Significance
Although resistance to glucocorticoids is a major clinical problem, the underlying mechanisms are unknown. It is known that glucocorticoid use can suppress adrenal androgen production. In population studies, animal models, and cell culture experiments, androgens are associated with several benefits in asthma, but neither androgen use in glucocorticoid-resistant asthma nor the genetic determinants of androgen responsiveness have been studied in humans. A missense-encoding variant in HSD3B1 is known to regulate conversion from adrenal precursors to potent androgens and clinical outcomes in prostate cancer. This is the first genetic evidence to our knowledge that implicates an androgen synthesis variant in resistance to glucocorticoids for asthma or any other inflammatory disease. Furthermore, this study demonstrates an adverse consequence of adrenal androgen suppression with glucocorticoid therapy.
Keywords: steroids, glucocorticoids, inflammation, androgens, HSD3B1
Abstract
Asthma resistance to glucocorticoid treatment is a major health problem with unclear etiology. Glucocorticoids inhibit adrenal androgen production. However, androgens have potential benefits in asthma. HSD3B1 encodes for 3β-hydroxysteroid dehydrogenase-1 (3β-HSD1), which catalyzes peripheral conversion from adrenal dehydroepiandrosterone (DHEA) to potent androgens and has a germline missense-encoding polymorphism. The adrenal restrictive HSD3B1(1245A) allele limits conversion, whereas the adrenal permissive HSD3B1(1245C) allele increases DHEA metabolism to potent androgens. In the Severe Asthma Research Program (SARP) III cohort, we determined the association between DHEA-sulfate and percentage predicted forced expiratory volume in 1 s (FEV1PP). HSD3B1(1245) genotypes were assessed, and association between adrenal restrictive and adrenal permissive alleles and FEV1PP in patients with (GC) and without (noGC) daily oral glucocorticoid treatment was determined (n = 318). Validation was performed in a second cohort (SARP I&II; n = 184). DHEA-sulfate is associated with FEV1PP and is suppressed with GC treatment. GC patients homozygous for the adrenal restrictive genotype have lower FEV1PP compared with noGC patients (54.3% vs. 75.1%; P < 0.001). In patients with the homozygous adrenal permissive genotype, there was no FEV1PP difference in GC vs. noGC patients (73.4% vs. 78.9%; P = 0.39). Results were independently confirmed: FEV1PP for homozygous adrenal restrictive genotype in GC vs. noGC is 49.8 vs. 63.4 (P < 0.001), and for homozygous adrenal permissive genotype, it is 66.7 vs. 67.7 (P = 0.92). The adrenal restrictive HSD3B1(1245) genotype is associated with GC resistance. This effect appears to be driven by GC suppression of 3β-HSD1 substrate. Our results suggest opportunities for prediction of GC resistance and pharmacologic intervention.
Since their discovery and introduction into clinical medicine about 70 y ago, glucocorticoids (GCs) have been recognized as eliciting a systemic antiinflammatory response, and currently play a major role in the treatment of severe asthma and other inflammatory disease processes (1). However, unresponsiveness to GC treatment is a major barrier to treatment of inflammatory disease processes, and the underlying mechanisms of this clinical entity have yet to be clearly elucidated (2). Management of GC-unresponsive disease represents a significant challenge for the treatment of asthma. Indeed, severe asthma is generally defined as asthma that remains symptomatic despite high-dose inhaled GC and/or systemic GC therapy (3).
Suppression of endogenous adrenal androgens and cortisol is a known consequence of systemic GC treatment. Substantial evidence suggests that stimulation of the androgen receptor (AR) expressed in the lung could be beneficial in asthma. Androgens inhibit human airway smooth muscle and fibroblast proliferation (4–6), promote airway smooth muscle relaxation (7), and inhibit both Th2 and Th1 inflammation in animal models of asthma (8, 9). Androgens are associated with better lung function in large healthy cohorts (10, 11) and in asthma (12). Increasing circulating levels of adrenal and gonadal androgens in males and females during adolescence are associated with improving asthma during adolescence (12). However, the role of GC-induced androgen suppression in the pathophysiology of severe, GC-resistant human asthma is not established.
The androgen dehydroepiandrosterone (DHEA) and its sulfate (DHEA-S) are secreted from the adrenal reticularis (13, 14), and together are the most abundant steroid in circulation. However, its function is not known. DHEA-S may have an immunomodulatory effect, but clinical results have been inconsistent, possibly because variations in DHEA-S metabolism are generally not taken into account (15). In peripheral tissues, DHEA is metabolized by the enzyme 3β-hydroxysteroid dehydrogenase-1 (3β-HSD1; encoded by HSD3B1) to potent downstream androgens (e.g., testosterone and dihydrotestosterone). A common missense-encoding variant in HSD3B1, rs1047303 (c.1245C > A, p.T367N), regulates biochemical function and clinical phenotypes. The HSD3B1(1245A) allele encodes for an adrenal restrictive enzyme that limits conversion from DHEA to downstream androgens, whereas the HSD3B1(1245C) allele encodes for an adrenal permissive enzyme that results in increased metabolic flux from DHEA to more potent androgens (16). Multiple studies from the United States, Japan, and Spain, now further confirmed in a phase 3 clinical trial, clearly show that men with advanced prostate cancer treated with castration who inherit the adrenal permissive 3β-HSD1 enzyme, which confers more rapid conversion from DHEA to potent androgens, have more rapid onset of androgen-driven disease progression (17–22). These studies establish that clear clinical phenotypes are associated with HSD3B1 genotypes that biochemically confer fast and slow metabolic flux to potent androgens. We hypothesized that the restrictive HSD3B1 genotype that disables DHEA-S conversion to potent androgens impairs FEV1PP specifically when GC treatment suppresses adrenal DHEA-S production, limiting substrate availability for 3β-HSD1 and possibly providing a mechanistic explanation for GC-resistant severe asthma in patients with this genotype.
Severe Asthma Research Programs (SARP) are comprehensive networks sponsored by the National Institutes of Health/National Heart, Lung, and Blood Institute that aimed to characterize and understand the pathobiology of severe asthma. Here, we determined FEV1PP in the SARP III cohort for patients with asthma treated with (GC) and without (noGC) daily oral GCs. Confirmatory studies were performed in patients with severe asthma from the SARP I&II study.
Methods
Subjects.
Individuals included in this analysis comprised a subset of 318 Caucasian (from 488 patients of all races) adults (aged >18 y) with asthma enrolled in the SARP III study for whom genotyping data and pulmonary function testing were available. We only included Caucasian patients in our study because of the numbers of patients available for analysis and the higher adrenal permissive HSD3B1(1245C) allele frequency of 30% to 40% in Caucasians, as opposed to ∼8% in people of African descent.
SARP III, a network of 11 clinical research centers across the United States, recruited 713 participants with asthma between 1 November 2012, and 1 October 2015. Of those 713 initially recruited participants with asthma, 526 were adults (aged >18 y), 60% had severe asthma as defined by the European Respiratory Society/American Thoracic Society criteria (3), and 17% (21% of adult Caucasians) were chronically treated with chronic oral and/or injectable GCs (i.e., systemic). The remaining 40% of patients with asthma did not meet the criteria for severe asthma and were classified as having nonsevere asthma. Among the 89 (17%) adult subjects treated with systemic GCs in SARP III, 55 received daily oral GCs only, 15 received daily oral GCs with additional GC injections, and 19 received intermittent GC injections alone. In the group that received intermittent GC injections alone, the majority (14 participants) received 1 to 3 injections per year. The rest (5 subjects) received more than 6 injections per year. While the suppressive effect of GCs is predictable with chronic daily GC use, intermittent GC injections might have an inconsistent effect on adrenal suppression, and accordingly, participants receiving such therapy were excluded from the analysis. Patients were also excluded if they were pregnant or breast-feeding during the initial characterization period, had a history of premature birth (<35 wk gestation), or had a diagnosis of any other chronic pulmonary disorder. All patients were not active smokers (past smokers should not have smoked within a year or exceeded 10 pack-years of tobacco use if >30 y of age, or <5 pack-years if <30 y of age) and were required to have evidence of bronchial hyperresponsiveness (defined as a PC20 methacholine value <16 mg/mL) or reversible airflow obstruction, as evidenced by an increase in FEV1 of 12% or greater after albuterol inhalation, ipratropium bromide inhalation, or both. Spirometry was performed according to the American Thoracic Society/European Respiratory Society guidelines (23, 24). The 2012 Global Lung Initiative standard reference equations were used to predict spirometric reference values (25). DHEA-S levels were analyzed at the University of Virginia Center for Research in Reproduction Ligand Core Laboratory, using the Siemens Immulite 2000 immunoassay system, which has a lower limit of detection for DHEA-S of 15 μg/dL Information on SARP III network, protocol, and characterization procedures have been published previously (26–28). All participants provided written informed consent. The institutional review board at each center approved the study. The study is listed on ClinicalTrials.gov.
To replicate primary findings, 184 Caucasian participants (from a total of 263 of all races) with severe asthma were selected from the SARP I&II cohorts. Similarly, SARP I&II is a National Institutes of Health/National Heart, Lung, and Blood Institute-sponsored multicenter study that recruited patients with asthma between 2001 and 2012 from 9 sites in the United States and 1 in the United Kingdom. However, as compared with SARP III, patients with asthma enrolled in SARP I&II were less likely to have severe asthma (40% vs. 60%) (28–30). In contrast to SARP III, severe asthma was defined in SARP I&II according to the initial ATS workshop definition of severe asthma (31).
Statistical Analysis.
Whole-genome sequence of 1,888 patients enrolled in SARP I, II, and III was released by the Trans-Omics for Precision Medicine (TOPMed) program (https://www.nhlbiwgs.org/) in its genotype call sets freeze 6a version. Whole-genome sequence with a read depth of 38× was performed on blood DNA, using Illumina HiSeq X technology. The TOPMed freeze 6a genotype call set includes 107,047 samples and 642M high-quality variants genomewide, >7.6M coding variants, and >350,000 loss-of-function variants. Genotype calling and quality control were performed by the Informatics Research Center, led by the University of Michigan.
Genotypes for variant rs1047303 (position chr1:119514623 [build GRCh38.p12]) in SARP I, II, and III were extracted with PLINK2 (32, 33) (https://www.cog-genomics.org/plink2). HSD3B1 genotypes were directly confirmed in 28 patients, using a method previously validated with 100% match (17).
Student’s t test was used for 2 group comparisons of continuous normally distributed variables. The means of the 3 different genotype groups were compared using the ANOVA test. Pairwise comparisons were performed using the Tukey–Kramer Honest Significant Differences (HSD) test. Otherwise, Wilcoxon’s rank sum test or Kruskal–Wallis one-way ANOVA were used when normality assumptions were not met. Categorical variables were compared using a χ2 test. We assumed an additive model of inheritance, in which predictor variables were an additive effect for the rs1047303 genotype (coded as the number of C alleles). Interaction terms were included between rs1047303 genetic variants and daily oral GC as the dependent variables of the prebronchodilator FEV1PP (pre-FEV1PP) post-FEV1PP. Models were fit under the assumption of a normal distribution for FEV1PP. All statistical analyses were conducted with R, version 3.5.3 (R Project for Statistical Computing, Vienna, Austria). A P value < 0.05 was considered statistically significant because only one position was tested.
Data Availability.
TOPMed genomic data and preexisting parent study phenotypic data are made available to the scientific community in study-specific accessions in the database of Genotypes and Phenotypes (https://www.ncbi.nlm.nih.gov/gap/).
Results
Baseline Characteristics.
Baseline characteristics of Caucasian SARP III participants with asthma and SARP I&II participants with severe asthma are listed in Table 1. Aside from higher body mass index (BMI) in participants with the CC compared with the AA genotype enrolled in SARP III, patients with the AA, AC, and CC genotypes had similar baseline characteristics. Other than the CC genotype being associated with higher FEV1/FVC ratio compared with AA and AC, all other characteristics were not significantly different in participants with severe asthma enrolled in SARP I, II, or III.
Table 1.
Baseline characteristics comparing the 2 SARP cohorts, SARP III vs. SARP I&II, stratified by HSD3B1(1245) genotypes*
| SARP III | SARP I& II | |||||||
| AA | AC | CC | P value | AA | AC | CC | P value | |
| N | 146 | 131 | 41 | 80 | 79 | 25 | ||
| Age, y | 49.5 ± 14.5 | 48.7 ± 13.8 | 47.8 ± 14.2 | 0.762 | 43.3 ± 11.2 | 46.3 ± 13.6 | 42.7 ± 13.7 | 0.26 |
| Female sex, n (%) | 92 (63.0) | 80 (61.1) | 31 (75.6) | 0.230 | 46 (57.5) | 47 (59.5) | 20 (80.0) | 0.11 |
| BMI† | 30.2 ± 6.2 | 31.8 ± 8.6 | 33.4 ± 9.8 | 0.038 | 30.3 ± 7.2 | 30.5 ± 7.5 | 29.9 ± 6.9 | 0.93 |
| Daily oral GC therapy, n (%) | 23 (15.8) | 24 (18.3) | 8 (19.5) | 0.787 | 35 (43.8) | 30 (38.0) | 10 (40.0) | 0.76 |
| Severe asthma, n (%)‡ | 82 (56.2) | 77 (58.8) | 20 (48.8) | 0.530 | 80 (100) | 79 (100) | 25 (100) | |
| FEV1/FVC ratio | 0.84 ± 0.12 | 0.84 ± 0.12 | 0.90 ± 0.10 | 0.011 | 0.61 ± 0.13 | 0.64 ± 0.12 | 0.71 ± 0.11 | 0.026 |
| Pre-FEV1, % of predicted value | 71.8 ± 19.3 | 72.6 ± 21.8 | 77.9 ± 16.3 | 0.224 | 57.4 ± 19.2 | 61.1 ± 21.6 | 67.3 ± 23.7 | 0.09 |
| Post-FEV1, % of predicted value | 80.8 ± 20.2 | 80.3 ± 20.8 | 88.0 ± 16.5 | 0.083 | 73.5 ± 19.2 | 76.3 ± 20.7 | 79.3 ± 24.8 | 0.43 |
GC, daily oral glucocorticoid therapy; FVC, forced vital capacity; Post-, postbronchodilator; Pre-, prebronchodilator.
Plus–minus values are means ± SD.
The BMI is the weight in kilograms divided by the square of the height in meters.
Analyzed SARP I&II cohort includes patients with severe asthma.
Baseline DHEA-S and FEV1PP.
FEV1PP was weakly associated with serum DHEA-S in both men and women. In the SARP III cohort, the R2 (proportion of FEV1PP variability explained by DHEA-S) was 0.04 for all races (n = 314; P < 0.001) and 0.04 (n = 203; P < 0.001) for Caucasians. Similarly, for severe asthma in SARP I&II, the R2 was 0.13 (n = 271; P < 0.001) for all races and 0.20 (n = 178; P < 0.001) in Caucasians (Fig. 1A and SI Appendix, Table S1). This association appears to be driven by patients with low DHEA-S (i.e., first quartile; SI Appendix, Fig. S1). Strikingly, no women and very few men with DHEA-S levels over 200 µg/dL in either cohort had a baseline FEV1PP of less than 75% in SARP III.
Fig. 1.
DHEA-S concentration is associated with FEV1PP and suppressed with daily systemic GC use. (A) In subjects with asthma, FEV1PP correlates significantly with DHEA-S levels in both Caucasian men (blue circles; R2 = 0.15; P < 0.001) and women (open red circles; R2 = 0.03; P = 0.047). (B) Chronic daily GC use is associated with DHEA-S suppression in SARP III in men and women.
DHEA-S Suppression Is Associated with Oral GC Use.
Daily oral GC therapy was commonly used in SARP III with median dose and duration that did not differ among the 3 HSD3B1 genotypes. The median duration was 12 mo for each of the 3 genotypes (P = 0.18 by Kruskal–Wallis test), and the median dose was 10 mg prednisone (P = 0.74 by Kruskal–Wallis test). Overall, 22.6% (29% of Caucasians) of adults with severe asthma enrolled in SARP III were treated with daily oral GC therapy (26) (SI Appendix, Table S2). Endogenous circulating cortisol declined significantly (P < 0.001) in patients receiving oral GCs, confirming both patient compliance and adrenal suppression (SI Appendix, Fig. S2). As expected, our analysis of DHEA-S from 314 adult participants with asthma enrolled in SARP III showed significantly lower plasma DHEA-S levels in patients treated with daily oral GC therapy, as opposed to those not receiving daily oral GCs for both men and women. Not surprisingly, DHEA-S decline occurs in men and women (Fig. 1B), irrespective of HSD3B1(1245) genotype (SI Appendix, Fig. S3), and there is no association between genotype and circulating testosterone (SI Appendix, Fig. S4). Overall, daily GC therapy was associated with a 70% decrease in DHEA-S compared with no GC use in SARPIII and SARPI/II (P < 0.001) (SI Appendix, Table S3). Circulating DHEA also decreased by about 70% with GC therapy (SI Appendix, Fig. S5).
HSD3B1(1245) Genotype and GC Resistance.
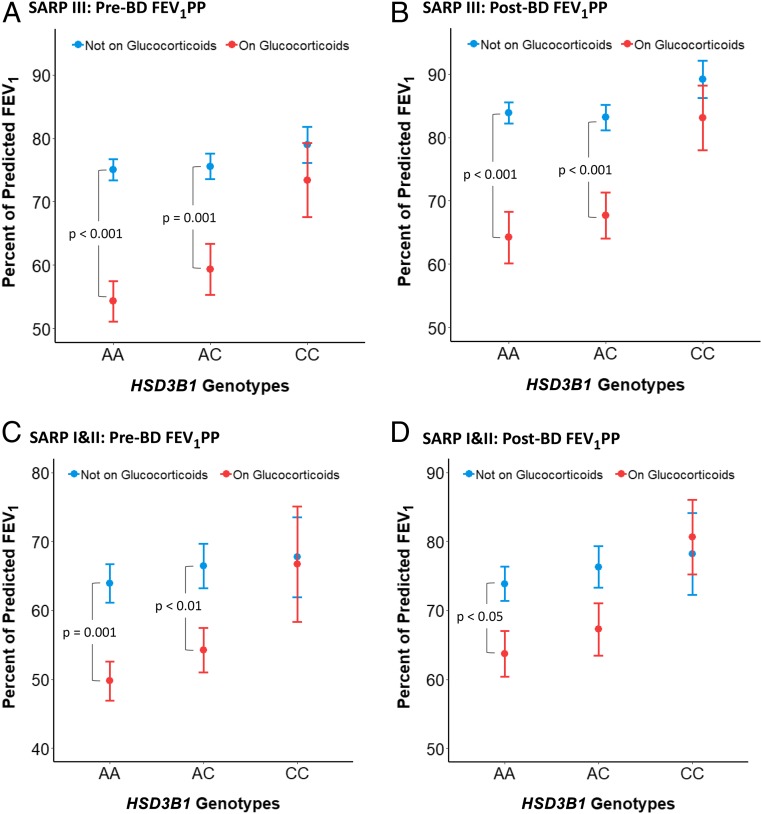
To test our hypothesis that the HSD3B1(1245A) adrenal restrictive allele is specifically associated with impaired lung function with GC treatment-mediated adrenal suppression, lung function was compared in 318 GC and noGC Caucasian patients enrolled in SARP III for whom HSD3B1(1245) genotype data were available. Statistical comparisons of GC and noGC FEV1PP by HSD3B1(1245) genotype, before and after bronchodilation (BD), are summarized in Table 2, and graphical representations are presented in Fig. 2 A and B. Overall, pre- or post-FEV1PP was not correlated with HSD3B1(1245) genotype in the noGC group. However, an interaction was present between daily GC use and HSD3B1(1245) genotype, suggesting that the effect of HSD3B1(1245) genotype depends on daily GC use. In patients with asthma homozygous for the HSD3B1(1245A) adrenal restrictive allele, pre-BD-FEV1PP (Fig. 2A) for GC-treated individuals is significantly worse than for noGC (54.3 vs. 75.1; P < 0.001). Post-BD-FEV1PP for GC-treated individuals with the homozygous adrenal restrictive genotype (Fig. 2B) is similarly worse than noGC (64.2 vs. 83.9; P < 0.001). In sharp contrast, for patients with asthma homozygous for the HSD3B1(1245C) adrenal permissive allele, the GC and noGC groups do not differ significantly for either pre-BD-FEV1PP (73.4 vs. 78.9; P = 0.41) or post-BD FEV1PP (83.1 vs. 89.2; P = 0.32). For patients with asthma who inherit the heterozygous genotype, the association of poorer outcomes in GC vs. noGC groups, although smaller in effect compared with the homozygous adrenal restrictive genotype, persists for pre-BD-FEV1PP (59.4 vs. 75.6; P = 0.001) and post-BD-FEV1PP (67.7 vs. 83.2; P < 0.001). This relationship also holds when the results were stratified by sex (SI Appendix, Fig. S6). Results from the asthma control test similarly show that outcomes in the GC group are worse for patients with asthma who inherit at least one copy of the adrenal restrictive HSD3B1(1245A) allele (SI Appendix, Fig. S7), and that neutrophil counts significantly decrease in those who do not inherit the adrenal restrictive allele (SI Appendix, Fig. S8).
Table 2.
Comparison of maximum post-BD-FEV1% between patients treated with and without daily oral GCs among the HSD3B1(1245) AA, AC, and CC genotypes*
| SARP III | SARP I & II | |||||
| HSD3B1(1245) Genotype | Not on GC | On GC | P value | Not on GC | On GC | P value† |
| AA | ||||||
| n | 123 | 23 | 45 | 35 | ||
| Pre-FEV1, % of predicted value | 75.1 ± 18.3 | 54.3 ± 15.3 | <0.001 | 63.4 ± 18.8 | 49.8 ± 17.1 | 0.001 |
| Post-FEV1, % of predicted value | 83.9 ± 18.8 | 64.2 ± 19.6 | < 0.001 | 73.5 ± 15.9 | 63.7 ± 18.4 | 0.024 |
| AC | ||||||
| n | 107 | 24 | 49 | 30 | ||
| Pre-FEV1, % of predicted value | 75.6 ± 21.2 | 59.4 ± 19.8 | 0.001 | 66.9 ± 22.3 | 54.3 ± 18.3 | 0.008 |
| Post-FEV1, % of predicted value | 83.2 ± 20.5 | 67.7 ± 17.9 | <0.001 | 77.2 ± 20.8 | 67.8 ± 20.3 | 0.07 |
| CC | ||||||
| n | 33 | 8 | 15 | 10 | ||
| Pre-FEV1, % of predicted value | 78.9 ± 16.3 | 73.4 ± 16.6 | 0.41 | 67.7 ± 22.5 | 66.7 ± 26.6 | 0.92 |
| Post-FEV1, % of predicted value | 89.2 ± 17.0 | 83.1 ± 14.4 | 0.32 | 78.2 ± 22.3 | 80.7 ± 16.2 | 0.76 |
Data are presented as mean ± SD for continuous variables and proportions or percentages for categorical variables.
P value comparing daily oral GC vs. no GC in each genotype group AA, AC, and CC.
Fig. 2.
The adrenal restrictive HSD3B1(1245A) allele is specifically associated with poor pulmonary function in GC-treated patients with severe asthma. In Caucasian AA genotype patients with asthma enrolled in SARP III, baseline prebronchodilator FEV1PP (Pre-BD FEV1PP) (A) and postbronchodilator FEV1PP (Post-BD FEV1PP) (B) is lower for those in the GC vs. no GC treatment groups. In contrast, for the CC genotype, there is no difference between GC and no GC treatment groups. Lower Pre-BD FEV1PP (C) and Post-BD FEV1PP (D) for AA genotype patients receiving GC also occurs in Caucasian patients with severe asthma enrolled in SARP I&II. Error bars indicate SEs.
To test the association between the HSD3B1 adrenal restrictive allele and lower FEV1PP with oral GC treatment in a different cohort, we assessed 184 Caucasian patients with severe asthma in SARP I&II. Mirroring the results in SARP III, patients homozygous for the HSD3B1(1245A) adrenal restrictive genotype in the GC group have significantly worse pre-BD-FEV1PP (49.8 vs. 63.4; P < 0.001) and post-BD-FEV1PP (63.7 vs. 73.5; P < 0.05) than noGC (Fig. 2 C and D). Patients homozygous for the HSD3B1(1245C) adrenal permissive genotype have pre-BD-FEV1PP and post-BD-FEV1PP that are statistically indistinguishable between GC and noGC groups, effectively replicating the findings in SARP III. FEV1PP for heterozygote patients are again intermediate between AA (adrenal restrictive) and CC (adrenal permissive) genotypes. Additional statistical comparisons for SARP III and SARP I/II are shown in SI Appendix, Fig. S9. We acknowledge that sample size is a limitation of our study.
The mechanisms by which androgens can benefit asthma have been described in detail in animal models. In particular, androgens directly inhibit airway inflammation (4, 8, 9, 15), airway smooth muscle, and fibroblast proliferation (4–7). Consistent with these data, we have recently shown that DHEA therapy improves lung function in low-DHEA-S women (34). Moreover, our additional analysis shows that all women in our study whose serum DHEA-S increased by more than 300 µg/dL had an increase in FEV1 (n = 8) compared with those who had less of an increase in DHEA-S (P = 0.018 by Fisher’s exact test). Furthermore, the plausibility of DHEA/DHEA-S benefit in asthma is further supported by 3β-HSD1 expression in the human lung (SI Appendix, Fig. S10). A model that summarizes the effect of the association between HSD3B1(1245) genotype, DHEA-S suppression with GC treatment and FEV1PP is shown in Fig. 3.
Fig. 3.
A model that explains physiologic effects of HSD3B1 inheritance on FEV1PP in patients with severe asthma. GC treatment suppresses adrenal DHEA, which may become a limiting substrate for 3β-HSD1, depending on HSD3B1 genotype. Adrenal permissive and adrenal restrictive alleles enable and limit metabolic flux through 3β-HSD1 and recovery of airflow.
Discussion
Severe asthma is defined as asthma that remains symptomatic and exacerbation-prone despite controlled high-dose inhaled ICS or systemic steroid treatment in conjunction with a second controller medication (3). Causes underlying severe asthma are heterogeneous (28, 30, 35, 36), and many patients are refractory, even to recently developed biological therapies (36). An aspect of severe asthma that is not commonly considered is that systemic GC therapy increases risk for low circulating levels of androgens, particularly DHEA-S (37).
Our study supports a model in which HSD3B1(1245) genotypes that confer less active conversion from adrenal precursors to potent androgens in peripheral tissues leads to a physiologic state of relative androgen deficiency that occurs specifically with DHEA-S suppression that is a consequence of systemic GC treatment. Strikingly, an HSD3B1(1245) allele-dose dependent effect appears to be clear and occurs for pre-BD-FEV1PP and post-BD-FEV1PP in both SARP III and SARP I&II.
In general, androgens require the AR to mediate much of their physiologic effects. Potent AR stimulation from adrenal DHEA/DHEA-S, which is available in circulation, requires enzymatic conversion by 3β-HSD1 to testosterone and dihydrotestosterone, which occurs in peripheral tissues. Given the variety of tissues in which AR is expressed, the association observed in our study may be attributable to androgen stimulation in several different cell types. Androgens have many effects that could be beneficial for the asthmatic airway. For example, DHEA-S inhibits human airway smooth muscle and fibroblast proliferation and may benefit airway epithelial to mesenchymal transition (4–6). Both DHEA-S and testosterone promote airway smooth muscle relaxation (7). Testosterone is associated with decreased Th2 and Th1 inflammation in animal models of asthma (8, 9). Epidemiologically, androgens are associated with better lung function in large healthy cohorts (10, 11) and in disease (12). Increasing circulating levels of adrenal and gonadal androgens in males and females during adolescence are believed to be associated with improving asthma during adolescence (12), and gonadal androgens in particular may be associated with gender-based differences in asthma incidence and severity in adulthood (12, 38–40). Notably, steroid metabolites downstream of DHEA-S are generated at the level of the target peripheral tissue and are generally not appreciable in circulation (41, 42).
These data suggest that androgen depletion, whether circulating and/or at the tissue level, could contribute to the pathophysiology of severe asthma and resistance to oral GC therapy. Our data confirm that low DHEA-S levels in asthma are associated with low lung function. However, we do not know whether this is a causal relationship, whether low androgen levels are simply a marker associated with patients with low lung function on more GCs, or both. We recently published a pilot study suggests that DHEA supplementation in women with low DHEA-S improves FEV1 (34).
Recent studies of prostate cancer have shown that the adrenal restrictive HSD3B1(1245A) allele limits conversion of DHEA to more potent androgens, whereas the adrenal permissive HSD3B1(1245C) allele increases tissue production of potent androgens (16, 17). The SARP studies provided an opportunity to study androgen metabolism effects in severe asthma. We therefore hypothesized that the restrictive allele, which impedes conversion from adrenal DHEA-S to testosterone and dihydrotestosterone, would be associated with lower lung function in severe asthma when systemic GCs are used and suppress substrate availability for 3β-HSD1. Data were analyzed in the SARP III cohort, enriched for severe asthma, and then validated in the patients with severe asthma in the SARP I&II cohort and are strikingly consistent with our hypothesis across both cohorts.
Limitations of this study include that it is restricted to Caucasian patients with asthma and the sample size, particularly for patients with adrenal permissive HSD3B1(1245C) genotypes. The low frequency of the adrenal permissive HSD3B1(1245C) allele in African-American subjects combined with the limited number of subjects did not allow for a sufficiently powered analysis in non-Caucasians. For example, only 1 African American participant with CC genotype was enrolled in SARP III, and 2 others in SARP I&II. The lower prevalence of the adrenal permissive C allele in African Americans could contribute to higher severity and risk from asthma, as well as GC resistance, in this patient population. Similarly, none of the 27 adult Hispanic participants with asthma enrolled in SARP III, and only 1 of the 34 adult Hispanics enrolled in SARP I&II, respectively, carried the CC genotype. Of note, the adrenal permissive allele frequency (i.e., the C allele) was 36% and 37% in adult Caucasian participants with asthma enrolled in SARP III and severe asthma enrolled SARP I&II. Those frequencies match the frequency reported for Caucasians in the 1000 Genomes Project and suggest the absence of any deviation from Hardy–Weinberg Equilibrium.
In conclusion, we have identified a genetic determinant associated with GC resistance and low lung function in asthma. Evidence that the adrenal restrictive HSD3B1(1245A) allele, which confers a lower level of prostate cancer adrenal androgen dependence by preventing local conversion of DHEA to more potent androgens, adversely affects lung function in GC-dependent severe asthma suggests that androgens have a central role in the pathophysiology of human severe asthma and response to systemic GC treatment. Our data provide genetic evidence that complements epidemiological data regarding a benefit of androgens on lung function. Our model is further supported by our data showing a positive relationship between circulating adrenal DHEA-S levels and lung function. Indeed, androgens cause airway smooth muscle relaxation and prevent both remodeling and inflammation in animal models of asthma. GC use suppresses endogenous androgen production, preventing the beneficial effects of androgens in human asthma. These data suggest the possibility that HSD3B1 genotype is predictive of which patients might benefit from systemic GC therapy alone and, for those who are resistant, who would benefit from androgen replacement in severe asthma.
Supplementary Material
Acknowledgments
We thank the patients and their families for their participation in the Severe Asthma Research Program; the site-based study coordinators and regulatory personnel for enrolling participants, the Data Safety and Monitoring Board for program oversight, and National Institute of Health/National Heart, Lung, and Blood Institute project officers and staff who administered the protocol. Supported by a grant from the National Heart, Lung, and Blood Institute Severe Asthma Research Program (U10 HL109250, P01 HL128192, and P01 HL101871 to Rainbow Babies and Children’s Hospital Virginia-Cleveland Consortium, and RO1 HL69170, U10 HL109250, K08 HL133381, P01 HL103453, P01 HL081064, R01CA172382, R01CA190289, R01 CA236780, and a grant from the Prostate Cancer Foundation to the Cleveland Clinic). We also thank the Harrington Discovery Institute of University Hospitals, Cleveland, for grant support.
Footnotes
Competing interest statement: Cleveland Clinic has applied for patents on HSD3B1.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1918819117/-/DCSupplemental.
References
- 1.Hench P. S., Slocumb C. H., Polley H. F., Kendal E. C., Effect of cortisone and pituitary adrenocorticotropic hormone (ACTH) on rheumatic diseases. J. Am. Med. Assoc. 144, 1327–1335 (1950). [DOI] [PubMed] [Google Scholar]
- 2.Barnes P. J., Adcock I. M., Glucocorticoid resistance in inflammatory diseases. Lancet 373, 1905–1917 (2009). [DOI] [PubMed] [Google Scholar]
- 3.Chung K. F., et al. , International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur. Respir. J. 43, 343–373 (2014) Erratum in: Eur Respir J. 43, 1216 (2014). [DOI] [PubMed] [Google Scholar]
- 4.Koziol-White C. J., et al. , DHEA-S inhibits human neutrophil and human airway smooth muscle migration. Biochim. Biophys. Acta 1822, 1638–1642 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mendoza-Milla C., et al. , Dehydroepiandrosterone has strong antifibrotic effects and is decreased in idiopathic pulmonary fibrosis. Eur. Respir. J. 42, 1309–1321 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Xu L., et al. , Effects and mechanism of dehydroepiandrosterone on epithelial-mesenchymal transition in bronchial epithelial cells. Exp. Lung Res. 40, 211–221 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Kouloumenta V., Hatziefthimiou A., Paraskeva E., Gourgoulianis K., Molyvdas P. A., Non-genomic effect of testosterone on airway smooth muscle. Br. J. Pharmacol. 149, 1083–1091 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cephus J. Y., et al. , Testosterone attenuates group 2 innate lymphoid cell-mediated airway inflammation. Cell Rep. 21, 2487–2499 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuseini H., et al. , Testosterone decreases house dust mite-induced type 2 and IL-17a-mediated airway inflammation. J. Immunol. 201, 1843–1854 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Svartberg J., Schirmer H., Medbø A., Melbye H., Aasebø U., Reduced pulmonary function is associated with lower levels of endogenous total and free testosterone. The Tromsø study. Eur. J. Epidemiol. 22, 107–112 (2007). [DOI] [PubMed] [Google Scholar]
- 11.Mohan S. S., et al. , Higher serum testosterone and dihydrotestosterone, but not oestradiol, are independently associated with favourable indices of lung function in community-dwelling men. Clin. Endocrinol. (Oxf.) 83, 268–276 (2015). [DOI] [PubMed] [Google Scholar]
- 12.DeBoer M. D., et al. , Effects of endogenous sex hormones on lung function and symptom control in adolescents with asthma. BMC Pulm. Med. 18, 58 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Auchus R. J., The physiology and biochemistry of adrenarche. Endocr. Dev. 20, 20–27 (2011). [DOI] [PubMed] [Google Scholar]
- 14.Sharifi N., Minireview: Androgen metabolism in castration-resistant prostate cancer. Mol. Endocrinol. 27, 708–714 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hazeldine J., Arlt W., Lord J. M., Dehydroepiandrosterone as a regulator of immune cell function. J. Steroid Biochem. Mol. Biol. 120, 127–136 (2010). [DOI] [PubMed] [Google Scholar]
- 16.Chang K. H., et al. , A gain-of-function mutation in DHT synthesis in castration-resistant prostate cancer. Cell 154, 1074–1084 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hearn J. W. D., et al. , HSD3B1 and resistance to androgen-deprivation therapy in prostate cancer: A retrospective, multicohort study. Lancet Oncol. 17, 1435–1444 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agarwal N., et al. , Independent validation of effect of HSD3B1 genotype on response to androgen-deprivation therapy in prostate cancer. JAMA Oncol. 3, 856–857 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hearn J. W. D., et al. , Association of HSD3B1 genotype with response to androgen-deprivation therapy for biochemical recurrence after radiotherapy for localized prostate cancer. JAMA Oncol. 4, 558–562 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shiota M., et al. , Association of missense polymorphism in HSD3B1 with outcomes among men with prostate cancer treated with androgen-deprivation therapy or abiraterone. JAMA Netw. Open 2, e190115 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia Gil S. R. R., et al. , Relationship between Mutations in the HSD3B1 Gene and Response Time to Androgen Deprivation Therapy in the Treatment of Prostate Cancer (European Society of Oncology Pharmacy, Nantes, France, 2018). [Google Scholar]
- 22.Hearn J. W. D., et al. , HSD3B1 genotype and clinical outcomes in metastatic castration-sensitive prostate cancer. JAMA Oncol., in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brusasco V., Crapo R., Viegi G.; American Thoracic Society ; European Respiratory Society , Coming together: The ATS/ERS consensus on clinical pulmonary function testing. Eur. Respir. J. 26, 1–2 (2005). [DOI] [PubMed] [Google Scholar]
- 24.Miller M. R., et al. ; ATS/ERS Task Force , Standardisation of spirometry. Eur. Respir. J. 26, 319–338 (2005). [DOI] [PubMed] [Google Scholar]
- 25.Quanjer P. H., et al. ; ERS Global Lung Function Initiative , Multi-ethnic reference values for spirometry for the 3-95-yr age range: The global lung function 2012 equations. Eur. Respir. J. 40, 1324–1343 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teague W. G., Phillips B. R., Fahy J. V. et al. , Baseline features of the severe asthma research program (SARP III) cohort: Differences with age. J. Allergy Clin. Immunol. Pract. 6, 545–554 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phipatanakul W., et al. ; Severe Asthma Research Program , Effects of age and disease severity on systemic corticosteroid responses in asthma. Am. J. Respir. Crit. Care Med. 195, 1439–1448 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moore W. C., et al. ; National Heart, Lung, Blood Institute’s Severe Asthma Research Program , Characterization of the severe asthma phenotype by the national Heart, lung, and blood institute’s severe asthma research program. J. Allergy Clin. Immunol. 119, 405–413 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jarjour N. N., et al. ; NHLBI Severe Asthma Research Program (SARP) , Severe asthma: Lessons learned from the national Heart, lung, and blood Institute severe asthma research program. Am. J. Respir. Crit. Care Med. 185, 356–362 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moore W. C., et al. ; National Heart, Lung, and Blood Institute’s Severe Asthma Research Program , Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am. J. Respir. Crit. Care Med. 181, 315–323 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.American Thoracic Society , Proceedings of the ATS workshop on refractory asthma: Current understanding, recommendations, and unanswered questions. Am. J. Respir. Crit. Care Med. 162, 2341–2351 (2000). [DOI] [PubMed] [Google Scholar]
- 32.Purcell S., et al. , PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81, 559–575 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang C. C., et al. , Second-generation PLINK: Rising to the challenge of larger and richer datasets. Gigascience 4, 7 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marozkina N., et al. , Dehydroepiandrosterone supplementation may benefit women with asthma who have low androgen levels: A pilot study. Pulm. Ther. 5, 213–220 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.The ENFUMOSA cross-sectional European multicentre study of the clinical phenotype of chronic severe asthma. European network for understanding mechanisms of severe asthma Eur. Respir. J. 22, 470–477 (2003). [DOI] [PubMed] [Google Scholar]
- 36.Denlinger L. C., et al. , Inflammatory and Co-morbid features of patients with severe asthma and frequent exacerbations. Am. J. Respir. Crit. Care Med. 195, 302–313 (2017). Erratum in: Am. J. Respir. Crit. Care Med 197, 971 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dorsey M. J., Cohen L. E., Phipatanakul W., Denufrio D., Schneider L. C., Assessment of adrenal suppression in children with asthma treated with inhaled corticosteroids: Use of dehydroepiandrosterone sulfate as a screening test. Ann. Allergy Asthma Immunol. 97, 182–186 (2006). [DOI] [PubMed] [Google Scholar]
- 38.Tantisira K. G., et al. ; Childhood Asthma Management Program Research Group , Airway responsiveness in mild to moderate childhood asthma: Sex influences on the natural history. Am. J. Respir. Crit. Care Med. 178, 325–331 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Becklake M. R., Kauffmann F., Gender differences in airway behaviour over the human life span. Thorax 54, 1119–1138 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tse S. M., et al. , Sex-specific risk factors for childhood wheeze and longitudinal phenotypes of wheeze. J. Allergy Clin. Immunol. 138, 1561–1568 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Labrie F., Extragonadal synthesis of sex steroids: Intracrinology. Ann. Endocrinol. (Paris) 64, 95–107 (2003). [PubMed] [Google Scholar]
- 42.Sabharwal N., Sharifi N., HSD3B1 genotypes conferring adrenal-restrictive and adrenal-permissive phenotypes in prostate cancer and beyond. Endocrinology 160, 2180–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
TOPMed genomic data and preexisting parent study phenotypic data are made available to the scientific community in study-specific accessions in the database of Genotypes and Phenotypes (https://www.ncbi.nlm.nih.gov/gap/).