Highlights
-
•
A model to assess drug interaction effects on drug induced respiratory depression.
-
•
Plasma oxycodone level AUC was 113 % higher when the two drugs were co-administered.
-
•
Diazepam showed synergistic effects on respiratory depression when given with oxycodone.
-
•
Combination of diazepam and oxycodone decreased resting arterial pO2 AUC by 17 %.
-
•
Combination of diazepam and oxycodone increased resting arterial pCO2 AUC by 25 %.
Keywords: Animal model, Opioids, Sedating psychotropic drugs, Respiratory depression, Arterial partial pressure of oxygen, Arterial partial pressure of carbon dioxide
Abstract
Opioids and benzodiazepines were frequently co-prescribed to patients with pain and psychiatric or neurological disorders; however, co-prescription of these drugs increased the risk for severe respiratory depression and death. Consequently, the U.S. Food and Drug Administration added boxed label warnings describing this risk for all opioids and benzodiazepines. Sedating psychotropic drugs with differing mechanisms of action (e.g., antipsychotics, antidepressants, non-benzodiazepine sedative-hypnotics, etc.) may be increasingly prescribed in place of benzodiazepines. Despite being marketed for years, many sedating psychotropic drugs have neither human nor animal data that quantify or qualify the potential for causing respiratory depression, either alone or in combination with an opioid. In this study, diazepam was selected as the benzodiazepine to detect any additive or synergistic effects on respiratory depression caused by the opioid, oxycodone. Pharmacokinetic studies were conducted at three doses with oxycodone (6.75, 60, 150 mg/kg) and with diazepam (2, 20, 200 mg/kg). Dose dependent decrease in arterial partial pressure of oxygen and increase in arterial partial pressure of carbon dioxide were observed with oxycodone. Diazepam caused similar partial pressure changes only at the highest dose. Further decreases in arterial partial pressure of oxygen and increases in arterial partial pressure of carbon dioxide consistent with exacerbated respiratory depression were observed in rats co-administered oxycodone 150 mg/kg and diazepam 20 mg/kg. These findings confirm previous literature reports of exacerbated opioid-induced respiratory depression with benzodiazepine and opioid co-administration and support the utility of this animal model for assessing opioid-induced respiratory depression and its potential exacerbation by co-administered drugs.
1. Introduction
Boxed Warnings were added to the drug labeling of opioids and benzodiazepines after a Food & Drug Administration (FDA) review found that “combined use of opioid medicines with benzodiazepines or other drugs that depress the central nervous system” can result in serious side effects including respiratory depression and death [[1], [2], [3], [4], [5]]. The number of patients dispensed an opioid in the U.S. outpatient retail setting in 2010 was 83 million [6]. Approximately 30 % of long-term opioids users were also regular users of sedative hypnotics that depress the central nervous system [7], and in 2014, about 10 % of opioid recipients in the US were co-prescribed a benzodiazepine [6]. In 2017, there were 47,600 deaths in the U.S. involving opioids alone or in combination with other drugs including 10,010 deaths (21 % of the total) in association with benzodiazepines [8]. A study at North Carolina State University found that in 2010, opioid analgesics were dispensed to 22.8 % of North Carolina’s 9.56 million residents, and 80 % of the patients under opioid analgesic treatment were also prescribed benzodiazepines concomitantly; overdose deaths among patients with concomitant treatment of benzodiazepines and opioid analgesics were nine times higher (7.0 per 10,000 person-years) than those treated with opioid analgesics alone (0.7 per 10,000 person-years) [9]. Many non-benzodiazepine sedating psychotropic drugs (SPDs) have long been in clinical use, but for some of these drugs there is little or no clinical or animal data on their potential to induce respiratory depression, either alone or in combination with an opioid [10]. Following the warning placed on opioid and benzodiazepine drug labels, other SPDs may be much more frequently co-prescribed with opioids. Therefore, defining the effect of these and other classes of drugs on respiratory depression when used alone or in combination with an opioid would enhance the ability of regulators to make informed decisions on future regulatory actions on these drugs.
Further investigation is also warranted given that findings from studies of opioids in combination with SPDs may or may not agree with results of other similar studies using different drugs within each class. For example, some investigators reported that diazepam (a benzodiazepine), midazolam (a benzodiazepine), or ketamine (an NMDA receptor antagonist) increased respiratory depression when co-administered with opioids [[11], [12], [13]], while others reported that diazepam co-administered with methadone (an opioid) in rats did not cause additional respiratory depression [14]; furthermore, from a clinical perspective, situations can be even more complicated in patients abusing multiple drugs or with intentional overdose, which may make diagnoses, treatments, and laboratory tests more challenging [15,16].
Direct measure of respiratory parameters, such as respiratory rate and expiratory volume, could be measured via plethysmography and used to define respiratory depression. However, in an animal model serial arterial blood collection via an indwelling cannula is practical and an expedient method for directly measuring the outcomes of respiratory function, arterial partial pressures of oxygen and carbon dioxide. This approach also has clinical implications as collecting arterial blood gas values is essential for monitoring and treating high-risk clinical patients for respiratory and acid–base status [17]. In addition, partial pressure of oxygen (pO2) and partial pressure of carbon dioxide (pCO2) have been widely used in animal studies on respiratory depression [[12], [13], [14],[17], [18], [19], [20], [21], [22]] and elevated pCO2 is considered a hallmark of respiratory depression [14]. Therefore, arterial pO2 and pCO2 were selected for monitoring respiration status in this study by using a portable blood gas testing device, that has been widely employed in clinical and preclinical analyses, and in the development of new blood gas analyzers [[23], [24], [25], [26], [27], [28], [29], [30], [31], [32]].
The present study was designed to establish an animal model that confirms reported effects on respiration of opioid and benzodiazepine co-administration and to add new information on blood drug concentrations at time points when blood pO2 and pCO2 were changed (PK/PD relationship). Establishing the ability of the model to discern gradations in respiratory depression creates a tool to generate knowledge on respiratory depression induced by marketed SPDs used alone or in conjunction with an opioid. The data will be used to support regulatory decision-making on the need for labeling changes or additional clinical studies for non-benzodiazepine SPDs.
2. Materials & methods
The general approach and design of the study are shown in Fig. 1.
Fig. 1.
Summary of study.
2.1. Animals
Previously, adult male Sprague-Dawley rats have been used in other opioid-diazepam combination studies [11]. In the present study, adult males with an indwelling femoral arterial cannula were obtained from Taconic Biosciences (Albany, NY, USA). All animal work was conducted under a protocol approved and overseen by the Food and Drug Administration’s White Oak Institutional Animal Care and Use Committee. Animal research was conducted in an AAALAC accredited facility in accordance with the Guide for the Care and Use of Laboratory Animals [8th Edition]. For the protection of the surgically implanted intraarterial cannulas, animals were individually housed on a 12-h light cycle with food and water access ad libitum. During experiments, rats were under constant observation and any clinical findings were recorded. To ensure the quality of PK data and prevent coprophagia, immediately after dosing rats were transferred to and continued to be individually housed in cages with a lifted wire grid bottom for the duration of each experiment.
2.2. Intraarterial cannula care
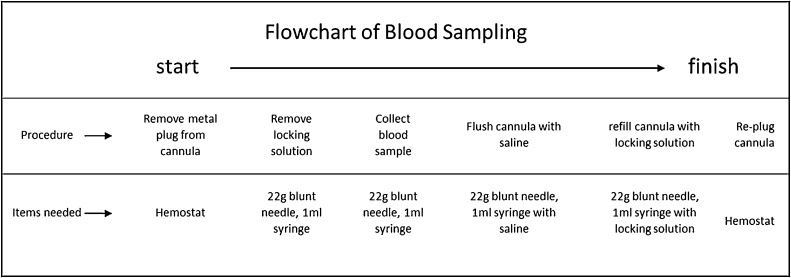
The procedure was completed on a draped large metal bench with the surface of the working area covered. The cannula care time needed for one animal was approximately 2 min. The care procedure was carried out by a 2-person team with one person holding the animal and the other person completing the following cannula care procedure: (1) remove the 22 g metal plug (Instech Laboratories, Inc, Plymouth Meeting, PA USA) from the external end of the cannula by using a hemostat; (2) aspirate locking solution from the cannula by using a 1-ml empty syringe with a 22 g blunt needle (Instech Laboratories, Inc, Plymouth Meeting, PA USA); (3) flush the cannula with 0.2 ml sterile saline using a 1-ml syringe with a 22 g blunt needle; (4) refill the cannula with 0.08 ml Locking solution (SAI Infusion Technologies, Lake Villa Township, IL) using a 1-ml syringe with a 22 g blunt needle; and (5) re-plug the cannula with its metal plug. Cannulas were flushed twice a week.
2.3. Treatment groups
The different treatment groups and group sizes for all experiments, including PK studies and combination drug interactions studies, are provided in Table 1.
Table 1.
Groups- oxycodone only, diazepam only, and combination treatments.
| Group | Rats | ||
|---|---|---|---|
| Oxycodone PKb | 6.75 mg/kg | Oxycodone | 6 |
| 60 mg/kg | Oxycodone | 6 | |
| 150 mg/kg | Oxycodone | 5a | |
| Diazepam PKb | 2 mg/kg | Diazepam | 6 |
| 20 mg/kg | Diazepam | 6 | |
| 200 mg/kg | Diazepam | 6 | |
| Oxycodone PK, repeat | 60 mg/kg | Oxycodone | 6 |
| Oxycodone PK, repeat | 150 mg/kg | Oxycodone | 6 |
| Oxycodone 150 mg/g + Diazepam 20 mg/kg | Combination study | Oxycodone + Diazepam | 18 |
| Oxycodone only | 17a | ||
One rat was removed due to a compromised arterial cannula.
PK: pharmacokinetics.
2.4. Blood sampling schedule for pharmacokinetics (PK), pO2 and pCO2 following oxycodone or diazepam treatment
Six animals were in each treatment group. As the focus of the study was commonly prescribed orally-administered drugs, the oral exposure route was selected for all drugs. Oxycodone was selected as the model opioid and PK studies were conducted at three dose levels (6.75, 60, 150 mg/kg) well below the LD50 [33]. For all PK studies, blood samples were collected prior to dosing and 15 min, 30 min, 1 h, 2 h, 3 h, 4 h, 6 h, and 8 h after dosing; the experiment concluded after blood collection and blood gas testing 8 h post-dosing. The pre-dosing sample was collected within 2 h before dosing and served as self-control for each animal. All blood samples were measured for drug concentrations, pO2, and pCO2. Oxycodone 150 and 60 mg/kg PK studies were repeated to confirm results. Diazepam was selected as a benzodiazepine positive control to demonstrate the ability of the model to differentiate an additive or synergistic effect from an effect solely due to oxycodone. PK studies with diazepam were conducted at three dose levels (2, 20, and 200 mg/kg), and blood samples were collected at the same time points and the same analyses performed as those described for oxycodone PK studies.
2.5. Dosing solutions and method
Oxycodone dosing solutions were prepared by dissolving 6.75 mg, 60 mg, or 150 mg oxycodone (United States Pharmacopeia, North Bethesda, Maryland) in 2 ml sterile water (Lonza, Basel, Switzerland) for PK and combination studies. Diazepam dosing solutions were prepared by dissolving 2 mg, 20 mg, or 200 mg diazepam (United States Pharmacopeia, North Bethesda, Maryland) in 4 ml of a 50:50 solution of PEG400 (J.T. Baker, Allentown, PA) and propylene glycol (Thermo Fisher Scientific, Waltham, Massachusetts) for PK studies; and 20 mg diazepam in 2 ml of a 50:50 solution of PEG400 and propylene glycol for the combination study. All doses were given as oral solutions using an 18 gauge oral gavage needle with a 1 ml syringe for oxycodone; and an 18 gauge oral gavage needle with a 1 ml (for combination study) or 3 ml (for PK studies) syringe for diazepam. The oral route of administration for opioids and other SPDs was chosen as it is the most common clinical route for these prescribed drugs [20,21].
2.6. Blood sampling schedule for PK, pO2 and pCO2 following combination treatments of oxycodone and diazepam
Diazepam was given 30−35 min after oxycodone dosing to deliver peak serum concentrations of both drugs at approximately the same time. Six animals were in each combined treatment group or control (oxycodone only) group. Since PK studies with diazepam alone demonstrated some transient respiratory depression at the highest dose (200 mg/kg), the dose of 20 mg/kg diazepam that caused no respiratory depression was selected to be used with 150 mg/kg oxycodone that caused definite opioid-induced respiratory depression. In all combination studies, blood samples were collected prior to drug treatment and 15 min, 30 min, 1 h, 2 h, 3 h after administration of diazepam for measurements of drug concentrations, pO2, and pCO2. Combination experiments concluded after sampling 3 h post- diazepam dosing. The combination study of oxycodone 150 mg/kg with diazepam 20 mg/kg was repeated twice for a total of three experiments.
2.7. Blood sampling method
Blood sampling was performed in the same environment and conditions as cannula care. The time needed for collecting one blood sample was approximately 2−3 min. The sampling procedure depicted in Fig. 2 was carried out by a 2-person team with one person holding the animal and the other person doing the blood sampling procedure as follows: (1) the 22 g metal plug of the intraarterial cannula was removed from the external end of the cannula by using a hemostat; (2) the locking solution was aspirated from the cannula by using a 1-ml empty syringe with a 22 g blunt needle; (3) 0.3 ml of blood was withdrawn from the cannula by using a 1-ml empty syringe with a 22 g blunt needle; immediately after blood sampling and before dispensing to the i-STAT cartridge for blood gas analysis, the blunt needle was removed from the sampling syringe to reduce chances of blood cell damage from the blood being pushed through the narrow needle; (4) after removal of the blunt needle, 2 drops of the blood were immediately dispensed into the sampling well of an i-STAT CG4+ cartridge (Abbott, Princeton, NJ, USA) for blood gas analysis and the rest of the blood sample was transferred to a 1 ml MiniCollect blood tube (K3E K3EDTA, Greiner Bio-One North America Inc., Monroe, NC USA), gently shaken, then kept on wet ice for drug concentration analysis; (5) the cannula was flushed using 0.2 ml saline by using a 1-ml syringe with a 22 g blunt needle; (6) the cannula was refilled with 0.08 ml locking solution by using a 1-ml syringe with a 22 g blunt needle; (7) and the metal plug was inserted back into the end of the cannula to re-seal it. In the case of a blocked cannula during the experimental procedure, the blood sample was collected after saline flushing and cannula re-opening; or the animal was removed from the study if the blocked cannula did not re-open after attempted saline flushing. Post-sampling blood gas analysis was a time limiting step of the experiment. Once the analysis started, running of the device was automated and internally controlled by the i-STAT analyzer itself and could not be interrupted or stopped.
Fig. 2.
Blood sampling procedure. Details of the repeated blood sampling process through an indwelling arterial cannula.
2.8. Blood gas analyses
To minimize the time lapse between blood sampling and blood gas analysis, prevent blood from coagulating, and reduce air exchange between the blood sample and the atmosphere, the blood gas analysis was done immediately after the sample was drawn. A VetScan i-STAT 1 Handheld Analyzer with CG4+ cartridges (Abbott, Princeton, NJ, USA) was used for the blood gas analyses. The cartridges were removed from the storage refrigerator and placed at room temperature the day before the study, so that the temperature of the cartridge would equilibrate and function normally during use. Immediately after collection of arterial blood from the pre-implanted cannula, the blunt needle was removed from the sample syringe, and 2 drops of blood were dispensed directly from the syringe into the sample well of the CG4+ cartridge until the blood reached the filling line of the cartridge. The sample well cover was then closed, and the cartridge inserted into the analyzer. The analysis procedure would start automatically once the cartridge was inserted into the i-STAT device and it took about 2 min for the procedure to complete. Results were automatically saved in the VetScan i-STAT 1 Handheld Analyzer and displayed on the screen of the device once the analysis was completed.
2.9. Plasma drug concentration measurements
Drug measurements were completed in an internal bioanalytical lab. Details of the analytical method are described elsewhere [34]. Briefly, a validated liquid chromatography tandem mass spectrometric method was used for plasma sample analysis. The bioanalytical method was developed for simultaneous determination of oxycodone and diazepam in rat plasma using deuterated internal standards (IS) with simple acetonitrile-based protein precipitation. The m/z 316.2–298.2 for oxycodone; m/z 285.1–154.1 for diazepam; m/z 322.2–304.2 for oxycodone-d6 and m/z 290.2–198.1 for diazepam-d5 were used for detection. The chromatographic separation was achieved on a Zorbax C18 column (2.1 × 50 mm, 3.5 μm, Agilent Technologies, Inc. Santa Clara, CA) at column temperature of 20 ± 5 °C. The mobile phase consisted of an ammonium formate-formic acid buffer and acetonitrile with a 2 min gradient program.
2.10. General observation
General physical condition of all animals including movement and consciousness were observed constantly during the entire dosing and sampling procedure until the conclusion of all procedures.
3. Statistical methods
Software used for statistical analyses was SigmaPlot version 12.0. ANOVA and t-test were used for statistical analyses. Details of statistical method for each analysis are described in corresponding sections.
4. Results
4.1. General observations
No mortality was observed in the experiments. All animals treated with oxycodone alone or oxycodone with diazepam had notably reduced activity starting a few minutes after dosing with oxycodone, alone, or in combination with diazepam. The reductions in activity after combination treatment lasted for approximately 4 h after oxycodone dosing (4.5 h after diazepam dosing) compared to approximately 3 h in rats treated with oxycodone alone. All animals with behavioral observation following diazepam treatment, at 20 mg/kg or 200 mg/kg, were less active or slightly lethargic starting a few minutes after dosing; activity reduction in rats of the 20 mg/kg group recovered within an hour after dosing while extended beyond 3 h in rats of the 200 mg/kg group. Rats treated with diazepam 2 mg/kg did not show behavioral changes. Two animals were removed from the study either prior to initiation or at the beginning of the study due to complications from obstruction of the arterial cannula that could not be resolved.
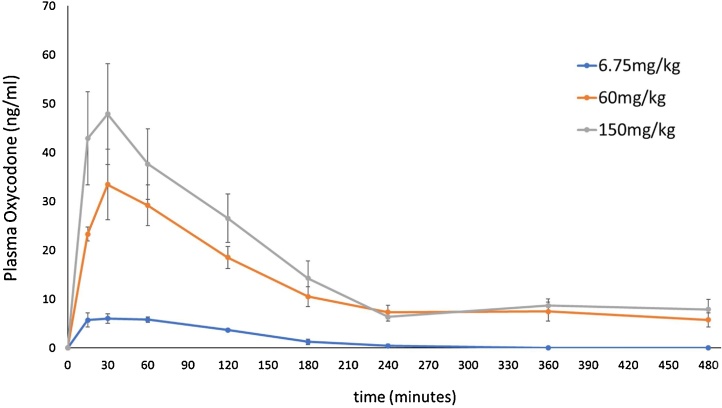
4.2. PK studies: plasma drug concentrations after treatment with oxycodone (Fig. 3)
Fig. 3.
Plasma oxycodone concentrations (ng/ml, mean ± SEM) after oral dosing with oxycodone.
Tmax was 30 min after dosing for all 3 dose levels (6.75, 60, and 150 mg/kg). The AUC (area under the curve) of the 6.75 mg/kg and 60 mg/kg groups were 89.83 % and 23.87 % lower than that of 150 mg/kg (high dose) group, respectively.
4.3. PK of repeat oxycodone 60 mg/kg (Supplemental Fig. 1)
The Tmax was the same as the Tmax of the original test, approximately 30 min after dosing; the PK profile curve was similar to the curve of the initial analysis with only slight variation.
4.4. PK of repeat oxycodone 150 mg/kg (Supplemental Fig. 2)
The PK profile curves of the primary and repeat tests were similar. There was no significant difference in post-treatment oxycodone levels between the 2 tests. Tmax of the repeat test was 60 min after treatment whereas Tmax of the primary test was 30 min after treatment (Supplemental Fig. 2). This difference of Tmax between the primary test and the repeat was taken into consideration when designing the dosing time for the combination studies with oxycodone + diazepam, so that the Cmax of both drugs could be reached at approximately the same time.
4.5. PD studies: blood pO2 and pCO2 after treatment with oxycodone (Fig. 4A and B)
Fig. 4.
A and B. Blood pO2 and pCO2 (mmHg, mean ± SEM) after oxycodone treatment. Upper panel (4A), pO2; lower panel (4B) pCO2.
One hour after dosing with oxycodone alone, pO2 levels were significantly lower than that of pre-treatment levels in all treatment groups of 6.75, 60, and 150 mg/kg (*p = 0.005, p = 008, p = 0.02, respectively, ANOVA) (Fig. 4A, upper panel). Post-treatment pCO2 levels were significantly higher than that of pre-treatment levels in the 150 mg/kg group (*p < 0.05, ANOVA), but not in treatment groups of 6.75 and 60 mg/kg (Fig. 4B, lower panel).
4.6. Reproducibility of the effect of oxycodone on blood gases (Supplemental Figs. 3A–B, 4A–B)
The curves of post-treatment pO2 levels of the primary and repeat tests were similar following treatment at both 60 mg/kg and at 150 mg/kg. At 60 mg/kg there was no significant difference between the tests. Post-treatment pO2 levels were significantly lower than the pre-treatment levels in both the primary and the repeat tests (p < 0.05, ANOVA). The curves of post-treatment pCO2 levels of the primary and repeat tests were similar.
With treatment at 150 mg/kg the curves of post-treatment pO2 levels of the primary and repeat tests were similar. Post-treatment pO2 levels were significantly lower than the pre- treatment levels in both the primary and the repeat tests (p < 0.001, ANOVA). The curves of post-treatment pCO2 levels of the primary and repeat tests were similar. Post-treatment pCO2 levels were significantly higher than the pre- treatment levels in both the primary and the repeat tests (p < 0.001, ANOVA).
4.7. PK studies: plasma drug concentrations after treatment with diazepam (Fig. 5)
Fig. 5.
Plasma diazepam concentrations (ng/ml, mean ± SEM) after diazepam oral dosing.
Tmax was 15 min after dosing for treatment groups 2 mg/kg and 20 mg/kg; and 120 min for the 200 mg/kg group. The mean post-treatment plasma diazepam concentrations of the 200 mg/kg group were significantly higher than that of the 20 mg/kg and 2 mg/kg groups (p < 0.001, ANOVA). There was no difference in post-treatment blood diazepam levels between the 2 mg/kg and 20 mg/kg groups (P = 0.19, ANOVA).
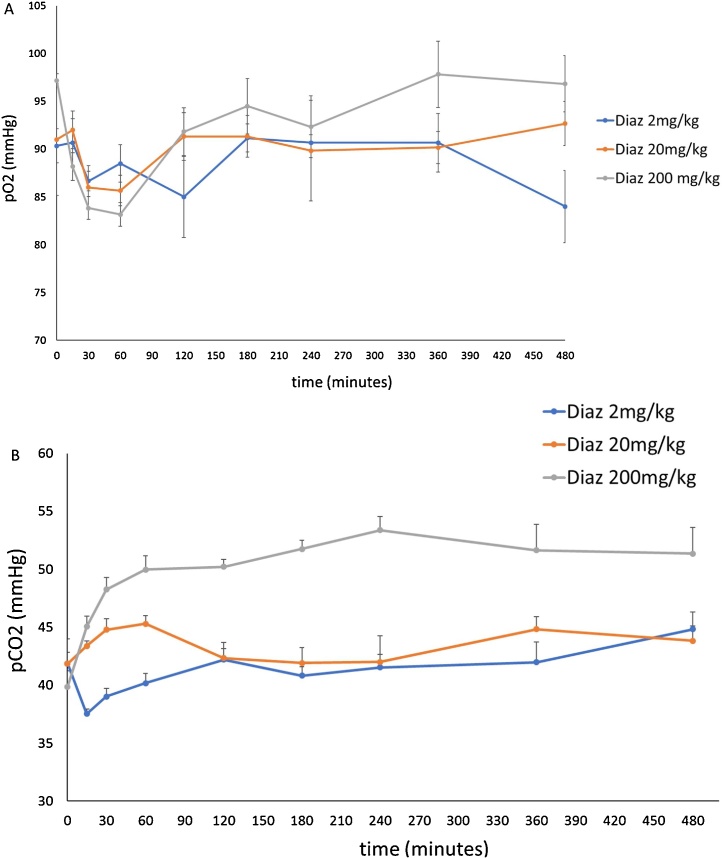
4.8. PD studies: blood pO2 and pCO2 after treatment with diazepam (Fig. 6A and B)
Fig. 6.
A and B. Blood pO2 and pCO2 (mmHg, mean ± SEM) after diazepam treatments (combination of 3 separate experiments). Upper panel (6A)-pO2; lower panel (6B)-pCO2.
The pO2 and pCO2 levels were not different from that of pre-treatment levels in the 2 mg/kg and 20 mg/kg groups; the post-treatment pO2 levels of the 200 mg/kg group were significantly lower (p < 0.001, ANOVA) and the post-treatment pCO2 levels were significantly higher than that of pre-treatment levels (p < 0.001, ANOVA). Post-treatment blood pCO2 levels in the 200 mg/kg group were significantly higher than that of the 20 mg/kg and 2 mg/kg groups (P < 0.001, ANOVA).
4.9. Selecting combination study timing and duration
Because it was the only dosing level to demonstrate a significant opioid-induced respiratory depression effect, 150 mg/kg was selected as the dose for oxycodone in combined opioid and benzodiazepine experiments. For diazepam, 200 mg/kg dosing had a significant depressing effect on respiration and, therefore, was not suitable for this study. A dosing concentration of 20 mg/kg that did not demonstrate respiratory depression was selected as the dose for diazepam in combined opioid and benzodiazepine experiments. In light of PD findings from the primary PK studies, it was decided that diazepam would be given 30−35 min after oxycodone dosing for the combination study, so that both drugs would reach Tmax at approximately the same time. From results of the preceding separate PK studies, blood concentrations of both drugs would have reached stable low levels at 180 min after dosing and pCO2 would have returned or would be approaching pre-treatment levels. Clinical observations also found that animals after treatment with either drug would have been largely recovered at 180 min after dosing. Therefore, the monitoring duration of blood drug concentrations and blood gases for the combination study was designated as 180 min after combined treatment.
4.10. Oxycodone PK profiles of combination treatment- oxycodone 150 mg/kg and diazepam 20 mg/kg
Oxycodone concentrations were increased when diazepam was administered 30−35 min after oxycodone (mean of three experiments). As presented in detail elsewhere [34], the AUC (area under the curve) of oxycodone concentrations of combination treatment (n = 18) was 113 % higher (p = 0.018, t-test) and Cmax was 100 % higher (p = 0.012, t-test) than those of rats receiving oxycodone only (n = 17).
4.11. Diazepam PK profiles of combination treatment- oxycodone 150 mg/kg and diazepam 20 mg/kg
Diazepam concentrations decreased when oxycodone was co-administered. As presented in detail elsewhere [34], the Cmax of combination treatment was 53.23 % lower than that of rats treated with diazepam 20 mg/kg alone; however, Tmax of combination treatment and diazepam treatment remained the same, both were 15 min after diazepam dosing.
The calculated PK parameters for oxycodone, diazepam and their combination are shown in Table 2.
Table 2.
Summary of Cmax and Tmax of treatment groups.
| Group | Cmax (ng/ml) | Tmax (min) | ||
|---|---|---|---|---|
| Oxycodone PK | 6.75 mg/kg | 7.0 | 30 | |
| 60 mg/kg* | 35.3 | 54 | ||
| 150 mg/kg** | 51.5 | 30 | ||
| Diazepam PK | 2 mg/kg | 22.6 | 30 | |
| 20 mg/kg | 286.5a | 18a | ||
| 200 mg/kg** | 1370.9 | 132 | ||
| Oxycodone PK, repeat | 60 mg/kg | 25.0 | 48 | |
| Oxycodone PK, repeat | 150 mg/kg | 45.0 | 78 | |
| Oxycodone 150 mg/g + Diazepem 20 mg/kg | Combined | oxycodone* | 102.3a | 78a |
| diazepam | 134.3a | 48a | ||
| Oxycodone (control) | 51.2a | 66a | ||
*p < 0.05, **P < 0.001: post treatment blood drug concentrations when compared with the lowest doses for PK studies; or when compared with controls for the combination study.
see reference [34].
4.12. Blood pO2 after treatment with oxycodone and diazepam (Fig. 7)
Fig. 7.
Blood pO2 (mmHg, mean ± SEM) after combination treatment of oxycodone 150 mg/kg and diazepam 20 mg/kg.
The AUC of pO2 levels of combination treatment (n = 18) was 17 % lower (p = 0.004, t-test) than controls treated with oxycodone only (n = 17); the lowest level of pO2 of the combination treatment group came at 60 min after oxycodone treatment (cf. 120 min for the oxycodone alone control group).
4.13. Blood pCO2 after treatment with oxycodone and diazepam (Fig. 8)
Fig. 8.
Blood pCO2 (mmHg, mean ± SEM) after combination treatment of oxycodone 150 mg/kg and diazepam 20 mg/kg.
The AUC of post dosing pCO2 levels with combination treatment (n = 18) was 25 % higher (p = 0.02, t-test) than that of controls (n = 17). The highest level of pCO2 in the combination treatment groups came at 60 min after combination treatment or 90 min after oxycodone treatment while the highest level of pCO2 of control animals occurred at 60 min after dosing with oxycodone alone. Increases in pCO2 were observed throughout the duration of the experiment (180 min).
5. Discussion
The objective of this study was to confirm previous work describing benzodiazepine exacerbation of opioid-induced respiratory depression and to test a rat model for assessing the respiratory depression potential, or risk, of different SPDs used alone or in combination with an opioid. A successful model requires sufficient sensitivity to detect relatively mild opioid-induced respiratory depression effects as well as additive or synergistic effects from a co-administered drug. Given the recent FDA product label changes and recognition of the exacerbated respiratory depression that could result from opioid use with a benzodiazepine, combined opioid-benzodiazepine exposure was the ideal positive control for a model of respiratory depression. Rats are a commonly used laboratory species especially in pharmacokinetic studies, are large enough to allow serial blood sampling, and have previously been used successfully in studies on respiratory depression [[35], [36], [37]].
Direct respiratory parameter measurements, such as rate and mean tidal volume as well as other inspiratory and expiratory parameters, were considered for assessing respiratory depression. Although methods are available in rats to obtain these measures, the complexity of the procedures suggested that this would not be the optimal approach. Alternatively, arterial partial pressures of oxygen and carbon dioxide are common clinical measures that reflect respiratory function, and that are used to evaluate clinical respiratory depression in patients [17,38,39]. The normal range and variability in these parameters are small and do not differ significantly between humans and rats [[40], [41], [42]]. The sensitivity to change appears to be high and relatively consistent across ranges measured in clinical conditions. For both rats and humans, most individuals have a normal pCO2 of approximately 40 mmHg with values below 35 mmHg indicating hyperventilation and above 45 mmHg hypoventilation [41,42]. For purposes of this model, demonstration of a change to hypoventilation and/or more severe hypoventilation were considered an indication of induced respiratory depression and/or exacerbated respiratory depression, respectively.
The first part of this study involved pharmacokinetic experiments designed to find optimal oxycodone and diazepam doses in rats to use for combination studies, to estimate optimal timing for dosing of the drugs in combination, and to acquire initial data on blood drug levels and blood gases (pO2 and pCO2) from groups of rats treated with a single drug, either oxycodone or diazepam, at three different dose levels. For combination exposures, oxycodone was given at 150 mg/kg, a dose that caused significant opioid-induced respiratory depression, and diazepam was administered at 20 mg/kg, a dose that had no respiratory depression effect.
It was noted that Tmax was reached between 30 min and 60 min after dosing in rats treated with oxycodone alone. This finding was taken into consideration in designing the combination study so that the Tmax of the two drugs would be reached at approximately the same time. The Tmax of diazepam 20 mg/kg was 18 min, therefore, diazepam was given 30−35 min after oxycodone treatment for the combination study with a target simultaneous Tmax between 45 and 60 min after oxycodone administration.
The clinical relevance of the dose levels of the two drugs was assessed in this study. For each drug, mean human Cmax and AUC data, for as many doses as available, were compiled from Clinical Pharmacology NDA reviews [internal communication] and published literature to estimate human single dose and steady-state exposures. Experimental rat data Cmax and AUC exposure data for each dose administered in the present rat experiments were compiled and approximate exposure equivalency between rat and human doses was assessed. Similar Cmax and AUC data in rats was then related to label dosing recommendation to see if exposures were clinically relevant. This process showed that a single dose of oxycodone at 150 mg/kg approximated a human single dose exposure of 40 mg PO and diazepam at 20 mg/kg reflected a human single dose exposure of 5–10 mg PO. Both of these exposures are within dosing recommendations and therefore were considered clinically relevant.
When co-administered, the Cmax of oxycodone was 100 % higher than that of controls treated with oxycodone alone and the Tmax of oxycodone of the combination treatment groups came at 78 min after oxycodone treatment while the Tmax of control animals treated with oxycodone alone came at 66 min after dosing.
The results obtained here are consistent with previous reported studies in the rat: treatment with diazepam at 20 mg/kg did not affect blood pCO2 values; however, the combination of diazepam with methadone caused a significantly greater increase in pCO2 than was seen with methadone alone [11]. Generally similar findings were observed with the combination of midazolam and buprenorphine [43]. Studies with other benzodiazepines show that different members of the family have different magnitudes of interaction with buprenorphine including no detected interaction [44].
Results of the present study confirmed literature reports [11] and mirrored the type of co-administration effects on respiratory depression that led to black box warnings for co-prescription of opioids and benzodiazepines. Additionally, blood drug concentrations and blood gases were measured at matching time points to understand the PK-PD relationship with co-administration. Although, the PK of oxycodone was significantly impacted by co-administration, the observed PD effects appear to exceed those anticipated by the change in PK. These findings suggest that with co-administration of oxycodone and diazepam changes in oxycodone PK work to exacerbate opioid-induced respiratory depression but there is also a direct exacerbating PD effect. These data support the potential clinical relevance of the model in assessing whether opioid-induced respiratory depression is exacerbated when used with different SPDs.
This study was designed for a specific purpose and may not be adequate for assessing respiratory depression in all circumstances particularly modeling of severe life-threatening respiratory depression. The goal was to define a model that could then be used with other SPDs to evaluate the respiratory depression potential of these SPDs for which little or no data currently exists. Oral absorption and availability of the drugs and the influence of different formulations are not likely to alter the occurrence of increased respiratory depression but could impact the relative risk assessment of the drugs based on the clinical relevance of exposures (depression seen at concentrations not commonly achieved clinically). However, the influence of dose, formulation, and availability should also be reflected in the measured plasma concentrations and can be adjudicated based on those measures. Differences in metabolism, uptake, or mechanistic pathway between rat and human could influence respiratory responses. Specifically, for oxycodone and diazepam, these do not appear to be significant factors for the immediate pharmacodynamic effect of interest (respiratory depression). Future use of the model would require case-by-case examination of these factors for other drugs.
The inclusion of some direct respiratory parameter measures could be used for assessing potential of acute severe respiratory depression. However, drug-induced acute severe respiratory depression is the exception and not the rule suggesting that any measure of significantly enhanced respiratory depression could indicate the potential for a more severe acute depression. The degree of change observed in blood gases with this model do not represent severe acute respiratory depression, and thus, may not predict the potential for that occurrence. The goal of this model was not to model severe acute respiratory depression but to measure the ability of drug combinations to change respiratory function, which it was able to do.
Due to the complexity of the sampling, in a single combination study, a maximum of six animals could be accommodated in the oxycodone-only control group and six in the combination group. The combination study of this experiment was completed a total of three times cumulatively providing n = 17 for the oxycodone only control group and n = 18 for the combination group. Results from individual experiments are similar but varied between experiments. The magnitude of the difference between oxycodone and oxycodone-diazepam pharmacokinetic and pharmacodynamic responses varied as much as 100 % from one experiment to another, but each experiment demonstrated a difference between the oxycodone group and oxycodone-diazepam groups. This variability particularly in oxycodone-alone responses demands a non-historic control for each combination experiment that also provides an interexperimental control for the oxycodone formulation that is made-up fresh for each experiment.
6. Conclusion
A rat model was successfully established to confirm previous literature reports by detecting exacerbating effects of benzodiazepines on opioid-induced respiratory depression when these drugs are co-administered. Concurrent blood drug concentrations and blood gas measures were analyzed to investigate the PK/PD relationship. That analysis supports both PK and direct PD interaction between oxycodone and diazepam that contribute to exacerbation of opioid-induced respiratory depression. This animal model is repeatable and sufficiently sensitive to detect opioid-induced respiratory depression as well as the exacerbating effects of a co-administered benzodiazepine. By recapitulating the known interaction of opioids and benzodiazepines, this model demonstrates potential for testing other drugs co-prescribed with opioids for their impact on opioid-induced respiratory depression.
Funding
The study was funded by U.S. Food and Drug Administration.
CRediT authorship contribution statement
Lin Xu: Methodology, Investigation, Data curation, Formal analysis, Visualization, Validation, Writing - original draft. Ashok Chockalingam: Methodology, Investigation. Sharron Stewart: Methodology, Investigation, Data curation, Validation. Katherine Shea: Methodology, Investigation. Murali K. Matta: Methodology, Investigation, Data curation, Validation. Suresh Narayanasamy: Methodology, Investigation, Data curation, Validation. Nageswara R. Pilli: Methodology, Investigation, Data curation, Validation. Donna A. Volpe: Methodology, Resources. James Weaver: Conceptualization, Methodology, Formal analysis, Writing - review & editing. Hao Zhu: Conceptualization, Methodology, Validation, Formal analysis. Michael C. Davis: Conceptualization, Methodology, Validation, Formal analysis. Rodney Rouse: Conceptualization, Methodology, Validation, Formal analysis, Resources, Data curation, Supervision, Project administration, Funding acquisition, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
Acknowledgment
None.
Footnotes
This article reflects the views of the authors and should not be construed to represent FDA’s views, policies or endorsements.
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.toxrep.2020.01.008.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.FDA Warns about Serious Risks and Death When Combining Opioid Pain or Cough Medicines with Benzodiazepines; Requires its Strongest Warning, 2016. https://www.fda.gov/Drugs/DrugSafety/ucm518473.htm.
- 2.Jones C.M., McAninch J.K. Emergency department visits and overdose deaths from combined use of opioids and benzodiazepines. Am. J. Prev. Med. 2015;49:493–501. doi: 10.1016/j.amepre.2015.03.040. [DOI] [PubMed] [Google Scholar]
- 3.Park T.W., Saitz R., Ganoczy D., Ilgen M.A., Bohnert A.S. Benzodiazepine prescribing patterns and deaths from drug overdose among US veterans receiving opioid analgesics: case-cohort study. BMJ. 2015;350:h2698. doi: 10.1136/bmj.h2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones C.M., Mack K.A., Paulozzi L.J. Pharmaceutical overdose deaths, United States. JAMA. 2010;309:657–659. doi: 10.1001/jama.2013.272. [DOI] [PubMed] [Google Scholar]
- 5.Jones C.M., Paulozzi L.J., Mack K.A., Centers for Disease Control and Prevention (CDC) Alcohol involvement in opioid pain reliever and benzodiazepine drug abuse-related emergency department visits and drug-related deaths—United States, 2010. MMWR Morb. Mortal. Wkly. Rep. 2014;63:881–885. [PMC free article] [PubMed] [Google Scholar]
- 6.Hwang Catherine, Kang Elizabeth, Kornegay Cynthia, Staffa Judy, Jones Christopher, McAninch Jana. Trends in the concomitant prescribing of opioids and benzodiazepines, 2002−2014. Am. J. Prev. Med. 2016;51(2):151–160. doi: 10.1016/j.amepre.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 7.Boudreau D., Von Korff M., Rutter C.M., Saunders K., Ray G.T., Sullivan M.D., Campbell C.I., Merrill J.O., Silverberg M.J., Banta-Green C., Weisner C. Trends in long-term opioid therapy for chronic non-cancer pain. Pharmacoepidemiol. Drug Saf. 2009;18(12):1166–1175. doi: 10.1002/pds.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Overdose Death Rates (Attached Supplemental Document: Drug Overdoses Data Document), Revised August 2018. National Institute of Drug Abuse 2018. https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates.
- 9.Dasgupta N., Funk M.J., Proescholdbell S., Hirsch A., Ribisl K.M., Marshall S. Cohort study of the impact of high-dose opioid analgesics on overdose mortality. Pain Med. 2016;17:85–98. doi: 10.1111/pme.12907. [DOI] [PubMed] [Google Scholar]
- 10.Jones Jermaine D., Mogali Shanthi, Comer Sandra D. Polydrug abuse: a review of opioid and benzodiazepine combination use. Drug Alcohol Depend. 2012;125(1–2):8–18. doi: 10.1016/j.drugalcdep.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCormick G.Y., White W.J., Zagon I.S., Lang C.M. Effects of diazepam on arterial blood gas concentrations and pH of adult rats acutely and chronically exposed to methadone. J. Pharmacol. Exp. Ther. 1984;230:353–359. [PubMed] [Google Scholar]
- 12.Papa N., Gueye Stephen W., Patricia Borron, Claire Risède, Françoise Monier, Marcel Buneaux, Frédéric Debray, Baud J. Buprenorphine and midazolam act in combination to depress respiration in rats. Toxicol. Sci. 2002;65(1):107–114. doi: 10.1093/toxsci/65.1.107. [DOI] [PubMed] [Google Scholar]
- 13.Hoffmann V.L., Vermeyen K.M., Adriaensen H.F., Meert T.F. Effects of NMDA receptor antagonists on opioid-induced respiratory depression and acute antinociception in rats. Pharmacol. Biochem. Behav. 2003;74:933–941. doi: 10.1016/s0091-3057(03)00020-0. [DOI] [PubMed] [Google Scholar]
- 14.Chevillarda Lucie, Declèvesa Xavier, Bauda Frédéric J., Risèdea Patricia, Mégarbanea Bruno. Respiratory effects of diazepam/methadone combination in rats: a study based on concentration/effect relationships. Drug Alcohol Depend. 2013;131:298–307. doi: 10.1016/j.drugalcdep.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 15.Giwa A., Oey E. The return of an old nemesis: survival after severe tricyclic antidepressant toxicity, a case report. Toxicol. Rep. 2018;5:357–362. doi: 10.1016/j.toxrep.2018.03.009. eCollection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Radke J.B., Kingery J.M., Maakestad J., Krasowski M.D. Diagnostic pitfalls and laboratory test interference after hydroxychloroquine intoxication: a case report. Toxicol. Rep. 2019;6:1040–1046. doi: 10.1016/j.toxrep.2019.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sood Pramod, Paul Gunchan, Puri Sandeep. Interpretation of arterial blood gas. Indian J. Crit. Care Med. 2010;14(2):57–64. doi: 10.4103/0972-5229.68215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanks G.W., Conno F., Cherny N. Morphine and alternative opioids in cancer pain: the EAPC recommendations. Br. J. Cancer. 2001;84:587–593. doi: 10.1054/bjoc.2001.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horn C.C., Kimball B.A., Wang H., Kaus J., Dienel S., Nagy A., Gathright G.R., Yates B.J., Andrews P.L. Why can’t rodents vomit? A comparative behavioral, anatomical, and physiological study. PLoS One. 2013;10(4):e60537. doi: 10.1371/journal.pone.0060537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ripamonti C., Zecca E., De Conno F. Pharmacological treatment of cancer pain: alternative routes of opioid administration. Tumori. 1998;84(3):289–300. doi: 10.1177/030089169808400302. [DOI] [PubMed] [Google Scholar]
- 21.Kirsh K., Peppin J., Coleman J. Characterization of prescription opioid abuse in the United States: focus on route of administration. J. Pain Palliat. Care Pharmacother. 2012;26(4):348–361. doi: 10.3109/15360288.2012.734905. [DOI] [PubMed] [Google Scholar]
- 22.Chevillard Lucie, Mégarbanea Bruno, Risède Patricia, Baud Frédéric J. Characteristics and comparative severity of respiratory response to toxic doses of fentanyl, methadone, morphine, and buprenorphine in rats. Toxicol. Lett. 2009;191:327–340. doi: 10.1016/j.toxlet.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 23.Subramanian R.K., Sidharthan A., Maneksh D., Ramalingam L., Manickam A.S., Kanthakumar P., Subramani S. Normative data for arterial blood gas and electrolytes in anesthetized rats. Indian J. Pharmacol. 2013;45(1):103–104. doi: 10.4103/0253-7613.106451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Izer Jenelle, Mattern Erin, Ellwanger Joy, Wilson Ronald. Comparison of arterial and venous blood-gas values in anesthetized Dorset cross-bred lambs (Ovis aries) using a point-of-care analyzer. Vet. Anaesth. Analg. 2019;46(2):209–213. doi: 10.1016/j.vaa.2018.12.003. Available online 21 January 2019. [DOI] [PubMed] [Google Scholar]
- 25.Sediame S., Zerah-Lancner F., d’Ortho M.P., Adnot S., Harf A. Accuracy of the i-STAT bedside blood gas analyser. Eur. Respir. J. 1999;14(1):214–217. doi: 10.1034/j.1399-3003.1999.14a36.x. [DOI] [PubMed] [Google Scholar]
- 26.Jacobs E., Vadasdi E., Sarkozi L., Colman N. Analytical evaluation of i-STAT Portable Clinical Analyzer and use by nonlaboratory health-care professionals. Clin. Chem. 1993;39(6):1069–1074. [PubMed] [Google Scholar]
- 27.Murthy J.N., Hicks J.M., Soldin S.J. Evaluation of i-STAT portable clinical analyzer in a neonatal and pediatric intensive care unit. Clin. Biochem. 1997;30(5):385–389. doi: 10.1016/s0009-9120(97)00046-5. [DOI] [PubMed] [Google Scholar]
- 28.Schneider J., Dudziak R., Westphal K., Vettermann J. The i-STAT analyzer. A new, hand-held device for the bedside determination of hematocrit, blood gases, and electrolytes. Anaesthesist. 1997;46(8):704–714. doi: 10.1007/s001010050457. [DOI] [PubMed] [Google Scholar]
- 29.Tschudi P.R. Evaluation of the portable blood analyser i-STAT. Schweiz. Arch. Tierheilkd. 1998;140(12):507–512. [PubMed] [Google Scholar]
- 30.Bardell D., West E., Mark Senior J. Evaluation of a new handheld point-of-care blood gas analyser using 100 equine blood samples. Vet. Anaesth. Analg. 2017;44(1):77–85. doi: 10.1111/vaa.12392. pii: S1467-2987(16)31392-7. [DOI] [PubMed] [Google Scholar]
- 31.Oyaert M., Van Maerken T., Bridts S., Van Loon S., Laverge H., Stove V. Analytical and pre-analytical performance characteristics of a novel cartridge-type blood gas analyzer for point-of-care and laboratory testing. Clin. Biochem. 2018;53:116–126. doi: 10.1016/j.clinbiochem.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 32.Prause G., Kaltenböck F., Doppler R. Preclinical blood gas analysis. 2. Experience with three blood gas analyzers in emergency care. Anaesthesist. 1998;47(6):490–495. doi: 10.1007/s001010050587. [DOI] [PubMed] [Google Scholar]
- 33.Oxycodone. https://www.drugbank.ca/drugs/DB00497.
- 34.Pilli Nageswara R., Narayanasamy Suresh, Xu Lin, Chockalingam Ashok, Shea Katherine I., Stewart Sharron, Rouse Rodney, Patel Vikram, Matta Murali K. A high-throughput bioanalytical assay to support pharmacokinetic interaction study of oxycodone and diazepam in Sprague Dawley rats. Internal communication. RSC Adv. 2020;10:886–896. doi: 10.1039/c9ra05785d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kiyatkin E.A. Respiratory depression and brain hypoxia induced by opioid drugs: morphine, oxycodone, heroin, and fentanyl. Neuropharmacology. 2019;151(June):219–226. doi: 10.1016/j.neuropharm.2019.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baby S.M., Gruber R.B., Young A.P., MacFarlane P.M., Teppema L.J., Lewis S.J. Bilateral carotid sinus nerve transection exacerbates morphine-induced respiratory depression. Eur. J. Pharmacol. 2018;834:17–29. doi: 10.1016/j.ejphar.2018.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liang X., Yong Z., Su R. Inhibition of protein kinase A and GIRK channel reverses fentanyl-induced respiratory depression. Neurosci. Lett. 2018;677:14–18. doi: 10.1016/j.neulet.2018.04.029. [DOI] [PubMed] [Google Scholar]
- 38.Walker H.K., Hall W.D., Hurst J.W. Clinical methods: the history, physical, and laboratory examinations. In: Trulock E.P. III, editor. Chapter 49 Arterial Blood Gases. 3rd edition. Butterworths; Boston: 1990. [PubMed] [Google Scholar]
- 39.David A. Kaufman. The American Thoracic Society. Interpretation of Arterial Blood Gases (ABGs). https://www.thoracic.org/professionals/clinical-resources/critical-care/clinical-education/abgs.php.
- 40.Thorson S.H., Marini J.J., Pierson D.J., Hudson L.D. Variability of arterial blood gas values in stable patients in the ICU. Chest. 1983;84(1):14–18. doi: 10.1378/chest.84.1.14. [DOI] [PubMed] [Google Scholar]
- 41.Svorc P., Petrášová D., Svorc P., Jr. Arterial pH and blood gas values in rats under three types of general anesthesia: a chronobiological study. Physiol. Res. 2018;67(5):721–728. doi: 10.33549/physiolres.933692. Epub 25 July 2018. [DOI] [PubMed] [Google Scholar]
- 42.O’Donnell Amy. 2008. Normal Values & Definitions 3 Steps to Abg Interpretation.https://www.aci.health.nsw.gov.au/__data/assets/pdf_file/0004/220675/ABG_poster_large.pdf [Google Scholar]
- 43.Gueye P.N., Borron S.W., Risede P., Monier C., buneauz F., Debray M., Baud F.J. Buprenorphine and midazolam act in combination to depress respiration in the rat. Toxicol. Sci. 2002;65:107–114. doi: 10.1093/toxsci/65.1.107. [DOI] [PubMed] [Google Scholar]
- 44.Pirnay S.O., Megarbane B., Borron S.W., Risede P., Monier C., Ricordel I., Baud F.J. Effects of various combinations of benzodiazepines with buprenorphine on arterial blood gases in rats. Basic Clin. Pharmacol. Toxicol. 2008;103:228–239. doi: 10.1111/j.1742-7843.2008.00273.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.