Inflammasomes are large multiprotein complexes composed of signal sensing platform proteins (e.g., NOD-, LRR- and pyrin domain-containing 3 [NLRP3], absent in melanoma 2 [AIM2] and NLR Family CARD Domain Containing 4 [NLRC4]), adaptor protein apoptosis-associated speck-like protein containing a CARD (ASC) and effector protein caspase-1. These complexes serve as signaling platforms that activate caspase-1, which in turn cleaves and processes inflammatory cytokines such as IL-1β and IL-18 and triggers the inflammatory response. Active caspase-1 also cleaves the pore-forming protein gasdermin D and generates the amino-terminal fragment, which oligomerizes and forms membrane pores in the plasma membrane, resulting in potassium efflux and pyroptosis. The subsequent potassium efflux leads to further NLRP3 activation.1 By inducing the robust inflammatory response and pyroptotic cell death, the inflammasome cascade contributes to tissue inflammation and destruction in many cardiovascular diseases including atherosclerosis. Thus, better understanding of the regulation of inflammasome and identification of the molecules responsible for inflammasome activation are critical for developing effective therapies to treat diseases in which activation of inflammasome is critically involved.
Inflammasome is activated by an array of intracellular and extracellular stresses including potassium efflux, metabolic stress, reactive oxygen species (ROS), ER stress and DNA damage. These stress conditions and factors, through various pathways, activate inflammasome by inducing the transcription or the posttranslational modifications (e.g., phosphorylation and ubiquitylation) of the key components in the inflammasome complex. Increasing evidence suggests that phosphorylation of the inflammasome components2–7 represents an important mechanism for inflammasome activation. The inflammasome activating signals induce a series of site-specific phosphorylation in different domains of inflammasome proteins (e.g., NLRP3, ASC) and alter their conformation, allowing their interaction, leading to inflammasome assembly and activation.
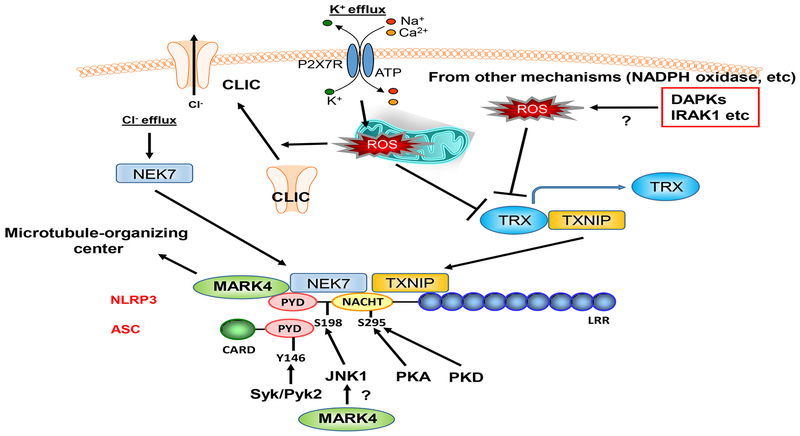
In this issue of ATVB, Li et al. identified microtubule-affinity regulating kinase 4 (MARK4) as a crucial upstream regulator for inflammasome activation in atherosclerosis. The authors show that in human atherosclerotic lesions, MARK4 expression was increased and that it co-localized with NLRP3 in macropahges. The expression of MARK4 and NLRP3 in the atherosclerotic lesions was associated with the production of active IL-1β and IL-18 within the plaque. In Ldlr-deficient mice that were fed a high-fat and cholesterol diet, Mark4 deficiency in bone marrow cells led to a reduction in lesion size and the levels of circulating IL-18. The authors further show that Mark4 deficiency in macrophages inhibited cholesterol crystal-induced NLRP3 inflammasome activation. Thus, these findings support a critical role of MARK4 in inflammasome activation and atherosclerotic plaque development. Although Li et al. have nicely shown the key role of MARK4 in regulating NLRP3 activation and the co-localization of MARK4 and NLRP3, the exact molecular mechanism how MARK4 regulates NLRP3 during the process of atherosclerosis remains unclear. MARK4 has been shown to induce c-Jun N-terminal Kinase (JNK) activation.8 JNK induces NLRP3 phosphorylation at S198 (S194 in mouse Nlrp3), which is critical for NLRP3 deubiquitination and the subsequent inflammasome assembly.7 Nlrp3 activation is disrupted in Nlrp3-S194A knock-in mice,7 also supporting the key role of this phosphorylation in vivo. Therefore, in addition to the microtubule-dependent mechanism,9 it is an attractive hypothesis that MARP4-mediated JNK activation is also involved in NLRP3 activation.
A number of kinases have been shown to regulate NLRP3 activation. One of the most established phosphorylation sites in NLRP3 is S295 located in the NACHT domain (Figure).10 Both protein kinase A (PKA) and protein kinase D (PKD) can phosphorylate this site, but with opposite consequences. While PKA-mediated S295 phosphorylation is inhibitory11, PKD-mediated S295 phosphorylation is necessary for NLRP3 activation6. The details of this discrepancy were discussed by Sandall et al.10 NLRP3 ubiquitination, which can be regulated by S295 phosphorylation, may play a role in PKA- and PKD-mediated regulation of NLRP3 activation.10
Figure. ROS, kinases, and inflammasome activation.
A number of kinsess can directly phosphorylate NLRP3 and ASC and activate inflammasome activation. Furthermore, ROS can activate NLRP3 activation via promoting TXNIP and NEK7 interaction with NLRP3. It is pssible that the ROS production induced by DAPKs and IRAK1 may contribute to NLRP3 activation initiated by these kinases activation. Lastly, MARK4 can bind to NLRP3 and activate it by driveing NLRP3 to the microtubule-organizing center. Please see the details in the text. TXNIP: thioredoxin-interacting protein (TXNIP), TRX: thioreoxin, NEK7: NIMA related kinase 7, CLIC: chloride intracellular channels, DAPK: Death associated proteik kinase, IRAK1: Interleukin 1 receptor associated kinase 1, PKA: Protein kinase A, PKD: Protein kinase D, Syk: Spleen associated tyrosine kinase, Pyk2: Proline-rich tyrosine kinase 2, PYD: Pyrin domain, CARD: Caspase activation and recruitment domain, LRR: Leucine-rich repeat, P2X7R: Purinergic Receptor P2X7.
In addition to the serine/threonine kinases, tyrosine kinases also contribute to the regulation of NLRP3 activation10 (Figure). Spleen tyrosine kinase (SYK), a nonreceptor protein tyrosine kinase (TK), plays a crucial role in signaling by specific immune receptors such as Toll-like receptors, C-type lectin receptors (CLRs), and NOD-like receptors (NLRs).12, 13 SYK can phosphorylate ASC, a component of the inflammasome complex, at Y146, which is critical for ASC oligomerization and caspase 1 activation.2–5 Proline-rich TK 2 (PYK2), another non-receptor protein TK with a role in integrin-induced cell migration, can directly phosphorylate ASC at Y146 and activate NLRP3.14 Briton’s tyrosine kinase (BTK) is another non-receptor TK, which also plays a crucial role in TLR signaling15, and induces ASC oligomerization and caspase-1 activation in a kinase activity-dependent manner, but the phosphorylation sites of NLRP3 and ASC remain unclear16, 17.
It is noteworthy to discuss that kinases can also regulate NLRP3 activation through ROS production. It has been reported that ROS induces inflammasome activation by regulating the interaction between thioredoxin (TRX)and TRX interacting protein (TXNIP) (Figure).18 TRX and TXNIP bind and mutually inhibit. However, after ROS stimulation, TRX releases TXNIP, and free TXNIP associates and activate NLRP3.19 ROS can also activate NLRP3 by regulating the interaction between NIMA related kinase 7 (NEK7) and NLRP3. NLRP3 agonists increase potassium efflux, and subsequently increase mitochondrial ROS (mtROS) production. mtROS induces chloride intracellular channels (CLIC) to translocate to the plasma membrane and subsequent chloride efflux, which promotes NEK7-NLRP3 association and NLRP3 activation.20 Interestingly, this is induced by a kinase activation independent mechanism. These data suggest that ROS takes in a key role for NLRP3 activation by multiple mechanisms.
The ROS-mediated NLRP3 activation reminds us of the fact that the molecular mechanisms underlying inflammasome regulation by several kinases remain unclear. The phosphorylation sites on NLRP3 induced by these kinases have not been identified. It is plausible that these kinases are somehow involved in activation of NLRP3 via increasing ROS (Figure). For example, Death-Associated Protein Kinase (DAPK) can associate with NLRP3 and induce IL-1β production.21 DAPK3 can also mediate TNFα-induced JNK, p38, and AKT activation via ROS production.22 Therefore, it is possible that DAPK activates NLRP3 via ROS or ROS-mediated JNK activation. Another example is interleukin-1 receptor-associated kinase 1 (IRAK1). Lin et al have reported that IRAK-1 associates with NLRP3 and increases NLRP3 activation and pyroptosis.23 Although they suggested that this is direct regulation by binding, it is well known that IRAK-1 can induce ROS,24, 25 and one cannot exclude the possible indirect involvement of ROS in IRAK-1-mediated NLRP3 activation. To clarify the binding site and to specifically inhibit the complex formation with NLRP3 in the absence of ROS production would be necessary to determine whether the direct or indirect effects of ROS production is critical for NLRP3 activation.
The roles of kinases in the dynamic regulation of NLRP3 activation by various stimuli and upstream regulators remain to be understood. Especially, the role of ROS in NLRP3 activation induced by the upstream so-called direct kinases remains unclear. This study has opened a door to future investigations on these aspects, and this will help us focus on the enigma of ROS-mediated kinase signaling and its functional consequences in the biology of inflammation.
Acknowledgement
We appreciate with Dr. Keigi Fujiwara for critical reading and comments.
Sources of Funding
Our research activities are supported by grants from the National Institute of Health (NIH) to YH. Shen (HL-HL131980 and HL143359) and J.-i. Abe (HL-130193, HL-123346, and HL-118462).
References
- 1.Liu X, Zhang Z, Ruan J, Pan Y, Magupalli VG, Wu H and Lieberman J. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535:153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gross O, Poeck H, Bscheider M, Dostert C, Hannesschlager N, Endres S, Hartmann G, Tardivel A, Schweighoffer E, Tybulewicz V, Mocsai A, Tschopp J and Ruland J. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature. 2009;459:433–436. [DOI] [PubMed] [Google Scholar]
- 3.Hara H, Tsuchiya K, Kawamura I, Fang R, Hernandez-Cuellar E, Shen Y, Mizuguchi J, Schweighoffer E, Tybulewicz V and Mitsuyama M. Phosphorylation of the adaptor ASC acts as a molecular switch that controls the formation of speck-like aggregates and inflammasome activity. Nat Immunol. 2013;14:1247–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yasukawa S, Miyazaki Y, Yoshii C, Nakaya M, Ozaki N, Toda S, Kuroda E, Ishibashi K, Yasuda T, Natsuaki Y, Mi-ichi F, Iizasa E, Nakahara T, Yamazaki M, Kabashima K, Iwakura Y, Takai T, Saito T, Kurosaki T, Malissen B, Ohno N, Furue M, Yoshida H and Hara H. An ITAM-Syk-CARD9 signalling axis triggers contact hypersensitivity by stimulating IL-1 production in dendritic cells. Nat Commun. 2014;5:3755. [DOI] [PubMed] [Google Scholar]
- 5.Lin YC, Huang DY, Wang JS, Lin YL, Hsieh SL, Huang KC and Lin WW. Syk is involved in NLRP3 inflammasome-mediated caspase-1 activation through adaptor ASC phosphorylation and enhanced oligomerization. J Leukoc Biol. 2015;97:825–835. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Z, Meszaros G, He WT, Xu Y, de Fatima Magliarelli H, Mailly L, Mihlan M, Liu Y, Puig Gamez M, Goginashvili A, Pasquier A, Bielska O, Neven B, Quartier P, Aebersold R, Baumert TF, Georgel P, Han J and Ricci R. Protein kinase D at the Golgi controls NLRP3 inflammasome activation. J Exp Med. 2017;214:2671–2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song N, Liu ZS, Xue W, Bai ZF, Wang QY, Dai J, Liu X, Huang YJ, Cai H, Zhan XY, Han QY, Wang H, Chen Y, Li HY, Li AL, Zhang XM, Zhou T and Li T. NLRP3 Phosphorylation Is an Essential Priming Event for Inflammasome Activation. Mol Cell. 2017;68:185–197 e6. [DOI] [PubMed] [Google Scholar]
- 8.Feng M, Tian L, Gan L, Liu Z and Sun C. Mark4 promotes adipogenesis and triggers apoptosis in 3T3-L1 adipocytes by activating JNK1 and inhibiting p38MAPK pathways. Biol Cell. 2014;106:294–307. [DOI] [PubMed] [Google Scholar]
- 9.Li X, Thome S, Ma X, Amrute-Nayak M, Finigan A, Kitt L, Masters L, James JR, Shi Y, Meng G and Mallat Z. MARK4 regulates NLRP3 positioning and inflammasome activation through a microtubule-dependent mechanism. Nat Commun. 2017;8:15986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sandall CF and MacDonald JA. Effects of phosphorylation on the NLRP3 inflammasome. Arch Biochem Biophys. 2019. [DOI] [PubMed] [Google Scholar]
- 11.Mortimer L, Moreau F, MacDonald JA and Chadee K. NLRP3 inflammasome inhibition is disrupted in a group of auto-inflammatory disease CAPS mutations. Nat Immunol. 2016;17:1176–1186. [DOI] [PubMed] [Google Scholar]
- 12.Lin YC, Huang DY, Chu CL and Lin WW. Anti-inflammatory actions of Syk inhibitors in macrophages involve non-specific inhibition of toll-like receptors-mediated JNK signaling pathway. Mol Immunol. 2010;47:1569–1578. [DOI] [PubMed] [Google Scholar]
- 13.Poeck H and Ruland J. SYK kinase signaling and the NLRP3 inflammasome in antifungal immunity. J Mol Med (Berl). 2010;88:745–752. [DOI] [PubMed] [Google Scholar]
- 14.Chung IC, OuYang CN, Yuan SN, Li HP, Chen JT, Shieh HR, Chen YJ, Ojcius DM, Chu CL, Yu JS, Chang YS and Chen LC. Pyk2 activates the NLRP3 inflammasome by directly phosphorylating ASC and contributes to inflammasome-dependent peritonitis. Sci Rep. 2016;6:36214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee KG, Xu S, Kang ZH, Huo J, Huang M, Liu D, Takeuchi O, Akira S and Lam KP. Bruton’s tyrosine kinase phosphorylates Toll-like receptor 3 to initiate antiviral response. Proc Natl Acad Sci U S A. 2012;109:5791–5796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murthy P, Durco F, Miller-Ocuin JL, Takedai T, Shankar S, Liang X, Liu X, Cui X, Sachdev U, Rath D, Lotze MT, Zeh HJ, 3rd, Gawaz M, Weber AN and Vogel S. The NLRP3 inflammasome and bruton’s tyrosine kinase in platelets co-regulate platelet activation, aggregation, and in vitro thrombus formation. Biochem Biophys Res Commun. 2017;483:230–236. [DOI] [PubMed] [Google Scholar]
- 17.Ito M, Shichita T, Okada M, Komine R, Noguchi Y, Yoshimura A and Morita R. Bruton’s tyrosine kinase is essential for NLRP3 inflammasome activation and contributes to ischaemic brain injury. Nat Commun. 2015;6:7360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou R, Tardivel A, Thorens B, Choi I and Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11:136–140. [DOI] [PubMed] [Google Scholar]
- 19.Abais JM, Xia M, Zhang Y, Boini KM and Li PL. Redox regulation of NLRP3 inflammasomes: ROS as trigger or effector? Antioxid Redox Signal. 2015;22:1111–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang T, Lang X, Xu C, Wang X, Gong T, Yang Y, Cui J, Bai L, Wang J, Jiang W and Zhou R. CLICs-dependent chloride efflux is an essential and proximal upstream event for NLRP3 inflammasome activation. Nat Commun. 2017;8:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chuang YT, Lin YC, Lin KH, Chou TF, Kuo WC, Yang KT, Wu PR, Chen RH, Kimchi A and Lai MZ. Tumor suppressor death-associated protein kinase is required for full IL-1beta production. Blood. 2011;117:960–970. [DOI] [PubMed] [Google Scholar]
- 22.Usui T, Okada M, Hara Y and Yamawaki H. Death-associated protein kinase 3 mediates vascular inflammation and development of hypertension in spontaneously hypertensive rats. Hypertension. 2012;60:1031–1039. [DOI] [PubMed] [Google Scholar]
- 23.Lin KM, Hu W, Troutman TD, Jennings M, Brewer T, Li X, Nanda S, Cohen P, Thomas JA and Pasare C. IRAK-1 bypasses priming and directly links TLRs to rapid NLRP3 inflammasome activation. Proc Natl Acad Sci U S A. 2014;111:775–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maitra U, Singh N, Gan L, Ringwood L and Li L. IRAK-1 contributes to lipopolysaccharide-induced reactive oxygen species generation in macrophages by inducing NOX-1 transcription and Rac1 activation and suppressing the expression of antioxidative enzymes. J Biol Chem. 2009;284:35403–5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holterman CE, Boisvert NC, Thibodeau JF, Kamto E, Novakovic M, Abd-Elrahman KS, Ferguson SSG and Kennedy CRJ. Podocyte NADPH Oxidase 5 Promotes Renal Inflammation Regulated by the Toll-Like Receptor Pathway. Antioxid Redox Signal. 2019;30:1817–1830. [DOI] [PubMed] [Google Scholar]