Significance
Crop damage by insect pests is predicted to increase as rising surface temperatures accelerate insect metabolism, population size, and range expansion. The plant defense hormone jasmonate promotes resistance to diverse herbivores, but how this wound signal impacts the plant’s ability to cope with a combination of herbivory and elevated temperature remains unknown. Here, we show that heat shock proteins contribute to enhanced jasmonate responses in tomato plants subjected to simulated heat waves. Herbivore-induced jasmonate signaling at elevated temperature, however, blocked stomatal opening and leaf hyponasty, leading to leaf overheating, reduced photosynthesis, and growth inhibition. Our results show how signal conflict between biotic and abiotic stress may exaggerate crop losses under warming conditions that accelerate herbivory, potentially jeopardizing food security.
Keywords: climate change, jasmonate, plant–insect interaction, stomata, heat shock protein 90
Abstract
As global climate change brings elevated average temperatures and more frequent and extreme weather events, pressure from biotic stresses will become increasingly compounded by harsh abiotic stress conditions. The plant hormone jasmonate (JA) promotes resilience to many environmental stresses, including attack by arthropod herbivores whose feeding activity is often stimulated by rising temperatures. How wound-induced JA signaling affects plant adaptive responses to elevated temperature (ET), however, remains largely unknown. In this study, we used the commercially important crop plant Solanum lycopersicum (cultivated tomato) to investigate the interaction between simulated heat waves and wound-inducible JA responses. We provide evidence that the heat shock protein HSP90 enhances wound responses at ET by increasing the accumulation of the JA receptor, COI1. Wound-induced JA responses directly interfered with short-term adaptation to ET by blocking leaf hyponasty and evaporative cooling. Specifically, leaf damage inflicted by insect herbivory or mechanical wounding at ET resulted in COI1-dependent stomatal closure, leading to increased leaf temperature, lower photosynthetic carbon assimilation rate, and growth inhibition. Pharmacological inhibition of HSP90 reversed these effects to recapitulate the phenotype of a JA-insensitive mutant lacking the COI1 receptor. As climate change is predicted to compound biotic stress with larger and more voracious arthropod pest populations, our results suggest that antagonistic responses resulting from a combination of insect herbivory and moderate heat stress may exacerbate crop losses.
In natural environments, plants often face multiple stresses concurrently and have evolved signaling networks to prioritize threats and allocate limited resources accordingly (1, 2). Plant responses to one stress may constrain the capacity for physiological adaptation to a second stress. For example, whereas plants mitigate heat stress by opening stomata to cool leaves through transpiration, stomata remain closed to prevent desiccation during a combination of heat and drought stress (1, 3, 4). The complex effects of higher average temperatures on species interactions underscores the need to better understand plant responses to concurrent abiotic and biotic stress (5). Among the emerging global biotic threats to plant productivity is temperature-driven increases in the range, size, and feeding voracity of insect herbivore populations (6, 7). How these changes in herbivore feeding behavior and dynamics will impact the ability of plants to cope with warmer climates remains largely unknown.
As a central regulator of the wound response, hormonally active jasmonates (JAs) confer durable resistance to insect herbivores and necrotrophic pathogens (8, 9). Tissue damage triggers the rapid production of JAs both at the site of wounding and systemically throughout the plant (10–12). Increased levels of the major receptor-active hormone, jasmonoyl-isoleucine (JA-Ile), herald the assembly of a high-affinity coreceptor complex consisting of the F-box protein CORONATINE INSENSITIVE1 (COI1) and JAZ transcriptional repressor proteins. JA-Ile–dependent JAZ degradation via the ubiquitin/26S proteasome pathway results in derepression of transcription factors that promote the expression of hundreds of defense-related genes (13). The core JA signaling pathway is subject to multiple layers of regulation, including dynamic control of COI1 protein levels. COI1 is stabilized by its interaction with the Skp1-like1 protein ASK1 and by assembly into the SCF (Skp1/Cullin1/F-box)-type ubiquitin ligase complex SCFCOI1 (14). COI1 stability is also controlled independently of ASK1 by the activity of the SGT1b–HSP70–HSP90 chaperone complex (15). Greater COI1 stability through these protein–protein interactions increases the strength of JA response outputs, including enhanced expression of JA-responsive genes (16).
Plants use a variety of mechanisms to sense and respond to a range of elevated temperatures (ETs) (17). Whereas extreme increases in temperature lead to heat shock responses triggered by protein misfolding and aggregation, more mild increases in ambient temperature promote a suite of morphological changes, including hypocotyl elongation, leaf hyponasty, decreased stomatal density, and early flowering. These adaptations to mild heat stress, collectively known as thermomorphogensis, are thought to promote cooling of affected plant tissues (17, 18). Recent studies suggest that the SGT1–HSP70–HSP90 complex participates in a thermosensory system for modulating multiple hormone signaling pathways and associated thermomorphogenic responses. These chaperones physically interact with and stabilize various hormone receptors, including proteins involved in the perception of auxin, gibberellic acid, and JA-Ile (16). At elevated temperature, SGT1b–HSP90 accumulates and stabilizes the auxin receptor TIR1, resulting in enhanced auxin sensitivity (19). Whether a similar mechanism promotes COI1 stability under elevated temperature remains to be investigated. Nevertheless, it has been reported that certain JA responses are controlled by SGT1–HSP70–HSP90 activity at normal growth temperatures. Mutations in SGT1, or treatment of wild-type (WT) plants with the HSP90 inhibitor geldanamycin (GDA), destabilize COI1, reduce JA responsiveness, and compromise wound-induced accumulation of JAs (16, 20). These collective studies suggest that JA signaling is integrated into a network of temperature sensing systems, possibly involving molecular chaperones that use COI1 as a client protein.
Here, we used the well-studied wound response of cultivated tomato (Solanum lycopersicum) to investigate reciprocal interactions between JA and elevated temperature-response pathways. We provide evidence that moderate heat stress enhances the strength of JA responses through the activity of HSP90, leading to stronger JA-dependent wound responses. Strikingly, induction of the JA pathway by insect feeding or mechanical wounding effectively blocked temperature-induced stomatal opening and hyponasty, leading to higher leaf temperatures, reduced photosynthesis, and inhibited growth. Our findings provide insights into temperature-sensitive nodes of the JA response pathway and further highlight a mechanism by which signal conflict between biotic and abiotic stress may exacerbate crop losses in a warming world.
Results
Elevated Temperature Enhances JA-Dependent Wound Responses but Accelerates Insect Feeding.
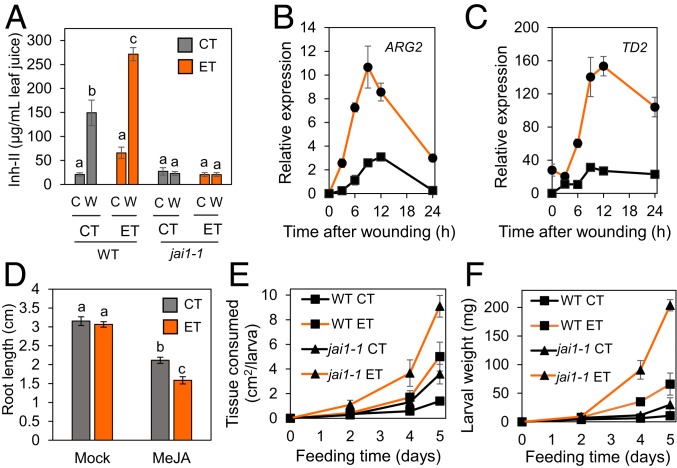
Previous studies showed that wound-induced accumulation of defensive proteinase inhibitor I (Inh-I) in tomato leaves is stimulated by increasing temperature, with a maximal rate of accumulation at about 36 °C (21). To determine whether proteinase inhibitor expression is enhanced under simulated heat wave conditions, 17-d-old plants grown under control temperature conditions (CT, 28 °C 16-h day/18 °C 8-h night) were either maintained at CT or were transferred to an ET regime (38 °C 16-h day/28 °C 8-h night). Plants were maintained at either CT or ET conditions for 5 d, at which time leaves were mechanically wounded and harvested 1 d later. Consistent with previous studies (21), the 5-d ET treatment enhanced wound-induced proteinase inhibitor II (Inh-II) levels in WT plants (Fig. 1A). Parallel experiments performed with the tomato jai1-1 mutant, which lacks the COI1 receptor (22, 23), demonstrated that the stimulatory effect of ET on Inh-II production was dependent on JA-Ile perception (Fig. 1A). We also observed that a shorter-duration heat wave (2-d exposure to ET prior to wounding) markedly increased the wound-induced expression of genes encoding additional JA-responsive defense proteins, ARGINASE2 (ARG2) and THREONINE DEAMINASE 2 (TD2) (24, 25) (Fig. 1 B and C). Moreover, phytohormone measurements showed that JA-Ile and its metabolic precursor, jasmonic acid, accumulated to higher levels in wounded leaves of ET-treated plants compared to CT controls (SI Appendix, Fig. S1). To further test the effect of ET on plant sensitivity to JA, we performed JA-induced root growth inhibition assays in the presence or absence of exogenous methyl jasmonic acid (MeJA), which is actively metabolized to JA-Ile in planta. Whereas mock-treated roots grew similarly under CT and ET conditions, exogenous MeJA inhibited root length to a greater extent under ET compared to CT conditions (Fig. 1D), suggesting that ET enhances plant responsiveness to JA.
Fig. 1.
Elevated temperature enhances JA responses and insect feeding. (A) Inh-II levels in leaves of wounded (W) and undamaged control (C) plants. Wild-type and jasmonate-insensitive1 (jai1-1) tomato plants grown for 17 d under the CT regime (28 °C 16 h light/18 °C 8 h dark) were transferred to either CT or ET (38 °C 16 h light/28 °C 8 h dark). Five days after transfer to CT or ET treatment chambers, leaves were mechanically wounded. Inh-II levels in the damaged leaves were measured 24 h after wounding. Data points represent the mean ± SE of four biological replicates. Lowercase letters denote significant differences (Tukey’s honestly significant difference [HSD] test P < 0.05). (B and C) Time course of wound-induced transcript accumulation of two JA-responsive genes, ARG2 (B) and TD2 (C). WT plants were grown as described in A and transferred to CT or ET treatment chambers for 2 d prior to wounding. Leaf tissue was harvested for RNA extraction at the indicated times after wounding. ARG2 and TD2 mRNA levels were determined by qPCR, with normalization to an ACTIN housekeeping gene. Data points represent the mean ± SE of four biological replicates. (D) Effect of ET on JA-mediated root growth inhibition. WT seeds were germinated at ambient temperature (22 °C) on filter paper prior to treatment with 1 mM MeJA or mock control (mock), followed by immediate transfer to CT or ET conditions for 2 d. Data points represent the mean ± SE of >30 seedlings. Lowercase letters denote significant differences (Tukey’s HSD test P < 0.05). (E and F) Insect feeding assays on WT and jai1-1 plants under CT or ET conditions. Seventeen-day-old WT and jai1-1 plants grown under CT conditions were transferred to CT or ET treatment chambers for 5 d prior to challenge with two M. sexta larva per leaf. Feeding proceeded at either CT or ET. The amount of leaf area consumed per larva (E) and larval weight gain (F) were measured at the indicated times after challenge. Data points represent the mean ± SE of three biological replicates.
The minor effect of ET treatment on the basal level (i.e., in unwounded leaves) of JA responses suggested that moderate heat stress may promote a physiological state that is more responsive to leaf wounding. To test this idea, plants that experienced a 5-d ET treatment were reacclimated to CT conditions for 1 h and then assessed for various wound responses. Wound-induced accumulation of JA and JA-Ile, as well as the expression of JA-responsive marker genes, was generally much higher in plants that received the ET pretreatment compared to control plants that were grown continuously at CT (SI Appendix, Fig. S2). Thus, simulated heat waves appear to prime tomato leaves for enhanced JA-dependent wound responses.
The stimulatory effect of ET on JA-dependent wound responses raised the possibility that moderate heat stress may increase plant resistance to chewing insects. In an initial experiment, WT plants grown under CT conditions were transferred to either CT or ET treatment chambers and then immediately challenged with neonate larvae of the native herbivore Manduca sexta. Contrary to our expectation, leaf consumption and weight gain by larvae at ET was much greater than that at CT (SI Appendix, Fig. S3). We next performed feeding bioassays in which WT and jai1-1 mutant plants were exposed for 5 d to ET (or maintained under CT conditions as a control) prior to insect infestation. Similar to the results obtained with simultaneous ET and herbivory treatments, WT plants that were exposed to ET prior to infestation remained highly susceptible to herbivory (Fig. 1 E and F). jai1-1 plants were compromised in resistance to feeding under CT conditions, and were even more susceptible than WT under ET conditions. These data indicate that an intact JA signaling pathway promotes host resistance under both CT and ET conditions. However, the enhanced wound-induced JA response at ET is apparently insufficient to overcome the stimulatory effect of ET on M. sexta feeding (7, 19, 20).
Wound-Induced Jasmonate Signaling at Elevated Temperature Inhibits Leaf Hyponasty.
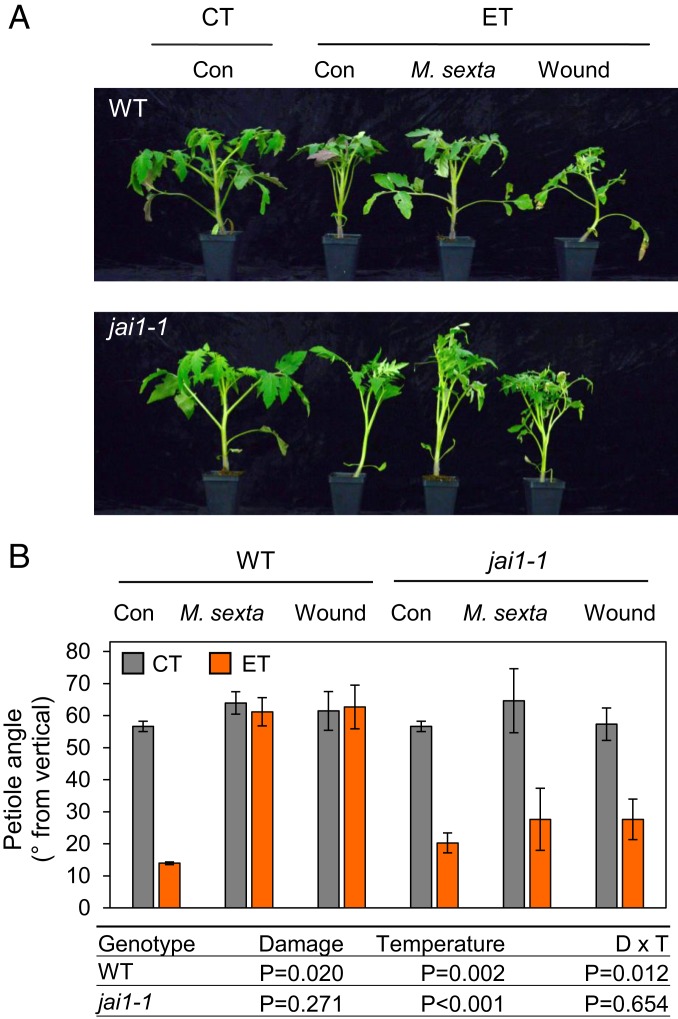
High temperature-induced developmental alterations in shoot architecture can provide protection against heat stress (17). Among the well-established morphological responses to ET is leaf elevation (hyponasty) from the soil surface, which serves to reduce leaf temperature (26, 27). We found that both WT and jai1-1 mutant plants exposed to ET conditions for 5 d exhibit leaf hyponasty (Fig. 2). To determine whether the onset of ET-induced hyponasty is modulated by JA signaling, WT and jai1-1 plants grown under CT conditions were transferred to CT or ET treatment chambers and simultaneously subjected to leaf damage either by infestation with neonate M. sexta larvae or daily mechanical wounding. Remarkably, both types of wound treatment strongly attenuated the hyponastic response in WT plants under ET conditions. Leaves of ET-treated jai1-1 plants that received mechanical or herbivore damage, however, maintained the hyponastic character of unwounded jai1-1 plants that were exposed to ET only (Fig. 2). These data show that ET-induced hyponasty is impaired by simultaneous wound stress, and that the antagonistic effect of wounding on this thermomorphogenic response requires the JA-Ile receptor COI1.
Fig. 2.
Wound-induced jasmonate signaling prevents leaf hyponasty in response to elevated temperature. (A) Representative photographs of undamaged control (Con) WT and jai1-1 plants 5 d after transfer to CT or ET treatment chambers. Other sets of plants were transferred to treatment chambers and simultaneously damaged by caterpillar feeding (M. sexta) or daily mechanical wounding (wound). (B) Quantitation of leaf hyponasty in plants treated as described in A. Following the treatment, the petiole angle of leaf 2 (second oldest) was measured from digital images. Data points represent the mean ± SE of four biological replicates. The table below B shows P values from two-way ANOVA tests of the effect of leaf damage (D), temperature (T), and the interaction of D × T for each of the two genotypes tested (WT and jai1-1).
Jasmonate Signaling Inhibits Stomatal Opening and Leaf Cooling Capacity at Elevated Temperature.
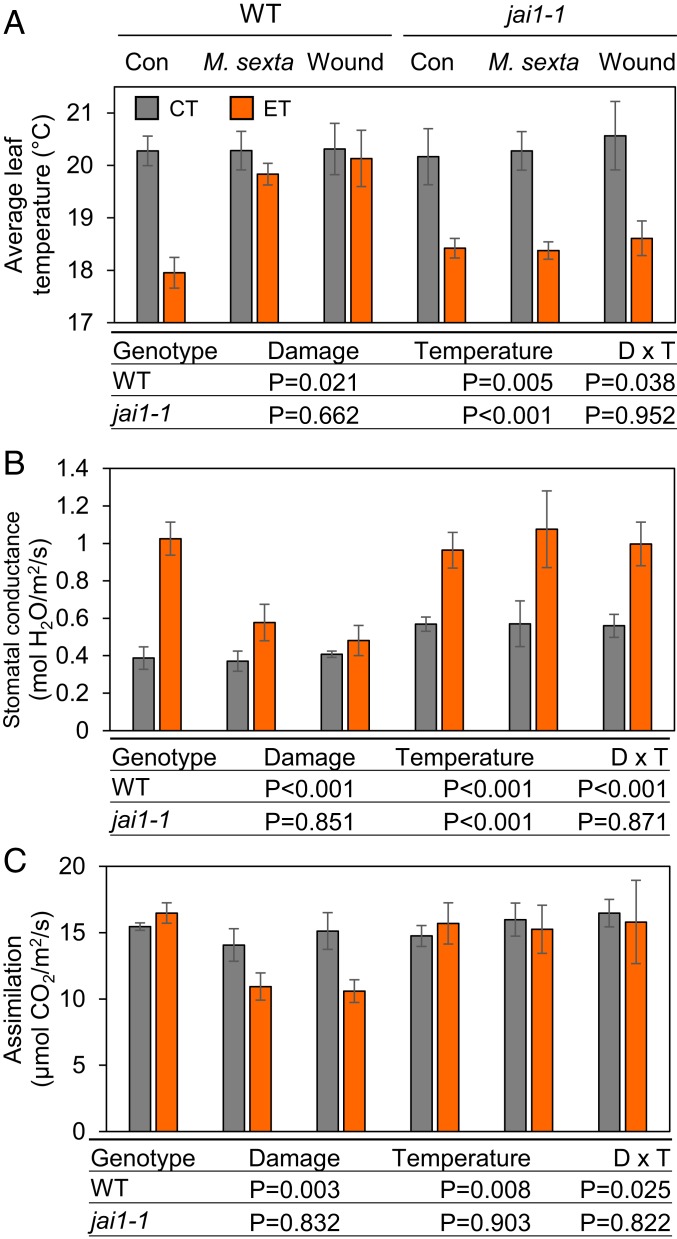
Plants mitigate elevated temperature stress under well-watered conditions by opening stomata to promote evaporative cooling (26, 27). The recent discovery that wounding triggers JA-induced stomatal closure in Arabidopsis at ambient temperatures (28), together with our data that ET conditions enhance JA-dependent wound responses (Fig. 1), raised the possibility that leaf cooling capacity under moderate heat stress may be compromised by leaf damage. To test this, we performed thermal imaging of WT and jai1-1 plants subjected to various temperature and wounding regimes. In the absence of wounding, a 5-d ET treatment increased the cooling potential of both WT and jai1-1 leaves; upon brief (1 h) acclimation of CT- and ET-treated plants to ambient temperature (∼22 °C), thermal imaging performed at ambient temperature revealed that leaves of ET-treated WT and jai1-1 plants were ∼2 °C cooler than leaves of plants subject to CT treatment (Fig. 3A). As observed for leaf hyponasty, this establishment of ET-induced leaf cooling was strongly attenuated in WT plants that were treated simultaneously with ET and either mechanical wounding or insect herbivory. Moreover, these wound treatments had no effect on the leaf cooling capacity of ET-treated jai1-1 plants (Fig. 3A and SI Appendix, Fig. S4). These data indicate that wound-induced JA signaling effectively prevents the leaf cooling response evoked by 5 d of moderate heat stress. Very similar results were obtained with WT and jai1-1 plants subjected to only 1 d of ET treatment (SI Appendix, Fig. S5).
Fig. 3.
Wound-induced jasmonate signaling prevents stomatal-based cooling responses to elevated temperature and represses photosynthesis. (A) Average temperature of leaves from WT and jai1-1 plants grown for 17 d under CT and then transferred to CT or ET conditions for 5 d. Control plants (Con) received no wounding at the time of transfer. Two other sets of plants were challenged with caterpillar larvae (M. sexta) or daily mechanical wounding (wound) at the time of transfer. Leaf temperatures were measured with a thermal camera after allowing all plants (CT and ET treatments) to acclimate to room temperature (22 °C) for ∼1 h. (B and C) Stomatal conductance (B) and photosynthetic carbon assimilation (C) of WT and jai1-1 plants grown as described in A. Measurements were made on undamaged areas from damaged leaves using a LI-6800 portable photosynthesis system (LI-COR). Data points represent the mean ± SE of four biological replicates. Tables below A–C show P values from two-way ANOVA tests of the effect of leaf damage (D), temperature (T), and the interaction of D × T for each of the two genotypes tested (WT and jai1-1).
The observation that leaf damage blocks ET-induced leaf cooling suggests an antagonistic interaction between stomata-based ET acclimation and leaf damage responses. To evaluate the combined effects of moderate heat stress and leaf damage on stomatal function, we measured stomatal conductance and net CO2 assimilation in WT and jai1-1 plants treated with ET, leaf damage (herbivory or mechanical wounding), or a combination of heat and wound stress. Indeed, ET treatment increased stomatal conductance (Fig. 3B) in undamaged WT and jai1-1 plants in comparison to CT controls. In WT plants, these ET-induced increases in gas exchange rates were abrogated by wounding, whereas in jai1-1 plants, stomatal conductance was largely unaffected by leaf damage (Fig. 3B). Net CO2 assimilation rates were similar in unwounded WT and jai1-1 plants under both CT and ET conditions. However, caterpillar herbivory and mechanical wounding caused a significant reduction in net CO2 assimilation in WT but not jai1-1 (Fig. 3C). Measurements of stomatal aperture using leaf epidermal peels confirmed that ET-induced stomatal opening is blocked by leaf damage in WT but not jai1-1 plants (SI Appendix, Fig. S6A). Elevated temperature conditions modestly increased intercellular CO2 levels (Ci) in both WT and jai1-1 leaves, but leaf damage had no physiologically relevant effects on Ci in either genotype (SI Appendix, Fig. S6B).
The inhibitory effect of wounding on CO2 assimilation at ET suggested that combined heat and wound stress may negatively impact plant biomass. Shoot fresh weight measurements showed that ET treatment alone reduced biomass by ∼30% in both WT and jai1-1 plants relative to CT controls. In WT plants, this reduction in biomass was further exacerbated in a COI1-dependent manner by herbivory or mechanical wounding imposed at the time of heat stress (SI Appendix, Fig. S6C). Direct measurements of leaf damage in these experiments showed that the reduced biomass is not accounted for by tissue loss to wounding or herbivory (SI Appendix, Supplemental Materials and Methods). These collective data indicate that wound-induced JA signaling under ET conditions interferes with multiple physiological processes, including hyponasty, stomatal-based leaf cooling, and photosynthesis.
To confirm that the inhibitory effects of leaf damage on responses to ET involve ligand-induced activation of COI1, WT plants were sprayed with coronatine, a potent COI1 receptor agonist (23), or a mock control solution at the time of transfer to CT and ET treatment chambers. We found that coronatine treatment recapitulated the inhibitory effects of leaf wounding on ET responses, including inhibition of leaf hyponasty, stomatal opening, and leaf cooling capacity (SI Appendix, Fig. S7). These collective data indicate that concurrent heat stress and activation of the JA signaling pathway by insect herbivory, wounding, or exogenous coronatine effectively impedes the ability of tomato to adapt to elevated temperature.
High Temperature Stabilizes COI1 through the Activity of HSP90.
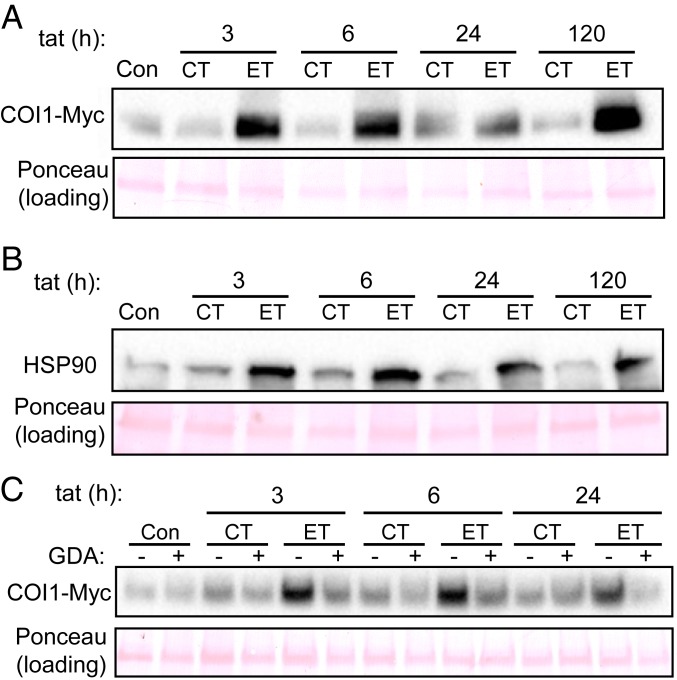
Previous studies with Arabidopsis showed that the JA-Ile receptor COI1 is a client protein of the SGT1b–HSP90 complex (16, 19). To test the hypothesis that ET-induced stimulation of JA responses in tomato is associated with stabilization of COI1, we used immunoblot analysis to assess COI1 protein levels in a transgenic line that expresses a functional c-Myc–tagged tomato COI1 (COI1-Myc) from the Cauliflower Mosaic Virus (CaMV) 35S promoter (29). Following transfer of CT-grown 35S::COI1-Myc plants to either ET or CT treatment chambers, COI1-Myc protein strongly accumulated within 3 h of ET treatment (Fig. 4A). Analysis of HSP90 levels in the same protein extracts revealed that native tomato HSP90 coaccumulated with COI1-Myc in response to ET treatment (Fig. 4B). In comparison to control plants maintained under CT conditions, both COI1-Myc and HSP90 levels remained elevated in ET-treated plants during the 5-d time course.
Fig. 4.
HSP90 levels correlate with COI1 accumulation at elevated temperature. (A) Western blot of COI1-Myc protein levels in leaves of 3-wk-old 35S::COI1-Myc plants grown under CT before transfer to either CT or ET treatment chambers. Immediately prior to (Con) or at the indicated time after transfer (tat), leaves were harvested for protein extraction. (B) Western blot of native HSP90 using the same protein extracts shown in A. (C) Effect of GDA treatment on ET-induced COI1 protein accumulation. Three-week-old 35S::COI1-Myc plants grown at CT were vacuum infiltrated with water (GDA −) or a solution containing 0.5 μM GDA (GDA +). One day after infiltration, plants were transferred to ET or CT treatment chambers for 24 h. Immediately prior to (Con) or at the indicated times after transfer (tat), leaves were harvested for protein extraction and Western blot analysis of COI1-Myc levels. Ponceau S staining of polyvinylidene fluoride membranes served as a control to assess equal protein loading.
We next used the highly specific HSP90 inhibitor, GDA, to test whether ET-induced COI1 accumulation depends on HSP90 activity. Leaves of CT-grown 35S::COI1-Myc plants were vacuum infiltrated with 0.5 µM GDA or water before drying at room temperature. Following recovery overnight at CT, plants were transferred to either CT or ET treatment chambers and COI1-Myc protein levels were measured over a 24-h period. COI1-Myc protein levels were induced 3 h after the shift to ET conditions and remained elevated for the duration of the time course. This inductive effect of ET on COI1 abundance was blocked by the GDA pretreatment (Fig. 4C). These findings support the notion that HSP90 activity is required for COI1 accumulation in response to moderate heat stress.
Inhibition of HSP90 Negates the Effects of Wounding on High Temperature Responses.
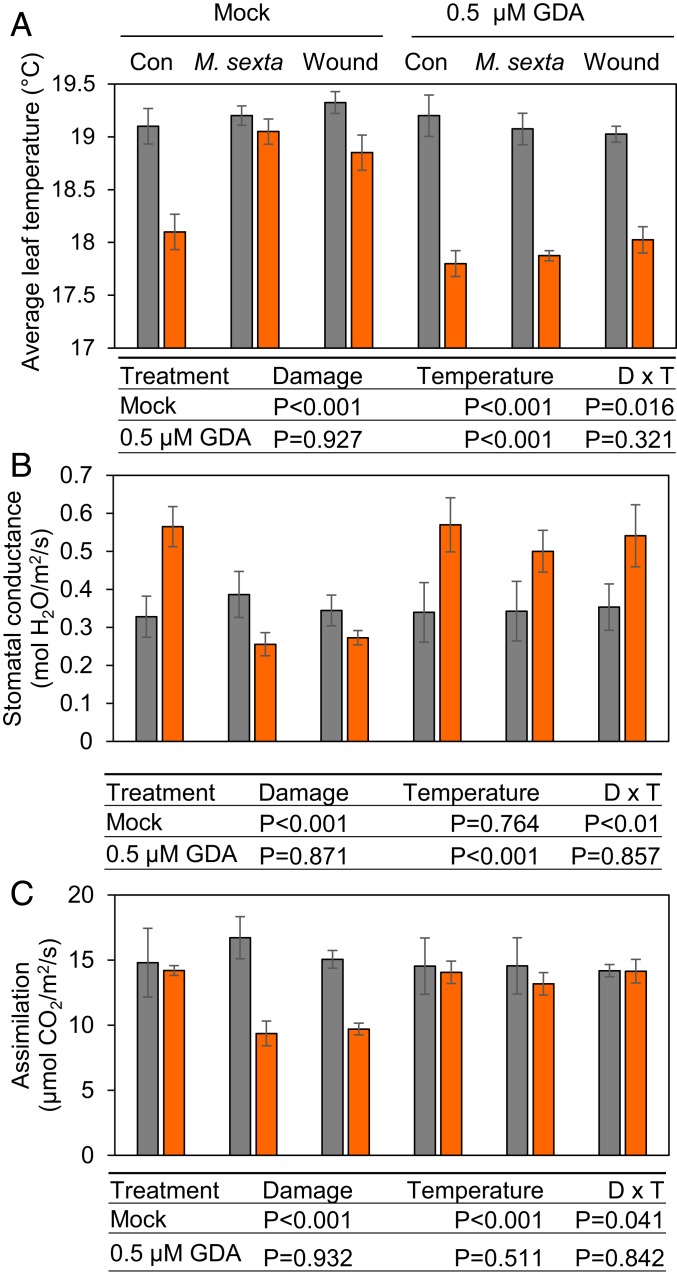
Our data suggested that HSP90, through its ability to increase COI1 accumulation at elevated temperature, promotes wound-induced JA responses (i.e., stomatal closure) that antagonize the plant’s capacity to adapt to moderate heat stress. This model predicts that inhibition of HSP90 by GDA will alleviate wound-induced inhibition of stomatal-based responses to high temperature. To test this hypothesis, leaves of 17-d-old WT plants grown under CT conditions were first infiltrated with a solution of GDA (or mock control), and plants were then transferred to CT or ET treatment chambers. Leaves were then damaged mechanically or challenged with M. sexta larvae. Leaf temperature, stomatal aperture, and gas exchange measurements were performed 1 d later, when stomatal-based cooling responses are active (SI Appendix, Fig. S5). In mock-control plants (vacuum infiltration of water), wounding of leaves at the time of transfer to ET inhibited the development of leaf cooling capacity (Fig. 5A), consistent with the results obtained without vacuum infiltration (Fig. 3A and SI Appendix, Fig. S5). Importantly, the ability of wounding to interfere with leaf cooling under ET conditions was suppressed by GDA pretreatment (Fig. 5A). GDA pretreatment also attenuated the wound-induced inhibition of stomatal conductance, photosynthetic carbon assimilation, and stomatal opening at ET (Fig. 5 B and C and SI Appendix, Fig. S8). We also found that GDA pretreatment effectively inhibited wound-induced accumulation of Inh-II protein under both CT and ET temperature regimes (SI Appendix, Fig. S9A). Likewise, experiments involving GDA application to germinated seedlings showed that the HSP90 inhibitor partially blocks JA-mediated root growth inhibition (SI Appendix, Fig. S9B). Thus, reduction in HSP90 activity by GDA phenocopies the effects of COI1 removal by jai1-1. These collective data support a scenario in which maintenance of COI1 activity by HSP90 enhances JA-dependent wound responses, which antagonize leaf cooling responses under conditions of mild heat stress.
Fig. 5.
The HSP90 inhibitor geldanamycin alleviates inhibition of high temperature-induced cooling responses by wounding. Leaves of WT plants were vacuum infiltrated with a solution containing 0.5 µM GDA or mock control (mock) and then transferred to CT or ET treatment chambers as described in the main text. Leaves were mechanically wounded across the apex of each leaflet at the beginning and end of the 24-h treatment period (wound) or were challenged with two neonate insect larvae per leaf (M. sexta). Control plants (Con) were not damaged. Physiological measurements (A–C) were made 24 h after the wound treatment or the beginning of the insect infestation. (A) Average leaf temperature was measured by thermal imaging after the 24-h treatment with temperature and damage. All plants were briefly (∼1 h) reacclimated to 22 °C for acquisition of thermal images. Data points represent the mean ± SE of four biological replicates. (B and C) GDA prevents wound-induced inhibition of stomatal conductance (B) and photosynthetic CO2 assimilation (C) at ET. Gas exchange was measured on undamaged regions of damaged leaves. Data points represent the mean ± SE of four biological replicates. ANOVA tables below A–C show P values assessing the effect of leaf damage (D), elevated temperature (T), and the interaction of D × T for each of the two chemical treatments tested (mock and GDA).
Discussion
Although it is well established that JAs promote resistance to a wide range of endothermic and ectothermic plant consumers (30, 31), little is known about how JA-triggered immune signaling is affected by warmer temperatures. The objective of this study was to use tomato as a model system to understand how plants respond to the combination of moderate heat stress and leaf damage inflicted by insect herbivory and, in the long term, to identify temperature-sensitive nodes of JA signaling. Our data indicate that moderate heat stress of tomato promotes a physiological state leading to more robust wound responses, including hyperaccumulation of JAs, increased expression of JA-responsive genes, and increased proteinase inhibitor accumulation. These findings are generally consistent with previous studies showing that exposure of Nicotiana tabacum to high temperature up-regulates the expression of the JA-responsive transcription factor MYC2, resulting in greater accumulation of the antiherbivore compound nicotine (32).
Because insect herbivores are poikilothermic (i.e., their body temperature varies with the environment), elevated temperatures are typically associated with increased metabolic rate, accelerated tissue consumption and, as a consequence, greater damage to plant tissues (7). Consistent with studies of the thermal ecology of M. sexta (33, 34), we found that consumption of WT tomato foliage by M. sexta larvae was dramatically accelerated at ET relative to CT conditions. That feeding activity on jai1-1 plants was even greater than that on WT indicates that JA signaling promotes antiinsect resistance under both temperature regimes. It is possible that temperature-dependent modulation of the amplitude of JA responses over a range of ambient conditions allows the plant to match its defense response to climate-associated changes in the intensity of insect feeding. Despite the increased amplitude of JA responses at ET, however, insect performance at ET as measured by larval weight gain and tissue consumption greatly exceeded that observed under control temperatures. Indeed, our bioassay data suggest that the extent to which resistance of WT plants is compromised by ET is greater than the effect of eliminating the JA pathway under normal temperature conditions (Fig. 1 E and F). Our results indicate that food security in the coming decades is thus likely to benefit from research aimed at identifying effective strategies to control insect herbivores in warming habitats.
Recent studies have established a role for the JA signaling pathway in damage-induced stomatal closure (28). In Arabidopsis, the GUARD CELL OUTWARD-RECTIFYING K+ (GORK) ion channel is activated by a COI1-dependent pathway in which the Ca2+ sensor kinase CBL1–CIPK5 complex phosphorylates GORK, leading to rapid stomatal closure. A role for JA in promoting stomatal closure suggests that stress-induced changes in JA levels may modulate gas exchange rates to restrict the expenditure of water resources (35). Because well-watered plants generally respond to elevated temperatures by opening stomata to promote evaporative leaf cooling (26, 27), we reasoned that damage-induced JA responses may interfere with this short-term adaptive strategy. Indeed, our results show that insect herbivory and mechanical wounding of tomato leaves under ET conditions results in COI1-dependent stomatal closure. Our data further indicate that the antagonistic interaction between leaf damage and heat stress has multiple negative consequences for the plant, including higher leaf temperature, lower stomatal conductance, lower photosynthetic carbon assimilation, and growth inhibition. Therefore, estimates of how climate warming will exacerbate crop losses due to insect pests (7) should take into consideration not only the effects of temperature on increased insect metabolic rate and population size, but also losses resulting from herbivore-induced decreases in the physiological performance of infested crops.
A key finding of our study was that the antagonism of ET-induced leaf cooling responses (e.g., stomatal closure) by leaf damage is linked to the activity of HSP90. Given that this wound-induced antagonism is dependent on COI1, together with the fact that COI1 is a client protein of HSP90 (16), the most straightforward interpretation of our data is that HSP90 stabilizes COI1 at ET to increase the plant’s sensitivity to bioactive JAs. This interpretation is consistent with the finding that ET promoted the coaccumulation of HSP90 and COI1, and that the HSP90 inhibitor GDA reduced COI1 accumulation under ET conditions. ET-induced HSP90 and the associated accumulation of COI1 seems to represent a previously unrecognized thermosensitive node in the jasmonate signaling pathway. In addition to, and synergistic with, increased COI1 protein levels, the stimulatory effect of ET on wound-induced accumulation of bioactive JAs may represent an additional thermosensitive component of JA signaling. It is possible that elevated hormone accumulation reflects a feed-forward activation of JA biosynthesis in response to elevated COI1 activity. The link between HSP90 activity and JA responses reported here is in agreement with previous work in wild tobacco showing that down-regulation of SGT1, which cooperatively interacts with HSP90, compromises wound-induced JA production and resistance to M. sexta attack (20). Interestingly, we also found that leaf damage inflicted by M. sexta larvae or mechanical wounding inhibited leaf hyponasty in a COI1-dependent manner. The central role of PIF (PHYTOCHROME INTERACTING FACTORS) transcription factors and auxin homeostasis in promoting leaf hyponasty (17) raises the possibility that a JA signaling component downstream of COI1 directly interferes with this thermomorphogenic response.
Several previous studies have reported that insect herbivory and JA signaling negatively impact photosynthesis in part by down-regulating the expression of photosynthesis genes and their corresponding proteins (36–38). We found that JA-dependent wound responses are associated with a significant decrease in CO2 assimilation at ET. While these damage responses also cause stomatal closure that could reduce internal CO2 levels, our measurements of intercellular CO2 concentrations (Ci) suggest that this is not the case. Future studies are required to understand the molecular mechanism of antagonism between JA and photosynthesis at ET.
Results from our study support a growing consensus (1) that cooccurring stresses present unique challenges to the plant. Under our CT conditions, limited insect herbivory and mechanical leaf damage had no obvious negative effects on plant growth beyond the loss of tissue at sites of damage. Under well-watered conditions and optimal temperatures, water loss through wound sites may present a low risk of severe water stress. However, because of a greater prevalence of drought at high temperatures and the risk of water loss through severed vasculature (39, 40), selective pressures associated with terrestrial habitats may have spurred the evolution of wound-associated thermosensory systems that not only enhance plant defenses under ET to keep pace with accelerated insect feeding, but also to mitigate water-deficient stress through genetically programmed stomatal closure. Insect herbivores may have evolved to coopt these host plant thermosensory systems to control leaf transpiration and thereby influence the temperature of their microenvironment. Ectothermic leaf-chewing herbivores can increase the temperature of the leaf surface by activating water-retention pathways resulting in temperature-driven increases in feeding, growth rate, and reproductive success (41). Conversely, phloem-feeding insects that avoid tissue damage can increase transpiration and cool leaves, possibly as a mechanism to reduce thermal stress (41). We suggest that HSP90-mediated stabilization of COI1 may provide a dynamic sensing mechanism to manage the balance between antiherbivore defense, photosynthesis, and water retention in response to fluctuating temperature.
Even as global average temperatures continue to rise and weather anomalies are more frequent and severe, agricultural production must double to meet the needs of the 11 billion people projected to coinhabit earth during this century. The stimulatory effect of elevated temperature on the metabolic demand of herbivore pests represents a challenge to agricultural productivity in the future. Recent models of the effects of climate change on insect populations suggest that each degree of warming will increase crop losses to insects by an additional 10 to 25% (7). These estimates generally do not account for the synergistic effects of compounding stress. Our findings suggest that the combined effects of insect herbivory and rising ambient temperatures will cause greater crop losses than either stress alone. Additional research is needed to disentangle the complex interactions of cooccurring stresses, which ultimately may inform new strategies to increase plant resilience to biotic stresses in a warming world.
Methods
S. lycopersicum (cv Castlemart) was used as the WT for all experiments with the exception of Western blot analysis of COI1 protein levels, in which case the MicroTom cultivar containing the 35S::COI1-Myc transgene (30) was used. Methodological details of plant growth conditions, chemical treatments, Western blot analysis, insect feeding, and gas exchange measurements are described in SI Appendix, Supplemental Materials and Methods.
Data Availability.
All data presented in the paper are described in the main text and SI Appendix. Biological materials are available from the corresponding author upon request.
Supplementary Material
Acknowledgments
We acknowledge Christian Danve M. Castroverde and Sheng Yang He for helpful discussions. This work was primarily funded by the Plant Resilience Institute at Michigan State University. Infrastructure support in the Plant Research Laboratory was provided by the Chemical Sciences, Geosciences and Biosciences Division, Office of Basic Energy Sciences, Office of Science, US Department of Energy through Grant DE-FG02-91ER20021. This publication was also made possible in part by a predoctoral training award to A.M.M. from Grant T32-GM110523 from the National Institute of General Medical Sciences of the National Institutes of Health. We also acknowledge support from the Michigan AgBioResearch Project MICL02278 from Michigan State University.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1913885117/-/DCSupplemental.
References
- 1.Suzuki N., Rivero R. M., Shulaev V., Blumwald E., Mittler R., Abiotic and biotic stress combinations. New Phytol. 203, 32–43 (2014). [DOI] [PubMed] [Google Scholar]
- 2.Zandalinas S. I., Mittler R., Balfagón D., Arbona V., Gómez-Cadenas A., Plant adaptations to the combination of drought and high temperatures. Physiol. Plant. 162, 2–12 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Carmo-Silva A. E., et al. , Decreased CO2 availability and inactivation of Rubisco limit photosynthesis in cotton plants under heat and drought stress in the field. Environ. Exp. Bot. 83, 1–11 (2012). [Google Scholar]
- 4.Rizhsky L., Liang H., Mittler R., The combined effect of drought stress and heat shock on gene expression in tobacco. Plant Physiol. 130, 1143–1151 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gornish E. S., Tylianakis J. M., Community shifts under climate change: Mechanisms at multiple scales. Am. J. Bot. 100, 1422–1434 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Raffa K. F., Powell E. N., Townsend P. A., Temperature-driven range expansion of an irruptive insect heightened by weakly coevolved plant defenses. Proc. Natl. Acad. Sci. U.S.A. 110, 2193–2198 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deutsch C. A., et al. , Increase in crop losses to insect pests in a warming climate. Science 361, 916–919 (2018). [DOI] [PubMed] [Google Scholar]
- 8.Howe G. A., Jander G., Plant immunity to insect herbivores. Annu. Rev. Plant Biol. 59, 41–66 (2008). [DOI] [PubMed] [Google Scholar]
- 9.Browse J., Jasmonate passes muster: A receptor and targets for the defense hormone. Annu. Rev. Plant Biol. 60, 183–205 (2009). [DOI] [PubMed] [Google Scholar]
- 10.Koo A. J. K., Howe G. A., The wound hormone jasmonate. Phytochemistry 70, 1571–1580 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toyota M., et al. , Glutamate triggers long-distance, calcium-based plant defense signaling. Science 361, 1112–1115 (2018). [DOI] [PubMed] [Google Scholar]
- 12.Mousavi S. A. R., Chauvin A., Pascaud F., Kellenberger S., Farmer E. E., GLUTAMATE RECEPTOR-LIKE genes mediate leaf-to-leaf wound signalling. Nature 500, 422–426 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Howe G. A., Major I. T., Koo A. J., Modularity in jasmonate signaling for multistress resilience. Annu. Rev. Plant Biol. 69, 387–415 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Yan J., et al. , The Arabidopsis F-box protein CORONATINE INSENSITIVE1 is stabilized by SCFCOI1 and degraded via the 26S proteasome pathway. Plant Cell 25, 486–498 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meldau S., Baldwin I. T., Wu J., For security and stability: SGT1 in plant defense and development. Plant Signal. Behav. 6, 1479–1482 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang X.-C., Millet Y. A., Cheng Z., Bush J., Ausubel F. M., Jasmonate signalling in Arabidopsis involves SGT1b-HSP70-HSP90 chaperone complexes. Nat. Plants 1, 15049 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casal J. J., Balasubramanian S., Thermomorphogenesis. Annu. Rev. Plant Biol. 70, 321–346 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Quint M., et al. , Molecular and genetic control of plant thermomorphogenesis. Nat. Plants 2, 15190 (2016). [DOI] [PubMed] [Google Scholar]
- 19.Wang R., et al. , HSP90 regulates temperature-dependent seedling growth in Arabidopsis by stabilizing the auxin co-receptor F-box protein TIR1. Nat. Commun. 7, 10269 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meldau S., Baldwin I. T., Wu J., SGT1 regulates wounding- and herbivory-induced jasmonic acid accumulation and Nicotiana attenuata’s resistance to the specialist lepidopteran herbivore Manduca sexta. New Phytol. 189, 1143–1156 (2011). [DOI] [PubMed] [Google Scholar]
- 21.Green T. R., Ryan C. A., Wound-induced proteinase inhibitor in tomato leaves: Some effects of light and temperature on the wound response. Plant Physiol. 51, 19–21 (1973). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li L., et al. , The tomato homolog of CORONATINE-INSENSITIVE1 is required for the maternal control of seed maturation, jasmonate-signaled defense responses, and glandular trichome development. Plant Cell 16, 126–143 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katsir L., Schilmiller A. L., Staswick P. E., He S. Y., Howe G. A., COI1 is a critical component of a receptor for jasmonate and the bacterial virulence factor coronatine. Proc. Natl. Acad. Sci. U.S.A. 105, 7100–7105 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen H., Wilkerson C. G., Kuchar J. A., Phinney B. S., Howe G. A., Jasmonate-inducible plant enzymes degrade essential amino acids in the herbivore midgut. Proc. Natl. Acad. Sci. U.S.A. 102, 19237–19242 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gonzales-Vigil E., Bianchetti C. M., Phillips G. N. Jr, Howe G. A., Adaptive evolution of threonine deaminase in plant defense against insect herbivores. Proc. Natl. Acad. Sci. U.S.A. 108, 5897–5902 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bridge L. J., Franklin K. A., Homer M. E., Impact of plant shoot architecture on leaf cooling: A coupled heat and mass transfer model. J. R. Soc. Interface 10, 20130326 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crawford A. J., McLachlan D. H., Hetherington A. M., Franklin K. A., High temperature exposure increases plant cooling capacity. Curr. Biol. 22, R396–R397 (2012). [DOI] [PubMed] [Google Scholar]
- 28.Förster S., et al. , Wounding-induced stomatal closure requires jasmonate-mediated activation of GORK K+ channels by a Ca2+ sensor-kinase CBL1-CIPK5 complex. Dev. Cell 48, 87–99.e6 (2019). [DOI] [PubMed] [Google Scholar]
- 29.Thines B., et al. , JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling. Nature 448, 661–665 (2007). [DOI] [PubMed] [Google Scholar]
- 30.Campos M. L., Kang J.-H., Howe G. A., Jasmonate-triggered plant immunity. J. Chem. Ecol. 40, 657–675 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu J., Baldwin I. T., New insights into plant responses to the attack from insect herbivores. Annu. Rev. Genet. 44, 1–24 (2010). [DOI] [PubMed] [Google Scholar]
- 32.Yang L., et al. , High temperature induces expression of tobacco transcription factor NtMYC2a to regulate nicotine and JA biosynthesis. Front. Physiol. 7, 465 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Casey T. M., Activity patterns, body temperature and thermal ecology in two desert caterpillars (Lepidoptera: Sphingidae). Ecology 57, 485–497 (1976). [Google Scholar]
- 34.Petersen C. H. R., Woods H. A., Kingsolver J., Stage-specific effects of temperature and dietary protein on growth and survival of Manduca sexta caterpillars. Physiol. Entomol. 25, 35–40 (2000). [Google Scholar]
- 35.Han X., et al. , Jasmonate negatively regulates stomatal development in Arabidopsis Cotyledons. Plant Physiol. 176, 2871–2885 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Havko N. E., et al. , Control of carbon assimilation and partitioning by jasmonate: An accounting of growth–defense tradeoffs. Plants (Basel) 5, E7 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwachtje J., Baldwin I. T., Why does herbivore attack reconfigure primary metabolism? Plant Physiol. 146, 845–851 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bilgin D. D., et al. , Biotic stress globally downregulates photosynthesis genes. Plant Cell Environ. 33, 1597–1613 (2010). [DOI] [PubMed] [Google Scholar]
- 39.Nabity P. D., Zavala J. A., DeLucia E. H., Indirect suppression of photosynthesis on individual leaves by arthropod herbivory. Ann. Bot. 103, 655–663 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang J. Y., et al. , The differential effects of herbivory by first and fourth instars of Trichoplusia ni (Lepidoptera: Noctuidae) on photosynthesis in Arabidopsis thaliana. J. Exp. Bot. 57, 527–536 (2006). [DOI] [PubMed] [Google Scholar]
- 41.Pincebourde S., Casas J., Narrow safety margin in the phyllosphere during thermal extremes. Proc. Natl. Acad. Sci. U.S.A. 116, 5588–5596 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data presented in the paper are described in the main text and SI Appendix. Biological materials are available from the corresponding author upon request.