Abstract
Epidemiology and health economics have systemic interdependencies. The identification of the economic outcomes of any disease is operated by overlapping its epidemiology with the economic functions of the impacted entities. This communication presents two epidemiologic-economic models designed to evaluate the economic burden of cystic echinococcosis and leishmaniasis in Veneto (Northeastern Italy). Following a One Health approach, the research integrates expertise from different disciplines and institutions and fulfilled its first stage by defining the relevant cost categories and the data collection strategy for the two diseases in the study area. The two models identify the relevant epidemiological factors and the economic outcomes of infections in both animals and humans. The results, visualized in flow charts indicating the types of costs associated with these zoonoses, will guide data collection and the epidemiologic and economic assessment in the next research stages. This experience shows that One Health methods, although still innovative or unusual in many scientific and professional contexts, can be applied by using relatively limited resources and already available professional skills.
Keywords: Epidemiologic-economic models, Cystic echinococcosis, Leishmaniasis, Neglected zoonosis, Epidemiology of parasitic zoonoses, Health economics, Animal health economics, One health economics
Highlights
-
•
An economic evaluation of c. echinococcosis and leishmaniasis in a hypo-endemic area was approached with systems-thinking.
-
•
The research team and an external network of experts integrated the necessary interdisciplinary knowledge.
-
•
Two epidemiologic-economic models were designed to identify relevant cost categories and data sources.
-
•
This experience shows that One Health methods can be applied with limited resources and skills already available.
1. Introduction: seeking a One Health approach for echinococcosis and leishmaniasis in a hypo-endemic area
This communication concerns the methodology applied to implement a research project on cystic echinococcosis (CE) and leishmaniasis in Veneto, a region of 4.9 million inhabitants in Northeastern Italy. CE is originated by infections with the larval stage of the tapeworm Echinococcus granulosus sensu lato. Canids (definitive hosts) harbour adult parasites, while larvae develop in several livestock species (intermediate hosts) and also in humans (accidental intermediate hosts) [[1], [2], [3], [4]]. The only agent of human leishmaniasis (HumL) in Italy is Leishmania infantum, a protozoan transmitted by Phlebotomus sandflies that can determine important illness, especially in non-fully immune-competent individuals. In the study area, as in all Southern Europe, dogs represent the main reservoir of this pathogen, which also causes canine leishmaniasis (CanL) [[5], [6], [7], [8]]. Both CE and leishmaniasis have high public health impacts worldwide [3,5], recognise domestic animals as reservoirs, and are considered emerging and hypo-endemic in Veneto, with verified circulation in animal populations [4,8,9] and human cases recorded [10,11].
The main objectives of the research – funded through the person-time costs of the experts from the different institutions involved and a small university grant – are (i) the evaluation of the costs currently bore by the population and the health services of the region for the two diseases, and (ii) the assessment of possible interventions. The research encompasses three stages: the first includes an analysis of the epidemiological characteristics of the two diseases in the region, the identification of their costs, and the design of a data collection strategy; the second consists in data collection and epidemiologic and economic assessments; and the last covers result analysis and evaluation of possible measures to inform public health decision-makers. The research approach is inspired by One Health concepts and practices [12,13], in particular, systems thinking and inter/trans-disciplinarity, which shaped both the selection of the heterogeneous expertise involved in the study and the work organization. Until now, the first stage of the project has been completed, by identifying, respectively for CE and leishmaniasis, the interrelations of the relevant disease impacts on animal and human health, the environment, and the socioeconomic domain. Here we present the methodological aspects of the project and two epidemiologic-economic models (EEMs) that describe, for each disease, the interdependencies of the outcomes and their economic consequences, up to the definition of data collection strategies for cost evaluation.
2. Integrated expertise and systemic interdependencies between epidemiology and health economics: the construction of epidemiologic-economic models
Inter/trans-disciplinarity and systems thinking are functionally interrelated and increasingly applied to health issues [[14], [15], [16], [17]] especially in the One Health context [12,15,[18], [19], [20], [21], [22], [23]]. In this study, they allowed to size the epidemiologic and socioeconomic complexity of CE and leishmaniasis through the integration of interdisciplinary expertise and the elaboration of two EEMs.
Seven specialists in human and veterinary parasitology, and economics, from different academic and health research institutions and hospitals form the research team (RT) covering the basic set of disciplines needed. The RT interacts with an interdisciplinary network (IDN) of professionals and scientists operating as an external advisory board that validates the main steps of the project. The IDN members have been selected through an analysis of the integrative expertise needed to widen the RT institutional and operational perspective in critical aspects of public health services' management, daily veterinary professional practice, CE and leishmaniasis control programs, and One Health research and intervention planning (Table 1).
Table 1.
Project's research team members (RT) and supervisors of the external interdisciplinary network (IDN): affiliations, background, expertise, and contributions.
| Member | Affiliation | Disciplinary background | Specific expertise | Contribution to the project |
|---|---|---|---|---|
| RT1 | Academic institute (Italy) | Vet. medicine, parasitology | Epidemiology of CE and CanL | Project planning, project coordination |
| RT2 | Academic institute (Italy) | Agricultural economics | Agricultural and animal health economics | Project planning, economic evaluation |
| RT3 | Academic institute (Italy) | Agricultural economics | Agricultural and animal health economics | Project planning, economic evaluation |
| RT4 | Academic institute (Italy) | Vet. medicine, epidemiology | Epidemiology and modelling | Conceptualization of epidemiologic aspects |
| RT5 | Vet. public health and research institute (Italy) | Vet. medicine, parasitology | Epidemiology and Diagnostics related to CE and CanL | Conceptualization of veterinary aspects |
| RT6 | Hospital research institute (Italy) | Hum. medicine, tropical diseases | Epidemiology and clinical aspects of Human CE | Conceptualization of human CE aspects |
| RT7 | Hospital research institute (Italy) | Hum. medicine, tropical diseases | Epidemiology and clinical aspects of HumL | Conceptualization of HumL aspects |
| IDN1 | Regional health authority (Veneto) | Hum. medicine, management | Preventive human health | Decision-makers perspective for human public health |
| IDN2 | Regional health authority (Veneto) | Vet. medicine, management | Veterinary public health | Decision-makers perspective for veterinary health |
| IDN3 | Local health authority (Verona District) | Vet. medicine | Veterinary public health, animal health | Perspective of local veterinary services |
| IDN4 | Private professional (Italy) | Vet. medicine | Professional veterinarian (pet sector) | Perspective of dog owners' and vet. private practice |
| IDN5 | National Institute of Health (Italy) | Epidemiology, public health | CE (international expert) | Perspective from a wide experience of CE control |
| IDN6 | National Institute of Health (Italy) | Epidemiology, public health | Leishmaniasis (international expert) | Perspective from a wide experience of leishmaniasis control |
| IDN7 | Academic institute (UK) | Vet. medicine, vet. economics, One Health | One Health (international expert) | One Health perspective and overall revision |
The EEMs explain the transmission mechanisms of CE and leishmaniasis in the region and their costs. Conceptually, the models are based on the interrelations between epidemiology and health economics [[24], [25], [26]]: the spread of pathogens in animals and humans and the distribution and intensity of symptoms cause efficiency losses that can be evaluated in economic terms by overlapping the disease epidemiology with the economic functions of the impacted entities [[27], [28], [29], [30]]. This exercise supports decision making for cost-effective health interventions.
The EEMs have been built as systems within the boundaries set by the study objectives, by identifying the relevant functional elements and the feedbacks determining the CE and Leishmaniasis outcomes and the related costs. This process resulted from the RT and IDN interaction. The first tentative models were submitted by the RT to the IDN members. Based on the comments received, revised versions were elaborated, resubmitted and then discussed between the RT and the IDN in a one-day meeting, where the final EEMs were approved.
Two flowcharts (Fig. 1, Fig. 2) show the cause-effect links within the EEMs. This visual approach, well-grounded in epidemiology [31] and many applications of the systems theory [32,33], boosted the transdisciplinary system-building process. The EEMs allow a qualitative identification of CE and leishmaniasis costs in the region and the type of data needed to develop the following stages of the project that will be validated by the IDN through similar iterative procedures.
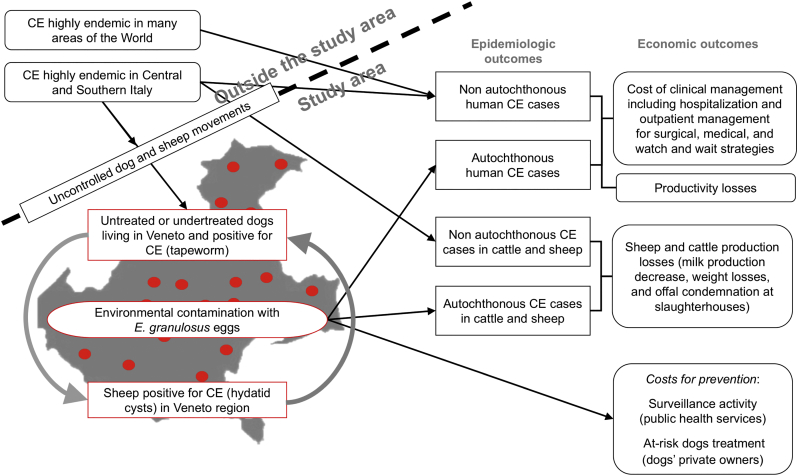
Fig. 1.
Epidemiologic-economic model flowchart of cystic echinococcosis in Veneto.
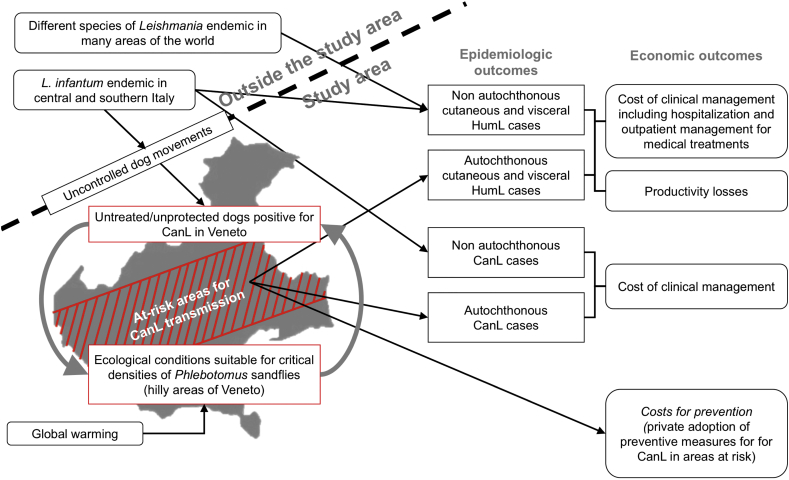
Fig. 2.
Epidemiologic-economic model flowchart of leishmaniasis in Veneto.
3. Cystic echinococcosis: epidemiologic-economic model and data collection strategy
In the CE flowchart (Fig. 1) the distinction between the epidemiological factors originating inside and outside the study area, represented by a map of the region, is indicated by a dashed line. The origin of the first CE animal infections in the region is unknown. Presently, around one hundred autochthonous cases of bovine CE are detected by local slaughterhouses every year [4]. Despite CE is supposed to affect mainly sheep in Veneto, no data on this species are available from slaughterhouses. Dogs positive for E. granulosus have been found in the last years through active surveillance [9]. Bovine CE is evenly distributed throughout the region [4], supporting the hypothesis of a moderately widespread environmental contamination of E. granulosus eggs (represented by red dots in the figure) that are a source of human infections. The cited studies prove the presence of an autochthonous cycle of the parasite (represented in Fig. 1 with the grey arrows connecting the boxes of positive dogs and positive sheep), hence possible local transmission to humans. Few human CE cases are treated in Veneto hospitals every year and the origin of such infections is largely unknown. The majority of patients come from other countries or Italian regions endemic for CE [10,[34], [35]].
Data collection aims at estimating the annual economic burden of CE in the region. Considering the emerging nature of the disease, hence its low prevalence in the region, the RT and the IDN agreed that data search will cover over ten years to detect temporal and spatial trends of the infection. Given the impossibility to distinguish with certainty the autochthonous from the non-autochthonous human cases, the IDN accepted the RT assumption that a CE patient resident in Veneto and born in the region or another non-endemic area will be considered an “autochthonous case”. For slaughtered cattle and sheep, animal movements can be tracked in the veterinary information system to operate this distinction.
For the evaluation of human CE costs, patients will be classified according to the three main disease management protocols: surgical, medical, and “watch and wait”. The relevant animal CE costs are related to weight losses, decreased milk production and offal condemnation in cattle and sheep, and to the treatment of dogs at risk. Table 2 shows a synthesis of the CE data collection strategy based on the relevant cost categories as settled between the RT and the IDN. Other possible costs related to the current level of the infection in the region have been considered negligible.
Table 2.
Data collection strategy for the cost evaluation of cystic echinococcosis and leishmaniasis in the analysed region.
| Type of cost | Data needed for the economic evaluation (analysed period 2001–2017) |
Data sources | |
|---|---|---|---|
| Cystic echinococcosis (CE) | Leishmaniasis | ||
| Costs for human health care; | Number of human CE cases in residents in the analysed region by age, sex, and nationality; distribution of liver CE, lung CE, and other potential localizations; | Number of human cases in residents in the analysed region by age, sex, and nationality; distribution of cutaneous and visceral leishmaniosis; | (a) |
| Diagnostic-therapeutic protocols applied and distribution of patients' management according to “watch and wait”, medical, and surgical treatment and respective outcomes; | Diagnostic-therapeutic protocols applied and distribution of patients' management according to the cutaneous and visceral forms and respective outcomes; | (a) (b) (c) | |
| Total cost per patient for clinical management, including diagnostics, hospitalization, surgery and postoperative follow up, medical treatments and patient monitoring; | Total cost per patient for clinical management, including diagnostics, hospitalization, post-hospitalization follow-up, medical treatments and patient monitoring; | (a) (b) (c) | |
| Productivity losses in the human population; | Patients' disability over time according to the therapeutic outcomes; gross domestic income per capita in the analysed region; | Patients' disability over time according to the therapeutic outcomes; gross domestic income per capita in the analysed region; | (a) (b) (c) (d) |
| Livestock production losses | Data on cattle and sheep heads, production, and production systems in the analysed region; | (c) (d) (e) | |
| Number of positive cases recorded in slaughterhouses for cattle and sheep (and respective livestock categories) from farms of the analysed region; | (f) | ||
| Weight losses and milk production decrease in affected animals; livestock and livestock product prices; | (c) (g) | ||
| Offal condemnations of positive animals at slaughterhouses; offal market prices; costs for offal disposal; | (c) (f) (g) | ||
| Costs for treatments of symptomatic dogs | Treatment protocols for symptomatic dogs; number of symptomatic dogs in the region; number of dogs treated in the region under the different protocols; | (c) (h) (i) (j) | |
| Unit cost of dog treatment for the different treatment protocols; | (h) (i) (k) | ||
| Costs for preventive treatment of exposed dogs | Definition of “at-risk” dog and standard preventive treatment protocol for at-risk dogs; number of at-risk dogs in the region; number of dogs subjected to preventive treatments; | Definition of a standard preventive treatment protocol; total number of dogs in the areas where the vector is present; number of dogs subjected to preventive treatments; | (c) (h) (i) (j) |
| Annual unit cost for dog treatment; | Annual unit cost for dog preventive treatment; | (h) (i) (k) | |
| Costs for surveillance of susceptible livestock species; | Notifications from slaughterhouses of infected animals coming from the analysed region: number and unit costs; | (i) (l) | |
| Investigations on farm outbreaks in the region by the public veterinary services: number and unit costs; | (i) (l) | ||
| Costs related to registrations, periodical reporting and maintenance of the information system; | (i) (l) | ||
| (a) Data from the hospital discharge records (HDRs) (b) Administrative and financial records of a reference hospital in the analysed region; (c) Information and data from the scientific literature; (d) Statistics on population, economy, and agriculture in the analysed region; (e) Data from the national veterinary information system; (f) Survey on Italian slaughterhouses receiving animals from the analysed region; (g) Agricultural price bulletins; |
(h) Interviews with private practice veterinarians managing animal health in farms and veterinary clinics for pets; (i) Interviews with veterinarians of the public health services (j) Data from the national canine registry; (k) Price lists of veterinary medicines; (l) Administrative and financial records from the veterinary public health services. |
||
4. Leishmaniasis: epidemiologic-economic model and data collection strategy
The leishmaniasis EEM also distinguishes the epidemiological factors originating within and outside the study area (Fig. 2).
The parasite was introduced from outside the region, but an autochthonous circulation of L. infantum (represented by the grey arrows connecting the boxes of positive dogs and sandfly high density in Fig. 2) has been demonstrated for many local canine populations [8,36].
Ecological conditions suitable for the development of the vector population can be found in the hilly parts of Veneto [37], where CanL endemicity is then favoured. In these zones, the territory of the municipalities with demonstrated parasite circulation in dogs (roughly represented by red diagonal stripes in Fig. 2) has been delimited as the area “at risk”. The rest of the region seems unapt for the development of critical sandfly densities. However, a progressive future expansion of the area at risk is possible because of global warming.
Few human cases of cutaneous and visceral HumL are reported each year from Veneto hospitals, but their origin is mostly uncertain [11]. The RT and IDN then agreed that one patient residing in a municipality within the area at risk and born in Veneto or another non-endemic region will be considered an “autochthonous” HumL case. A distinction between cutaneous and visceral forms will be applied for the HumL cost evaluation. Costs for CanL concern the treatment of symptomatic dogs and the prevention of CanL in the areas at risk. The RT and IDN decisions about leishmaniasis data collection strategy and main cost categories to be considered are summarised in Table 2.
5. Final considerations
The holistic approach of One Health supports the idea that economic evaluations of health interventions should consider the complexity of the human, animal and environmental context to a broad extent, hence the need for a methodological shift toward inter/trans-disciplinarity and systems thinking [38]. Fulfilling its first stage, this research shows the feasibility of such a shift, even with small-scale projects and limited financial resources. The elaboration of the two EEMs tested the possibility of setting innovative procedures between researchers and institutions from different fields. Three aspects of this exercise should be underlined:
-
1.
the integration of expertise resulting in an effective interdisciplinary work to identify the main cost categories and plan data collection for a comprehensive economic evaluation;
-
2.
the cooperation within and between the RT and the IDN leading to the validation of the EEMs in a wider perspective than that achievable by a mono-disciplinary institution or group;
-
3.
the optimization of available resources, i.e. individual skills, professional roles and institutions already working on different aspects of the same problems, but usually not coordinated toward specific goals, especially when dealing with neglected zoonoses like CE and leishmaniasis.
The definitions of “autochthonous” human cases for the two diseases, based on information from veterinary surveillance (e.g. the delimitation of areas at-risk for HumL based on CanL endemicity) are examples of fruitful interdisciplinary expertise integration and institutional cooperation driven by a One Health approach. The first results of this project indicate that One Health methods, although still pioneering or unusual in many scientific and professional contexts, can be implemented with limited resources and already available capacities.
Author contributions
MC, MA, and RC conceived the project and drafted the manuscript. AA, GC, MD, FG, and FT contributed to the development of the epidemiologic-economic models and manuscript revision and editing.
Declaration of Competing Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Acknowledgments
Authors are grateful to Giuseppina Napoletano, Michele Brichese, Fabrizio Cestaro, Roberto Zampiccoli, Adriano Casulli, Luigi Gradoni, Barbara Haesler for their contribution as independent experts in the fine-tuning of the EEMs, and to Matteo Legnardi and Matteo Mosconi for providing useful insights and for their help in practical organization of the research work.
Author Statement
Massimo Canali, Maurizio Aragrande, and Rudi Cassini conceived the project and drafted the manuscript. Andrea Angheben,Gioia Capelli, Michele Drigo, Federico Gobbi, and Francesca Tamarozzi contributed to the development of the epidemiologic-economic models and manuscript revision and editing.
Funding
The information provided in this communication is issued by the the project “Evaluation of policy measures to control two emerging parasitic diseases (Cystic Echinococcosis and Leishmaniasis) in Veneto Region using a One Health approach” (cod. BIRD174940), granted in the framework of the Departmental integrated research budget of the University of Padova, year 2017.
References
- 1.WHO-IWGE Meeting of the WHO informal working group on echinococcosis (WHO-IWGE), Geneva, Switzerland, 15–16 December 2016. 2017. http://apps.who.int/iris/bitstream/handle/10665/254869/WHO-HTM-NTD-NZD-2017.01-eng.pdf?sequence=1 Geneva, pp 1-27.
- 2.Otero-Abad B., Torgerson P.R. A systematic review of the epidemiology of echinococcosis in domestic and wild animals. PLoS Negl. Trop. Dis. 2013;7:1–13. doi: 10.1371/journal.pntd.0002249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rinaldi L., Alvarez Rojas C.A., Torgerson P.R., Harandi M.F., Romig T., Antolova D., Schurer J.M., Lahmar S., Cringoli G., Magambo J., Jenkins E.J. Global distribution of alveolar and cystic echinococcosis. Adv. Parasitol. 2017;95:315–493. doi: 10.1016/bs.apar.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Cassini R., Mulatti P., Zanardello C., Simonato G., Signorini M., Cazzin S., Tambalo P.G., Cobianchi M., Pietrobelli M., Capelli G. Retrospective and spatial analysis tools for integrated surveillance of cystic echinococcosis and bovine cysticercosis in hypo-endemic areas. Geospat. Health. 2014;8:509. doi: 10.4081/gh.2014.40. [DOI] [PubMed] [Google Scholar]
- 5.Okwor I., Uzonna J. Social and economic burden of human Leishmaniasis. Am. J. Trop. Med. Hyg. 2016;94:489–493. doi: 10.4269/ajtmh.15-0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gradoni L., López-Vélez R., Mokni M., editors. Manual on Case Management and Surveillance of the Leishmaniases in the WHO European Region. World Health Organization; Geneva: 2017. https://www.who.int/leishmaniasis/resources/978-92-89052-51-1/en/ (accessed December 26, 2018) [Google Scholar]
- 7.WHO Surveillance of leishmaniasis in the WHO European Region, 2016 and Global leishmaniasis surveillance update, 1998–2016. WHO Wkly. Epidemiol. Rec. 2018;93:521–540. https://www.who.int/leishmaniasis/resources/who_wer9340/en/ (accessed December 26, 2018) [Google Scholar]
- 8.Cassini R., Signorini M., Frangipane di Regalbono A., Natale A., Montarsi F., Zanaica M., Brichese M., Simonato G., Borgato S., Babiker A., Pietrobelli M. Preliminary study of the effects of preventive measures on the prevalence of canine leishmaniosis in a recently established focus in northern Italy. Vet. Ital. 2013;49:157–161. [PubMed] [Google Scholar]
- 9.Cassini R., Simonato G., Mulatti P., Ravagnan S., Danesi P., Pascotto E., Breda T., Brichese M., Pietrobelli M., Capelli G. A new approach to outbreak management for bovine Cystic Echinococcosis cases in hypo-endemic areas. Vet. Parasitol. Reg. Stud. Reports. 2019;16:100269. doi: 10.1016/j.vprsr.2019.100269. [DOI] [PubMed] [Google Scholar]
- 10.Piseddu T., Brundu D., Stegel G., Loi F., Rolesu S., Masu G., Ledda S., Masala G. The disease burden of human cystic echinococcosis based on HDRs from 2001 to 2014 in Italy. PLoS Negl. Trop. Dis. 2017;11 doi: 10.1371/journal.pntd.0005771. (e0005771 doi:10.1371/journal.pntd.0005771) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mannocci A., La Torre G., Chiaradia G., Waure De C., Mainelli M., Cernigliaro A., Bruno S., Ricciardi G.W. Epidemiology and direct medical costs of human Leishmaniasis in Italy. J. Prev. Med. Hyg. 2007;48 [PubMed] [Google Scholar]
- 12.Zinsstag J., Schelling E., Waltner-Toews D., Whittaker M., Tanner M. CABI; Wallingford: 2015. One Health: The Theory and Practice of Integrated Health Approaches. [Google Scholar]
- 13.Rüegg S.R., Häsler B., Zinsstag J. A Handbook for the Evaluation of One Health. Wageningen Academic Publishers; Wageningen: 2018. Integrated approaches to health. [Google Scholar]
- 14.Choi B.C.K., Pak A.W.P. Multidisciplinarity, interdisciplinarity and transdisciplinarity in health research and policy: 1. Definitions, objectives, and evidence of effectiveness. Clin. Investig. Med. 2006;29:351–364. [PubMed] [Google Scholar]
- 15.Hitziger M., Esposito R., Canali M., Aragrande M., Häsler B., Rüegg S.R. Knowledge integration in one health policy formulation, implementation and evaluation. Bull. World Health Organ. 2018;96:211–218. doi: 10.2471/BLT.17.202705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hitziger M., Aragrande M., Berezowski J.A., Canali M., Del Rio Vilas V., Hoffmann S., Igrejas G., Keune H., Lux A., Bruce M., Palenberg M.A., Pohl C., Radeski M., Richter I., Robledo Abad C., Salerno R.H., Savic S., Schirmer J., Vogler B.R., Rüegg S.R. EVOLvINC: EValuating knOwLedge INtegration capacity in multistakeholder governance. Ecol. Soc. 2019;24:36. [Google Scholar]
- 17.de Savigny D., Taghreed A. World Health Organization; Genève: 2009. Systems Thinking for Health Systems Strengthening. https://www.who.int/alliance-hpsr/resources/9789241563895/en/ (accessed December 25, 2018) [Google Scholar]
- 18.Rüegg S., Rosenbaum Nielsen S., Buttigieg L., Santa M., Aragrande M., Canali M., Ehlinger T., Chantziaras I., Boriani E., Radeski M., Bruce M., Häsler B. A systems approach to evaluate one health initiatives. Front. Public Heal. 2018;5:1–18. doi: 10.3389/fvets.2018.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rüegg S.R., McMahon B.J., Häsler B., Esposito R., Nielsen L.R., Ifejika Speranza C., Ehlinger T., Peyre M., Aragrande M., Zinsstag J., Davies P., Mihalca A.D., Buttigieg S.C., Rushton J., Carmo L.P., De Meneghi D., Canali M., Filippitzi M.E., Goutard F.L., Ilieski V., Milićević D., O’Shea H., Radeski M., Kock R., Staines A., Lindberg A. A blueprint to evaluate one health. Front. Public Health. 2017;5:20. doi: 10.3389/fpubh.2017.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rüegg S.R., Häsler B., Rosenbraun Nielsen L., Buttigieg S.C., Santa M., Aragrande M., Canali M., Queenan K., Chantziaras I., Boriani E. A one health evaluation framework. In: Rüegg S.R., Häsler B., Zinsstag J., editors. Integr. Approaches to Heal. A Handb. Eval. One Heal. Wageningen Academic Publishers; Wageningen (NL): 2018. pp. 38–85. [Google Scholar]
- 21.Buttigieg S.C., Savic S., Cauchi D., Lautier E., Canali M., Aragrande M. Brucellosis control in Malta and Serbia: a one health evaluation. Front. Vet. Sci. 2018;5:147. doi: 10.3389/fvets.2018.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laing G., Aragrande M., Canali M., Savic S., De Meneghi D. Control of cattle ticks and tick-borne diseases by Acaricide in Southern Province of Zambia: a retrospective evaluation of animal health measures according to current one health concepts. Front. Public Health. 2018;6:45. doi: 10.3389/fpubh.2018.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Canali M., Aragrande M., Cuevas S., Cornelsen L., Bruce M., Rojo-Gimeno C., Häsler B. The economic evaluation of one health. In: Rüegg S.R., Häsler B., Zinsstag J., editors. Integr. Approaches to Heal. A Handb. Eval. One Heal. Wageningen Academic Publishers; Wageningen (NL): 2018. pp. 170–226. [Google Scholar]
- 24.Howe K.S. Conceptual affinities in veterinary epidemiology and economics. Acta Vet. Scand. Suppl. 1988;84:347–349. http://www.ncbi.nlm.nih.gov/pubmed/3232635 (accessed December 25, 2018) [PubMed] [Google Scholar]
- 25.Perrings C., Castillo-Chavez C., Chowell G., Daszak P., Fenichel E.P., Finnoff D., Horan R.D., Kilpatrick A.M., Kinzig A.P., Kuminoff N.V., Levin S., Morin B., Smith K.F., Springborn M. Merging economics and epidemiology to improve the prediction and Management of Infectious Disease. Ecohealth. 2014;11:464–475. doi: 10.1007/s10393-014-0963-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geoffard P.-Y., Philipson T. Rational epidemics and their public control. Int. Econ. Rev. (Philadelphia). 1996;37:603. [Google Scholar]
- 27.Roberts R.R., Mensah E.K., Weinstein R.A. A guide to interpreting economic studies in infectious diseases. Clin. Microbiol. Infect. 2010;16:1713–1720. doi: 10.1111/j.1469-0691.2010.03366.x. [DOI] [PubMed] [Google Scholar]
- 28.Häsler B., Howe K.S., Di Labio E., Schwermer H., Stärk K.D.C. Economic evaluation of the surveillance and intervention programme for bluetongue virus serotype 8 in Switzerland. Prev. Vet. Med. 2012;103:93–111. doi: 10.1016/j.prevetmed.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 29.Aragrande M., Canali M. Animal health and price transmission along livestock supply chains. OIE Rev. Sci. Tec. 2017;36:87–96. doi: 10.20506/rst.36.1.2612. [DOI] [PubMed] [Google Scholar]
- 30.Zinsstag J., Dürr S., Penny M.A., Mindekem R., Roth F., Menendez Gonzalez S., Naissengar S., Hattendorf J. Transmission dynamics and economics of rabies control in dogs and humans in an African city. Proc. Natl. Acad. Sci. U. S. A. 2009;106:14996–15001. doi: 10.1073/pnas.0904740106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joffe M., Gambhir M., Chadeau-Hyam M., Vineis P. Causal diagrams in systems epidemiology. Emerg. Themes Epidemiol. 2012;9(1) doi: 10.1186/1742-7622-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meadows D.H. A Primier; Earthscan, London: 2009. Thinking in Systems. https://wtf.tw/ref/meadows.pdf (accessed December 25, 2018) [Google Scholar]
- 33.Anderson V., Johnson L. Pegasus Communications; Cambridge: 1997. Systems Thinking Basics : From Concepts to Causal Loops. [Google Scholar]
- 34.Brundu D., Piseddu T., Stegel G., Masu G., Ledda S., Masala G. Retrospective study of human cystic echinococcosis in Italy based on the analysis of hospital discharge records between 2001 and 2012. Acta Trop. 2014;140:91–96. doi: 10.1016/j.actatropica.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 35.Centro di Riferimento Regionale Vaccinazioni Internazionali - Regione Veneto Echinococco, Newsletter N.5, Regione Veneto, Venice. 2013. https://viaggiatori.aulss9.veneto.it/docs/MalattieInfettive/Viaggiatori_Internazionali/News/News-13/news5-2013.pdf
- 36.Maroli M., Rossi L., Baldelli R., Capelli G., Ferroglio E., Genchi C., Gramiccia M., Mortarino M., Pietrobelli M., Gradoni L. The northward spread of leishmaniasis in Italy: evidence from retrospective and ongoing studies on the canine reservoir and phlebotomine vectors. Tropical Med. Int. Health. 2008;13:256–264. doi: 10.1111/j.1365-3156.2007.01998.x. [DOI] [PubMed] [Google Scholar]
- 37.Signorini M., Cassini R., Drigo M., Frangipane di Regalbono A., Pietrobelli M., Montarsi F., Stensgaard A.-S. Ecological niche model of Phlebotomus perniciosus, the main vector of canine leishmaniasis in North-Eastern Italy. Geospat. Health. 2014;9:193–201. doi: 10.4081/gh.2014.16. [DOI] [PubMed] [Google Scholar]
- 38.Kock R., Queenan K., Garnier J., Rosenbaum Nielsen L., Buttigieg S., De Meneghi D., Holmberg M., Zinsstag J., Rüegg S., Häsler B. Health solutions: Theoretical foundations of the shift from sectoral to integrated systems. In: Rüegg S.R., Häsler B., Zinsstag J., editors. Integr. Approaches to Heal. A Handb. Eval. One Heal. Wageningen Academic Publishers; Wageningen (NL): 2018. pp. 22–37. [Google Scholar]