Abstract
Background
Patients with recurrent glioma after prior radiotherapy have a poor prognosis. Carbon ion beam radiotherapy offers highly conformal dose distributions and more complex biological radiation effects eventually resulting in optimized normal tissue sparing and improved outcome. The aim of this study was to analyze toxicity, local control and overall survival after reirradiation of recurrent high-grade glioma with carbon ion radiotherapy.
Methods
Between 10/2015 and 12/2018, 30 patients (median age: 59 years) with recurrent high-grade glioma were reirradiated with carbon ion beams and retrospectively analyzed. Diagnosis of recurrent glioma was based on magnetic resonance imaging. Thirteen patients had repeated resection prior to reirradiation and 24 patients underwent additional chemotherapy. The median initial radiation dose was 60 Gy and the median time interval between the initial and repeated radiotherapy was 10 months. The reirradiation dose was 45 Gy (relative biological effectiveness) applied in 15 fractions. All patients received regular follow-up imaging after reirradiation. Kaplan-Meier estimation, log rank test and Cox regression analysis were used for statistical assessment.
Results
Applying common toxicity criteria, there were no grade 5 or 4 adverse events, while 8 patients showed grade 3 adverse events. The median follow-up after reirradiation was 11 months and the median overall survival after diagnosis of recurrent high-grade glioma was 13 months. The 6-, 12- and 24-month overall survival rates after diagnosis of recurrent high-grade glioma were 76%, 50% and 19%, respectively. Upon multivariate Cox regression analysis, a Ki67 score of the initial tumor histology of less than 20% was prognostic. Repeated resection or chemotherapy for the recurrent disease did not result in significantly prolonged survival.
Conclusion
Carbon ion reirradiation in recurrent high-grade glioma is safe and feasible. No radiation-associated grade 4 toxicities were documented and treatment was tolerated well.
Keywords: glioma, glioblastoma, reirradiation, carbon ion beam therapy, C12, particle beam therapy, radiotherapy
Introduction
Primary tumors of the central nervous system (CNS) had an estimated standardized incidence of 8/100.000 in men and 5.6/100.000 in women in Germany in 2016. Two thirds were glioblastoma.1 Standard of care includes fractionated radiotherapy up to 60 Gy in 30–33 fractions with concomitant and adjuvant chemotherapy after initial maximal safe resection or biopsy only.2 Despite intensified treatments, progression is common and management for progressive disease includes systemic therapies, surgery and reirradiation.2 Median overall survival (OS) after reirradiation for recurrent glioma lies between 6 and 10.7 months.3–6 Carbon ions have different radiobiological effects that might have the potential to overcome radioresistence.7,8 For example, carbon ions were able to eradicate hypoxic and stem cell-like tumor cells and create an antiangiogenic and less immunosuppressive state.9 Furthermore, due to the specific energy deposition and the Bragg-Peak, carbon ions offer improved normal tissue sparing. Therefore, carbon ion beam radiotherapy might be more effective in eliminating tumor cells while showing less adverse events than photon beam radiotherapy. First clinical results for combined photon and carbon ion treatment have shown encouraging results in a small heterogeneous collective with primary high-grade glioma10,11 and therefore could also be a promising approach for recurrent anaplastic astrocytoma and glioblastoma. Recent developments in prognostic scoring systems for patient selection for reirradiation might help to further optimize the management in recurrent glioma.3,4 It was our aim to retrospectively assess the outcome and toxicity of patients with recurrent high-grade glioma consecutively treated with carbon ion beam reirradiation at the Marburg Ion-Beam Therapy Center.
Materials and Methods
Patients’ Characteristics
Between October 2015 and December 2018 30 patients from Marburg and Gießen University Hospital with recurrent glioma World Health Organization (WHO) grade 3 and 4 after prior multimodal treatment including radiotherapy were reirradiated with carbon ion beams at the Marburg Ion-Beam Therapy Center. Diagnosis of tumor progression was primarily based on magnetic resonance imaging (MRI) including perfusion sequences. Further patients’ characteristics are found in Table 1.
Table 1.
Patients’ Characteristics
| Parameter | N | % |
|---|---|---|
| Gender | ||
| Male | 14 | 47 |
| Female | 16 | 53 |
| Age, years | ||
| Median | 59 | |
| Mean | 56 | |
| Range | 28-76 | |
| WHO grade initially | ||
| 3 | 7 | 23 |
| 4 | 23 | 77 |
| Time interval between initial RT and ReRT, months | ||
| Median | 10 | |
| Range | 3-154 | |
| Temozolomide during initial RT | ||
| Yes | 26 | 87 |
| No | 4 | 13 |
| IDH mutation initially | ||
| Yes | 3 | 10 |
| No | 25 | 83 |
| n.a. | 2 | 7 |
| TP53 mutation initially | ||
| Yes | 13 | 43 |
| No | 8 | 27 |
| n.a. | 9 | 30 |
| EGFR mutation initially | ||
| Yes | 12 | 40 |
| No | 12 | 40 |
| n.a. | 6 | 20 |
| Ki67 initially | ||
| <10 | 5 | 17 |
| 10-20 | 14 | 47 |
| >20 | 5 | 17 |
| n.a. | 6 | 20 |
| MGMT mutation initially | ||
| Yes | 17 | 57 |
| No | 12 | 40 |
| n.a. | 1 | 3 |
| ATRX loss initially | ||
| Yes | 20 | 67 |
| No | 4 | 13 |
| n.a. | 6 | 20 |
| Repeated surgery prior to ReRT | ||
| Biopsy | 4 | 13 |
| Partial resection | 7 | 23 |
| Gross total resection | 6 | 20 |
| No | 13 | 43 |
| Chemotherapy after ReRT | ||
| Yes | 24 | 80 |
| No | 6 | 20 |
| Volume CTV ReRT, ccm | ||
| Median | 52.25 | |
| Range | 6.5-224 | |
| Karnofsky Performance Score at ReRT | ||
| 100% | 0 | 0 |
| 90% | 2 | 7 |
| 80% | 12 | 40 |
| 70% | 11 | 37 |
| 60% and below | 5 | 17 |
Abbreviations: ATRX, alpha-thalassemia/mental retardation syndrome X-linked expression; EGFR, epidermal growth factor receptor; IDH, Isocitrat dehydrogenase; n.a., not available; ReRT, reirradiation; RT, radiotherapy.
Initial Radiation Treatment
The median initial radiotherapy dose was 60 Gy (range: 37.5–61.2 Gy, one of the patients received hypofractionated 37.5 Gy in single doses of 2.5 Gy) and patients were initially treated with low linear energetic transfer (LET) irradiation in addition to surgical and/or chemotherapeutic procedures. Tumor treating fields were not part of the standard treatment.
Repeated Radiation Treatment
For patient immobilization a thermoplastic head-mask was used. Computed tomography (CT, 3‐mm slices) was used for the 3‐dimensional treatment planning. For precise contouring a T1-weighted contrast enhanced MRI was 3‐dimensionally registered to the planning CT. The gross tumor volume (GTV) was defined as the contrast enhancement on a T1 contrast enhanced MRI. The clinical target volume (CTV) was defined as a 3–5 mm expansion to the GTV. The planning target volume (PTV) was defined as a 3 mm margin to the CTV. The median volume of the CTV was 52 ccm (range, 7–224 ccm). Treatment planning was performed with Siemens Syngo.via PT Planning software. The treatment was performed at the Marburg Ion-Beam Therapy Center with C12 carbon ion beams via an active raster scanning method. The prescribed dose was normalized to the median dose of the target volume. Furthermore, the PTV was encompassed within the 95–107% isodose level of the prescribed dose. The dose of reirradiation was 45 Gy (relative biological effectiveness, RBE) in 15 fractions, 3 Gy (RBE) each fraction, 5 fractions each week. The equivalent doses in 2 Gy (EQD2) calculated with an α/β of 2 Gy for organs at risk and 10 Gy for the tumor are according to the linear quadratic model 56.25 Gy (RBE) and 48.8 Gy (RBE), respectively, resulting in an applied total dose of the initial and repeated radiotherapy of median 116.25 Gy (RBE) and 108.8 Gy (RBE), respectively. Daily patient positioning was performed with orthogonal in-room x-ray imaging. For organs at risk a recovery of 25–50% of the applied initial radiotherapy dose depending on the interval between initial radiotherapy and reirradiation was assumed.
Statistical Design and Classifications
The primary endpoint of this retrospective analysis was toxicity; the secondary endpoints were local control (LC) and OS. Time estimates refer to the end of reirradiation, except for OS, which refers to the MRI diagnosis of recurrent disease. MRI included perfusion imaging. LC was defined as the absence of local tumor progression including all cases of stable disease (less than 50% tumor mass reduction), partial remission (tumor mass reduction of at least 50%) and complete remission (requiring no detectable disease). Survival analyses were carried out with I.B.M. SPSS 25 using Kaplan-Meier estimation, log rank test and Cox regression analysis. Adverse events (AE) were classified according to the common toxicity criteria for adverse events version 4 (CTCAE V.4). The first follow-up examination including MRI of the brain was 4–8 weeks after finishing radiotherapy and every 3 months thereafter.
Ethics
The local ethics committee approved the study (Marburg, Germany, study number 166/18). All patients gave written informed consent. This study was conducted in accordance with the Declaration of Helsinki.
Data Sharing Statement
Due to legal aspects of the patients’ informed consent, sharing of data is not possible.
Results
Adverse Events
None of the patients developed CTCAE grade 5 or 4 adverse events, while CTCAE grade 3 adverse events were observed in 8 patients. In terms of CTCAE grade 3 toxicity, two patients developed fatigue not relievable by rest and concentration impairment 8 weeks after reirradiation. There was one case of dysphasia with impaired ability to read 12 weeks after the treatment. The symptoms did respond to corticosteroid treatment. However, tumor progression as underlying reason could not be excluded by means of MRI. In two patients involuntary movements of the left limbs and seizures limiting the activities of daily living were reported. These symptoms developed 4 and 8 weeks after therapy and responded to anticonvulsant treatment in both cases. One patient developed incomplete hemiparesis 3 weeks after reirradiation, requiring an assistive device, there was no response to corticosteroid treatment. The MRI in this patient showed edema without clear signs of tumor progression or necrosis. Nevertheless, the symptoms in this case may be tumor-related, as tumor progression was seen on another MRI 6 weeks later. Two patients developed central nervous system necrosis CTCAE grade 3 after 4 and 9 months, respectively. One of them showed motor deficits of the right arm and the other became symptomatic with intracranial pressure, showing nausea and vomiting. Both responded to corticosteroid treatment. None of the patients received bevacizumab after reirradiation.
Survival and Local Control
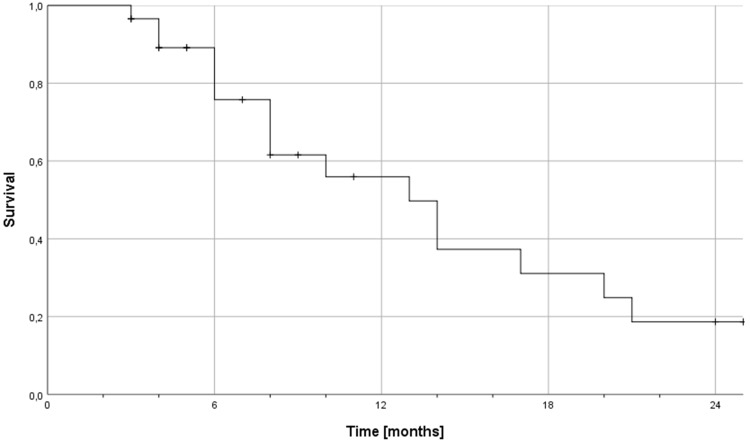
The median time interval between the initial and reirradiation was 10 months. The median follow-up after reirradiation was 11 months (range: 1–23 months) and the median OS after diagnosis of recurrent high-grade glioma 13 months (range: 3–25 months). For glioma WHO grade 3 and 4 the median OS after diagnosis of recurrent disease was 17 months (range: 4–25 months) and 12 months (range: 3–25), respectively. The according 6-, 12- and 24-month OS rates after diagnosis of recurrent disease were 76%, 50% and 19%, respectively (Figure 1). Seven patients showed LC at the last follow-up MRI. The median LC time after finishing reirradiation was 7 months (range: 2–23 months) and the 6- and 12-month LC rates after finishing reirradiation were 45% and 30%, respectively. Pure out-of-field progression was seen in 3 patients.
Figure 1.
Kaplan-Meier estimation of overall survival after diagnosis of recurrent disease in 30 patients with recurrent high-grade glioma reirradiated with carbon ion beams.
Prognostic Factors for OS and LC
In univariate analyses of factors impacting OS a Ki67 score of the initial tumor histology of less than 20% (median not reached versus 8 months, p=0.002) and an age younger than 56 years (median not reached versus 8 months, p=0.016) were positive prognostic factors. Repeated maximal safe resection (median 13 months without versus 14 months with surgery, p=0.115) and chemotherapy after reirradiation (median 6 months without versus 14 months with chemotherapy, p=0.087) did not result in significantly prolonged survival. Furthermore, the presence of WHO grade 3 versus 4 histology did not result in a significantly longer OS (median 12 months versus median 9 months, p=0.578). In multivariate Cox regression analysis only a Ki67 score of the initial tumor histology of less than 20% was prognostic. Repeated maximal safe resection and additional chemotherapy did not result in a significantly prolonged survival. Further parameters are found in Tables 2 and 3.
Table 2.
Univariate Analyses on Overall Survival (Log Rank Test)
| Parameter | p-value |
|---|---|
| Age > or ≤ 56 years | 0.016* |
| Gender | 0.905 |
| Repeated maximal safe resection before ReRT | 0.115 |
| Chemotherapy after ReRT | 0.087 |
| Histopathology WHO grade 3 versus 4 | 0.578 |
| KPS > or ≤ 70 | 0.670 |
| CTV > or ≤ 30 ccm | 0.666 |
| CTV > or ≤ 50 ccm | 0.753 |
| Ki67 ≥ or < 10% | 0.058 |
| Ki67 ≥ or < 20% | 0.002* |
| IDH mutation initially | 0.252 |
| TP53 mutation initially | 0.655 |
| EGFR mutation initially | 0.533 |
| MGMT mutation initially | 0.495 |
| ATRX loss initially | 0.875 |
Note: * p < 0.05.
Abbreviations: ATRX, alpha-thalassemia/mental retardation syndrome X-linked expression; CTV, clinical target volume; EGFR, epidermal growth factor receptor; IDH, Isocitrat dehydrogenase; KPS, Karnofsky Performance Score; MGMT, O-6-methylguanine-DNA methyltransferase; ReRT, reirradiation; TP53, Tumor protein 53; WHO, World Health Organization.
Table 3.
Multivariate Analysis on Overall Survival (Cox Regression Analysis, Stepwise Backwards)
| Parameter | p-value |
|---|---|
| Age > or ≤ 56 years | 0.58 |
| Gender | 0.297 |
| Repeated maximal safe resection before ReRT | 0.446 |
| Chemotherapy after ReRT | 0.92 |
| KPS > or ≤ 70 | 0.423 |
| CTV > or ≤ 50 ccm | 0.371 |
| Ki67 ≥ or < 20% | 0.001* |
| Histopathology WHO grade 3 versus 4 | 0.148 |
Note: *p < 0.05.
Abbreviations: CTV, clinical target volume; KPS, Karnofsky Performance Score; ReRT, reirradiation; WHO, World Health Organization.
In univariate analyses on factors impacting LC a Ki67 score of the initial tumor histology of less than 20% (p=0.014, median 4 months above versus 9 months below) as well as an initial IDH mutation (p=0.044, median 10 months with versus 5 months without) were positive prognostic factors. The presence of WHO grade 3 versus 4 histology did not result in significantly longer local control (median 8 months versus median 5 months, p=0.63). In multivariate Cox regression analysis only a Ki67 score of the initial tumor histology of less than 20% showed significance (p=0.02). Further parameters are found in Tables 4 and 5.
Table 4.
Univariate Analyses on Local Control (Log Rank Test)
| Parameter | p-value |
|---|---|
| Age > or ≤ 56 years | 0.145 |
| Gender | 0.642 |
| Repeated maximal safe resection before ReRT | 0.258 |
| Histopathology WHO grade 3 versus 4 | 0.63 |
| Chemotherapy after ReRT | 0.974 |
| KPS > or ≤ 70 | 0.212 |
| CTV > or ≤ 30 ccm | 0.776 |
| CTV > or ≤ 50 ccm | 0.314 |
| Ki67 ≥ or < 10% | 0.197 |
| Ki67 ≥ or < 20% | 0.014* |
| IDH mutation initially | 0.044* |
| TP53 mutation initially | 0.452 |
| EGFR mutation initially | 0.117 |
| MGMT mutation initially | 0.846 |
| ATRX loss initially | 0.260 |
Note: *p < 0.05.
Abbreviations: ATRX, alpha-thalassemia/mental retardation syndrome X-linked expression; CTV, clinical target volume; EGFR, epidermal growth factor receptor; IDH, Isocitrat dehydrogenase; KPS, Karnofsky Performance Score; MGMT, O-6-methylguanine-DNA methyltransferase; ReRT, reirradiation; TP53, Tumor protein 53; WHO, World Health Organization.
Table 5.
Multivariate Analysis on Local Control (Cox Regression Analysis, Stepwise Backwards)
| Parameter | p-value |
|---|---|
| Age > or ≤ 56 years | 0.309 |
| Gender | 0.863 |
| Repeated maximal safe resection before ReRT | 0.628 |
| Chemotherapy after ReRT | 0.328 |
| KPS > or ≤ 70 | 0.612 |
| CTV > or ≤ 50 ccm | 0.634 |
| Ki67 ≥ or < 20% | 0.02* |
| Histopathology WHO grade 3 versus 4 | 0.684 |
Note: *p < 0.05.
Abbreviations: CTV, clinical target volume; KPS, Karnofsky Performance Score; ReRT, reirradiation; WHO, World Health Organization.
Discussion
We analyzed all patients with recurrent high-grade glioma consecutively treated with carbon ion beams at Marburg Ion-Beam Therapy Center between October 2015 and December 2018 and being referred by Marburg and Gießen University Hospital. It was our aim to retrospectively assess the treatment results in our patients and help finding ways to improve the outcome in this challenging disease. To our knowledge, this is the first report on clinical outcomes after reirradiation with carbon ion beams in recurrent glioma WHO grade 3 and 4.
To our knowledge, the only report on proton beam reirradiation for recurrent glioma is from Galle et al, who reported on 20 patients. The median survival after reirradiation was 10.2 months in grade 3 glioma and 8.2 months in glioblastoma.12 In 2011 Minniti et al reported on 36 patients treated with stereotactic reirradiation and concomitant temozolomide for recurrent glioblastoma. The dose of reirradiation was 37.5 Gy, applied in 15 fractions. The median OS after reirradiation was 9.7 months and the 6- and 12-months survival rates were 84% and 33%, respectively.13 Fogh et al reported on 147 patients being treated with hypofractionated stereotactic radiation therapy (H-SRT) applying median doses of 35 Gy in 3.5 Gy single fractions.14 The authors reported survival times after the initiation of H-SRT of 10 months for grade 3 tumors and 11 months for grade 4 tumors. Krauze et al reported a median OS of 6 months after reirradiation in 31 patients reirradiated for recurrent glioma at the National Cancer Institute between 2008 and 2016 with median 30 Gy.4 Furthermore, Scholtyssek et al reported on reirradiation applying median total doses of 36 Gy in single doses of 2–5 Gy.15 In their cohort the median OS from the start of reirradiation was 7.7 months and the OS rates at 6- and 12-months were 60% and 24%, respectively. In comparison, our results with a median OS of 13 months after diagnosis of recurrent high-grade glioma and 6-, 12- and 24-months OS rates of 76%, 50% and 19%, respectively, are superior. However, one should take the time interval of eventually 2–4 weeks between diagnosis of recurrent high-grade glioma and start of reirradiation as well as the higher reirradiation dose applied in our cohort into account.
In the report by Galle et al 10% of the patients developed unspecified radiation necrosis.12 Minniti et al reported mainly moderate to severe fatigue (41%) and neurological deterioration due to radiation necrosis in 8% (the latter was treated with dexamethasone) in their cohort.13 In the study by Scholtyssek et al MRI follow-up examinations were not performed on a routine base and no grade 3 or 4 AE reported.15 In addition, Krauze et al did not observe any grade 3–5 AE during follow-up and reported mainly fatigue and alopecia as AE.4 Fogh et al reported that none of their patients required hospitalization or surgery due to toxicity, while 1 patient developed grade 3 headaches.14 However, even though treatment was generally well tolerated in our cohort and none of the patients was diagnosed with CTC grade 5 or 4 AE, 8 patients (27%) developed CTC grade 3 AE, requiring medical intervention. This higher rate of CTC grade 3 AE might be due to the higher dose applied in our cohort as well as a RBE variation within the prescription volume.
In the report by Combs et al on reirradiation in recurrent glioma, the extent of resection and age at primary diagnosis were – among others - significant factors for OS.3 In addition, Minniti et al reported initial MGMT methylation status to be the only prognostic factor for OS using multivariate analysis.13 However, in the study by Scholtyssek et al on reirradiation in 64 patients with recurrent high-grade glioma, multivariate analysis on OS revealed female gender, age < 50 years, WHO grade 3 histology, KPS ≥70% and complete resection prior to reirradiation to be positive prognostic factors.15 Concurrent chemotherapy, the time interval between initial and reirradiation and the PTV volume were not prognostic. The data reported by Fogh et al showed younger age at diagnosis, smaller GTV and an increasing number of lesions to be prognostic for OS in multivariate analysis.14 In contrast our data revealed only initial Ki67 score < 20% to be a positive prognostic factor for OS. In addition, our cohort was relatively homogenous in terms of KPS and only a small number of patients underwent repeated gross total resection, which might explain the lack of statistical difference. These differences between the mentioned reports might be due to generally relative small patient numbers or the different reirradiation techniques or doses. In addition, the limited patient number in our cohort might affect the multivariate analysis performed.
Finally, the limitations of our analysis were the retrospective character and the limited patient number. Furthermore, patients had different radiation doses during the initial radiotherapy, some underwent surgery and had different approaches to systemic therapy during their treatments for initial and recurrent disease. However, this study is the first analysis reporting carbon ion beam reirradiation in recurrent high-grade glioma and that in addition has a reasonable number of patients treated with a homogenous reirradiation approach.
Conclusions
Carbon ion beam reirradiation in recurrent high-grade glioma is safe and feasible. No radiation-associated grade 5 or 4 adverse events were documented and the treatment was generally tolerated well. However, 8 patients developed grade 3 adverse events, which were treated with medical interventions. Further investigations including prospective trials on carbon ion beam reirradiation in recurrent glioma are warranted.
Acknowledgment
We thank Melanie Maron for data management.
Funding Statement
This research did not receive external funding.
Abbreviations
AE, Adverse events; CNS, central nervous system; CT, Computed tomography; CTC, common toxicity criteria; CTV, clinical target volume; EQD2, equivalent dose in 2 Gy; GTV, gross tumor volume; H-SRT, hypofractionated stereotactic radiation therapy; IDH, Isocitrat dehydrogenase; LC, local control; LET, linear energetic transfer; MRI, magnetic resonance imaging; OS, overall survival; PTV, planning target volume; RBE, Relative biological effectiveness; WHO, World Health Organization.
Author Contributions
HH, FE, SL, REC and JD developed and planned the study. HH, FE and SL performed data management. HH, FE, and SL performed data analysis. HH, FE, SL, AJD, MS and BC performed data interpretation. HH, FE, and SL drafted the manuscript. JD, REC, ADJ, MS, ands BC critically revised the manuscript for important intellectual content. All authors read and approved the final manuscript and agreed to be accountable for all aspects of the work.
Disclosure
Barbara Carl reports personal fees from Brainlab and B. Braun, outside the submitted work. Jürgen Debus reports grants from Viewray Inc, CRI The Clinical Research Institute GmbH, Accuracy International Sarl, RaySearch Laboratories AB, Vision RT Limited, Merck Serono GmbH, Astellas Pharma GmbH, Astra Zeneca GmbH, Siemens Healthcare GmbH, Solution Akademie GmbH, Ergomed PLC Surrey Research Park, Quintiles GmbH, Pharmaceutical Research Associates GmbH, Boehringer Ingelheim Pharma GmbH&CoKG, PTW Freiburg Dr. Pychlau GmbH, and Nanobiotix S.A., outside the submitted work. All other authors declare no conflicts of interest.
References
- 1.Robert-Koch-Institut. Krebs in Deutschland 2011/2012. Gesundheitsberichterstattung des Bundes. 10;2015. doi: 10.17886/rkipubl-2015-004 [DOI] [Google Scholar]
- 2.Stupp R, Brada M, van den Bent MJ, Tonn J-C, Pentheroudakis G, ESMO Guidelines Working Group. High-grade glioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol off J Eur Soc Med Oncol. 2014;25(Suppl 3):iii93–iii101. doi: 10.1093/annonc/mdu050 [DOI] [PubMed] [Google Scholar]
- 3.Combs SE, Niyazi M, Adeberg S, et al. Re-irradiation of recurrent gliomas: pooled analysis and validation of an established prognostic score-report of the Radiation Oncology Group (ROG) of the German Cancer Consortium (DKTK). Cancer Med. 2018;7(5):1742–1749. doi: 10.1002/cam4.1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krauze AV, Peters C, Cheng J, et al. Re-irradiation for recurrent glioma- the NCI experience in tumor control, OAR toxicity and proposal of a novel prognostic scoring system. Radiat Oncol Lond Engl. 2017;12(1):191. doi: 10.1186/s13014-017-0930-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arvold ND, Shi DD, Aizer AA, et al. Salvage re-irradiation for recurrent high-grade glioma and comparison to bevacizumab alone. J Neurooncol. 2017;135(3):581–591. doi: 10.1007/s11060-017-2611-9 [DOI] [PubMed] [Google Scholar]
- 6.Fokas E, Wacker U, Gross MW, Henzel M, Encheva E, Engenhart-Cabillic R. Hypofractionated stereotactic reirradiation of recurrent glioblastomas: a beneficial treatment option after high-dose radiotherapy? Strahlenther Onkol Organ Dtsch Rontgengesellschaft Al. 2009;185(4):235–240. doi: 10.1007/s00066-009-1753-x [DOI] [PubMed] [Google Scholar]
- 7.Schlaff CD, Krauze A, Belard A, O’Connell JJ, Camphausen KA. Bringing the heavy: carbon ion therapy in the radiobiological and clinical context. Radiat Oncol Lond Engl. 2014;9(1):88. doi: 10.1186/1748-717X-9-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karger CP, Peschke P. RBE and related modeling in carbon-ion therapy. Phys Med Biol. 2017;63(1):01TR02. doi: 10.1088/1361-6560/aa9102 [DOI] [PubMed] [Google Scholar]
- 9.Chiblak S, Tang Z, Lemke D, et al. Carbon irradiation overcomes glioma radioresistance by eradicating stem cells and forming an antiangiogenic and immunopermissive niche. JCI Insight. 2019;4(2). doi: 10.1172/jci.insight.123837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mizoe J-E, Tsujii H, Hasegawa A, et al. Phase I/II clinical trial of carbon ion radiotherapy for malignant gliomas: combined X-ray radiotherapy, chemotherapy, and carbon ion radiotherapy. Int J Radiat Oncol Biol Phys. 2007;69(2):390–396. doi: 10.1016/j.ijrobp.2007.03.003 [DOI] [PubMed] [Google Scholar]
- 11.Combs SE, Bruckner T, Mizoe J-E, et al. Comparison of carbon ion radiotherapy to photon radiation alone or in combination with temozolomide in patients with high-grade gliomas: explorative hypothesis-generating retrospective analysis. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2013;108(1):132–135. doi: 10.1016/j.radonc.2013.06.026 [DOI] [PubMed] [Google Scholar]
- 12.Galle JO, McDonald MW, Simoneaux V, Buchsbaum JC. Reirradiation with proton therapy for recurrent gliomas. Int J Part Ther. 2015;2(1):11–18. doi: 10.14338/THEIJPT-14-00029.1 [DOI] [Google Scholar]
- 13.Minniti G, Armosini V, Salvati M, et al. Fractionated stereotactic reirradiation and concurrent temozolomide in patients with recurrent glioblastoma. J Neurooncol. 2011;103(3):683–691. doi: 10.1007/s11060-010-0446-8 [DOI] [PubMed] [Google Scholar]
- 14.Fogh SE, Andrews DW, Glass J, et al. Hypofractionated stereotactic radiation therapy: an effective therapy for recurrent high-grade gliomas. J Clin Oncol off J Am Soc Clin Oncol. 2010;28(18):3048–3053. doi: 10.1200/JCO.2009.25.6941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scholtyssek F, Zwiener I, Schlamann A, et al. Reirradiation in progressive high-grade gliomas: outcome, role of concurrent chemotherapy, prognostic factors and validation of a new prognostic score with an independent patient cohort. Radiat Oncol Lond Engl. 2013;8:161. doi: 10.1186/1748-717X-8-161 [DOI] [PMC free article] [PubMed] [Google Scholar]