Abstract
Background
Scientists are trying to discover how to repair cartilage defects in knee osteoarthritis (KOA). In our previous study, we found a fibrocartilage-rich cover over the defective portions of cartilage after administering leukocyte-rich platelet-rich plasma (LR-PRP). This study aimed to investigate the efficacy of multiple injections of LR-PRP for the treatment of KOA and determine an LR-PRP treatment protocol for KOA in actual clinical practice.
Hypothesis
We hypothesized that using abundant LR-PRP would improve outcomes in patients with KOA.
Study design
Prospective, cross-sectional, interventional, randomized trial.
Methods
Intra-articular LR-PRP injections were administered to 50 knees. Patients received six injections of LR-PRP in total, which were administered at 4-week intervals. Patients were evaluated based on clinical outcomes, including visual analog scale (VAS) scores, Knee injury and Osteoarthritis Outcome Scores (KOOS), and magnetic resonance images (MRI) and radiographic findings before treatment and at 3 and 6 months after treatment. We investigated the recurrence of pain and presence/absence of MRI changes. Furthermore, we examined the Outcome Measures In Rheumatology-Osteoarthritis Research Society International (OMERACT-OARSI) responder criteria.
Results
The mean improvement rate, as assessed by VAS, was 61.6% (P < .0001). Concerning OMERACT-OARSI, 37 of 50 knees (74%) were considered responders. There was a significant difference in the follow-up MRI findings, as assessed by the MRI Osteoarthritis Knee Score for bone marrow lesions (P < .007). No significant difference in osteoarthritis grade was observed.
Conclusion
Our LR-PRP procedure resulted in 74% of knees being classified as responders, regardless of the degree of knee deformation. Multiple injections of LR-PRP was effective for advanced grades of KOA. Thus, based on the results of our study, we believe that LR-PRP should be implemented as an additional conservative treatment option for non-operative management of OA.
Trial registration
Japan Medical Association Center for Clinical Trials (JMA-XXX).
Keywords: Knee osteoarthritis, Leukocyte-rich platelet-rich plasma, Knee injury and osteoarthritis outcome score, Outcome measures in rheumatology-osteoarthritis research society international, Magnetic resonance imaging osteoarthritis knee score, Bone mallow lesion, Multiple injection
What is known about the subject: Recent reports suggest that leukocyte-rich platelet-rich plasma (LR-PRP) may be beneficial for long-term relief of osteoarthritis (OA) symptoms.
What this study adds to existing knowledge: Our LR-PRP procedure was effective in patients with knee OA, regardless of the degree of knee deformation. Therefore, we suggest that LR-PRP injections should be added as an additional conservative treatment option for management of OA.
1. Introduction
Osteoarthritis (OA) is a multifactorial chronic condition characterized by the destruction of articular cartilages, which leads to joint space loss.1 Significant efforts are being made by scientists to discover how to repair cartilage defects in knee OA (KOA). There are several operative (microfracture, osteochondral, and tissue engineered grafts) and non-operative (single-molecule agents, hyaluronic acid, and corticosteroid injections) treatment strategies for cartilage repair and management of KOA pain.
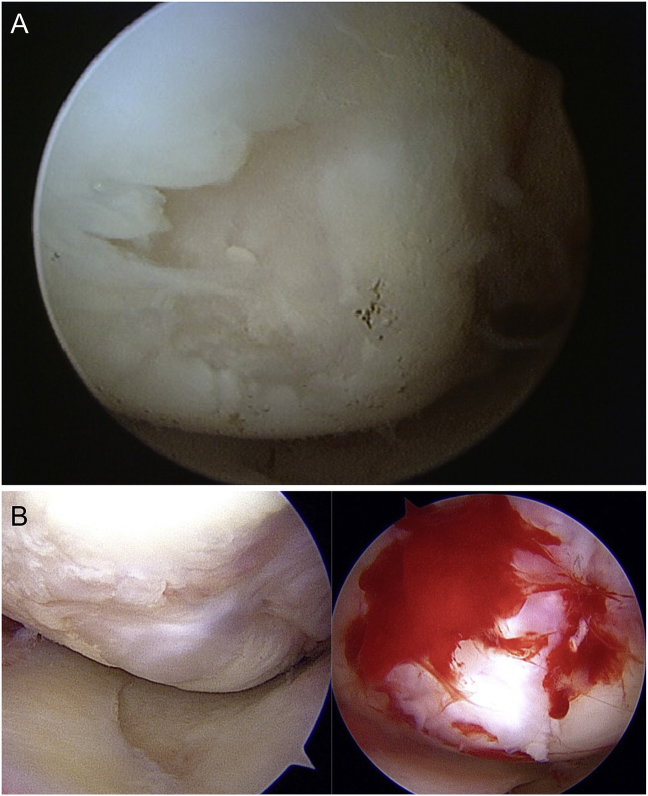
Currently, there is high demand for immunomodulatory biological approaches to treat cartilage defects and delay progressive OA.2 Reports suggest that platelet-rich plasma (PRP) may promote healing and improve osteoarthritis (OA) symptoms for a longer duration.3 Cartilage regeneration using synovial stem cells has also been reported.4 Multiple injections of PRP have an anti-inflammatory effect on the synovium in a short period of time, this effect lasts for a long time and has a cartilage protective effect. However, there are basic research reports that this effect cannot be seen with a single injection.5 We previously found on arthroscopy that cartilage defects were covered with rich fibrocartilage after 7 months of treatment with leukocyte-rich platelet-rich plasma (LR-PRP) and PRP (Fig. 1). There is also a similar basic research report supporting this findings.6
Fig. 1.
Fibrocartilage-rich cover over the defective portion of the cartilage.
(a) Medial femoral condyle before treatment; (b) Arthroscopic findings 7 months after PRP treatment.
PRP, platelet-rich plasma.
Although PRP treatment is said to be effective for OA patients with low-grade inflammation,7 as per the Kellgren-Lawrence (K-L) classification,8 a large proportion of patients with clinical OA with severe pain are those with advanced-grade inflammation. Based on reports that multiple injections may be effective for advanced-grade inflammation7 and the fact that formation of rich fibrocartilage was noted after about 6 months (as described above), injections were administered six times every 4 weeks for 6 months. Therefore, we hypothesized that abundant LR-PRP may cause fibrocartilage-rich cover over the defective portion of the cartilage in KOA patients with advanced-grade inflammation.
In this study, we investigated the efficacy of multiple injections of LR-PRP for treatment of KOA. Moreover, we determined an LR-PRP treatment protocol for KOA in actual clinical practice.
2. Method
This was a prospective, interventional, clinical trial. It was approved by the Institutional Ethics Review Board of the Japanese Association for the Promotion of State of Art in Medicine Ethics Review Committee (Approval no.: KE-06). In addition, the study was conducted in accordance with the law for ensuring the safety of regenerative medicine, after examination by specific committees and authorization by the Ministry of Health Labor and Welfare for the use of regenerative medicine.9 This study was registered in the clinical trial registry of the clinical Japan Medical Association Center for Clinical Trials (JMA-IIA00351). This study was conducted between June 2018 and April 2019.
2.1. Patient selection
Subjects for this treatment were recruited using advertisements on homepages and social networking services, radio broadcasting, health magazines, and in-clinic posters. All patients with a diagnosis of KOA were screened. All patients provided written informed consent after receiving an explanation of the potential benefits of PRP, the treatment procedure, and follow-up.
The inclusion criteria were: 1) long-standing difficulties in performing activities of daily living, and 2) a desired level of activity that could not be reached despite continued treatment with hyaluronic acid injections, steroid injections, and/or NSAIDs at various clinics and hospitals. The exclusion criteria were: 1) a history of a systemic disorder, such as rheumatoid arthritis, malignant cancer, haematological disease, infection, or immunodeficiencies, 2) recent intra-articular injection of corticosteroids and HA in the past 2 weeks, and 3) recent administration of anti-cancer drugs or immunosuppressive drugs.
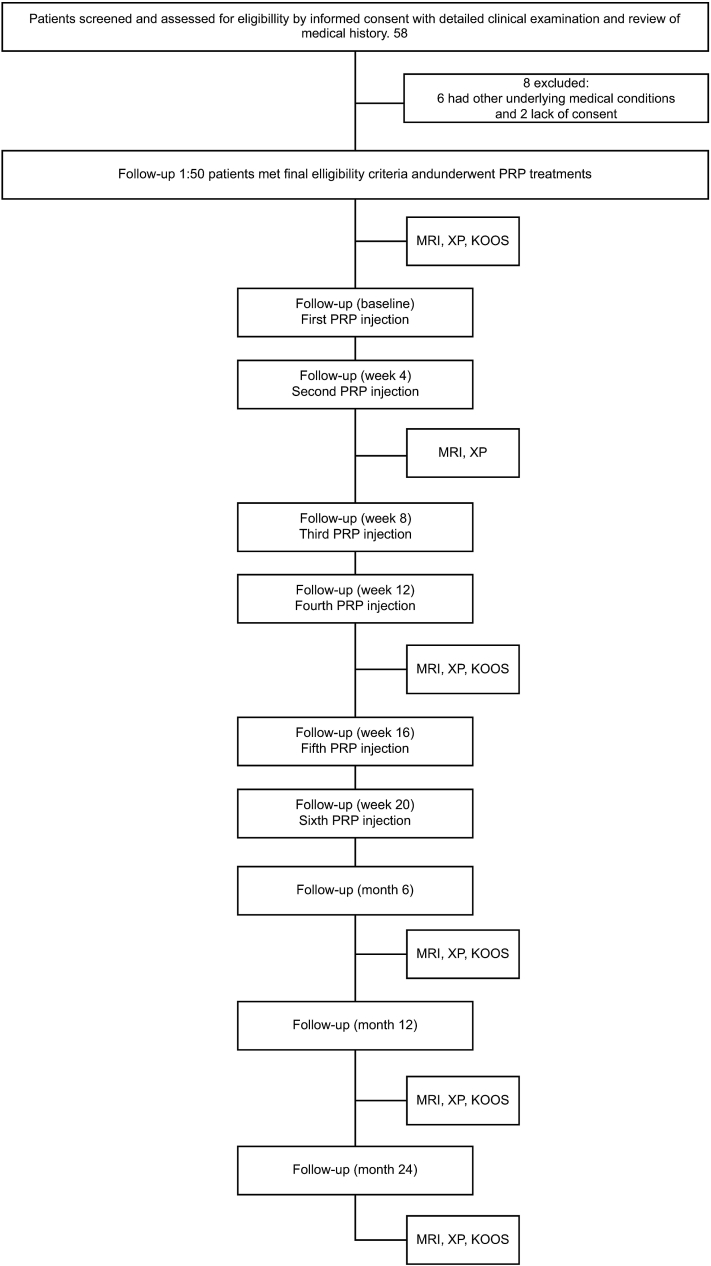
The number of patients screened and the treatment protocol are shown in Fig. 2. Although 58 knees were screened, the treatment was indicated for a total of 50 knees (eight were excluded).
Fig. 2.
Patient follow-up.
MRI, magnetic resonance imaging; X–P, radiographic findings; KOOS, Knee Injury and Osteoarthritis Outcome Score; PRP, platelet-rich plasma.
2.2. Processing PRP
2.2.1. Preparation of PRP (LR-PRP)
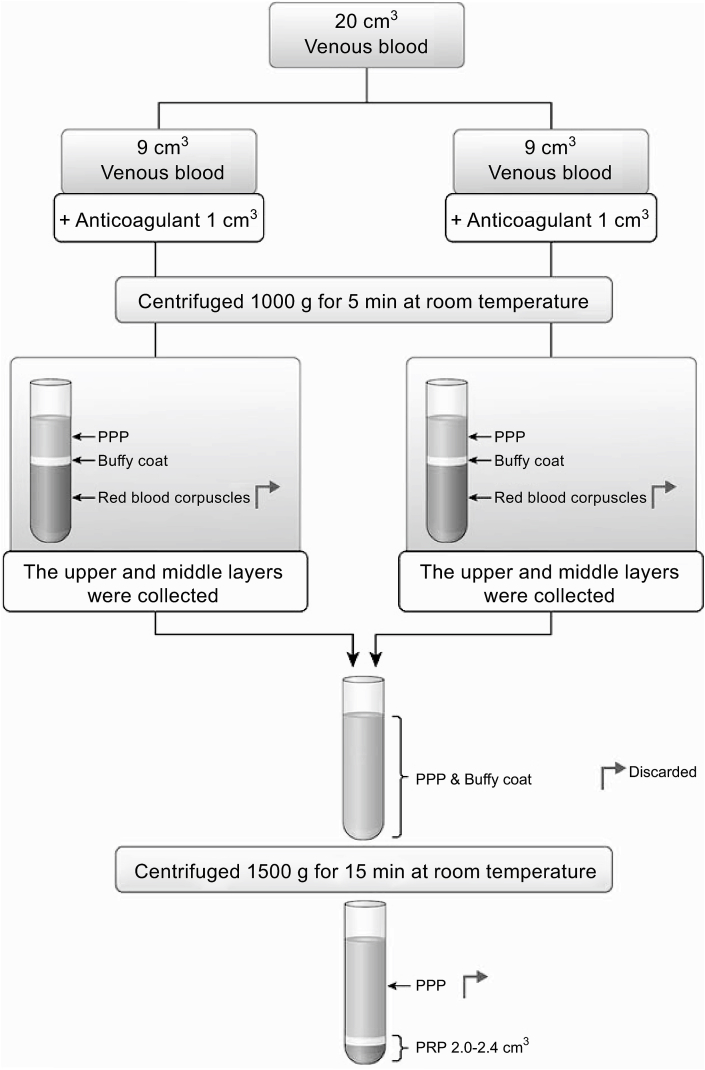
We creatively designed a preparation protocol for PRP. Based on a report by Shin et al.,10 we chose a method of centrifuging whole blood to obtain the highest concentration ratio. First, the 20-cm3 blood samples drawn from the patients were divided into two samples of 9 cm3 and placed in sterilized 10-cm3 glass tubes. Next, 1 cm3 of anticoagulant (sodium citrate solution) was added to each tube containing the samples and the tube was immediately centrifuged at 1000 G for 5 min at room temperature (Tabletop Centrifuge 2420; KUBOTA Corporation, Tokyo, Japan). The centrifugation allowed blood separation into three distinct layers. At the bottom of the glass tube, red blood corpuscles were present. At the top of the glass tube, the acellular plasma layer was obtained, containing circulating plasmatic molecules and a few platelets [platelet-poor plasma (PPP)]. Between these two layers was an intermediate layer with an increased platelet concentration, representing a buffy coat. Using a sterile syringe with an 18-gauge-long needle, the PPP, buffy coat with PRP, and some red blood corpuscles were aspirated (i.e., the upper and middle layers were collected after centrifuging) from both samples and collected into one dry glass tube without anticoagulant. This tube was then centrifuged at 1500 G for 15 min at room temperature. This second centrifugation again allowed the blood to separate into three distinct layers, with a platelet concentrate at the bottom of the tube. The PPP on the top was aspirated and discarded, leaving just enough serum to maintain the buffy coat as a middle layer and thin red blood corpuscles as a bottom layer. The glass tubes were then gently shaken to obtain ready-to-use PRP. We obtained approximately 2.4 cm3 of PRP from 20 cm3 of blood per injection (Fig. 3). Three 10 cc glass tubes and two syringes were used per injection.
Fig. 3.
Preparation of PRP (LR-PRP)
We modified the standard procedure for obtaining PRP and obtained approximately 2.4 cm3 of PRP
PRP, platelet-rich plasma; LR-PRP, leukocyte-rich platelet-rich plasma; PPP, platelet-poor plasma.
2.2.2. PRP injection
Six ultrasonography (US)-guided intra-articular injections were administered to every patient by a well-trained doctor (not involved in outcome assessment) at 4-week intervals.
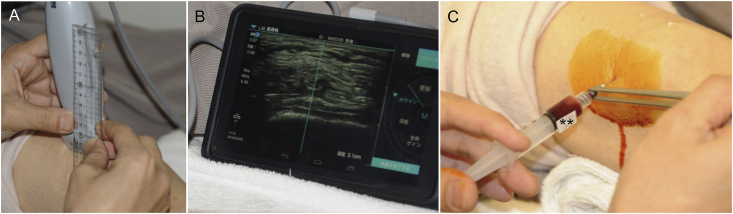
During the procedure, the patient's knee was kept in a slight bent position at about 20°, and the injection was administered in a sterile condition using a 25-G needle via a suprapatellar approach from the outside of the knee with US guidance (SonoSite iViz®; Fuji Film SonoSite Inc., Tokyo, Japan).
In cases of knee effusion, the suprapatellar pouch of the knee was observed, and the joint fluid was aspirated as much as possible, while confirming the presence or absence of the accumulation of synovial fluid (Fig. 4). Next, the suction syringe was replaced with a PRP syringe and about 2.4 mL of PRP was gently and smoothly injected.
Fig. 4.
PRP injection technique with ultrasonography guidance
(a) Inspection probe and ruler; (b) On the screen, a 21-G needle is inserted in the suprapatellar capsule in a parallel projection; (c) PRP injection is performed ensuring there is no resistance.
PRP, platelet-rich plasma.
2.3. Generation of platelet concentrates and haematology measurements
During the preparation of PRP using the abovementioned protocol, each residue sample prepared under the same conditions was examined using a haemocytometer (Microsemi LC-66; HORIBA Ltd., Kyoto, Japan). To avoid platelet activation, the measurement of samples was performed at room temperature as soon as possible after loading the haemocytometer chamber. The results from the analysis were assessed based on the PAW classification, which classifies PRP according to platelet count, activation, and white blood cell count,11 and the Mishra Sports Medicine PRP classification system, which classifies PRP according to the same criteria as the PAW.12
2.4. Treatment and evaluation
After administration of injections, the patients did not have any restrictions and were allowed to resume activities of daily living. Patients were clinically evaluated via subjective and objective assessments using KOOS and VAS at baseline and at 12- and 24-weeks post-treatment to determine the primary clinical outcomes of PRP treatment. After 12 and 24 weeks, radiographic imaging and MRI were performed.
Demographic and clinical characteristic data, including patient age, sex, race, OA grade according to the K-L classification, and body mass index (BMI), were measured in all patients. Regression analysis was performed to identify variables that affected treatment response, such as OA grade, BMI, age, and preoperative pain. In addition, the correlations between the leukocyte concentration rate and treatment outcome and between the platelet concentration rate and treatment outcome were analysed. To investigate the number of times PRP should be administered, we examined the average number of doses required to reduce the pain by 20% and 50% or more. A visual analogue scale (VAS) ruler was used to evaluate pain and determine improvement. The VAS used a scale of 0 mm–100 mm, with 0 mm representing no pain and 100 mm representing the worst possible pain to quantify subjective pain.
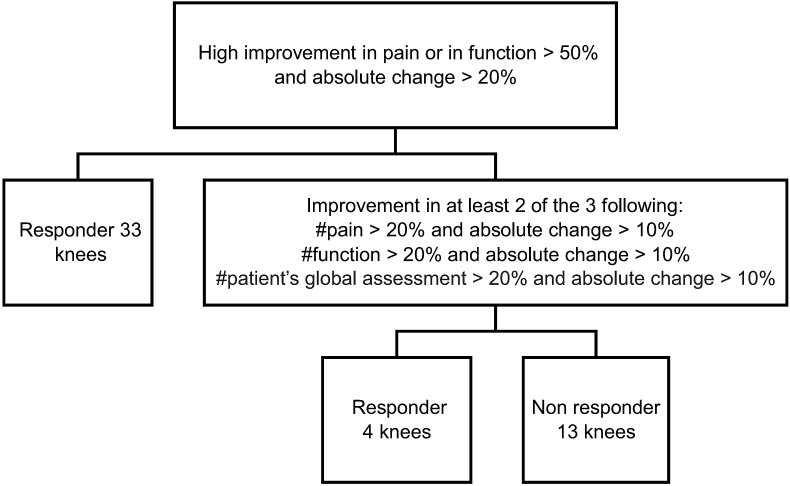
The Outcome Measures In Rheumatology-Osteoarthritis Research Society International (OMERACT-OARSI) responder's criteria13 was used to determine treatment effect (Fig. 5).
Fig. 5.
OMERACT-OARSI flow chart (50 knees)
Details of responders (Grade 1: 4/4; Grade 2: 5/8; Grade 3: 17/23; Grade 4: 11/15).
OMERACT-OARSI, Outcome Measures In Rheumatology-Osteoarthritis Research Society International.
2.5. Clinical assessment
Clinical assessments included the following: 1) knee injury and osteoarthritis outcome scores (KOOS) including KOOS-total (T), symptoms (S), pain (P), activity (A), sports (Sp), and quality of life (Q)14; 2) VAS scores15; 3) radiographic findings; and 4) MRI (0.3 T open-type instrument; HITACHI Medico Airis Bent, Tokyo, Japan) findings. The bone marrow lesion (BML) area was assessed on MRI using the MRI Osteoarthritis Knee Score (MOAKS).16 The MOAKS BML is scored for 15 regions (two patellar, six femoral, and seven tibial) based on BML size (none = 0; <33% of region = 1; 33–66% of region = 2; >67% = 3) for a maximum score of 15 × 3 = 45 per knee. In addition, the nature, duration, and severity of any adverse events related to this study protocol was assessed.
2.6. Statistical analyses
Continuous data are reported as the mean ± standard deviation (SD). Welch's t-tests were performed for group comparisons, and paired t-tests were performed to evaluate within-group changes. All statistical analyses were performed using SPSS version 22.0 for Windows (IBM Corp., Armonk, NY). A P-value of ≤0.05 was considered statistically significant.
3. Results
The average age of the patients (six men and 38 women) was 67.2 ± 9.6 years (range, 36–84 years), and the average BMI was 25.3 kg/m2(19.6–33.8). There were 42 Japanese and two Brazilian patients. There were 22 cases of left KOA and 28 of right KOA. Thus, there were six patients with OA in both knees. The degree of KOA according to the K-L classification was Grade I in four knees, Grade II in eight knees, Grade III in 23 knees, and Grade IV in 15 knees.
For the PRP used in this study, the mean platelet concentration was 4.8-fold that in whole blood, and the mean white blood cell concentration was 2.6-fold that in whole blood. Hence, the overall average LR-PRP classification according to MISHRA was Type 1-B and P3-Aα according to PAW.
The mean improvement rate as per the VAS was 61.6% (P < 0.0001). The average VAS scores before treatment and after each PRP injection were as follows: 5.8 cm (before treatment), 48 mm (after first injection), 44 mm (after second injection), 41 mm (after third injection), 34 mm (after fourth injection), 30 mm (after fifth injection), and 31 mm (after sixth injection). VAS scores significantly improved after the first injection in Grade II patients (P = 0.0053) and after the second injection in Grade III and IV patients (P = 0.0039 and P = 0.0003, respectively). There were 47 knees (94%) with ≥20% improvement in VAS scores. On an average, 2.3 doses were required for a 20% improvement. Similarly, there were 37 knees (74%) with ≥50% improvement in VAS scores. On average, 3.5 doses were required for a 50% improvement. On average, 4.8 doses were required for the highest improvement in VAS score, depending on the OA grade as per the K-L classification: K-L Grade I, 4.5; K-L Grade II, 5.1; K-L Grade III, 4.5; K-L Grade IV, 5.0.
The improvements in mean KOOS at baseline and at the 3- and 6-month follow-ups were as follows: KOOS-T: 56.2 to 65.1 to 69.1; KOOS–S: 55.3 to 65.9 to 70.1; KOOS–P: 53.6 to 63.4 to 70.2; KOOS-A: 68.3 to 76.6 to 79.1; KOOS-Sp: 33.7 to 41.6 to 46.0; KOOS-Q: 34.4 to 48.7 to 50.9 (all P < 0.01). There were significant differences in KOOS (all P < 0.0001), including KOOS-Sp (before vs. 3 months, P = 0.005).
There was no correlation between age and VAS score improvement rate (r = 0.134, P = 0.125), or between BMI and VAS score improvement rate (r = −0.055, P < 0.0001). Similarly, no correlation was found between the concentration of leukocytes (r = 0.153, P < 0.0001) and platelets (r = −0.135, P < 0.0001).
According to the OMERACT-OARSI, responses were observed in 37 knees (74%), and no response was observed in 13 knees (26%) (Fig. 5). There were no obvious adverse events. However, discomfort and pain around the knee joint occurred in 39 of 50 patients (58%) and disappeared within a few days.
Follow-up MRIs and radiographic imaging were performed at 3 and 6 months after treatment. There were no cases where deformity was aggravated on radiographic findings. The mean grade of BMLs as per the MOAKS classification was 7.44 before administration, 7.22 after 3 months, and 6.6 after 6 months. Significant changes occurred in BML between pre-treatment and after 3 months (P = 0.040), between pre-treatment and after 6 months (P = 0.0070), and between 3 months and 6 months (P = 0.020). In contrast, no significant differences in osteoarthritis grade was observed.
4. Discussion
This study found that the mean improvement rate following LR-PRP therapy, as measured by VAS, was 61.6% (P < 0.0001). Furthermore, there were significant differences in all subsections of the KOOS, and according to the OMERACT-OARSI, 37 knees (74%) were responsive and 13 knees (26%) were not (Fig. 5). On follow-up MRIs, significant changes occurred in BML. In contrast, no significant differences in osteoarthritis grade were observed.
PRP treatment reports vary depending on the components of PRP. We believe that remodelling by macrophages is essential for the repair of the degenerating knee joint cartilage with a cartilage defect. Accordingly, based on a report by Shin et al.,10 we chose a method of centrifuging whole blood to obtain the highest concentration ratio. However, despite maintaining the surrounding conditions and using samples from the same person, the finished PRP had a different concentration each time. Likewise, even when a commercially available separation kit was used, it was still difficult to achieve the same PRP concentration each time, as the effects of environmental variables influence the outcome.17,18 However, considering that we are using human blood samples, such variations are inevitable.
Most patients showed significant changes (pre-treatment and post-treatment) in VAS scores and KOOS. There was no clear significant difference between severity of deformity and treatment outcome, and good treatment results were confirmed even when the K-L classification grade was advanced. Based on our purified LR-PRP preparation and administration method, LR-PRP administration shows promise as a temporary treatment for many OA patients. There are large variations in the literature regarding the dosing schedule of PRP injections for KOA.19, 20, 21, 22
In a previous study, we were surprised to find that cartilage defects were covered with rich fibrocartilage on arthroscopy after 7 months of treatment with LR-PRP and PRF. Hence, we hypothesized that abundant LR-PRP may cause a fibrocartilage-rich cover over the defective portion of the cartilage and abundant PRP and long-term administration would be effective for advanced KOA, as it was for cartilage defects. This findings may be thought of as supporting Fang's et al. report.6
With regard to our administration method, referring to previous reports,23 we decided to administer six injections at 4-week intervals. Based on our results, we determined whether we should have administered PRP over a longer or shorter duration. To reach 50% or more patient satisfaction with regard to degree and symptom improvement, four injections or more appeared to be necessary. In our study, the number of doses required for an improvement rate of 20% and 50%, and maximum improvement were significantly increased. Therefore, the more frequent the administration, the better the recovery of pain. Some reports have elucidated the efficacy of multiple PRP injections and one study reported the effectiveness of multiple PRP injections for low grade KOA.5 Cook et al.20 reported that patients with more severe KOA need four, five, or six injections to get maximal relief. The results of our research strongly support their findings.
According to some reports,24,25 bone marrow oedema on MRI is considered to be caused by a cortical break. In our study, there were significant differences in MRI findings before and after 6 months. These findings suggest the restoration of a cortical break for 6 months. Therefore, we can assume that the exposed part of the subchondral bone is covered for 6 months. Temporary covering of cartilage defects with fibrocartilage and prevention of subchondral bone exposure are believed to lead to an improvement in pain and function.
4.1. Limitations
This study had several limitations. The cleanliness level at our hospital's PRP production site corresponded to class 10000 of the NASA standard. We worked in a clean environment, but the PRP preparation method used in this study was the open technique. Accordingly, the contamination risk may have been increased.
Compositional variation was believed to be not only due to homeostasis, but also the preparation procedure. Therefore, future studies with more precise efforts regarding the accuracy of the method are required.
Next, the single-centre design and low number of cases, the lack of a placebo control group, and a rather short-term follow-up limits the statistical strength of our conclusions. Therefore, we will consider including a placebo group in future studies. Although this study did not include a control group and does not have significant statistical power, the results indicate real-world evidence, and they warrant further validation in future studies.
In conclusion, the results of our PRP procedure was satisfactory, regardless of the degree of knee deformation. PRP is a source of autologous and safe growth factors and has the potential to cause a paradigm shift in conservative treatment protocols for knee osteoarthritis. It can improve subjective pain and KOOS results. Therefore, we suggest that PRP should be administered in the clinical setting as an additional conservative treatment option for KOA patients before considering TKA.
Unblinded ethical approval statement
This was a prospective, interventional, clinical trial. It was approved by the Institutional Ethics Review Board of the Ministry of Health Labor and Welfare (approval no.: KE-06).
Unblinded clinical trial registration statement
This study was registered in the clinical Japan Medical Association Center for Clinical Trials (JMA-IIA00351).
Author contributions
The author, Masahiko Kemmochi, contributed to the conception and design of the study, or the acquisition of data, or the analysis and interpretation of data.
The author, Masahiko Kemmochi, contributed to the drafting of the article or revising it critically for important intellectual content.
The author, Masahiko Kemmochi, approved the approval of the final version of the manuscript to be submitted.
Role of the funding source
The study sponsors had no involvement in this research.
Ethics approval of research on humans
This prospective interventional clinical trial was approved by the Institutional Ethics Review Board of the Ministry of Health Labor and Welfare (approval no.: KE-06). All patients provided written informed consent after receiving an explanation about the potential benefits of PRP, the treatment procedure, and follow-up.
Data availability
The data is available from Masahiko Kemmochi upon reasonable request.
Declaration of competing interest
The author declares that there are no conflicts of interest.
Acknowledgments
None.
References
- 1.Pereira D., Peleteiro B., Araújo J., Branco J., Santos R.A., Ramos E. The effect of osteoarthritis definition on prevalence and incidence estimates: a systematic review. Osteoarthr Cartil. 2011;19:1270–1285. doi: 10.1016/j.joca.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 2.Zhu Y., Yuan M., Meng H.Y. Basic science and clinical application of platelet-rich plasma for cartilage defects and osteoarthritis: a review. Osteoarthr Cartil. 2013;21:1627–1637. doi: 10.1016/j.joca.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 3.Kennedy M.I., Whitney K., Evans T., LaPrade R.F. Platelet-rich plasma and cartilage repair. Curr Rev Musculoskelet Med. 2018;11:573–582. doi: 10.1007/s12178-018-9516-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sekiya I., Muneta T., Horie M., Koga H. Arthroscopic transplantation of synovial stem cells improves clinical outcomes in knees with cartilage defects. Clin Orthop Relat Res. 2015;473:2316–2326. doi: 10.1007/s11999-015-4324-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chouhan D.K., Dhillon M.S., Patel S., Bansal T., Bhatia A., Kanwat H. Multiple platelet-rich plasma injections versus single platelet-rich plasma injection in early osteoarthritis of the knee: an experimental study in a Guinea pig model of early knee osteoarthritis. Am J Sports Med. 2019 Aug;47(10):2300–2307. doi: 10.1177/0363546519856605. [DOI] [PubMed] [Google Scholar]
- 6.Fang D, Jin P, Huang Q, Yang Y, Zhao J, Zheng L. Platelet-rich plasma promotes the regeneration of cartilage engineered by mesenchymal stem cells and collagen hydrogel via the TGF-β/SMAD signaling pathway. J Cell Physiol. 2019 Feb 15;234(9):15627–15637. doi: 10.1002/jcp.28211. Submitted for publication. [DOI] [PubMed] [Google Scholar]
- 7.Huang P.H., Wang C.J., Chou W.Y., Wang J.W., Ko J.Y. Short-term clinical results of intra-articular PRP injections for early osteoarthritis of the knee. Int J Surg. 2017;42:117–122. doi: 10.1016/j.ijsu.2017.04.067. [DOI] [PubMed] [Google Scholar]
- 8.Whitney K.E., Liebowitz A., Bolia I.K. Current perspectives on biological approaches for osteoarthritis. Ann N Y Acad Sci. 2017;1410:26–43. doi: 10.1111/nyas.13554. [DOI] [PubMed] [Google Scholar]
- 9.Konomi K., Tobita M., Kimura K., Sato D. New Japanese initiatives on stem cell therapies. Cell Stem Cell. 2015;16:350–352. doi: 10.1016/j.stem.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 10.Shin H.S., Woo H.M., Kang B.J. Optimisation of a double-centrifugation method for preparation of canine platelet-rich plasma. BMC Vet Res. 2017;13:198. doi: 10.1186/s12917-017-1123-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeLong J.M., Russell R.P., Mazzocca A.D. Platelet-rich plasma: the PAW classification system. Arthroscopy. 2012;28:998–1009. doi: 10.1016/j.arthro.2012.04.148. [DOI] [PubMed] [Google Scholar]
- 12.Mishra A., Harmon K., Woodall J., Vieira A. Sports medicine applications of platelet rich plasma. Curr Pharmaceut Biotechnol. 2012;13:1185–1195. doi: 10.2174/138920112800624283. [DOI] [PubMed] [Google Scholar]
- 13.Bellamy N., Kirwan J., Boers M. Recommendations for a core set of outcome measures for future phase III clinical trials in knee, hip, and hand osteoarthritis. Consensus development at OMERACT III. J Rheumatol. 1997;24:799–802. [PubMed] [Google Scholar]
- 14.Roos E.M., Roos H.P., Lohmander L.S., Ekdahl C., Beynnon B.D. Knee injury and osteoarthritis outcome score (KOOS)--development of a self-administered outcome measure. J Orthop Sport Phys Ther. 1998;28:88–96. doi: 10.2519/jospt.1998.28.2.88. [DOI] [PubMed] [Google Scholar]
- 15.Breivik H., Borchgrevink P.C., Allen S.M. Assessment of pain. Br J Anaesth. 2008;101:17–24. doi: 10.1093/bja/aen103. [DOI] [PubMed] [Google Scholar]
- 16.Hunter D.J., Guermazi A., Lo G.H. Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI Osteoarthritis Knee Score) Osteoarthr Cartil. 2011;19:990–1002. doi: 10.1016/j.joca.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobayashi Y., Saita Y., Nishio H. Leukocyte concentration and composition in platelet-rich plasma (PRP) influences the growth factor and protease concentrations. J Orthop Sci. 2016;21:683–689. doi: 10.1016/j.jos.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 18.Oudelaar B.W., Peerbooms J.C., Huis In 't Veld R., Vochteloo A.J.H. Concentrations of blood components in commercial platelet-rich plasma separation systems: a review of the literature. Am J Sports Med. 2019;47:479–487. doi: 10.1177/0363546517746112. [DOI] [PubMed] [Google Scholar]
- 19.Cole B.J., Karas V., Hussey K., Pilz K., Fortier L.A. Hyaluronic acid versus platelet-rich plasma: a prospective, double-blind randomized controlled trial comparing clinical outcomes and effects on intra-articular biology for the treatment of knee osteoarthritis. Am J Sports Med. 2017;45:339–346. doi: 10.1177/0363546516665809. [DOI] [PubMed] [Google Scholar]
- 20.Cook C.S., Smith P.A. Clinical update: why PRP should be your first choice for injection therapy in treating osteoarthritis of the knee. Curr Rev Musculoskelet Med. 2018;11:583–592. doi: 10.1007/s12178-018-9524-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Milants C., Bruyère O., Kaux J.F. Responders to platelet-rich plasma in osteoarthritis: a technical analysis. BioMed Res Int. 2017;2017:7538604. doi: 10.1155/2017/7538604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raeissadat S.A., Rayegani S.M., Hassanabadi H. Knee osteoarthritis injection choices: platelet-rich plasma (PRP) versus hyaluronic acid (a one-year randomized clinical trial) Clin Med Insights Arthritis Musculoskelet Disord. 2015;8:1–8. doi: 10.4137/CMAMD.S17894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Görmeli G., Görmeli C.A., Ataoglu B., Çolak C., Aslantürk O., Ertem K. Multiple PRP injections are more effective than single injections and hyaluronic acid in knees with early osteoarthritis: a randomized, double-blind, placebo-controlled trial. Knee Surg Sport Traumatol Arthrosc. 2017;25:958–965. doi: 10.1007/s00167-015-3705-6. [DOI] [PubMed] [Google Scholar]
- 24.Jimenez-Boj E., Nöbauer-Huhmann I., Hanslik-Schnabel B. Bone erosions and bone marrow edema as defined by magnetic resonance imaging reflect true bone marrow inflammation in rheumatoid arthritis. Arthritis Rheum. 2007;56:1118–1124. doi: 10.1002/art.22496. [DOI] [PubMed] [Google Scholar]
- 25.Schett G. Bone marrow edema. Ann N Y Acad Sci. 2009;1154:35–40. doi: 10.1111/j.1749-6632.2009.04383.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data is available from Masahiko Kemmochi upon reasonable request.