Abstract
The aim of this study was to examine toxic effects of tranexamic acid (TXA) on the viability of human chondrocytes. Our hypothesis was that TXA damages human chondrocytes. Chondrocytes were cultured from donated human knee joints. TXA was added to these cultures. Toxicity were analyzed by using LDH und XTT Elisa assays, light microscopy and fluorescence microscopy. The results show that TXA damages human chondrocytes in vitro. We cannot recommend the use of TXA in hemiarthroplasty of the hip or unicompartmental knee arthroplasty in higher concentrations.
Keywords: Human chondrocytes, Tranexamic acid, Toxicity, Viability, Cell damage
1. Introduction
Tranexamic acid (TXA) is a hemostatic agent which generates its antihemorrhagic effect by inhibiting the fibrinolytic characteristics of plasmin. It binds to plasminogen and inhibits its activation to plasmin.1,2
Originally used to treat bleeding as a cause of local or generalized hyperfibrinolysis it is now widely accepted as a prophylactic application in orthopedic procedures such as arthroplasty of the hip and knee or anterior cruciate ligament reconstruction.3, 4, 5 Several studies have shown that administration of TXA benefits in reduced blood loss and transfusion rates.6, 7, 8 Although the systemic intravenous application of TXA has been proven to be a safe method without increasing the incidence of thromboembolic events, intraarticular application seems to be an effective alternative especially for high-risk patients. Ultimately the ideal application route remains unclear.9, 10, 11, 12
However, only few studies have investigated the possible toxicity of TXA on human chondrocytes when administered topically.13, 14, 15, 16, 17 Especially in procedures where native cartilage remains preserved, such as hemiarthroplasty of the hip or unicompartmental knee replacement, possible toxic effects of TXA could be devastating.
The aim of the present work was to examine the toxicity of TXA towards human chondrocytes in vitro. We aimed to investigate the effects of short- and long-term exposure and to establish a possible safe dose. The hypothesis of that study was that TXA damages human chondrocytes in vitro in a dose and time depended way.
2. Methods
Tissue culture plastic ware was obtained from BD (Germany). Culture medium, phosphate buffer saline (PBS), trypsin, fetal calf serum (FCS) and all other reagents were obtained from Invitrogen (Germany).
2.1. Chondrocyte isolation and culture
Chondrocyte isolation was performed as described previously.18 Human cartilage was obtained from 7 patients undergoing arthroplasty of the knee and hip (mean age 67,86 years, five males, three females, age range 60–79). Donors were excluded when presenting infectious signals or tumorous diseases. All patients were informed and provided written informed consent to participate in the study. Experimental protocols were approved by the local ethics committee (3769-05/13). Human cartilage was minced and digested in medium containing 1 mg/ml of pronase (Roche, Germany) for 30 min at 37 °C. Digestion medium was discarded and the tissue was digested with medium containing 1 mg/ml clostridial collagenase (Roche, Germany) at 37 °C for 12 h. Next, the digested solution was filtered (70 μm Nylon, BD Falcon, Germany) and centrifuged at 1200 rpm for 10 min. The cell pellet was washed three times with phosphate buffer saline (PBS). Then, chondrocytes were suspended in DMEM Hams-F12 with 10% FCS, 1% penicillin/streptomycin and cultured at 37 °C in 95% air and 5% CO2
2.2. Chondrocytes treatment
Chondrocytes were seeded on 48-well plates at a sub-confluent density of 20.000 cells/cm2 and cultured for 48 h to allow attachment of the cells. Then they were exposed to TXA (Tranexamsäure Carino 100 mg/ml Injektionslösung, PZN: 10816978, CARINOPHARM GmbH, Elze, Germany) concentrations of 0 mg/ml, 6,25 mg/ml, 12,5 mg/ml, 25 mg/ml, 50 mg/ml and 100 mg/ml. Total length of exposure were 336 h. Medium was exchanged every 48 h containing TXA in the above mentioned concentrations, DMEM Hams-F12 with 10% FCS and 1% penicillin/streptomycin.
2.3. Visualization of defect cell structures and cell death
Chondrocytes, cultured on chamber slides with a density of 2 × 104 cells/cm,2 were incubated with the above mentioned TXA concentrations over a period of 4 h, 24 h and 48 h. After the washing procedure, cells were stained with fluorescein and propidium iodide (PI) (20 μl/ml) for 15 min. Documentation was performed immediately by light microscopy and fluorescence microscopy (microscope CKX 41, Olympus, Hamburg, Germany).
2.4. XTT Elisa
The XTT assay (Cytotoxicity Detection Kit II (XTT), Roche Molecular Biochemicals, Switzerland) was used for the spectrophotometric quantification of cell growth and viability. This is based on the cleavage of the tetrazolium salt XTT which forms a formazan dye by metabolic active cells. This conversation only occurs in viable cells. An increase in overall XTT activity is, in direct proportion, the result of an increase in the number of living cells. Cells, grown in a 96-well tissue culture plates, were treated with TXA concentrations und length of exposure shown above. After washing with PBS twice, cells were incubated with the XTT solution for 4 h. After this incubation period, formazan solution is formed, and this is spectrophotometrically quantified using an ELISA plate reader.
2.5. LDH Elisa
LDH activity is a marker of cell death. Release of LDH indicates the loss of membrane plasma integrity. The cytotoxicity was determined by quantifying the lactate dehydrogenase activity in the medium. 100 μl supernatant was added to 100 μl of reagent from the test kit (Cytotoxicity Detection Kit, Roche Molecular Biochemicals, Switzerland) on a 96-well measuring plate. Activity was determined by the colorimetric measurement of the reduction of sodium pyruvate in the presence of NADH and expressed as the percentage of total enzyme activity liberated from chondrocytes in the presence of TXA.
2.6. Statistical Analysis
Statistical Analysis was performed using Microsoft Office Excel. Graphics were designed using Microsoft Office Excel. A p value of <0.05 was considered to be significant.
3. Results
3.1. Light microscopy
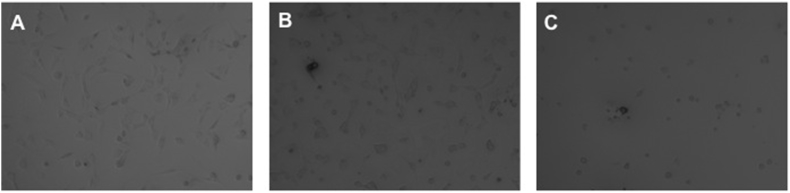
Defect cell structures were registrated with increasing TXA concentrations and length of exposure. After an incubation time of 24 h and a TXA concentration above 12,5 mg/ml Chondrocytes were globular, shrunken and showed partial losses of cell contacts (Fig. 1A). Above 25 mg/ml cells were drastically reduced, shrunken und showed no adhesion contacts at all (Fig. 1B and C).
Fig. 1.
Light microscopy images showing effect of TXA on chondrocytes after 24 h exposure time. A 12,5 mg/ml, B 25 mg/ml, C 50 mg/ml. One representative image is shown.
3.2. Visualization of defect cell structures and cell death
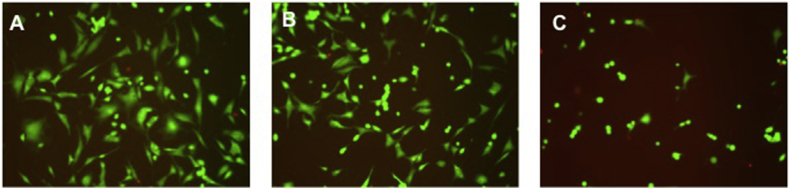
Fluorescein and PI staining of chondrocytes visualized a reduction of vital cells (green coloring) and emerging of necrotic cells (red coloring) with increasing TXA concentrations and length of exposure. TXA concentrations above 25 mg/ml induced cell necrosis and vanishing of vital cells (Fig. 2B, C). Rarely any necrotic cells were detected after exposure to TXA concentrations below 25 mg/ml (Fig. 2A).
Fig. 2.
Live-dead images of chondrocytes after 24 h TXA exposure visualized by fluorescence microscopy. A 12,5 mg/ml, B 25 mg/ml, C 50 mg/ml. Vital cells stain green (fluorescein positive and PI negative), necrotic cells stain red (fluorescein negative and PI positive). One representative picture is shown.
3.3. XTT Elisa
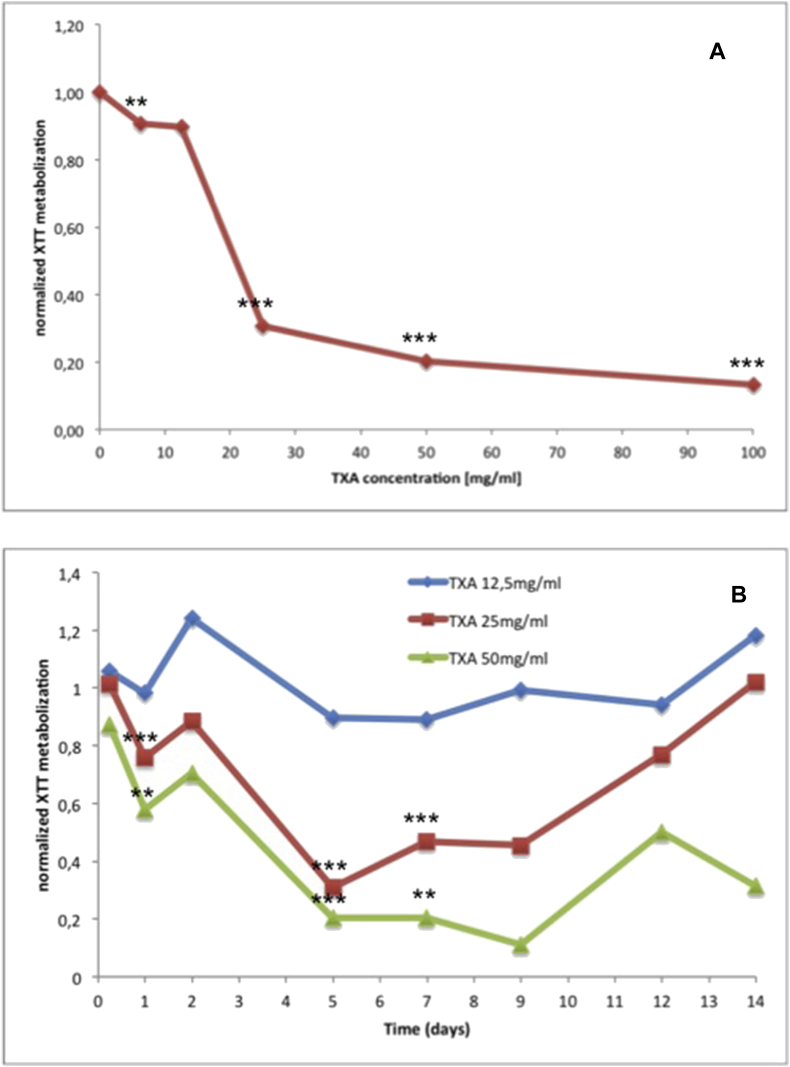
XTT activity was indirect proportional with increasing TXA concentrations and length of exposure. Cells showed decreased metabolization of XTT and thus decreased viability when confronted with increasing TXA concentrations over a longer period of time (Fig. 3A and B).
Fig. 3.
A. XTT Assay for determination of viability. Graph showing the correlation of XTT metabolism of chondrocytes and TXA concentration after 5 days of exposure. B. XTT Assay for determination of viability. Graph showing the correlation of XTT metabolism of chondrocytes and length of exposure to TXA at different concentrations.
When administered with a TXA concentration of 25 mg/ml XTT activity was increasing again after five days of exposure. This revealed the regeneration potential of chondrocytes and the toxic threshold of TXA concentrations below 25 mg/ml (Fig. 3B).
3.4. LDH Elisa
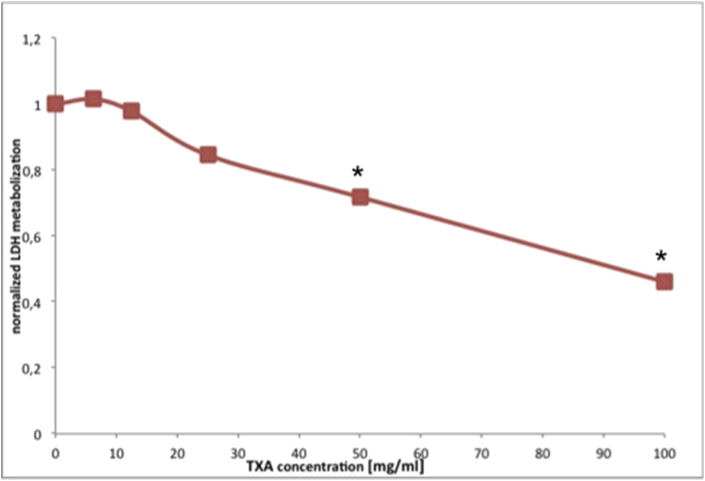
LDH activity decreased already after TXA exposure for 2,5 min. Hence LDH was completely released after 2,5 min which indicates that the toxic effect of TXA starts immediately (Fig. 4).
Fig. 4.
LDH Assay for determination of toxicity. Graph showing the correlation of LDH metabolism of human chondrocytes and TXA concentration after 2.5 min exposure.
4. Discussion
The hypothesis of this study was confirmed by the results: TXA has chondrotoxic characteristics which are directly proportional to increasing concentration and length of exposure. However, concentrations below 25 mg/ml seem to allow a recovery of chondrocytes over a longer observation period. This identified toxic threshold is relevant for clinical practice, since TXA concentrations easily surpass this limit when administered topically.13,19 On the other hand when administered systemically concentrations measured in the synovial fluid are far below this critical limit.1,20
Although numerous studies were published who examined the effects of TXA in vitro this is one of the few studies which investigated the effects towards human chondrocytes.15, 16, 17,21
Besides that we found no study which utilized such a broad observation period. We detected toxic effects of TXA as early as 2,5 min after exposition as well as long-term impacts after 336 h. Total intraarticular elimination time of TXA remains unclear. Especially treatment with high-dose TXA up to 100 mg/ml can lead to remaining residuals up to 24 h and longer.1,20 Our findings differ partly with the results of Goderecci et al. who found no toxic effects after 10 min but significant effects after 48 h of exposure to TXA.17
We identified a toxic threshold of TXA at a concentration of 25 mg/ml. Chondrocytes seemed to regenerate when exposed to lower concentrations over time. These results align to a great extend within the literature. Parker et al. established a safe dose at concentrations between 10 and 20 mg/ml using 2D and 3D human chondrocyte models.15 Tutle et al. found no toxic potential of TXA when exposed to bovine cartilage explants and murine chondrocytes at concentrations up to 25 mg/ml.13 More recently a comprehensive study by McLean et al. confronted human periarticular tissue with TXA and observed toxic effects at 50 mg/ml.16 Sitek et al. found not toxic effects on human chondrocyte gel drafts when exposed to TXA concentrations between 10 and 20 mg/ml.22 No chondrotoxicity was observed by Ambra et al. who treated porcine osteochondral plugs with low doses of TXA (1; 2; 4 mg/ml).14 Marmotti et al. reported no toxic effects of TXA towards human synovial biopsies.21
Ultimately we cannot recommend the use of intraarticular administration of TXA when human cartilage is left untouched such is the case in hemiarthroplasty of the hip, unicompartmental knee arthroplasty or patellofemoral joint replacement. We even would suggest performing a patellar joint replacement or patelloplasty when large doses of TXA are used.
There are limitations of this study. Chondrocyte cultures were obtained from osteoarthritic knee joints and thus viability could be affected beforehand. Also we do not know the clearance and distribution of TXA in the joint space. Our study cannot give answer to the exact TXA damage mechanism on a cellular basis and how chondrocytes in a biological environment react. Further human clinical trials and in vivo studies are needed to clarify final recommendations for the topical use of TXA.
5. Conclusions
The data generated by this study clearly shows toxic effects of TXA towards human chondrocytes in vitro. This toxicity directly correlates with increasing concentration and length of exposure. Damage occurs as early as 2,5 min after exposure. The toxic threshold of TXA seems to be above a concentration of 25 mg/ml. Based on this data the use of intra-articular TXA in the presence of native cartilage tissue seems to be critical. These findings need to be supported by in vivo investigations before drawing definite clinical conclusions.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for profit sectors.
Declaration of competing interest
None.
References
- 1.No Authors Listed. Prescribing Information Issued Aug 2014. Tranexamsäure Carino 100 Mg/ml Amp PZN: 10816978.
- 2.No authors listed https://www.drugs.com/pro/tranexamic-acid-injection.html (medically reviewed on Oct 1, 2018)
- 3.Sukeik M., Alshryda S., Haddad F.S., Mason J.M. Systematic review and metaanalysis of the use of tranexamic acid in total hip replacement. J Bone Jt Surg Br Vol. 2011;93-B:39–46. doi: 10.1302/0301-620X.93B1.24984. [DOI] [PubMed] [Google Scholar]
- 4.Stowers M.D.J., Aoina J., Vane A., Poutawera V., Hill A.G., Munro J.T. Tranexamic acid in knee surgery study — a multicentered, randomized, controlled trial. J Arthroplast. 2017;32(11):3379–3384. doi: 10.1016/j.arth.2017.05.058. [DOI] [PubMed] [Google Scholar]
- 5.Karaaslan F., Karaolu S., Yurdakul E. Reducing intra-articular hemarthrosis after arthroscopic anterior cruciate ligament reconstruction by the administration of intravenous tranexamic acid: a prospective, randomized controlled trial. Am J Sports Med. 2015;43:2720–2726. doi: 10.1177/0363546515599629. [DOI] [PubMed] [Google Scholar]
- 6.Sassoon A., Nam D., Jackups R. Tranexamic acid: optimal blood loss management in surface replacement arthroplasty. Bone Joint Lett J. 2016;98-B:173–178. doi: 10.1302/0301-620X.98B2.36776. [DOI] [PubMed] [Google Scholar]
- 7.Xie J., Hu Q., Ma J., Huang Q., Pei F. Multiple boluses of intravenous tranexamic acid to reduce hidden blood loss and the inflammatory response following enhanced recovery primary total hip arthroplasty: a randomised clinical trial. Bone Joint Lett J. 2017;99-B:1442–1449. doi: 10.1302/0301-620X.99B11.BJJ-2017-0488.R1. [DOI] [PubMed] [Google Scholar]
- 8.Henry D.A., Carless P.A., Moxey A.J. Anti fibrinolytic use for minimising perioperative allogeneic blood transfusion (review) Cochrane Database Syst Rev. 2011;3 doi: 10.1002/14651858.CD001886.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yuan Z.F., Yin H., Ma W.P., Xing D.L. The combined effect of administration of intravenous and topical tranexamic acid on blood loss and transfusion rate in total knee arthroplasty: combined tranexamic acid for TKA. Bone Jt Res. 2016;5:353–361. doi: 10.1302/2046-3758.58.BJR-2016-0001.R2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tzatzairis T.K., Drosos G.I., Kotsios S.E. Intravenous vs. topical tranexamic acid in total knee arthroplasty without tourniquet application: a randomized controlled study. J Arthroplast. 2016;31:2465–2470. doi: 10.1016/j.arth.2016.04.036. [DOI] [PubMed] [Google Scholar]
- 11.Panteli M., Papakostidis C., Dahabreh Z., Giannoudis P.V. Topical tranexamic acid in total knee replacement: a systematic review and meta-analysis. The Knee. 2013;20(5):300–309. doi: 10.1016/j.knee.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 12.Seo J.-G., Moon Y.-W., Park S.-H., Kim S.-M., Ko K.-R. The comparative efficacies of intra-articular and IV tranexamic acid for reducing blood loss during total knee arthroplasty. Knee Surg Sport Traumatol Arthrosc. 2013;21:1869–1874. doi: 10.1007/s00167-012-2079-2. [DOI] [PubMed] [Google Scholar]
- 13.Tuttle J.R., Feltman P.R., Ritterman S.A., Ehrlich M.G. Effects of tranexamic acid cytotoxicity on in vitro chondrocytes. Am J Orthoped. 2015;44:E497–E502. [PubMed] [Google Scholar]
- 14.Ambra L.F., de Girolamo L., Niu W., Phan A., Spector M., Gomoll A.H. No effect of topical application of tranexamic acid on articular cartilage. Knee Surg Sport Traumatol Arthrosc. 2017 doi: 10.1007/s00167-017-4746-9. [DOI] [PubMed] [Google Scholar]
- 15.Parker J.D., Lim K.S., Kieser D.C., Woodfield T.B.F., Hooper G.J. Is tranexamic acid toxic to articular cartilage when administered topically? Bone Jt J. 2018;100-B(3):404–412. doi: 10.1302/0301-620X.100B3.BJJ-2017-1135.R1. [DOI] [PubMed] [Google Scholar]
- 16.McLean M., McCall K., Smith I.D.M. Tranexamic acid toxicity in human periarticular tissues. Bone Jt Res. 2019 doi: 10.1302/2046-3758.81.BJR-2018-0181.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goderecci R., Giusti I., Necozione S. Short exposure to tranexamic acid does not affect, in vitro, the viability of human chondrocytes. Eur J Med Res. 2019 Feb 22;24(1):15. doi: 10.1186/s40001-019-0373-x. https://doi: 10.1186/s40001-019-0373-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Röhner E., Kolar P., Seeger J.B. Toxicity of antiseptics against chondrocytes: what is best for the cartilage in septic joint surgery? Int Orthop. 2011;35(11):1719–1723. doi: 10.1007/s00264-010-1178-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mao Z., Yue B., Wang Y., Yan M., Dai K. A comparative, retrospective study of peri-articular and intra-articular injection of tranexamic acid for the management of postoperative blood loss after total knee arthroplasty. BMC Muscoskelet Disord. 2016;17:438. doi: 10.1186/s12891-016-1293-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahlberg A., Eriksson O., Kjellman H. Diffusion of tranexamic acid to the joint. Acta Orthop Scand. 1976;47:486–488. doi: 10.3109/17453677608988725. [DOI] [PubMed] [Google Scholar]
- 21.Marmotti A., Mattia S., Mangiavini L. Tranexamic acid effects on cartilage and synovial tissue: an in vitro study for a possible safe intra-articular use. J Biol Regul Homeost Agents. 2016;30(Suppl 1):33–40. [PubMed] [Google Scholar]
- 22.Sitek P., Wysocka-Wycisk A., Kpski F. PRP-fibrinogen gel-like chondrocyte carrier stabilized by TXA-preliminary study. Cell Tissue Bank. 2013;14:133–140. doi: 10.1007/s10561-011-9290-0. [DOI] [PubMed] [Google Scholar]