Abstract
Polymethylmethacrylate (PMMA) represents the current gold standard as an antibiotic delivery carrier in orthopaedic surgery. Despite the accepted use of local antibiotic carriers, there aren't any conclusive data comparing PMMA to Bone Graft Substitutes. The aim of this in vitro study was to compare the elution profile of gentamicin from various preparations of PMMA cements and Herafill beads. All cements had high initial elution during the first hour which then slowly decreased, Herafill beads on the other hand showed its higher elution around the eighth hour. Herafill, in general, presented the highest elution of gentamicin regardless of its input amount.
Keywords: Gentamicin, In vitro release, Palacos, Copal, PMMA, Herafill
1. Introduction
Osteomyelitis is a bone infection by microorganisms and despite advances in antibiotics and operative techniques, remains an orthopaedic challenge. While most acute bone infections are usually successfully treated with systemically administered antibiotics, chronic infections and infections in the presence of foreign materials usually require operative treatment with debridement, removal of metals, systemic antibiotics, and very often adjunctive locally administered antibiotics.1,2
Local antibiotic delivery for prevention and treatment of bone infections was introduced in clinical practice many decades ago.3,4 Local antibiotic administration provides high concentrations of antibiotics while minimizing systemic toxicity. Therefore, they are considered to be safe and free of systemic side effects. Furthermore, they can minimize hospitalization time and treatment costs.5 Antibiotic-loaded bone cement (polymethylmethacrylate, PMMA) is the most widely used material and represents the current gold standard as an antibiotic delivery carrier in orthopaedic surgery.
Antibiotic loading of bone cement can be performed during the mixing procedure (ad hoc mixed) or during production of the powder component (premixed). Premixed bone cements with antibiotics are available since the beginning of the 1990s. But still, manual blending of a premixed cement with a second antibiotic is a common procedure among orthopedic surgeons.6
The main disadvantage of PMMA is its lack of biodegradability with the need of surgical removal. This has led to the development of biodegradable materials.7 Biodegradable materials have been studied during the last two decades and include proteins (collagen, gelatin, thrombin, etc.), bone grafts and substitutes, and synthetic polymers. These biodegradable carriers have the ability to elution high local concentrations of antibiotics with serum concentrations in safe margins, without the need of additional surgery to remove them.8 In specific, Bone Graft Substitutes (BGS) are porous calcium-based products with osteoconductive properties and they are gradually replaced by new bone. These materials where designed to mimic bone properties. The most attractive are calcium phosphate-based materials due to their similarity to the bone composition.
The choice of the loaded antibiotic is of paramount importance because it has to fulfill certain requirements, such as to be heat stable and resistant to polymerization reaction and of course to have broad antibacterial spectrum. As such, aminoglycosides and vancomycin are the most commonly used antibiotics and especially gentamicin takes precedence over the other antibiotics.9 Gentamicin is an effective and safe commonly used antibiotic which is active against a wide range of bacterial infections, mostly Gram-negative bacteria including Pseudomonas, Proteus, Escherichia coli, Klebsiella pneumoniae, Enterobacter aerogenes, and the Gram-positive Staphylococcus.10
Despite the accepted use of local antibiotic carriers as therapy in orthopaedic surgery, there aren't any conclusive clinical data comparing BGS to PMMA bone cement in osteomyelitis treatment. The aim of this in vitro study was to compare the elution profile of gentamicin from various preparations of cements (either premixed or ad hoc mixed home-made) and one ready-to-use gentamicin-loaded BGS, as monotherapy, or in combination with other antibiotics. in order to determine a) whether there is significant difference in their elution profile and b) whether there is significant difference in the elution profile between PMMA cement and a calcium sulfate-based bone graft substitute (Herafill beads) that contains the same concentration of gentamicin.
2. Materials and methods
2.1. Groups of gentamicin-loaded specimens
In our experiments, we compared one commercially available ready-to-use gentamicin-loaded BGS (Group A), three commercially available premixed PMMA cements (Groups B-D) and four ad hoc mixed home-made PMMA cements (Groups F-G). In specific, the study materials are enlisted below:
-
•
Group A: HERAFILL® beads G containing 1% Gentamicin (Herafill beads G)
-
•
Group B: PALACOS® R + G containing 1.2% Gentamicin (Palacos R + G)
-
•
Group C: COPAL® G + V containing 1.2% Gentamicin and 5% Vancomycin (Copal G + V)
-
•
Group D: COPAL® G + C containing 2.4% Gentamicin and 2.4% Clindamycin (Copal G + C)
-
•
Group E: home-made PALACOS® R + G containing 1% Gentamicin (Palacos R + G 1%)
-
•
Group F: home-made PALACOS® R + G containing 1.2% Gentamicin (Palacos R + G 1.2%)
-
•
Group G: home-made PALACOS® R + G + V containing 1.2% Gentamicin and 5% Vancomycin (Palacos R + G + V)
-
•
Group H: home-made PALACOS® R + G containing 2.4% Gentamicin (Palacos R + G 2.4%)
The premixed PMMA bone cements PALACOS® R, PALACOS® R + G, COPAL® G + V and COPAL® G + C and the ready-to-use BGS HERAFILL® beads G, were all donated from Heraeus Medical GmbH, Werheim, Germany.
2.2. Home-made PMMA cements preparation
Gentamicin was added as gentamicin sulfate and was purchased by SIGMA-ALDRICH (Merck KGaA, Darmstadt, Germany). Vancomycin was added as vancomycin hydrochloride and was purchased by Mylan (Generics Pharma Hellas LTD, Athens, Greece). All home-made specimens were prepared in a sterile operating theater according to the manufacturer's instructions. For group E (Palacos R + G 1%), 0.5g gentamicin was added to 40g PALACOS® R so that the gentamicin concentration would be the same as the one in the Herafill. For group F (Palacos R + G 1.2%) 0.5g gentamicin sulfate was added to 40g PALACOS® R (to reach the concentration of the premixed PALACOS® R + G). For group G (Palacos R + G + V), 0.5g gentamicin and 2.0g vancomycin were added to 40g PALACOS® R (concentrations equivalent to the Copal G + V). Finally, for group H (Palacos R + G 2.4%), 1g gentamicin was added to 40g PALACOS® R so that the gentamicin concentration would be the same as the one in the Copal G + C. The monomer liquid was poured into a mixing bowl and then the powder cement was added. They were mixed for 1 min at room temperature until the beginning of the dough phase. Then the paste was pressed in silicon bead shaped moulds with similar dimensions (5 cm × 7 cm × 7 cm) to Herafill, in order to allow the polymerization process and obtain identical beads for all the groups of PMMA cements. After the beads were solidified, they were removed from the moulds and were stored at room temperature until further use.
2.3. In vitro gentamicin elution
One specimen (bead) per group was placed in a falcon tube containing 25 ml 1 × PBS (Biosera, Nuaille, France) at pH 7.4, under sterile conditions. The falcon tubes were then placed on a shaker in an incubator set at 37 °C for 20 days. At certain time points (1, 8, 24, 48, 72, 96, 120, 144, 168, 192, 216, 240, 288, 384, 480 h) 2 ml aliquots were taken from each group of specimens. The rest was discarded and replaced with 25 ml of fresh sterile 1 × PBS. The aliquots were stored in −20 °C until gentamicin concentration measurement. Each experiment was carried out in sixplicate to ensure reproducibility of the results. The concentration of gentamicin was measured in the COBAS INTEGRA 400 plus clinical analyzer (Roche Hellas, Athens, Greece) using a quantitative fluorescent polarizing immunoassay. The lowest limit of detection for this immunoassay is 0.04μg/mL.
2.4. Statistical analysis
A sample size of 6 specimen was used for each experimental group of our study. All results are displayed as mean ± standard deviation. We performed T-test analysis between groups in order to determine differences in the antibiotic elution. A p-value of <0.05 was considered significant. The statistical analysis was conducted using SPSS 21.0 software (SPSS Inc. Chicago, Il, USA).
3. Results
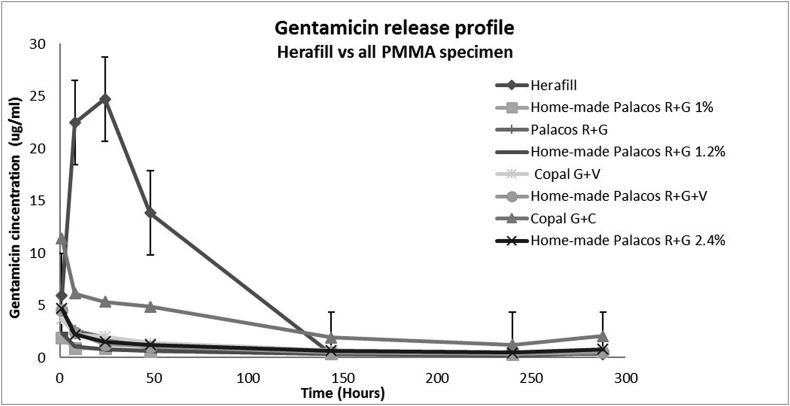
The mean concentration of gentamicin elution of all specimen at 1, 8, 24, 48, 144, 240 and 288 h of incubation is presented in Table 1. All PMMA cement beads had high initial elution during the first hour which then slowly decreased, Herafill on the other hand showed its higher elution around the eighth hour and the peak concentration at 24 h. Herafill, in general, had the highest in vitro elution of gentamicin compared to the PMMA specimen regardless of the input amount of the antibiotic (Fig. 1).
Table 1.
Mean (±standard deviation) concentration of gentamicin elution for the seven time points (1, 8, 24, 48, 144, 240 and 288 h) from Herafill and PMMA cements of the study.
| Time (hours) | Mean gentamicin elution |
|||||||
|---|---|---|---|---|---|---|---|---|
| Herafill (ug/ml) | Palacos R + G (ug/ml) | Copal G + V (ug/ml) | Copal G + C (ug/ml) | Home-made Palacos R + G 1% (ug/ml) | Home-made Palacos R + G 1.2% (ug/ml) | Home-made Palacos R + G + V (ug/ml) | Home-made Palacos R + G 2.4% (ug/ml) | |
| 1 | 5.91 ± 0.76 | 4.39 ± 0.67 | 3.69 ± 0.75 | 11.39 ± 1.7 | 1.88 ± 0.24 | 2.34 ± 0.26 | 4.54 ± 0.82 | 4.67 ± 0.51 |
| 8 | 22.46 ± 0.42 | 2.59 ± 0.28 | 2.21 ± 0.33 | 6.13 ± 0.56 | 0.92 ± 0.25 | 1.11 ± 0.09 | 2.38 ± 0.06 | 2.21 ± 0.19 |
| 24 | 24.72 ± 3.55 | 1.92 ± 0.71 | 2.03 ± 0.20 | 5.34 ± 0.34 | 0.88 ± 0.26 | 0.75 ± 0.12 | 1.26 ± 0.05 | 1.51 ± 0.23 |
| 48 | 13.81 ± 0.36 | 0.91 ± 0.07 | 1.43 ± 0.43 | 4.86 ± 1.01 | 0.63 ± 0.03 | 0.61 ± 0.06 | 1.05 ± 0.21 | 1.22 ± 0.12 |
| 144 | 0.33 ± 0.17 | 0.37 ± 0.18 | 0.68 ± 0.41 | 1.92 ± 0.26 | 0.38 ± 0.06 | 0.33 ± 0.11 | 0.52 ± 0.11 | 0.67 ± 0.21 |
| 240 | 0.34 ± 0.17 | 0.39 ± 0.01 | 0.48 ± 0.25 | 1.25 ± 0.21 | 0.32 ± 0.13 | 0.23 ± 0.16 | 0.38 ± 0.08 | 0.47 ± 0.32 |
| 288 | 0.35 ± 0.15 | 0.57 ± 0.13 | 0.56 ± 0.14 | 2.07 ± 0.13 | 0.48 ± 0.12 | 0.44 ± 0.16 | 0.52 ± 0.08 | 0.80 ± 0.20 |
Fig. 1.
The elution profile of gentamicin from Herafill and all PMMA specimens for 288 h.
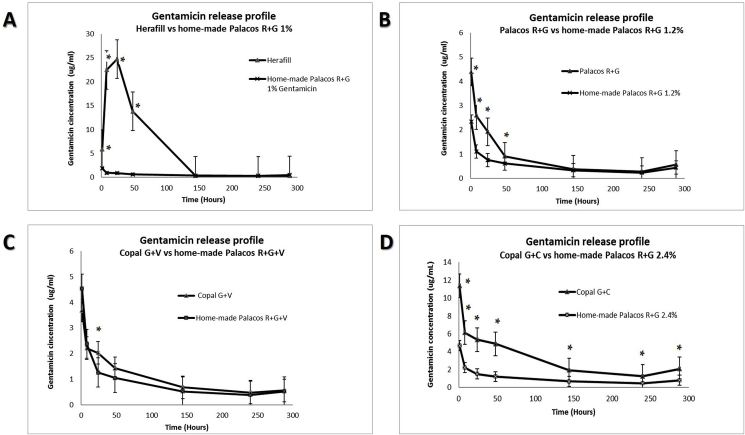
In specific, gentamicin elution from Herafill was significantly higher compared to the home-made Palacos R + G 1% and this significant difference lasted for at least 48 h (Fig. 2A). Palacos R + G showed significantly higher gentamicin elution compared to the home-made Palacos R + G 1.2% which was kept almost double for 24 h (Fig. 2B). Copal G + V showed slightly better gentamicin elution profiles than the home-made Palacos R + G + V at all seven time points but differences were not statistically significant (Fig. 2C). Copal G + C showed superior gentamicin elution profile compared to our home-made control Palacos R + G 2.4% containing only gentamicin (Fig. 2D). The p-values of T test analysis for the seven time points are presented in Table 2.
Fig. 2.
A) The elution profile of gentamicin from Herafill and home-made Palacos R + G both containing 1% gentamicin for 288 h. B) The elution profile of gentamicin from Palacos R + G and home-made Palacos R + G both containing 1.2% gentamicin for 288 h. C) The elution profile of gentamicin from Copal G + V and home-made Palacos R + G + V both containing 1.2% gentamicin and 5% vancomycin for 288 h. D) The elution profile of gentamicin from Copal G + C and home-made Palacos R + G both containing 2.4% gentamicin for 288 h * indicates statistically significant difference.
Table 2.
T-test analysis results from the comparison of Herafill and the PMMA cements of the study.
| Time (Hours) | Specimen comparison | p-value | Specimen comparison | p-value | Specimen comparison | p-value | Specimen comparison | p-value |
|---|---|---|---|---|---|---|---|---|
| 1 | Herafill | 0.001 | Palacos R + G | 0.008 | Copal G + V | 0.255 | Copal G + C | 0.003 |
| Home-made Palacos R + G 1% | Home-made Palacos R + G 1.2% | Home-made Palacos R + G + V | Home-made Palacos R + G 2.4% | |||||
| 8 | Herafill | <0.001 | Palacos R + G | 0.001 | Copal G + V | 0.424 | Copal G + C | <0.001 |
| Home-made Palacos R + G 1% | Home-made Palacos R + G 1.2% | Home-made Palacos R + G + V | Home-made Palacos R + G 2.4% | |||||
| 24 | Herafill | <0.001 | Palacos R + G | 0.049 | Copal G + V | 0.003 | Copal G + C | <0.001 |
| Home-made Palacos R + G 1% | Home-made Palacos R + G 1.2% | Home-made Palacos R + G + V | Home-made Palacos R + G 2.4% | |||||
| 48 | Herafill | <0.001 | Palacos R + G | 0.004 | Copal G + V | 0.241 | Copal G + C | 0.003 |
| Home-made Palacos R + G 1% | Home-made Palacos R + G 1.2% | Home-made Palacos R + G + V | Home-made Palacos R + G 2.4% | |||||
| 144 | Herafill | 0.214 | Palacos R + G | 0.776 | Copal G + V | 0.547 | Copal G + C | 0.003 |
| Home-made Palacos R + G 1% | Home-made Palacos R + G 1.2% | Home-made Palacos R + G + V | Home-made Palacos R + G 2.4% | |||||
| 240 | Herafill | 0.571 | Palacos R + G | 0.816 | Copal G + V | 0.571 | Copal G + C | 0.024 |
| Home-made Palacos R + G 1% | Home-made Palacos R + G 1.2% | Home-made Palacos R + G + V | Home-made Palacos R + G 2.4% | |||||
| 288 | Herafill | 0.623 | Palacos R + G | 0.850 | Copal G + V | 0.612 | Copal G + C | 0.020 |
| Home-made Palacos R + G 1% | Home-made Palacos R + G 1.2% | Home-made Palacos R + G + V | Home-made Palacos R + G 2.4% |
4. Discussion
The results of this study suggest that our practice of adding gentamicin manually to PMMA cement in the operating theater produces on the one hand inferior antibiotic elution at concentration of 1.2% and 2.4% (without the presence of vancomycin) but on the other hand superior antibiotic elution when vancomycin is present to the mix. This observation is in agreement with the findings of Frew et al., who had also compared the elution of gentamicin from their also home-made Palacos R + G 1.2% cement containing vancomycin with the Copal G + V.11 Frew et al. found that the addition of 2 g vancomycin powder to gentamicin-impregnated bone cement by hand significantly increases the elution of both antibiotics compared with commercially prepared cements containing vancomycin.11 On the other hand, Boelch et al. found no influence of vancomycin addition on gentamicin elution even for PALACOS® R + G (0.5 g gentamicin premixed bone cement) manually blended with 2.0 g vancomycin.6 Furthermore, Herafill is obviously superior in gentamycin elution as compared with any other preparations, if bulk concentrations are necessary right from the beginning. therefore, is superior for clinical use due to its other advantages. To the best of our knowledge, this is the first study comparing the gentamicin in vitro elution profile of Herafill to PMMA cement beads containing the same amount of antibiotic. Herafill revealed a much higher gentamicin elution for at least 48 h as compared to ad hoc mixed Palacos R + G 1%.
PMMA is a widely used cement that, when loaded with antibiotics, has been shown to reduce infection rates. However, PMMA is not resorbable and requires a second surgery for removal. Alternatively, BGS present the advantage of being completely reabsorbed. Calcium sulfate-based, gentamicin-loaded BGS offer an expedient extension of the treatment of osteomyelitis. In specific, the effectiveness and safety of Herafill have already been investigated in a couple of studies which all end in highlighting its overall reliability as bone substitute.12, 13, 14 Most recently, in a recent German study, the use of Herafill in parallel with systemic administration of antibiotics achieved a remission rate of 85% for recurrent infections of osteomyelitis that had been unsuccessfully treated by the primary implantation of a PMMA chain and systemic antibiotics, encouraging the use of Herafill for treatment of recurrent osteomyelitis.15
The ad hoc addition of antibiotic powder to PMMA cement has been a standard practice in arthroplasty surgery for many years. Due to the antibiotic resistance crisis, ad hoc addition of antibiotics such as gentamicin and vancomycin to PMMA cements has been crucial the last years. Our study aided our understanding of gentamicin elution profile of calcium sulfate beads compared to that of PMMA cement beads and of premixed PMMA cement beads compared to that ad hoc mixed PMMA bead cements.
5. Conclusions
This was an in vitro study mimicking the in vivo elution conditions of gentamicin and our conclusions could be further confirmed in a clinical setting where the materials will be evaluated in the actual environment that will be exposed to exert their therapeutic effect. On the other hand, our simple approach provides a suitable tool for the evaluation of all the bulk of PMMA products currently in market.
Declaration of competing interest
None.
References
- 1.Lalidou F., Kolios G., Tavridou A., Drosos G.I. Bone grafts as carriers for local antibiotic delivery for the treatment and prevention of bone infections. Surg Technol Int. 2014;25:239–245. [PubMed] [Google Scholar]
- 2.Zalavras C.G., Patzakis M.J., Holtom P. Local antibiotic therapy in the treatment of open fractures and osteomyelitis. Clin Orthop Relat Res. 2004:86–93. doi: 10.1097/01.blo.0000143571.18892.8d. [DOI] [PubMed] [Google Scholar]
- 3.Buchholz H.W., Engelbrecht H. [Depot effects of various antibiotics mixed with Palacos resins]. Der Chirurg. Zeitschrift fur alle Gebiete der operativen Medizen. 1970;41:511–515. [PubMed] [Google Scholar]
- 4.Henry S.L., Ostermann P.A., Seligson D. The prophylactic use of antibiotic impregnated beads in open fractures. J Trauma. 1990;30:1231–1238. doi: 10.1097/00005373-199010000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Lalidou F., Kolios G., Drosos G.I. Bone infections and bone graft substitutes for local antibiotic therapy. Surg Technol Int. 2014;24:353–362. [PubMed] [Google Scholar]
- 6.Boelch S.P. Loading with vancomycin does not decrease gentamicin elution in gentamicin premixed bone cement. J Mater Sci Mater Med. 2017;28:104. doi: 10.1007/s10856-017-5915-6. [DOI] [PubMed] [Google Scholar]
- 7.Hake M.E. Local antibiotic therapy strategies in orthopaedic trauma: practical tips and tricks and review of the literature. Injury. 2015;46:1447–1456. doi: 10.1016/j.injury.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 8.McLaren A.C. Alternative materials to acrylic bone cement for delivery of depot antibiotics in orthopaedic infections. Clin Orthop Relat Res. 2004:101–106. doi: 10.1097/01.blo.0000143554.56897.26. [DOI] [PubMed] [Google Scholar]
- 9.Webb J.C., Spencer R.F. The role of polymethylmethacrylate bone cement in modern orthopaedic surgery. J Bone Jt Surg. 2007;89:851–857. doi: 10.1302/0301-620X.89B7.19148. British volume. [DOI] [PubMed] [Google Scholar]
- 10.Chang Y., Tai C.L., Hsieh P.H., Ueng S.W. Gentamicin in bone cement: a potentially more effective prophylactic measure of infection in joint arthroplasty. Bone Jt Res. 2013;2:220–226. doi: 10.1302/2046-3758.210.2000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frew N.M., Cannon T., Nichol T., Smith T.J., Stockley I. Comparison of the elution properties of commercially available gentamicin and bone cement containing vancomycin with 'home-made' preparations. Bone Jt J. 2017;99-b:73–77. doi: 10.1302/0301-620X.99B1.BJJ-2016-0566.R1. [DOI] [PubMed] [Google Scholar]
- 12.Coraca-Huber D., Hausdorfer J., Fille M., Nogler M., Kuhn K.D. Calcium carbonate powder containing gentamicin for mixing with bone grafts. Orthopedics. 2014;37:e669–672. doi: 10.3928/01477447-20140728-50. [DOI] [PubMed] [Google Scholar]
- 13.Coraca-Huber D.C. Antibiotic-loaded calcium carbonate/calcium sulfate granules as co-adjuvant for bone grafting. J Mater Sci Mater Med. 2015;26:5344. doi: 10.1007/s10856-014-5344-8. [DOI] [PubMed] [Google Scholar]
- 14.Marczak D., Synder M., Sibinski M., Okon T., Kowalczewski J. The use of calcium carbonate beads containing gentamicin in the second stage septic revision of total knee arthroplasty reduces reinfection rate. The Knee. 2016;23:322–326. doi: 10.1016/j.knee.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Gramlich Y., Walter G., Gils J., Hoffmann R. [Early results of adjuvant topical treatment of recurrent osteomyelitis with absorbable antibiotic carriers] Zeitschrift fur Orthopadie und Unfallchirurgie. 2017;155:35–44. doi: 10.1055/s-0042-112228. [DOI] [PubMed] [Google Scholar]