Abstract
Purpose:
To determine cataract surgeon viewpoints on the efficacy of available therapies/preventatives for two common sequelae of cataract surgery; inflammation and posterior capsular opacification (PCO).
Methods:
Cataract surgeons practicing worldwide specializing in adult, pediatric and veterinary patients were interviewed between March and August 2018.
Results:
Ocular inflammation following cataract surgery is treated by either corticosteroids and/or nonsteroidal anti-inflammatories (NSAIDs). Adult and pediatric cataract surgeons are satisfied with current treatments whereas this inflammation is still considered a problem by some in veterinary practice due to its slow resolution. YAG laser therapy is the PCO treatment of choice for adult cataract surgeons and they are generally pleased with its outcome. However, pediatric cataract surgeons find YAG problematic, especially in patients under 6 years of age, and invasive surgery is often needed to correct PCO/visual axis opacification (VAO). Veterinary ophthalmologists report that YAG is not effective for PCO in animals, especially dogs, due to the density of the fibrotic plaques. 86% of adult and 100% of veterinary and pediatric cataract surgeons surveyed agree that effective anti PCO therapeutics would improve clinical care.
Conclusion:
Surgeons treating human patients are pleased with the available treatments for ocular inflammation following cataract surgery, although some veterinary ophthalmologists disagree. The surgeons surveyed agree that PCO/VAO remains an unsolved problem in pediatric and veterinary cataract surgery while the long term outcome of adult cataract surgery could be improved by additional attention to this issue.
Keywords: Cataract surgery, Inflammation, Posterior capsular opacification, PCO, Interview, Opinion, Therapeutics
Introduction:
Cataracts, a major cause of blindness worldwide[1–3], are efficiently treated by surgery followed by implantation of an artificial intraocular lens (IOL)[2]. However, cataract surgery triggers acute ocular inflammation which can be painful and slows visual recovery[2,4]. Inflammation is currently treated by either anti-inflammatory eye drops which are plagued by low patient compliance[5] or installation of anti-inflammatories into the eye at the time of surgery (“drop-less” cataract surgery)[6]. While this acute inflammation usually resolves quickly in the absence of infection, low-level inflammation can persist for months post surgery and may exacerbate other ocular pathologies such as uveitis and glaucoma [7–10]. Then, months to years following cataract surgery, a significant proportion of cataract patients experience an apparent recurrence of their cataract as Posterior Capsular Opacification (PCO)[11–14].
PCO develops due to the presence of a mixture of scar-producing myofibroblasts and aberrant lens fiber cells in the optical axis[11]. While the rates of PCO in human patients as determined within the first year of surgery have greatly diminished over the past 10 years due to the widespread introduction of “square edge” IOLs made of hydrophobic materials, late-onset PCO still occurs in a significant number of adult patients which limits the long-term outcome of cataract surgery[15–20]. Late onset PCO becomes particularly problematic as multifocal and accommodating IOLs intended to restore the full range of high-resolution vision enter common clinical use as even slight amounts of PCO can greatly impair the performance of these lenses [21–23]. Further, short term PCO rates in infants and animals undergoing cataract surgery are still high as the remnant lens cells have a higher proliferative and migratory potential than is typical in age-related human cataract patients[24–27]. Animals also tend to develop relatively dense PCO plaques, perhaps due to the high prevalence of lens-induced uveitis in veterinary patients due to their more advanced cataract phenotypes at surgery[26,28].
In adult humans, PCO is routinely treated non-invasively by YAG laser capsulotomy[2,12,29]. However, while YAG therapy for PCO is highly effective in this population, it can result in retinal detachment, macular edema, glaucoma, IOL damage, and IOL dislocation[30–34], especially when high laser power is used to treat dense PCO[30]. Although these complications are relatively uncommon (0.5-2% of patients undergoing YAG), and could be resulting from the cataract surgery itself and not YAG capsulotomy per se, it has been pointed out that these potentially blinding sequelae still affect significant numbers of people as both cataract surgery and YAG therapy for PCO are common[35]. Outside of clinical considerations, YAG laser capsulotomy is also a cost for these patients and their health insurers[36]. Further, YAG laser therapy is problematic in young children both due to their inability to sit at the instrument for the procedure and a tendency to form dense PCO[37], while YAG is generally not performed on animals due to both their tendency to form dense PCO and cost[26,30].
For these reasons, researchers have sought to further reduce PCO rates by a variety of approaches. Attempts have been made to prevent PCO through further improvements in IOL design and surgical techniques such as “Bag in the lens”[35,38,39]. However, safety concerns, cost, and the high level of surgical expertise often needed for the most effective approaches have prevented their universal incorporation into clinical practice[35,39–42]. As PCO is caused by proliferation, migration, and differentiation/transdifferentiation of lens epithelial cells (LECs) remaining behind post cataract surgery, numerous attempts have been made to interfere with the behavior of the remnant LECs post cataract surgery. The role of IOL material in PCO development has been investigated leading to a consensus that hydrophobic IOLs lead to lower short term PCO rates due to better adherence of the IOL to the lens capsule, although this is not a universal solution as hydrophobic IOLs are more prone to glistening and can exacerbate ocular inflammation[43,44]. Surface modification of IOLs with bioactive substances such as heparin have been attempted in order to improve IOL biocompatibility. While these approaches can improve IOL-triggered inflammation, their success in preventing PCO is mixed [44–47]. Attempts have also been made to block LEC division with anti-proliferative agents or to kill the LECs entirely[48,49], but, in many cases, these approaches are plagued by safety concerns and are often not effective long-term as they seldom can target every remnant LEC[48]. Further, it has been pointed out that modern IOL designs rely on the remnant lens cells to secure the implant’s long term position in the eye, while the inadvertent loss of all lens epithelial cells following cataract surgery results in “dead bag syndrome” in which the IOL fails to remain centered in the optical axis[50,51].
It has been proposed that PCO may be best prevented by treatments that encourage the remnant LECs to differentiate into lens fibers which will remain at the capsular bag periphery to both fix the IOL in place while being less migratory and permanently postmitotic[52–54]. A few reports have suggested that small capsulotomy cataract surgery/refilling of the capsular bag with a fiber differentiation promoting compound may be effective, but some fibrotic cells are still produced [52,55]. In recent years, we have gained a significant cell biological understanding of what pathways drive remnant lens epithelial cells towards fiber cell differentiation versus transdifferentiation into fibrosis producing myofibroblasts [56–61]. To date though, pharmacological antagonists of these pathways have shown limited success in preventing PCO, although most of the tested therapies only targeted one of the many signal transduction pathways likely involved in PCO pathogenesis[62–67]. However, the feasibility of continuing to develop our deepening understanding of PCO pathogenesis into FDA approved anti-PCO therapies is impaired by a perception by some that PCO is not still an issue of clinical concern[35].
Thus, we undertook a survey of cataract surgeons who treat a breadth of patients including adults, children, and animals to discuss the clinical challenges they encounter and the types of therapeutic interventions that would enhance the long term efficacy of cataract surgery.
Methods:
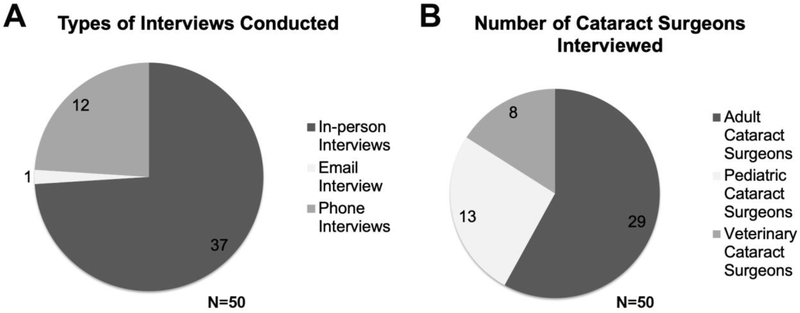
Institutional review board review was conducted at the University of Delaware and this project was deemed “not human subjects research” as it consisted of “Information-gathering interviews where questions focus on things, products, or policies”. Practicing cataract surgeons (50) were recruited through direct email contacts (supplemental figure 1), ARVO-connect, the online community for the Association for Research in Vision and Ophthalmology (supplemental figure 2), and direct conference contacts at the American Association for Pediatric Ophthalmology and Strabismus (AAOPS) Annual Meeting-2018-Washington DC, USA, The Association for Research in Vision and Ophthalmology (ARVO) Annual Meeting-2018, Honolulu, Hawaii, USA, Centro de Oftalmología Barraquer, Investigación, Barcelona, Spain, the Ryan Veterinary Hospital - Penn Vet - University of Pennsylvania, USA, and private practice offices in Newark, Delaware and Plymouth, Pennsylvania, USA to participate in in person, phone and e-mail interviews (Figure 1A and 1B). Only people who self-identified as board-certified ophthalmologists who perform cataract surgery as the major portion of their clinical duties were surveyed for this study. Typical interview questions are listed below and the same questions were asked to each cataract surgeon who participated in this study.
Figure 1:
(A) 50 cataract surgeons were interviewed in person, by email, and over the phone and (B) included cataract surgeons treating human adults and children as well as animals.
Questions
What are your top three concerns regarding post-surgical management after cataract surgery?
How do you manage post cataract surgical inflammation? Are you satisfied with the current standard of care for post cataract surgical inflammation treatment? Any alternatives that you would prefer?
How many Posterior Capsular Opacification (PCO) cases do you get per year? Do you still think PCO is a clinical problem?
How long does PCO take to develop after surgery in your patients (based on your experiences)?
How do you treat PCO? How many patients get subsequent consequences such as macular edema and retinal detachment? (you can use a percentage of patients out of the total number of PCO patients)
Are you satisfied with the treatment protocol for PCO? What do you desire or what changes would you do?
If there was a non-surgical approach to treating PCO/post-surgical ocular inflammation would you use it? What type of target would you prefer?
-
Which drug delivery system would you prefer? (Ointment/injection during surgery.)
This question was least discussed during the interview session.
• Do you have any colleagues that could help us out with our market research? Please leave contact information below either yours to reach out later or that of your colleagues interested in participating in this type of interview.
Results:
What are your top 3 concerns for post-surgical management after cataract surgery?
Post cataract surgical inflammation and PCO are major patient management concerns post cataract surgery (PCS)
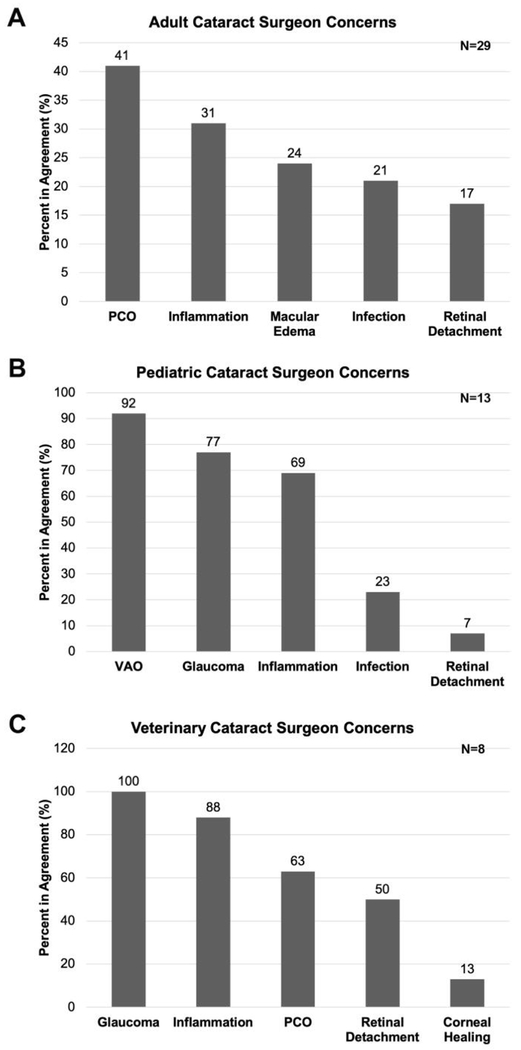
Cataract surgeons were asked about their major concerns with patient management post cataract surgery (PCS) (Figure 2). 41% of adult cataract surgeons have stated that PCO is one of their top three major post cataract surgical complications and 31% of adult cataract surgeon mentioned that inflammation is a major patient management concern PCS. Macular edema is a major concern of 24% of adult cataract surgeons while post-surgical infection (21%) and retinal detachment (17%) were also major concerns of some of adult cataract surgeons interviewed (Figure 2A).
Figure 2:
Cataract surgeon opinion on their major concerns regarding post cataract surgical side effects. A- adult cataract surgeons; B- pediatric cataract surgeons; C- veterinary cataract surgeons.
92% of pediatric cataract surgeons surveyed reported that visual axis opacification (VAO) is their major concern PCS while 77% of pediatric surgeons felt that post-surgical glaucoma was among their top three major concerns. Post-surgical inflammation was a major concern for 69% of pediatric surgeons interviewed while fewer surgeons gave post-surgical infection (23%) and retinal detachment (7%) as major management concerns for pediatric cataract patients (Figure 2B).
In contrast to adult and pediatric cataract surgeons, 100% of veterinary cataract surgeons report that glaucoma is among their top three major concerns PCS while 88% mentioned inflammation among their major clinical concerns after cataract surgery. 63% of veterinary cataract surgeons report that PCO is a long term clinical issue affecting their patient’s vision PCS, while 50% of them report that retinal detachment is a major PCS complication. In contrast to human ophthalmologists, 13% of veterinary ophthalmologists mentioned that corneal healing can be an issue PCS in their patients (Figure 2C).
How do you manage to post cataract surgical inflammation? Are you satisfied with the current standard of care for post cataract surgical inflammation treatment? Any alternatives that you would prefer?
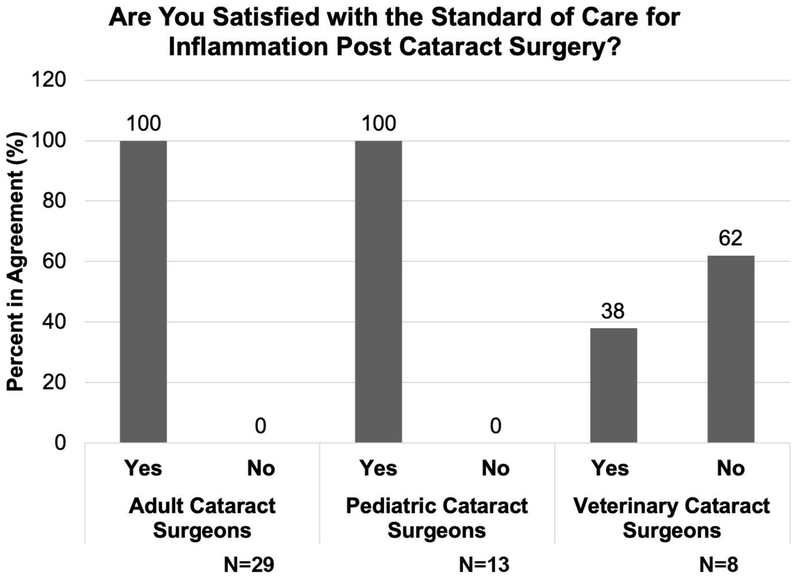
Steroidal and nonsteroidal anti-inflammatory agents (NSAIDs) are the standard of care for the management of inflammation post cataract surgery
Steroidal and nonsteroidal anti-inflammatory agents (NSAIDs) are regularly administered to treat and/or prevent post cataract surgical inflammation. Cataract surgeons treating both adult and pediatric human patients are quite satisfied with the current standard of care for the management of post cataract surgical inflammation. In contrast, 62% of veterinary cataract surgeons are dissatisfied with this standard of care as animals, such as dogs, often need to be treated with anti-inflammatory agents for 3 to 6 months PCS for a complete recovery as they often present at surgery with phacolytic uveitis[68,69]. Thus, veterinary cataract surgeons expressed an interest in trying out alternatives if they are made available. (Figure 3).
Figure 3:
Cataract surgeon opinion on the current standard of care for the management of post cataract surgical inflammation. Left- adult cataract surgeons; Middle- pediatric cataract surgeons; Right- veterinary cataract surgeons.
How many Posterior Capsular Opacification (PCO) cases do you get per year?
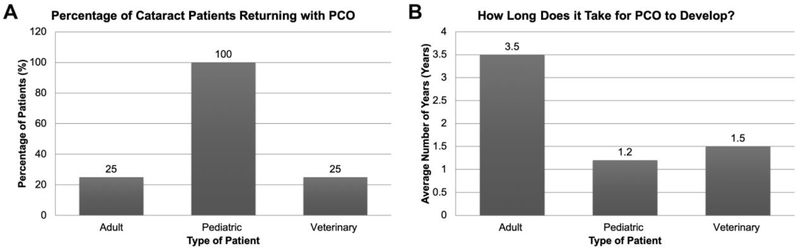
25% of adult and veterinary patients, and almost 100% of pediatric patients develop clinically significant PCO/VAO post cataract surgery (PCS)
Most adult cataract surgeons surveyed estimated that approximately 25% of the cataract patients they treat return with clinically significant PCO within 10 years of cataract surgery. Cataract surgeons from developing countries such as South Africa and Peru have stated that 50% of their patients develop clinically significantly PCO in their clinical settings. In contrast, few cataract surgeons from developed countries experience 10% or fewer PCO patients per year. On the other hand, pediatric cataract surgeons said that almost 100% of pediatric patients develop PCO/VAO within a few months to years after cataract surgery. Some veterinary cataract surgeons report that 100% of their patients develop PCO, especially dogs. However, not all PCO impairs vision enough to hinder animals from performing regular activities like walking or finding food. Thus, about 25% of animal owners bring their animals to the veterinary cataract surgeon for behaviorally significant PCO (Figure 4A).
Figure 4:
(A) Cataract surgeon estimates on the percentage of treated cataract patients returning with PCO (B) Cataract surgeon estimates of the time it takes to develop clinically significant PCO post cataract surgery.
How long does PCO take to develop after surgery in your patients (based on your experience)?
Pediatric patients develop PCO quickly compared to adult and veterinary patients
Surgeons who treat adult human patients feel that it takes between 2 to 5 years (average 3.5 years) to develop PCO PCS. Pediatric surgeons have informed us that it takes 12- 16 months for pediatric patients to develop PCO/VAO. However, several pediatric cataract surgeons have seen PCO/VAO development as early as 4 weeks PCS. Veterinary cataract surgeons report that behaviorally significant PCO develops in about 1-2 years PCS in younger dogs (Figure 4B), although many older dogs do not live long enough PCS for this to be a concern.
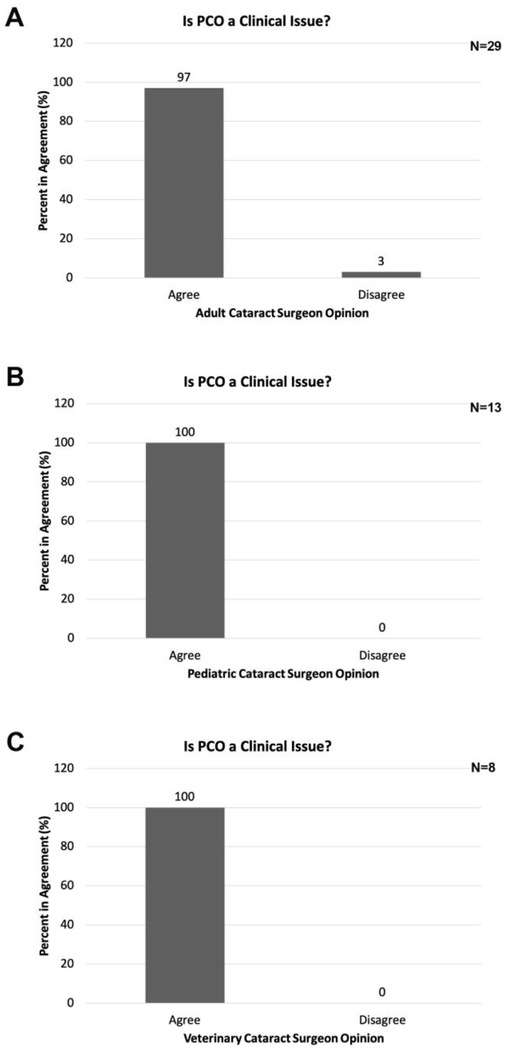
Do you still think PCO is a clinical problem?
PCO is still clinically important
Since all cataract surgeons report PCO in their practice, next we asked if they consider PCO as a significant, unsolved clinical issue and found that 100% of pediatric and veterinary cataract surgeons and 97% of adult cataract surgeons surveyed agreed with this statement. The sole disagreeing cataract surgeon who treats adult patients has told us that PCO is not clinically important since 1% or less of their patients develop PCO and this is easily treated by YAG when it occurs (Figure 5).
Figure 5:
Percentage of cataract surgeons who report that PCO is still a clinical issue in their practice. A- adult cataract surgeons; B- pediatric cataract surgeons; C- veterinary cataract surgeons.
How do you treat PCO?
YAG laser capsulotomy is the treatment of choice for PCO in adults while this is less used in pediatric and veterinary patients
Cataract surgeons specializing in adult patients reported that YAG laser therapy is the treatment of choice for PCO. While pediatric cataract surgeons told us that YAG is sometimes possible in older children when the posterior capsule is intact, YAG is less feasible in younger or developmentally delayed children unable to sit still for the procedure as this requires placement of an anesthetized child at the instrument. Even when YAG is possible, children often develop dense PCO which cannot be removed by YAG and still require a posterior capsulotomy and anterior vitrectomy. Further, pediatric patients often develop PCO-like symptoms even if they underwent posterior capsulotomy during the initial cataract surgery due to the growth of cells across the anterior hyaloid membrane (visual axis opacification (VAO)). Due to the location of the aberrant cells, VAO must be treated by anterior vitrectomy[70–72]. In contrast, all veterinary cataract surgeons interviewed stated that there is no practical treatment for PCO in animals (Table 1).
Table 1:
Cataract surgeons’ opinion on the current treatment to manage PCO.
| Question | Response from cataract surgeons |
|---|---|
| How do you treat PCO? | Adult- YAG laser therapy Pediatric- Sometimes YAG is possible when the posterior capsule is intact, but VAO may require anterior vitrectomy. Veterinary- There is no practical treatment for PCO in animals. |
How many patients get subsequent consequences such as macular edema and retinal detachment? (you can use a percentage of patients out of the total number of PCO patients)
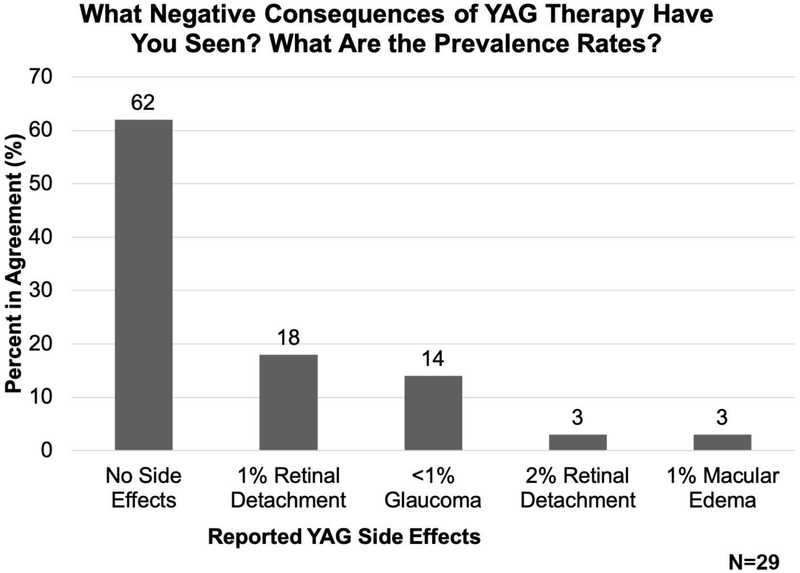
Most adult cataract surgeons surveyed reported that they have not seen side-effects following YAG laser capsulotomy, while others stated that although the negative consequences of YAG laser capsulotomy are often minimal, important side effects still occur
YAG laser is the treatment of choice for adult PCO among the cataract surgeons surveyed. As some negative consequences of YAG therapy have been reported[30–32,34], we asked adult cataract surgeons whether their patients have experienced any negative consequences after YAG laser therapy and found that 62% of adult cataract surgeons report that they have not seen any side effects following YAG laser therapy. However, 18% of adult cataract surgeons state that they have seen approximately 1% of patients experience retinal detachment following YAG laser therapy in their clinical practice, whereas 3% of adult cataract surgeons report a 2% retinal detachment rate, and a 1% rate of macular edema after YAG laser therapy. 14% of adult cataract surgeons surveyed reported that they have seen glaucoma after YAG laser treatment, but this occurs in 1% or less of all patients (Figure 6).
Figure 6:
Adult cataract surgeon estimates of the prevalence of negative consequences of YAG therapy based on their clinical experience.
Are you satisfied with the treatment protocol for post cataract surgical inflammation and PCO? What do you desire or what changes would you do? If there was a new non-surgical approach to treating PCO/post-surgical ocular inflammation would you use it?
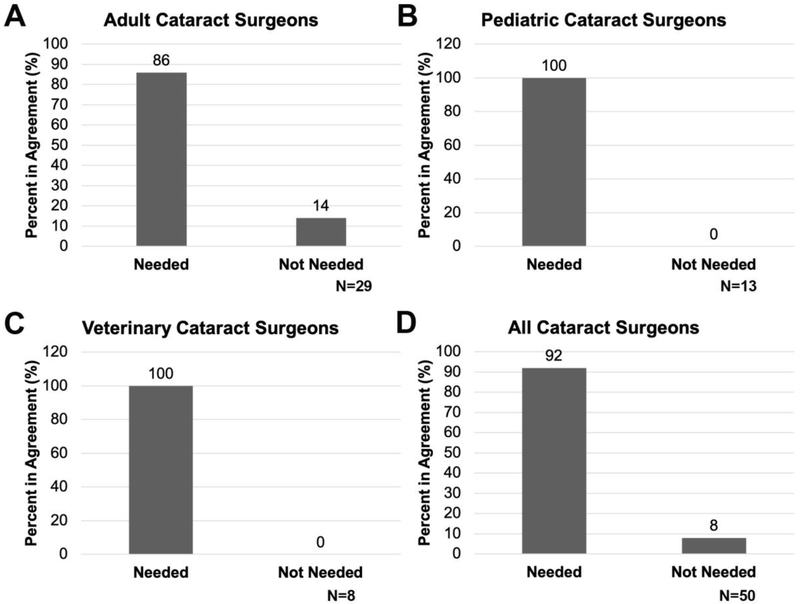
Most of the cataract surgeons surveyed are interested in new ways to prevent PCO while they are generally satisfied with the standard of care for inflammation prevention post cataract surgery
Finally, we asked if cataract surgeons would be interested in incorporating anti PCO therapeutics into their clinical practice and found that all pediatric and veterinary cataract surgeons showed interest in preventive anti PCO therapeutics (Figure 7B and C). However, 14% of adult cataract surgeons think that YAG laser is adequate to treat PCO and thus do not feel that additional preventive anti PCO measures are necessary (Figure 7A). Overall, cataract surgeons treating humans feel that standard of care is sufficient for the treatment of inflammation associated with cataract surgery (Figure 3), however, those who treat animals would like additional therapeutic options for inflammation as this is not adequately controlled in their patients with the current standard of care (Figure 3).
Figure 7:
The percentage of cataract surgeons who report that new PCO therapies are needed. A- adult cataract surgeons; B- pediatric cataract surgeons; C- veterinary cataract surgeons; D- all cataract surgeons.
Discussion:
The development of extracapsular cataract surgery followed by intraocular lens implantation is one of the most significant advances in modern medicine, taking cataract from being the major cause of human blindness and low vision that it was through most of human history, to a condition that can be treated with a quick outpatient procedure[73]. However, even the most robust surgical intervention has the potential for negative sequelae[2,11,12].
Most cataract surgeons surveyed are satisfied with the treatments available for ocular inflammation following cataract surgery
Cataract surgery is one of the most commonly performed surgeries in the world and has been highly successful for decades[5]. However, cataract surgery results in acute ocular inflammation arising as a normal response to the surgical wound[74]. While high levels of inflammation can be an important sign of infection, uncontrolled “sterile” ocular inflammation is undesirable as it opacifies the ocular media and can lead to several other complications notably uveitis, secondary glaucoma, macular edema and even retinal detachment[5,7,75]. Thus, its post cataract surgical management is of paramount importance. Currently, corticosteroids or nonsteroidal anti-inflammatory agents (NSAIDs), or a combination of both, are the treatment of choice in the management of inflammation post cataract surgery[5,76]. In general, steroids are more effective in managing inflammation than NSAIDs, however, in some cases, NSAIDs might be sufficient in routine patients undergoing cataract surgery[77].
Due to the importance of inflammation management post cataract surgery, we first asked cataract surgeons if they see post-surgical inflammation in their clinical settings. All of the cataract surgeons have informed us that inflammation is a common side effect that they see following cataract surgery which is consistent with reports that most if not all cataract patients develop inflammation post cataract surgery[5,75,76]. Next, we have asked them about their preferred methods to manage post-surgical inflammation. Consistent with the literature, some of the cataract surgeons we surveyed prefer to use corticosteroids, while others apply NSAIDs or a combination of both[5]. Most surveyed adult and pediatric cataract surgeons stated that they were satisfied with the current standard of care for inflammation management post cataract surgery. However, veterinary cataract surgeons were interested in additional ways to prevent inflammation post cataract surgery as it can take a few months to resolve inflammation PCS in dogs, which is time-consuming and not cost-effective. This observation is consistent with the literature which suggests that a high proportion of dogs receive cataract surgery after the onset of phacolytic uveitis resulting from mature cataract which must be treated to prevent side effects such as inflammation and glaucoma[78].
Most cataract surgeons surveyed feel that PCO is still an important clinical problem
As surgical therapies for cataract were being developed, intracapsular lens removal became the therapy of choice since extracapsular lens extraction was plagued by PCO as proliferation, migration, and differentiation/transdifferentiation of the remnant lens epithelial cells resulted in a rapid recurrence of visual symptoms which could only be treated with subsequent invasive surgery[73]. Later, attempts to develop intraocular lens prostheses were greatly slowed by high rates of PCO, however, the advent of YAG laser capsulotomy made this treatable by a non-invasive office procedure[29]. This has been bolstered by the development of square edge IOLs which have reduced PCO rates in adults to the single digits when measured within the six months to a year after cataract surgery[79–81], allowing extracapsular lens extraction followed by IOL implantation to become the standard of care for cataract treatment. However, there is some controversy about the global use of square edge IOLs as their design results in pseudophakic dysphotopsia which is the most prevalent reason patients are dissatisfied with cataract surgery[82,83]. Further, it has been noted that the growing popularity of “premium” IOLs which correct vision at all distances make PCO more clinically important in adults, while YAG laser capsulotomy is not without side effects[84]. A new surgical technique bag-in the-lens (BIL) has shown promising results preventing PCO in both adult and pediatric patients compared to the traditional lens-in-the bag (LIB) procedure. However, this approach has not widely adopted in the USA due to the lack of clinical studies in the USA, and the specialized technical expertise needed [41,42,85–90].
These reports have led to some controversy as to whether PCO is a “solved” clinical problem or still should be the focus of both basic science and drug development efforts due to its high importance. Most adult cataract surgeons surveyed stated that about 25% of adult patients undergoing cataract surgery develop clinically significant PCO within 2- 5 years post cataract surgery. The remaining surgeon reports PCO rates below 1% due to their routine use of “posterior optic capture/bag in the lens” surgery in which a posterior capsulotomy is performed at surgery and the remanent lens cells are trapped at the capsule periphery by placing the IOL in Berger’s space[91]. Surgeons practicing in developing countries reported higher PCO rates compared to developed countries due to lower access to improved IOLs and modern cataract surgical techniques. YAG laser therapy is the treatment of choice for adults presenting with PCO, and the surgeons surveyed are generally satisfied with the clinical outcomes of YAG laser therapy although they acknowledge that some patients do develop undesirable sequelae such as macular edema and retinal detachment. These opinions closely correlate with published reports that show that the long-term (1-10 years post surgery) PCO rates are still 20-70% in adults[2,35]. The relatively low rates of severe negative outcomes observed after YAG is also borne out in the literature which reports rates of 1-3%[2,29]. Several cataract surgeons informed us that YAG laser capsulotomy is not a financial burden for patients as Medicare and government health systems cover or subsidize YAG therapy, although this opinion does not consider the cost to these health care systems or patient copays. For instance, YAG laser capsulotomy to treat PCO was the 10th most costly ambulatory procedure performed on Medicare patients in 2016[35]. The adult cataract surgeons surveyed are generally satisfied with YAG laser therapy as a treatment for PCO, although it is less available in developing countries[92]. Overall, 86% of adult cataract surgeons surveyed would be interested in pharmacological methods to robustly prevent PCO, although some adult cataract surgeons emphasized the need for improved IOL designs and surgical techniques as other ways to improve the visual outcomes for their patients. However, the pediatric cataract surgeons interviewed told us that the reality is quite different for their patients. They stated that almost 100 percent of pediatric patients undergoing traditional extracapsular lens extraction followed by IOL implantation develop PCO within the first year following surgery which is supported by reports in the literature[79,93]. Even in cases where prophylactic posterior capsulotomy is performed at the time of cataract extraction, the response of the remnant lens epithelial cells to surgery is still a problem because they can migrate onto the anterior hyaloid and/or cause phimosis of the anterior capsulotomy resulting in visual axis opacification (VAO)[72,94]. While children over 6 years of age can often be treated with YAG laser capsulotomy, younger children or those with developmental delays can not sit still at the instrument[93]. The pediatric cataract surgeons surveyed also noted that VAO/PCO treatment is problematic in young children as this requires general anesthesia, and YAG instruments optimized for treating anesthetized children are generally not available. Further, YAG is often counter-indicated when the PCO is dense or when VAO involving the anterior hyaloid is present[70,93]. In these cases, invasive posterior capsulotomies are done and/or anterior vitrectomies are required to remove the opacification, and even then VAO can re-occur [71,93,95,96]. All pediatric cataract surgeons surveyed stated that they would be interested in exploring new approaches to prevent PCO and/or VAO, including the use of pharmacological agents to prevent these conditions, due to the need for increasing the safety and efficacy, while reducing the cost, for cataract treatment in children.
The veterinary cataract surgeons surveyed noted that animals (dogs, cats, and horses) tend to get PCO earlier and progress faster than humans, with PCO rates approaching 100% which is consistent with the literature[97]. However, veterinary surgeons also note that PCO is often less clinically significant as animals have shorter life expectancies and most pets only need sufficient vision to navigate their surroundings and find food, so have less need for excellent visual acuity. Even with that, 25% of their owners bring their animals back to the veterinary cataract surgeon reporting a recurrence of visual symptoms. However, few animals are treated for PCO. First, YAG laser therapy is not effective for most animal PCO cases due to both the tendency for animal PCO to be denser than that seen in humans, as it forms more aggressively and must be severe before the owner notes a reduction in their pet’s vision[97]. Second, as only 25% of veterinary patients develop clinically significant PCO by the end of their life, and YAG lasers are costly, YAG lasers are uncommon in veterinary practices. Thus, all veterinary cataract surgeons surveyed are very interested in preventive anti-PCO therapeutics.
These interviews suggest that most cataract surgeons are satisfied with the current standard of care for post-surgical inflammation while the majority surveyed felt that new approaches to prevent PCO would be clinically useful, although the relatively small number of surgeons interviewed here (50 total across three different practice types; adult, pediatric and veterinary) could mean the market for new PCO prevention strategies is not as large as suggested here. However, as major progress has been made in recent years towards understanding the pathophysiology of PCO[11,56,58,59,98], there is likely sufficient information about PCO pathogenesis to identify new anti-PCO therapeutics which would be of particular use in veterinary and pediatric cataract surgery. Simultaneously, new surgical approaches such as “bag in the lens” and further refinements in IOL design have clinical promise in PCO prevention for all patient populations. Thus, the future looks bright for approaches to reduce the incidence of PCO, improving the long term effectiveness of cataract surgery.
Supplementary Material
4. Acknowledgments:
We thank all the cataract surgeons who participated in our study, our collaborators Dr. Ralph Michael and Justin C D’Antin, Centro de Oftalmología Barraquer, Barcelona, Spain and Jing Jin, MD, AI DuPont Hospital for Children for their critical reading of this manuscript.
1. Declaration of funding: This study was supported by the National Science Foundation-funded- NSF I-Corps Entrepreneurial program run by the Horn Entrepreneurship program at the University of Delaware to MHS, SGN, and MKD; National Eye Institute Grant EY EY015279 to MKD; A summer research doctoral travel award from the Office of Graduate and Professional Education, The University of Delaware and an ARVO travel grant supported travel for MHS. None of the funding sources had any role in the decision to submit this article for publication.
2.
Declaration of financial/other relationships: No financial disclosures in medicine exist for any of the authors. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
- [1].Khairallah M, Kahloun R, Bourne R, Limburg H, Flaxman SR, Jonas JB, Keeffe J, Leasher J, Naidoo K, Pesudovs K, Price H, White RA, Wong TY, Resnikoff S, Taylor HR, Number of People Blind or Visually Impaired by Cataract Worldwide and in World Regions, 1990 to 2010, Invest. Ophthalmol. Vis. Sci 56 (2015) 6762–6769. doi: 10.1167/iovs.l5-17201. [DOI] [PubMed] [Google Scholar]
- [2].Liu Y-C, Wilkins M, Kim T, Malyugin B, Mehta JS, Cataracts, The Lancet. 390 (2017) 600–612. doi: 10.1016/S0140-6736(17)30544-5. [DOI] [PubMed] [Google Scholar]
- [3].Lee CM, Afshari NA, The global state of cataract blindness., Curr. Opin. Ophthalmol 28 (2017) 98–103. doi: 10.1097/ICU.0000000000000340. [DOI] [PubMed] [Google Scholar]
- [4].Chan E, Mahroo OAR, Spalton DJ, Complications of cataract surgery, Clin. Exp. Optom 93 (2010) 379–389. doi: 10.1111/j.l444-0938.2010.00516.x. [DOI] [PubMed] [Google Scholar]
- [5].Juthani VV, Clearfield E, Chuck RS, Non-steroidal anti-inflammatory drugs versus corticosteroids for controlling inflammation after uncomplicated cataract surgery., Cochrane Database Syst. Rev 7 (2017) CD010516. doi: 10.1002/14651858.CD010516.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lindstrom RL, Galloway MS, Grzybowski A, Liegner JT, Dropless Cataract Surgery: An Overview., Curr. Pharm. Des 23 (2017) 558–564. doi: 10.2174/1381612822666161129150628. [DOI] [PubMed] [Google Scholar]
- [7].Abbouda A, Tortorella P, Restivo L, Santoro E, De Marco F, La Cava M, Follow-Up Study of Over Three Years of Patients with Uveitis after Cataract Phacoemulsification: Outcomes and Complications., Semin. Ophthalmol 31 (2016) 532–541. doi: 10.3109/08820538.2015.1009554. [DOI] [PubMed] [Google Scholar]
- [8].Bhutto I, Lutty G, Understanding age-related macular degeneration (AMD): Relationships between the photoreceptor/retinal pigment epithelium/Bruch’s membrane/choriocapillaris complex, NEW INSIGHTS Etiol. Treat. MACULAR Degener 33 (2012) 295–317. doi: 10.1016/j.mam.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Teh BL, Megaw R, Borooah S, Dhillon B, Optimizing cataract surgery in patients with age-related macular degeneration., Surv. Ophthalmol 62 (2017) 346–356. doi: 10.1016/j.survophthal.2016.12.003. [DOI] [PubMed] [Google Scholar]
- [10].Diagourtas A, Petrou P, Georgalas I, Oikonomakis K, Giannakouras P, Vergados A, Papaconstantinou D, Bleb failure and intraocular pressure rise following Nd: Yag laser capsulotomy., BMC Ophthalmol. 17 (2017) 18. doi: 10.1186/sl2886-017-0408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wormstone IM, Wang L, Liu CSC, Posterior capsule opacification., Exp. Eye Res 88 (2009) 257–269. doi: 10.1016/j.exer.2008.10.016. [DOI] [PubMed] [Google Scholar]
- [12].Awasthi N, Guo S, Wagner BJ, Posterior capsular opacification: a problem reduced but not yet eradicated., Arch. Ophthalmol. Chic. Ill 1960. 127 (2009) 555–562. doi: 10.1001/archophthalmol.2009.3. [DOI] [PubMed] [Google Scholar]
- [13].Vasavada AR, Praveen MR, Posterior Capsule Opacification After Phacoemulsification: Annual Review., Asia-Pac. J. Ophthalmol. Phila. Pa 3 (2014) 235–240. doi: 10.1097/APO.0000000000000080. [DOI] [PubMed] [Google Scholar]
- [14].Marcantonio JM, Vrensen GF, Cell biology of posterior capsular opacification., Eye Lond. Engl 13 ( Pt 3b) (1999) 484–488. doi: 10.1038/eye.1999.126. [DOI] [PubMed] [Google Scholar]
- [15].Mencucci R, Favuzza E, Boccalini C, Gicquel J-J, Raimondi L, Square-edge intraocular lenses and epithelial lens cell proliferation: implications on posterior capsule opacification in an in vitro model, BMC Ophthalmol. 15 (2015) 5. doi: 10.1186/1471-2415-15-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Opthalmology Times, Square-edge design of lens effective against PCO, (2015). http://www.ophthalmologytimes.com/ophthalmology/square-edge-design-lens-effective-against-pco.
- [17].Kent Christopher, IOLs: How Much Does Material Matter?, Rev. Opthalmology (2008). https://www.reviewofophthalmology.com/article/iols-how-much-does-material-matter.
- [18].Bellucci R, An Introduction to Intraocular Lenses: Material, Optics, Haptics, Design and Aberration, in: ESASO Course Ser., 2013: pp. 38–55. doi: 10.1159/000350902. [DOI] [Google Scholar]
- [19].Zhao Y, Yang K, Li J, Huang Y, Zhu S, Comparison of hydrophobic and hydrophilic intraocular lens in preventing posterior capsule opacification after cataract surgery: An updated meta-analysis, Medicine (Baltimore). 96 (2017) e8301–e8301. doi: 10.1097/MD.0000000000008301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Li Y, Wang J, Chen Z, Tang X, Effect of hydrophobic acrylic versus hydrophilic acrylic intraocular lens on posterior capsule opacification: meta-analysis., PloS One. 8 (2013) e77864. doi: 10.1371/journal.pone.0077864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lindstrom Richard L., PCO treatment would disrupt cataract surgery, Ocul. Surg. News US Ed (2017). https://www.healio.com/ophthalmology/cataract-surgery/news/print/ocular-surgery-news/%7B3ab570b1-a3fb-4eac-85c6-510c02f86c90%7D/pco-treatment-would-disrupt-cataractsurgery.
- [22].Nishi Y, Mireskandari K, Khaw P, Findl O, Lens refilling to restore accommodation., J. Cataract Refract. Surg 35 (2009) 374–382. doi: 10.1016/j.jcrs.2008.10.054. [DOI] [PubMed] [Google Scholar]
- [23].Ong HS, Evans JR, Allan BDS, Accommodative intraocular lens versus standard monofocal intraocular lens implantation in cataract surgery., Cochrane Database Syst. Rev (2014) CD009667. doi: 10.1002/14651858.CD009667.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Li Y, Yan Q, Wolf NS, Long-term caloric restriction delays age-related decline in proliferation capacity of murine lens epithelial cells in vitro and in vivo., Invest. Ophthalmol. Vis. Sci 38 (1997) 100–107. [PubMed] [Google Scholar]
- [25].Dawes LJ, Duncan G, Wormstone IM, Age-related differences in signaling efficiency of human lens cells underpin differential wound healing response rates following cataract surgery., Invest. Ophthalmol. Vis. Sci 54 (2013) 333–342. doi: 10.1167/iovs.12-10425. [DOI] [PubMed] [Google Scholar]
- [26].Cook Cynthia, Canine Cataract Surgery, Cataract Refract. Surg. Today (2018). https://crstoday.com/articles/2008-jul/crst0708_06-php/. [Google Scholar]
- [27].Nasisse MP, Dykstra MJ, Cobo LM, Lens capsule opacification in aphakic and pseudophakic eyes., Graefes Arch. Clin. Exp. Ophthalmol. Albrecht Von Graefes Arch. Klin. Exp. Ophthalmol 233 (1995)63–70. [DOI] [PubMed] [Google Scholar]
- [28].Brookshire HL, English RV, Nadelstein B, Weigt AK, Gift BW, Gilger BC, Efficacy of COX-2 inhibitors in controlling inflammation and capsular opacification after phacoemulsification cataract removal., Vet. Ophthalmol 18 (2015) 175–185. doi: 10.1111/vop.12159. [DOI] [PubMed] [Google Scholar]
- [29].Karahan E, Er D, Kaynak S, An Overview of Nd:YAG Laser Capsulotomy., Med. Hypothesis Discov. Innov. Ophthalmol. J 3 (2014) 45–50. [PMC free article] [PubMed] [Google Scholar]
- [30].Beale AB, Salmon J, Michau TM, Gilger BC, Effect of ophthalmic Nd:YAG laser energy on intraocular lenses after posterior capsulotomy in normal dog eyes., Vet. Ophthalmol 9 (2006) 335–340. doi: 10.1111/j.1463-5224.2006.00473.x. [DOI] [PubMed] [Google Scholar]
- [31].Burq MA, Taqui AM, Frequency of retinal detachment and other complications after neodymium:Yag laser capsulotomy., JPMA J. Pak. Med. Assoc 58 (2008) 550–552. [PubMed] [Google Scholar]
- [32].Wesolosky JD, Tennant M, Rudnisky CJ, Rate of retinal tear and detachment after neodymium:YAG capsulotomy., J. Cataract Refract. Surg 43 (2017) 923–928. doi: 10.1016/j.jcrs.2017.03.046. [DOI] [PubMed] [Google Scholar]
- [33].Kruijt B, van den Berg TJTP, Optical Scattering Measurements of Laser Induced Damage in the Intraocular Lens, PLOS ONE. 7 (2012) e31764. doi: 10.1371/journal.pone.0031764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Trinavarat A, Atchaneeyasakul L, Udompunturak S, Neodymiurm:YAG laser damage threshold of foldable intraocular lenses11Alcon Laboratories (Thailand), Bausch & Lomb (Thailand), Maxim Intercontinental, A View, and Interfocus (Thailand) helped in providing the IOLs used in the study., J. Cataract Refract. Surg 27 (2001) 775–780. doi: 10.1016/S0886-3350(00)00855-5. [DOI] [PubMed] [Google Scholar]
- [35].Sabbagh Leslie PCO: What’s Wrong With Doing a YAG, Rev. Opthalmology (2018). https://www.reviewofophthalmology.com/article/pco-whats-wrong-with-doing-a-yag. [Google Scholar]
- [36].Aaronson A, Grzybowski A, Tuuminen R, The health economic impact of posterior capsule opacification in Finland comparing the two single-piece intraocular lenses: a cost-consequence analysis, Acta Ophthalmol. (Copenh.) 0 (2019). doi: 10.1111/aos.14139. [DOI] [PubMed] [Google Scholar]
- [37].Fan DSP, Yip WWK, Yu CBO, Rao SK, Lam DSC, Updates on the surgical management of paediatric cataract with primary intraocular lens implantation., Ann. Acad. Med. Singapore 35 (2006)564–570. [PubMed] [Google Scholar]
- [38].Gimbel HV, Neuhann T, Continuous curvilinear capsulorhexis., J. Cataract Refract. Surg 17 (1991) 110–111. [DOI] [PubMed] [Google Scholar]
- [39].Tassignon M-J, Gobin L, Mathysen D, Van Looveren J, De Groot V, Clinical outcomes of cataract surgery after bag-in-the-lens intraocular lens implantation following ISO standard 11979-7:2006., J. Cataract Refract. Surg 37 (2011) 2120–2129. doi: 10.1016/j.jcrs.2011.06.025. [DOI] [PubMed] [Google Scholar]
- [40].Mohammadpour M, Erfanian R, Karimi N, Capsulorhexis: Pearls and pitfalls, Saudi J. Ophthalmol 26 (2012) 33–40. doi: 10.1016/j.sjopt.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Gobin L, Ní Dhubhghaill S, Tassignon M-J, Technical Specifications of the Bag-in-the-Lens Implant, in: Tassignon M-J, Ní Dhubhghaill S, Van Os L (Eds.), Innov. Implant. Tech. Bag---Lens Cataract Surg, Springer International Publishing, Cham, 2019: pp. 45–60. doi: 10.1007/978-3-030-03086-5_6. [DOI] [Google Scholar]
- [42].Altenburg A, Ni Dhubhghaill SS, Tassignon M-J, Bean-shaped Ring Segments as a Capsule Enhancement Tool in Complex Bag-in-the-Lens Intraocular Lens Implantation., J. Refract. Surg. Thorofare NJ 1995. 33 (2017) 454–459. doi: 10.3928/1081597X-20170504-08. [DOI] [PubMed] [Google Scholar]
- [43].Perez-Vives C, Biomaterial Influence on Intraocular Lens Performance: An Overview., J. Ophthalmol 2018 (2018) 2687385. doi: 10.1155/2018/2687385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Huang Q, Cheng GP-M, Chiu K, Wang G-Q, Surface Modification of Intraocular Lenses., Chin. Med. J. (Engl.) 129 (2016) 206–214. doi: 10.4103/0366-6999.173496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Kang S, Choi JA, Joo C-K, Comparison of posterior capsular opacification in heparin-surface-modified hydrophilic acrylic and hydrophobic acrylic intraocular lenses., Jpn. J. Ophthalmol 53 (2009) 204–208. doi: 10.1007/sl0384-008-0646-3. [DOI] [PubMed] [Google Scholar]
- [46].Krall EM, Arlt EM, Jell G, Strohmaier C, Moussa S, Dexl AK, Prospective Randomized Intraindividual Comparison of Posterior Capsule Opacification After Implantation of an IOL With and Without Heparin Surface Modification., J. Refract. Surg. Thorofare NJ 1995. 31 (2015) 466–472. doi: 10.3928/1081597X-20150623-05. [DOI] [PubMed] [Google Scholar]
- [47].Spangberg M, Kihlstrom I, Bjorklund H, Bjurstrom S, Lydahl E, Larsson R, Improved biocompatibility of intraocular lenses by heparin surface modification: a 12-month implantation study in monkeys., J. Cataract Refract. Surg 16 (1990) 170–177. [DOI] [PubMed] [Google Scholar]
- [48].Walker TD, Pharmacological attempts to reduce posterior capsule opacification after cataract surgery-a review., Clin. Experiment. Ophthalmol 36 (2008) 883–890. doi: 10.1111/j.1442-9071.2009.01921.x. [DOI] [PubMed] [Google Scholar]
- [49].Abdelwahab MT, Kugelberg M, Kugelberg U, Zetterstrom C, After-cataract evaluation after using balanced salt solution, distilled deionized water, and 5-fluorouracil with a sealed-capsule irrigation device in the eyes of, J. Cataract Refract. Surg 32 (2006) 1955–1960. doi: 10.1016/j.jcrs.2006.07.018. [DOI] [PubMed] [Google Scholar]
- [50].Moreno-Montañés J, Barrio-Barrio J, De-Nova E, Werner L, Lens Epithelial Cell Death Secondary to Acanthamoeba Keratitis: Absence of Capsular Bag Opacification Six Years after Cataract Surgery, Case Rep. Ophthalmol 2 (2011) 354–359. doi: 10.1159/000334785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Spalton DJ, Russell SL, Evans-Gowing R, Eldred JA, Wormstone IM, Effect of total lens epithelial cell destruction on intraocular lens fixation in the human capsular bag, J. Cataract Refract. Surg 40 (2014) 306–312. doi: 10.1016/j.jcrs.2013.06.030. [DOI] [PubMed] [Google Scholar]
- [52].Gwon A, Lens regeneration in mammals: a review., Surv. Ophthalmol 51 (2006) 51–62. doi: 10.1016/j.survophthal.2005.11.005. [DOI] [PubMed] [Google Scholar]
- [53].Saika S, Relationship between posterior capsule opacification and intraocular lens biocompatibility, Prog. Retin. Eye Res 23 (2004) 283–305. doi: 10.1016/j.preteyeres.2004.02.004. [DOI] [PubMed] [Google Scholar]
- [54].David Spalton FRCS, Preventing PCO, (2011). https://www.eyeworld.org/article-preventing-pco. [Google Scholar]
- [55].Servick K, Stem cell approach for cataracts challenged, Science. 356 (2017) 1318. doi: 10.1126/science.356.6345.1318. [DOI] [PubMed] [Google Scholar]
- [56].Mamuya FA, Wang Y, Roop VH, Scheiblin DA, Zajac JC, Duncan MK, The Roles of α(V) Integrins in Lens EMT and Posterior Capsular Opacification, J. Cell. Mol. Med 18 (2014) 656–670. doi: 10.1111/jcmm.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Shu DY, Wojciechowski MC, Lovicu FJ, Bone Morphogenetic Protein-7 Suppresses TGFbeta2-Induced Epithelial-Mesenchymal Transition in the Lens: Implications for Cataract Prevention., Invest. Ophthalmol. Vis. Sci 58 (2017) 781–796. doi: 10.1167/iovs.16-20611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].de longh RU, Wederell E, Lovicu FJ, McAvoy JW, Transforming growth factor-beta-induced epithelial-mesenchymal transition in the lens: a model for cataract formation., Cells Tissues Organs. 179 (2005) 43–55. doi: 10.1159/000084508. [DOI] [PubMed] [Google Scholar]
- [59].Wang Y, Mahesh P, Wang Y, Novo SG, Shihan MH, Hayward-Piatkovskyi B, Duncan MK, Spatiotemporal dynamics of canonical Wnt signaling during embryonic eye development and posterior capsular opacification (PCO)., Exp. Eye Res 175 (2018) 148–158. doi: 10.1016/j.exer.2018.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Shu DY, Wojciechowski M, Lovicu FJ, ERK1/2-mediated EGFR-signaling is required for TGFbeta-induced lens epithelial-mesenchymal transition., Exp. Eye Res 178 (2018) 108–121. doi: 10.1016/j.exer.2018.09.021. [DOI] [PubMed] [Google Scholar]
- [61].de longh RU, Duncan MK, Growth Factor Signaling in Lens Fiber Differentiation, in: Lens Epithelium Posterior Capsul. Opacification, Springer, Springer, Tokyo, 2014. [Google Scholar]
- [62].Wertheimer C, Brandlhuber U, Kook D, Mayer WJ, Laubichler P, Wolf A, Kampik A, Eibl-Lindner K, Erufosine, a phosphoinositide-3-kinase inhibitor, to mitigate posterior capsule opacification in the human capsular bag model., J. Cataract Refract. Surg 41 (2015) 1484–1489. doi: 10.1016/j.jcrs.2015.02.034. [DOI] [PubMed] [Google Scholar]
- [63].Dong N, Tang X, Xu B, miRNA-181a inhibits the proliferation, migration, and epithelial-mesenchymal transition of lens epithelial cells., Invest. Ophthalmol. Vis. Sci 56 (2015) 993–1001. doi: 10.1167/iovs.14-15860. [DOI] [PubMed] [Google Scholar]
- [64].Wertheimer C, Liegl R, Kernt M, Mayer W, Docheva D, Kampik A, Eibl-Lindner KH, EGF receptor inhibitor erlotinib as a potential pharmacological prophylaxis for posterior capsule opacification., Graefes Arch. Clin. Exp. Ophthalmol. Albrecht Von Graefes Arch. Klin. Exp. Ophthalmol 251 (2013) 1529–1540. doi: 10.1007/s00417-013-2257-z. [DOI] [PubMed] [Google Scholar]
- [65].Wertheimer C, Kueres A, Siedlecki J, Braun C, Kassumeh S, Wolf A, Mayer W, Priglinger C, Priglinger S, Eibl-Lindner K, The intraocular lens as a drug delivery device for an epidermal growth factor-Receptor inhibitor for prophylaxis of posterior capsule opacification., Acta Ophthalmol. (Copenh.) 96 (2018) e874–e882. doi: 10.1111/aos.l3759. [DOI] [PubMed] [Google Scholar]
- [66].Wertheimer C, Siedlecki J, Kook D, Mayer WJ, Wolf A, Klingenstein A, Kampik A, Eibl-Lindner K, EGFR inhibitor Gefitinib attenuates posterior capsule opacification in vitro and in the ex vivo human capsular bag model., Graefes Arch. Clin. Exp. Ophthalmol. Albrecht Von Graefes Arch. Klin. Exp. Ophthalmol 253 (2015) 409–417. doi: 10.1007/s00417-014-2875-0. [DOI] [PubMed] [Google Scholar]
- [67].Sureshkumar J, Haripriya A, Muthukkaruppan V, Kaufman PL, Tian B, Cytoskeletal drugs prevent posterior capsular opacification in human lens capsule in vitro., Graefes Arch. Clin. Exp. Ophthalmol. Albrecht Von Graefes Arch. Klin. Exp. Ophthalmol 250 (2012) 507–514. doi: 10.1007/s00417-011-1869-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Coster Martin, Lifting the Veil: Canine Cataracts & Cataract Surgery, The MSPCA-Angell. (2019). https://www.mspca.org/angell_services/lifting-the-veil-canine-cataracts-cataract-surgery/. [Google Scholar]
- [69].Phacolytic Uveitis, in: Clin. Atlas Canine Feline Ophthalmic Dis, John Wiley & Sons, Ltd, 2015: pp. 220–221. doi: 10.1002/978111884080l.ch105. [DOI] [Google Scholar]
- [70].Vasavada AR, Trivedi RH, Nath VC, Visual axis opacification after AcrySof intraocular lens implantation in children., J. Cataract Refract. Surg 30 (2004) 1073–1081. doi: 10.1016/j.jcrs.2003.08.020. [DOI] [PubMed] [Google Scholar]
- [71].Trivedi RH, Wilson MEJ, Bartholomew LR, Lal G, Peterseim MM, Opacification of the visual axis after cataract surgery and single acrylic intraocular lens implantation in the first year of life., J. AAPOS Off. Publ. Am. Assoc. Pediatr. Ophthalmol. Strabismus 8 (2004) 156–164. doi: 10.1016/S1091853103003197. [DOI] [PubMed] [Google Scholar]
- [72].Shrestha UD, Shrestha MK, Visual Axis Opacification in Children Following Paediatric Cataract Surgery., JNMA J. Nepal Med. Assoc 52 (2014) 1024–1030. [PubMed] [Google Scholar]
- [73].Olson RJ, Cataract Surgery From 1918 to the Present and Future-Just Imagine!, Am. J. Ophthalmol 185 (2018) 10–13. doi: 10.1016/j.ajo.2017.08.020. [DOI] [PubMed] [Google Scholar]
- [74].Kohnen T, Treating inflammation after lens surgery, J. Cataract Refract. Surg 41 (2015) 2035. doi: 10.1016/j.jcrs.2015.10.056. [DOI] [PubMed] [Google Scholar]
- [75].McColgin AZ, Heier JS, Control of intraocular inflammation associated with cataract surgery., Curr. Opin. Ophthalmol 11 (2000) 3–6. [DOI] [PubMed] [Google Scholar]
- [76].Colin J, The role of NSAIDs in the management of postoperative ophthalmic inflammation., Drugs. 67 (2007) 1291–1308. doi: 10.2165/00003495-200767090-00004. [DOI] [PubMed] [Google Scholar]
- [77].Grzybowski* Andrzej and Kanclerz Piotr, The Role of Steroids and NSAIDs in Prevention and Treatment of Postsurgical Cystoid Macular Edema, Curr. Pharm. Des 24 (2018) 4896–4902. doi: 10.2174/1381612825666190206104524. [DOI] [PubMed] [Google Scholar]
- [78].Biros DJ, Gelatt KN, Brooks DE, Kubilis PS, Andrew SE, Strubbe DT, Whigham HM, Development of glaucoma after cataract surgery in dogs: 220 cases (1987-1998)., J. Am. Vet. Med. Assoc 216(2000)1780–1786. [DOI] [PubMed] [Google Scholar]
- [79].Vasavada AR, Raj SM, Shah GD, Nanavaty MA, Posterior capsule opacification after lens implantation: incidence, risk factors and management, Expert Rev. Ophthalmol 8 (2013) 141+. http://link.galegroup.com.udel.idm.oclc.org/apps/doc/A323937672/AONE?u=udel_main&sid=AONE&xid=797e3d42 (accessed November 16, 2018). [Google Scholar]
- [80].Auffarth GU, Golescu A, Becker KA, Völcker HE, Quantification of posterior capsule opacification with round and sharp edge intraocular lenses, Ophthalmology. 110 (2003) 772–780. doi: 10.1016/S0161-6420(02)01980-2. [DOI] [PubMed] [Google Scholar]
- [81].Buehl W, Findl O, Effect of intraocular lens design on posterior capsule opacification, J. Cataract Refract. Surg 34 (2008) 1976–1985. doi: 10.1016/j.jcrs.2008.07.029. [DOI] [PubMed] [Google Scholar]
- [82].Olson Randall J., Demystifying Dysphotopsia, (205AD). https://www.reviewofophthalmology.com/article/demystifying-dysphotopsia.
- [83].Masket S, Fram NR, Cho A, Park I, Pham D, Surgical management of negative dysphotopsia., J. Cataract Refract. Surg 44 (2018) 6–16. doi: 10.1016/j.jcrs.2017.10.038. [DOI] [PubMed] [Google Scholar]
- [84].MacRae Scott M. MD, Zheleznyak Len MS, Yoon Geunyoung PHD, https://www.ophthalmologymanagement.com/issues/2013/february-2013/fine-tuning-premium-iols, Fine-Tuning Prem. IOLs Opthalmology Manag; (n.d.). [Google Scholar]
- [85].Nystrom A, Almarzouki N, Magnusson G, Zetterberg M, Phacoemulsification and primary implantation with bag-in-the-lens intraocular lens in children with unilateral and bilateral cataract., Acta Ophthalmol. (Copenh.) 96 (2018) 364–370. doi: 10.1111/aos.l3626. [DOI] [PubMed] [Google Scholar]
- [86].Tassignon M-J, De Veuster I, Godts D, Kosec D, Van den Dooren K, Gobin L, Bag-in-the-lens intraocular lens implantation in the pediatric eye., J. Cataract Refract. Surg 33 (2007) 611–617. doi: 10.1016/j.jcrs.2006.12.016. [DOI] [PubMed] [Google Scholar]
- [87].Tassignon M-JBR, De Groot V, Vrensen GFJM, Bag-in-the-lens implantation of intraocular lenses., J. Cataract Refract. Surg 28 (2002) 1182–1188. [DOI] [PubMed] [Google Scholar]
- [88].De Groot V, Leysen I, Neuhann T, Gobin L, Tassignon M-J, One-year follow-up of bag-in-the-lens intraocular lens implantation in 60 eyes., J. Cataract Refract. Surg 32 (2006) 1632–1637. doi: 10.1016/j.jcrs.2006.05.007. [DOI] [PubMed] [Google Scholar]
- [89].De Groot V, Tassignon M-JBR, Vrensen GFJM, Effect of bag-in-the-lens implantation on posterior capsule opacification in human donor eyes and rabbit eyes., J. Cataract Refract. Surg 31 (2005) 398–405. doi: 10.1016/j.jcrs.2004.04.061. [DOI] [PubMed] [Google Scholar]
- [90].Tassignon M-J, Bartholomeeusen E, Rozema JJ, Jongenelen S, Mathysen DGP, Feasibility of multifocal intra-ocular lens exchange and conversion to the bag-in-the-lens implantation., Acta Ophthalmol. (Copenh.) 92 (2014) 265–269. doi: 10.1111/aos.l2093. [DOI] [PubMed] [Google Scholar]
- [91].Time is ripe to rethink posterior optic capture techniques, Ocul. Surg. News US Ed. (2017). https://www.healio.com/ophthalmology/cataract-surgery/news/print/ocular-surgery-news/%7B276b30a0-873d-4b38-a1b0-d7a701657fc2%7D/time-is-ripe-to-rethink-posterior-optic-capture-techniques.
- [92].Findl O, Buehl W, Bauer P, Sycha T, Interventions for preventing posterior capsule opacification., Cochrane Database Syst. Rev (2010) CD003738. doi: 10.1002/14651858.CD003738.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Batur M, Gül A, Seven E, Can E, Yaşar T, Posterior Capsular Opacification in Preschool- and School-Age Patients after Pediatric Cataract Surgery without Posterior Capsulotomy, Turk. J. Ophthalmol 46 (2016) 205–208. doi: 10.4274/tjo.24650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Khaja WA, Verma M, Shoss BL, Yen KG, Visual Axis Opacification in Children, Ophthalmology. 118 (2011) 224–225. doi: 10.1016/j.ophtha.2010.08.030. [DOI] [PubMed] [Google Scholar]
- [95].Petrie I, Lacmanovic Loncar V, Surgical technique and postoperative complications in pediatric cataract surgery: retrospective analysis of 21 cases., Croat. Med. J 45 (2004) 287–291. [PubMed] [Google Scholar]
- [96].Hutcheson KA, Drack AV, Ellish NJ, Lambert SR, Anterior hyaloid face opacification after pediatric Nd:YAG laser capsulotomy., J. AAPOS Off. Publ. Am. Assoc. Pediatr. Ophthalmol. Strabismus 3 (1999) 303–307. [DOI] [PubMed] [Google Scholar]
- [97].Gift BW, English RV, Nadelstein B, Weigt AK, Gilger BC, Comparison of capsular opacification and refractive status after placement of three different intraocular lens implants following phacoemulsification and aspiration of cataracts in dogs., Vet. Ophthalmol 12 (2009) 13–21. doi: 10.1111/j.1463-5224.2009.00667.x. [DOI] [PubMed] [Google Scholar]
- [98].Jiang J, Shihan MH, Wang Y, Duncan MK, Lens Epithelial Cells Initiate an Inflammatory Response Following Cataract Surgery, Invest. Ophthalmol. Vis. Sci 59 (2018) 4986–4997. doi: 10.1167/iovs.18-25067. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.