Abstract
Objective.
Little is known about the frequency and severity of hand dysfunction in individuals with multiple sclerosis (MS). Hence, we sought to determine the extent that quantitative tests of hand function detect changes over time, evaluate their relationship to global disability measures, and identify predictors of hand function.
Methods.
One-hundred and forty-seven individuals with MS were included (96 women, 84 relapsing-remitting MS [RRMS]) along with 35 age-and-sex matched controls. Quantitative tests of hand function (grip strength, pinch strength, 9 hole peg test [9HPT], finger tapping) and leg strength were acquired and normalized to age and sex. Expanded Disability Status Scale (EDSS) and timed 25 foot walk were also obtained. Spearman correlations, multivariate regression models and mixed effects linear regression were used for analysis.
Results.
Our cohort had an EDSS of 3.6±2.2 (median±SD) and age 44.6±11.9 years. Follow up time was up to 5 years. At baseline, 14/63 individuals with progressive MS (PMS) required more than twice as much time to complete the 9HPT using their dominant hand, compared to controls. Similarly, 11 individuals with PMS had less than 50% of grip strength and 6 had less than 50% of pinch strength, compared to controls. Additionally, 7 individuals with PMS were found to be at least 50% slower than controls in finger tapping. Over two years, 27/85 individuals with MS had more than 20% worsening in their 9HPT results from baseline (17 RRMS, 10 PMS) and 37/74 (20 RRMS, 17 PMS) had more than 20% worsening in their grip strength compared to baseline.
Conclusions.
Hand function is commonly impaired in individuals with MS. Assessing hand dysfunction with dynamometry and the 9HPT could help improve the precision of detecting changes in hand function over time in MS, and may be more sensitive in detecting changes in PMS. These quantitative tests may be useful as outcome measures in clinical trials using neuroprotective or reparative therapies and rehabilitative interventions.
Keywords: multiple sclerosis, motor control, hand function, outcome measure, rehabilitation
INTRODUCTION
Multiple sclerosis (MS) is a central nervous system disease that can cause many neurological deficits and lead to disability over time. The global disability in MS is often evaluated by rating scales such as the Expanded Disability Status Scale (EDSS) score.1 However, limited information is provided by the EDSS and other rating scales about what specific impairments are causing the functional disability seen in individuals with MS. Moreover, the EDSS is heavily influenced by an individual’s ability to ambulate with little emphasis on other important functional domains like hand function.
Recent studies have attempted to explore the effects of specific medications on upper extremity function.2,3 However, little is known about the frequency and severity of hand dysfunction in individuals with MS despite how disabling these deficits can be and how they negatively impact an individual’s quality of life especially in those with more advanced disease. Hand dysfunction - along with ambulation, fatigue, and cognitive dysfunction – has been associated with increased unemployment which highlights the importance of gaining a better understanding of how common and severe are these deficits in MS.4 Strength and coordination appear to be distinct functional dimensions that affect hand function, although currently there is limited data available on how to best measure these hand functions.
Hence, there is a great need for more clinical biomarkers of disability that have the capability of detecting disease progression or intervention responsiveness especially with the emerging putative neuroreparative therapies. The goal of this study is to determine the extent that quantitative tests of hand function detect changes over time, evaluate their relationship to global disability measures, and identify predictors of hand function.
METHODS
Participants
Hand function data was collected as part of a longitudinal study of functional abilities in people with MS at the Johns Hopkins MS Center from October 2004 to December 2009. Age and gender matched healthy controls were recruited by convenience sampling at the Kennedy Krieger Institute. The study was approved by the Institutional Review Board of Johns Hopkins Medical Institute and Kennedy Krieger Institute. Written informed consent was obtained from all study participants.
One-hundred and forty-seven individuals with clinically definite MS according to the 2005 McDonald criteria5 were included in this cross sectional study; 84 with relapsing-remitting MS (RRMS) and 63 with progressive MS (PMS; primary and secondary progressive combined). Ninety-five of the above individuals with MS had longitudinal data available over at least two years (53 RRMS and 42 PMS).
Individuals who had experienced an MS relapse within the preceding two months either by clinical judgment or as evidenced by gadolinium enhancement on magnetic resonance imaging (MRI) were not included. Participants were also excluded if they had orthopedic or other neurologic conditions that might interfere with study procedures. We enrolled 35 age and sex matched healthy controls without neurological disease and with no known limitation in hand function.
Quantitative Measures
The following quantitative tests of hand function (i.e., strength and coordination) were obtained bilaterally for all study participants: grip strength was measured using a hand-held dynamometer (Lafayette Industries), pinch strength was obtained using a mechanical pinch gauge (B&L Engineering), finger tapping was assessed by using a digital finger tapping test (Western Psychological Services), and a nine-hole-peg test (9HPT) was obtained using a Purdue Pegboard (Rolyan, Sammons Preston). We chose grip and pinch strength and 9HPT because they are known to have strong interrater and test-retest reliability in persons with MS.6–8 Finger tapping was chosen because it has been shown to reliably distinguish motor dysfunction due to cerebellar problems or weakness from healthy controls.9 Hip flexion strength was obtained as a measure of lower extremity function using a Microfet2 hand-held dynamometer (Hoggan Health Industries, West Jordan, UT) with the same methods as previously published.10 Timed 25 foot walk test (T25FW) was obtained as a measure of ambulation and calculated using a stopwatch.7,10 We chose these lower extremity tests because hip flexion strength can be reliably quantified, it is a common site of weakness in MS and walking is an important functional limitation.10
The average of two trials was calculated and recorded for all measures except for the finger tapping test, where the average of five trials was used as per established protocols.11 Test-retest reliability has previously been shown to be highest in hand function tests when the mean of multiple trials was used rather than one trial or the highest score of multiple trials.8 As a measure of overall disability, all MS patients had a comprehensive neurological exam with assignment of EDSS by Neurostatus-certified neurologists.
Statistics
Statistical analyses were done with STATA software version 12.0 and with R-software (open source free software http://www.r-project.org). For analysis of the cross-sectional data, we chose to assess the ‘worse side’, regardless of hand dominance, and used Spearman’s rank order correlation tests and multivariate regression. For comparison of MS and healthy control participants the data was age and sex matched. When comparing hand function tests in the MS cohort, the MS participants ‘worse side’ was used, normalized to control data and matched for age, sex and hand dominance. Correlations were done with the other hand function tests obtained on the same side. To evaluate the hand function tests, multivariate regression models were done using the following dependent variables: age, sex, disease type (RRMS versus PMS), disease duration, hip flexor strength (weakest side used), EDSS and walking speed (T25FW).
For analysis of the longitudinal data, different models were evaluated through cross-validation and by comparing goodness-of-fit metrics such as R-squared. The cross validation was conducted as follows: We first randomly divided the data sets into a training set and a testing set. Training set included 2/3 of the subjects and the testing set included the remaining 1/3. We used the training set to fit the candidate models and use the fitted coefficients to predict hand function scores on the testing dataset. The mean square error (MSE) of predicted versus observed scores for each model was calculated. We repeated the procedure 50 times and selected the model with the smallest average MSE as the best fit. Additionally, we conducted a backward step-wise regression starting from the full model with the following variables considered: age, sex, disease type, disease duration, baseline hand function, change in hand function over the first year, leg strength, EDSS (at baseline, one year and two years), and T25FW. In each step, a variable was dropped based on Akaike information criteria, which penalizes the number of variables in the model (model complexity) while maximizing the likelihood of obtaining the observed data from the model.12,13 For model fitting constraint, subjects with missing values were removed resulting in 95 patients with complete longitudinal datasets. We chose to analyze the longitudinal data using the most conservative approach, using results from the dominant hand, as this is the side that is most important from a functional point of view.
RESULTS
Study Population
There were a total of 147 participants with MS who volunteered for the cross-sectional portion of this study. This cohort had a mean age of 44.6±11.9 years (mean±SD), mean disease duration of 11.4±9.7 years, and 65% were females. These study participants had a broad range of disability (EDSS 0–8) and included 84 with RRMS and 63 with PMS. The mean age for individuals with RRMS is 38.7±10.7 years, mean disease duration of 7.3±6.3 years, and 73% are female; the mean age for individuals with PMS is 52.6±8.2 years old, mean disease duration of 17.0±10.7 and 56% are female.
In our longitudinal cohort of 95 MS participants there was a mean age of 44.8±11.5 years (mean±SD), mean disease duration of 10.7±9.2 years, and 62% were females. This cohort similarly represented a wide range of disability (EDSS: 0–7.5) and included 53 with RRMS and 42 with PMS. The mean longitudinal follow up was 2.1±1.2 years. Additional study participants’ characteristics are presented in Table 1. Table 2 summarizes our MS cohort’s quantitative hand function tests raw data.
Table 1:
Demographics of Study Population
| Total | Longitudinal Cohort | Controls | |
|---|---|---|---|
| n | 147 | 95 | 35 |
| Age | 44.6 (20–69) | 44.8 (20–69) | 43.5 (25–69) |
| Gender | 51 Male | 36 Male | 15 Male |
| 96 Female | 59 Female | 20 Female | |
| Handedness | 121 right | 81 right | 26 right |
| 20 left | 11 left | 2 left | |
| 6 unknown | 3 unknown | 7 unknown | |
| EDSS (baseline) | 3.6 (0–8) | 3.5 (0–7.5) | N/A |
| Disease Type | 84 RRMS | 53 RRMS | N/A |
| 63 PMS | 42 PMS | ||
| Symptom Duration | 11.4 (0–44) | 10.7 (0–43) | N/A |
Mean values (range in parentheses) for Age, EDSS and Symptom Duration. n=sample size; EDSS=Expanded Disability Status Scale; RRMS=relapsing remitting multiple sclerosis; PMS=progressive multiple sclerosis; N/A=not applicable
Table 2.
(Cross-Sectional): Quantitative hand function tests divided by age, gender, disease and disability, in individuals with multiple sclerosis
| Grip Strength | Pinch | 9HPT | Tapping | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Dom | n | Non-Dom | n | Dom | n | Non-Dom | n | Dom | n | Non-Dom | n | Dom | n | Non-Dom | |
| Total | 147 | 72.2 (26.8) | 146 | 66.1 (27.8) | 129 | 17.2 (5.4) | 127 | 16.0 (5.6) | 144 | 26.3 (16.5) | 145 | 28.6 (24.5) | 129 | 45.3 (10.7) | 128 | 41.6 (11.6) |
| By Age | ||||||||||||||||
| <45 | 71 | 77.5 (26.8) | 71 | 71.7 (28.5) | 65 | 18.4 (5.1) | 65 | 17.6 (5.6) | 70 | 25.5 (21.2) | 70 | 24.3 (13.8) | 65 | 48.2 (9) | 64 | 45 (8.4) |
| ≥45 | 76 | 67.3 (26.1) | 75 | 60.7 (26.2) | 64 | 16 (5.4) | 62 | 14.4 (5.2) | 74 | 27 (10.5) | 75 | 32.6 (30.9) | 64 | 42.4 (11.5) | 64 | 38.2 (13.4) |
| By Gender | ||||||||||||||||
| Male | 51 | 96.4 (24.7) | 50 | 85 (33.4) | 43 | 21.8 (4.7) | 42 | 19.7 (6.2) | 49 | 32.4 (25) | 50 | 32.9 (28.6) | 43 | 46.9 (10.9) | 43 | 41.5 (12.6) |
| Female | 96 | 59.3 (17.4) | 96 | 56.2 (17.7) | 86 | 14.9 (4) | 85 | 14.2 (4.3) | 95 | 23.1 (8.2) | 95 | 26.4 (21.8) | 86 | 44.5 (10.5) | 85 | 41.7 (11.2) |
| By Disease | ||||||||||||||||
| RRMS | 84 | 78.3 (27.5) | 84 | 72.5 (28.9) | 75 | 18.3 (5.3) | 75 | 17.2 (5.6) | 84 | 22.4 (15) | 84 | 22.2 (8.8) | 75 | 49.2 (7.4) | 74 | 45.9 (7.5) |
| PMS | 63 | 66 (24.7) | 62 | 58.5 (25.3) | 54 | 16 (5.3) | 52 | 14.5 (5.4) | 60 | 31.6 (17.5) | 61 | 37.8 (35.4) | 54 | 40.5 (11.9) | 54 | 35.8 (13.9) |
| By Disability | ||||||||||||||||
| EDSS (<4) | 101 | 73.9 (26.3) | 100 | 69.9 (28.4) | 86 | 17.8 (5.2) | 85 | 17 (5.4) | 99 | 22.8 (9.5) | 100 | 22.6 (8.2) | 86 | 48 (8) | 85 | 44.4 (9.1) |
| EDSS (>4) | 46 | 68.5 (27.9) | 46 | 57.8 (24.7) | 43 | 16 (5.6) | 42 | 14.1(5.6) | 45 | 33.9 (24.5) | 45 | 42.1 (39.2) | 43 | 39.8(13) | 43 | 36.1 (14.1) |
Mean values presented with standard deviations in parentheses. RRMS=relapsing remitting multiple sclerosis; PMS=progressive multiple sclerosis; EDSS=Expanded Disability Status Scale; n=sample size; Dom=dominant side; Non-Dom=non-dominant side; 9HPT=9 hole peg test.
Our healthy controls included 20 women and 15 men with a mean age of 43.5±9.7 years (range: 25–64). Quantitative hand function and hip flexion strength were also obtained in this control population (data not shown).
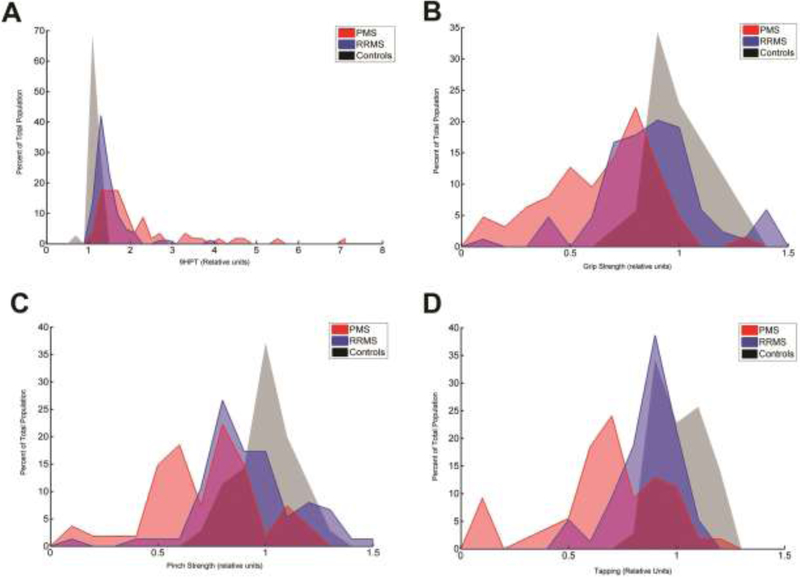
Abnormal Hand Function is Common in MS
A large percentage of MS patients in our cohort had abnormalities in hand function (Figure 1). The 9HPT was found to be the most frequently abnormal quantitative hand test within the cohort. In individuals with PMS, 23.3% required at least double the time to complete the 9HPT compared to healthy controls and in some patients it took them up to 10 times longer than controls to complete the test. In 44.6% of all MS patients (RRMS and PMS), the 9HPT completion time were more than 2 standard deviations (SD) worse compared to age and gender matched controls.
Figure 1:
Histogram of hand function tests. The data is plotted to show, on the y-axis, percent occurrence for the four normalized hand function tests, among the three groups (progressive MS [PMS] in red, relapsing remitting MS [RRMS} in blue, and healthy controls in gray). A. 9 hole peg test (9HPT) time. B. Grip Strength. C. Pinch Strength. D. Number of taps from the tapping test.
Grip strength was also found to be abnormal in a large percentage of MS patients. In individuals with PMS, 17.5% had less than 50% of their expected grip strength when compared to healthy controls. In 31.3% of all MS patients, grip strength was more than 2 SD weaker than controls. The magnitude of pinch strength findings were less than what was found for grip strength. In individuals with PMS, 11.1% had less than 50% pinch strength compared to controls. In 26.1% of the entire MS cohort, pinch strength fell outside the range of 2 SD of controls. Finger tapping results were also abnormal in this cohort. In individuals with PMS, 13% tapped at least 50% slower than controls. In 22.2% of all MS patients, finger tapping was more than 2 SD slower than controls.
Grip Strength and 9HPT are Distinct Measures of Hand Function
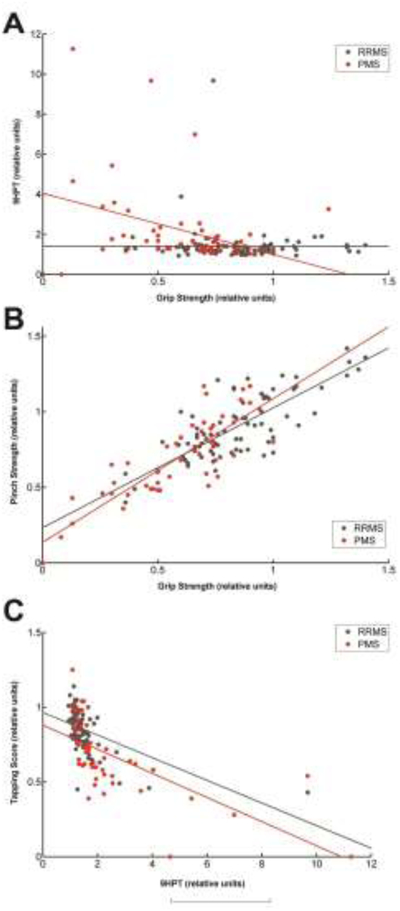
Interestingly, we found that some hand function tests were more closely related to one another than others. Grip strength and 9HPT were only modestly related in the PMS group (r= −0.412, p= 0.0069 for PMS) and did not correlate well in the RRMS group (r=0.023, p= 0.824) (Figure 2A). By contrast, grip strength and pinch strength were strongly correlated for both PMS and RRMS groups (r=0.817, p< 0.0001 in PMS; r= 0.696, p< 0.0001 in RRMS) (Figure 2B). In addition, finger tapping correlated well with 9HPT in both MS groups, although this correlation was stronger in the PMS group (r=−0.673, p< 0.0001 in PMS; r=−0.506, p< 0.0001 in RRMS). In the following analyses we therefore focused on the two most unique hand measurements, grip strength and 9HPT. Overall, these two hand functions separately correlated with the other hand functions the best (Table 3).
Figure 2:
Scatterplots showing the relationship between two hand function tests in multiple sclerosis subtypes. The data is plotted to show the relationship of various hand function tests among the two groups of MS patients (progressive MS [PMS] in red, relapsing remitting MS [RRMS] in black). A. 9 hole peg test (9HPT) time and Grip Strength. B. Pinch Strength and Grip Strength. C. Number of taps from the tapping test and 9HPT.
Table 3.
(Cross Sectional): Spearman correlation coefficients among the four hand function measures
| Grip Strength† | Pinch Strength† | Tapping† | 9HPT† | |||||
|---|---|---|---|---|---|---|---|---|
| Grip Strength* | -- | 0.696 | 0.8166 | 0.1969 | 0.6857 | 0.0228 | −0.4122 | |
| Pinch Strength* | 0.6806 | 0.8448 | -- | 0.1252 | 0.7134 | 0.1737 | −0.5166 | |
| Tapping* | 0.231 | 0.5124 | 0.1434 | 0.5659 | -- | −0.5061 | −0.673 | |
| 9HPT* | −0.026 | −0.4821 | 0.0262 | −0.5352 | −0.5777 | −0.7993 | -- | |
worse/weaker side.
data matching the worse/weaker side of the other test. Gray highlight shows significant values (p<0.05). 9HPT=9 hole peg test; RRMS=relapsing remitting multiple sclerosis; PMS=progressive multiple sclerosis.
Grip strength and 9HPT are related to different clinical measurements
For both grip strength and 9HPT, most of the variability in hand function was not accounted for by the dependent variables of age, disease type, disease duration, EDSS or walking speed (adjusted R2 0.626 for grip strength and 0.235 for 9HPT; p< 0.0001 for grip strength, p=0.0002 for 9HPT test). Gender and hip flexor strength were the only significant contributors to the grip strength regression model (Table 4). For the 9HPT regression model, EDSS and gender were the only significant contributors (Table 4).
Table 4.
(Cross Sectional): Regression model outcomes for Grip Strength and 9HPT
| Worst Grip Strength | Worst 9HPT | |||||
|---|---|---|---|---|---|---|
| Age | −0.002 | 0.232 | 0.992 | −0.470 | 0.310 | 0.134 |
| Gender | 25.345 | 5.161 | 0.000 | 13.826 | 6.906 | 0.049 |
| Symptom Duration | −0.123 | 0.306 | 0.689 | 0.162 | 0.409 | 0.694 |
| Disease Subtype | 0.907 | 5.931 | 0.879 | −1.354 | 7.937 | 0.865 |
| Worse Hip Flexion | 0.878 | 0.195 | 0.000 | 0.016 | 0.260 | 0.951 |
| EDSS | −0.158 | 1.690 | 0.926 | 5.291 | 2.262 | 0.022 |
| T25W | 0.683 | 0.528 | 0.2 | 0.281 | 0.706 | 0.692 |
Adjusted r squared values -- Worse grip: 0.570, worse 9HPT: 0.162. Gray highlight shows significant values (p<0.05). EDSS=Expanded Disability Status Scale; T25FW=Timed 25 foot walk; Coeff=coefficient; Stdev=standard deviation; p=p value; 9HPT=9 hole peg test.
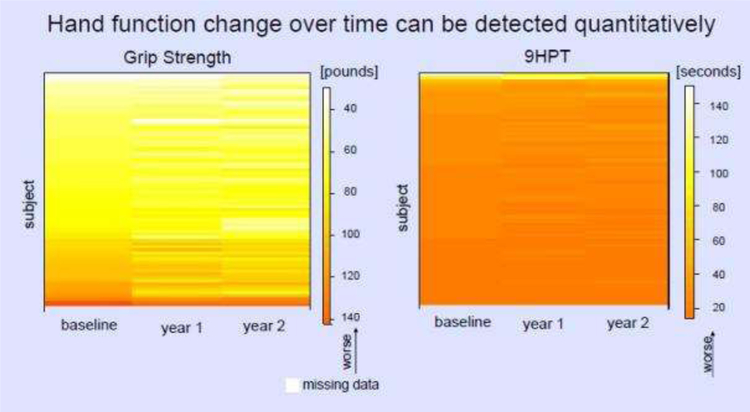
Hand Function Change Over Time can be Quantified
Figure 3 shows the variation in grip strength and 9HPT over the two year period. We further quantified this change. For grip strength, the mean change was −4.65 pounds (median −1.25, SD 11.15, max +18.5, min −53.0). The time to complete the 9HPT ranged from 12.1 seconds slower to 36.7 seconds faster after two years (mean 0.99 seconds faster, median 0.5, SD 4.91).
Figure 3:
Heat maps showing the variation in hand function (left: grip strength, right: 9 hole peg test [9HPT]) over time using colors to represent the magnitude of change. Lighter colors represent greater impairment. Each row represents one subject over three visits in the 2-year observation period.
Hand Function at Baseline Predicts Change in Hand Function Over Time
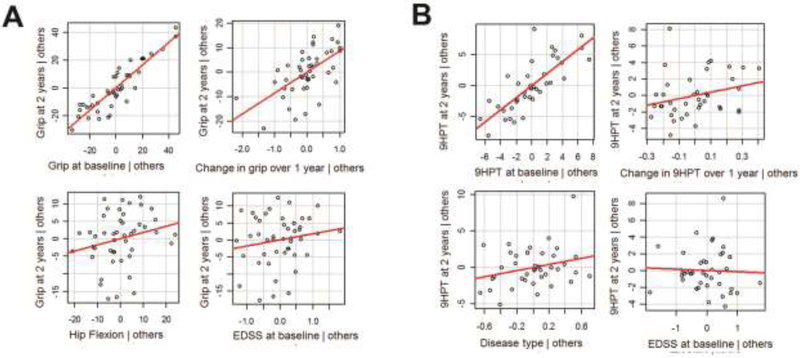
Table 5 shows the results of our step-wise regression analysis for hand function. 86% of the variability in grip strength over two years (R2 = 0.857) was explained by sex, grip strength at baseline and grip strength change over one year. For the 9HPT, 96% of the variability over two years (R2 = 0.959) was explained by baseline 9HPT, change in 9HPT over one year, disease type and EDSS. The factors predicting 9HPT at two years were baseline 9HPT, change in 9HPT over one year as well as disease type and EDSS, though the latter two only contributed minimally. Figure 4 shows the most predictive models. Grip strength at baseline and 9HPT at baseline have the highest correlation with grip strength at 2 years and 9HPT at 2 years, respectively.
Table 5.
(longitudinal): Predictor values for Dominant-side Grip Strength/9HPT after 2 years regression models
| Coefficient | p value | Confidence Interval | |
|---|---|---|---|
| Grip Strength After 2 years | |||
| Gender | −11.16 | 0.014 | −19.982; −2.337 |
| Age | −0.15 | 0.315 | −0.447; 0.146 |
| Grip at baseline | 0.97 | 0.000 | 0.796; 1.151 |
| Change in grip over 1 year | 7.08 | 0.000 | 4.037; 10.130 |
| Disease type | −0.83 | 0.803 | −7.449; 5.791 |
| EDSS | 1.97 | 0.037 | 0.126; 3.813 |
| Disease duration | −0.33 | 0.092 | −0.706; 0.054 |
| Worse hip flexion | 0.08 | 0.420 | −0.121; 0.286 |
| 9HPT after 2 years | |||
| Gender | 0.78 | 0.428 | −1.174; 2.729 |
| Age | 0.06 | 0.170 | −0.027; 0.152 |
| 9HPT at baseline | 0.84 | 0.000 | 0.731; 0.956 |
| Change in 9HPT over 1 year | 3.84 | 0.009 | 0.984; 6.687 |
| Disease type | 2.28 | 0.033 | 0.195; 4.367 |
| EDSS | 0.85 | 0.021 | 0.131; 1.568 |
| Timed 25 foot walk | 0.06 | 0.604 | −0.159; 0.271 |
| Disease duration | −0.12 | 0.030 | −0.224; −0.012 |
| Worse hip flexion | −0.01 | 0.777 | −0.080; 0.060 |
The strongest models after cross-validation of multiple models are shown above. Overall Grip Strength model: Adjusted R-squared = 0.863; p< 0.0001, n=69. 9HPT model: Adjusted R-squared =0.953; p< 0.0001; n=63. Gray highlight shows significant values (p<0.05). EDSS=Expanded Disability Status Scale; 9HPT=9 hole peg test
Figure 4:
Hand function at baseline predicts change over time. Correlation of different variables with grip strength (A) and 9 hole peg test [9HPT] (B) at 2 years. Hand function at baseline (upper left) and to a lesser degree change in hand function over 1 year (upper right) best predicts hand function at 2 years. Hip flexion (A left lower), disease type (B left lower) and Expanded Disability Status Scale [EDSS] (right lower) show a weaker correlation.
DISCUSSION
Rating scales assessing the clinical function and disability of MS patients typically focus on walking. However, data presented here show that hand function is frequently impaired in individuals with MS. It is therefore important to consider assessing hand function when following individuals with MS both in a clinical setting as well as in clinical trials. We found that grip strength and coordination measures like the 9HPT are not highly correlated. We therefore propose that both tests should be included when assessing MS patients as they provide unique information about a person’s hand function. Both tests are inexpensive, portable and can easily be done in various settings of patient care. We found a relationship between grip strength and leg strength in our cohort. Since it is difficult to obtain objective quantitative measurements of leg strength, grip strength could also be considered as a surrogate marker that may be used when assessing global motor strength in MS patients and may play an important role in assessing changes in people with PMS who cannot walk but are losing function in the upper extremities.
Our data not only show that hand dysfunction in MS can be detected compared to controls (i.e., more than 2 SD difference) in a cross-sectional cohort that is different from controls, but also that clinically relevant change can be detected over time. The most robust quantitative measures of hand function in this study proved to be grip strength and 9HPT. These quantitative tests provide unique information about an individuals hand function and can be used to detect relevant changes over time. Previous studies in MS patients have shown that a change of 20% or more for the 9HPT and of 5.5 kg (12 pounds) or more for grip strength reliably indicate a true change in function for an individual and are outside of the range of day-to-day variability.14,15 Many individuals in our study fall outside of that range when assessed over 2 years. These findings suggest that grip strength and 9HPT can be used to detect clinically significant changes in hand function over time. In this study we also found that hand function after two years is strongly related to hand function at baseline and at one year. This indicates that baseline hand function tests may be sensitive enough to give us information about what to expect in the future. Exploring the idea of using hand function as a predictor outcome for global disability is an important direction for future research.
There remains an urgent need for clinical outcome measures that detect clinically meaningful changes in individuals with PMS since rating scales (i.e., EDSS) and paraclinical tests (i.e., MRI) do not change significantly over a short interval of time. Our study shows that quantitative measures of hand function, specifically grip strength and 9HPT, demonstrate robust clinically relevant changes in PMS over a relatively short time period; therefore, we propose that these measures along with lower extremity quantitative sensorimotor measures be considered in future PMS clinical trials especially when putative neurorepairative or neuroprotective agents are being studied.16
While the cohort evaluated in our study includes individuals with progressive as well as relapsing MS and spans a broad range of disability as measured by EDSS, our findings may not be relevant for all MS patients, since MS is a heterogenous disease. Analysis of different subgroups of individuals (i.e., high EDDS, low EDSS, relapsing vs. progressive subtypes with different levels of progression) may give additional insights. Following patients for longer than two years would add valuable information about the longitudinal changes in individuals with MS; this is something we are actively pursuing. In the future, it would also be interesting to incorporate a patient’s perception of their hand function, its impact on home and work life and their perception of change in function over time.17,18 The ABILHAND questionnaire, for example, measures patient-perceived difficulties in performing manual ADLs. This was originally validated in patients with rheumatoid arthritis and was recently shown to be useful and valid in patients with MS.17 Lastly, further investigation and corroboration of the findings in this study are necessary to assess which hand function test may be the most useful as an outcome measure in clinical trials of neuroprotective or neuroreparative therapies.
Highlights.
Hand function is commonly impaired in individuals with MS.
The most robust measures in this study proved to be grip strength and 9HPT.
Grip strength and 9HPT detect changes in hand function over time in MS.
Hand function at baseline predicts change in hand function over time.
Acknowledgements:
The authors wish to thankfully acknowledge all of our participants.
Study Funding: Supported by Project Restore and NIH-NCMRR
Footnotes
Author Disclosures:
Dr. Newsome participated in scientific advisory boards for Biogen, Genentech, Celgene, EMD Serono and is an advisor for Gerson Lehrman Group. He has received grant/research funding (paid directly to Institution): Biogen, Genentech, Department of Defense, National MS Society, Patient Centered Outcomes Research Institute
Dr. von Geldern has received grant/research support from Novartis and Patient Centered Outcomes Research Institute.
Dr. Shou reports no disclosures.
Mrs. Baynes reports no disclosures.
Mr. Marasigan reports no disclosures.
Dr. Calabresi has received Honoria for consulting from Disarm Therapeutics and Biogen and is PI on grants to JHU from Annexon, MedImmune, Biogen, and Sanofi
Dr. Zackowski reports no disclosures
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983. November;33(11):1444–1452. [DOI] [PubMed] [Google Scholar]
- 2.Fox E, et al. Ocrelizumab reduces progression of upper extremity impairment in patients with primary progressive multiple sclerosis: Findings from the phase III randomized ORATORIO trial. Mult Scler 2018. December;24(14):1862–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Savin Z, et al. Effect of Fampridine-PR (prolonged released 4-aminopyridine) on the manual functions of patients with Multiple Sclerosis. J Neurol Sci 2016. January;360:102–109. [DOI] [PubMed] [Google Scholar]
- 4.Julian LJ, et al. Employment in multiple sclerosis. J Neurology 2008. September;255(9):1354–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polman CH, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol 2005. December;58(6):840–846. [DOI] [PubMed] [Google Scholar]
- 6.Lamers I, et al. Upper limb assessment in multiple sclerosis: a systematic review of outcome measures and their psychometric properties. Arch Phys Med Rehabil 2014. June;95(6):1184–200 [DOI] [PubMed] [Google Scholar]
- 7.Paltamaa J, et al. Reliability of physical functioning measures in ambulatory subjects with MS. Physiother Res Int 2005. 10(2):93–109. [DOI] [PubMed] [Google Scholar]
- 8.Mathiowetz V, et al. Reliability and validity of grip and pinch strength evaluations. The Journal of Hand Surgery 1984. March;9(2):222–226. [DOI] [PubMed] [Google Scholar]
- 9.Shimoyama I, et al. The Finger-Tapping Test: A Quantitative Analysis. Arch Neurol 1990. June;47(6):681–684. [DOI] [PubMed] [Google Scholar]
- 10.Newsome SD, et al. Quantitative measures detect sensory and motor impairments in multiple sclerosis. J Neurol Sci 2011. June;305(1–2):103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spreen O and Strauss E. A compendium of neuropsychological tests, 2nd edition; 1998. New York: Oxford University Press. [Google Scholar]
- 12.Akaike H Likelihood of a model and information criteria. Journal of Econometrics 1981. 16:3–14. [Google Scholar]
- 13.Bedrick EJ and Tsai CL. Model Selection for Multivariate Regression in Small Samples. Biometrics 1994. 50:226–231. [Google Scholar]
- 14.Schwid SR, et al. Quantitative functional measures in MS: what is a reliable change? Neurology 2002. April;58(8):1294–1296. [DOI] [PubMed] [Google Scholar]
- 15.Polman CH and Rudick RA. The multiple sclerosis functional composite: a clinically meaningful measure of disability. Neurology 2010. April;74(S3):S8–S15. [DOI] [PubMed] [Google Scholar]
- 16.Zackowski KM, et al. Quantitative sensory and motor measures detect change over time and correlate with walking speed in individuals with multiple sclerosis. Mult Scler Relat Disord 2015. January;4(1):67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barrett L, et al. Can the ABILHAND handle manual ability in MS? Mult Scler 2013. May;19(6):806–815. [DOI] [PubMed] [Google Scholar]
- 18.Mokkink LB, et al. The Arm Function in Multiple Sclerosis Questionarre (AMSQ): development and validation of a new tool using IRT methods. Disabil Rehabil March 2015. December;37(26):2445–2451. [DOI] [PubMed] [Google Scholar]