Significance
There is a pressing need for new antiinfluenza therapeutic agents. We show that a molecularly engineered banana lectin (carbohydrate-binding protein) has broad-spectrum activity against all influenza strains tested, including drug-resistant and currently circulating strains; is safe upon repeated administration in mice; and, moreover, is efficacious at treating lethal influenza infection via clinically pertinent routes of administration. We demonstrate that the lectin binds to the viral hemagglutinin glycoprotein and exerts its primary antiviral effect via inhibition of an early stage of the viral life cycle, viral membrane fusion to the host endosomal membrane. Our findings indicate that this engineered lectin, which has a mechanism of action quite distinct from the presently available agents, has potential as an antiinfluenza agent.
Keywords: influenza virus, hemagglutinin, membrane fusion, lectin, antiviral
Abstract
There is a strong need for a new broad-spectrum antiinfluenza therapeutic, as vaccination and existing treatments are only moderately effective. We previously engineered a lectin, H84T banana lectin (H84T), to retain broad-spectrum activity against multiple influenza strains, including pandemic and avian, while largely eliminating the potentially harmful mitogenicity of the parent compound. The amino acid mutation at position 84 from histidine to threonine minimizes the mitogenicity of the wild-type lectin while maintaining antiinfluenza activity in vitro. We now report that in a lethal mouse model H84T is indeed nonmitogenic, and both early and delayed therapeutic administration of H84T intraperitoneally are highly protective, as is H84T administered subcutaneously. Mechanistically, attachment, which we anticipated to be inhibited by H84T, was only somewhat decreased by the lectin. Instead, H84T is internalized into the late endosomal/lysosomal compartment and inhibits virus–endosome fusion. These studies reveal that H84T is efficacious against influenza virus in vivo, and that the loss of mitogenicity seen previously in tissue culture is also seen in vivo, underscoring the potential utility of H84T as a broad-spectrum antiinfluenza agent.
Influenza viruses pose a major threat to public health worldwide, causing both seasonal epidemics, which infect millions and kill ∼500,000 people annually, and pandemics, which are unpredictable and have the potential to kill millions, as was illustrated most recently in 2009 with the “swine flu” pandemic and most dramatically in 1918 with the emergence of the “Spanish flu” that resulted in 50 million deaths. Unfortunately, current prevention and treatment modalities, although helpful, suffer from distinct limitations and are at best only moderately effective. Although it clearly reduces both the number and severity of cases, vaccination is often underutilized and its effectiveness varies from year to year (1). Effectiveness was estimated in the 2017 to 2018 United States season to be only ∼25% against influenza A subtype H3N2 viruses, which comprised ∼69% of infections (2).
At present, although there are a number of antiinfluenza agents currently in phase II or III clinical trials (3), only three classes of drugs are approved to treat influenza virus infection in the United States: Adamantanes, neuraminidase (NA) inhibitors, and as of October 2018, a new class with the sole member baloxavir marboxil, a cap-dependent endonuclease protein inhibitor (4). Adamantanes are obsolete in practice due to overwhelmingly high levels of resistance to them among circulating influenza A strains and due to their intrinsic inactivity against influenza B strains. The current standard of care treatment is oseltamivir, a member of the NA inhibitor class. Oseltamivir is associated with a moderate decrease in mortality among adults hospitalized for influenza, but it must be initiated within 48 h of illness onset to be effective and only shortens the duration of symptoms by about 1 d in otherwise healthy adults (5, 6). Although oseltamivir resistance among circulating strains has generally been low since the emergence of the 2009 pandemic H1N1, resistance does occur and can be high in certain seasons (5, 7).
With the twin threats of another influenza pandemic and emergence of antiviral resistance ever present and a paucity of new classes of antiinfluenza drugs introduced since the NA inhibitors in 1999, there is a strong unmet need for new antiinfluenza therapeutics, particularly ones that are broad-spectrum given the high genetic and antigenic diversity of influenza. Lectins, or carbohydrate-binding proteins, have been proposed as potential antiviral agents due to their propensity to specifically bind the types of glycans present at high densities in glycoproteins on the surfaces of viruses. Indeed, influenza viruses are potential targets of lectins because they possess two major glycoproteins, hemagglutinin (HA) and NA, which mediate key steps of the viral life cycle, including attachment to sialic acid-containing cellular receptors and fusion of the viral membrane with the endosomal membrane (HA), assembly of new virus particles (HA and NA) (8–10), and release of virus particles by cleavage of sialic acid residues (NA). However, the development of lectins for clinical use has been limited by their mitogenicity and hence potential for inflammatory side effects (11).
We have previously demonstrated that mitogenicity can be removed from a banana lectin (BanLec), while maintaining broad-spectrum antiviral activity, by engineering a single amino acid mutation at position 84 from histidine to threonine (H84T BanLec, H84T) (12). H84T is highly efficacious against pandemic, epidemic, and avian influenza in vitro and against lethal influenza virus infection in vivo when administered intranasally, yet its safety and efficacy via other clinically important routes of administration remain to be determined. In addition, although H84T is known to bind high-mannose type N-glycans, these glycans are abundantly present on both HA and NA (13), and thus it is not clear whether HA or NA is the key viral component antagonized by H84T. We therefore set out to determine the efficacy of systemically and subcutaneously delivered H84T, as well as the mechanism of action of H84T. Here, we report that in a lethal mouse model, H84T is indeed nonmitogenic as predicted by the in vitro data, and both early and delayed therapeutic administration of H84T intraperitoneally are highly protective, as is H84T administered subcutaneously. Mechanistically, attachment is only somewhat inhibited by H84T, incommensurate with the overall inhibitory effect of H84T, which was unexpected based on our observation that wild-type (WT) BanLec inhibits HIV attachment to cells. Instead, H84T, which can bind to HA, is internalized into the late endosomal/lysosomal compartment and inhibits the HA-mediated step of fusion with the endosome. Taken together, these studies reveal that H84T is efficacious against influenza virus in vivo, even when given up to 72 h after challenge, and that it inhibits virus–endosome fusion, underscoring its potential use as a new broad-spectrum antiinfluenza agent with a mechanism of action that is quite distinct from that of the currently available inhibitors.
Results
H84T Is Active Against Oseltamivir-Resistant and Influenza B Viruses and Synergizes with Oseltamivir In Vitro.
Our previous work demonstrates that H84T has robust activity against diverse influenza viruses in vitro, including A/California/04/2009 (H1N1)pdm09, A/California/07/2009 (H1N1)pdm09, A/New York/18/2009 (H1N1)pdm09, A/Perth/16/2009 (H3N2), A/Duck/MN/1525/1981 (H5N1), and the 1918 H1N1 pandemic strain, with half-maximum effective concentration (EC50) values of 1 to 4 μg/mL (∼32 to 130 nM) versus H1N1 virus, 0.06 to 0.1 μg/mL (∼2 to 3 nM) versus H3N2 virus, and 5 to 11 μg/mL (∼160 to 350 nM) versus H5N1 virus (12). Antiinfluenza activity is dependent on carbohydrate binding, as the D133G BanLec mutant lacking carbohydrate-binding activity has no antiinfluenza activity. Because influenza strains resistant to the standard-of-care therapy, oseltamivir, do circulate in certain years (7, 14, 15), we sought to evaluate whether H84T is effective against oseltamivir-resistant viruses. Indeed, H84T inhibited replication of A/Mississippi/3/2001-H275Y (H1N1), a strain reported to be oseltamivir-resistant (16–18), in Madin-Darby canine kidney (MDCK) cells, with an EC90 value of 0.074 μg/mL (2.4 nM), as compared to >100 μg/mL for oseltamivir. H84T treatment also decreased spreading infection of two other oseltamivir-resistant viruses in tissue culture, A/Texas/12/2007 (H3N2) and A/Louisiana/08/2013 (H1N1)pdm09 (SI Appendix, Fig. S1), in which NA inhibitor resistance is conferred by the E119V and H275Y mutations, respectively, and adamantane resistance conferred by the S31N mutation (19, 20). Thus, H84T is highly effective even against oseltamivir- and adamantane-resistant strains.
As combination therapy with both oseltamivir and H84T could be a potential strategy to treat oseltamivir-susceptible strains, we sought to determine whether synergy exists between oseltamivir and H84T. Treatment of MDCK cells infected with A/California/07/2009 (H1N1)pdm09, A/Perth/16/2009 (H3N2), A/Duck/MN/1525/1981 (H5N1), and B/Brisbane/60/2008 with both oseltamivir and H84T resulted in a virus yield reduction that exceeded one log10 when compared to either individual drug alone, identifying possible synergy between oseltamivir and H84T for all four viruses tested (21) (SI Appendix, Tables S1–S4). We further examined the effect of the drug combination using 3D analysis (MacSynergy II) (22) and found that the combination of oseltamivir and H84T has minor but significant synergy against both A/California/07/2009 (H1N1)pdm09 and A/Perth/16/2009 (H3N2) (SI Appendix, Figs. S2 and S3). Thus, H84T shows some, but not striking, synergy with oseltamivir.
Since influenza B infections cannot be distinguished clinically from influenza A infections (23), antiinfluenza agents that have activity against both influenza A and B are particularly attractive for empiric treatment of influenza-like illnesses. We therefore sought to evaluate whether H84T is efficacious against influenza B virus infection. We found that H84T has EC50 concentrations by neutral red assay of 0.15 and 0.33 μg/mL (∼4.8 and 11 nM) against influenza B viruses B/Brisbane/60/2008 (Victoria lineage) and B/Florida/4/2006 (Yamagata lineage), respectively, and reduces spreading infection of B/Brisbane/60/2008 in MDCK cells (SI Appendix, Fig. S4), indicating that H84T is indeed active against influenza B viruses and has promise as an antiinfluenza A and B agent.
Intranasal Administration of H84T Protects Against Lethal H3N2 Influenza Virus Infection.
We previously reported that intranasal administration of H84T protects mice against otherwise lethal H1N1 influenza virus infection (12). To assess whether H84T is also efficacious against another influenza virus strain in vivo, we inoculated C57BL/6J mice via aerosol with a lethal dose of A/Hong Kong/8/1968 (H3N2) influenza virus and treated them with 0.1 mg/kg of H84T intranasally once daily for 5 d beginning 4 h postinfection (SI Appendix, Fig. S5). Greater than 80% of H84T-treated mice survived otherwise lethal influenza virus infection, as compared to <10% of placebo-treated animals, extending the results of our previous study and indicating that H84T has robust activity against the two major contemporary subtypes of influenza A in vivo.
H84T, but Not WT BanLec, Is Well-Tolerated In Vivo.
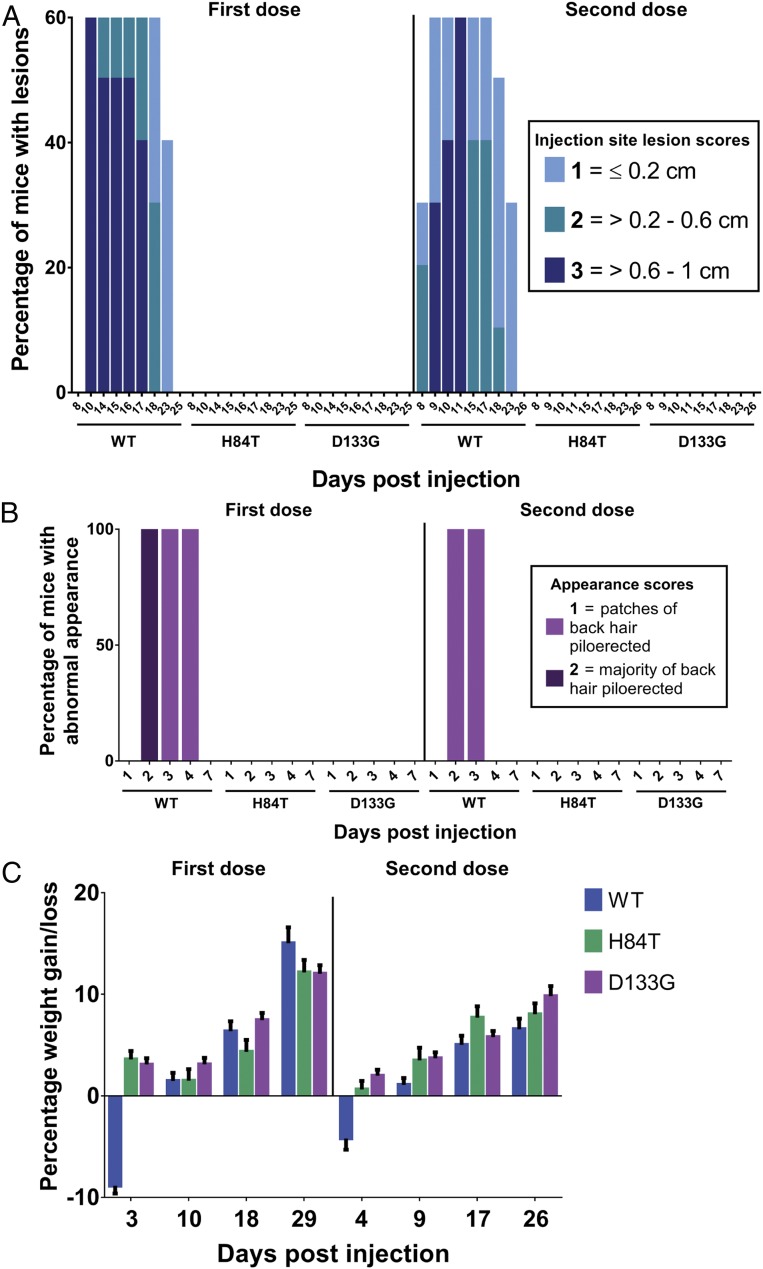
Given that H84T is highly efficacious against influenza infection in vitro and in vivo via intranasal administration, we sought to further characterize its safety and efficacy in vivo when administered systemically. In BALB/c mice, we found that intraperitoneal administration of WT BanLec in two 50-mg/kg doses 1 mo apart resulted in prolonged injection site lesions, characterized as erythematous papules up to a centimeter in size, in 60% of the mice lasting for up to 23 d postinjection (Fig. 1A); piloerection of back hair for up to 4 d postinjection in all of the mice (Fig. 1B); and reversible but notable weight loss of an average of ∼10% of body weight (Fig. 1C). These findings support in vivo the in vitro finding that WT BanLec is mitogenic and give a stark description of how mitogenicity manifests in vivo. In clear contrast, intraperitoneal administration of H84T was very well tolerated, resulting in no injection site lesions, no piloerection of back hair, and no weight loss upon administration of either dose, similar to administration of D133G BanLec, which in vitro lacks both mitogenicity and antiviral activity due to a mutation in the carbohydrate-binding site and so was not expected to elicit an inflammatory reaction. Thus, H84T appears to be nonmitogenic both in vitro and in vivo, in contrast to WT BanLec, which is poorly tolerated in mice, consistent with its mitogenicity in vitro.
Fig. 1.
Systemic H84T is very well tolerated in mice. BALB/c mice were injected intraperitoneally with two 50-mg/kg doses of WT, H84T, or D133G BanLec administered 1 mo apart (n = 10 per group). Injection site lesions (A) and appearances (B) were scored, and weight measured (C) for all mice for 59 d postinjection. In C, the data represent the mean for all mice in each group and error bars the SEM.
H84T Distributes to the Lung.
We next determined the pharmacokinetic profile of H84T in mice dosed intraperitoneally with 5, 15, and 50 mg/kg of the lectin in a single injection by measuring the concentration of H84T postinjection in both the plasma and lung (SI Appendix, Fig. S6A). H84T showed a dose-dependent increase in total drug exposure in plasma with a terminal half-life ranging from 33.5 to 238 h in plasma and from 25 to 32 h in the lung (SI Appendix, Tables S5 and S6). Total exposure levels (AUClast) of H84T in the lung, a key target organ for influenza treatment, exceed plasma levels significantly, but in a nonlinear fashion, with an ∼200-fold increase at 5 mg/kg, ∼100-fold at 15 mg/kg, and ∼30-fold at 50 mg/kg. Lower doses deliver a greater portion of H84T to the lung and might allow for minimizing systemic exposure if proven efficacious. Furthermore, H84T remains in the lung for greater than 72 h at the dose levels tested (SI Appendix, Fig. S6A). The long terminal half-life and prolonged presence of H84T in the lung raises the possibility that H84T could be administered as a single-dose treatment, or at least be given at long intervals. In a parallel assessment of the distribution of H84T, we found that H84T distributes to the lung, as well as the liver and spleen, but not to the brain or kidney in mice dosed intraperitoneally with 50 mg/kg of H84T (SI Appendix, Fig. S6B).
Therapeutic Administration of H84T Protects Against Lethal Influenza Virus Infection.
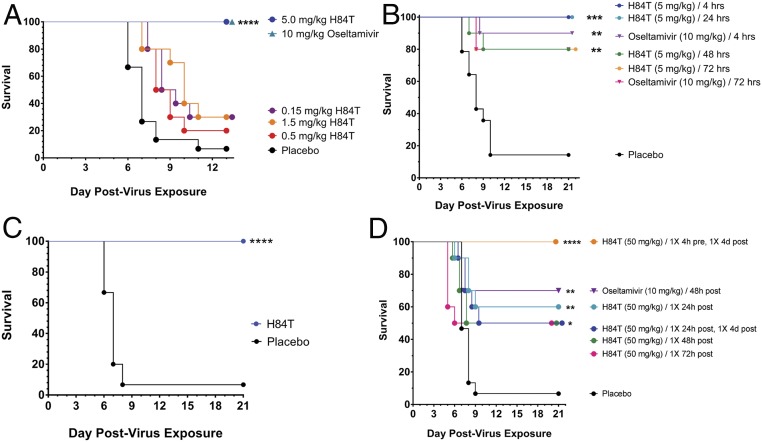
To assess the therapeutic potential of H84T delivered systemically, we inoculated BALB/c mice intranasally with a lethal dose of influenza and treated them with H84T delivered intraperitoneally once daily for 5 d beginning 4 h postinoculation (Fig. 2A). All mice treated with H84T at 5 mg/kg once daily survived, as compared to <10% of placebo-treated animals. Furthermore, mice treated at 5 mg/kg once daily up to 24 h postchallenge all survived, as compared to ∼90% treated with oseltamivir twice daily and <20% treated with PBS. Strikingly, 80% of mice were still protected when treatment began 48 or 72 h postchallenge (Fig. 2B). A key remaining question was whether H84T-treated mice would mount an antibody response to the therapeutic protein that would limit the efficacy of future H84T treatment (24). We found that mice do develop antibodies against H84T (SI Appendix, Fig. S7), consistent with the finding by others that people develop antibodies to WT BanLec (25) and with our finding that human sera from three healthy donors also contained antibodies to H84T (SI Appendix, Fig. S8). However, even mice with anti-H84T antibodies generated at a time of prior H84T administration are 100% protected from lethal influenza infection by H84T treatment (Fig. 2C). These mice also suffered no histological or biochemical damage to the lung, liver, spleen, blood, or other organs.
Fig. 2.
Systemic H84T treatment is highly efficacious against lethal influenza virus infection in mice. (A–C) Therapeutic efficacy of H84T administered intraperitoneally in BALB/c mice challenged intranasally with two times the 50% mouse lethal dose of A/WSN/HA(NC/2099-N225G)/1933 (H1N1). (A) Survival of mice following intraperitoneal treatment with H84T (0.15–5 mg/kg) once daily for 5 d beginning 4 h postchallenge. (B) Survival of mice following intraperitoneal treatment with H84T (5 mg/kg) once daily for 5 d beginning 4, 24, 48, or 72 h postchallenge, or treatment with oseltamivir (10 mg/kg) beginning 4 or 72 h postchallenge. (C) Survival of mice following intraperitoneal treatment with H84T (50 mg/kg) on a single occasion 21 d before challenge, then once daily for 5 d (5 mg/kg/d) beginning 4 h postchallenge. Placebo mice received PBS 21 d prechallenge and once daily for 5 d beginning 4 h postchallenge. (D) Therapeutic efficacy of H84T administered subcutaneously in BALB/c mice challenged intranasally with a lethal dose of A/WSN/HA(NC/2099-N225G)/1933 (H1N1). Survival of mice following subcutaneous treatment with a regimen of either one or two 50-mg/kg doses of H84T within 4 d postinfection. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. Kaplan–Meier survival curves for A to D were compared by the log-rank (Mantel–Cox) test followed by pairwise comparison using the Gehan–Breslow–Wilcoxon test.
H84T confers robust antiinfluenza protection when delivered intraperitoneally, but in a clinical setting intravenous administration, which is analogous to intraperitoneal administration, of H84T would likely be limited to hospitalized patients. On the other hand, subcutaneous administration of H84T, if efficacious, would provide the important advantage of being feasible in the outpatient setting. We therefore examined whether subcutaneous administration of H84T conferred protection against lethal influenza virus infection in mice (Fig. 2D). BALB/c mice were inoculated intranasally with a lethal dose of influenza virus and survival of the mice monitored after treatment with one or two 50-mg/kg doses of H84T. H84T provided 100% protection from challenge when administered in a two-dose regimen at 4 h preinfection and 4 d postinfection, as compared to <10% survival in the placebo-treated group. In addition, two doses of H84T provided 60% protection when administered at 24 h and 96 h (4 d) postinfection, and a single dose at 24, 48, or 72 h provided 50% protection. Oseltamivir, used as the positive control, protected 70% of mice when administered twice daily for 5 d starting 48 h postinfection. Thus, it appears that subcutaneous administration of H84T holds therapeutic promise as well.
H84T Binds to HA Specifically, but Only Minorly Decreases Attachment of Influenza Virus.
A detailed mechanistic understanding of H84T’s ability to inhibit influenza replication is key to developing it as a potential new therapeutic (26, 27). H84T is known to bind to high-mannose structures and perhaps other types of N-glycans such as can be found on the influenza virus glycoproteins HA and NA. To ascertain whether H84T interacts with HA, we performed ELISAs using recombinant HA from A/California/07/2009 (H1N1)pdm09 and A/Perth/16/2009 (H3N2) derived from baculovirus, with the D133G BanLec mutant as a control, as it does not bind carbohydrates. Insect-derived glycoproteins are glycosylated in a distinct manner as compared to mammalian glycoproteins, in that they lack high-mannose N-glycans and instead contain paucimannose N-glycans (28). However, it should be noted that high-mannose and paucimannose N-glycans are structurally similar, with paucimannose N-glycans containing terminal mannose residues like high-mannose N-glycans, although fewer of them. Furthermore, BanLec is known to interact with paucimannose N-glycans (29), although not as strongly as with high-mannose N-glycans. Therefore, we reasoned that determining whether H84T could bind to baculovirus-derived HA would be an informative first step. We observed that H84T binds HA in a concentration-dependent manner, and D133G shows much reduced binding to HA (SI Appendix, Fig. S9A), which suggests that H84T could potentially inhibit HA-mediated steps and that carbohydrate binding activity is important for binding to HA. To further assess whether binding to HA was dependent on carbohydrate binding, we performed a series of competition ELISAs with several different carbohydrates. The known H84T ligand methyl α-d-mannopyranoside was able to inhibit binding of H84T to HA in a concentration-dependent manner (SI Appendix, Fig. S9B). Unsurprisingly, it took a relatively large concentration of the sugar to compete for HA binding since there are predicted to be a large number of interaction sites for H84T on the HA glycans (13, 30). A higher concentration of the sugar was required to inhibit binding to H3 than to H1 HA. The binding to HA was specific, since galactose, known to not be a ligand for H84T (12), was not able to inhibit binding to HA (SI Appendix, Fig. S9C). Yeast mannan, which contains more mannose residues than does methyl α-d-mannopyranoside (31), was able to inhibit binding to HA at 10-fold lower concentrations (SI Appendix, Fig. S10).
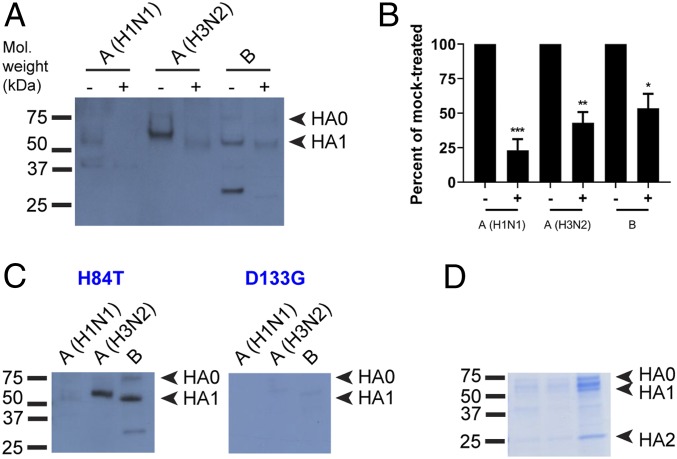
The above experiments suggested that H84T does indeed bind to HA, but given the differences between insect and mammalian glycosylation, we sought to determine whether H84T could also bind to viral-derived HA produced in mammalian cells, and furthermore, to which subunits it might bind. Influenza HA is comprised of two subunits; HA0 is the full-length peptide that, upon activation of the virus by cellular proteases, is cleaved into HA1, which forms the head group, and HA2, which forms most of the stalk. We performed lectin blot analysis with viral lysates from three strains (A/Florida/3/2006 [H1N1], A/Perth/16/2009 [H3N2], and B/Brisbane/60/2008) produced in chicken egg culture (Fig. 3). Lectin blot analysis is similar to Western blot analysis, except that blots are probed first with lectins, after which primary and secondary antibodies are directed against the lectins in order to determine with which blotted proteins a particular lectin interacts. Viral proteins from the lysates were incubated overnight with either PNGase F, a bacterial enzyme that cleaves all N-glycans from glycoproteins, or PBS (mock), after which the lysates were resolved by reducing SDS/PAGE and blotted. Blots were then probed with H84T prior to antibody detection of BanLec (Fig. 3A and SI Appendix, Fig. S11). When the proteins were incubated with PBS, H84T bound to both HA0 and HA1 in all three strains, but did not appear to bind to HA2 in any of the strains. In contrast, when the proteins were incubated with PNGase F, the proteins migrated faster, demonstrating that deglycosylation had occurred, and there was a marked reduction of H84T binding to HA0 and HA1, indicating that H84T binding to HA is carbohydrate-dependent. The percentage of binding of H84T to HA1 in the PNGase F- versus mock-treated group for each strain was calculated by comparing band intensity, with mock-treated set at 100% (Fig. 3B). Not all binding was abolished, likely due to incomplete deglycosylation by PNGase F, although some lectins are also known to interact with molecules in a nonglycan-mediated manner (32). D133G showed dramatically less binding than did H84T (Fig. 3 C and D), underscoring that the ability to bind carbohydrates is important for binding to HA.
Fig. 3.
H84T binds to virus-derived HA. (A) Lectin blot. Virus particles from A/Florida/3/2006 (H1N1), A/Perth/16/2009 (H3N2), and B/Brisbane/60/2008 were lysed for protein extraction. Proteins were incubated with PNGaseF to remove N-glycans or with PBS (mock) overnight, then resolved by reducing SDS/PAGE and blotted. Blots were incubated with 100 nM H84T, followed by primary and secondary antibody incubations to detect BanLec bound to viral proteins. Note that the lower band seen in the influenza B lanes does not appear to be HA2 as it does not run at the correct size. Mol. weight, molecular weight. (B) Percent binding of H84T to PNGase- versus mock-treated HA1 in A, relative to mock-treated set at 100%. Data in A and B are representative of three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001, as compared to the mock-treated group. Error bars denote the SEM. +, PNGase F-treated; −, mock-treated. (C) Lectin blots were performed as in A, without PNGase F digestion. Blots were incubated with 100 nM H84T or D133G BanLec. Data are representative of three independent experiments. (D) Proteins were resolved as in C but not transferred and gels stained with a Coomassie-based reagent.
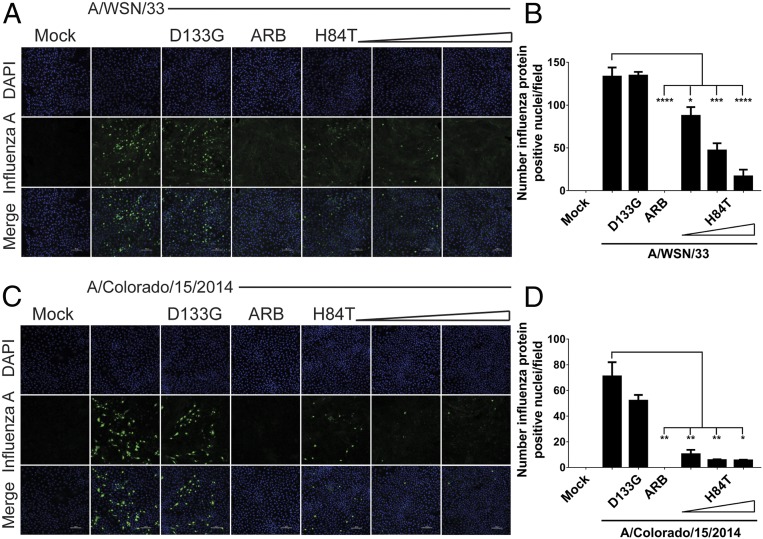
HA mediates both early and late stages of the virus life cycle, including attachment and entry, fusion, and assembly, whereas NA, another potential target for H84T, primarily mediates late stages, including assembly and budding (9). To understand whether H84T exerts its primary inhibitory effect against influenza virus replication early or late in the influenza virus life cycle, we assessed whether viral protein expression was reduced by H84T. Arbidol (ARB), a viral fusion inhibitor that locks HA in a nonfusogenic conformation (33), was used as a positive control for inhibition of early-stage infection. A dose-dependent reduction in viral protein expression was observed at 5 h postinfection (hpi) with A/WSN/1933 (H1N1) (Fig. 4 A and B), suggesting that H84T inhibits a step at or before viral protein translation. Inhibition was dependent on carbohydrate binding, as D133G did not block viral protein expression. H84T also inhibited a step at or before protein translation 5 hpi with a virus pseudotyped with the HA and NA of A/Colorado/15/2014 (H3N2), a strain representative of the 3C.2a clade of H3N2 viruses that encompassed the majority of the ∼69% of clinical influenza infections caused by H3N2 strains in the 2017 to 2018 season, for which vaccine effectiveness was only ∼25% (2) (Fig. 4 C and D). To ensure that the decrease in viral protein expression observed could not be attributed to toxicity, we measured cytotoxicity of H84T on MDCK cells. At 24 h posttreatment, there was no decrease in cell viability at the EC50 concentration for H1N1 virus (∼32 to 130 nM), and minimal decreases above it, suggesting that cytotoxicity could not account for the observed decrease in viral protein expression (SI Appendix, Fig. S12).
Fig. 4.
H84T prevents influenza virus protein expression in MDCK cells. MDCK cells were pretreated for 1 h with H84T (0.1, 1, or 10 μM in A; 0.05, 0.1, or 1 μM in C), 10 μM D133G, or 40 μM of the fusion inhibitor ARB and infected with A/WSN/1933 (H1N1) (A) or A/Colorado/15/2014 (H3N2) (C) (MOI = 0.5) for 5 h at 37 °C. (A and C) Representative immunofluorescent micrographs of influenza A antigen staining (green) and nuclei (blue) 5 h postinfection. Data for A and C are representative of three and four independent experiments, respectively. Scale bars indicate 100 μm. (B and D) Quantitation of the number of cells with influenza protein-positive nuclei per field, as assessed by a trained observer in a blinded fashion. Statistical analyses were performed by t test. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001, as compared to the infected, untreated group. Error bars denote the SEM.
We next sought to further delineate the step at which H84T inhibits influenza virus replication, hypothesizing that H84T would inhibit influenza virus at the attachment or fusion steps, since we previously found that those (especially the former) are the steps at which WT BanLec inhibits HIV infection (30). To investigate whether attachment was decreased by H84T, we infected MDCK cells for 1 h at 4 °C with A/WSN/1933 (H1N1) that had been preincubated for 30 min with concentrations of H84T from 1 to 10,000 nM, the timing and temperature allowing the virus only to attach but not progress to postattachment steps. After washing cells to remove excess virus, we collected the cells and extracted whole-cell RNA. We then measured the amount of cell-associated virus, which represents the amount of virus that has undergone attachment, by qRT-PCR. We observed that cells infected with virus that had been preincubated with H84T showed a minor decrease in the amount of cell-associated virus, to a much lesser extent than did cells infected with virus that had been preincubated with the monoclonal antibody H17-L19, known to inhibit attachment of this strain (34) (SI Appendix, Fig. S13A). In parallel, H84T also could not inhibit hemagglutination of turkey red blood cells, which depends on interactions between HA and red blood cells that are akin to those between HA and the cell during attachment, by the virus (SI Appendix, Fig. S13B). These results suggest that attachment is only minorly inhibited by H84T, which was surprising as this was the step at which HIV was predominantly inhibited by WT BanLec (30).
H84T Inhibits Endosomal Fusion of Influenza Virus.
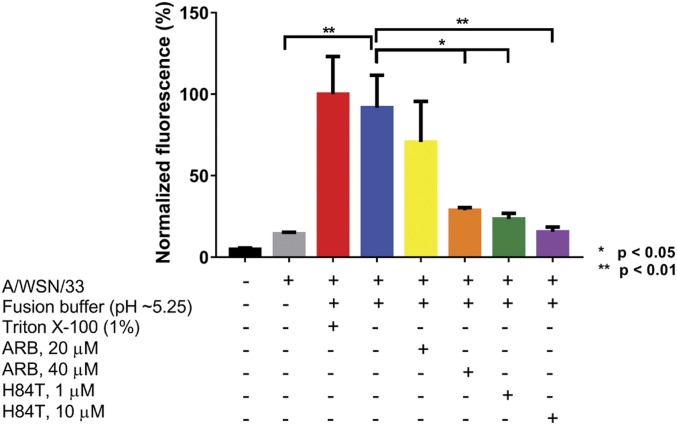
Given that inhibition of attachment does not appear to be the major mechanism of action of H84T, we next investigated whether fusion is decreased by H84T (Fig. 5). Dequenching of the fluorescent membrane probe R18 from R18-labeled virus was measured in MDCK cells pretreated with the fusion inhibitor ARB, as a positive control, or H84T. When incorporated into the viral membrane at a high molarity, R18 quenches itself, but when fusion of the viral membrane with the plasma membrane is induced by a low pH fusion buffer, R18 diffuses laterally across the much larger plasma membrane, becoming dequenched and fluorescing (35–37). Triton X-100, as a detergent, induces maximal dequenching and the highest fluorescence of R18. Labeled virus was allowed to attach to cells in the presence of ARB or H84T before the addition of fusion buffer, after which fluorescence dequenching was measured. In cells incubated with virus, fusion buffer alone increased fluorescence to levels near to those from Triton X-100–treated cells, indicating that fusion with the plasma membrane had occurred. Treatment with 40 μM ARB or 1 and 10 μM H84T significantly reduced fluorescence dequenching upon exposure to low pH, which suggests that H84T may act by inhibiting viral fusion.
Fig. 5.
H84T inhibits influenza virus fusion. MDCK cells were pretreated for 1 h at 37 °C with 1 or 10 μM H84T or 20 or 40 μM of the fusion inhibitor ARB and incubated with octadecyl R18-labeled A/WSN/1933 for 1 h at 4 °C to allow attachment only. Excess virus was removed and virus fusion with the cell membrane was triggered at 37 °C using pH ∼5.25 fusion buffer. Fluorescence dequenching of R18 was monitored on a microplate fluorometer with excitation and emission wavelengths of 560 and 590 nm, respectively. Maximal dequenching was achieved with 1% Triton X-100 set at 100%. Bars represent the mean values from duplicate wells from two independent experiments and error bars represent the SEM. Statistical analyses were performed by t test. *P < 0.05, **P < 0.01, comparing groups indicated by brackets.
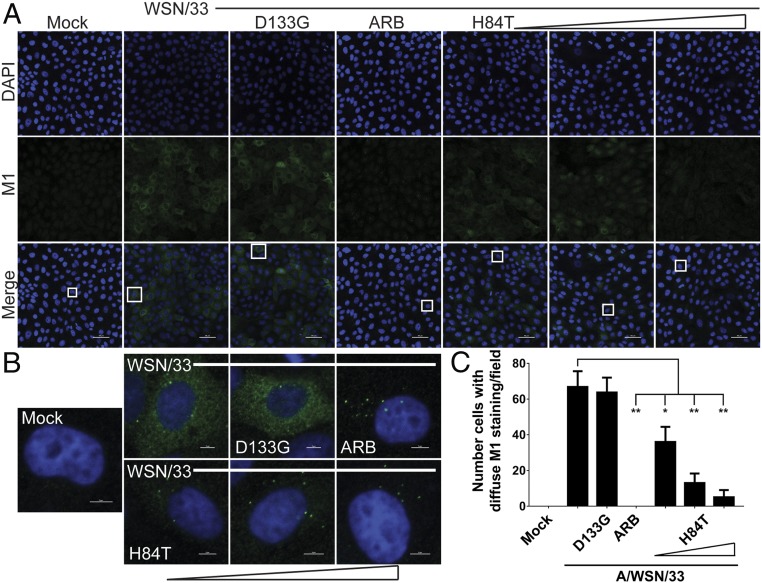
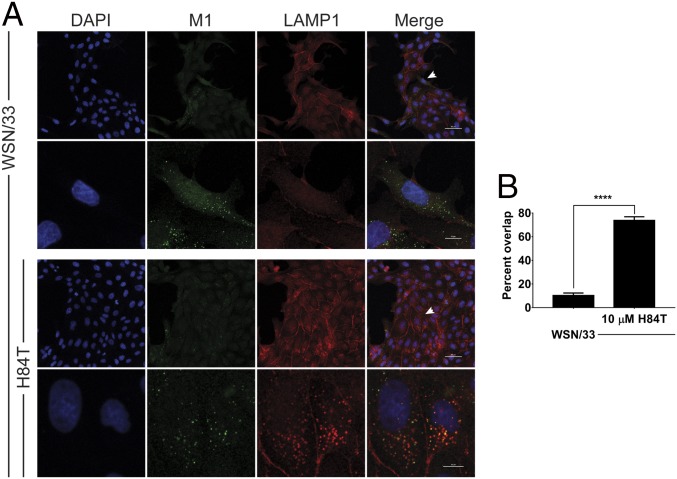
If fusion of influenza virus is inhibited by H84T, we reasoned that the next step in the virus life cycle would also be inhibited, namely uncoating of the viral ribonucleoproteins. With uncoating, the viral matrix protein (M1), which forms the scaffolding between the viral membrane and the viral ribonucleoproteins, dissociates from its prefusion location beneath the membrane and disperses throughout the cytoplasm of the cell. Detection of diffuse M1 by immunocytochemistry has thus been used at early infection time points (2.5 h, before protein translation leads to the production of more M1) to determine in which cells uncoating, and thus fusion immediately before it, has occurred (38). After 2.5 h of infection with A/WSN/1933 (MOI 0.5), many MDCK cells displayed diffuse cytoplasmic staining of M1, indicating that uncoating had occurred in these cells (Fig. 6 A and B). As expected, the number of cells with diffuse M1 staining was significantly decreased by treatment with ARB. H84T also reduced the number of cells with diffuse M1 staining in a dose-responsive manner, indicating that H84T prevents events (such as fusion) that occur before (or at) uncoating of the virus (Fig. 6C). Instead of diffuse M1 staining, virtually all of the ARB-treated infected cells and increasing numbers of infected cells treated with H84T in a dose-dependent manner exhibited perinuclear, punctate staining of M1 (Fig. 6B), consistent with localization in the late endosome. The reduction in diffuse staining of M1 was observed both when the cells were pretreated with H84T and when only the virus was pretreated with H84T, suggesting that preconditioning of cells is not required for H84T to exert its antiinfluenza activity. We performed these studies with two more recently circulating strains, A/Florida/3/2006 (H1N1) and A/Perth/16/2009 (H3N2), and again observed the same decrease in diffuse M1 staining and increase in perinuclear punctate M1 staining with H84T treatment (SI Appendix, Fig. S14). These results suggest that H84T prevents exit of influenza viruses from the endosome. Indeed, colocalization between M1 in the M1+ punctae and the late endosomal/lysosomal marker LAMP1 is high in H84T-treated cells as compared to untreated infected cells (Fig. 7). These results indicate that uncoating is inhibited by H84T, consistent with a block at the step of fusion and restriction of the virus to the endosome.
Fig. 6.
H84T reduces influenza virus uncoating. MDCK cells were pretreated for 1 h with 0.1, 1, or 10 μM H84T, 10 μM D133G, or 40 μM of the fusion inhibitor ARB and infected with A/WSN/1933 (MOI 0.5) for 2.5 h at 37 °C. (A) Representative immunofluorescent micrographs of M1 antigen staining (green) and nuclei (blue) 2.5 h postinfection. Scale bars indicate 50 μm. (B) Micrographs of single cells from A, as denoted by the boxes in A. Scale bars indicate 5 μm. Data for A and B are representative of 15 independent experiments. (C) Quantitation of the number of cells with diffuse cytoplasmic M1 staining per field, as assessed by a trained observer in a blinded fashion. Statistical analyses were performed by t test. *P < 0.05 and **P < 0.01, as compared to the infected, untreated group. Error bars represent the SEM.
Fig. 7.
H84T restricts influenza virus to the late endosomal/lysosomal compartment. (A) Representative immunofluorescent micrographs of M1 (green) and LAMP1 antigen staining (red) and nuclei (blue). Following 1 h pretreatment with H84T (10 μM), MDCK cells were incubated with A/WSN/1933 (H1N1) at 4 °C for 1 h to allow only for attachment, excess virus was removed, treatment-containing medium was replaced, and the cells were incubated at 37 °C for an additional 3 h. The second and fourth rows are micrographs of one or two cells from the first and third rows, respectively, indicated by the arrows. Data are representative of five independent experiments. Scale bars indicate 50 μm in the first and third rows and 10 μm in the second and fourth rows. (B) Quantitation of the percent of M1+ punctae that are also LAMP1+ (percent overlap). Statistical analyses were performed by t test. ****P < 0.0001, as compared to the infected, untreated group. Error bars represent the SEM.
H84T Is Internalized into the Late Endosomal/Lysosomal Compartment.
Because H84T inhibits fusion between the virus and the endosomal membrane, we hypothesized that it might be internalized into the endosome in infected cells, but not in uninfected cells, via binding to HA. However, interestingly, H84T treatment alone increases the size of LAMP1+ punctae in cells relative to untreated cells (SI Appendix, Fig. S15), suggesting that H84T may be internalized even in the absence of virus, as has been demonstrated for other lectins (39, 40). As predicted, immunofluorescent staining for BanLec revealed that H84T is internalized into punctae that resemble late endosomes/lysosomes (SI Appendix, Fig. S16) in both infected and uninfected cells; D133G is internalized into cells to a dramatically lesser extent. To examine whether H84T is internalized into the late endosomal/lysosomal compartment in the absence of influenza virus infection, we next treated cells with His-tagged H84T or D133G and performed immunofluorescent staining for the His tag and LAMP1. Colocalization between the His tag and LAMP1 was high in H84T-treated cells (SI Appendix, Fig. S17), indicating that H84T localizes to the late endosome/lysosome, whereas D133G-treated cells exhibited minimal His tag staining, consistent with the low degree of BanLec staining in D133G-treated cells (SI Appendix, Fig. S16). In rare D133G-treated cells, His tag staining resembled that in H84T-treated cells and colocalization between the His tag and LAMP1 was high (SI Appendix, Fig. S17, Inset), suggesting that in the rare cases that D133G is internalized, it localizes to the same compartment as does H84T. Given that both M1 and LAMP1, as well as H84T and LAMP1, colocalize to a high degree in H84T-treated cells, it is likely that H84T and the virus also colocalize in the late endosomal/lysosomal compartment, ideally positioning H84T for inhibition of fusion.
Discussion
Due to their ability to bind to glycan structures, especially high-mannose preferentially expressed on the surface of pathogenic viruses, lectins have been considered as potential antiviral therapeutics (41). In the case of influenza, cyanovirin-N (CV-N), surfactant protein (SP)-A, SP-D, and mannose-binding lectin are among the lectins shown to have activity in vitro or in vivo (42–44). We have demonstrated that an engineered banana lectin, H84T BanLec, is highly active against an array of influenza virus strains. While it is somewhat difficult to compare the overall sensitivity of influenza viruses to the different lectins without head-to-head comparisons, H84T BanLec would appear to be similar in vitro and equal to, or perhaps better than most previously tested lectins (e.g., CV-N EC50 = 0.005 to >10 μg/mL against tested influenza virus strains). It is notable that H84T is quite broad-spectrum, capable of inhibiting multiple oseltamivir-resistant as well all other influenza virus strains tested in this and our previous study (12); H84T inhibits both influenza A and B type viruses and a representative of the currently circulating 3C.2a clade of H3N2 viruses, which proved especially deadly in the 2017 to 2018 Northern Hemisphere influenza season (2). The finding that there is some synergy between H84T and oseltamivir raises the possibility of combination therapy, which might be more effective than single agents alone and limit the development of resistance.
Paralleling the reduced mitogenicity of H84T compared to WT BanLec that we previously demonstrated in vitro, systemic administration of H84T does not result in the robust inflammatory response seen after administration of the WT lectin, indicating that we have truly removed mitogenicity from H84T by substituting one specific amino acid for another. Mitogenicity has primarily been addressed as an in vitro concept, with studies examining mitogenicity of lectins in vivo focusing on local rather than systemic effects (45). However, through our study, we have been able to characterize in vivo the effects of a systemically delivered mitogenic versus nonmitogenic lectin. Lessening mitogenic activity of BanLec via the H84T mutation has removed a major hurdle in the development of an antiviral lectin for clinical use, since mitogenicity has been viewed as the biggest drawback for most lectins (11). In addition, we have shown that H84T BanLec is very safe when delivered by the intraperitoneal and subcutaneous routes, with this and our previous study (12) showing that it is also safe when administered intranasally.
H84T delivered systemically by the intraperitoneal route possesses a long terminal half-life, indicating that infrequent or even single dosing could potentially be efficacious, which would be clinically advantageous. However, intravenous administration, which is largely analogous to intraperitoneal administration in the mouse, would not be ideal for all clinical situations (e.g., treating outpatients with influenza virus infections). Oral administration is not a feasible clinical strategy, as it is known that alimentary lectins do not effectively distribute to the blood (46–48), but as we have shown, subcutaneous and intranasal administration are efficacious and have potential as eventual treatments in the outpatient setting. Thus, H84T BanLec shows promise for use in both hospitalized patients and outpatients. It is notable that administration of H84T can be delayed for up to 72 h and still offer 80% protection by the intraperitoneal route and 50% protection by the subcutaneous route. People have antibodies against banana lectin (25), which means that they would potentially develop or already possess antibodies against H84T, but this is not expected to limit activity given that the efficacy of H84T against influenza infection was not lessened in mice with anti-H84T antibodies. It is worth pointing out that the class of antibody that people develop against bananas is IgG4 (25), which is widely thought to be tolerogenic and so potentially less likely to disrupt therapeutic efficacy of H84T. Of course, whether humans develop therapeutically limiting antibodies to H84T would ultimately need to be determined in humans.
Our work indicates that H84T binds to HA specifically, as binding to baculovirus-derived HA can be inhibited by a known ligand of BanLec but not by a noninteracting monosaccharide and as carbohydrate digestion by PNGase F reduces binding to virus-derived HA. Furthermore, we demonstrate that H84T functions as an antiinfluenza agent primarily via fusion inhibition. Carbohydrate binding is essential for both binding and fusion inhibition, as the carbohydrate binding site mutant D133G does not bind as efficiently and is not inhibitory to fusion. A key question to address was where HA might be bound by H84T, as both its head (HA1) and stalk (HA2) regions are glycosylated with high-mannose type N-glycans (13), among other glycans, which are ligands for H84T. Neutralizing antibodies that function as fusion inhibitors typically bind the stalk of HA (49–51), which contains the fusion peptide and other components necessary for fusion (52). However, work by others indicates that an inhibitor that binds to the head of HA can target fusion (53) and it is known that the antiinfluenza lectin cyanovirin-N binds HA1 (43), so it was not wholly unexpected that H84T would bind HA1. A potential limitation of H84T is that it may require that its target be glycosylated and pandemic strains are sometimes less heavily glycosylated than seasonal strains, but some glycosylation is virtually always present, even in pandemic strains (13). Interestingly, the number of glycosites that a certain strain possesses seems to correlate with the EC50 concentration of H84T against that strain. Contemporary H3 viruses have more glycosites than contemporary H1 viruses (13) and H84T has a lower EC50 concentration against the former strains, which correlates with the higher amount of methyl α-d-mannopyranoside needed to compete for binding to H3 versus H1 HA. In addition, the amount of binding to strains as determined by lectin blot generally correlated with the EC50 concentrations of H84T against those strains, with the most binding to the H3N2 strain, followed by binding to influenza B, then the H1N1 strain. Of course, multiple other strains of each subtype would need to be evaluated to further clarify this issue. It is presently not possible to assess whether binding of H84T correlates with the number of high-mannose N-glycans in particular on certain HA molecules, as the number of high-mannose glycans on specific HAs cannot be predicted by the sequence of the protein and has to be empirically determined (54), but overall it appears that binding to HA by H84T correlates with the number of glycans on HA.
H84T treatment, regardless of infection status, enlarges the LAMP1+ compartment, consistent with the observation that many lectins are internalized by endocytosis and that some induce the formation of large lysosomes (39, 40). H84T, and to a much lesser extent D133G, localize to the late endosome/lysosome. Although H84T is internalized into the endosomal compartment and enlarges it, the fact that fusion at the plasma membrane is inhibited by H84T (Fig. 5), in addition to uncoating at the endosomal membrane, suggests that the antiinfluenza activity of H84T does not depend primarily on alteration of the endosomal compartment. Consistent with these observations, preconditioning of the endosomal compartment with H84T is not required for viral inhibition, since either cells or virus can be pretreated with H84T and the lectin is still highly active against uncoating. We therefore posit that binding of H84T to HA is important for the inhibitory effect of H84T. We propose a model in which H84T, internalized in both the presence and absence of virus, likely by endocytosis, accumulates in the late endosomal/lysosomal compartment where fusion occurs and is thus positioned at a key watch point to interact with HA and thereby prevent entry of virus into the cytoplasm. Since H84T disrupts fusion of the virus and prevents its release into the cytoplasm, it is reasonable to think that the virus might be degraded once it reaches the lysosome, accounting for the ability of H84T to decrease influenza virus replication overall.
Our data demonstrate that H84T BanLec has potential as a new antiviral against influenza viruses. It is very well tolerated and nonmitogenic in vivo and is highly efficacious against otherwise lethal influenza virus infection via three distinct routes of administration, even when treatment is delayed. H84T is highly broad-spectrum in that it inhibits replication of all oseltamivir-resistant and -sensitive strains and all influenza A and B strains tested, highlighting its promise as an empiric drug for influenza illness, which will be critically needed in the event of a new pandemic influenza virus or increasing resistance to neuraminidase inhibitors. H84T is broad-spectrum not only in its antiinfluenza activity, but also in the sense that it inhibits HIV and hepatitis C virus as reported previously (12), and Ebola (55), among other viruses. The present study, along with our previous work, underscores the value of engineering antiviral lectins to be nonmitogenic and safe in vivo, as H84T and perhaps eventually other similarly engineered lectins could be leveraged as a therapeutic arsenal against a diverse array of viruses.
Materials and Methods
H84T and D133G BanLec Purification.
Recombinant His-tagged H84T and D133G were prepared from Eschrichia coli as previously described (12), except that non–His-tagged H84T, which was used in experiments unless otherwise stated, was prepared using a Sephadex G-75 column instead of a Ni-NTA agarose column, as described in SI Appendix.
Cells, Viruses, and Antiinfluenza Antibodies.
MDCK cells were maintained in DMEM (Gibco) supplemented with 10% FBS and 1% penicillin/streptomycin at 37 °C in 5% CO2. Influenza virus infection was performed on monolayers of MDCK cells in virus growth buffer (DMEM supplemented with 0.187% BSA and 25 mM Hepes). Supernatant from 293T cells transfected with eight influenza gene plasmids was used to propagate A/WSN/33 (H1N1) on MDCK cells, as previously described (56). Recombinant virus containing HA and NA genes from A/Colorado/15/2014 (H3N2) and other genes from A/Puerto Rico/8/1934 (H1N1) was engineered by reverse genetics and propagated on MDCK-SIAT1 cells to limit adaptation and glycan changes. Additional strains and antiinfluenza antibodies are indicated in figure legends and in SI Appendix.
ELISA.
For ELISA, 96-well ELISA plates (Thermo Fisher Scientific) were coated overnight with 100-ng recombinant H1 or H3 HA proteins from Influenza A/California/07/2009 (H1N1)pdm09 and Influenza A/Perth/16/2009 (H3N2), respectively, produced in baculovirus, for determination of H84T binding to HA. To determine whether human sera contained anti-H84T antibodies, 100 ng of H84T were used to coat plates. ELISA experiments were performed as described in SI Appendix.
Lectin Blot Analysis.
Lectin blot analysis was performed as described previously (57) and as described in the legend of Fig. 3. Further details are provided in SI Appendix.
Virus Infection and Immunofluorescence Staining.
MDCK cells were plated in eight-well chamber slides, pretreated for 1 h with 0.05 to 10 μM H84T, 10 μM D133G, or 40 μM ARB, and infected at 37 °C with A/WSN/1933 (H1N1), A/Perth/2009 (H3N2), or A/Florida/2006 (H1N1) at a multiplicity of infection (MOI) of 0.5 in the presence of H84T, D133G, or ARB. Where indicated, virus alone (rather than cells) was pretreated with H84T, D133G, or ARB and uncoating examined. M1/LAMP1 colocalization studies were performed as described in Fig. 7. Spreading infections of B/Brisbane/60/2008 were performed following pretreatment, removal of excess virus following 1 h of incubation, and replacement of treatment-containing medium at MOI = 0.05 for 16 to 18 h. Infected cells were washed twice in virus growth buffer and once in PBS before fixation in 2% PFA/PBS. Immunofluoresence assays were then performed as described in SI Appendix.
Neutral Red Assay for Assessment of Antiinfluenza B Activity.
Neutral red assays were performed to determine the 50% effective concentrations (EC50) of H84T against B/Brisbane/60/2008 (Victoria lineage) and B/Florida/4/2006 (Yamagata lineage) and 50% cytotoxic concentration (CC50) on MDCK cells, as described in SI Appendix.
In Vitro Cytotoxicity in MDCK Cells.
Monolayers of MDCK cells in 96-well plates were treated for 24 h with H84T or D133G BanLec (0 to 15 μM) in 100 μL medium. Cytotoxicity was evaluated by MTT assay (Roche) as described in SI Appendix.
Cell-Associated Virus Assay.
Virus was incubated with 0, 1, 10, 100, 1,000, or 10,000 nM H84T or with 10 μg/mL H17-L19 monoclonal antibody for 30 min at room temperature, after which the virus-H84T mixture was incubated with MDCK cells for 1 h at 4 °C, allowing only for attachment. Following three washes with cold virus growth buffer, cells were collected in TRIzol reagent (Thermo Fisher Scientific) and whole-cell RNA extracted. To measure the relative amount of cell-associated virus normalized to GAPDH expression, qRT-PCR was performed using primers specific to influenza A M1 and to GAPDH. Primer sequences are provided in SI Appendix.
Hemagglutination Inhibition Assay.
Hemagglutination inhibition assays were carried out using turkey red blood cells as described in SI Appendix.
Virus-Cell Fusion Assay.
MDCK cells were pretreated for 1 h with 1 or 10 μM H84T or 20 or 40 μM of the fusion inhibitor arbidol (ARB, Cayman Chemical), incubated with octadecyl rhodamine (R18, Sigma-Aldrich)-labeled A/WSN/1933 virus for 1 h at 4 °C to allow attachment of the virus, then washed three times in virus growth buffer and once in PBS to remove unbound virus. Fluorescence dequenching was assessed as previously described (37) and as described in SI Appendix.
Safety of Intraperitoneal H84T.
Eight-week-old female BALB/c mice were injected intraperitoneally with two 50-mg/kg doses of WT, H84T, or D133G BanLec administered 1 mo apart (n = 10 per group) and weighed and scored for appearance and injection site lesions.
Pharmacokinetics of Single-Dose Intraperitoneal H84T.
To determine the dose-dependent pharmacokinetic profile of H84T following a single intraperitoneal dose, groups of 44 5-wk-old male CFW mice were injected intraperitoneally on a single occasion with 5, 15, or 50 mg/kg H84T in PBS. Plasma and lung samples from multiple time points were processed and analyzed by ELISA as described in SI Appendix.
Distribution of Single-Dose Intraperitoneal H84T.
To determine the distribution of H84T following a single intraperitoneal dose, 15-wk-old female 129svev/B6 mice were injected intraperitoneally with 50 mg/kg H84T. Organs were harvested at multiple time points and processed for analysis by Western blot, as further described in SI Appendix.
Therapeutic Efficacy of Systemic H84T Against Influenza in BALB/c Mice.
Virus.
Influenza A/WSN/HA(NC/2099-N225G)/1933 virus, produced as described in SI Appendix and as described previously (56, 58).
Experimental design.
For the intraperitoneal efficacy study, groups of 10 mice were treated by the intraperitoneal route, as indicated in Fig. 2. Control groups included mice treated with 10 mg/kg/d oseltamivir twice daily for 5 d beginning 4, 24, 48, or 72 hpi, or treated with PBS as placebo (15 mice) twice daily for the first 5 d postinfection, then once daily for the next 3 d to match treatment beginning at 4 and 72 hpi. For the subcutaneous efficacy study, mice were treated by subcutaneous injection using a 50-mg/kg dose of H84T either once or twice within 4 d postinfection. A group of 10 mice was given oseltamivir by oral gavage twice daily for 5 d with 10 mg/kg of oseltamivir, starting 48 hpi. Control mice treated with PBS as placebo were dosed according to the same regimen as H84T. Further details are provided in SI Appendix.
Administration of BanLec for Assessment of Anti-BanLec Antibody Production.
Eight-week-old female BALB/c mice (Taconic) were anesthetized with isoflurane then injected intraperitoneally with either 50 mg/kg V66D/H84T/A100D BanLec, a variant of H84T engineered to increase solubility for biochemical assays, or with PBS (n = 5 per group). Twenty-one days later, mice received a second injection of either BanLec or PBS as before. Mice were euthanized 2 d after the second administration by the University of Michigan Unit for Laboratory Animal Medicine. Mouse sera were collected and stored frozen prior to use for detection of anti-BanLec antibodies by ELISA (see ELISA in Materials and Methods).
Therapeutic Efficacy of Intranasal H84T Against H3N2 Influenza Virus.
The efficacy of intranasal H84T in C57BL/6J mice challenged with A/Hong Kong/8/1968 (H3N2) was assessed as described in SI Appendix. Virus was delivered by aerosolization as previously described (59). For each group, n = 12.
Animal Care and Use.
Animal studies were conducted in accordance with institutional policies as described in SI Appendix.
Quantitation of Immunofluorescence Staining.
ImageJ was used to quantitate the number and diameter of punctae in immunofluorescence micrographs, and the percent overlap of punctae in different color channels, as described in SI Appendix.
Statistics.
All statistical analyses were performed using GraphPad Prism 6 and 7. Specific tests used are noted in figure legends. All t tests performed were two-tailed.
Materials and Data Availability.
H84T must be obtained through material transfer agreement. All data are available in the main text and SI Appendix.
Supplementary Material
Acknowledgments
We thank A. Krafft and the Influenza Drug Development and Diagnostic Development Program at the National Institute of Allergy and Infectious Diseases for support for testing of H84T against influenza virus in vitro and in vivo; S. Bedi from the University of Michigan for providing supernatants from influenza plasmid-transfected cells for virus propagation; S. Hensley from the University of Pennsylvania for providing H17-L19 antibody and 3C.2a virus; S. Yalavarthi and J. Knight from the University of Michigan for providing human donor sera; and E. Martens from the University of Michigan for providing galactose and yeast mannan. This work was supported by grants to D.M.M. from the Defense Threat Reduction Agency (HDTRA1-15-1-0067) and the University of Michigan MTRAC (Michigan Translational Research and Commercialization Life Sciences). E.M.C.-D. was supported by training grants from the University of Michigan Medical Scientist Training Program (T32 GM07863) and the Molecular Mechanisms of Microbial Pathogenesis Training Program (T32 AI007528) from the National Institutes of Health, and by a National Research Service Award (1F31AI136615-01) from the National Institute of Allergy and Infectious Diseases.
Footnotes
Competing interest statement: D.M.M. is an inventor on a patent for H84T BanLec and the founder of the company Virule, which aims to commercialize H84T BanLec.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1915152117/-/DCSupplemental.
References
- 1.Osterholm M. T., Kelley N. S., Sommer A., Belongia E. A., Efficacy and effectiveness of influenza vaccines: A systematic review and meta-analysis. Lancet Infect. Dis. 12, 36–44 (2012). [DOI] [PubMed] [Google Scholar]
- 2.Flannery B., et al. , Interim estimates of 2017-18 seasonal influenza vaccine effectiveness—United States, February 2018. MMWR Morb. Mortal. Wkly. Rep. 67, 180–185 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koszalka P., Tilmanis D., Hurt A. C., Influenza antivirals currently in late-phase clinical trial. Influenza Other Respir. Viruses 11, 240–246 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayden F. G., et al. ; Baloxavir Marboxil Investigators Group , Baloxavir marboxil for uncomplicated influenza in adults and adolescents. N. Engl. J. Med. 379, 913–923 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Jefferson T., Jones M., Doshi P., Del Mar C., Neuraminidase inhibitors for preventing and treating influenza in healthy adults: Systematic review and meta-analysis. BMJ 339, b5106 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oxford J. S., Antivirals for the treatment and prevention of epidemic and pandemic influenza. Influenza Other Respir. Viruses 1, 27–34 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poland G. A., Jacobson R. M., Ovsyannikova I. G., Influenza virus resistance to antiviral agents: A plea for rational use. Clin. Infect. Dis. 48, 1254–1256 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takizawa N., Momose F., Morikawa Y., Nomoto A., Influenza A virus hemagglutinin is required for the assembly of viral components including bundled vRNPs at the lipid raft. Viruses 8, 249 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ali A., Avalos R. T., Ponimaskin E., Nayak D. P., Influenza virus assembly: Effect of influenza virus glycoproteins on the membrane association of M1 protein. J. Virol. 74, 8709–8719 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chlanda P., et al. , Structural analysis of the roles of influenza A virus membrane-associated proteins in assembly and morphology. J. Virol. 89, 8957–8966 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huskens D., Vermeire K., Vandemeulebroucke E., Balzarini J., Schols D., Safety concerns for the potential use of cyanovirin-N as a microbicidal anti-HIV agent. Int. J. Biochem. Cell Biol. 40, 2802–2814 (2008). [DOI] [PubMed] [Google Scholar]
- 12.Swanson M. D., et al. , Engineering a therapeutic lectin by uncoupling mitogenicity from antiviral activity. Cell 163, 746–758 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tate M. D., et al. , Playing hide and seek: How glycosylation of the influenza virus hemagglutinin can modulate the immune response to infection. Viruses 6, 1294–1316 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meijer A., et al. ; European Influenza Surveillance Scheme , Oseltamivir-resistant influenza virus A (H1N1), Europe, 2007-08 season. Emerg. Infect. Dis. 15, 552–560 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dharan N. J., et al. ; Oseltamivir-Resistance Working Group , Infections with oseltamivir-resistant influenza A(H1N1) virus in the United States. JAMA 301, 1034–1041 (2009). [DOI] [PubMed] [Google Scholar]
- 16.Smee D. F., Julander J. G., Tarbet E. B., Gross M., Nguyen J., Treatment of oseltamivir-resistant influenza A (H1N1) virus infections in mice with antiviral agents. Antiviral Res. 96, 13–20 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lackenby A., et al. , Emergence of resistance to oseltamivir among influenza A(H1N1) viruses in Europe. Euro Surveill. 13, 8026 (2008). [DOI] [PubMed] [Google Scholar]
- 18.Sheu T. G., et al. , Surveillance for neuraminidase inhibitor resistance among human influenza A and B viruses circulating worldwide from 2004 to 2008. Antimicrob. Agents Chemother. 52, 3284–3292 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simonsen L., et al. , The genesis and spread of reassortment human influenza A/H3N2 viruses conferring adamantane resistance. Mol. Biol. Evol. 24, 1811–1820 (2007). [DOI] [PubMed] [Google Scholar]
- 20.Bright R. A., Shay D. K., Shu B., Cox N. J., Klimov A. I., Adamantane resistance among influenza A viruses isolated early during the 2005-2006 influenza season in the United States. JAMA 295, 891–894 (2006). [DOI] [PubMed] [Google Scholar]
- 21.Schinazi R. F., Peters J., Williams C. C., Chance D., Nahmias A. J., Effect of combinations of acyclovir with vidarabine or its 5′-monophosphate on herpes simplex viruses in cell culture and in mice. Antimicrob. Agents Chemother. 22, 499–507 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prichard M. N., Shipman C. Jr, A three-dimensional model to analyze drug-drug interactions. Antiviral Res. 14, 181–205 (1990). [DOI] [PubMed] [Google Scholar]
- 23.Su S., et al. , Comparing clinical characteristics between hospitalized adults with laboratory-confirmed influenza A and B virus infection. Clin. Infect. Dis. 59, 252–255 (2014). [DOI] [PubMed] [Google Scholar]
- 24.Baker M. P., Reynolds H. M., Lumicisi B., Bryson C. J., Immunogenicity of protein therapeutics: The key causes, consequences and challenges. Self Nonself 1, 314–322 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koshte V. L., Aalbers M., Calkhoven P. G., Aalberse R. C., The potent IgG4-inducing antigen in banana is a mannose-binding lectin, BanLec-I. Int. Arch. Allergy Immunol. 97, 17–24 (1992). [DOI] [PubMed] [Google Scholar]
- 26.Matrosovich M. N., Matrosovich T. Y., Gray T., Roberts N. A., Klenk H.-D., Neuraminidase is important for the initiation of influenza virus infection in human airway epithelium. J. Virol. 78, 12665–12667 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Su B., et al. , Enhancement of the influenza A hemagglutinin (HA)-mediated cell-cell fusion and virus entry by the viral neuraminidase (NA). PLoS One 4, e8495 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi X., Jarvis D. L., Protein N-glycosylation in the baculovirus-insect cell system. Curr. Drug Targets 8, 1116–1125 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kanagawa M., et al. , Structural basis for multiple sugar recognition of Jacalin-related human ZG16p lectin. J. Biol. Chem. 289, 16954–16965 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swanson M. D., Winter H. C., Goldstein I. J., Markovitz D. M., A lectin isolated from bananas is a potent inhibitor of HIV replication. J. Biol. Chem. 285, 8646–8655 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cuskin F., et al. , Human gut Bacteroidetes can utilize yeast mannan through a selfish mechanism. Nature 517, 165–169 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Komath S. S., Kavitha M., Swamy M. J., Beyond carbohydrate binding: New directions in plant lectin research. Org. Biomol. Chem. 4, 973–988 (2006). [DOI] [PubMed] [Google Scholar]
- 33.Kadam R. U., Wilson I. A., Structural basis of influenza virus fusion inhibition by the antiviral drug Arbidol. Proc. Natl. Acad. Sci. U.S.A. 114, 206–214 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Doud M. B., Hensley S. E., Bloom J. D., Complete mapping of viral escape from neutralizing antibodies. PLoS Pathog. 13, e1006271 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoekstra D., de Boer T., Klappe K., Wilschut J., Fluorescence method for measuring the kinetics of fusion between biological membranes. Biochemistry 23, 5675–5681 (1984). [DOI] [PubMed] [Google Scholar]
- 36.Gilbert J. M., Mason D., White J. M., Fusion of Rous sarcoma virus with host cells does not require exposure to low pH. J. Virol. 64, 5106–5113 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chu V. C., Whittaker G. R., Influenza virus entry and infection require host cell N-linked glycoprotein. Proc. Natl. Acad. Sci. U.S.A. 101, 18153–18158 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Banerjee I., et al. , Influenza A virus uses the aggresome processing machinery for host cell entry. Science 346, 473–477 (2014). [DOI] [PubMed] [Google Scholar]
- 39.Poretz R. D., et al. , Lectin-induced accumulation of large lysosomes in cultured fibroblasts. Exp. Cell Res. 128, 133–142 (1980). [DOI] [PubMed] [Google Scholar]
- 40.Qaddoumi M., Lee V. H. L., Lectins as endocytic ligands: An assessment of lectin binding and uptake to rabbit conjunctival epithelial cells. Pharm. Res. 21, 1160–1166 (2004). [DOI] [PubMed] [Google Scholar]
- 41.François K. O., Balzarini J., Potential of carbohydrate-binding agents as therapeutics against enveloped viruses. Med. Res. Rev. 32, 349–387 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smee D. F., et al. , Treatment of influenza A (H1N1) virus infections in mice and ferrets with cyanovirin-N. Antiviral Res. 80, 266–271 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O’Keefe B. R., et al. , Potent anti-influenza activity of cyanovirin-N and interactions with viral hemagglutinin. Antimicrob. Agents Chemother. 47, 2518–2525 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holmskov U., Thiel S., Jensenius J. C., Collections and ficolins: Humoral lectins of the innate immune defense. Annu. Rev. Immunol. 21, 547–578 (2003). [DOI] [PubMed] [Google Scholar]
- 45.Izbicka E., Dunstan C. R., Horn D., Adams R., Mundy G. R., Mitogenic lectin concanavalin A induces calvarial bone formation in vivo via indomethacin-sensitive pathway. Calcif. Tissue Int. 60, 204–209 (1997). [DOI] [PubMed] [Google Scholar]
- 46.Wang Q., Yu L. G., Campbell B. J., Milton J. D., Rhodes J. M., Identification of intact peanut lectin in peripheral venous blood. Lancet 352, 1831–1832 (1998). [DOI] [PubMed] [Google Scholar]
- 47.Kilpatrick D. C., Pusztai A., Grant G., Graham C., Ewen S. W., Tomato lectin resists digestion in the mammalian alimentary canal and binds to intestinal villi without deleterious effects. FEBS Lett. 185, 299–305 (1985). [DOI] [PubMed] [Google Scholar]
- 48.Pusztai A., Greer F., Grant G., Specific uptake of dietary lectins into the systemic circulation of rats. Biochem. Soc. Trans. 17, 481–482 (1989). [Google Scholar]
- 49.Corti D., et al. , A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science 333, 850–856 (2011). [DOI] [PubMed] [Google Scholar]
- 50.Wei C.-J., et al. , Elicitation of broadly neutralizing influenza antibodies in animals with previous influenza exposure. Sci. Transl. Med. 4, 147ra114 (2012). [DOI] [PubMed] [Google Scholar]
- 51.Kallewaard N. L., et al. , Structure and function analysis of an antibody recognizing all influenza A subtypes. Cell 166, 596–608 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lear J. D., DeGrado W. F., Membrane binding and conformational properties of peptides representing the NH2 terminus of influenza HA-2. J. Biol. Chem. 262, 6500–6505 (1987). [PubMed] [Google Scholar]
- 53.Shen C., et al. , A multimechanistic antibody targeting the receptor binding site potently cross-protects against influenza B viruses. Sci. Transl. Med. 9, eaam5752 (2017). [DOI] [PubMed] [Google Scholar]
- 54.Stadlmann J., et al. , Comparative glycoproteomics of stem cells identifies new players in ricin toxicity. Nature 549, 538–542 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Covés-Datson E. M., et al. , Inhibition of Ebola virus by a molecularly engineered banana lectin. PLoS Negl. Trop. Dis. 13, e0007595 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hoffmann E., Neumann G., Kawaoka Y., Hobom G., Webster R. G., A DNA transfection system for generation of influenza A virus from eight plasmids. Proc. Natl. Acad. Sci. U.S.A. 97, 6108–6113 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sato T., Lectin-probed Western blot analysis. Methods Mol. Biol. 1200, 93–100 (2014). [DOI] [PubMed] [Google Scholar]
- 58.Mishin V. P., Novikov D., Hayden F. G., Gubareva L. V., Effect of hemagglutinin glycosylation on influenza virus susceptibility to neuraminidase inhibitors. J. Virol. 79, 12416–12424 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kirkpatrick C. T., et al. , Inducible lung epithelial resistance requires multisource reactive oxygen species generation to protect against viral infections. MBio 9, e00696-18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.