Key Points
A small molecular activator of PKM2 attenuates allergic airways disease in mice.
Activation of PKM2 decreases IL-1β–induced airway inflammation.
PKM2 activation decreases IL-1β–induced nuclear phosphorylation of STAT3.
Abstract
Asthma is a chronic disorder characterized by inflammation, mucus metaplasia, airway remodeling, and hyperresponsiveness. We recently showed that IL-1–induced glycolytic reprogramming contributes to allergic airway disease using a murine house dust mite model. Moreover, levels of pyruvate kinase M2 (PKM2) were increased in this model as well as in nasal epithelial cells from asthmatics as compared with healthy controls. Although the tetramer form of PKM2 converts phosphoenolpyruvate to pyruvate, the dimeric form of PKM2 has alternative, nonglycolysis functions as a transcriptional coactivator to enhance the transcription of several proinflammatory cytokines. In the current study, we examined the impact of PKM2 on the pathogenesis of house dust mite–induced allergic airways disease in C57BL/6NJ mice. We report, in this study, that activation of PKM2, using the small molecule activator, TEPP46, augmented PKM activity in lung tissues and attenuated airway eosinophils, mucus metaplasia, and subepithelial collagen. TEPP46 attenuated IL-1β–mediated airway inflammation and expression of proinflammatory mediators. Exposure to TEPP46 strongly decreased the IL-1β–mediated increases in thymic stromal lymphopoietin (TSLP) and GM-CSF in primary tracheal epithelial cells isolated from C57BL/6NJ mice. We also demonstrate that IL-1β–mediated increases in nuclear phospho-STAT3 were decreased by TEPP46. Finally, STAT3 inhibition attenuated the IL-1β–induced release of TSLP and GM-CSF, suggesting that the ability of PKM2 to phosphorylate STAT3 contributes to its proinflammatory function. Collectively, these results demonstrate that the glycolysis-inactive form of PKM2 plays a crucial role in the pathogenesis of allergic airways disease by increasing IL-1β–induced proinflammatory signaling, in part, through phosphorylation of STAT3.
Introduction
Asthma is a complex pulmonary disorder that is characterized by mucus metaplasia, airways hyperresponsiveness and remodeling and is accompanied by a chronic inflammatory process controlled by cells of the innate and adaptive immune system (1). The precise metabolic alterations that are induced in structural or immune cells that promote the disease processes remain incompletely understood. However, glycolytic reprogramming has been shown to be important in the regulation of immune cell activation and differentiation (1, 2). Our laboratory recently described that IL-1–induced glycolytic reprogramming contributes to allergic inflammation, airway remodeling, and airways hyperresponsiveness in a mouse model of house dust mite (HDM)–induced allergic airway disease (3). Moreover, enhanced glycolysis was shown to be required for the IL-1β–mediated release of the pleiotropic cytokines thymic stromal lymphopoietin (TSLP) and GM-CSF, two major epithelium-derived inflammatory mediators implicated in the pathogenesis of asthma. Levels of lactate were also increased in sputum of asthmatics, and significant correlations were observed between lactate and IL-1β. Moreover, lactate levels were elevated in subjects with neutrophilic asthma who had poor disease control (3), suggesting that increased glycolysis may be a feature of severe asthma.
During glycolysis, glucose is converted into pyruvate, which can be further metabolized in the mitochondria to produce ATP via oxidative phosphorylation. Pyruvate kinase (PK) catalyzes the final, rate-limiting step in glycolysis, the formation of pyruvate from phosphoenolpyruvate (PEP) while generating two molecules of ATP per glucose molecule. Pyruvate can also be converted into lactate under hypoxic conditions (anaerobic glycolysis) or in the presence of oxygen (aerobic glycolysis) in metabolically active cells such as cancer cells (4, 5). The PK family consists of four isoforms, which are encoded by two distinct genes. The Pkrl gene encodes the isoforms PKL and PKR, which are expressed in the liver and RBCs, respectively, and the PK muscle isozymes M1 (PKM1) and M2 (PKM2), which are derived from alternative splicing of the PKM gene (6, 7). PKM1 naturally occurs in a highly active tetrameric form and is expressed in many differentiated tissues, such as the muscle and the brain (8), whereas PKM2 can adopt monomer, dimer, or tetramer structural forms that dictate its intracellular function (9, 10). PKM2 is highly expressed during embryonic development as well as in proliferating cells (9). Tetrameric PKM2 has a high binding affinity to its substrate, PEP, prompting PKM2 glycolytic activity (11). In contrast, PKM2 in its dimer form has a low binding affinity to PEP and can translocate into the nucleus, where it acts as a transcriptional coactivator to enhance transcription of multiple proinflammatory cytokines (12). PKM2 has been shown to phosphorylate STAT3, which, in turn, augments its transcriptional activity (13). PKM2-linked STAT3 activation was recently shown to contribute to LPS-induced lung injury (14).
We previously showed that, in mice with HDM-induced airway disease, levels of PKM2 were increased compared with controls. Similarly, primary nasal epithelial cells derived from asthmatics also displayed increased PKM2 protein levels compared with cells from healthy controls. These observations of increases in PKM2 in settings of allergic airway disease, along with its dichotomous role as a glycolysis enzyme (glycolytic kinase) or proinflammatory mediator, led us to investigate whether a small molecule activator of PKM2, which stabilizes tetrameric PKM2 to promote conversion of PEP to pyruvate, affects HDM-induced allergic airways disease and IL-1β–induced inflammation.
In this study, we show that activation of the glycolysis function of PKM2 with the small molecule activator 6-[(3-Aminophenyl)methyl]-4,6-dihydro-4-methyl-2-(methylsulfinyl)-5H-Thieno[2′,3′:4,5]pyrrolo[2,3-d]pyridazin-5-one (TEPP46) exerts an anti-inflammatory effect in models of HDM- or IL-1β–induced lung inflammation in association with diminished activation of STAT3.
Materials and Methods
Reagents and Abs
All reagents were from Sigma-Aldrich unless otherwise noted.
Mouse studies
Age-matched 8–10-wk-old male and female wild-type (WT) C57BL/6NJ mice were bred at the University of Vermont. All animal experiments were approved by the Institutional Animal Care and Use Committee. To induce allergic airways disease, mice were sensitized intranasally with 10 μg of HDM (XPB70D3A2.5, lot 348718; volume, 2.5 ml/vial; endotoxin, 1140 endotoxin units per vial; Der p 1 levels, 144.9 mcg/vial; dry weight, 17 mg/vial; protein 2.92 mg/vial; Greer Laboratories, Lenoir, NC) in week 1 (day 1), resensitized in week 2 (day 8), followed by five consecutive challenges in week 3 (day 15–19). Moreover, mice were i.p. injected once per day with 25 or 50 mg/kg TEPP46 (Cayman Chemicals) on days 14–19. HDM was dissolved in saline, whereas TEPP46 was dissolved in 100% DMSO and further diluted 1:1 in a 0.5% carboxymethylcellulose solution in water. Therefore, the vehicle control groups were exposed to saline and received DMSO (50%) in carboxymethylcellulose solution (50%). Mice were harvested on day 20, 24 h after the last HDM installation.
In separate experiments, WT mice were i.p. injected with 50 mg/kg TEPP46 at day 1, followed by a second injection after 24 h of 50 mg/kg TEPP46 at day 2, together with intranasal administration of 1 μg of IL-1β (R&D Systems). IL-1β was dissolved in 0.1% BSA in PBS. Mice were harvested 6 or 24 h after the IL-1β administration.
Bronchoalveolar lavage fluid processing
Mice were euthanized, and bronchoalveolar lavage (BAL) was performed using 1 ml PBS. BAL was collected, and total cells were counted manually using a hemocytometer. BAL was centrifuged at 500 × g for 10 min at 4°C. Supernatant was stored at −80°C for further analysis. The cells were resuspended in 5% BSA in PBS and subsequently transferred to slides using a Cytospin, fixed in 100% methanol for 5 min, and stained with the Hema 3 kit (Thermo Fisher Scientific, Kalamazoo, MI). Total macrophages, neutrophils, eosinophils, and lymphocytes were analyzed by counting a total of 300 cells per slide by two independent investigators blinded to the identity of the samples.
Cell culture
Primary mouse tracheal epithelial (MTE) cells were isolated from WT C57BL/6NJ mice (purchased from The Jackson Laboratory, Bar Harbor, ME) and cultured as previously described (15, 16). WT MTE cells were grown on 12-well Transwell inserts to confluency, followed by overnight starvation and pretreated with 100 μM TEPP46 (Cayman Chemicals) for 1 h prior to stimulation with 10 ng/ml IL-1β (R&D Systems) for 24 h. In all cell experiments, MTE cells were treated at the apical and basolateral side. In HDM-treated MTE cell experiments, cells were washed 24 h after IL-1β treatment and incubated with DMEM/F12 medium for 2 h before stimulation with 50 μM HDM (XPB70D3A2.5, lot 348718; volume, 2.5 ml/vial; endotoxin, 1140 endotoxin units per vial, Der p 1 levels, 144.9 mcg/vial; dry weight, 17 mg/vial; protein 2.92 mg/vial; Greer Laboratories) for an additional 2 h. Cells were harvested for protein or mRNA, and medium was collected for analysis of lactate and cytokine levels. To examine the contribution of phosphorylation of STAT3, MTE cells were treated with Stattic (17) (Abcam, Cambridge, U.K.) at the indicated concentrations for 1 h prior to IL-1β treatment for 24 h.
Assessment of mucus metaplasia and collagen deposition
Left lung lobes were fixed in 4% paraformaldehyde in PBS, embedded in paraffin, and sectioned. Airway mucus was analyzed via Periodic acid–Schiff stain. Collagen deposition was assessed via Masson’s trichrome stain. The intensity of the staining was evaluated by scoring of slides by two independent blinded investigators.
Western blotting
Protein concentrations in cell and tissue lysates were determined by Bio-Rad DC Protein Assay Kit (Bio-Rad Laboratories, Hercules, CA). Proteins were resolved using reducing sodium dodecyl sulfate SDS-PAGE, then transferred to polyvinylidene difluoride membranes, followed by incubation with the indicated primary Ab. PKM1 (no. 7067), PKM2 (no. 4053), p-STAT3 (no. 8119), STAT3 (no. 4904), inhibitory κ B kinase ε (IKKε; no. 3416), and histone H3 (no. 4499) Abs were obtained from Cell Signaling Technology (Danvers, MA). β-Actin Ab was acquired from Sigma-Aldrich. Subsequently, membranes were incubated with peroxidase-conjugated secondary Abs and visualized using chemiluminescence (Pierce, Rockford, IL). Nonreducing gel electrophoresis assays in the presence of the disuccinimidyl suberate cross-linker (Thermo Fisher Scientific) were performed to evaluate the formation of tetrameric PKM2. Densitometric analyses were performed using ImageJ software. Values were normalized to corresponding β-actin bands.
PKM activity assay
PKM activity assay was performed using a PK activity kit according to the manufacturer’s protocol (BioVision). Briefly, lung tissues or MTE cells were homogenized in PBS, and lysates were normalized to equal protein concentrations. Equal volumes (total volume of 50 μl) of normalized lung tissue or cell lysates were used in the assay. The relative fluorescence units, which displays the rate of pyruvate yield, was normalized and expressed as relative fluorescence units per minute per μg of protein.
Preparation of nuclear extracts
For fractionation, MTE cells were stimulated with 10 ng/ml IL-1β (R&D Systems) for 24 h. Fractionation of nuclear and cytosolic extracts was performed by using the Nuclear and Cytoplasmic Extraction Kit (Thermo Fisher Scientific) according to manufacturer’s protocol, followed by Western blotting.
Lactate measurements
Lactate levels were measured in cell culture medium with a lactate assay kit (Eton Bioscience) according to manufacturer’s instructions. Equal volumes of cell culture medium was used in 10-kDa Amicon Ultra Centrifugal Filters (MilliporeSigma). Samples were deproteinized by centrifugation for 1 h at 14,000 × g at 4°C.
Cell viability assay
MTE cells were gently washed twice in ice-cold PBS and stained with a Crystal Violet dye (0.5% Crystal Violet solution in 20% methanol) for 20 min at room temperature. After incubation, the staining solution was carefully removed, and the cells were washed four times with distilled water. Subsequently, 10% acetic acid was added to the cells for 30 s while shaking. Finally, 100 μl of the acetic acid solution per well was transferred to a 96-well plate, and the OD was measured at a wavelength of 595 nm. In addition, a Calcein AM Assay Kit (Cayman Chemicals) was used according to manufacturer’s instructions. Cell survival was expressed as percentage of survival compared with untreated control cultures.
Real-time quantitative PCR
Total RNA was extracted using miRNeasy columns (QIAGEN, Valencia, CA) according to the manufacturer’s protocol. First-strand cDNA was synthesized from 1 μg RNA and reverse transcribed for gene analysis using SYBR Green (Bio-Rad Laboratories). cDNA from the samples were amplified by real-time quantitative PCR with specific primers for Tslp, Csf2, Cxcl1, Ccl20, Muc5AC, and Col1a1. The data were normalized to Ppia (also known as cyclophilin A). The primer sequences are listed in Supplemental Table I.
ELISA
CCL20, TSLP, GM-CSF, KC/CXCL1, IL-33, and IL-1β were detected by ELISA kits (R&D Systems, Minneapolis, MN) in normalized lung tissue lysates or supernatants from cell cultures according to the manufacturer’s instructions.
Statistical analysis
Data are expressed as means ± SEM. All cell experiments were performed at least three times with n = 3 per group. Significant differences between groups were determined using the GraphPad Prism software (GraphPad) by two-way ANOVA with a Tukey post hoc test for multiple comparisons. Any p values lower than 0.05 were accepted as significant.
Results
Activation of the glycolysis function of PKM2 with TEPP46 attenuates airway inflammation, mucus metaplasia, and subepithelial collagen in mice with HDM-induced allergic airways disease
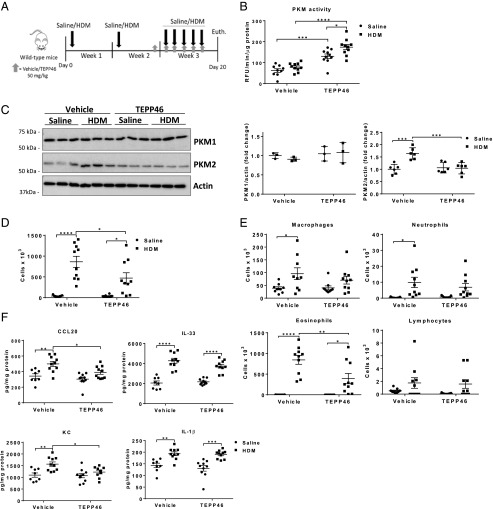
To investigate the role of PKM2 in the pathogenesis of HDM-induced allergic airways disease, C57BL6/NJ mice were sensitized with HDM once in week 1 and in week 2, followed by five consecutive challenges in week 3 to induce allergic airways disease (Fig. 1A). To promote PKM2 glycolytic activity, mice were injected i.p. once per day with TEPP46 during the HDM challenges (days 14–19), starting the day prior to the HDM challenges in week 3 (Fig. 1A). Administration of TEPP46 resulted in elevated activity of PKM in lung tissue from saline control animals, and further increases in PKM activity were observed in lungs from mice exposed to HDM, indicating that TEPP46 augmented PKM2 activity (Fig. 1B). Although protein levels of PKM1 did not differ between the groups, we observed slight increases in PKM2 expression in HDM-exposed mice (Fig. 1C), consistent with our previous observations, and these increases in PKM2 expression were no longer observed in mice receiving TEPP46. No statistically significant differences in overall PKM activity were observed in lungs from saline or HDM-treated mice in the absence of TEPP46 (Fig. 1B, vehicle groups), despite observed increases in PKM2 expression in mice exposed to HDM (Fig. 1C), suggesting that the increased expression of PKM2 does not contribute to its enhanced activity as a glycolysis enzyme, converting PEP to pyruvate. Next, we assessed the extent of inflammation by total and differential immune cell counts in the BAL fluid. Activation of PKM2 by TEPP46 attenuated the HDM-mediated increases in total cells in the BAL fluid, reflected by a decrease in eosinophils (Fig. 1D, 1E), whereas the number of neutrophils, macrophages, and lymphocytes were comparable between the HDM-treated groups. To further investigate the impact of activation of PKM2 on the extent of HDM-induced allergic airway inflammation, we evaluated protein levels of various cytokines in lung tissue homogenates. HDM-mediated increases of the cytokines CCL20 and KC were attenuated upon PKM2 activation (Fig. 1F). Small, but not statistically significant, decreases in IL-33 were observed in HDM-exposed mice that received TEPP46, compared with the respective control group, whereas IL-1β levels did not change (Fig. 1F). Together, these results show that PKM2 activation, using TEPP46, attenuates HDM-induced inflammatory cytokines in the lung.
FIGURE 1.
Activation of PKM2 by TEPP46 attenuates proinflammatory cytokines in mice with HDM-induced allergic airway disease. (A) Schematic depicting the exposure regimen. Mice were sensitized twice with 10 μg of HDM or saline on days 1 and 8. Mice were treated with 50 mg/kg TEPP46 i.p. daily, starting on day 14. Mice were challenged with HDM on days 15–19 and euthanized 24 h after the final HDM challenge. (B) Assessment of PKM activity in lung tissue homogenates. (C) Representative Western blots and quantification for total PKM1 and PKM2 levels. β-Actin; loading control (n = 3–6 per group). (D and E) Total and differential cell counts in BAL fluid. (F) Measurements of CCL20, IL-33, KC, and IL-1β in lung tissue homogenates by ELISA. (A, B, and D–F) n = 8–10 per group. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 (two-way ANOVA).
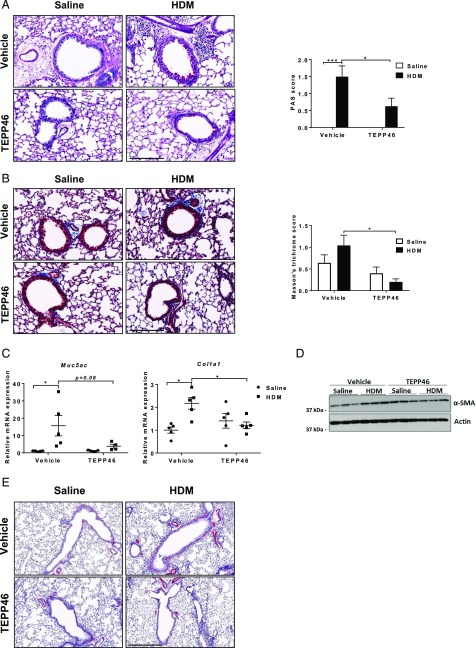
We next evaluated the impact of PKM2 activation on airway remodeling by assessing mucus metaplasia, subepithelial collagen, and α–smooth muscle actin (α-SMA). Results in Fig. 2A–E demonstrate that administration of TEPP46 attenuated HDM-mediated increases in mucus metaplasia, subepithelial collagen, and α-SMA content and decreased expression of Muc5AC and Col1a1 mRNAs. These results demonstrate that activation of PKM2 by TEPP46 attenuates airway remodeling in mice with HDM-induced allergic airways disease.
FIGURE 2.
Activation of PKM2 by TEPP46 attenuates mucus metaplasia, subepithelial collagen, and markers of airway remodeling in mice with HDM-induced allergic airway disease. (A) Assessment and quantification of mucus metaplasia by Periodic acid–Schiff (PAS) staining intensity and (B) collagen deposition by Masson’s trichrome staining. Scale bar, 200 μm. (C) mRNA expression of Muc5AC, and Col1a1, normalized to Ppia. (D) Representative Western blots for SMA levels and the loading control β-actin. (E) Assessment of α-SMA staining around large airways (n = 5 per group). Scale bar, 300 μm. *p < 0.05, ***p < 0.001 (two-way ANOVA).
Activation of PKM2 by TEPP46 attenuates IL-1β–mediated proinflammatory responses in mouse lungs
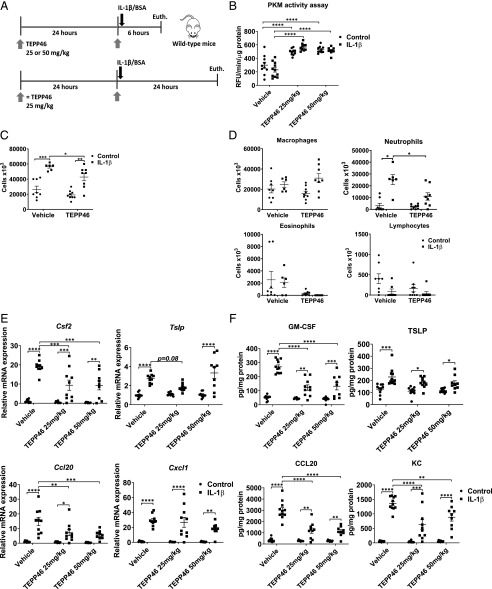
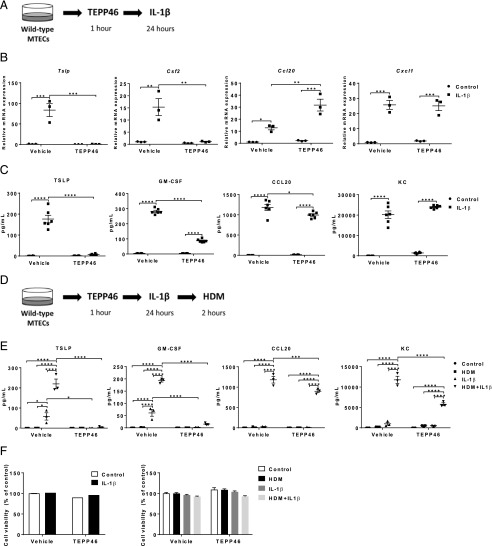
We have previously shown that increases in glycolysis promote proinflammatory responses in airway epithelial cells exposed to IL-1β by increasing the production of the proinflammatory cytokines TSLP, GM-CSF, KC, and CCL20 (3). Results in Fig. 1F demonstrate similar increases in IL-1β levels in lung tissue from HDM-exposed mice receiving vehicle or TEPP46, suggesting that TEPP46 does not regulate expression of IL-1β. To examine whether PKM2 activity affects the responsiveness of lungs to IL-1β, WT mice were i.p. injected with TEPP46 prior to intranasal IL-1β instillation for either 6 or 24 h (Fig. 3A). TEPP46 increased the total PKM activity in WT mice, with no further increases being observed in response to IL-1β (Fig. 3B) after 6 h, and similar results were observed after 24 h (data not shown). As expected, IL-1β elicited increases in total cell counts, reflected by neutrophils 24 h after intranasal administration, without affecting airway eosinophils, macrophages, and lymphocytes (Fig. 3C, 3D). In animals receiving TEPP46, the IL-1β–mediated increases in total cell counts and neutrophils were diminished (Fig. 3C, 3D). In agreement with these findings, mRNA expression levels of Csf2 and Ccl20 and the respective protein levels of GM-CSF and CCL20 were significantly attenuated in mice treated with TEPP46 prior to IL-1β instillation for 6 h (Fig. 3E, 3F), whereas there was less to no effect on Cxcl1 and Tslp mRNA and protein levels (KC and TSLP). Dosages of 25 or 50 mg/kg of TEPP46 were similar in their ability to induce PKM activity and dampen proinflammatory responses (Fig. 3B, 3E, 3F). Collectively, these results demonstrate that PKM2 activation decreases select IL-1β–induced inflammatory responses in the lung.
FIGURE 3.
PKM2 activation attenuates the release of proinflammatory cytokines following intranasal administration of IL-1β. (A) Schematic depicting the pretreatment with 25 or 50 mg/kg TEPP46 prior to intranasal administration of 1 μg of IL-1β for either 6 or 24 h. The total cell count and cell differentials in the BAL fluid reflect 24 h after IL-1β treatment, the other results shown are obtained 6 h post–IL-1β. (B) Assessment of PKM activity in lung tissue homogenates. (C and D) Total and differential cell counts in BAL fluid. (E) mRNA expression of proinflammatory cytokine genes in lung tissue homogenates. Results were normalized to the housekeeping gene Ppia. (F) Levels of proinflammatory mediators TSLP, GM-CSF, KC, and CCL20 in lung tissue homogenates by ELISA (n = 8–11 per group). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 (two-way ANOVA).
TEPP46 decreases nuclear translocation of PKM2 and dampens IL-1β–mediated proinflammatory responses in mouse tracheal basal cells
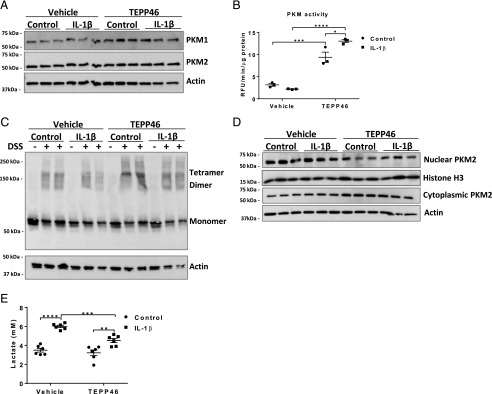
Airway epithelial cells are important contributory cells to allergic airway disease, as these cells release a number of mediators that promote innate and adaptive immune responses (18, 19). We previously demonstrated that IL-1β–induced glycolysis is critical for the release of TSLP and other asthma-relevant cytokines by epithelial cells and that IL-1β–induced glycolysis also primes these cells to elicit augmented proinflammatory responses to HDM (3). PKM2 in its dimer form has a low binding affinity to PEP and can translocate into the nucleus, where it acts as a transcriptional coactivator to enhance transcription of multiple proinflammatory cytokines. We, therefore, next determined whether IL-1β affects the status and/or nuclear presence of PKM2 in epithelial cells and whether PKM2 activation affects the response to IL-1β. Primary MTE cells were pretreated with TEPP46 for 1 h, prior to IL-1β stimulation for 24 h. This experimental regimen did not result in apparent changes in expression levels in PKM1 and PKM2 (Fig. 4A). However, TEPP46 augmented overall PKM glycolytic activity in control cells, and a further enhancement of PKM activity occurred when cells were treated with TEPP46 in combination with IL-1β (Fig. 4B). PKM2 is active as a glycolysis enzyme in its tetramer form and loses its activity as a glycolytic kinase in the dimer form (11). Instead, dimeric PKM2 has been shown to translocate into the nucleus, where it acts as a protein kinase to induce phosphorylation of STAT3, augmenting STAT3 transcriptional activity and leading to increased expression of proinflammatory mediators and increased expression of glycolysis enzymes including glucose transporter 1 (GLUT-1), thereby promoting glycolytic reprogramming (20). IL-1β led to a slight attenuation of PKM2 tetramers in MTE cells, whereas TEPP46 increased PKM2 tetramers in both control and IL-1β–treated cells, relative to the respective vehicle groups (Fig. 4C). We did not observe an increase in nuclear PKM2 24 h postadministration of IL-1β. However, TEPP46 diminished the nuclear presence of PKM2 and increased its cytoplasmic localization, in both control and IL-1β–stimulated cells (Fig. 4D). Consistent with the attenuation of glycolytic reprogramming, TEPP46 administration led to a decrease in IL-1β–mediated lactate secretion (Fig. 4E).
FIGURE 4.
TEPP46 augments PKM activity and PKM2’s cytosolic presence and attenuates IL-1β–mediated lactate secretion in primary MTE cells. MTE cells were treated with 100 μM TEPP46 for 1 h prior to stimulation with 10 ng/ml IL-1β for 24 h. (A) Representative Western blot of total PKM1 and PKM2 levels and β-actin. (B) PKM activity assay in MTE cells and (C) representative Western blot for tetrameric, dimeric, and monomeric PKM2 and the loading control β-actin. MTE cells were incubated in the presence or absence (first lane of each condition) of the disuccinimidyl suberate (DSS) cross-linker to evaluate the formation of the isoforms of PKM2. (D) Representative Western blots of nuclear and cytosolic extracts of PKM2 (n = 3 per group). (E) Lactate levels in supernatants of MTE cells. Experiments were performed at least three times. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 (two-way ANOVA).
To investigate whether activation of PKM2 attenuates proinflammatory cytokine release from airway epithelial cells, MTE cells were pretreated with TEPP46, followed by stimulation with IL-1β (Fig. 5A). Strikingly, PKM2 activation strongly attenuated the IL-1β–induced mRNA and protein levels of TSLP and Csf2 (GM-CSF), respectively, whereas it modestly or did not affect CCL20 or Cxcl1 (KC) (Fig. 5B, 5C). As was stated earlier, exposure to IL-1β primes MTE cells to subsequent stimulation with HDM, leading to augmented release of proinflammatory cytokines. We therefore pretreated primary MTE cells with TEPP46, followed by stimulation with IL-1β for 24 h. Cells were then washed and exposed to HDM for 2 h (Fig. 5D). In agreement with our previous observations, prior exposure to IL-1β leads to potent HDM-stimulated release of TSLP, GM-CSF, KC, and CCL20. TEPP46 almost completely abolished TSLP and GM-CSF in this sequential exposure regimen and significantly decreased KC and CCL20 (Fig. 5E). TEPP46 treatment alone or in combination with IL-1β or IL-1β plus HDM did not induce cell death (Fig. 5F), demonstrating that the decreased cytokine production is not due to a loss of cell survival. Collectively, these data demonstrate that TEPP46 diminishes IL-1β and HDM-mediated proinflammatory responses in epithelial cells in association with increases in PKM2 cytosolic presence and enhanced PKM glycolytic activity.
FIGURE 5.
Activation of PKM2 attenuates IL-1β–mediated proinflammatory responses in primary MTE cells and the release of proinflammatory mediators following subsequent exposure to HDM. (A) Schematic depicting the pretreatment with 100 μM TEPP46, followed by stimulation of 10 ng/ml IL-1β for 24 h. (B) mRNA expression of Tslp, Csf2, Cxcl1, and Ccl20 in MTE cells. Ppia is used as housekeeping gene. (C) Proinflammatory cytokine mediators TSLP, GM-CSF, KC, and CCL20 in cell culture supernatants of MTE cells were detected by ELISA. (D) Schematic depicting the pretreatment with 100 μM TEPP46, followed by stimulation of 10 ng/ml IL-1β for 24 h. Media was replaced and exposed to HDM (50 μg/ml) for an additional 2 h. (E) Proinflammatory cytokine mediators TSLP, GM-CSF, KC, and CCL20 in cell culture supernatants of MTE cells. (F) Cell survival was evaluated by Crystal Violet staining (left) and Calcein AM Assay (right) in MTE cells (n = 3–6 per group). Experiments were performed at least three times. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 analyzed (two-way ANOVA).
PKM2-mediated phosphorylation of STAT3 contributes to IL-1β–mediated proinflammatory signaling in epithelial cells
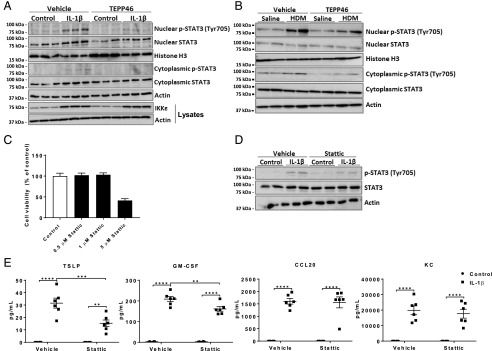
It has been previously described that nuclear PKM2 phosphorylates STAT3, thereby augmenting the production of proinflammatory cytokines, including IL-6 and IL-1β (11, 20). We therefore addressed whether PKM2 contributed to STAT3 activation and, in turn, whether STAT3 promoted IL-1β–induced proinflammatory signaling in mouse epithelial cells. Despite the lack of observed increases in nuclear PKM2 in response to IL-1β (Fig. 4D), IL-1β elicited strong increases in nuclear p-STAT3 using an Ab directed against phosphorylation of tyrosine 705, the residue known to be phosphorylated by PKM2 (11) (Fig. 6A). Total content of STAT3 in the nucleus was also increased in epithelial cells exposed to IL-1β (Fig. 6A). Exposure to TEPP46 led to a strong diminution of nuclear p-STAT3 (Fig. 6A), consistent with the aforementioned role of PKM2 as an STAT3 kinase (20). Similarly, phosphorylation of nuclear STAT3 was also increased in lung tissues from mice with HDM-induced allergic airways disease and was diminished in mice also treated with TEPP46 (Fig. 6B). We previously showed data suggesting that IKKε is a critical mediator in IL-1β–induced glycolysis. In this study, we show that the IL-1β–induced expression levels of IKKε were unaffected when MTE cells were pretreated with TEPP46, suggesting that the effect of TEPP46 on diminishing STAT3 phosphorylation may be downstream or independent of IKKε (Fig. 6A). To further corroborate the role of STAT3 in promoting IL-1β–induced proinflammatory responses, we used the STAT3 inhibitor, Stattic (17, 20), in vitro. Concentrations greater than 1 μM Stattic caused marked epithelial cell death (Fig. 6C). Nonetheless, a concentration of 0.5 μM Stattic diminished IL-1β–mediated phosphorylation of STAT3 in whole-cell lysates (Fig. 6D) and attenuated the IL-1β–mediated increases of TSLP and GM-CSF without affecting the other cytokines (Fig. 6E). All together, these data suggest that activation of PKM2 as a glycolytic kinase by TEPP46 diminishes the proinflammatory responses induced by IL-1β in lung epithelial cells or in mice with allergic airway disease and that the increased kinase activity of PKM2 toward STAT3 in these settings may, in part, contribute to PKM2-linked inflammation.
FIGURE 6.
PKM2-mediated phosphorylation of STAT3 contributes to IL-1β–mediated proinflammatory signaling in epithelial cells. (A) Representative Western blots of total and phosphorylated STAT3 in nuclear and cytosolic extracts from MTE cells and total IKKε levels in whole-cell lysates. (B) Representative Western blots of total and phosphorylated STAT3 in nuclear and cytosolic extracts from HDM- or saline-treated lung tissues. (C) Impact of Stattic on survival of MTE cells was evaluated by a Calcein AM Assay. (D) Representative Western blots of total and phosphorylated STAT3 in whole-cell lysates from control or IL-1β–stimulated MTE cells pretreated with Stattic or vehicle control. (E) Proinflammatory mediators TSLP, GM-CSF, KC, and CCL20 in cell culture supernatants of MTE cells after treatment for 1 h with 0.5 μM Stattic, followed by stimulation of 10 ng/ml IL-1β for 24 h. Experiments were conducted at least three times. **p < 0.01, ***p < 0.001, ****p < 0.0001 analyzed (two-way ANOVA).
Discussion
Allergic airway disease is associated with chronic inflammation and airway remodeling, processes that are metabolically demanding. During glycolysis, some of the carbons derived from glucose are used to allow for biosynthetic processes. In addition, glycolysis has also been linked to proinflammatory responses in immune cells (21, 22). Our laboratory has previously shown that glycolysis is a feature of allergic asthma in association with neutrophilic inflammation and steroid-resistant disease and that IL-1 is an important driver of glycolysis in settings of allergic airways disease in mice (3). In addition to these observations, increases in aerobic glycolysis have been shown to promote T cell activation (23) and to promote T cell effector function (21). Increases in glycolysis also have been implicated in LPS-induced airway smooth muscle cell proliferation (24) and in IL-33–mediated increases in cytokine production in mast cells (25). Asthma-associated, single-nucleotide polymorphisms within the orosomucoid-like 3 (ORMDL3) locus have been implicated in disease susceptibility. A recent study showed that ablation of ORMDL3 attenuated IL-1–mediated endoplasmic reticulum stress and cytokine responses in A549 lung epithelial cells in association with alterations in glycolysis and glucose metabolism genes (26), indicating a potential link between glycolysis and asthma susceptibility. Increases in glycolysis, basal and maximal respiration, and oxidative stress were demonstrated in airway epithelial cells and platelets from obese asthmatics (who tend to have more severe disease), in comparison with lean asthmatics and healthy subjects (27). Notably, increases in airway lactate were demonstrated in asthmatics with a high fraction of exhaled NO, in association with elevated expression of inducible NO synthase and arginase 2, and suggests a link between enhanced glycolysis, arginine metabolism, and a high fraction of exhaled NO asthma phenotype (28). Nonetheless, the precise signals and settings that elicit the glycolysis-associated proinflammatory responses in lung epithelial cells remain unclear. In the current study, we demonstrate the importance of the glycolytic enzyme PKM2 in promoting inflammation and airway remodeling in mice with HDM-induced allergic airway disease. PKM2 has generated substantial interest because of its impact on glycolytic reprogramming in activated immune cells and tumor cells and its emerging role as a proinflammatory mediator (9, 12). In this study, we demonstrate that activation of the glycolysis function of PKM2 with TEPP46 augments PK activity in lung tissue and airway epithelial cells and dampens inflammation, evidenced by attenuated airway eosinophilia and airway remodeling in mice with HDM-induced allergic airways disease. Moreover, administration of TEPP46 attenuated IL-1β–induced airway neutrophilia in mice and significantly reduced IL-1β–mediated expression of proinflammatory cytokines and lactate. These results, in addition to our previous results (3), show that enhanced glycolysis is important for the amplification of allergen-induced proinflammatory responses and show the importance that PKM2 plays in regulating this process.
Results in this study point to the importance of glycolysis in the secretion of specific proinflammatory mediators from airway epithelial cells, notably TSLP and GM-CSF. Activation of PKM2 with TEPP46 almost completely abolished expression of both cytokines, whereas Stattic also attenuated the IL-1β–mediated release of these cytokines (Figs. 5C, 6E). These findings are in line with our previous study in which we demonstrated that inhibition of IKKε (IKBKE) or TANK-binding kinase 1 (TBK1), two kinases critical in promoting IL-1β–induced glycolysis, also virtually abolished secretion of TSLP and GM-CSF (3). The importance of TSLP in asthma has been extensively studied (22, 29–32). TSLP is primarily expressed in epithelial cells and acts on both innate and adaptive immune cells, thereby promoting Th2 immunity and steroid resistance (33). Overexpression of TSLP results in the development of severe airway inflammation and airway hyperresponsiveness (29, 32). The U.S. Food and Drug Administration has granted breakthrough-therapy designation for tezepelumab, a TSLP-blocker, in patients with severe asthma. Blocking TSLP may prevent the release of other proinflammatory cytokines by immune cells, resulting in the prevention of asthma exacerbations and improved asthma control (34). Similarly, GM-CSF has been shown to activate macrophages and promote eosinophil migration, differentiation, and survival in addition to its function in the differentiation and maturation of dendritic cells (35). Our present data showing that activation of PKM2 with TEPP46 preferentially attenuates TSLP and GM-CSF while modestly or not affecting CCL20 and KC suggest that avenues to attenuate glycolysis, or to activate PKM2 in an environment in which IL-1 signaling is operative, will be attractive strategies to dampen TSLP and GM-CSF.
The proinflammatory role of PKM2 has been extensively studied in tumor and immune cells, and a number of transcription factors have been demonstrated to mediate the proinflammatory effect of PKM2 (36–38). In macrophages and tumor cells, LPS induces nuclear binding of PKM2 to hypoxia-inducible factor 1–α (HIF-1α) (20, 39). In tumor cells, an interaction between PKM2 and Jumonji C domain–containing dioxygenase (JMJD5) also has been shown (37). These interactions with PKM2 have been linked to the transcription of glycolysis genes including LDHA and GLUT-1. Moreover, nuclear PKM2 was also shown to phosphorylate STAT3 (13, 36), which, in turn, augments IL-1β and IL-6 production (20). In line with the latter findings, the present observations that IL-1β or HDM led to increases in phosphorylation of STAT3, which were attenuated by TEPP46, suggest the importance of PKM2 in promoting STAT3 phosphorylation. Our findings that inhibition of STAT3 attenuated release of TSLP and GM-CSF in airway basal cells strongly suggest that the ability of PKM2 to phosphorylate STAT3 contributes to its proinflammatory function. Findings from the current study using an activator of PKM2 are in line with another study showing that activation of the glycolysis function of PKM2 attenuated the proinflammatory phenotype of macrophages from patients with atherosclerotic coronary artery disease (20) and inhibited the PKM2–HIF-1α complex (39), leading to decreased IL-1β production and glycolysis and proinflammatory genes (38, 39). Moreover, it has been shown that inhibition of STAT3, by Stattic, attenuated inflammatory injury in LPS-challenged mice (14).
PKM2 and PKM1 are both encoded by the same PKM gene; however, they represent different splicing products (exon 9 for PKM1 and exon 10 for PKM2). Unlike PKM1, PKM2 is not a constitutive stable tetrameric enzyme, and can be allosterically regulated by fructose-1,6-bisphosphate (FBP) to enhance tetramer formation. The PKM2 tetramer can be converted to dimers following a number of posttranslational modifications that include phosphorylation (40, 41), acetylation (42, 43), oxidation (44), hydroxylation (37, 38), ubiquitination (45), glycosylation (46), methylation (47), and sumoylation (48) in response to various stimuli. Interestingly, epidermal growth factor (EGF)–activated ERK2 binds directly to PKM2 and can induce phosphorylation of PKM2 at serine 37 in association with its nuclear translocation and increases in transcriptional activation of GLUT-1 and LDHA (49, 50). The EGF receptor (EGFR) is of notable interest because of its role of type 2 inflammatory responses in allergic airways disease, including mucus metaplasia (51–53). Additional studies will be required to elucidate whether EGFR activation contributed to phosphorylation, and subsequently, the inactivation of PKM2 that was observed in the current study. Other studies have demonstrated that PKM2 can be inactivated following oxidation of cysteine 358 (44). Changes in the oxidative environment and notably cysteine oxidations accompany allergic airway disease and lead to activation of EGFR in cells (51). Similarly, changes in the oxidative environment also control dendritic cell activation and T cell subsets (54). Additional studies will also be required to address whether oxidative events regulate PKM2 activity in this study.
Small molecule activators of PKM2, such as TEPP46 (also known as ML265) and DASA-58, have been developed to stabilize PKM2 in the tetramer configuration. TEPP46 activates PKM2 by binding to the dimer–dimer interface between two subunits of PKM2, which stabilizes tetrameric PKM2 to promote conversion of PEP to pyruvate, hence increasing its glycolytic activity. TEPP46 is highly selective in its ability to activate PKM2 (55), because it does not affect recombinant PKM1 in vitro (10) and has no significant effect in PKM2 knockout models (39, 56). These observations suggest that the effects observed by TEPP46 in this study are due to the activation of the glycolysis function of PKM2 and not due to off-target effects, although additional studies will be required to corroborate the lack of off-target effects. Paradoxically, although activation of PKM2 dampened inflammation, ablation of PKM2 also elicited anti-inflammatory effects. As discussed earlier, the TEPP46-induced PKM2 tetramer inhibited LPS-induced expression of IL-1β and other HIF-1α–dependent genes in macrophages (20, 39), whereas macrophages lacking PKM2 also showed reduced expression of IL-1β and Ldha mRNAs in response to LPS. These findings suggest that dimeric PKM2 has a proinflammatory gain of function and that strategies to either remove PKM2 all together or to promote its glycolysis kinase function elicit similar anti-inflammatory effects. Another limitation of the current manuscript is that experiments were performed in mice only. Further studies using human samples will be required to fully understand the contribution of PKM2 to proinflammatory responses in epithelial cells or airways from asthmatics.
Altogether, our results demonstrate that the glycolysis-inactive form of PKM2 plays a crucial role in the pathogenesis of allergic airway disease in association with enhancing IL-1β–induced proinflammatory signaling, in part through phosphorylation of STAT3, and notably the upregulation of Tslp and Csf2 genes. PKM2, therefore, could be a novel potential target for the development of anti-inflammatory therapies for the treatment of IL-1 high, glycolysis-associated asthma.
Supplementary Material
Acknowledgments
We are grateful to Iris van Gerwen for technical help with the preparation of the Masson’s trichrome staining and Nic Hutt for advice toward the cell differentials. We acknowledge the College of Medicine Shared Instrumentation Award, which was used to purchase the Leica Biosystems Aperio VERSA whole-slide imaging system.
This work was supported by National Institutes of Health R01 HL137268 and an unrestricted grant from Chiesi.
The online version of this article contains supplemental material.
- BAL
- bronchoalveolar lavage
- EGF
- epidermal growth factor
- EGFR
- EGF receptor
- GLUT-1
- glucose transporter 1
- HDM
- house dust mite
- HIF-1α
- hypoxia-inducible factor 1–α
- IKKε
- inhibitory κ B kinase ε
- MTE
- mouse tracheal epithelial
- PEP
- phosphoenolpyruvate
- PK
- pyruvate kinase
- PKM1
- PK muscle isozyme M1
- PKM2
- PK muscle isozyme M2
- α-SMA
- α–smooth muscle actin
- TEPP46
- 6-[(3-Aminophenyl)methyl]-4,6-dihydro-4-methyl-2-(methylsulfinyl)-5H-Thieno[2′,3′:4,5]pyrrolo[2,3-d]pyridazin-5-one
- TSLP
- thymic stromal lymphopoietin
- WT
- wild-type.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Fahy J. V. 2015. Type 2 inflammation in asthma--present in most, absent in many. Nat. Rev. Immunol. 15: 57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Domblides C., Lartigue L., Faustin B. 2018. Metabolic stress in the immune function of T cells, macrophages and dendritic cells. Cells 7: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qian X., Aboushousha R., van de Wetering C., Chia S. B., Amiel E., Schneider R. W., van der Velden J. L. J., Lahue K. G., Hoagland D. A., Casey D. T., et al. 2018. IL-1/inhibitory κB kinase ε-induced glycolysis augment epithelial effector function and promote allergic airways disease. J. Allergy Clin. Immunol. 142: 435–450.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doherty J. R., Cleveland J. L. 2013. Targeting lactate metabolism for cancer therapeutics. J. Clin. Invest. 123: 3685–3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ward P. S., Thompson C. B. 2012. Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer Cell 21: 297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noguchi T., Inoue H., Tanaka T. 1986. The M1- and M2-type isozymes of rat pyruvate kinase are produced from the same gene by alternative RNA splicing. J. Biol. Chem. 261: 13807–13812. [PubMed] [Google Scholar]
- 7.Noguchi T., Yamada K., Inoue H., Matsuda T., Tanaka T. 1987. The L- and R-type isozymes of rat pyruvate kinase are produced from a single gene by use of different promoters. J. Biol. Chem. 262: 14366–14371. [PubMed] [Google Scholar]
- 8.Mazurek S. 2011. Pyruvate kinase type M2: a key regulator of the metabolic budget system in tumor cells. Int. J. Biochem. Cell Biol. 43: 969–980. [DOI] [PubMed] [Google Scholar]
- 9.Christofk H. R., Vander Heiden M. G., Harris M. H., Ramanathan A., Gerszten R. E., Wei R., Fleming M. D., Schreiber S. L., Cantley L. C. 2008. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature 452: 230–233. [DOI] [PubMed] [Google Scholar]
- 10.Anastasiou D., Yu Y., Israelsen W. J., Jiang J. K., Boxer M. B., Hong B. S., Tempel W., Dimov S., Shen M., Jha A., et al. 2012. Pyruvate kinase M2 activators promote tetramer formation and suppress tumorigenesis. [Published erratum appears in 2012 Nat. Chem. Biol. 8: 1008.] Nat. Chem. Biol. 8: 839–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao X., Wang H., Yang J. J., Liu X., Liu Z. R. 2012. Pyruvate kinase M2 regulates gene transcription by acting as a protein kinase. Mol. Cell 45: 598–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alves-Filho J. C., Pålsson-McDermott E. M. 2016. Pyruvate kinase M2: a potential target for regulating inflammation. Front. Immunol. 7: 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Demaria M., Poli V. 2012. PKM2, STAT3 and HIF-1α: the Warburg’s vicious circle. JAKSTAT 1: 194–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu K., Yang Y., Lin L., Ai Q., Dai J., Fan K., Ge P., Jiang R., Wan J., Zhang L. 2018. Caloric restriction mimetic 2-deoxyglucose alleviated inflammatory lung injury via suppressing nuclear pyruvate kinase M2-signal transducer and activator of transcription 3 pathway. Front. Immunol. 9: 426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu R., Smith D. 1982. Continuous multiplication of rabbit tracheal epithelial cells in a defined, hormone-supplemented medium. In Vitro 18: 800–812. [DOI] [PubMed] [Google Scholar]
- 16.Alcorn J. F., Guala A. S., van der Velden J., McElhinney B., Irvin C. G., Davis R. J., Janssen-Heininger Y. M. 2008. Jun N-terminal kinase 1 regulates epithelial-to-mesenchymal transition induced by TGF-beta1. J. Cell Sci. 121: 1036–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McMurray J. S. 2006. A new small-molecule Stat3 inhibitor. Chem. Biol. 13: 1123–1124. [DOI] [PubMed] [Google Scholar]
- 18.Holtzman M. J., Byers D. E., Alexander-Brett J., Wang X. 2014. The role of airway epithelial cells and innate immune cells in chronic respiratory disease. Nat. Rev. Immunol. 14: 686–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hahn C., Islamian A. P., Renz H., Nockher W. A. 2006. Airway epithelial cells produce neurotrophins and promote the survival of eosinophils during allergic airway inflammation. J. Allergy Clin. Immunol. 117: 787–794. [DOI] [PubMed] [Google Scholar]
- 20.Shirai T., Nazarewicz R. R., Wallis B. B., Yanes R. E., Watanabe R., Hilhorst M., Tian L., Harrison D. G., Giacomini J. C., Assimes T. L., et al. 2016. The glycolytic enzyme PKM2 bridges metabolic and inflammatory dysfunction in coronary artery disease. J. Exp. Med. 213: 337–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michalek R. D., Gerriets V. A., Jacobs S. R., Macintyre A. N., MacIver N. J., Mason E. F., Sullivan S. A., Nichols A. G., Rathmell J. C. 2011. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J. Immunol. 186: 3299–3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lambrecht B. N., Hammad H., Fahy J. V. 2019. The cytokines of asthma. Immunity 50: 975–991. [DOI] [PubMed] [Google Scholar]
- 23.Ostroukhova M., Goplen N., Karim M. Z., Michalec L., Guo L., Liang Q., Alam R. 2012. The role of low-level lactate production in airway inflammation in asthma. Am. J. Physiol. Lung Cell. Mol. Physiol. 302: L300–L307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang L., Ma C., Wang X., He S., Li Q., Zhou Y., Liu Y., Zhang M., Yu X., Zhao X., et al. 2019. Lipopolysaccharide-induced proliferation and glycolysis in airway smooth muscle cells via activation of Drp1. J. Cell. Physiol. 234: 9255–9263. [DOI] [PubMed] [Google Scholar]
- 25.Caslin H. L., Taruselli M. T., Haque T., Pondicherry N., Baldwin E. A., Barnstein B. O., Ryan J. J. 2018. Inhibiting glycolysis and ATP production attenuates IL-33-mediated mast cell function and peritonitis. Front. Immunol. 9: 3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y., Willis-Owen S. A. G., Spiegel S., Lloyd C. M., Moffatt M. F., Cookson W. O. C. M. 2019. The ORMDL3 asthma gene regulates ICAM1 and has multiple effects on cellular inflammation. Am. J. Respir. Crit. Care Med. 199: 478–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Winnica D., Corey C., Mullett S., Reynolds M., Hill G., Wendell S., Que L., Holguin F., Shiva S. 2019. Bioenergetic differences in the airway epithelium of lean versus obese asthmatics are driven by nitric oxide and reflected in circulating platelets. Antioxid. Redox Signal. 31: 673–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu W., Comhair S. A. A., Janocha A. J., Lara A., Mavrakis L. A., Bennett C. D., Kalhan S. C., Erzurum S. C. 2017. Arginine metabolic endotypes related to asthma severity. PLoS One 12: e0183066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.West E. E., Kashyap M., Leonard W. J. 2012. TSLP: a key regulator of asthma pathogenesis. Drug Discov. Today Dis. Mech. 9: e83–e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smelter D. F., Sathish V., Thompson M. A., Pabelick C. M., Vassallo R., Prakash Y. S. 2010. Thymic stromal lymphopoietin in cigarette smoke-exposed human airway smooth muscle. J. Immunol. 185: 3035–3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Demehri S., Morimoto M., Holtzman M. J., Kopan R. 2009. Skin-derived TSLP triggers progression from epidermal-barrier defects to asthma. PLoS Biol. 7: e1000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou B., Headley M. B., Aye T., Tocker J., Comeau M. R., Ziegler S. F. 2008. Reversal of thymic stromal lymphopoietin-induced airway inflammation through inhibition of Th2 responses. J. Immunol. 181: 6557–6562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ziegler S. F., Roan F., Bell B. D., Stoklasek T. A., Kitajima M., Han H. 2013. The biology of thymic stromal lymphopoietin (TSLP). Adv. Pharmacol. 66: 129–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gauvreau G. M., O’Byrne P. M., Boulet L. P., Wang Y., Cockcroft D., Bigler J., FitzGerald J. M., Boedigheimer M., Davis B. E., Dias C., et al. 2014. Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. N. Engl. J. Med. 370: 2102–2110. [DOI] [PubMed] [Google Scholar]
- 35.Shi Y., Liu C. H., Roberts A. I., Das J., Xu G., Ren G., Zhang Y., Zhang L., Yuan Z. R., Tan H. S., et al. 2006. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and T-cell responses: what we do and don’t know. Cell Res. 16: 126–133. [DOI] [PubMed] [Google Scholar]
- 36.Yang P., Li Z., Li H., Lu Y., Wu H., Li Z. 2015. Pyruvate kinase M2 accelerates pro-inflammatory cytokine secretion and cell proliferation induced by lipopolysaccharide in colorectal cancer. Cell. Signal. 27: 1525–1532. [DOI] [PubMed] [Google Scholar]
- 37.Wang H. J., Hsieh Y. J., Cheng W. C., Lin C. P., Lin Y. S., Yang S. F., Chen C. C., Izumiya Y., Yu J. S., Kung H. J., Wang W. C. 2014. JMJD5 regulates PKM2 nuclear translocation and reprograms HIF-1α-mediated glucose metabolism. Proc. Natl. Acad. Sci. USA 111: 279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luo W., Hu H., Chang R., Zhong J., Knabel M., O’Meally R., Cole R. N., Pandey A., Semenza G. L. 2011. Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell 145: 732–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palsson-McDermott E. M., Curtis A. M., Goel G., Lauterbach M. A., Sheedy F. J., Gleeson L. E., van den Bosch M. W., Quinn S. R., Domingo-Fernandez R., Johnston D. G., et al. 2015. Pyruvate kinase M2 regulates Hif-1α activity and IL-1β induction and is a critical determinant of the warburg effect in LPS-activated macrophages. [Published erratum appears in 2015 Cell Metab. 21: 347.] Cell Metab. 21: 65–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hitosugi T., Kang S., Vander Heiden M. G., Chung T. W., Elf S., Lythgoe K., Dong S., Lonial S., Wang X., Chen G. Z., et al. 2009. Tyrosine phosphorylation inhibits PKM2 to promote the Warburg effect and tumor growth. Sci. Signal. 2: ra73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Christofk H. R., Vander Heiden M. G., Wu N., Asara J. M., Cantley L. C. 2008. Pyruvate kinase M2 is a phosphotyrosine-binding protein. Nature 452: 181–186. [DOI] [PubMed] [Google Scholar]
- 42.Lv L., Li D., Zhao D., Lin R., Chu Y., Zhang H., Zha Z., Liu Y., Li Z., Xu Y., et al. 2011. Acetylation targets the M2 isoform of pyruvate kinase for degradation through chaperone-mediated autophagy and promotes tumor growth. Mol. Cell 42: 719–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bhardwaj A., Das S. 2016. SIRT6 deacetylates PKM2 to suppress its nuclear localization and oncogenic functions. Proc. Natl. Acad. Sci. USA 113: E538–E547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anastasiou D., Poulogiannis G., Asara J. M., Boxer M. B., Jiang J. K., Shen M., Bellinger G., Sasaki A. T., Locasale J. W., Auld D. S., et al. 2011. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science 334: 1278–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu K., Li F., Han H., Chen Y., Mao Z., Luo J., Zhao Y., Zheng B., Gu W., Zhao W. 2016. Parkin regulates the activity of pyruvate kinase M2. J. Biol. Chem. 291: 10307–10317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Y., Liu J., Jin X., Zhang D., Li D., Hao F., Feng Y., Gu S., Meng F., Tian M., et al. 2017. O-GlcNAcylation destabilizes the active tetrameric PKM2 to promote the Warburg effect. Proc. Natl. Acad. Sci. USA 114: 13732–13737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu F., Ma F., Wang Y., Hao L., Zeng H., Jia C., Wang Y., Liu P., Ong I. M., Li B., et al. 2017. PKM2 methylation by CARM1 activates aerobic glycolysis to promote tumorigenesis. [Published erratum appears in 2017 Nat. Cell Biol. 19: 1442.] Nat. Cell Biol. 19: 1358–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spoden G. A., Morandell D., Ehehalt D., Fiedler M., Jansen-Dürr P., Hermann M., Zwerschke W. 2009. The SUMO-E3 ligase PIAS3 targets pyruvate kinase M2. J. Cell. Biochem. 107: 293–302. [DOI] [PubMed] [Google Scholar]
- 49.Yang W., Xia Y., Ji H., Zheng Y., Liang J., Huang W., Gao X., Aldape K., Lu Z. 2011. Nuclear PKM2 regulates β-catenin transactivation upon EGFR activation. [Published erratum appears in 2017 Nature 550: 142.] Nature 480: 118–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang W., Zheng Y., Xia Y., Ji H., Chen X., Guo F., Lyssiotis C. A., Aldape K., Cantley L. C., Lu Z. 2012. ERK1/2-dependent phosphorylation and nuclear translocation of PKM2 promotes the Warburg effect. [Published erratum appears in 2013 Nat. Cell Biol. 15: 124.] Nat. Cell Biol. 14: 1295–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hristova M., Habibovic A., Veith C., Janssen-Heininger Y. M., Dixon A. E., Geiszt M., van der Vliet A. 2016. Airway epithelial dual oxidase 1 mediates allergen-induced IL-33 secretion and activation of type 2 immune responses. J. Allergy Clin. Immunol. 137: 1545–1556.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burgel P. R., Nadel J. A. 2008. Epidermal growth factor receptor-mediated innate immune responses and their roles in airway diseases. Eur. Respir. J. 32: 1068–1081. [DOI] [PubMed] [Google Scholar]
- 53.Hamilton L. M., Torres-Lozano C., Puddicombe S. M., Richter A., Kimber I., Dearman R. J., Vrugt B., Aalbers R., Holgate S. T., Djukanović R., et al. 2003. The role of the epidermal growth factor receptor in sustaining neutrophil inflammation in severe asthma. Clin. Exp. Allergy 33: 233–240. [DOI] [PubMed] [Google Scholar]
- 54.Tan P. H., Sagoo P., Chan C., Yates J. B., Campbell J., Beutelspacher S. C., Foxwell B. M., Lombardi G., George A. J. 2005. Inhibition of NF-kappa B and oxidative pathways in human dendritic cells by antioxidative vitamins generates regulatory T cells. J. Immunol. 174: 7633–7644. [DOI] [PubMed] [Google Scholar]
- 55.Boxer M. B., Jiang J. K., Vander Heiden M. G., Shen M., Skoumbourdis A. P., Southall N., Veith H., Leister W., Austin C. P., Park H. W., et al. 2010. Evaluation of substituted N,N′-diarylsulfonamides as activators of the tumor cell specific M2 isoform of pyruvate kinase. J. Med. Chem. 53: 1048–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qi W., Keenan H. A., Li Q., Ishikado A., Kannt A., Sadowski T., Yorek M. A., Wu I. H., Lockhart S., Coppey L. J., et al. 2017. Pyruvate kinase M2 activation may protect against the progression of diabetic glomerular pathology and mitochondrial dysfunction. Nat. Med. 23: 753–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.