Abstract
The mismatch negativity (MMN) is an event-related potential component seen in response to unexpected “novel” stimuli, such as in an auditory oddball task. The MMN is of wide interest and application, but the neural responses that generate it are poorly understood. This is in part due to differences in design and focus between animal and human oddball paradigms. For example, one of the main explanatory models, the “predictive error hypothesis”, posits differences in timing and selectivity between signals carried in auditory and prefrontal cortex. However these predictions have not been fully tested because 1) noninvasive techniques used in humans lack the combined spatial and temporal precision necessary for these comparisons, and 2) single-neuron studies in animal models, which combine necessary spatial and temporal precision, have not focused on higher order contributions to novelty signals. In addition, accounts of the MMN traditionally do not address contributions from subcortical areas known to be involved in novelty detection, such as the amygdala. To better constrain hypotheses and to address methodological gaps between human and animal studies, we recorded single neuron activity from the auditory cortex, dorsolateral prefrontal cortex, and basolateral amygdala of two macaque monkeys during an auditory oddball paradigm modeled after that used in humans. Consistent with predictions of the predictive error hypothesis, novelty signals in prefrontal cortex were generally later than in auditory cortex, and were abstracted from stimulus-specific effects seen in auditory cortex. However, we found signals in amygdala that were comparable in magnitude and timing to those in prefrontal cortex, and both prefrontal and amygdala signals were generally much weaker than those in auditory cortex. These observations place useful quantitative constraints on putative generators of the auditory oddball-based MMN, and additionally indicate that there are subcortical areas, such as the amygdala, that may be involved in novelty detection in an auditory oddball paradigm.
Introduction
An organism’s ability to extract patterns from the world, and to quickly detect deviation from these predictions, is key to survival (Friston, 2009). The auditory oddball task is one of the dominant paradigms used to examine mechanisms of novelty detection (Naatanen et al., 2012; Nelken, 2014). In this task, an auditory stimulus is presented repeatedly as a “standard” and is infrequently interleaved with a different “deviant” or “oddball” auditory stimulus. The response to the deviant is greater than the response to the standard, or to the response of the deviant stimulus when played as a standard. This early difference in response is referred to as the “mismatch negativity” (MMN) in scalp-based event related potentials. Though the neural basis of this potential is poorly understood, the MMN is of wide use and interest as an index of early sensory processing and novelty sensitivity, and is of clinical interest as a potential index of symptoms of psychiatric disorders.
To examine the basis of the auditory oddball mediated MMN, human studies using noninvasive measures (fMRI, EEG, MEG) have suggested that it has both temporal and frontal cortical generators. According to the predictive error hypothesis of the MMN, sensory and frontal generators have distinct functions (Garrido, Kilner, Stephan, & Friston, 2009; Naatanen, Teder, Alho, & Lavikainen, 1992). While the standard is being repeated, a stimulus specific memory is built up in sensory (auditory) cortex, and this memory drives an expectation. On the other hand, frontal cortex represents whether there is a difference between the expected and actual stimulus (Garrido et al., 2009; Giard, Perrin, Pernier, & Bouchet, 1990; Naatanen et al., 1992). This hypothesis predicts that frontal novelty signals should arise later than those in auditory cortex, and additionally that the signal in prefrontal cortex be abstracted from any stimulus selective activity seen in auditory cortex. It has been difficult to test this hypothesis in humans using noninvasive techniques because they do not combine the precise temporal and spatial resolution needed to compare timecourses with certainty (Deouell, 2007; Rinne, Alho, Ilmoniemi, Virtanen, & Naatanen, 2000; Tse & Penney, 2008). In addition, to compare stimulus specificity, one must be able to compare the novelty response between stimulus types across areas, which is not commonly done. Human electrocortocogtraphy (ECoG) studies have also been used to examine the correlates of oddball and novelty detection, and have confirmed frontal and sensory components. However, heterogeneity of clinical electrode placement within and between patients makes it difficult to systematically compare magnitude and timecourse of early novelty signals between areas (Durschmid, Edwards, et al., 2016; Edwards, Soltani, Deouell, Berger, & Knight, 2005; Rosburg et al., 2005). In contrast, animal models of auditory oddball paradigms do have the necessary spatial and temporal specificity to compare signals across areas. However, these studies have primarily been concerned with mechanisms of oddball in early auditory processing (cochlear nucleus through auditory cortex), and not activity from other areas (Ayala, Perez-Gonzalez, Duque, Nelken, & Malmierca, 2012; Fishman, 2014; Javitt, Steinschneider, Schroeder, Vaughan, & Arezzo, 1994; Parras et al., 2017; Ulanovsky, Las, & Nelken, 2003; Yarden & Nelken, 2017). In addition, the majority of animal studies choose neuron-specific “standard” and “deviant” stimuli based on the receptive fields of the neuron being recorded at the time. This tuned design has been undeniably powerful for other questions (e.g. mechanisms of adaptation and effects of pharmacological manipulations at a single-neuron level), but may not be ideal for connecting to the larger body of human literature where, by definition, identical standard and deviant stimuli are used for all areas. This distinction appears especially important when questions pertain to the comparison of timecourse and magnitude of novelty signal between areas.
Largely separate from accounts involving the cortical substrates of the MMN in oddball, a body of work has established correlates of novelty and salience detection in subcortical nuclei such as the amygdala and nucleus accumbens (NAc) (Balderston, Schultz, & Helmstetter, 2013; Blackford, Buckholtz, Avery, & Zald, 2010; Bradley et al., 2015; Zaehle et al., 2013). For example, a human intracranial study suggested robust auditory oddball-based MMN in NAc (Durschmid, Zaehle, et al., 2016). Amygdala activation in auditory oddball has been reported in at least one fMRI study (Czisch et al., 2009), but an intracranial study suggested that auditory oddball novelty signal may only arise in the amygdala during active detection (Kropotov et al., 2000). There has thus been renewed interest in these as nuclei that can affect, either directly or indirectly, the MMN, with the understanding that source localization techniques used in human noninvasive studies can miss subcortical generators. However, it is unclear whether these areas’ signals are as fast or as robust as those of the hypothesized cortical generators. The human intracranial studies described above are suggestive, but cannot systematically compare the signals in amygdala to that of other areas due to the necessary heterogeneity of clinical placement. Studies in animal models would be able to address magnitude and timing differences between these and more established generators but extant studies have largely focused on examining oddball responses in early auditory areas. The goal of the present experiment, therefore, was to systematically compare the single neuron correlates of an early auditory oddball signal in auditory cortex, dorsolateral prefrontal cortex and basolateral amygdala. This approach, drawing on a substantial number of neurons (~600 per area afforded by a multichannel approach), allows us to address predictions of the predictive error account in auditory and prefrontal cortex and evaluate whether the account should be extended to areas such as the amygdala. This comparison, with the advantage that it is modeled after human studies, provides useful quantitative constraints for future accounts of the brain bases of auditory oddball-mediated MMN.
Materials and Methods
Subjects, surgical procedures, and neurophysiological data acquisition
Experiments were carried out on two adult male rhesus macaques (Macaca mulatta). All experimental procedures were approved by the National Institute of Mental Health Animal Care and Use Committee and followed Guide for the Care and Use of Laboratory Animals. Before data acquisition, monkeys were implanted with titanium head posts for head restraint. In a separate procedure, monkeys were fitted with custom acrylic chambers oriented to allow vertical grid access to the left dorsolateral prefrontal cortex (dorsal bank of the principal sulcus extending ventral, >1 mm away from arcuate sulcus, roughly 46/8Ad), the lateral portion of the amygdala (entire dorsoventral extent, primarily basolateral amygdala), and auditory cortex (primarily A1 but including small portions of lateral belt areas). Recording areas were verified though a T1 scan of grid coverage with respect to underlying anatomical landmarks (Fig. 1B–C), combined with maps of frequency reversals and response latencies of single neurons to determine A1 location and extent (Camalier, D’Angelo, Sterbing-D’Angelo, de la Mothe, & Hackett, 2012). Recordings were made using either 16 or 24 channel laminar “V-trodes” (Plexon, Inc, Dallas TX; 200-300 μm contact spacing, respectively), which allowed identification of white matter tracts, further allowing identification of electrode location with respect to sulci and gyri. Electrodes were advanced to their target location (NAN microdrives, Nazareth, Israel) and allowed to settle for at least 1 hour before recording.
Figure 1. Task design, recording locations and trajectory.
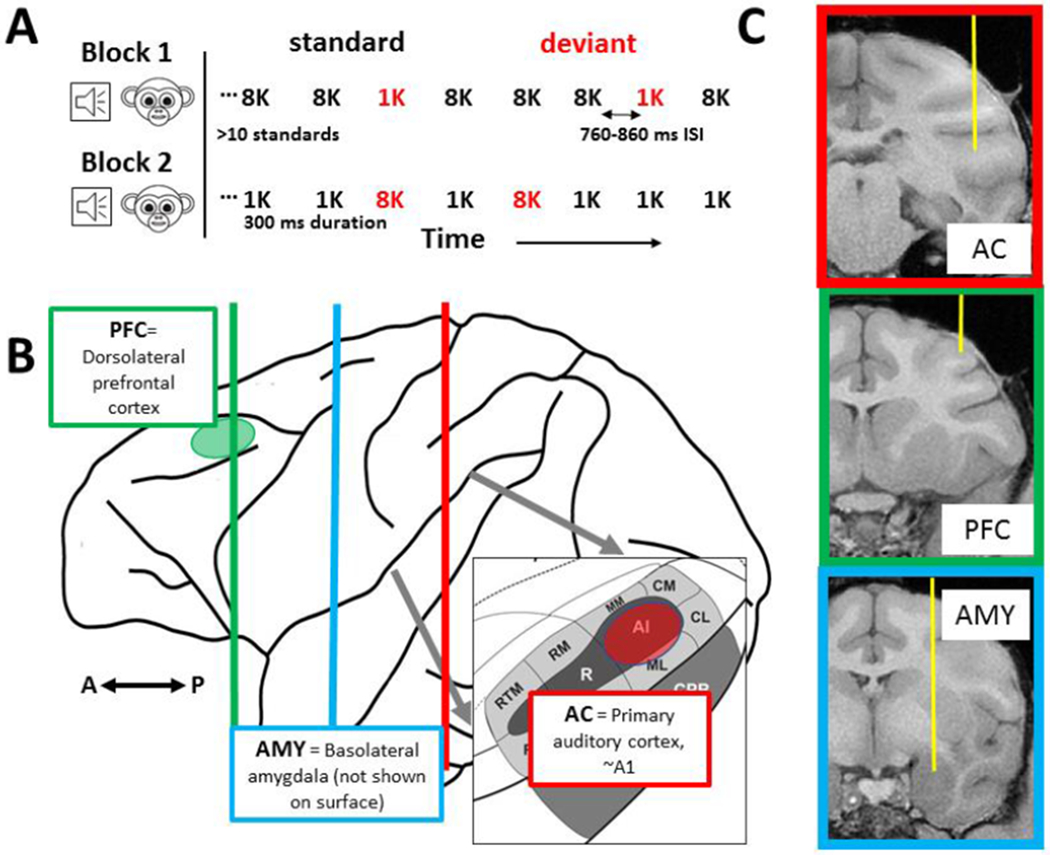
A. Details of the “flip-flop” oddball task. The 1 kHz and 8 kHz stimuli were consistently used for all areas. B. Schematic of macaque brain with locations of recording areas shown on surface (where possible) in red (AC) and green (dlPFC). Vertical lines indicate sagittal slices shown in panel C. C. Exemplar recording trajectory for each area reconstructed from T1-weighted scan – all areas were recorded with multichannel laminar probes oriented vertically which allowed for a comparison of ~600 neurons per area.
Multichannel spike and local field potential recordings were acquired with a 64 channel data acquisition system. Spike signals were amplified, filtered (0.3-8 kHz), and digitized at ~24.4 kHz. Spikes were initially sorted online on all channels using real-time window discrimination. Digitized waveforms (snippets) and timestamps of stimulus events were saved for final sorting (Plexon offline sorter V 3.3.5). The units were also graded according to isolation quality (single or multi neurons). Single and multiunits were analyzed separately, but the patterns of results were similar, and so were combined. The acquisition software interfaced directly with the stimulus delivery system and both systems were controlled by custom software (OpenWorkbench and OpenDeveloper, controlling a RZ2, RX8, Tucker Davis Technologies (TDT) System 3, Alachua, FL).
Stimuli, experimental design, and statistical analysis
We utilized a “flip-flop” auditory oddball design used in intracranial studies (e.g. Naatanen, Gaillard, & Mantysalo, 1978; Ulanovsky et al., 2003). This paradigm controls for differences in activity driven by the stimulus selectivity of neurons unrelated to stimulus type (type: standard vs deviant, Fig. 1A). Since our questions were primarily concerned with comparisons of neural responses in auditory cortex and higher-order areas, the stimuli used were constant across all areas and neurons and chosen to drive responses in higher order areas such as the amygdala and prefrontal cortex, and thus were more complex than pure tones. The stimuli, 300 ms 1 kHz and 8 kHz square waves, are perceptually distinct, spectrally nonoverlapping wideband stimuli likely to activate large parts of the auditory cortex. The spectrum of a square waves contains the odd harmonics of the fundamental frequency, such that the 1kHz square wave contains 1kHz and harmonics of 3, 5, 7, 9, up to ~33 kHz, and the 8 kHz square wave contains 8 kHz and a functional harmonic of 24 kHz, as macaques can hear up to about 35 kHz (Hauser, Burton, Mercer, & Ramachandran, 2018; Jackson, Heffner, & Heffner, 1999; Recanzone, Guard, & Phan, 2000). These stimuli have minimal spectral overlap, which is thought to be important for evoking MMN correlates (see Khouri & Nelken, 2015). The sounds were 300 ms in duration (with 5 ms cos2 onset/offset ramps to minimize spectral splatter) and they were presented from a speaker 10 cm from the contralateral (right) ear calibrated to 60 dB SPL.
The “flip-flop” design has two blocks, one in which the 1 kHz pulse is the standard and the 8 kHz the deviant, and the other in which standard and deviant identities are reversed. The flip-flop design critically allows the ability to dissociate any stimulus specificity of the response from actual novelty; the measure of most interest is the difference between the response to a stimulus when it is presented as a standard vs to identical stimulus when it is a presented as a deviant (DEV-STD). If this comparison is positive, then the response to a stimulus presented as a deviant is greater than when it is presented as a standard. Each block in the flip-flop design contained 270 standards and 30 deviants (10% deviant probability) presented in randomized order with an inter-onset interval between 760-860 ms. Ten additional standards were added to the beginning of each block to ensure a stable standard trace (Fig. 1). The deviant stimulus identity of the first block (1 kHz or 8 kHz) was switched across days to eliminate any order effects. During the oddball task, head-restrained monkeys sat quietly in a primate chair, watching a soundless video (common in human oddball studies) in a double-walled acoustically isolated sound booth (Industrial Acoustics Company, Bronx, NY). Oddball data were from 21 sessions in monkey 1 and 55 sessions in monkey 2, and each monkey was presented with a single session a day.
All data analysis was performed with custom scripts in Matlab (Natick, MA). Code and data are available upon reasonable request to the corresponding author. Data from the first 10 standards was discarded, and then spikes evoked by each stimulus (1 kHz vs 8 kHz) and type (standard vs deviant) combination were calculated over time. For each stimulus, 270 presentations of it as standard and 30 as a deviant included in analyses (600 presentations in total). For all analyses, mean evoked spike rates were converted to z-scores using the baseline mean and standard deviation (−150-0 ms prestimulus).
ANOVA models
To establish the population of auditory-responsive cells (here defined as responding to any of the stimuli in any context), we performed a 100 ms sliding window ANOVA on the evoked activity of each neuron (timebin advanced from 0-250 ms in 20 ms increments), including the factors of stimulus frequency (1 vs 8 kHz) and type (deviant vs standard) and their interaction. If any factor was significant in any window (p<0.01, FDR corrected for multiple comparisons), the neuron was considered task responsive. For the population-based analysis of oddball selectivity by area and stimulus (Fig. 4–5), we used an analysis of a mixed within-between effects ANOVA (3x2x2; area x stimulus x type). In this case, neuron identity was specified as a “random” effect in the model and was nested under area, where area is the region recorded from. To ensure these effects were not driven by results from an individual monkey, we re-ran the tests with monkey identity as a factor in the model, and effects did not change (see results). To establish oddball selectivity at the level of individual neurons, if a neuron exhibited a significant response to type, and response to deviant > standard, or a significant interaction and one of the stimuli had a deviant response > standard response (p<0.01, Bonferroni corrected as appropriate), then it was considered to exhibit deviance selectivity analogous to the mismatch negativity. To be conservative, we refer to this firing-rate based deviance selectivity as simply “oddball selectivity”. It has the same pattern as the MMN measured by continuous methods such as EEG but is not derived from a continuous measure. Note this measure is similar to “stimulus-specific adaptation” (SSA) used in other reports, but as the basis of this is probably not strictly adaptation, especially in higher-order areas examined here, we prefer to use descriptive nomenclature to avoid confusion.
Figure 4. Population response by stimulus across areas.
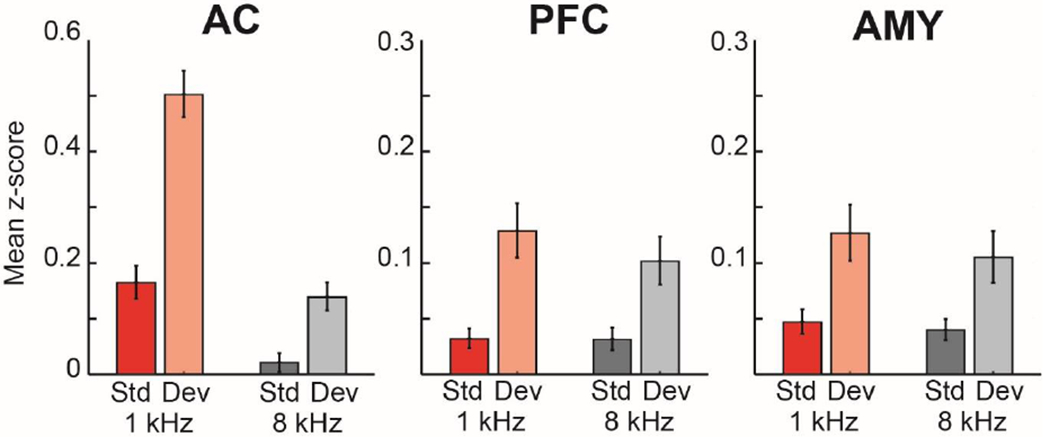
Average response magnitude for each stimulus, type (standard vs deviant), and area. Consistent with the exemplar responses in Fig. 2, responses are on average larger in AC than PFC and AMY. Error bars are SEM across cells.
Figure 5. Population novelty signal across areas.

Average novelty signal, computed as a neuron’s response to a stimulus as a standard subtracted from its response as a deviant (DEV-STD). Average magnitude of novelty signal is shown separately for each stimulus and area, error bars are SEM. All areas show a population-based novelty signal, but only auditory cortex shows a signal whose magnitude is affected by driving stimulus (sig difference denoted as a star).
For the individual-neuron based analysis of time course of deviance detection (Fig. 6A), we measured the first timebin that exhibited a selective response to stimulus type for each responsive neuron in each area (type x stim, 30 ms windows sliding at 10 ms (p<0.05, Bonferroni corrected). For the population-based analysis of the time course of novelty detection (Fig. 7B–D), we used a sliding ANOVA (mixed within-between, stimulus x type x neuron identity) on response in 30 ms bins with 10 ms intervals and looked for periods with a significant effect of type (p<0.01, Bonferroni corrected). To examine whether adaptation exists and is different between areas (Fig. 8), we calculated the evoked response as a function of time (either presentation number since the beginning of each block or presentation number following deviant) for each responsive neuron. To quantify this time course, we fit an exponential decay function (R=a*exp(b*t), where presentation #= t) (After Antunes, Nelken, Covey, & Malmierca, 2010; Ulanovsky, Las, Farkas, & Nelken, 2004) to each neuron’s time course for each stimulus, and then determined whether the population of decay constants (b) were less than 0 via a one-tailed t-test. If adaptation was indicated, ANOVAs on the population of b values was done to determine whether adaptation was greater for some stimuli. For the analysis of within block adaptation, this was done for the first 27 presentations. This covers the majority of the adaptation, and includes the same number of stimuli for standards as deviants. For the analyses relative to the last deviant, adaptation was calculated for the first 6 presentations of standards following a deviant. Consistent with a population-based approach done by other studies (see above), these results were confirmed by a bootstrap test on 1000 fits done to averages of 70 neurons (chosen randomly from population), and the results were identical.
Figure 6. Individual neuron-based magnitude of novelty signal (response as deviant – response as standard) and novelty selectivity for each stimulus and area.
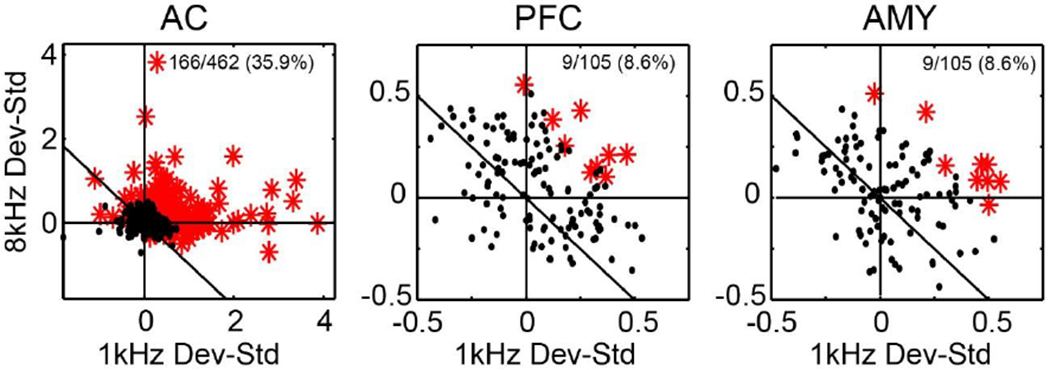
Single neurons which exhibited a significant novelty signal are shown in red. This fraction is lower in PFC and AMY but greater than chance, and magnitude in these areas is lower than in AC (see text). Note that at level of individual neurons, generally responses in AC are skewed towards stronger novelty responses for 1 kHz, and there is less stimulus selectivity in PFC and AMY.
Figure 7. Latency of deviance signal across areas.

A-C. Average PSTHs of DEV-STD differences for each area (shaded bars are SEM). Earliest 30 ms bin in which differences were significant denoted as vertical dotted line. Plots are smoothed for visualization purposes only. D. Cumulative distribution function of latencies of individual neuron sensitivity to stimulus type for each area. Mean of each area denoted as vertical dotted line.
Figure 8. Adaptation effects across areas.
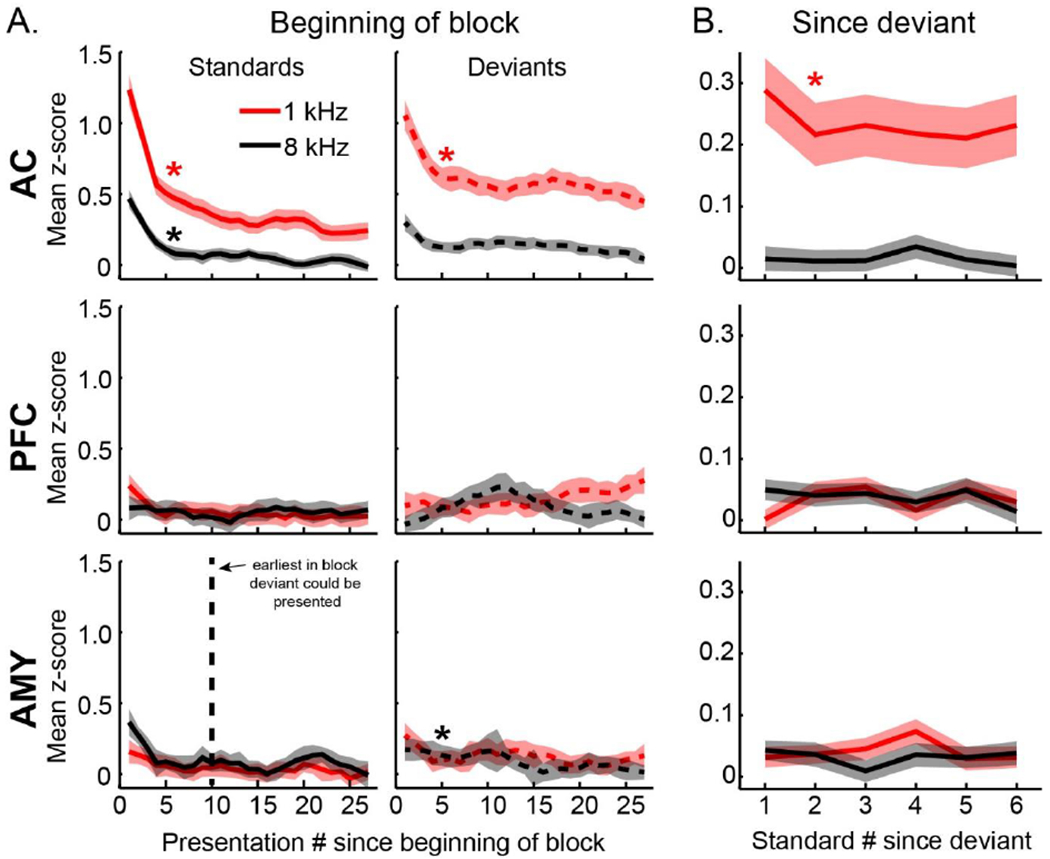
A. Adaptation since block start. Average response to a standard (solid) and deviant (dotted) plotted by ordinal presentation number since beginning of block for each area, stimulus, and type (shaded bars are SEM). Significant adaptation is denoted with a star. Vertical dotted line indicates the earliest that a deviant could be presented within a block (all blocks started with >10 standards). B. Adaptation since deviant. Average response to standard after presentation of a deviant for each area and stimulus (shaded bars are SEM). Plots are smoothed for visualization purposes only.
Results
We recorded neurons from primary auditory cortex (AC, n=690), dorsolateral prefrontal cortex (PFC, n=598) and the basolateral amygdala (AMY, n=627), while monkeys were presented with a flip-flop auditory oddball paradigm (Fig. 1A). In all three areas, we found neurons that responded more strongly to the stimulus when presented as an oddball (responses from exemplar individual neurons in Fig. 2). Note that generally both baseline firing rates and responses were weaker in amygdala and prefrontal cortex, so subsequent analyses focus on the normalized differences in firing rates expressed as a z-score. To compare novelty responsiveness and stimulus selectivity across areas, we first identified the fraction of neurons that were generally auditory responsive under these oddball task conditions. In auditory cortex 462 neurons were responsive to the task stimuli in any context (67%). Smaller proportions were responsive in prefrontal cortex (105, 18%) and amygdala (105, 17%). This analysis was done using a fixed window (0-250 ms) chosen to correspond to the period of MMN generation in the primate (Gil-da-Costa, Stoner, Fung, & Albright, 2013). To ensure that this was not due to the choice of analysis window, we examined larger or smaller sliding windows (50, 200 ms) and the pattern of results did not change, though the fixed window yielded more conservative estimates of responsiveness.
Figure 2. Example neuron responses.
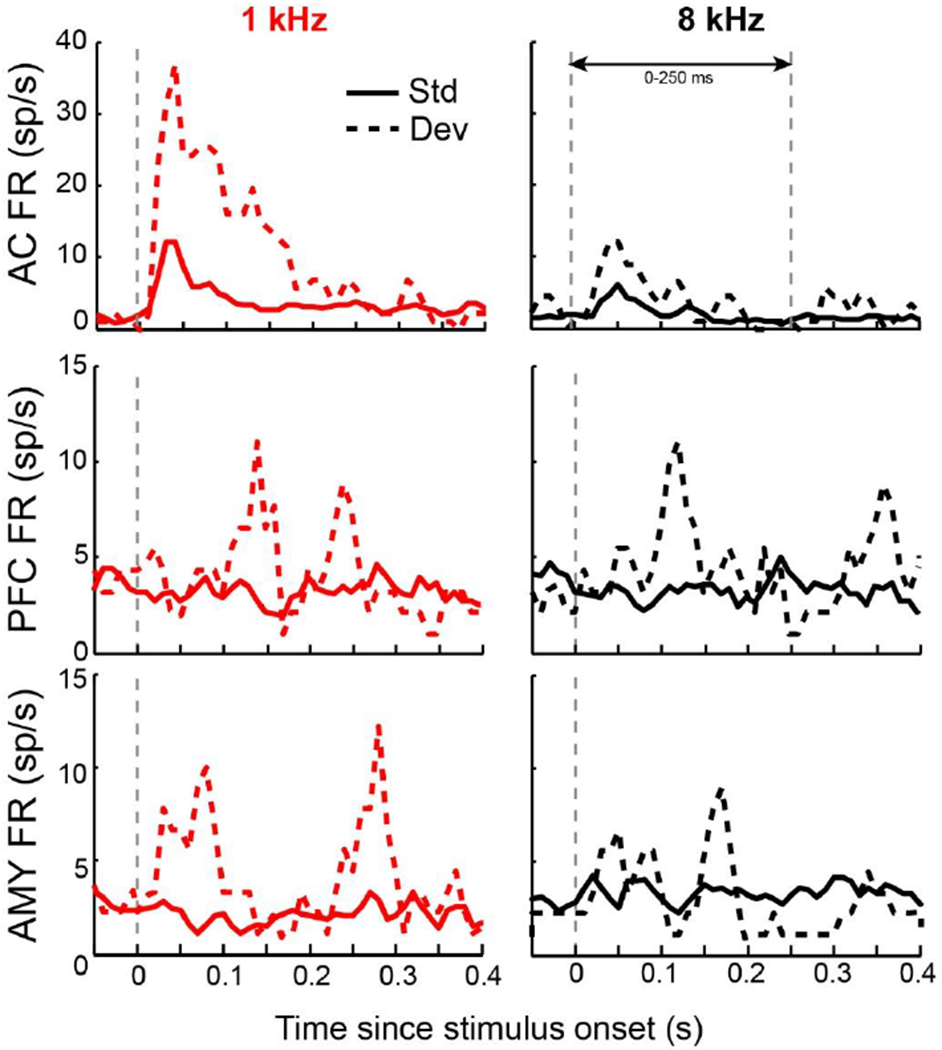
Peristimulus time histograms of average firing rate (FR) of an example single neuron response from each area (auditory cortex: AC, dorsolateral prefrontal cortex: PFC and lateral amygdala: AMY) to each stimulus and type combination. Each neuron shown is novelty selective. Note that in the prefrontal cortex and amygdala baseline firing rates and evoked activity are generally lower than in auditory cortex. PSTHs are smoothed for visualization purposes only.
The MMN measured at the scalp surface presumably represents activity of a neural population. While there is no direct mapping of neural activity to ERP components, a straightforward approach is to examine the magnitude of population activity of task responsive neurons and whether that population activity carries a novelty signal, and whether this novelty signal magnitude is different across areas or different with stimulus. For visualization and for a more direct comparison to human studies, we first collapse across all stimulus types to show average oddball response across areas (all deviants-all standards: Fig. 3), and the overall deviance response was approximately twice as strong in AC than in PFC or AMY (significant effect of area F(2,676)=15.4; p<0.001, between area post-hoc tests AC vs PFC: F(1,573)=14.8; p=0.001, AC vs AMY: F(1,573)=16.8; p<0.001, PFC vs AMY area: n.s., p=0.56). To better understand what is driving this pattern, we first examined the magnitude of evoked responses, and saw that responses were approximately twice as strong in auditory cortex than in prefrontal cortex and amygdala (significant effect of area F(2,676)=7.2; p<0.001, between area post-hoc tests AC vs PFC: F(1,572)=7.4; p=0.006, AC vs AMY: F(1,676)=7.0; p=0.009, PFC vs AMY area: n.s., p=0.69). When evoked responses were divided by stimulus and type (Fig. 4) note that auditory cortex responded more strongly to 1 kHz than to 8 kHz. We next examined the magnitude of novelty signal by stimulus for each area, which, as above can be measured as DEV-STD. We saw that the novelty signal was dependent on the stimulus in auditory cortex – it was stronger for the 1 kHz than for the 8 kHz stimulus (Fig. 5). However, this stimulus selectivity was not present in prefrontal cortex and amygdala – novelty signals were the same magnitude irrespective of driving stimulus (overall effect of type F(1,676)=58.2; p<0.001; AC stimulus F(1,468)=66.1, p<0.001; type F(1,468)=190.8, p<0.001; and stim x type F(1,468)=51.3, p<0.001; PFC type F(1,104)=41.0, p<0.001; AMY type F(1,104)=25.5, p<0.001 all other factors in PFC and amygdala p>0.1). These results persist when monkey was included as a factor, and there were no significant interactions with monkey factor (e.g. stim x type x monkey; stimulus (overall effect of type F(1,2027)=11.3; p<0.001; AC stimulus F(1,1401)=34.5, p<0.001; type F(1,1401)=8.1, p=0.004; and stim x type F(1,1401)=8.8, p=0.003; PFC type F(1,309)=9.13, p=0.002; AMY type F(1,309)=12.6, p<0.001 all other factors in PFC and amygdala, and critical interactions with monkey p>0.05). Thus at a population level the average novelty signal in auditory cortex was robust and stimulus dependent, and in amygdala and prefrontal cortex it was smaller (though significant) and not stimulus dependent.
Figure 3. Population oddball response by area, collapsed by stimulus.

Measure shown is average response to both deviants -average response to both standards calculated for each responsive neuron in each area (AC n=462, PFC n=105, AMY n=105). Note that the overall deviance response is approximately twice as large in AC than in PFC and AMY. Error bars are SEM across cells.
For a more complete picture of what is driving this this population level signal, we next asked whether the novelty signal is driven by a few or many neurons in each area (e.g. a weak signal in prefrontal cortex could be a few neurons carrying a signal comparable in magnitude to that of auditory cortex or many neurons carrying a weak signal). In auditory cortex a substantial number of neurons show a novelty signal for at least one of the stimuli (Fig. 6: 166/462 = 35.9%). However, few single neurons in prefrontal cortex and amygdala showed a similar profile (PFC: 9/105 = 8.6%, AMY: 9/105 = 8.6%), and the magnitude of the signal is again smaller relative to that in auditory cortex. Thus, at the level of single neurons few neurons are carrying a strong signal in in the amygdala and prefrontal cortex – it is a weak signal carried by many that leads to the overall novelty population response. Note that again, these results were not dependent on window choice – they did not change substantially with smaller (0-100 ms) or larger (0-400 ms) fixed or sliding windows.
For a given neuron, there is no predictive relationship between stimulus selectivity and deviance selectivity in these areas. We first examine whether the presence of deviance selectivity (ANOVA significant for stimulus type; STD vs DEV) is a function of stimulus selectivity (significant for stimulus frequency; 1kHz vs 8kHz). In AC, stimulus frequency affects magnitude of deviance response, it does not strongly affect whether deviance is signaled (of the 166 units that show a deviance selectivity, only 21 (12%) show it for only one of the stimuli, split fairly evenly between the two stimuli (13 units for 1kHz only, 8 units for 8 kHz only). In PFC and AMY, all neurons that show deviance selectivity show it for both stimuli (1kHz and 8kHz). Second, we examined the reverse - whether the presence of stimulus selectivity affected deviance selectivity. Again, a neuron exhibiting stimulus selectivity does not correspond to a neuron exhibiting deviance selectivity – in AC 248 neurons were stimulus selective of which only about half (87, or 54%) were also deviance selective. Additionally, 58 neurons were deviance selective, but not stimulus selective, and 21 neurons were deviance selective for only one stimulus (described above). In PFC and AMY, the number of individually responsive neurons is quite low, and in PFC 8 neurons were stimulus selective of which only 2 of these (25%) were also deviance selective, 7 additional neurons were deviance selective but not stimulus selective, and no neurons had deviance selectivity to only one stimulus. In AMY it was very similar - 8 neurons were stimulus selective, of which only 1 of these (12%) was also deviance selective, 8 additional neurons were deviance selective but no stimulus selective, and again no neurons had deviance selectivity to only one stimulus. Thus while stimulus selectivity affects the magnitude of the single unit response in AC, it is not predictive of whether a neuron exhibits deviance selectivity in AC, AMY or PFC.
We next wanted to examine a prediction of the predictive error hypothesis of the MMN, whether the novelty signal emerges first from sensory cortex. To do this we compare the timecourse of the novelty signal between auditory cortex, prefrontal cortex and amygdala, again using two approaches, one based on population level activity and the second based on single neurons, to more fully understand what is driving the population activity. As an estimate of how novelty signal evolves across population in each area, we calculated the population average PSTHs for each stimulus x type combination over all the responsive neurons and examined when response to deviants differed from response to standards at a population level (Fig. 7A–C). In auditory cortex, the earliest difference was detected in the bin spanning 10-40 ms. In amygdala it was later, 30-60 ms. In prefrontal cortex, no 30 ms bin reached significance. The lack of an onset time in this analysis in prefrontal cortex probably due to the diffuse nature of the deviance signal in prefrontal cortex and was not due to the size of the analysis bin. We repeated the analysis with shorter and longer bins and prefrontal cortex did not reach significance in any bin from 10-150 ms. While this population approach is informative about when the signal generally is arising, it may obscure heterogeneous but important dynamics (e.g. a smaller population of prefrontal neurons respond before auditory cortex but the majority are later). To examine whether there are any fast dynamics that are obscured by averages, we analyzed latencies on a single-neuron basis. Here, we calculated the latency at which the deviance signal (e.g. selectivity to type) arose in each area on an individual neuron basis, using 30 ms bins to increase temporal resolution (Fig. 7D, as cumulative distribution functions). The 30 ms bins increases the overall number of neurons that carry a novelty signal in each area (relative to the fixed bins in Fig. 6 above), but importantly, as stated above, the overall trends between areas are the same. According to this single neuron analysis, sensitivity to type was still earlier in auditory cortex than amygdala and prefrontal cortex, and amygdala and PFC did not differ (AC=56.6 ms, n=332; PFC = 128.9 ms, n=36; AMY=137.2 ms, n=46; ANOVA sig for area F(2,411)=57.4; p<0.001, post hoc t-tests AC< PFC (t(366) = −7.6, p<0.001), AC<AMY t(376) = −9.1, p<0.001, PFC=AMY t(80) = 0.45, p = 0.64). Earliest novelty sensitivity latencies in auditory cortex were still well earlier than those in prefrontal cortex and amygdala. Taken together, these analyses suggest that a deviance signal occurs first in auditory cortex, and later emerges in prefrontal cortex and amygdala, and that the population signal is a veridical reflection of dynamics occurring at the single neuron level.
As a final way to examine differences in how the novelty signal evolves across these areas, we compared the dynamics of response adaptation between areas. Neural responses may adapt at the beginning of the block, and also after a deviant is presented, and the existence and time course of adaptation may change with level of processing and brain region. For example, responses that are strongly sensory driven may show strong adaptation effects, but one would not expect adaptation in higher-order responses that presumably reflect more generalized novelty processing. To address this, we analyzed adaption of the responses to standards and deviants in two ways. First we examined standard and deviant adaptation across blocks (Fig. 8A). Consistent within-block adaptation occurred in auditory cortex for three of the stimuli (mean and t-test on fit beta values: 1 kHz standard mean=−0.036, t(461)=−10.9, p<0.001; 8 kHz standard mean=−0.016, t(461)=−4.7, p<0.001, 1 kHz deviant mean=−0.03, t(461)=−4.2, p<0.016, 8 kHz deviant mean=−0.006, t-test ns, p=0.056). In auditory cortex, adaptation was faster for the standards and faster for the 1 kHz stimulus (2x2 stim x type within-neuron ANOVA stim F(1,900.2)=16.4, p<0.001, type F(1,472.5)=17.2, p<0.001, stim x type ns (p>0.1)). Adaptation was not observed in prefrontal cortex, and it was only observed in amygdala for the 8 kHz deviant (amygdala 8 kHz deviant mean=−0.02, t(104)=−3.6 p<0.001, all other p>0.1). Note that this within block adaptation did not contribute to the novelty signal described above, as the first deviant was not presented until at least 10 standards had been presented (dotted line), after the within-block adaptation had occurred. A second type of adaptation is the effect that the deviant has on subsequent standards (Fig. 8B). We found that post-deviant adaptation occurred in auditory cortex for the 1 kHz stimulus (mean=−0.02, t(461)=−3.3, p<0.001), but not for other stimuli or areas (all other t-tests p>0.1). Note that the lower responses in amygdala and prefrontal cortex, as well as auditory cortex 8 kHz, may make adaptation difficult to detect. Generally however, it appears that stimulus-specific characteristics of adaptation seen in auditory cortex were not present in prefrontal cortex or amygdala, consistent with the more generalized novelty signal these higher-order areas appear to carry.
Discussion
The overall goal of this experiment was to leverage the spatial and temporal specificity of a primate model to address outstanding questions about novelty processing. Specifically, we compared the magnitude, timing, and stimulus specificity of auditory oddball-based novelty signals in auditory cortex, dorsolateral prefrontal cortex, and the basolateral amygdala. These data were recorded under identical conditions from a substantial number of neurons in the alert macaque, so even subtle effects could be captured and compared. This approach allows us to test predictions made by the predictive error/sensory memory model of the MMN, which posits that a frontal generator exists which is sensitive to a change in the expected sensory stream, and that this signal should be both abstracted from and later than the one in auditory cortex (Garrido et al., 2009; Giard et al., 1990; Naatanen et al., 1992). We find that, compared to the novelty signal seen in auditory cortex, the signal seen in dorsolateral prefrontal cortex and basolateral amygdala is smaller in magnitude, longer in latency, and not stimulus dependent. This is generally consistent with predictions of the predictive error hypothesis in that novelty signals in prefrontal cortex should be generally later than in auditory cortex, as well as abstracted from stimulus specific effects seen in auditory cortex. However, the fact that signals in amygdala were comparable in magnitude and timing to those in prefrontal cortex, and both prefrontal and amygdala signals were generally much weaker than those in auditory cortex, is not wholly consistent with the account.
The approach we present here allows more precise cross area comparisons than previous noninvasive or ECoG approaches. We see that novelty response in prefrontal cortex and amygdala has properties that are strikingly similar to each other yet do not appear to be simply inherited from the signal seen in auditory cortex. First, the stimulus specificity seen in auditory cortex was no longer significant in prefrontal cortex or the amygdala. Second, the within-block adaptation and post-deviance adaptation seen in auditory cortex were also no longer observed. This suggests that prefrontal cortex and amygdala have, to some degree, an abstracted deviance signal distinct from auditory cortex. These areas signal “difference”, but they do not reflect low level properties of the driving stimuli (e.g. spectral overlap). The timing of the difference signal is also consistent with that interpretation. The prefrontal difference signal lags that of auditory cortex by an average of ~100 ms, and the amygdala difference signal lags auditory cortex by between 20-100 ms. Note that a single neuron deviance signal which emerges in AC starting in a time window of 10-40 ms is quite consistent with estimates that primate MMN (and human) emerges around 50 ms (Gil-da-Costa et al., 2013) as well as early response latencies generally in AC (Camalier et al., 2012), These observations place useful quantitative constraints on putative generators of the auditory oddball-based MMN, and indicate that subcortical areas, such as the amygdala, may need to be included in future explanatory accounts of auditory oddball.
Previously there have been only indirect measures suggesting that frontal MMN generators are later than sensory generators (Deouell, 2007; Rinne et al., 2000; Tse & Penney, 2008). Human intracranial studies of auditory oddball paradigms have supported the existence of separate frontal and temporal components (Durschmid, Edwards, et al., 2016; Edwards et al., 2005; El Karoui et al., 2015; Kropotov et al., 2000; Liasis, Towell, Alho, & Boyd, 2001; Rosburg et al., 2005). However, due to the heterogeneity of the clinical placement of monitoring electrodes within and between patients, it has been difficult to compare the magnitude and timing of the deviance signal between these areas. In addition, our study extends our understanding of putative substrates of the oddball-based novelty processing to the amygdala. This builds on a hypothesized role of the amygdala in salience detection (Blackford et al., 2010). Here, we show that amygdala correlates of novelty are comparable in magnitude, timing, and response characteristics to those seen in prefrontal cortex. Previous accounts may have been biased towards cortical generators because noninvasive scalp-based measures are less sensitive to deep generators. This new finding may require an update of the accounts of the generators underlying the MMN.
Experiments in both rodents (Parras et al., 2017; Taaseh, Yaron, & Nelken, 2011; Ulanovsky et al., 2003; Yarden & Nelken, 2017) and primates (Fishman, 2014; Fishman & Steinschneider, 2012; Javitt et al., 1994) have examined single neuron correlates of the MMN in the early auditory hierarchy (cochlear nucleus through auditory cortex). The novelty signal in auditory cortex that we observed in this study had a magnitude, time course, and stimulus dependent characteristics consistent with what has been seen in population-based analyses of single neuron studies in auditory cortex (Farley, Quirk, Doherty, & Christian, 2010; Nieto-Diego & Malmierca, 2016; Parras et al., 2017; Ulanovsky et al., 2004; Ulanovsky et al., 2003). There are two methodological differences worth noting between our study and these studies, driven by the aim of our study, which was to compare the characteristics and timing of two putative higher-order generators of the deviance signal, to the deviance signal seen in auditory cortex. First, the stimuli used in this study were wideband and kept constant for all of the data collected, instead of the previous studies’ method of using pure tones whose identity is chosen to carefully flank the individual tuning of the neurons studied (and “population” responses collapsed across stimuli later). Thus we would expect the population responses in our study to be more comparable to human studies in which all the data are collected using the same stimuli for all of the areas. Second, the majority of the single neuron studies were performed under anesthesia (but see Farley et al., 2010; Parras et al., 2017). While deviance detection, especially the mismatch negativity, can be elicited under anesthesia, the anesthetic state may affect higher order areas and minimize top-down effects seen from, for example, prefrontal cortex, which does not exhibit robust responses under deep anesthesia. Such top down effects have also been seen on the MMN when during some kinds of attention manipulations, though the mechanisms of this effect are still hotly debated (Rinne, Sarkka, Degerman, Schroger, & Alho, 2006).
Utilizing the spatial and temporal specificity of a macaque model, this study overcomes limitations in our understanding from earlier findings derived from noninvasive methods and human intraoperative recordings, though there are multiple aspects of these findings that merit further investigation. The MMN-indexed deviance signal is commonly thought to contain an adaptation component (suppression of the expected), and/or a surprise component (enhancement of the unexpected; see Fishman 2014). Note that detection of deviance is of substantial evolutionary importance and therefore it is likely supported by a broad network of areas whose magnitude, network recruitment, and mechanism (e.g. adaptation vs surprise) may depend on task type, attentional state, behavioral requirements, and even anesthetic depth (MacLean, Blundon, & Ward, 2015; Nourski et al., 2018; Warbrick, Reske, & Shah, 2013). Even in the “simple” passive auditory oddball paradigm used here and in other studies, the relative contributions of each of these mechanisms (adaptation vs surprise) to the recorded signal has been a matter of intense debate, especially in the early auditory hierarchy (Fishman, 2014; Parras et al., 2017).
While the “error prediction” model posits that the frontal activation is an important generator of the MMN, the “adaptation only” hypothesis (e.g. Fishman, 2014), would posit that it is epiphenomenal. Our study finds that the signals in amygdala and prefrontal cortex have characteristics described by the error prediction hypothesis, but that the strength in both areas is weaker than that of auditory cortex. Therefore, an important future direction will be to establish causal links between prefrontal cortex and amygdala and the deviance signal seen in auditory cortex and at the scalp to be able to disambiguate between these accounts, especially using a combination of single unit and local field potential data compared with the extracranial MMN which has bene demonstrated (Gil-da-Costa et al., 2013). An earlier study reports that patients with prefrontal lesions exhibit a smaller scalp-measured mismatch negativity (Alain, Woods, & Knight, 1998; Alho, Woods, Algazi, Knight, & Naatanen, 1994). Though this would seem to imply a causal relationship of the prefrontal cortex to the scalp-recorded MMN, it is difficult to compare the magnitude of ERP components when there is substantial difference in damage to the underlying cortex. Due to its shared primate homology and established MMN (Gil-da-Costa et al., 2013) the macaque model is an ideal model to test these questions using reversible inactivation. In addition, other areas should be explored that are hypothesized to play a role such as the NAc and ACC. Towards that end, these data provide a significant advance in our conceptual framework of how deviance is processed outside of the early subcortical to cortical auditory hierarchy and open new directions of investigation in one of the dominant deviance detection paradigms, the auditory oddball paradigm.
Acknowledgments:
The authors would like to thank Dr. Israel Nelken and Dr. Brian Scott for valuable feedback, Dr. Richard Saunders for surgical assistance, Anna Leigh Brown and Jess Jacobs for assistance in data collection, and the NIH Section on Instrumentation assisted in custom manufacture of recording chambers and grid. Research was supported by NIMH DIRP ZIA MH002928-01 to BA and ZIA MH001101-25 to MM.
Footnotes
Conflict of interest: None.
References
- Alain C, Woods DL, & Knight RT (1998). A distributed cortical network for auditory sensory memory in humans. Brain Research, 812(1-2), 23–37. [DOI] [PubMed] [Google Scholar]
- Alho K, Woods DL, Algazi A, Knight RT, & Naatanen R (1994). Lesions of frontal cortex diminish the auditory mismatch negativity. Electroencephalography and Clinical Neurophysiology, 91(5), 353–362. [DOI] [PubMed] [Google Scholar]
- Antunes FM, Nelken I, Covey E, & Malmierca MS (2010). Stimulus-specific adaptation in the auditory thalamus of the anesthetized rat. PloS One, 5(11), e14071. doi: 10.1371/journal.pone.0014071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala YA, Perez-Gonzalez D, Duque D, Nelken I, & Malmierca MS (2012). Frequency discrimination and stimulus deviance in the inferior colliculus and cochlear nucleus. Front Neural Circuits, 6, 119. doi: 10.3389/fncir.2012.00119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balderston NL, Schultz DH, & Helmstetter FJ (2013). The effect of threat on novelty evoked amygdala responses. PloS One, 8(5), e63220. doi: 10.1371/journal.pone.0063220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackford JU, Buckholtz JW, Avery SN, & Zald DH (2010). A unique role for the human amygdala in novelty detection. NeuroImage, 50(3), 1188–1193. doi: 10.1016/j.neuroimage.2009.12.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Costa VD, Ferrari V, Codispoti M, Fitzsimmons JR, & Lang PJ (2015). Imaging distributed and massed repetitions of natural scenes: spontaneous retrieval and maintenance. Human Brain Mapping, 36(4), 1381–1392. doi: 10.1002/hbm.22708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camalier CR, D’Angelo WR, Sterbing-D’Angelo SJ, de la Mothe LA, & Hackett TA (2012). Neural latencies across auditory cortex of macaque support a dorsal stream supramodal timing advantage in primates. Proceedings of the National Academy of Sciences of the United States of America, 109(44), 18168–18173. doi: 10.1073/pnas.1206387109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czisch M, Wehrle R, Stiegler A, Peters H, Andrade K, Holsboer F, & Samann PG (2009). Acoustic oddball during NREM sleep: a combined EEG/fMRI study. PloS One, 4(8), e6749. doi: 10.1371/journal.pone.0006749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deouell LY (2007). The frontal generator of the mismatch negativity revisited. Journal of Psychophysiology, 21(3-4), 188–203. doi: 10.1027/0269-8803.21.34.188 [DOI] [Google Scholar]
- Durschmid S, Edwards E, Reichert C, Dewar C, Hinrichs H, Heinze HJ, … Knight RT (2016). Hierarchy of prediction errors for auditory events in human temporal and frontal cortex. Proceedings of the National Academy of Sciences of the United States of America, 113(24), 6755–6760. doi: 10.1073/pnas.1525030113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durschmid S, Zaehle T, Hinrichs H, Heinze HJ, Voges J, Garrido MI, … Knight RT (2016). Sensory Deviancy Detection Measured Directly Within the Human Nucleus Accumbens. Cerebral Cortex, 26(3), 1168–1175. doi: 10.1093/cercor/bhu304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards E, Soltani M, Deouell LY, Berger MS, & Knight RT (2005). High gamma activity in response to deviant auditory stimuli recorded directly from human cortex. Journal of Neurophysiology, 94(6), 4269–4280. doi: 10.1152/jn.00324.2005 [DOI] [PubMed] [Google Scholar]
- El Karoui I, King JR, Sitt J, Meyniel F, Van Gaal S, Hasboun D, … Naccache L (2015). Event-Related Potential, Time-frequency, and Functional Connectivity Facets of Local and Global Auditory Novelty Processing: An Intracranial Study in Humans. Cerebral Cortex, 25(11), 4203–4212. doi: 10.1093/cercor/bhu143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley BJ, Quirk MC, Doherty JJ, & Christian EP (2010). Stimulus-specific adaptation in auditory cortex is an NMDA-independent process distinct from the sensory novelty encoded by the mismatch negativity. Journal of Neuroscience, 30(49), 16475–16484. doi: 10.1523/JNEUROSCI.2793-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman YI (2014). The mechanisms and meaning of the mismatch negativity. Brain Topography, 27(4), 500–526. doi: 10.1007/s10548-013-0337-3 [DOI] [PubMed] [Google Scholar]
- Fishman YI, & Steinschneider M (2012). Searching for the mismatch negativity in primary auditory cortex of the awake monkey: deviance detection or stimulus specific adaptation? Journal of Neuroscience, 32(45), 15747–15758. doi: 10.1523/JNEUROSCI.2835-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K (2009). The free-energy principle: a rough guide to the brain? Trends Cogn Sci, 13(7), 293–301. doi: 10.1016/j.tics.2009.04.005 [DOI] [PubMed] [Google Scholar]
- Garrido MI, Kilner JM, Stephan KE, & Friston KJ (2009). The mismatch negativity: a review of underlying mechanisms. Clinical Neurophysiology, 120(3), 453–463. doi: 10.1016/j.clinph.2008.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giard MH, Perrin F, Pernier J, & Bouchet P (1990). Brain generators implicated in the processing of auditory stimulus deviance: a topographic event-related potential study. Psychophysiology, 27(6), 627–640. [DOI] [PubMed] [Google Scholar]
- Gil-da-Costa R, Stoner GR, Fung R, & Albright TD (2013). Nonhuman primate model of schizophrenia using a noninvasive EEG method. Proceedings of the National Academy of Sciences of the United States of America, 110(38), 15425–15430. doi: 10.1073/pnas.1312264110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser SN, Burton JA, Mercer ET, & Ramachandran R (2018). Effects of noise overexposure on tone detection in noise in nonhuman primates. Hearing Research, 357, 33–45. doi: 10.1016/j.heares.2017.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson LL, Heffner RS, & Heffner HE (1999). Free-field audiogram of the Japanese macaque (Macaca fuscata). Journal of the Acoustical Society of America, 106(5), 3017–3023. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Steinschneider M, Schroeder CE, Vaughan HG Jr., & Arezzo JC (1994). Detection of stimulus deviance within primate primary auditory cortex: intracortical mechanisms of mismatch negativity (MMN) generation. Brain Research, 667(2), 192–200. [DOI] [PubMed] [Google Scholar]
- Khouri L, & Nelken I (2015). Detecting the unexpected. Current Opinion in Neurobiology, 35, 142–147. doi: 10.1016/j.conb.2015.08.003 [DOI] [PubMed] [Google Scholar]
- Kropotov JD, Alho K, Naatanen R, Ponomarev VA, Kropotova OV, Anichkov AD, & Nechaev VB (2000). Human auditory-cortex mechanisms of preattentive sound discrimination. Neuroscience Letters, 280(2), 87–90. [DOI] [PubMed] [Google Scholar]
- Liasis A, Towell A, Alho K, & Boyd S (2001). Intracranial identification of an electric frontal-cortex response to auditory stimulus change: a case study. Brain Research: Cognitive Brain Research, 11(2), 227–233. [DOI] [PubMed] [Google Scholar]
- MacLean SE, Blundon EG, & Ward LM (2015). Brain regional networks active during the mismatch negativity vary with paradigm. Neuropsychologia, 75, 242–251. doi: 10.1016/j.neuropsychologia.2015.06.019 [DOI] [PubMed] [Google Scholar]
- Naatanen R, Gaillard AW, & Mantysalo S (1978). Early selective-attention effect on evoked potential reinterpreted. Acta Psychologica, 42(4), 313–329. [DOI] [PubMed] [Google Scholar]
- Naatanen R, Kujala T, Escera C, Baldeweg T, Kreegipuu K, Carlson S, & Ponton C (2012). The mismatch negativity (MMN)--a unique window to disturbed central auditory processing in ageing and different clinical conditions. Clinical Neurophysiology, 123(3), 424–458. doi: 10.1016/j.clinph.2011.09.020 [DOI] [PubMed] [Google Scholar]
- Naatanen R, Teder W, Alho K, & Lavikainen J (1992). Auditory attention and selective input modulation: a topographical ERP study. Neuroreport, 3(6), 493–496. [DOI] [PubMed] [Google Scholar]
- Nelken I (2014). Stimulus-specific adaptation and deviance detection in the auditory system: experiments and models. Biological Cybernetics, 108(5), 655–663. doi: 10.1007/s00422-014-0585-7 [DOI] [PubMed] [Google Scholar]
- Nieto-Diego J, & Malmierca MS (2016). Topographic Distribution of Stimulus-Specific Adaptation across Auditory Cortical Fields in the Anesthetized Rat. PLoS Biology, 14(3), e1002397. doi: 10.1371/journal.pbio.1002397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nourski KV, Steinschneider M, Rhone AE, Kawasaki H, Howard MA 3rd, & Banks MI (2018). Auditory Predictive Coding across Awareness States under Anesthesia: An Intracranial Electrophysiology Study. Journal of Neuroscience, 38(39), 8441–8452. doi: 10.1523/JNEUROSCI.0967-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parras GG, Nieto-Diego J, Carbajal GV, Valdes-Baizabal C, Escera C, & Malmierca MS (2017). Neurons along the auditory pathway exhibit a hierarchical organization of prediction error. Nat Commun, 8(1), 2148. doi: 10.1038/s41467-017-02038-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recanzone GH, Guard DC, & Phan ML (2000). Frequency and intensity response properties of single neurons in the auditory cortex of the behaving macaque monkey. Journal of Neurophysiology, 83(4), 2315–2331. [DOI] [PubMed] [Google Scholar]
- Rinne T, Alho K, Ilmoniemi RJ, Virtanen J, & Naatanen R (2000). Separate time behaviors of the temporal and frontal mismatch negativity sources. NeuroImage, 12(1), 14–19. doi: 10.1006/nimg.2000.0591 [DOI] [PubMed] [Google Scholar]
- Rinne T, Sarkka A, Degerman A, Schroger E, & Alho K (2006). Two separate mechanisms underlie auditory change detection and involuntary control of attention. Brain Research, 1077(1), 135–143. doi: 10.1016/j.brainres.2006.01.043 [DOI] [PubMed] [Google Scholar]
- Rosburg T, Trautner P, Dietl T, Korzyukov OA, Boutros NN, Schaller C, … Kurthen M (2005). Subdural recordings of the mismatch negativity (MMN) in patients with focal epilepsy. Brain, 128(Pt 4), 819–828. doi: 10.1093/brain/awh442 [DOI] [PubMed] [Google Scholar]
- Taaseh N, Yaron A, & Nelken I (2011). Stimulus-specific adaptation and deviance detection in the rat auditory cortex. PloS One, 6(8), e23369. doi: 10.1371/journal.pone.0023369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse CY, & Penney TB (2008). On the functional role of temporal and frontal cortex activation in passive detection of auditory deviance. NeuroImage, 41(4), 1462–1470. doi: 10.1016/j.neuroimage.2008.03.043 [DOI] [PubMed] [Google Scholar]
- Ulanovsky N, Las L, Farkas D, & Nelken I (2004). Multiple time scales of adaptation in auditory cortex neurons. Journal of Neuroscience, 24(46), 10440–10453. doi: 10.1523/JNEUROSCI.1905-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulanovsky N, Las L, & Nelken I (2003). Processing of low-probability sounds by cortical neurons. Nature Neuroscience, 6(4), 391–398. doi: 10.1038/nn1032 [DOI] [PubMed] [Google Scholar]
- Warbrick T, Reske M, & Shah NJ (2013). Do EEG paradigms work in fMRI? Varying task demands in the visual oddball paradigm: Implications for task design and results interpretation. NeuroImage, 77, 177–185. doi: 10.1016/j.neuroimage.2013.03.026 [DOI] [PubMed] [Google Scholar]
- Yarden TS, & Nelken I (2017). Stimulus-specific adaptation in a recurrent network model of primary auditory cortex. PLoS Computational Biology, 13(3), e1005437. doi: 10.1371/journal.pcbi.1005437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaehle T, Bauch EM, Hinrichs H, Schmitt FC, Voges J, Heinze HJ, & Bunzeck N (2013). Nucleus accumbens activity dissociates different forms of salience: evidence from human intracranial recordings. Journal of Neuroscience, 33(20), 8764–8771. doi: 10.1523/JNEUROSCI.5276-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]


