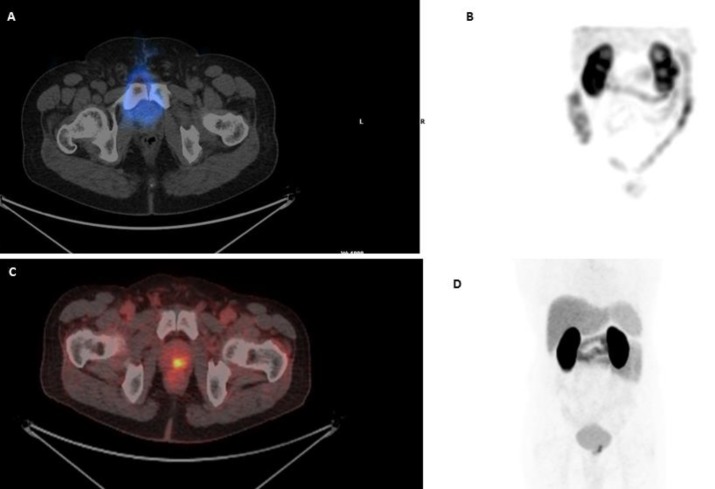
Our study showed that 99mTc-PSMA scan is as sensitive as 68Ga-PSMA scan in patients with prostate cancer in terms of detection of abnormality, lymph node metastasis or skeletal involvement, however it is less sensitive in detection of lesions in prostatic bed (Figure 1).
Figure 1.
99mTc-PSMA SPECT/CT transaxial view (A) of the pelvis and planar anterior view (B) shows no PSMA uptake in the prostate gland. 68Ga-PSMA PET/CT fused images (C) and whole body scan (D) shows focal small PSMA avidity in the prostate gland
Our results are concordant with the results of a joint study from South Africa, Netherland and Belgium which showed that 99mTc-PSMA scan might be valuable in imaging of prostate malignancy although with poor efficiency in small sized lesions and its utilization was not suggested in patients with small volume disease. In the same study all lymph nodes greater than 10 mm were detected while only 28% of the lymph nodes less than 10 mm were detected by 99mTc-PSMA scan (10) .
In another study head to head comparison of qualitative and semi-quantitative performance of 99mTc-EDDA/HYNIC-iPSMA SPECT/CT and 68Ga-PSMA-11 PET/CT was done in 23 patients and the published data were suggestive of comparable findings between the two tracers supporting the use of 99mTc-PSMA SPECT/CT in patients with progressive metastatic castration-resistant prostate cancer (11) .
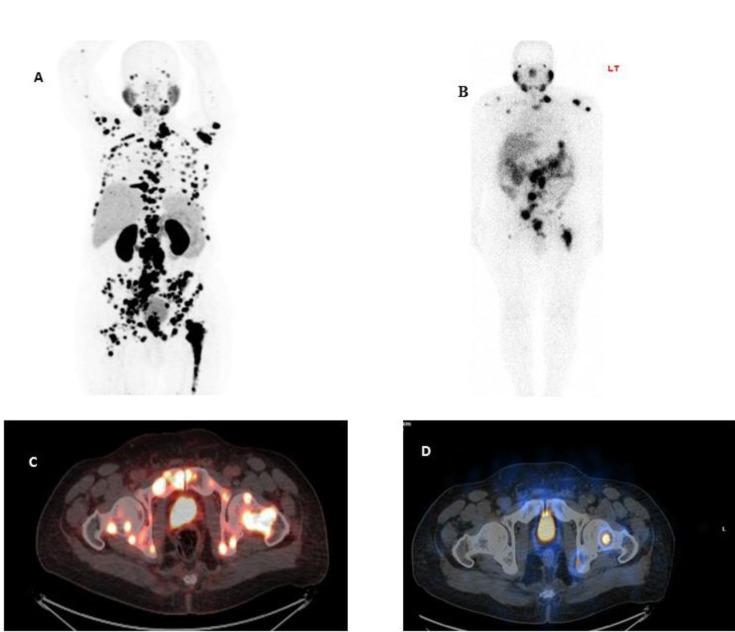
Our study, however showed that the mean number of detected lesions in 99mTc-PSMA scan were significantly less than 68Ga-PSMA scan (10.52±10.99 vs 26.07±27.5 respectively) (Figure 2).
Figure 2.
68 Ga-PSMA PET-MIP image (A) and fused PET/CT image of the pelvic region (C) shows extensive multiple bone and lymphatic metastases. 99m Tc-PSMA anterior planar whole body (B) and corresponding SPECT/CT slice (D) shows fewer PSMA avid lesions
Lawal et al demonstrated superiority of 68Ga-PSMA versus 99mTc-PSMA in terms of number of detected lesions and in the published data accordingly 46 lesions were detected in 68Ga-PSMA while 99mTc-PSMA SPECT-CT detected only 36 lesions which were localized to the prostate, lymph nodes and bones (10) .
In our study, although the number of detected lesions was higher in 68Ga-PSMA PET/CT compared to 99mTc-PSMA SPECT/CT, there was no statistically significant difference in terms of localization of abnormality in patients with PSA level of >0.5ng/ml. Therefore 99mTc-PSMA imaging offers a good alternative in the absence of 68Ga-PSMA PET Imaging and it is also cost effective enabling many centers to proceed with prostate cancer imaging work up. 99mTc-PSMA SPECT/CT may also be considered in selection of patients suitable for 177Lu-PSMA therapy and radio guided surgery (8) .
Conclusion
99mTc-PSMA SPECT/CT is a cost effective alternative to 68Ga-PSMA PET/CT with no significant difference in M staging. The mean number of detected lesions was significantly higher in 68Ga-PSMA PET/CT compared to 99mTc-PSMA SPECT/CT. Although 68Ga-PSMA PET/CT was more successful in detection of prostate bed lesions compared to 99mTc-PSMA scan, detection of lymph nodes and bone metastases was not significantly different. Finally, no patient with PSA of >2.1 ng/ml had discordant result between two sets of images.
References
- 1.Litwin MS, Tan HJ. The Diagnosis and Treatment of Prostate Cancer: A Review. Jama. 2017 Jun;317(24):2532–42. doi: 10.1001/jama.2017.7248. [DOI] [PubMed] [Google Scholar]
- 2.Beheshti M, Haim S, Zakavi R, Steinmair M, Waldenberger P, Kunit T, et al. Impact of 18F-choline PET/CT in prostate cancer patients with biochemical recurrence: influence of androgen deprivation therapy and correlation with PSA kinetics. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2013 Jun;54(6):833–40. doi: 10.2967/jnumed.112.110148. [DOI] [PubMed] [Google Scholar]
- 3.Afshar-Oromieh A, Babich JW, Kratochwil C, Giesel FL, Eisenhut M, Kopka K, et al. The Rise of PSMA Ligands for Diagnosis and Therapy of Prostate Cancer. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2016 Oct;57(Suppl 3):79S–89S. doi: 10.2967/jnumed.115.170720. [DOI] [PubMed] [Google Scholar]
- 4.Rahbar K, Afshar-Oromieh A, Jadvar H, Ahmadzadehfar H. PSMA Theranostics: Current Status and Future Directions. Molecular imaging. 2018 Jan-Dec;17:1536012118776068. doi: 10.1177/1536012118776068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kratochwil C, Schmidt K, Afshar-Oromieh A, Bruchertseifer F, Rathke H, Morgenstern A, et al. Targeted alpha therapy of mCRPC: Dosimetry estimate of (213)Bismuth-PSMA-617. European journal of nuclear medicine and molecular imaging. 2018 Jan;45(1):31–7. doi: 10.1007/s00259-017-3817-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Afshar-Oromieh A, Hetzheim H, Kratochwil C, Benesova M, Eder M, Neels OC, et al. The Theranostic PSMA Ligand PSMA-617 in the Diagnosis of Prostate Cancer by PET/CT: Biodistribution in Humans, Radiation Dosimetry, and First Evaluation of Tumor Lesions. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2015 Nov;56(11):1697–705. doi: 10.2967/jnumed.115.161299. [DOI] [PubMed] [Google Scholar]
- 7.Kasperzyk JL, Finn SP, Flavin R, Fiorentino M, Lis R, Hendrickson WK, et al. Prostate-specific membrane antigen protein expression in tumor tissue and risk of lethal prostate cancer. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2013 Dec;22(12):2354–63. doi: 10.1158/1055-9965.EPI-13-0668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robu S, Schottelius M, Eiber M, Maurer T, Gschwend J, Schwaiger M, et al. Preclinical Evaluation and First Patient Application of 99mTc-PSMA-I&S for SPECT Imaging and Radioguided Surgery in Prostate Cancer. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2017 Feb;58(2):235–42. doi: 10.2967/jnumed.116.178939. [DOI] [PubMed] [Google Scholar]
- 9.Su HC, Zhu Y, Hu SL, Liu C, Lin GW, Dai B, et al. The Value of (99m) Tc-PSMA SPECT/CT-Guided Surgery for Identifying and Locating Lymph Node Metastasis in Prostate Cancer Patients. Annals of surgical oncology. 2019 Feb;26(2):653–9. doi: 10.1245/s10434-018-6805-y. [DOI] [PubMed] [Google Scholar]
- 10.Lawal IO, Ankrah AO, Mokgoro NP, Vorster M, Maes A, Sathekge MM. Diagnostic sensitivity of 99m Tc- HYNIC PSMA SPECT/CT in prostate carcinoma: A comparative analysis with 68Ga- PSMA PET/CT. The Prostate. 2017 Aug;77(11):1205–12. doi: 10.1002/pros.23379. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Perez FO, Davanzo J, Lopez-Buenrostro S, Santos-Cuevas C, Ferro-Flores G, Jimenez-Rios MA, et al. Head to head comparison performance of (99m)Tc-EDDA/HYNIC-iPSMA SPECT/CT and (68)Ga-PSMA-11 PET/CT a prospective study in biochemical recurrence prostate cancer patients. American journal of nuclear medicine and molecular imaging. 2018;8(5):332–40. [PMC free article] [PubMed] [Google Scholar]
- 12.Janssen JC, Meissner S, Woythal N, Prasad V, Brenner W, Diederichs G, et al. Comparison of hybrid (68)Ga-PSMA-PET/CT and (99m)Tc-DPD-SPECT/CT for the detection of bone metastases in prostate cancer patients: Additional value of morphologic information from low dose CT. European radiology. 2018 Feb;28(2):610–9. doi: 10.1007/s00330-017-4994-6. [DOI] [PubMed] [Google Scholar]
- 13.Thomas L, Balmus C, Ahmadzadehfar H, Essler M, Strunk H, Bundschuh RA. Assessment of Bone Metastases in Patients with Prostate Cancer-A Comparison between (99m) Tc-Bone-Scintigraphy and [(68) Ga] Ga-PSMA PET/CT. Pharmaceuticals (Basel, Switzerland). 2017 Jul;10:3. doi: 10.3390/ph10030068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Langsteger W, Rezaee A, Pirich C, Beheshti M. (18)F-NaF-PET/CT and (99m)Tc-MDP Bone Scintigraphy in the Detection of Bone Metastases in Prostate Cancer. Seminars in nuclear medicine. 2016 Nov;46(6):491–501. doi: 10.1053/j.semnuclmed.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Lengana T, Lawal IO, Boshomane TG, Popoola GO, Mokoala KMG, Moshokoa E, et al. 68Ga-PSMA PET/CT Replacing Bone Scan in the Initial Staging of Skeletal Metastasis in Prostate Cancer: A Fait Accompli? Clinical genitourinary cancer. 2018;16(5):392–401. doi: 10.1016/j.clgc.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 16.Su HC, Zhu Y, Ling GW, Hu SL, Xu XP, Dai B, et al. Evaluation of 99mTc-labeled PSMA-SPECT/CT imaging in prostate cancer patients who have undergone biochemical relapse. Asian journal of andrology. 2017 May-Jun;19(3):267–71. doi: 10.4103/1008-682X.192638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong HS, Leung J, Bartholomeusz D, Sutherland P, Le H, Nottage M, et al. Comparative study between (68) Ga-prostate-specific membrane antigen positron emission tomography and conventional imaging in the initial staging of prostate cancer. Journal of medical imaging and radiation oncology. 2018 Dec;62(6):816–22. doi: 10.1111/1754-9485.12791. [DOI] [PubMed] [Google Scholar]
- 18.Eiber M, Maurer T, Souvatzoglou M, Beer AJ, Ruffani A, Haller B, et al. Evaluation of Hybrid (6)(8)Ga-PSMA Ligand PET/CT in 248 Patients with Biochemical Recurrence After Radical Prostatectomy. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2015 May;56(5):668–74. doi: 10.2967/jnumed.115.154153. [DOI] [PubMed] [Google Scholar]
- 19.Perera M, Papa N, Christidis D, Wetherell D, Hofman MS, Murphy DG, et al. Sensitivity, Specificity, and Predictors of Positive (68)Ga-Prostate-specific Membrane Antigen Positron Emission Tomography in Advanced Prostate Cancer: A Systematic Review and Meta-analysis. European urology. 2016 Dec;70(6):926–37. doi: 10.1016/j.eururo.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 20.Natarajan A, Agrawal A, Murthy V, Bakshi G, Joshi A, Purandare N, et al. Initial experience of Ga-68 prostate-specific membrane antigen positron emission tomography/computed tomography imaging in evaluation of biochemical recurrence in prostate cancer patients. World journal of nuclear medicine. 2019 Jul-Sep;18(3):244–50. doi: 10.4103/wjnm.WJNM_47_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rauscher I, Maurer T, Beer AJ, Graner FP, Haller B, Weirich G, et al. Value of 68Ga-PSMA HBED-CC PET for the Assessment of Lymph Node Metastases in Prostate Cancer Patients with Biochemical Recurrence: Comparison with Histopathology After Salvage Lymphadenectomy. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2016 Nov;57(11):1713–9. doi: 10.2967/jnumed.116.173492. [DOI] [PubMed] [Google Scholar]
- 22.Fendler WP, Eiber M, Beheshti M, Bomanji J, Ceci F, Cho S, et al. (68)Ga-PSMA PET/CT: Joint EANM and SNMMI procedure guideline for prostate cancer imaging: version 10. European journal of nuclear medicine and molecular imaging. 2017 Jun;44(6):1014–24. doi: 10.1007/s00259-017-3670-z. [DOI] [PubMed] [Google Scholar]