Significance
While it has long been known that vertebrates rely on compensatory responses to gravity with changing body orientation, this topic has not been previously studied in invertebrates. Here, we use synchrotron X-ray imaging and radio-tracing to demonstrate that body orientation has dramatic effects on hemolymph and air distribution in grasshoppers, and that grasshoppers exhibit similar physiological responses to gravity as vertebrates. Our findings suggest that gravity-driven cardiovascular responses may be ancient and widely shared among animals, opening the door for invertebrates as model systems for investigation of cellular and systemic mechanisms of gravity responses. Furthermore, future physiological studies of arthropods should control for body position.
Keywords: gravity, invertebrate, insect, cardiovascular system, respiratory system
Abstract
Gravity is one of the most ubiquitous environmental effects on living systems: Cellular and organismal responses to gravity are of central importance to understanding the physiological function of organisms, especially eukaryotes. Gravity has been demonstrated to have strong effects on the closed cardiovascular systems of terrestrial vertebrates, with rapidly responding neural reflexes ensuring proper blood flow despite changes in posture. Invertebrates possess open circulatory systems, which could provide fewer mechanisms to restrict gravity effects on blood flow, suggesting that these species also experience effects of gravity on blood pressure and distribution. However, whether gravity affects the open circulatory systems of invertebrates is unknown, partly due to technical measurement issues associated with small body size. Here we used X-ray imaging, radio-tracing of hemolymph, and micropressure measurements in the American grasshopper, Schistocerca americana, to assess responses to body orientation. Our results show that during changes in body orientation, gravity causes large changes in blood and air distribution, and that body position affects ventilation rate. Remarkably, we also found that insects show similar heart rate responses to body position as vertebrates, and contrasting with the classic understanding of open circulatory systems, have flexible valving systems between thorax and abdomen that can separate pressures. Gravitational effects on invertebrate cardiovascular and respiratory systems are likely to be widely distributed among invertebrates and to have broad influence on morphological and physiological evolution.
In vertebrates, gravity effects are commonly experienced when an animal changes body orientation, resulting in changing pressures throughout the body governed by principles of fluid mechanics, with pressure differences directly proportional to vertical height (1–5). Many insects change body positions routinely and often, especially during locomotion; for example, ants and roaches routinely walk up and down trees and walls (6). Because the circulatory system does not have a primary role in oxygen delivery (7), the consequences of gravity-driven hemolymph flow may be less consequential than in vertebrates; however, hemolymph provides water, ions, nutrients, and hormones to all tissues, and disruption of hemolymph supply to a tissue is deleterious (8). Changes in fluid distribution may also affect an organism’s center of gravity, requiring compensatory neuromuscular adjustments to maintain balance and coordinated movement (8).
We studied the effect of body orientation on air sac and tracheal morphology within the grasshopper, Schistocerca americana. Similar to many other insects, this grasshopper perches, moves, flies, and feeds in head-up, head-down, prone, and even upside-down positions (personal observations). During other studies of tracheal system function at Argonne National Laboratory using synchrotron X-ray imaging (9), we were surprised to observe dramatic changes in air sac volume occurring in real time within ketamine-anesthetized grasshoppers in different body orientations. In the present study, we quantified the effect of body orientation on tracheal morphology, using synchrotron X-ray imaging of live grasshoppers and microtomography of recently killed grasshoppers, and assessed effects of orientation on hemolymph distribution, heart and ventilation rates, and hemolymph pressure gradients.
Results
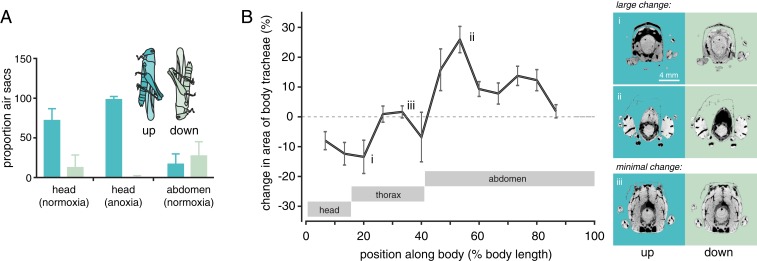
We recorded synchrotron X-ray video of alert, unanesthetized grasshoppers kept for approximately 5 min in head-up, head-down, or prone positions, and then measured the proportion of air sacs and tracheae in the head and abdominal tip from individual video frames (SI Appendix, Fig. S1). This analysis showed clearly that the tracheal system responded dramatically to body position (Fig. 1A). For head-up animals, air sacs in the head were greatly expanded, whereas air sacs in the caudal tip of the abdomen were compressed and mostly invisible; head-down animals showed the opposite pattern, and prone animals tended to be intermediate (Fig. 1A). Adult and third instar juvenile S. americana showed generally similar effects of body position on air sac volumes, despite differing 20× in body mass (SI Appendix, Table S1). The fraction of X-ray images occupied by tracheae was unaffected by body orientation (SI Appendix, Table S1), likely because the changes in hemolymph pressure were insufficient to induce tracheal collapse (10). We then anesthetized animals by perfusing a chamber with N2 gas. Anesthesia exacerbated the effect of body position, causing air sacs in the head to be even more expanded and those in the abdominal tip to be nearly completely compressed (Fig. 1A and SI Appendix, Table S2), demonstrating that awake grasshoppers actively resist the effects of body position on the tracheal system.
Fig. 1.
Body orientation affects the distribution of air in grasshoppers. (A) Proportion of the body occupied by air sacs as a function of body orientation in alert and anesthetized grasshoppers. In the head-up position, the proportion of X-ray images occupied by air sacs in the head were increased and those in the caudal abdomen were decreased (2-way ANOVA, using individual as random effect, significant position*location interaction effect; F1,7 = 34.5; n = 8; P < 0.001). The effect of body orientation on the head air sacs was exacerbated when animals were anesthetized by anoxia (2-way ANOVA, significant condition*position effect; F1,6 = 15.9; n = 6; P = 0.007). (B) Effect of body position (head-down vs. head-up) on the percentage of a region occupied by the tracheal system of recently killed grasshoppers. Grasshoppers killed and imaged in the head-down position had reduced tracheal system in cranial regions and an expanded tracheal system in caudal regions. Locations responded differently to orientation change, as indicated statistically by a highly significant interaction between orientation and location, using a 2-way ANOVA on logit-transformed proportions with individual as a random factor (F24,169 = 2.066; P = 0.0042). Image pairs to the Right of Fig. 1B are head-up (Left) and head-down (Right) microcomputed tomography images from identical locations (for larger images, see SI Appendix, Fig. S1). Dark pixels represent tracheal air space. Eight locusts were measured in each orientation; means and 95% confidence limits are shown in this and subsequent images.
To provide a more comprehensive examination of the effect of body orientation on tracheal system anatomy, we followed these experiments with tomographic imaging studies of grasshoppers killed in both the head-up and head-down positions, assessing the percentage of body area occupied by the respiratory system (air sacs plus tracheae) from image slices at 13 locations along the body (Fig. 1B and SI Appendix, Fig. S2 and Table S3). Compared with head-up animals, the head and prothoracic regions of head-down animals had a smaller fraction of their body occupied by the respiratory system, whereas the tracheal system was expanded in the entire abdomen (Fig. 1B).
Is the reciprocal expansion and compression of cranial and caudal air sacs induced by changes in body orientation caused by gravitational effects on blood flow? As a direct test of this hypothesis, we quantified hemolymph distribution within alert, unanesthetized adult grasshoppers using a 3H-labeled inulin, which remains unmetabolized in the hemolymph (11).
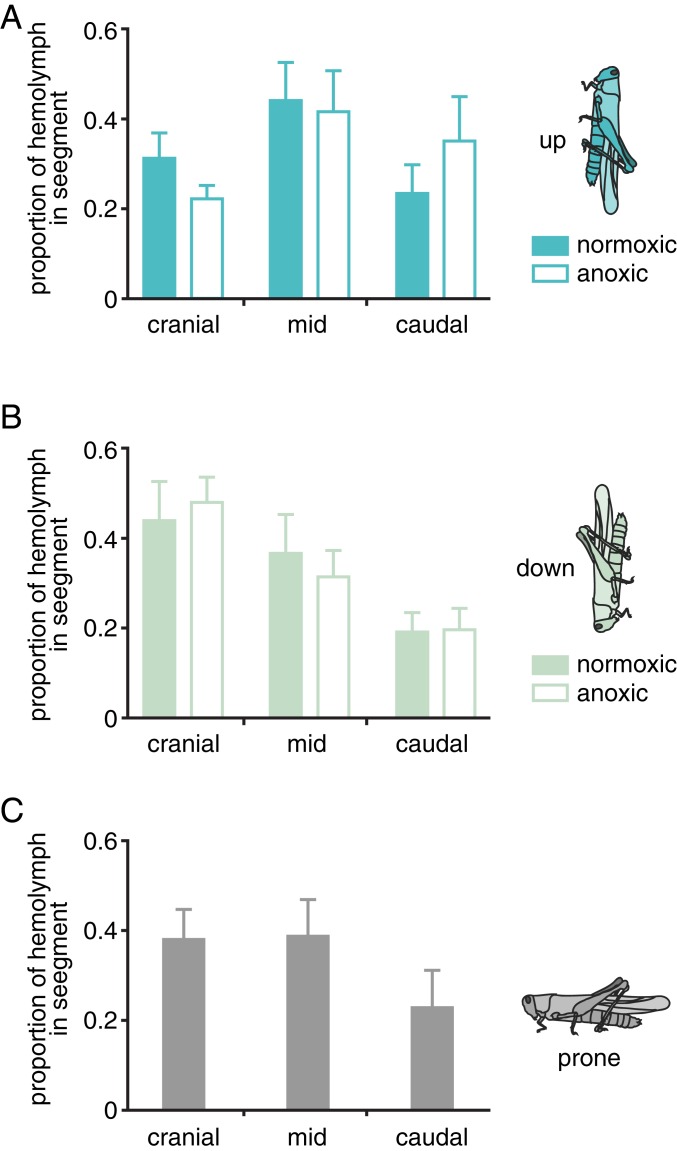
As predicted, grasshoppers in the head-down position had more blood in their cranial third and less in their middle and caudal thirds compared with grasshoppers in the head-up position (Fig. 2 and SI Appendix, Tables S4–S6). Treatment with N2-anesthesia amplified this effect, resulting in greater effects of body orientation on hemolymph distribution (Fig. 2 and SI Appendix, Table S7). Thus, changes in body orientation are driven by gravity-induced shifts in blood distribution that cause reciprocal shifts in tracheal air distribution. Furthermore, the result that gravity effects are greater in anesthetized animals suggests that unanesthetized animals actively resist gravity-driven shifts in hemolymph.
Fig. 2.
Body orientation is associated with downward movement of hemolymph. Proportion of hemolymph in S. americana in cranial, middle, and caudal thirds of the body, for animals in a head-up (A), head-down (B), or prone (C) position. Prone animals were only tested in normoxia. Orientation (head-up vs. head-down) significantly affected hemolymph distribution (3-way ANOVA with animal as a random factor, significant orientation*segment interaction; F1,96 = 7.40; P < 0.01; SI Appendix, Tables S4 and S5) and anoxia significantly affected hemolymph distribution in the head-up animals (2-way ANOVA; F1,34 = 16.53; P = 0.0003; SI Appendix, Table S7). n = 57 grasshoppers in total; N’s for specific treatments are shown in SI Appendix, Table S6).
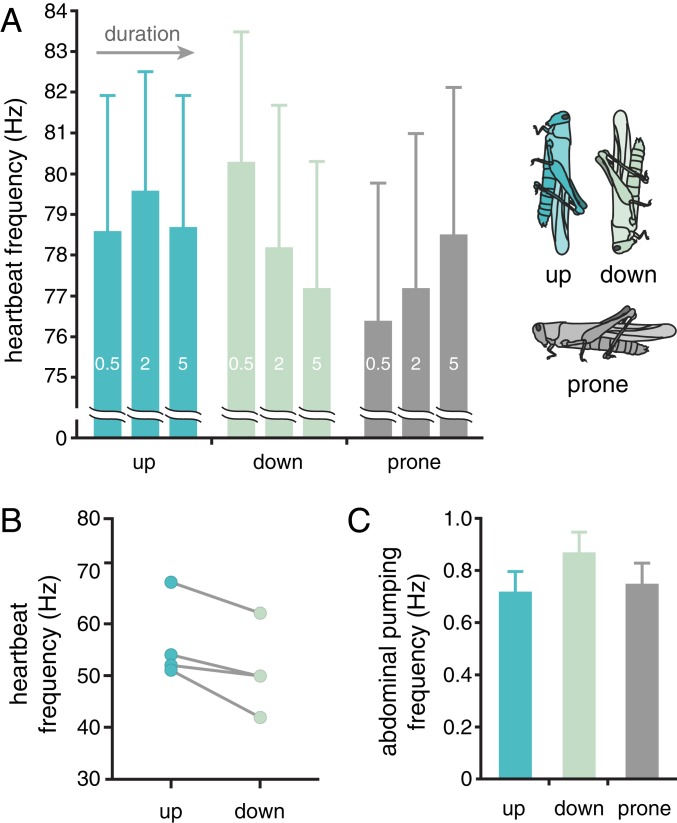
Vertebrates counteract gravity effects on blood partly by varying heart rates in response to baroreceptor cardiac reflexes (1). Because the heart of grasshoppers pumps primarily in a cranial direction (12), we hypothesized that heart rates would be higher in head-up than head-down positions to counteract gravity effects on hemolymph. We tested the effect of body orientation on heart rate by direct visualization of the heart through the cuticle (after removing the wings), using a microscope. We found that heart rates were significantly affected by orientation. Grasshoppers in a head-down position exhibited progressively lower heart rates with time (Fig. 3A), and head-down grasshoppers had lower heart rates than head-up grasshoppers after 5 min in position (Fig. 3A and SI Appendix, Table S8). We also measured heart rates for some of the same animals presented in Fig. 1 by analyzing synchrotron X-ray video of longitudinal tracheae adjacent to the heart, using a custom Python program; these tracheae exhibited a rhythmic pulsing corresponding to heart rate (SI Appendix, Fig. S3 and Movie S2). Again, heart rates were significantly higher for grasshoppers in the head-up position (Fig. 3B and SI Appendix, Table S9).
Fig. 3.
Effect of body orientation on heart and ventilation rates of grasshoppers. (A) Heart rates for grasshoppers placed into head-down, head-up, or prone position (in random order). Heart movements were observed with a microscope through the dorsal cuticle after removal of wings. Heart rate fell with time in the head-down but not other orientations (linear mixed effect model with individual as a random variable, significant orientation*time effect; F4,112 = 2.92; P = 0.024). Note that the large confidence limits in 3A reflect large interindividual variation; within animals, trends were more consistent (SI Appendix, Table S8). After 5 min, heart rate was significantly lower in the head-down than the head-up position (F2,118 = 3.42; P = 0.036). (B) Body orientation significantly affected heart rates of 4 locusts measured using synchrotron X-ray video of the pulses of the cardiac tracheae (paired t test, t1,3 = 3.52; n = 4; P = 0.036). (C) Ventilation rates were significantly affected by orientation (F2,96 = 4.23; P = 0.017, ANOVA with individual as random factor), with higher rates for animals in the head-down position.
The changes in air sac volumes with orientation suggest that body position might affect gas exchange. Indeed, changes in body orientation caused significant changes in ventilation rates, with grasshoppers in the head-down position having higher rates of abdominal pumping (Fig. 3C). Plausibly, compression of the head air sacs in head-down grasshoppers reduces gas exchange in the head, and the increase in ventilation rates in this position is a compensatory response.
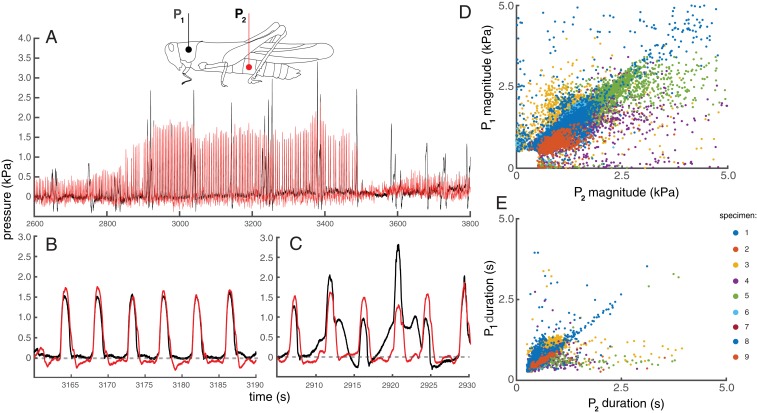
Vertebrates also use valves in their veins to resist gravity-driven blood flow (3, 5). Functional valving between body segments could provide a key active mechanism for insects to resist gravity effects. Classically, open circulatory systems are depicted as consisting of a single open hemocoel, although appendages and antennae are well-known to receive augmented circulation by accessory pumps (8, 13). However, grasshoppers appear to be able to compartmentalize pressures within various body regions, as evidenced by the observation that air sacs exhibit very different behaviors in the thorax and abdomen of grasshoppers (9). As a direct test of functional valving and compartmentalization, we simultaneously measured hemolymph pressures in the abdomen and thorax for alert, unanesthetized grasshoppers in various orientations. Grasshoppers produced regular pulses in pressures on the order of 1 to 10 kPa in both locations. In some cases, pressure pulses were strongly correlated and were equal in magnitude and duration, consistent with an open hemocoel between abdomen and thorax (Fig. 4B). However, often pressures in the thorax and abdomen differed strongly in magnitude and duration, and overall, pressures in the 2 compartments were only weakly correlated (Fig. 4 C–E and SI Appendix, Fig. S4 and Table S11), consistent with recent measurements in beetles (14). Body position (head up or head down) did not significantly affect hemolymph pressures in the thorax or abdomen, demonstrating that active regulatory mechanisms permit blood pressure regulation despite gravity-driven blood flow (SI Appendix, Figs. S5 and S6 and Table S12).
Fig. 4.
Comparison of hemolymph pressures in live grasshoppers. (A) Hemolymph pressures measured in the thorax and abdomen of a prone grasshopper over the course of 20 min. Timing and magnitude of thoracic and abdominal pressure pulses were sometimes highly congruent (B), and other times were quite different (C). Thoracic vs. abdominal pulse magnitudes (D) and durations (E) for 9 locusts (identified by color). Overall, thoracic and abdominal pulse characteristics were not well correlated. The median and modal R2 value for plots of thoracic vs. abdominal pulse magnitudes within individuals was 0.59 (SI Appendix, Table S11). For individual specimen plots, see SI Appendix, Fig. S4.
Discussion
Gravity has striking effects on many aspects of grasshopper physiology, including air sac inflation, hemolymph distribution, heart rates, and ventilation rates. The difference in air sac image content of the head is ∼20% (head down) vs. 75% (head up), with anoxia causing approximately a 30% further increase head air sac sizes (Fig. 1). Similarly, hemolymph content of the head approximately doubled in head-down relative to head-up animals, with anoxia causing a further 30% increase in the magnitude of the change (Fig. 2). Although the increased heart rate in head-up animals, regional pressure separation, and greater changes in tracheal and hemolymph systems during anoxia all suggest active regulatory processes that resist gravity effects, such regulatory systems are clearly unable to completely prevent gravity-driven hemolymph flow and the resulting changes in tracheal system morphology.
Gravity effects act on fluid and air flows within the insect despite ongoing processes that drive circulation, including heart function and pressure gradients between the thorax and abdomen that likely drive some fluid flow. Pressure gradients between the thorax and abdomen can be either positive or negative, and absolute values average 350 Pa (SI Appendix, Fig. S7). In theory, these pressure differences between the thorax and abdomen could result from differences in hydrostatic pressure related to height, viscous shear effects arising from hemolymph flow, or functional compartmentalization of the body. Because pressures were not significantly affected by body orientation (SI Appendix, Fig. S6), we conclude that hydrostatic pressure differences between the abdomen and thorax are not the dominant determinant of measured pressure differences. Precise calculations associated with viscous shear require a detailed understanding of the flow patterns and morphology inside the hemocoel, which are not available. However, we can assess whether it is likely that these pressure gradients could result from viscous loss associated with hemolymph flow using Hagen–Poiseuille theory. The pressure drop due to viscosity in a pipe is , where is dynamic viscosity (taken as 2.3 cP [15]), is average flow speed, is the length of a theoretical pipe between thorax and abdomen (taken as 1.5 cm), and is cross-sectional area of that pipe. To estimate the area, we consider a cylindrical grasshopper with a 5-mm radius, which would have a cross-sectional area of 79 mm2. Assuming that 20% of the area is hemolymph (this would appear to be a maximum possible value based on visual inspection of X-ray images), the cross-sectional area would be about 16 mm2. To generate the mean pressure gradient of 350 Pa by viscous flow would require a flow rate of 6 m⋅s−1, nearly 3 orders of magnitude above the maximum flow rate reported for the grasshopper heart (9.5 mm⋅s−1; ref. 16). Assuming that flow rates between the thorax and abdomen are no higher than the maximum flow rates within grasshopper hearts, the cross-sectional area between these segments would have to be constricted greatly to 0.024 mm2, a small value roughly the size of a medium-sized tracheal tube, for viscous shear to explain the pressure differences between thorax and abdomen. While these calculations have many uncertainties, these estimates support the hypothesis that the thorax and abdomen of insects can at times become partially or completely isolated as fluidic chambers. This hypothesis, known as functional compartmentalization, has also been suggested by 2 prior studies (9, 14).
While to our knowledge the effects of body orientation on volumes, blood flows, and pressures have not been studied in any other terrestrial invertebrates, it is likely that the patterns we observed for grasshoppers are widespread. Possibly such body orientation effects are restricted to insects with air sacs, as we found that the tracheae did not change in volume; even if so, air sacs occur broadly in many insect orders (17). However, the presence of functional compartmentalization of hemolymph pressures in beetles without air sacs (14) suggests that such insects may also exhibit mechanisms for counteracting such effects. We found similarly strong gravity effects on ∼60 mg third instar animals as in ∼1 g adults. However, it is plausible that responses to gravity effects are graded and decreasing in smaller insects. In land crabs, changes in hemolymph volume produce changes in both heart rate and the frequency of dorsoventral pumping in a manner compatible with baroreceptor reflex responses, suggesting that cardiovascular responses to gravity and orientation may also occur in crustaceans (18, 19).
The finding that body position strongly affects physiology raises questions about how gravity sensors are linked to physiological responses in insects. Such gravity sensors include the chordotonal organs, which can be distributed across the body, and Johnston’s organs, found in the antennae (20, 21). Conceivably, the response of these sensors could be linked to the control of cardiorespiratory functions, as occurs in the vestibular systems of vertebrates (22).
The cardiovascular systems of vertebrates and invertebrates demonstrate many homologies, including morphological similarities and widespread use of similar developmental genes (23). Developmental, physiological, and gene expression responses to variation in gravity have been observed in a wide variety of organisms, including plants, insects, mammals, and microbes (24–27). However, until now it has not been appreciated that invertebrates can provide systems-level as well as cellular models for gravity responses. Our study also provides a possibly general mechanism in insects and terrestrial arthropods: gravity-driven advective respiratory and circulatory transport. Future studies of terrestrial invertebrate physiology should control for variation associated with body orientation, explore the consequences of these gravity effects on function, and examine the molecular components of baroreceptor control mechanisms.
Materials and Methods
Experimental Design.
To test the effect of body orientation on air sacs and tracheae in the head and caudal abdomen, live grasshoppers (S. americana) were imaged with synchrotron X-rays in both the head-up and head-down orientation (in arbitrary order), conducted at beamline 32-ID at the Advanced Photon Source, Argonne National Laboratory. The proportion of the head or abdomen occupied by air sacs and tracheae was calculated by analyzing these videos (SI Appendix, Fig. S1). The head regions of other grasshoppers in a head-up or head-down position were imaged after animals were anesthetized with N2. In all cases, the effect of orientation was tested with ANOVA after proportions were logit-transformed to satisfy assumptions of normality. All statistical analyses were performed using R and the nmle and lme4 packages (28, 29).
To assess how body orientation affected the volume of the tracheal system throughout the body of grasshoppers, animals were killed in either the head-up or head-down position and imaged with microcomputed tomography. At 13 locations along the body, the proportion of the body occupied by the tracheal system was calculated from cross-sectional slices of the tomographic data. The hypothesis that orientation affects air distribution was tested by factorial ANOVA, using animal as a random factor, testing for an interactive effect of orientation and location on the proportion of that region occupied by the tracheal system.
To assess how body orientation affected hemolymph distribution, hemolymph was labeled using radiolabeled inulin. Animals were placed randomly in head-up, head-down, or prone positions, with some of the head-up and head-down grasshoppers anesthetized with nitrogen gas. After 10 min, animals were frozen in liquid N2 and cut into 3 pieces, and the hemolymph content of each third was measured by scintillation counting. The effect of body position and anesthesia on hemolymph distribution was tested by 3-way ANOVA, after logit-transforming proportions to achieve data normality, using individual as a random factor.
To measure effects of body orientation on heart rate, grasshoppers were mounted on a custom device that enabled rapid changes in body orientation while observing the heart through the dorsal cuticle with a microscope. Heart rates for each grasshopper were measured in the head-up, head-down, and prone positions, with order randomized. Heart rates were counted 3 times: 0.5, 2, and 5 min after moving the grasshopper into position. Data were analyzed with a linear mixed model, using individual as random factor. We were also able to measure heart rates in the head-up and head-down orientation for 4 of the grasshoppers used at Argonne National Laboratory by counting the pulsing of the cardiac tracheae; these data were analyzed by a paired t test.
To measure body orientation effects on ventilation rate, we measured abdominal pumping rate in 3 positions: prone, head-up, and head-down. Each individual was used once and acclimated to 1 of 3 prior positions (prone, head-up, or head-down) for at least 20 min. Then, its position was set to prone, head-up, or head-down; for animals remaining in the same position, the cylinder was handled so that stress effects would be comparable across the treatments. We tested for an effect of orientation and prior orientation on ventilation rates during the first minute and for the full 5 min after position change using a linear model with animal as a random factor.
For all dependent parameters, we first tested full linear mixed models including body mass, sex, and sampling time, but because these parameters never improved model fit, they are not reported here.
Animals.
S. americana Drury were maintained and X-ray imaged as previously described (30) at the Advanced Photon Source, Argonne National Laboratory, using settings that minimized harm (31). Briefly, monochromatic X-rays (E = 25 keV) and a sample-to-scintillator distance of 0.7 m were used to create phase-contrast enhanced images, which were recorded with a videocamera (Cohu 4920, Cohu, San Diego, CA). We used adults, both males and females (0.81 to 1.13 g), as well as third instars (0.078 to 0.140 g). Other experiments (hemolymph distribution, visually-observed heart rate, abdominal pumping rate) were conducted at Arizona State University, and others (hemolymph pressure, microtomography) were conducted at Virginia Tech. At Arizona State University and Virginia Tech, air temperatures were 25 ± 1 °C and 23 ± 1 °C, within 3 °C of temperatures at the Advanced Photon Source (which averaged 23 °C).
Effects of Body Orientation and Anesthesia on Air Sacs and Tracheae (Synchrotron X-Ray Imaging of Live Grasshoppers).
For synchrotron X-ray imaging, animals were placed in X-ray translucent polyimide film containers (Kapton, Dupont) that allowed perfusion of N2 for anesthesia (Movie S1). Body orientation (head-up, head-down, prone) was adjusted using externally controlled stepper motors, allowing us to obtain X-ray videos within approximately 5 min of transferring animals to a new orientation. Simultaneous visible-light and X-ray video data were recorded and synchronized as previously described (9), allowing us to confirm that animals were quiescent during X-ray recordings. Body orientations were examined in arbitrary order. We recorded video for approximately 5 min in each orientation, scanning the grasshopper to obtain video of the entire animal.
We obtained X-ray videos of the head and caudal abdomen for 5 adults and 7 third-instar grasshoppers in both head-up and head-down orientations. Afterward, we anesthetized grasshoppers by perfusing N2 through their chambers; we waited for 5 min after they were motionless and again captured X-ray video of their heads. For the effects of anesthesia, we obtained images of heads from 5 adults in head-up and head-down positions. Video frames were captured on tape and analyzed with ImageJ software (32). The outline of the animal, air sacs, and tracheae (defined as having a tubular form and lengths more than 5× diameter) were traced in ImageJ, and the proportion of the area of the body within each image occupied by air sacs and tracheae was calculated (SI Appendix, Fig. S1). For head images of adults, the field of view covered about one-third of the head; we focused all views on the central head, between the eyes, keeping the eyes out of the field of view. For the caudal abdomen images of adults, the field of view included the epiproct and approximately the most posterior 2 abdominal segments (Fig. 1A). For juveniles, the field of view for the head included the entire head; for the caudal abdomen view, we analyzed approximately the back third of the abdomen. We analyzed 1 to 3 views per individual per position, and averaged values when multiple views were measured. Three of the third instars lacked any air sacs in the head, and so were excluded from the air sac analysis. Proportions were logit-transformed (33), and the interaction between orientation and region was analyzed using a 2-way ANOVA with individual as a random factor, using the aov function in R, as we had data for each individual in each condition. For analyzing the effect of N2 anesthesia on air sacs in the head, we used a 2-way ANOVA, as different individuals were measured at each condition.
Tomography of Recently Killed Grasshoppers in Head-Up and Head-Down Positions.
Adult grasshoppers (n = 8; mass = 1.75 ± 0.21 g) were killed and imaged using a benchtop microcomputed tomography scanner (Skyscan 1172, Bruker). Grasshoppers were placed in 6.35-mm-diameter, 0.007-mm-thick polyimide tubes (Kapton, Dupont) and surrounded in thick multipurpose automotive grease (Valvoline, Lexington KY) to minimize specimen motion during imaging. We took special care to keep at least 1 of the thoracic spiracles unobstructed by grease, so that the internal tracheal lumen remained at ambient pressure. The specimens were imaged in both head-up and head-down orientations to observe gravity effects on internal morphology. Scans were taken with a camera-to-specimen distance of 113.7 mm and a nominal image resolution of 23.8 µm/pixel. Reconstructed images were 1,024 × 1,024 pixels, representing a field of view of 13.8 × 13.8 mm and 10.8 µm/pixel resolution. Images of the insect’s cross-section were selected at 13 equidistant points along each specimen’s body. Each image was then digitally manipulated using ImageJ (34) to exclude grease and the Kapton tube, leaving only the anatomical features of the internal morphology (SI Appendix, Fig. S2). The ratio of tracheal volume to overall body volume was calculated by determining the amount of air-filled area inside the body (representing the tracheae and air sacs) and dividing by the overall body outline area in pixels at the 13 equidistant points along the body (Fig. 2). At each of the 13 locations, 10 consecutive cross-sectional images were measured (representing a total span of 0.11 mm along the body), and the average taken as the representative value for that location. We tested for a significant interaction between location and orientation using a 2-way ANOVA with individual as a random factor, using the aov function in R.
Effect of Body Orientation on Hemolymph Distribution.
To test whether body position affected the distribution of hemolymph between head and abdomen, we injected 10 µL grasshopper Ringer’s (35) containing 0.25 mC ml−1 3H-methoxy inulin (Perkin-Elmer, Waltham, MA) into the hemocoel of adult grasshoppers, using a Hamilton microsyringe through the soft tissue between abdominal terga 2 and 3. Thirty to 60 min later, grasshoppers were placed behind an opaque sheet in the head-up or head-down position within plastic tubes that prevented them from altering their orientation, either in normal air or anesthetized with a continuous stream of N2 gas. An additional group of animals was placed in a prone position (ventral side down), but only in normoxia. Approximately 15 min later, animals were frozen in liquid nitrogen and cut into thirds (cranial, middle, caudal), and the carcass segments pulverized under liquid nitrogen with a mortar and pestle. Hemolymph and pulverized tissue samples were homogenized in detergent-containing simple grasshopper saline (100 mM sucrose, 150 mM NaCl, 15 mM glycine, 5 mM CaCl, 10 mM MgSO4, 10 mM NaHCO3, 0.2% TritonX). After centrifugation, 3H-counts of 100 µL aliquots of supernatants were measured using scintillation mixture and a Beckman liquid scintillation counter. Preliminary experiments demonstrated that levels of 3H-methoxy inulin measured in samples of hemolymph obtained from a cut in the cervix (neck) region were stable 0.25 to 12 h after injection. We analyzed effects of orientation on hemolymph distribution using a 3-way ANOVA, with animal as a random factor.
Effect of Body Orientation on Heart Rate.
The heart is suspended by the alary muscles within a membrane that includes the paired longitudinal cardiac tracheae. Heart rates of grasshoppers visualized with X-ray could be counted from the pulsing of these tracheae (Movie S2). We used a custom Python program to count the beats (SI Appendix, Fig. S3), and tested for an effect of orientation on the heart rates for the 4 grasshoppers that had clear heart images in both a head-up and head-down orientation using a paired t test.
We also examined the effect of body orientation on heart rate using a separate group of grasshoppers in the laboratory at Arizona State University by direct counting of heart rate through the dorsal cuticle. The wings were trimmed from adult male S. americana, with an age approximately 1 mo past adulthood. Animals were lightly restrained in a custom cotton cylinder and mounted within a clear plastic tube, with cotton packing obscuring the grasshopper’s eyes to reduce the possibility that they could visually sense the human observer. Five grasshoppers were mounted on a rack that could be easily rotated so that grasshoppers could be placed into a prone, head-up, or head-down position. Heartbeats could be observed through a translucent part of the cuticle on the thorax between the bases of the 2 wings, using a dissecting microscope mounted horizontally when measuring head-up or head-down rates and vertically for measuring heart rates of prone grasshoppers. Grasshoppers were first placed in a prone position for 5 min and then rotated to a head-down orientation for 5 min and then to a head-up orientation for 5 min. Heart rates were counted for 15 s immediately after the rotation and 2 and 5 min after the position change. Data were analyzed using a paired t test.
Effect of Body Orientation on Abdominal Pumping Rate.
Grasshoppers were placed in plastic transparent cylinders, with their translation restricted by cotton balls placed at the cranial and caudal ends. Opaque tape covered the cylinder in the head region to prevent visual disturbance of the grasshopper, but the abdomen was clearly visible and free to move. Each individual was used once and acclimated to 1 of 3 prior positions: prone, head-up, or head-down, for at least 20 min. Then, its position was set to either prone, head-up, or head-down; for animals remaining in the same position, the cylinder was handled so that stress effects would be comparable across the treatments. Abdominal pumping rate was observed for 1 min at the prior position and for 5 min after its position change, with pump cycles recorded using a custom-made electronic counter connected to a data acquisition device (UI2, Sable System International, Las Vegas, NV). Specifically, a pump cycle was noted whenever the abdomen compressed (expiration). For abdominal pumping recording and analysis, we used ExpeData software (UI2, Sable System International, Las Vegas, NV). We tested for significant effects on ventilation rates, using linear mixed models with individual as a random factor, using the nlme function in R. Because there was no significant effect of prior orientation or sex, and no significant interaction between orientation and prior orientation on ventilation rates, we simply reported the significant orientation effects.
Measurement of Pressures in Thoracic and Abdominal Compartments.
To examine the effect of body orientation on hemolymph pressures, pressure transducers were inserted into the hemocoel at 2 different locations in the hemocoels of adult grasshoppers (n = 9; length: 44.25 ± 1.31 mm; mass: 1.50 ± 0.15 g). Pressure measurements and analyses follow previously described methods (14). Hemocoel pressures were measured with two 420-µm-diameter Fabry–Pérot fiberoptic pressure sensors (TSD174A, Samba Sensors, Sweden) connected to a control unit (Samba 202, MPMS100A-2, Samba Sensors). To insert the sensors, 2 small holes were drilled (using a 1-mm-diameter bit) along the midaxillary line of the thorax and abdomen to a depth of ∼2 mm. The drilled volume was cleared of tissue with a probe and allowed to fill with hemolymph, and then the sensor was inserted. To ensure that no contact with tissue occurred during recordings, the sensors were slowly pushed into the hole until a slight resistance was encountered (assumed to be tissue), and then the sensor was pulled back approximately half the total distance. During this process, the pressure signal was monitored; pressures spiked to very high values (>∼35 kPa) when tissue was encountered and then fell once the sensor was pulled back. Once inserted, the holes were rapidly self-sealing due to coagulation effects of the hemolymph in contact with air. The grasshopper was then secured to a custom 3D-printed mount, with long slits along the floor and openings along the back wall to allow the legs to protrude and for the body to be secured while also allowing the abdomen to move laterally and pump freely. The walls were designed to be more than double the height of the animal, minimizing visual stimuli and reducing animal movement. The mount also served as a mounting location to anchor the pressure sensors and minimize aberrant pressure signals from sensor motion. The mount was secured to a rotatable platform, allowing the insect to be oriented in different positions.
Grasshoppers were cold anesthetized (∼4 °C) during and for 30 min after sensor implantation. Then grasshoppers were allowed an additional 30 min to warm to room temperature. Once acclimated, grasshoppers were cycled among prone, head up, prone, and head down manipulations, with each position held for 30 min. The order of the first manipulation after the prone position was chosen at random. Pressure traces were analyzed using a custom code (Matlab, Natick MA) to identify individual pulse times, durations, and magnitudes. Individual pressure pulses were compared directly in the 2 different locations within the hemocoel.
To test the role of gravity in causing hemolymph pressure changes with alterations in body orientation, we compared our measured pressures to calculated expected values based on the change in fluid height above the sensor after orientation changes, using P = ρgh, where P is pressure, ρ is hemolymph density (using the value reported for Manduca sexta larvae, 1.02 ± 0.03 g⋅mL−1 [15]), g is gravitational acceleration (9.81 m⋅s−2), and h is height (SI Appendix, Fig. S5). These calculations assume that hemolymph pressures reflect static fluidic pressures only. This assumption neglects contributions from possible flows of hemolymph, which would entail an additional dynamic pressure component, calculated as Pd = 1/2ρv2, where ρ is hemolymph density and v is flow speed. To provide a quantitative evaluation of this assumption requires knowledge of hemolymph flow speeds within the hemocoel, but such measurements are not available for any insect. However, flow speeds in the hemolymph are unlikely to be higher than the fast flows produced in the heart, which have been measured for this species (16). Using the maximum reported speed of flow in the grasshopper heart, 9.5 mm/s, the corresponding dynamic pressure would be 0.046 Pa. The pressure changes and differences across segments recorded in the hemocoel in this study were much greater, on order of 1 kPa for pressure pulses, and on order of 50 Pa for differences during body orientation trials, suggesting that the contribution of dynamic pressure to the total hemolymph pressure is negligible.
Data Materials and Availability.
All data used in this manuscript are available in the manuscript and SI Appendix.
Supplementary Material
Acknowledgments
Elizabeth Alanis helped analyze the X-ray images, and Alex Deriy was helpful with setup for synchrotron X-ray imaging. We thank Jessica Aviles and Jacob Sieve for help with tomography experiments. This research was supported by NSF Integrative Organismal Systems (IOS) 1558052 and NSF Emerging Frontiers in Research and Innovation (EFRI) 0938047. Use of the Advanced Photon Source, an Office of Science User Facility operated for the US Department of Energy Office of Science by Argonne National Laboratory, was supported by the US Department of Energy under Contract No. DE-AC02-06CH11357.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1915424117/-/DCSupplemental.
References
- 1.Pilowsky P. M., Goodchild A. K., Baroreceptor reflex pathways and neurotransmitters: 10 years on. J. Hypertens. 20, 1675–1688 (2002). [DOI] [PubMed] [Google Scholar]
- 2.Lillywhite H. B., Donald J. A., Neural regulation of arterial blood pressure in snakes. Physiol. Zool. 67, 1260–1283 (1994). [Google Scholar]
- 3.Lillywhite H. B., Circulatory adaptations of snakes to gravity. Am. Zool. 27, 81–95 (1987). [Google Scholar]
- 4.Seymour R. S., Hargens A. R., Pedley T. J., The heart works against gravity. Am. J. Physiol. 265, R715–R720 (1993). [DOI] [PubMed] [Google Scholar]
- 5.Hicks J. W., Badeer H. S., Gravity and the circulation: “open” vs. “closed” systems. Am. J. Physiol. 262, R725–R732 (1992). [DOI] [PubMed] [Google Scholar]
- 6.Full R. J., Tullis A., Energetics of ascent: Insects on inclines. J. Exp. Biol. 149, 307–317 (1990). [DOI] [PubMed] [Google Scholar]
- 7.Harrison J. F., Woods H. A., Roberts S. P., Ecological and Environmental Physiology of Insects (Oxford University Press, New York, 2012). [Google Scholar]
- 8.Chapman R. F., Simpson S. J., Douglas A. E., The Insects: Structure And Function (Cambridge Press, New York: ), ed. 5, 2013). [Google Scholar]
- 9.Harrison J. F., et al. , How locusts breathe. Physiology (Bethesda) 28, 18–27 (2013). [DOI] [PubMed] [Google Scholar]
- 10.Webster M. R., De Vita R., Twigg J. N., Socha J. J., Mechanical properties of tracheal tubes in the American cockroach (Periplaneta americana). Smart Mater. Struct. 20, 094017 (2011). [Google Scholar]
- 11.Harrison J. M., Temperature effects on intra- and extracellular acid-base status in the American locust, Schistocerca nitens. J. Comp. Physiol. B 158, 763–770 (1988). [Google Scholar]
- 12.Sinclair B. J., et al. , Synchrotron x-ray visualisation of ice formation in insects during lethal and non-lethal freezing. PLoS One 4, e8259 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pass G., Accessory pulsatile organs: Evolutionary innovations in insects. Annu. Rev. Entomol. 45, 495–518 (2000). [DOI] [PubMed] [Google Scholar]
- 14.Pendar H., Aviles J., Adjerid K., Schoenewald C., Socha J. J., Functional compartmentalization in the hemocoel of insects. Sci. Rep. 9, 6075 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kenny M. C., Giarra M. N., Granata E., Socha J. J., How temperature influences the viscosity of hornworm hemolymph. J. Exp. Biol. 221, jeb186338 (2018). [DOI] [PubMed] [Google Scholar]
- 16.Lee W. K., Socha J. J., Direct visualization of hemolymph flow in the heart of a grasshopper (Schistocerca americana). BMC Physiol. 9, 2 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harrison J. F., Wasserthal L. T., Chapman R. F., “Gaseous exchange” in The Insects: Structure and Function, Simpson S. J., Douglas A. E., Eds. (Cambridge University Press, New York, 2013), pp. 501–545. [Google Scholar]
- 18.Wilkens J. L., Young R. E., Regulation of pulmonary blood flow and of blood pressure in a mangrove crab (Goniopsis cruentata). J. Exp. Biol. 162, 297–316 (1992). [Google Scholar]
- 19.Burggren W. W., Pinder A. W., McMahon B. R., Doyle M., Wheatly M., Heart rate and hemolymph pressure responses to hemolymph volume changes in the land crab Cardisoma guanhumi–Evidence for baroreflex regulation. Physiol. Zool. 63, 167–181 (1990). [Google Scholar]
- 20.Beckingham K. M., Texada M. J., Baker D. A., Munjaal R., Armstrong J. D., “Genetics of graviperception in animals” in Advances in Genetics (Academic Press, 2005), vol. 55, pp. 105–145. [DOI] [PubMed] [Google Scholar]
- 21.Matsuo E., Kamikouchi A., Neuronal encoding of sound, gravity, and wind in the fruit fly. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 199, 253–262 (2013). [DOI] [PubMed] [Google Scholar]
- 22.Lan Y., et al. , Additive role of the vestibular end organ and baroreceptors on the regulation of blood pressure in rats. Korean J. Physiol. Pharmacol. 17, 367–373 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monahan-Earley R., Dvorak A. M., Aird W. C., Evolutionary origins of the blood vascular system and endothelium. J. Thromb. Haemost. 11 (suppl. 1), 46–66 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herranz R., et al. , Spaceflight-related suboptimal conditions can accentuate the altered gravity response of Drosophila transcriptome. Mol. Ecol. 19, 4255–4264 (2010). [DOI] [PubMed] [Google Scholar]
- 25.Hoson T., Wakabayashi K., Role of the plant cell wall in gravity resistance. Phytochemistry 112, 84–90 (2015). [DOI] [PubMed] [Google Scholar]
- 26.Hoson T., Plant growth and morphogenesis under different gravity conditions: Relevance to plant life in space. Life (Basel) 4, 205–216 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kohn F. P. M., Ritzmann R., Gravity and neuronal adaptation, in vitro and in vivo-from neuronal cells up to neuromuscular responses: A first model. Eur. Biophys. J. 47, 97–107 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Team R. C., R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna, Austria, 2013). [Google Scholar]
- 29.Pinheiro J., Bates D., DebRoy S., Sarkar D.; R. C. Team , nlme: Linear and Nonlinear Mixed Effects Models. (R package version 3.1-131, 2017).
- 30.Greenlee K. J., et al. , Synchrotron imaging of the grasshopper tracheal system: Morphological components of tracheal hypermetry and the effect of age and stage on abdominal air sac volumes and convection. Am. J. Physiol. Regul. Integr. Comp. Physiol. 297, R1343–R1350 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Socha J. J., Westneat M. W., Harrison J. F., Waters J. S., Lee W. K., Real-time phase-contrast x-ray imaging: A new technique for the study of animal form and function. BMC Biol. 5, 6 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rasband W. S., ImageJ (U. S. National Institutes of Health, Bethesda, Maryland, 2012). [Google Scholar]
- 33.Warton D. I., Hui F. K. C., The arcsine is asinine: The analysis of proportions in ecology. Ecology 92, 3–10 (2011). [DOI] [PubMed] [Google Scholar]
- 34.Schneider C. A., Rasband W. S., Eliceiri K. W., NIH image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanrahan J. W., Meredith J., Phillips J. E., Brandys D., “Methods for the study of transport and control in insect hindgut” in Measurement of Ion Transport and Metabolic Rate in Insects, Bradley T. J., Miller T. A., Eds. (Springer-Verlag, New York, 1984), pp. 19–67. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.