Significance
Large conductance calcium-activated potassium (BK) channels are ubiquitously expressed and alter cellular excitability. These channels are formed by four pore-forming α subunits whose biophysical and pharmacological properties are modulated by regulatory β and γ subunits. LINGO1 is a protein, previously shown to be upregulated in both Parkinson’s disease and Essential Tremor. Consequently, we investigated its effects on BK channels and demonstrate that LINGO1 associates with these channels in human cerebellum. LINGO1 causes BK channels to inactivate and to open at more negative potentials. Furthermore, coexpression of BK with LINGO1 also led to a reduction in BK channels in the membrane. Our data support the idea that LINGO1 is a regulatory subunit of BK channels.
Keywords: BK channels, leucine-rich repeat containing proteins, LINGO1, accessory subunits, Parkinson’s disease
Abstract
LINGO1 is a transmembrane protein that is up-regulated in the cerebellum of patients with Parkinson’s disease (PD) and Essential Tremor (ET). Patients with additional copies of the LINGO1 gene also present with tremor. Pharmacological or genetic ablation of large conductance Ca2+-activated K+ (BK) channels also result in tremor and motor disorders. We hypothesized that LINGO1 is a regulatory BK channel subunit. We show that 1) LINGO1 coimmunoprecipitated with BK channels in human brain, 2) coexpression of LINGO1 and BK channels resulted in rapidly inactivating BK currents, and 3) LINGO1 reduced the membrane surface expression of BK channels. These results suggest that LINGO1 is a regulator of BK channels, which causes a “functional knockdown” of these currents and may contribute to the tremor associated with increased LINGO1 levels.
LINGO1 is one of four leucine rich repeat and immunoglobin-like (LRRIG) domain-containing proteins predominantly expressed in the central nervous system (1–3) and may play a role in both Essential Tremor (ET) and Parkinson’s disease (PD), since it is up-regulated in the cerebellum of these patients (4–6). Furthermore, a recent study demonstrated that adults with an extra copy of the LINGO1 gene also present with tremor, suggesting that increased levels of LINGO1 protein could be a causative factor in tremor (7).
Calcium-activated potassium (BK) channels are widely expressed, transmembrane proteins that govern smooth muscle (8) and neuronal excitability (9). Pharmacological blockade (10–12), or molecular ablation (13, 14), of these channels also induces tremor. For example, ingestion of indole diterpenoids (11, 12), the causative agents of “Rye Grass Staggers” (10), induces motor impairment and tremor (12). Similarly, global null BK−/− (13) and cerebellar Purkinje cell-specific BK−/− mice (14) exhibit motor impairment and tremor. It is clear, therefore, that inhibition of BK channels, or enhanced expression of LINGO1, are both associated with motor disorders and tremor.
The biophysical and pharmacological properties of BK channels are modulated by regulatory β1−4 (15–17) and γ1–4 subunits (18, 19), which are expressed in a tissue-dependent manner. Similar to LINGO1, BKγ subunits are leucine-rich repeat containing (LRRC) proteins but lack an Ig1 domain of LINGO1 and contain 6 rather than 12 extracellular LRRC domains. BKγ subunits have been shown to shift BK channel activation to negative voltages (18, 19). Since LINGO1 shares a number of structural features with BKγ subunits (18, 19), we hypothesized that it may also be a novel regulatory subunit of BK channels. Here, we show that LINGO1 induced rapid inactivation of BK channels, shifted their activation to more negative potetials, and reduced their expression in the plasma membrane. Moreover, it coimmunoprecipitated with BK channels in lysates of human cerebellar tissues. These data support the hypothesis that LINGO1 is a regulatory, accessory subunit, which functionally “knocks down” BK channels.
Coexpression of LINGO1 Induces Inactivation
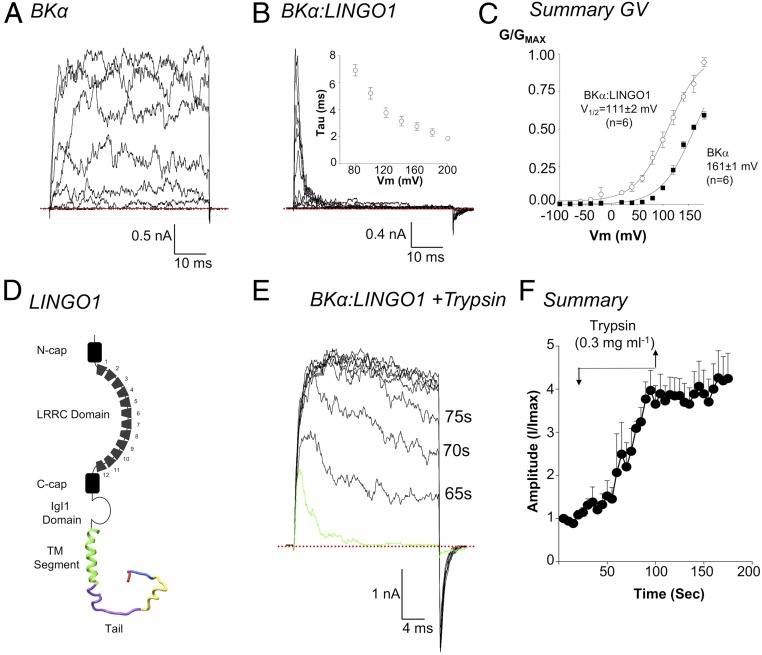
In our first series of experiments, we compared BK currents in excised patches from HEK cells transfected with BKα cDNA only, with those cotransfected with BKα and LINGO1 cDNA. As shown in Fig. 1A, large, noisy, sustained outward currents were recorded from BKα only inside-out patches, in response to depolarizing steps of up to +200 mV (Ca2+ concentration on the cytosolic side, [Ca2+]i, = 100 nM). When conductance/voltage (G/V) curves were constructed (Fig. 1C, filled squares) and fitted with a Boltzmann function (solid line, Fig. 1C), it was apparent that these currents were half maximally activated (V1/2) at +161 ± 1 mV (n = 6), in agreement with previous studies (20). However, when recordings were made from cells cotransfected with cDNA for BKα and LINGO1 (BKα:LINGO1, Fig. 1B), the currents differed markedly in several respects: 1) The sustained BK currents were absent and, instead, rapidly and completely inactivating currents were recorded (Fig. 1B, [Ca2+]i = 100 nM). Inactivation of BKα:LINGO1 currents was faster at more positive potentials (Fig. 1 B, Inset). These currents are reminiscent of the transient BK currents recorded in murine Purkinje neurons (21, 22), which may be due to LINGO1 or other regulatory BK subunits. 2) The BKα:LINGO1 tail currents deactivated more slowly than BK controls. 3) Their activation V1/2 was shifted by ∼ −50 mV (open symbols, Fig. 1C) compared to BKα alone, which was similar to the effect of γ subunits on BK channels (18, 19). 4) BKα:LINGO1 cotransfection reduced the amplitude of BK currents, since ∼5% of patches had currents >1 nA at +160 mV (peak current 451 ± 135 pA, mean ± SEM, n = 71), compared to ∼75% of BK controls (peak current 4,988 ± 632 pA mean ± SEM, n = 43, P < 0.001, ANOVA Tukey’s multiple comparison). 5) The inactivation V1/2 of BKα:LINGO1 currents shifted ∼ −80 mV when [Ca2+]i was increased from 100 nM to 1 μM (SI Appendix, Fig. S1 A–C).
Fig. 1.
Coexpression of BKα and LINGO1 cDNA produces inactivating currents that activate at negative potentials. A shows a typical record of an inside-out patch from a HEK cell transiently expressing BKα channels and exposed to 100 nM Ca2+. Currents were evoked by stepping from −100 mV to +200 mV for 50 ms from a holding potential of −60 mV. Patches were repolarized to −80 mV to elicit tail currents. B shows typical inactivating currents recorded using the same protocol from a patch obtained from HEK cells cotransfected with BKα and LINGO1 cDNA. As shown by Inset in B, inactivation had an apparent voltage sensitivity (n = 6). (C) When GV curves were constructed from these currents and fitted with a Boltzmann function (solid lines), BKα:LINGO1 currents activated at significantly more negative potentials (n = 6) compared to BKα channels (P < 0.001). D shows the main structural features of the LINGO1 protein including the extracellular domains (black), the transmembrane domain (green), and the intracellular tail (purple, yellow, blue, and red). (E) Application of trypsin (0.3 mg·mL−1) gradually removed inactivation of BKα:LINGO1 currents and increased current amplitude evoked by a step to +160 mV. The green line represents a control current, prior to application of Trypsin. F shows a summary of four similar experiments in which the time course of the effects of trypsin were recorded. Currents remained significantly increased above control after trypsin treatment (P < 0.05). The red dotted lines represent zero current.
To confirm that the inactivating currents were carried through BK channels, we assessed the effects of external iberiotoxin (IbTx) on whole-cell recordings of cells transfected with either BKα or BKα:LINGO1 cDNA (SI Appendix, Fig. S2). Cumulative addition of increasing concentrations of IbTx blocked both sustained and inactivating whole-cell currents in a concentration-dependent manner, confirming that both currents were due to activation of BK channels.
To assess if LINGO1 could cause inactivation when both BKα and β subunits were expressed, we cotransfected with cDNA for LINGO1, BKα, and either BKβ1 or BKβ4. Currents from these patches also showed inactivation (SI Appendix, Fig. S3), although the rate of inactivation was slower than those recorded in BKα:LINGO1 patches. When evoked by a step to +200 mV in 100 nM Ca2+, BKαβ1:LINGO1 currents inactivated with a τi of 17 ± 5 ms (n = 5) compared to 1.9 ± 0.2 ms in LINGO1:BKα (n = 7, unpaired t test, P < 0.05). Even when the ratio of BKβ1 cDNA and LINGO1 cDNA was increased to 5:5:1 (250 ng·mL−1 BKβ1, 250 ng·mL−1 LINGO1, 50 ng·mL−1 BKα), there was no further change in the rate of inactivation at +200 mV (τi = 18 ± 3 ms, n = 11, unpaired t test). Similarly, coexpression of BKαβ4:LINGO1 resulted in currents which inactivated in 100 nM Ca2+ at +200 mV with τi = 18 ± 3 ms (n = 8).
Inactivation Was Abolished When the Distal C Terminus of LINGO1 Was Absent
Although the crystal structure of the extracellular domain of LINGO1 has been solved (23), little is known about the structure of the transmembrane domain or the proposed intracellular tail. Therefore, we created a homology model of LINGO1, in which residues 551–583 formed a single transmembrane helix (green helix, Fig. 1D). In contrast, residues 584–620 (shown in purple, yellow, blue, and red, Fig. 1D) were intracellular, adopting a disordered loop structure that could possibly behave as an inactivating particle, as has been shown in some BKβ subunits (24–26) and Shaker channels (27). To test this, we enzymatically digested the tail by applying trypsin (0.3 mg·mL−1) to the cytosolic surface of patches coexpressing BK and LINGO1. Steps to +160 mV (Fig. 1E, green trace) evoked rapidly inactivating currents and small, slowly deactivating tail currents were apparent upon repolarization to −80 mV. However, after ∼60 s in the continued presence of trypsin, the BKα:LINGO1 current amplitude increased, inactivation was practically abolished, and deactivation was faster (τ = 0.4 ± 0.3 ms compared to 3.0 ± 0.3 ms before trypsin, P < 0.05, paired t test). Fig. 1F summarizes four experiments in which peak BK current amplitude was plotted before, during, and after trypsin digestion. Current amplitude was irreversibly increased ∼fourfold by trypsinization.
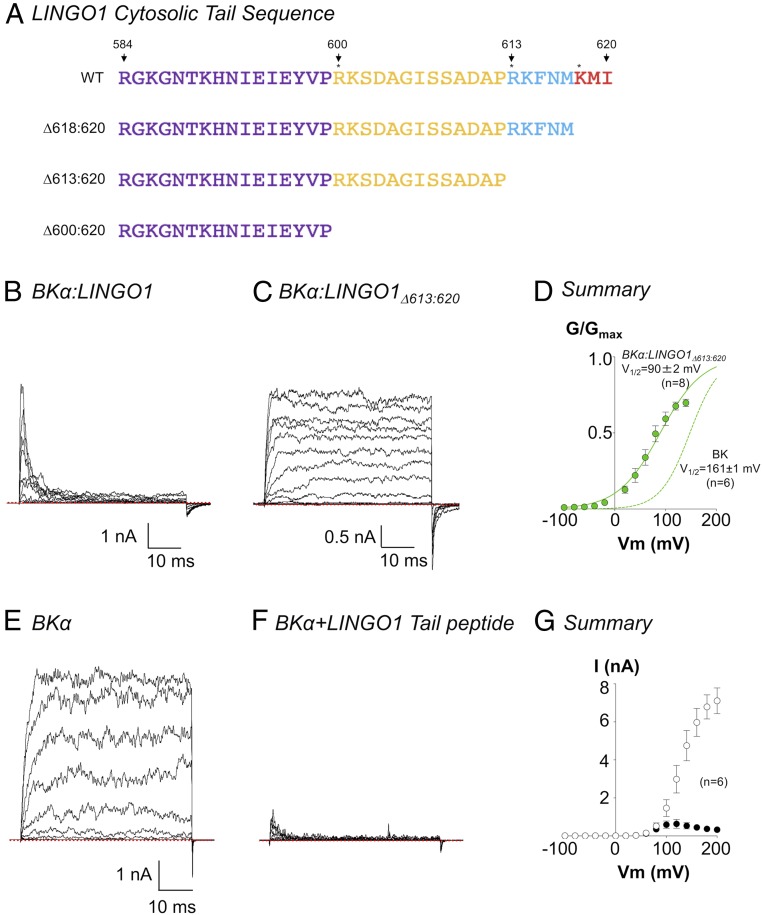
Since trypsin is likely to cleave the cytosolic tail at arginine or lysine residues, as marked by the asterisks in Fig. 2A, we made a series of LINGO1 C terminus deletion constructs at these sites (Δ600:620, Δ613:620, Δ618:620) and found that inactivation was practically abolished in constructs with at least the last eight amino acids removed. Fig. 2C shows a typical family of currents, recorded in 100 nM Ca2+, from BKα:LINGO1Δ613:620 in response to a series of voltage steps from −100 mV to 200 mV. When compared to the full-length BKα:LINGO1 construct (Fig. 2B), inactivation was abolished, but as shown in the summary GV curve (green symbols, Fig. 2D), the negative shift in V1/2 was retained (compare BKα, green dashed line). As shown in SI Appendix, Figs. S4 and S5B, all three deletion constructs retained the negative shift in activation V1/2, supporting the idea that truncated LINGO1 proteins still trafficked to the membrane and associated with BK channels.
Fig. 2.
Inactivation of BKα channels by LINGO1 depends on the terminal eight amino acids of LINGO1. (A) Amino acid sequence of residues 584:620 in LINGO1. Colors correspond to those in the model shown in Fig. 1. Asterisks mark most likely trypsin cleavage sites, which were used to design the deletion constructs shown. B shows a typical family of BKα:LINGO1 currents recorded as per Fig. 1B. C shows the currents evoked by the same voltage protocol in a deletion construct, which lacked the terminal eight amino acids of LINGO1. D shows the summary data (n = 8) for this deletion construct recorded in 100 nM Ca2+ and fitted with a Boltzmann (solid line). The activation V1/2 was significantly more negative than that recorded in cells transfected with BKα cDNA alone (dashed green line, P < 0.001). A typical family of currents from a patch containing BKα channels before (E) and during (F) application of tail peptide RKFNMKMI (30 μM) to the cytosolic surface of the patch. A summary of the inhibitory effect on this peptide on the IV relationship, in six similar patches is shown in G. Open and closed circles in G represent absence and presence of 30 μM RKFNMKMI, respectively.
To test if the last eight amino acids in the C terminus of LINGO1 were sufficient to cause inactivation, we made a synthetic peptide (peptide acid with a free N terminus and the C terminus terminated by a free carboxyl group) consisting of these eight residues (RKFNMKMI) and applied it to the cytosolic surface of patches expressing BKα-only channels. This peptide mimicked the inactivation observed when the full-length LINGO1 protein was coexpressed with BKα channels. Fig. 2E shows a family of BK currents recorded in 100 nM Ca2+ and evoked by a series of depolarizing steps from −100 mV to +200 mV, prior to the application of the synthetic peptide. In the presence of 30 μM of the peptide, the current amplitude was reduced approximately fivefold and the resultant currents inactivated (Fig. 2F). Fig. 2G shows a summary of six similar experiments in which the current amplitude (measured in the last 5 ms of each voltage step) was plotted in the absence (open symbols) and presence (closed symbols) of 30 μM RKFNMKMI. Under these conditions, BK current amplitude was significantly reduced at voltages >100 mV (P < 0.05, paired t test).
LINGO1 Reduces the Cell Surface Expression of BK Channels
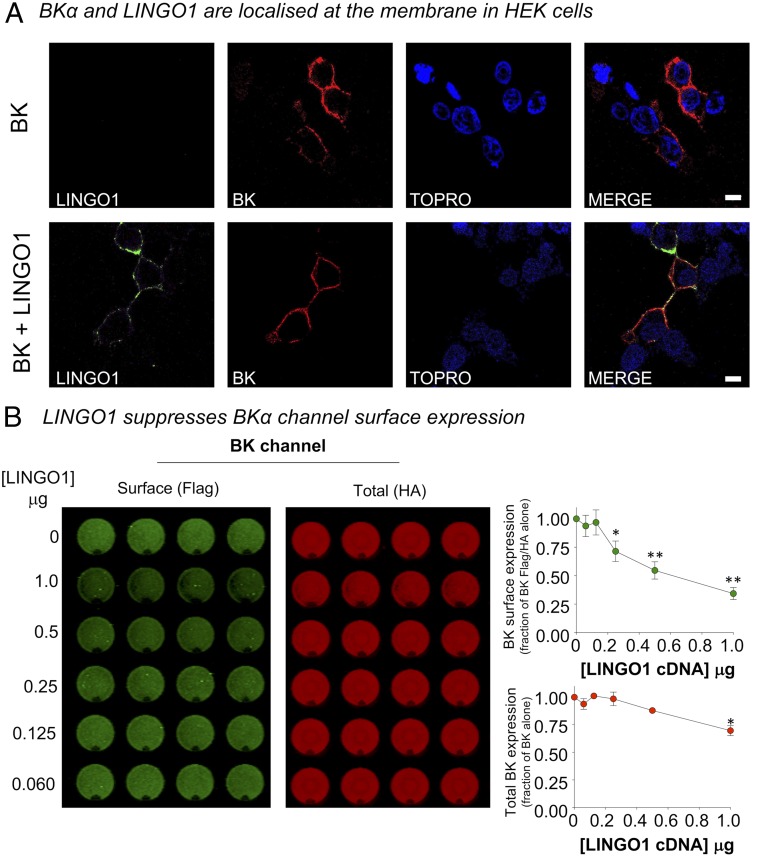
We next used immunocytochemistry experiments to further confirm that LINGO1 and BKα expressed in the membranes of HEK cells, transiently transfected with BKα and LINGO1 cDNA. As shown in Fig. 3 A, Upper, only BKα channels were detected in the membranes of HEK cells transfected with BKα cDNA. In contrast, when LINGO1 was cotransfected with BKα, both proteins were present. However, it appeared that BKα channel expression in the membrane was lower when cotransfected with LINGO1 cDNA (Fig. 3 A, Lower), which may contribute to the reduced amplitude of the BK currents recorded in the BKα:LINGO1 cotransfected cells. An on-cell Western (OCW) assay, using HEK cells transfected with BKα channel cDNA encoding an intracellular HA tag and an extracellular FLAG tag, was used to compare BKα expression in the absence and presence of LINGO1. It is clear from Fig. 3B that both surface and total BKα expression were reduced by LINGO1. BK channel surface expression (Fig. 3B, green symbols), as a function of total BKα expression, was also significantly reduced in a concentration-dependent manner when cotransfected with LINGO1. However, total BKα expression was only reduced in cells cotransfected with 1 μg of LINGO1 cDNA (Fig. 3B, red symbols). To verify that the reduction of BK channel plasmalemmal and total expression with increasing concentrations of LINGO1 cDNA was not a consequence of nonspecific saturation of synthesis/trafficking, we assayed cell surface expression of the BK channel in the presence of the transmembrane BKβ1 subunit. Over the same concentration range as used for LINGO1 (60–1,000 ng), in OCW assays coexpression of BKβ1 resulted in a significant increase in BK channel surface expression (∼2.5-fold above that of BK alone using 1,000 ng of BKβ1), with no significant effect on total BK expression (SI Appendix, Fig. S7). Thus, LINGO1 and BKβ1 have opposite effects on BKα cell surface expression.
Fig. 3.
LINGO1 suppresses cell surface expression of BKα channels. (A) Representative confocal sections from HEK293 cells expressing BK channels alone (Upper) or coexpressed with LINGO1 (Lower) in HEK293 cells. Cells were fixed and immunostained for LINGO1 and BKα channels and nuclear stain (TOPRO). (Scale bar, 7 μm.) (B) Representative experiment (Left) from an OCW assay to detect cell surface expression of BK channels in HEK293 cells in the presence of different concentrations of LINGO1 cDNA, run in quadruplicate. Surface expression (Flag-) was determined in nonpermeabilized cells probing for the extracellular Flag- epitope on the BKα channel N terminus. Total BKα expression was determined in the same well after cell permeabilization and probing for the -HA epitope on the intracellular C terminus of the BK channels. Quantification of BK channel surface expression, expressed as a fraction of the Flag/HA ratio in the absence of LINGO1 (Upper), and total BKα channel expression (Lower) expressed as a fraction of total BK channel in absence of LINGO1. Data are mean ± SEM from four to seven independent experiments in each group. *P < 0.05, **P < 0.01 vs. BKα alone ANOVA with post hoc Tukey test.
To further quantify plasmalemmal BK expression, we performed an additional series of electrophysiology experiments on cells in which BKα cDNA was cotransfected alone, or with equimolar concentrations (100 ng·mL−1) of WT LINGO1 cDNA, or each of the three LINGO1 tail deletion constructs (SI Appendix, Fig. S6 A–F). Pipettes with resistances of 4–5 MΩ were used for all of these experiments. All currents were evoked by steps to +160 mV, in the presence of 100 nM Ca2+. The amplitude of currents in patches from cells expressing BKα:LINGO1 (SI Appendix, Fig. S6B) were 90% smaller than BKα alone (SI Appendix, Fig. S6A), and the mean current amplitude (SI Appendix, Fig. S6G) was 451 ± 135 pA (n = 71) and 4,988 ± 632 pA (n = 43), respectively (P < 0.001, ANOVA, Tukey’s multiple comparison test). An important point to note is that of the 71 patches recorded from cells transfected with BKα:LINGO1, no currents were detected in 26 patches (∼37%). Furthermore, trypsin (0.3 mg·mL−1) was applied to six of these “blank” patches and failed to unmask any currents. In contrast, zero “blank” patches were detected in the 43 patches taken from cells transfected with just BKα cDNA. However, application of trypsin to 12 other patches containing inactivating BKα:LINGO1 channels (SI Appendix, Fig. S6C) increased mean current amplitude ∼fourfold (from 503 ± 128 pA to 1,762 ± 380 pA, P < 0.001, paired t test, SI Appendix, Fig. S6G). Thus, in the absence of inactivation, the BKα:LINGO1 current amplitude was still reduced by ∼65% compared to patches taken from cells transfected with just BKα cDNA (P < 0.001, ANOVA, Tukey’s multiple comparisons test). These data are in agreement with the effects of equimolar BKα:LINGO1 cDNA cotransfection on surface BK expression, shown in our OCW experiments (65.7 ± 5%, Fig. 3B). The amplitude of currents recorded (under exactly the same conditions as BKα currents) from the LINGO1 tail deletion constructs BKα:LINGO1Δ618:620, BKα:LINGO1Δ613:620, and BKα:LINGO1Δ600:620 (SI Appendix, Fig. S6 D–F, respectively) were similar in amplitude to trypsinized BKα:LINGO1 currents and, thus, were also ∼65–70% smaller than BKα (SI Appendix, Fig. S6G). These were all significantly smaller than BKα currents (P < 0.001, ANOVA, Tukey’s multiple comparisons test) but were not significantly different to each other, or to the trypsin-treated patches.
LINGO1 Coimmunoprecipitates with BK in Human Cerebellar Tissues
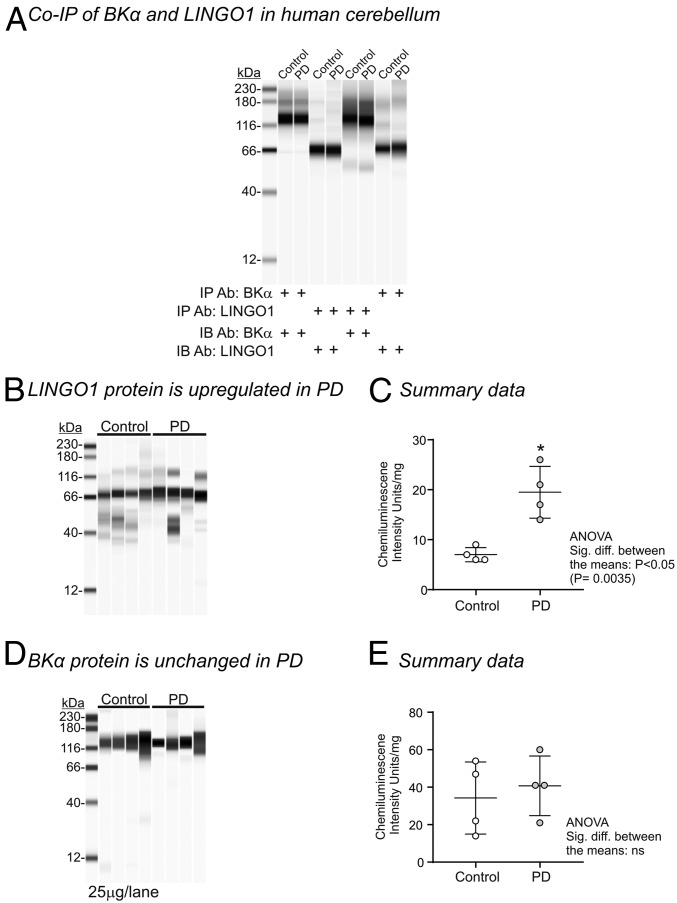
Having established that LINGO1 behaved as a modulator of BK channels in HEK cells, we next examined if it interacted with BK channels in the membranes of human cerebellum. Since LINGO1 has previously been shown to be up-regulated in the cerebellum of PD patients (5), we obtained postmortem cerebellar samples from both PD patients and age-matched controls. As shown in Fig. 4A, LINGO1 and BKα proteins coimmunoprecipitated in both control and PD samples, providing support that both of these proteins closely associate in the membranes of native human tissues. We also confirmed that LINGO1 was significantly elevated in PD samples compared to control (Fig. 4 B and C), in agreement with previous studies (4, 5). However, when BK protein in the membrane was quantified, there was no significant difference between controls and PD cerebellar samples (Fig. 4 D and E).
Fig. 4.
BKα and LINGO1 proteins in human cerebellum. (A) Representative Wes analysis of BKα and LINGO1 immunoprecipitates showing an interaction between BKα and LINGO1 in cerebellum from PD patients and age-matched controls. Equal volumes of each elution were loaded into each lane. Representative Wes analysis (B) and the summary data (C) showing that LINGO1 protein levels are up-regulated in cerebellum samples from PD patients (25 μg of protein per lane). Representative Wes analysis (D) and the summary data (E) showing that BKα protein levels are unchanged in cerebellum samples from PD patients (25 μg protein per lane).
Discussion
In the present study, we found that 1) LINGO1 coexpression with BK channels resulted in rapidly inactivating, slowly deactivating and negatively activating BK currents; 2) LINGO1 reduced plasmalemmal expression of BK channels in HEK cells; and 3) LINGO1 and BK coimmunoprecipitated in cerebellar tissues from both PD patients and age-matched controls. These data suggest that LINGO1 is a regulatory subunit of BK channels.
LINGO proteins and γ subunits are both LRRC proteins, but they differ in several respects. First, the LINGO1 protein has <20% sequence identity to the γ subunits. Second, the LINGO proteins have 12 extracellular LRRC domains, compared to 6 in the γ subunits. Third, the LINGO proteins have an IgI1 domain, which is absent in the γ subunits. Finally, in contrast to the γ subunits, the LINGO1–3 proteins share a KMI sequence at the distal end of the tail and all three proteins have a net positive charge in this region, as illustrated in the sequence alignment shown in SI Appendix, Fig. S8A. Interestingly, LINGO4 lacks this KMI motif and only has one positive charge in this region, compared to three in LINGO1–3, leading us to speculate that its effects on BK channel inactivation are likely to differ from the other LINGO family members.
Although the extracellular domains of LINGO1 differ significantly from the γ subunits, the phylogenetic tree (SI Appendix, Fig. S8B) based on the sequence alignment of the LRRPs transmembrane region and part of the intracellular tail (SI Appendix, Fig. S9) suggests that each of the four LINGO proteins are evolutionarily close to the BKγ subunits.
The presence of net positive charge and a hydrophobic region in the C terminus of LINGO1 suggests that this protein shares some similarities with the N terminus of inactivating BKβ subunits (25, 28, 29). These subunits are thought to induce N terminus inactivation via a mechanism, which shares some similarities to that observed in voltage-dependent K+ channels (27, 30), whereby the open channel becomes blocked by a tethered peptide. Our results with LINGO1 are consistent with such a mechanism of action, since 1) LINGO1 slowed down deactivation of the BK channels, suggesting that it prevents channel closure following inactivation; 2) application of trypsin to the cytosolic surface of BKα:LINGO1 patches, irreversibly abolished inactivation; 3) deletion of the charged and hydrophobic regions (BKα:LINGOΔ613:620) from the C terminus practically abolished inactivation (Fig. 2C); and 4) application of a peptide identical to the last eight residues of LINGO1 mimicked inactivation (Fig. 2F). However, caution is called for in the interpretation of the last point, since basic peptides can have promiscuous blocking effects. Although we have not studied the mechanism of block/unblock in any detail, it is interesting to note that the BKα:LINGO1 tail currents were inwardly rectifying (SI Appendix, Fig. S1 D and E), suggesting that LINGO1 unblocks much more rapidly at very negative potentials. However, the precise blocking mechanism clearly warrants further investigation.
Deletion of the terminal three residues of the LINGO1 C terminus (Δ618–620) greatly reduced inactivation of BK channels (SI Appendix, Fig. S4), suggesting that the KMI sequence, common to LINGO1–3 (SI Appendix, Fig. S8A), contributes to inactivation. The involvement of a triplet of residues in inactivation has also been shown in BKβ2 subunits, where an N terminus FIW deletion mutant abolished the inactivation of BKβ2 channels (25). However, in LINGO1, other residues are also clearly involved since inactivation was observed with the LINGO1Δ618–620 mutant at potentials positive to +160 mV and in higher [Ca2+]i (SI Appendix, Fig. S4A). For example, the τi at +200 mV, in 1 μM Ca2+, was 2.6 ± 0.5 ms (n = 6) in this mutant, compared to 1.2 ± 0.3 ms in WT LINGO1 (n = 5, P < 0.05, unpaired t test). However, the deletion of the terminal 8 residues (Δ613–620, SI Appendix, Fig. S4C) and 21 residues (Δ600–620, SI Appendix, Fig. S4E) of LINGO1 completely abolished LINGO1-mediated inactivation of BK channels. These results support the idea that the inactivation domain resides in the distal C terminus of LINGO1.
BKα:LINGO1 currents shared some features of inactivating BKβ subunits, but there were a number of obvious differences. Thus, the time dependence of inactivation (τi) of BKα:LINGO1 currents was faster (τi ∼ 5 ms in 100 nM Ca2+ at +100 mV) than BKβ2 currents (∼200 ms and ∼50 ms at +100 mV in 100nM Ca2+ and 10 μM Ca2+, respectively; ref. 31). However, it is important to note that the experiments presented here were carried out at 37 °C, compared to room temperature in the Wallner et al. study. Although τi measured in BKα:LINGO1 channels was more similar to that observed with BKαβ3b channels (τi ∼ 1 ms at +100 mV in 100 nM Ca2+; ref. 32), it has been established that BKαβ3b currents fail to completely inactivate, in contrast to that observed with BKα:LINGO1 currents.
A feature of BKα:LINGO1 currents was a −50 mV negative shift in the activation V1/2 in 100 nM Ca2+ compared to BKα alone (20). This is similar to the shift observed previously in BKγ3 channels (18, 19) in the absence of Ca2+. Interestingly, the negative shift in V1/2 was retained in the C terminus deletion mutants as illustrated in SI Appendix, Figs. S4 and S5B, suggesting that this region does not contribute to the shift in activation V1/2 observed in LINGO1:BK channels. Thus, the extracellular domain or the transmembrane and intracellular membrane-flanking residues of LINGO1 might contribute to the observed shift in V1/2. In BKγ subunits, the F273 residue of the TM domain and a cluster of positively charged membrane flanking residues contribute significantly to the negative shift in activation of γ1 subunits (33, 34), so it is tempting to speculate that similar residues could play a role in the LINGO1-mediated negative shift of BK channel activation, but this will require confirmation.
Although previous studies on BKγ subunits have demonstrated that they augment BK current at physiological potentials, it is likely that the functional effects of LINGO1 will be complicated by its ability to inactivate the channel, as shown in SI Appendix, Fig. S1. Consequently, as illustrated in SI Appendix, Fig. S10H, the availability of BKα:LINGO1 current will not only depend on the [Ca2+]i, the presence and stoichiometry of other regulatory subunits, but also the resting potential. Future experiments will be directed at examining how LINGO1 changes the “available” BK current at physiological potentials in BK channels, in the absence and presence of regulatory subunits.
It is clear from the data obtained from postmortem cerebellar tissue that LINGO1 and BK coimmunoprecipitated, suggesting that in human brain, these two proteins are also intimately associated. It is also important to note that LINGO1 protein levels were significantly elevated in all four PD patient samples compared to age-matched controls, in agreement with previous studies (4–6). However, in contrast to the reduction in BK channel expression observed with LINGO1 in HEK cells, we found no evidence that BK channel protein expression was altered in the cerebellar samples from the four PD patient samples used in this study. Unfortunately, we were unable to ascertain if tremor was present in these deceased PD patients, and it therefore remains a possibility that the LINGO1 levels recorded in these patients were insufficient to down-regulate BK expression. Nevertheless, the results of a recent study from a family with an extra copy of the LINGO1 gene (7) suggests that elevated LINGO1 expression can result in tremor and this may, at least in part, be due to its effects on neuronal BK channels.
In summary, we have demonstrated that LINGO1 is a regulatory BK channel subunit that could be involved with the tremor involved in patients with increased LINGO1 levels.
Methods
Electrophysiology.
Experiments were performed on BKα subunits and LINGO1 transiently expressed in HEK cells and studied with either the inside out or whole-cell configurations of the patch clamp technique. The concentrations of Ca2+ in each experiment applied to the cytosolic face of the channel are shown in each figure. See SI Appendix, SI Materials and Methods for details. All data were expressed as the mean ± SEM.
Molecular Biology and Cell Culture.
Cell surface expression of BKα subunits in the presence and absence of LINGO1 was determined using OCW assay with epitope tagged BKα subunits expressed in HEK293 cells.
Human Samples and Westerns.
BKα and LINGO1 protein expression and coimmunoprecipitation experiments were carried out on postmortem human cerebellum homogenates from PD patients and age-matched unaffected controls and determined by Wes analysis.
Data Availability Statement.
All data discussed in the paper will be made available to readers upon request.
Supplementary Material
Acknowledgments
We thank Dr. Tim Webb for cloning the rabbit β subunits. This study was funded as part of the Borders and Regions Airway Training Hub project by the European Union (EU), under the Interreg VA Programme, managed by the Special EU Programmes Body (to K.D.T., G.P.S., and M.A.H.). Human cerebellar tissue samples were obtained from the NIH Neurobiobank at the University of Maryland, Baltimore, MD, and the Human Brain and Spinal Fluid Resource Centre, Los Angeles, CA.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1916715117/-/DCSupplemental.
References
- 1.Barrette B., Vallières N., Dubé M., Lacroix S., Expression profile of receptors for myelin-associated inhibitors of axonal regeneration in the intact and injured mouse central nervous system. Mol. Cell. Neurosci. 34, 519–538 (2007). [DOI] [PubMed] [Google Scholar]
- 2.Inoue H., et al. , Inhibition of the leucine-rich repeat protein LINGO-1 enhances survival, structure, and function of dopaminergic neurons in Parkinson’s disease models. Proc. Natl. Acad. Sci. U.S.A. 104, 14430–14435 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Llorens F., et al. , Developmental analysis of Lingo-1/Lern1 protein expression in the mouse brain: Interaction of its intracellular domain with Myt1l. Dev. Neurobiol. 68, 521–541 (2008). [DOI] [PubMed] [Google Scholar]
- 4.Kuo S. H., et al. , Lingo-1 expression is increased in essential tremor cerebellum and is present in the basket cell pinceau. Acta Neuropathol. 125, 879–889 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delay C., et al. , Increased LINGO1 in the cerebellum of essential tremor patients. Mov. Disord. 29, 1637–1647 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Tan E. K., LINGO1 and essential tremor: Linking the shakes. Linking LINGO1 to essential tremor. Eur. J. Hum. Genet. 18, 739–740 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alakbarzade V., et al. , Copy number variation of LINGO1 in familial dystonic tremor. Neurol. Genet. 5, e307 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brayden J. E., Nelson M. T., Regulation of arterial tone by activation of calcium-dependent potassium channels. Science 256, 532–535 (1992). [DOI] [PubMed] [Google Scholar]
- 9.Adams P. R., Constanti A., Brown D. A., Clark R. B., Intracellular Ca2+ activates a fast voltage-sensitive K+ current in vertebrate sympathetic neurones. Nature 296, 746–749 (1982). [DOI] [PubMed] [Google Scholar]
- 10.Imlach W. L., et al. , The molecular mechanism of “ryegrass staggers,” a neurological disorder of K+ channels. J. Pharmacol. Exp. Ther. 327, 657–664 (2008). [DOI] [PubMed] [Google Scholar]
- 11.Fletcher L. R., Harvey I. C., An association of a Lolium endophyte with ryegrass staggers. N. Z. Vet. J. 29, 185–186 (1981). [DOI] [PubMed] [Google Scholar]
- 12.Gallagher R. T., Hawkes A. D., The potent tremorgenic neurotoxins lolitrem B and aflatrem: A comparison of the tremor response in mice. Experientia 42, 823–825 (1986). [DOI] [PubMed] [Google Scholar]
- 13.Meredith A. L., Thorneloe K. S., Werner M. E., Nelson M. T., Aldrich R. W., Overactive bladder and incontinence in the absence of the BK large conductance Ca2+-activated K+ channel. J. Biol. Chem. 279, 36746–36752 (2004). [DOI] [PubMed] [Google Scholar]
- 14.Cheron G., et al. , Purkinje cell BK channel ablation induces abnormal rhythm in deep cerebellar nuclei and prevents LTD. Nat. Sci. Rep. 8, 4220 (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knaus H. G., et al. , Tremorgenic indole alkaloids potently inhibit smooth muscle high-conductance calcium-activated potassium channels. Biochemistry 33, 5819–5828 (1994). [DOI] [PubMed] [Google Scholar]
- 16.Uebele V. N., et al. , Cloning and functional expression of two families of beta-subunits of the large conductance calcium-activated K+ channel. J. Biol. Chem. 275, 23211–23218 (2000). [DOI] [PubMed] [Google Scholar]
- 17.Brenner R., et al. , Vasoregulation by the beta1 subunit of the calcium-activated potassium channel. Nature 407, 870–876 (2000). [DOI] [PubMed] [Google Scholar]
- 18.Yan J., Aldrich R. W., LRRC26 auxiliary protein allows BK channel activation at resting voltage without calcium. Nature 466, 513–516 (2010). [DOI] [PubMed] [Google Scholar]
- 19.Yan J., Aldrich R. W., BK potassium channel modulation by leucine-rich repeat-containing proteins. Proc. Natl. Acad. Sci. U.S.A. 109, 7917–7922 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Webb T. I., et al. , Molecular mechanisms underlying the effect of the novel BK channel opener GoSlo: Involvement of the S4/S5 linker and the S6 segment. Proc. Natl. Acad. Sci. U.S.A. 112, 2064–2069 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khaliq Z. M., Gouwens N. W., Raman I. M., The contribution of resurgent sodium current to high-frequency firing in Purkinje neurons: An experimental and modeling study. J. Neurosci. 23, 4899–4912 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benton M. D., Lewis A. H., Bant J. S., Raman I. M., Iberiotoxin-sensitive and -insensitive BK currents in Purkinje neuron somata. J. Neurophysiol. 109, 2528–2541 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mosyak L., et al. , The structure of the Lingo-1 ectodomain, a module implicated in central nervous system repair inhibition. J. Biol. Chem. 281, 36378–36390 (2006). [DOI] [PubMed] [Google Scholar]
- 24.Xia X. M., Ding J. P., Lingle C. J., Molecular basis for the inactivation of Ca2+- and voltage-dependent BK channels in adrenal chromaffin cells and rat insulinoma tumor cells. J. Neurosci. 19, 5255–5264 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xia X. M., Ding J. P., Lingle C. J., Inactivation of BK channels by the NH2 terminus of the beta2 auxiliary subunit: An essential role of a terminal peptide segment of three hydrophobic residues. J. Gen. Physiol. 121, 125–148 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ding J. P., Li Z. W., Lingle C. J., Inactivating BK channels in rat chromaffin cells may arise from heteromultimeric assembly of distinct inactivation-competent and noninactivating subunits. Biophys. J. 74, 268–289 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoshi T., Zagotta W. N., Aldrich R. W., Biophysical and molecular mechanisms of Shaker potassium channel inactivation. Science 250, 533–538 (1990). [DOI] [PubMed] [Google Scholar]
- 28.Lingle C. J., Zeng X. H., Ding J. P., Xia X. M., Inactivation of BK channels mediated by the NH(2) terminus of the β3b auxiliary subunit involves a two-step mechanism: Possible separation of binding and blockade. J. Gen. Physiol. 117, 583–606 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benzinger G. R., Xia X.-M., Lingle C. J., Direct observation of a preinactivated, open state in BK channels with β2 subunits. J. Gen. Physiol. 127, 119–131 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rettig J., et al. , Inactivation properties of voltage-gated K+ channels altered by presence of β-subunit. Nature 369, 289–294 (1994). [DOI] [PubMed] [Google Scholar]
- 31.Wallner M., Meera P., Toro L., Molecular basis of fast inactivation in voltage and Ca2+-activated K+ channels: A transmembrane beta-subunit homolog. Proc. Natl. Acad. Sci. U.S.A. 96, 4137–4142 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xia X. M., Ding J. P., Zeng X. H., Duan K. L., Lingle C. J., Rectification and rapid activation at low Ca2+ of Ca2+-activated, voltage-dependent BK currents: Consequences of rapid inactivation by a novel beta subunit. J. Neurosci. 20, 4890–4903 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Q., Fan F., Kwak H. R., Yan J., Molecular basis for differential modulation of BK channel voltage-dependent gating by auxiliary γ subunits. J. Gen. Physiol. 145, 543–554 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Q., Guan X., Yen K., Zhang J., Yan J., The single transmembrane segment determines the modulatory function of the BK channel auxiliary γ subunit. J. Gen. Physiol. 147, 337–351 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data discussed in the paper will be made available to readers upon request.