Abstract
OBJECTIVES
The purpose of this study was to evaluate the sensitivity of multiparametric cardiac magnetic resonance imaging (CMR) for the detection of acute cardiac allograft rejection (ACAR).
BACKGROUND
ACAR is currently diagnosed by endomyocardial biopsy, but CMR may be a noninvasive alternative because of its capacity for regional myocardial structure and function characterization.
METHODS
Fifty-eight transplant recipients (mean age 47.0 ± 14.7 years) and 14 control subjects (mean age 47.7 ± 16.7 years) were prospectively recruited from August 2014 to May 2017 and underwent 97 CMR studies (83 transplant recipients, 14 control subjects) for assessment of global left ventricular function and myocardial T2, T1, and extracellular volume fraction (ECV). CMR studies were divided into 4 groups on the basis of biopsy grade: control subjects (n = 14), patients with no ACAR (no history of ACAR; n = 36), patients with past ACAR (history of ACAR; n = 24), and ACAR+ patients (active grade ≥1R ACAR; n = 23).
RESULTS
Myocardial T2 was significantly higher in patients with past ACAR compared with those with no ACAR (51.0 ± 3.8 ms vs. 49.2 ± 4.0 ms; p = 0.02) and in patients with no ACAR compared with control subjects (49.2 ± 4.0 ms vs. 45.2 ± 2.3 ms; p < 0.01). ACAR+ patients demonstrated increased T2 compared with the no ACAR group (52.4 ± 4.7 ms vs. 49.2 ± 4.0 ms, p < 0.01) but not compared with the past ACAR group. In contrast, ECV was significantly elevated in ACAR+ patients compared with transplant recipients without ACAR regardless of history of ACAR (no ACAR: 31.5 ± 3.9% vs. 26.8 ± 3.3% [p < 0.01]; past ACAR: 31.5 ± 3.9% vs. 26.8 ± 4.0% [p < 0.01]). Receiver operating characteristic curve analysis revealed that a combined model of age at CMR, global T2, and global ECV was predictive of ACAR (area under the curve = 0.84).
CONCLUSIONS
The combination of CMR-derived myocardial T2 and ECV has potential as a noninvasive tissue biomarker for ACAR. Larger studies during acute ACAR are needed for continued development of multiparametric CMR for transplant recipient surveillance.
Keywords: cardiac MRI, CMR, ECV, extracellular volume fraction, heart transplantation, rejection, T1 mapping, T2 mapping
Acute cardiac allograft rejection (ACAR) is a cell-mediated and/or antibody-mediated response against a transplanted heart and is among the leading causes of morbidity and mortality following transplantation, requiring treatment in 44% of recipients within the first 5 years (1). Although advances in immunosuppression have decreased the incidence of ACAR, it remains a primary concern for 5 to 6 years following transplantation (1-3).
The current gold standard screening tool for ACAR is endomyocardial biopsy (EMB), typically performed 10 to 15 times during the first year post-transplantation (4). EMB grade guides treatment, which is typically reserved for moderate to severe cases (grade ≥2R acute cellular rejection [ACR] or ≥pAMR2 antibody-mediated rejection [AMR]). Despite sensitivity approaching 98% (5), EMB is invasive (potential for complications such as hematoma, pseudoaneurysm, arrhythmia), expensive, and limited because of sampling error (6,7).
Cardiac magnetic resonance imaging (CMR) has shown promise as a noninvasive alternative ACAR screening tool because of its ability to provide information on global and regional cardiac tissue structure (8-16), notably edema (through T2 mapping) and interstitial expansion or fibrosis (pre- and post-gadolinium [Gd] contrast T1 mapping to quantify extracellular volume fraction [ECV]). T2 mapping has shown the most consistent correlation to ACAR (8-12), and there is evidence that T1 and ECV may be increased as well (12-15). Nevertheless, a comprehensive assessment of structural (T2, T1, ECV) changes in transplant recipients and their associations with ACAR has not been performed to date.
The goal of this study was to evaluate the sensitivity of multiparametric CMR for the noninvasive detection of ACAR. We hypothesized that T2, T1, and ECV can detect significant structural differences 1) between healthy control subjects and transplant recipients without history of ACAR; and 2) between transplant recipients with and without evidence of ACAR.
METHODS
STUDY COHORT AND DESIGN.
Fifty-eight cardiac transplant recipients and 14 age- and sex-matched healthy control subjects were prospectively recruited for CMR from August 2014 through June 2017 at a single tertiary care medical center. All transplant recipients receiving transplantation follow-up care were approached, regardless of time since transplantation or center at which transplantation was performed. The 58 transplant recipients underwent up to 4 CMR studies. Exclusion criteria included age <18 years (n = 0), inability to obtain written consent (declined participation, impaired cognition, language barrier; n = 27), and contraindications to CMR (e.g., pregnancy, non-retracted lead; n = 39). In addition, only patients with CMR studies performed within 3 months following EMB were included in this study. Patient EMB reports and cardiology notes were reviewed for evidence of myocarditis, myocardial infarction, infiltrative disease, or any condition known to influence CMR parameters; these patients (n = 2) were excluded. Patients who received treatment for ACAR between EMB and CMR (n = 0) were also excluded. Cardiology notes and prior coronary angiography and intravascular ultrasound reports were also reviewed for diagnosis of cardiac allograft vasculopathy (CAV). The study was approved by the Institutional Review Board, and all participants provided written informed consent.
CMR ACQUISITION.
All CMR studies were performed on 1.5-T magnetic resonance systems (Aera/Avanto, Siemens Healthcare, Erlangen, Germany) and included electrocardiographically gated 2-dimensional cine steady-state free precession (SSFP) imaging, T2 mapping, and pre- and post-Gd contrast T1 mapping. Two-dimensional cine SSFP images of the entire heart were obtained in short-axis (8- to 12-slice stack) and long-axis (2-chamber, 4-chamber, and 3-chamber) orientations. T2 and T1 maps were acquired during breath holding at 3 identical short-axis locations at the base, middle, and apex of the left ventricle. Participants with glomerular filtration rates ≤30 ml/min did not receive Gd contrast.
T2 mapping was based on the successive acquisition of 3 T2-prepared SSFP images with varying T2 preparation times (0, 24, and 55 ms) (17). Further imaging parameters were as follows: echo time, 1.1 to 1.4 ms; repetition time, 2.2 to 2.6 ms; spatial resolution, 1.5 to 2.1 × 2.0 to 2.5 mm; slice thickness, 8 mm; flip angle, 70°.
T1 mapping consisted of single-shot modified Look-Locker inversion recovery images before and 15 min following Gd contrast administration (0.1 mmol/kg Magnevist/Gadavist, Bayer, Leverkusen, Germany) (18). Pre- and post-Gd modified Look-Locker inversion recovery sequences occurred over 11 heartbeats in a 5(3)3 pattern. Imaging parameters were as follows: echo time, 1.0 to 1.3 ms; repetition time, 2.5 to 4.2 ms; spatial resolution, 1.0 to 2.1 × 1.5 to 2.5 mm; slice thickness, 8 mm; flip angle, 35°. Imaging reconstruction included the calculation of parametric left ventricular (LV) T2 and T1 maps.
CMR POST-PROCESSING.
Measures of global LV function were calculated from short- and long-axis cine SSFP images using commercial software (cvi42 version 5.3.6, Circle, Calgary, Alberta, Canada).
For all regional analyses, cardiac segments were divided according to the American Heart Association 16-segment model (19). Segmental T2 and T1 values were calculated from T2 and T1 maps using commercial software (cvi42 version 5.3.6). ECV was calculated during post-processing using patient hematocrit level obtained on the day of CMR to generate segmental ECV maps using the formula: ECV = (ΔR1 myocardium/ΔR1 blood) × (1 – hematocrit), where R1 = 1/T1 and ΔR1 is the change in relaxation rate between pre- and post-Gd contrast images (20). Global (averaged over all 16 segments) and peak (maximum segmental average of all 16 segments) T2, T1, and ECV were calculated.
All post-processing analysis was performed by a single reviewer (R.S.D.). Interobserver reliability was evaluated in a subgroup of patients by a second independent reviewer (A.A.R.), who was blinded to the analyses of the first reviewer.
DEFINITION OF ACAR AND STUDY GROUPS.
EMBs were obtained as part of routine clinical care by right heart catheterization using a standardized approach by multiple cardiologists. Samples were graded according to the revised International Society for Heart & Lung Transplantation cardiac allograft biopsy grading system for ACR (0R, 1R, 2R, and 3R) (6) and AMR (pAMR0, pAMR1[h/i], pAMR2, and pAMR3) (21). CMR studies were grouped into the ACAR+ group if an EMB within 1 week of CMR revealed histological evidence of ACAR (International Society for Heart & Lung Transplantation grade ≥1R ACR or ≥pAMR1 AMR). CMR studies performed on patients without positive biopsy within 1 week (ACAR−) were separated on the basis of history of significant (treated) ACAR into 1 of 2 groups: past ACAR if they had an episode of ≥2R ACR or ≥pAMR2 AMR in the past or no ACAR if they had never had an episode of ≥2R ACR or ≥pAMR2 AMR.
DATA AND STATISTICAL ANALYSIS.
Descriptive statistics and Pearson correlation analysis were performed between demographic data and CMR parameters for all transplant recipients. Interobserver reliability was assessed between 2 independent observers (R.S.D., with 1 year of CMR post-processing experience; A.A.R., with 3 years of experience) using intraclass correlation analysis (intraclass correlation coefficient >0.90 indicates excellent reliability) in 15 patients.
To compare the global, peak, and segmental distribution of T2, T1, and ECV between patient cohorts (control subjects, patients with no ACAR, patients with past ACAR, and ACAR+ patients), a Lilliefors test was first used to determine parameter normality. If significant differences were found in global or peak segmental measures, additional analyses were performed on all 16 segmental values. Comparisons among the 4 cohorts were performed using generalized estimating equation linear regression models with an exchangeable working correlation matrix and robust standard errors to account for repeated measures.
Exploratory receiver operating characteristic (ROC) curve analyses using cluster resampling were conducted on CMR parameters showing significant differences between the ACAR− and ACAR+ groups on the basis of EMB results (gold standard) within 3 months of CMR. CMR parameters that were the strongest independent predictors of ACAR, along with basic demographic information (age at CMR, time since transplantation, sex), were evaluated using binary logistic regression to determine if a combination of factors was predictive of ACAR.
A sensitivity analysis was performed to determine if CAV affected CMR parameters; patients with CAV were removed from the sample, and parameters were tested for notable changes.
Statistical analysis was conducted in SPSS version 23 (IBM, Armonk, New York) and Stata version 14 (StataCorp, College Station, Texas), and p values <0.05 were considered to indicate statistical significance.
RESULTS
STUDY COHORT.
Fifty-eight transplant recipients (mean age 47.0 ± 14.7 years, 47% women) underwent 83 CMR studies (n = 38 with 1, n = 16 with 2, n = 3 with 3, and n = 1 with 4 CMR studies). As shown in Table 1, 37 CMR studies (45%) were performed between 1-month and 1-year post-transplantation, 32 (39%) between 1 and 6 years, and 14 (17%) ≥7 years post-transplantation. Demographic data and global LV function parameters are summarized in Table 2. Transplant recipients demonstrated significantly lower stroke volume and higher heart rate and cardiac output compared with control subjects but showed no difference in ejection fraction (transplant recipients 61.4 ± 11.2% vs. control subjects 64.5 ± 7.2%).
TABLE 1.
Timing of Cardiac Magnetic Resonance Imaging and Endomyocardial Biopsy
| Endomyocardial Biopsy Timing | ||||
|---|---|---|---|---|
| n | ≤1 Week | 1 Week to 1 Month | 1 to 3 Months | |
| <1 yr | 37 (45%) | 37 | 0 | 0 |
| 1–6 yrs | 32 (39%) | 14 | 8 | 10 |
| >7 yrs | 14 (17%) | 6 | 3 | 5 |
| Total | 83 | 57 | 11 | 15 |
Of the 83 cardiac magnetic resonance imaging scans performed in 58 transplant recipients, 45% were performed during the first year post-transplantation, 39% during years 1 to 6 post-transplantation, and 17% more than 7 years post-transplantation. The majority of transplant recipients underwent endomyocardial biopsy within 1 week of cardiac magnetic resonance imaging (endomyocardial biopsy timing ≤1 week).
TABLE 2.
Demographic Data and Left Ventricular Function Parameters
| Transplant Recipients (n = 58) |
Control Patients (n = 14) |
p Value* | |
|---|---|---|---|
| Age at CMR (yrs) | 47.0 ± 14.7 | 47.7 ± 16.7 | NS |
| Time from Tx to CMR (yrs) | 3.8 ± 4.6 | ||
| Sex (female) | 27 (47) | 5 (36) | NS |
| Race | |||
| White | 33 (57) | ||
| Black | 16 (28) | ||
| Hispanic | 5 (9) | ||
| Asian | 3 (5) | ||
| Native American | 1 (2) | ||
| Height (cm) | 164.7 ± 12.1 | ||
| Weight (kg) | 82.7 ± 14.9 | ||
| BMI (kg/m2) | 28.2 ± 5.5 | ||
| Tx indication | |||
| DCM | 28 (48) | ||
| IHD | 13 (22) | ||
| HCM | 3 (5) | ||
| ARVC | 2 (3) | ||
| Other | 12 (21) | ||
| Cold ischemic time (min) | 202.2 ± 51.5 | ||
| CAV diagnosis | 6 (8 CMR studies) | ||
| Other cardiac disease | 0 | ||
| Left ventricular function | 83 CMR studies | 14 CMR studies | |
| Stroke volume (ml) | 75.6 ± 22.6 | 89.3 ± 19.5 | 0.04 |
| Heart rate (beats/min) | 92.3 ± 13.4 | 61.2 ± 6.4 | <0.01 |
| Cardiac output (l/min) | 6.9 ± 1.8 | 5.3 ± 1.5 | <0.01 |
| Ejection fraction (%) | 61.4 ± 11.2 | 64.5 ± 7.2 | NS |
| End-diastolic myocardial mass (g) | 85.4 ± 26.0 | 96.5 ± 40.3 | NS |
Values are mean ± SD or n (%).
Independent-samples t test probability values comparing the 2 groups. The bold values indicate statistically significant differences.
ARVC = arrhythmogenic right ventricular cardiomyopathy; BMI = body mass index; CAV = cardiac allograft vasculopathy; CMR = cardiac magnetic resonance imaging; DCM = dilated cardiomyopathy; HCM = hypertrophic cardiomyopathy; IHD = ischemic heart disease; Tx = transplantation.
CMR ACQUISITION AND INTEROBSERVER AGREEMENT.
Among the 83 CMR studies performed on transplant recipients, 81 had complete T2 data (1 without T2 sequences, 1 poor image quality), 81 had complete T1 data (2 without T1 sequences), and 57 had complete ECV data (2 without T1 sequences, 24 without Gd contrast).
Intraclass correlation analysis of 15 studies demonstrated excellent interobserver agreement for T2 (>0.95), T1 (0.91), and ECV (>0.95).
ACAR.
Biopsy-proven ACAR was detected in 23 transplant recipients (ACAR+ group; n = 20 grade 1R, n = 1 grade pAMR1H+, n = 2). The 60 remaining CMR studies were divided into no ACAR (n = 36) and past ACAR (n = 24) groups.
GLOBAL AND SEGMENTAL T2, T1, AND ECV.
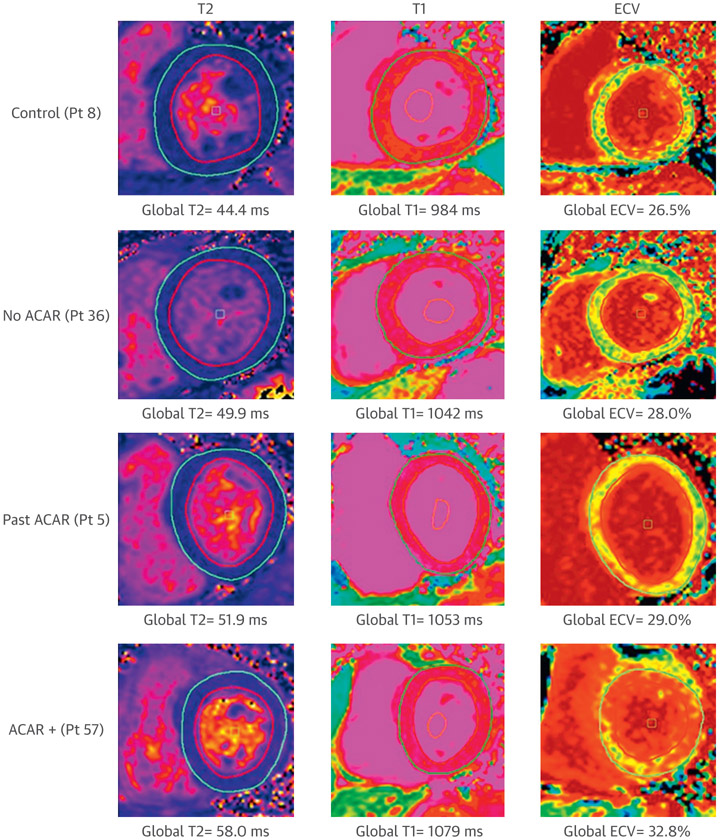
Representative CMR examples from each of the 4 groups are shown in Figure 1, which illustrate increases in T2, T1, and ECV from control subjects to ACAR+. Comparison of mean global and peak segmental T2, T1, and ECV among the 4 groups (control subjects, patients with no ACAR, patients with past ACAR, and ACAR+ patients) are summarized in Table 3 and Figures 2 and 3.
FIGURE 1. Representative Patient Examples for Multiparametric Magnetic Resonance Imaging for 4 Cohorts.
(Left) T2 maps show Left ventricular segmentation contours used for analysis and progressively increasing T2 from a control subject to an ACAR+ patient (top to bottom). (Middle) T1 maps demonstrated subtle increases from a control subject to an ACAR+ patient. (Right) Extracellular volume fraction (ECV) maps demonstrated increased myocardial ECV for an ACAR+ patient. ACAR = acute cardiac allograft rejection.
TABLE 3.
Comparison of Mean Global and Peak Segmental T2, T1, and Extracellular Volume Fraction
| (1) Control Subjects (n = 14) |
(2) No ACAR (n = 36) |
(3) Past ACAR (n = 24) |
(4) ACAR+ (n = 23) |
Linear Regression p Value | ||||
|---|---|---|---|---|---|---|---|---|
| 1 vs. 2 | 2 vs. 3 | 2 vs. 4 | 3 vs. 4 | |||||
| Global T2 | 45.2 ± 2.3 | 49.2 ± 4.0 | 51.0 ± 3.8 | 52.4 ± 4.7 | <0.01 | 0.02 | <0.01 | 0.21 |
| Peak segmental T2 | 49.2 ± 2.4 | 54.5 ± 4.4 | 55.7 ± 4.6 | 57.9 ± 6.4 | <0.01 | 0.52 | 0.04 | 0.09 |
| Global T1 | 993.8 ± 34.1 | 1,031.1 ± 45.8 | 1,072.8 ± 71.2 | 1,059.4 ± 63.6 | <0.01 | <0.01 | 0.13 | 0.24 |
| Peak segmental T1 | 1,064.2 ± 48.2 | 1,094.7 ± 57.9 | 1,156.1 ± 89.1 | 1,137.0 ± 81.9 | 0.06 | <0.01 | 0.08 | 0.38 |
| Global ECV | 25.9 ± 2.7 | 26.8 ± 3.3 | 26.8 ± 4.0 | 31.5 ± 3.9 | 0.22 | 0.80 | <0.01 | <0.01 |
| Peak segmental ECV | 29.6 ± 3.4 | 30.2 ± 3.5 | 31.1 ± 4.7 | 35.9 ± 4.8 | 0.43 | 0.85 | <0.01 | <0.01 |
Values are mean ± SD. The bold values indicate statistically significant differences.
ACAR = acute cardiac allograft rejection; ECV = extracellular volume fraction.
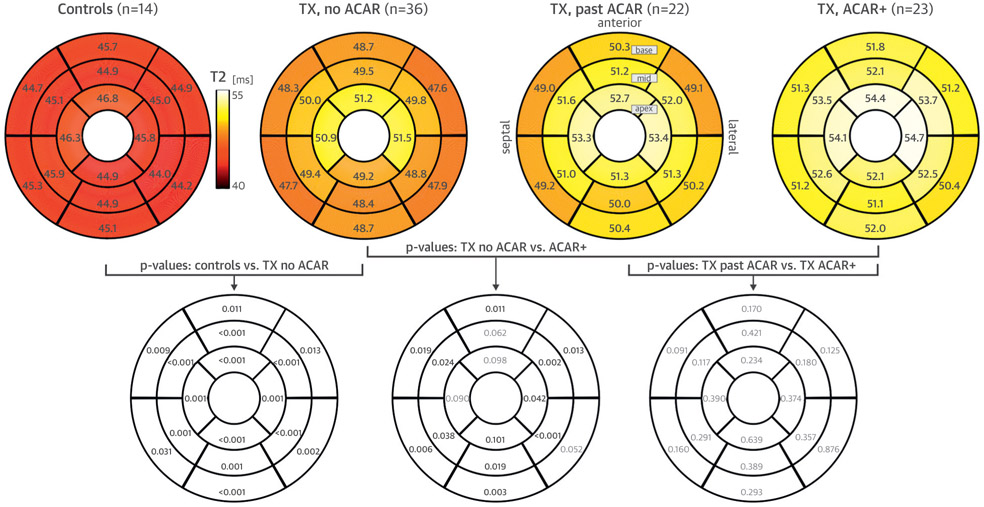
FIGURE 2. Segmental T2 Comparisons.
(Top row) Regional distribution of myocardial T2 (American Heart Association 16-segment plot) for the 4 study cohorts (control subjects, patients with no acute cardiac allograft rejection [ACAR], patients with past ACAR, and ACAR+ patients). All numbers represent the mean T2 over all subjects in each cohort. (Lower row) Segmental differences among groups (significant shown in bold). TX = transplant recipient.
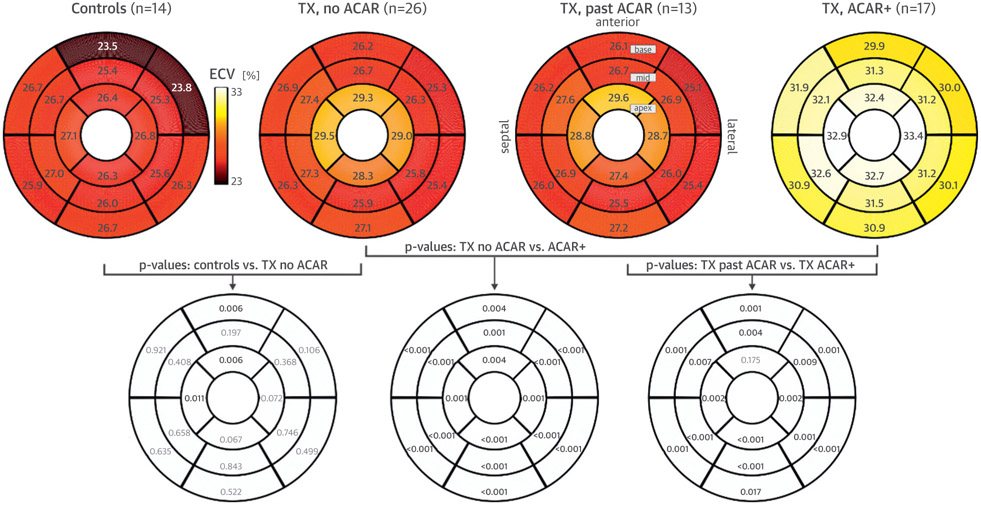
FIGURE 3. Segmental Extracellular Volume Fraction Comparisons.
(Top row) Regional distribution of myocardial extracellular volume fraction (ECV) (American Heart Association 16-segment plot) for the 4 study cohorts (control subjects, patients with no acute cardiac allograft rejection [ACAR], patients with past ACAR, and ACAR+ patients). All numbers represent the mean ECV over all subjects in each cohort. (Lower row) Segmental differences among groups (significant shown in bold). TX = transplant recipient.
Global and peak segmental T2 showed progressively higher values from healthy control subjects to patients with no ACAR to those with past ACAR to ACAR+ patients (Table 3). Both parameters were significantly higher in patients with no ACAR compared with healthy control subjects. Global T2 was significantly higher in patients with past ACAR compared with those with no ACAR. In addition, patients experiencing active ACAR (ACAR+) had increased T2 compared with the no ACAR group but not compared with recipients with history of ACAR (past ACAR). Segmental T2 followed the same pattern (Figure 2). All 16 segments demonstrated higher T2 values in patients with no ACAR compared with healthy control subjects, and 12 of 16 segments showed higher T2 values in ACAR+ patients compared with those with no ACAR.
Global T1 was significantly higher in patients with no ACAR compared with healthy control subjects, and global and peak segmental T1 were significantly higher in patients with past ACAR patients compared with those with no ACAR. Nevertheless, ACAR+ patients did not demonstrate significantly higher T1 values compared with recipients without ACAR (Table 3).
Global, peak segmental, and segmental ECV in all 16 segments (Table 3, Figure 3) were significantly elevated in ACAR+ patients compared with recipients without ACAR regardless of history of ACAR (no ACAR and past ACAR).
In a subgroup analysis of CMR studies performed within 1 week of EMB (n = 57) (column 4 in Table 1), global T2 was significantly higher in patients with no ACAR patients compared with control subjects (p = 0.02), but the differences between the no ACAR group and both past ACAR and ACAR+ groups were no longer significant. Global and peak T1 demonstrated no significant differences among groups. With respect to ECV, ACAR+ patients demonstrated significantly higher global and peak ECV than patients with no ACAR (p < 0.01 and p < 0.01) and those with past ACAR (p < 0.01 and p = 0.01), mirroring results in the full cohort.
SENSITIVITY ANALYSIS FOR IMPACT OF CAV.
Eight CMR studies were performed in patients diagnosed with CAV. A sensitivity analysis showed no significant impact of CAV status on any CMR parameters.
ROC CURVE ANALYSES.
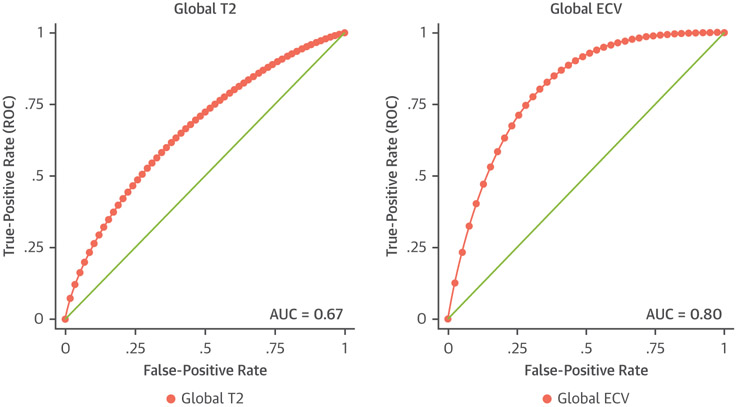
Global ECV had the highest area under the curve (AUC) of all parameters at 0.80 (Figure 4), achieving 100% sensitivity and 60% specificity at global ECV of 26.8%. The highest AUC for T2 was 0.67, which reached 75% sensitivity and 60% specificity at global T2 of 51.5 ms. Global T1 demonstrated an AUC of 0.53. Logistic regression determined that age at CMR, global T2, and global ECV were the factors most predictive of ACAR. ROC curve analysis of a combined model of these factors generated an AUC of 0.84.
FIGURE 4. Receiver Operating Characteristic Curve Analyses of Global T2 and Global Extracellular Volume Fraction.
Receiver-operating characteristic (ROC) curves for global T2 and global extracellular volume fraction (ECV). Global ECV demonstrated the highest combined sensitivity and specificity for detection of acute cardiac allograft rejection, illustrated by the greatest area under the curve (AUC).
DISCUSSION
Our study demonstrated 4 main findings: 1) T2 and T1 were higher in all transplant recipients compared with control subjects (even for patients without history of ACAR); 2) T2 and T1 were higher in recipients with history of ACAR compared with patients without history; 3) T2 was elevated in ACAR+ patients compared with patients without history of ACAR; and 4) ECV was elevated in ACAR+ patients compared with recipients without active ACAR, regardless of ACAR history. ROC curve analysis confirmed the potential for multiparametric CMR within 3 months of EMB to differentiate between patients with and without ACAR.
These findings demonstrate that several structural changes are present in the transplanted heart, which are further altered by ACAR. Elevation of T2 and T1 values in all transplanted patients, even without history of ACAR, compared with control subjects suggests that all transplanted hearts display more myocardial edema and interstitial fibrosis at baseline. Even though T2 and T1 are validated measures of edematous and fibrotic change respectively, the two measures overlap, making it difficult to isolate independent cellular mechanisms (13). Higher T2 and T1 in transplant recipients compared with control subjects support several prior studies (13,22), including previous findings by our group (22), but conflict with another prospective study (15).
Additional increases in T2 and T1 with history of ACAR suggest a higher degree of edema and fibrosis, even in the absence of evidence of active ACAR. In ACAR+ patients, T2 was significantly higher compared with patients without histories of ACAR, but not when compared with patients with histories of ACAR. Our results are similar to previous studies showing increased T2 in active ACAR (8-13) but demonstrated that the significant difference in T2 is not upheld if the patient has a history of ACAR. These findings suggest that history of ACAR and active ACAR increase T2 to a similar degree; therefore, T2 may not be a sensitive marker of edematous changes that occur during mild ACAR in patients with histories of ACAR. In addition, these differences were no longer significant in the subgroup analysis of CMR studies performed within 1 week of EMB, possibly because of decreased sample size.
ECV, in contrast, was significantly elevated in ACAR+ patients compared with patients without active ACAR, regardless of history. ECV is a technique for measurement of interstitial expansion, reflecting diffuse myocardial fibrosis and edema due to an acute or chronic cardiovascular insult (23,24), such as ACAR (14,25). Irrespective of the etiology, cardiovascular stress leads to a common pathway of matrix metalloproteinase activation, cytokines, and neurohormones, which causes collagen synthesis and fibrotic remodeling (23,26,27). Unlike T2, ECV was not elevated in recipients with history of ACAR, suggesting different structural processes identified by these 2 parameters. These findings show that ECV may be superior to T2 at distinguishing active ACAR, especially in patients with significant history of ACAR.
Evaluation by ROC curve analysis revealed that global T2 and particularly global ECV were each independently effective at screening for ACAR. Using the cutoff global ECV of 26.8% yielded 100% sensitivity and 60% specificity for detection of any grade of ACAR. Even though these parameters are strong independent predictors of ACAR, they cannot individually compare with the 98% sensitivity of EMB (5). Use of multiple CMR parameters in combination to form a multiparametric model, however, takes advantage of the diverse information distinct sequences convey regarding myocardial structure. By combining age at CMR, global T2, and global ECV into a logistic regression model, multiparametric CMR was able to achieve an AUC of 0.84, demonstrating the potential of CMR to detect ACAR.
Even though our results suggest that ECV may be as effective or superior to T2 for detection of ACAR, it is important to note that calculation of ECV requires intravenous contrast, which is contraindicated in many patients, most commonly by chronic kidney disease. For example, in this study, 29% of the CMR studies were performed without intravenous contrast, preventing calculation of ECV in these patients. T2 and pre-contrast T1, however, do not require contrast and can be acquired safety in all patients undergoing CMR.
Because quantitative CMR parameters should be evaluated in context, consideration of prior significant ACAR in heart transplant recipients seemed prudent, given strong evidence that acute episodes increase T2 (10,12) and possibly influence other measures. Separating hearts without history of moderate or severe (grade ≥2R) ACAR from hearts with considerable history of ACAR allowed us to capture a set of CMR studies in healthy transplanted hearts at baseline for comparison with control subjects and to evaluate the relationship between prior and active ACAR.
Because CAV is a possible consequence of repeated ACAR (28), patients with CAV were not excluded. Instead, we performed a sensitivity analysis by removing studies performed on patients diagnosed with CAV and performing the same analyses. We found no change in significant results, so patients with CAV were included in the final cohort.
In contrast to many CMR studies concentrating on ACAR, our study defined ACAR as International Society for Heart & Lung Transplantation grade ≥1R instead of grade ≥2R. Studies have grouped grade 1R with 0R as low-grade or no ACAR (8,10,13,15) because it resolves on its own 60% of the time and is rarely treated (3,6). Additionally, some CMR studies that have looked at grade 1R separately have found no significant differences between grades 0R and 1R (9,11). Nevertheless, grade 1R confers worse long-term graft function and progresses to higher grade ACAR in approximately 20% of cases (3,29). Our ACAR+ group, composed mostly of grade 1R biopsies, demonstrated significantly higher ECV values compared with our ACAR− group, suggesting that mild ACAR increases interstitial expansion. Distinguishing grade 1R from grade 0R ACAR using CMR shows continuing improvement of CMR accuracy and reliability, supporting possible future use as a noninvasive surrogate for EMB.
STUDY LIMITATIONS.
The number of CMRs performed within 1 week of grade ≥2R EMB was a limitation. Low enrollment of patients during acute grade ≥2R ACAR reflects both decreasing incidence of moderate to severe ACAR (because of improving immunosuppressive therapies) and difficulty scanning these patients in a timely manner upon admission for suspected ACAR. In addition, limited CMR studies performed in patients with AMR precluded further comparisons of CMR parameters between patients with ACR and AMR. Future studies with routine CMRs during the first year post-transplantation and collaboration among centers would likely increase the pool of CMR studies performed during acute episodes of ACAR, allowing more extensive comparison of grade 1R and ≥2R ACR, as well as evaluation for differences between ACR and AMR.
Another limitation of our study is inconsistent timing between EMB and CMR. Although the majority of CMR studies were performed within 1 week of EMB (including the positive biopsies), some were performed up to 3 months following negative biopsy results in patients without clinical suspicion for ACAR at time of CMR. Further investigation with standardized timing of CMR and EMB would help confirm our findings.
This study was also limited by focusing on CMR structural sequences without including measures to evaluate regional LV function. Several CMR methods are being developed to detect regional LV function, including strain and tissue phase mapping (30-32). In addition to CMR measures of function, comparison with echocardiography is another future direction. Studies of ACAR detection by echocardiography-derived parameters have been mixed (8,33,34) because of limited spatial resolution and structural analysis, but its measures show strong potential. Furthermore, studies comparing CMR and echocardiographic findings with those of other noninvasive modalities (AlloMap, DNA, biomarkers) would enhance the comprehensive detection of ACAR.
CONCLUSIONS
Multiparametric CMR is sensitive to structural changes in heart transplant recipients and shows great promise for detection of ACAR. Given its safety profile and noninvasive approach, it may be particularly well used when subclinical disease is suspected. As CMR continues to develop, longitudinal studies assessing the optimal timing of EMB and CMR surveillance, complete with patient outcome monitoring and cost-effectiveness analysis, are warranted.
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE: In addition to well-established roles for detailed assessment of cardiac anatomy, valvular disease, and ischemic heart disease, clinicians should consider CMR for characterization of global and regional myocardial structure following heart transplantation. T2 and ECV are significantly elevated in patients with ACAR, and combining these measures increases the sensitivity for detection of rejection. CMR may serve as a noninvasive and cheaper alternative to EMB, particularly in patients in whom there is a low to moderate index of suspicion for rejection.
TRANSLATIONAL OUTLOOK: The findings of this study advance the utility of multiparametric CMR in the detection of ACAR following heart transplantation. This study also demonstrates that history of rejection influences CMR measures and should be considered in future research. Because CMR is unique in its capacity to assess regional myocardial structure and function and is less invasive and expensive than EMB, further study is warranted. A longitudinal study with multiple CMRs during the first year post-transplantation corresponding to acquisition of EMBs would be most valuable for evaluating which combination of factors is most predictive of rejection. Related longitudinal directions include assessment of optimal timing for EMB and CMR surveillance and cost-effectiveness analysis. In addition, comparing CMR findings with echocardiography and tissue biomarkers would improve multimodality sensitivity for rejection.
Acknowledgments
This work was funded by grant R01 HL117888 from the National Heart, Lung, and Blood Institute.
ABBREVIATIONS AND ACRONYMS
- ACAR
acute cardiac allograft rejection
- ACR
acute cellular rejection
- AMR
antibody-mediated rejection
- AUC
area under the curve
- CAV
cardiac allograft vasculopathy
- CMR
cardiac magnetic resonance imaging
- ECV
extracellular volume fraction
- EMB
endomyocardial biopsy
- Gd
gadolinium
- LV
left ventricular
- ROC
receiver operating characteristic
- SSFP
steady-state free precession
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
REFERENCES
- 1.Stehlik J, Edwards LB, Kucheryavaya AY, et al. The registry of the International Society for Heart and Lung Transplantation: twenty-eighth adult heart transplant report–2011. J Heart Lung Transplant 2011;30:1078–94. [DOI] [PubMed] [Google Scholar]
- 2.Lund LH, Edwards LB, Dipchand AI, et al. The registry of the International Society for Heart and Lung Transplantation: thirty-third adult heart transplant report–2016; focus theme: primary diagnostic indications for transplant. J Heart Lung Transplant 2016;35:1158–69. [DOI] [PubMed] [Google Scholar]
- 3.Brunner-LaRocca HP, Sutsch G, Schneider J, Folath F, Kiowski W. Natural course of moderate cardiac allograft rejection (International Society for Heart Transplantation grade 2) early and late after transplantation. Circulation 1996;94:1334–8. [DOI] [PubMed] [Google Scholar]
- 4.Hamour IM, Burke MM, Bell AD, Panicker MG, Banerjee R, Banner NR. Limited utility of endomyocardial biopsy in the first year after heart transplantation. Transplantation 2008;85: 969–74. [DOI] [PubMed] [Google Scholar]
- 5.From AM, Maleszewski JJ, Rihal CS. Current status of endomyocardial biopsy. Mayo Clin Proc 2011;86:1095–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stewart S, Winters GL, Fishbein MC, et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant 2005; 24:1710–20. [DOI] [PubMed] [Google Scholar]
- 7.Fishbein MC, Kobashigawa J. Biopsy-negative cardiac transplant rejection: etiology, diagnosis, and therapy. Curr Opin Cardiol 2004;19:166–9. [DOI] [PubMed] [Google Scholar]
- 8.Marie PY, Carteaux JP, Angioi M, et al. Detection and prediction of acute heart transplant rejection: preliminary results on the clinical use of a black blood magnetic resonance imaging sequence. Transplant Proc 1998;30:1933–5. [DOI] [PubMed] [Google Scholar]
- 9.Marie PY, Angioi M, Carteaux JP, et al. Detection and prediction of acute heart transplant rejection with the myocardial T2 determination provided by a black-blood magnetic resonance imaging sequence. J Am Coll Cardiol 2001;37: 825–31. [DOI] [PubMed] [Google Scholar]
- 10.Bonnemains L, Villemin T, Escanye JM, et al. Diagnostic and prognostic value of MRI T2 quantification in heart transplant patients. Transpl Int 2014;27:69–76. [DOI] [PubMed] [Google Scholar]
- 11.Usman AA, Taimen K, Wasielewski M, et al. Cardiac magnetic resonance T2 mapping in the monitoring and follow-up of acute cardiac transplant rejection: a pilot study. Circ Cardiovasc Imaging 2012;5:782–90. [DOI] [PubMed] [Google Scholar]
- 12.Butler CR, Thompson R, Haykowsky M, Toma M, Paterson I. Cardiovascular magnetic resonance in the diagnosis of acute heart transplant rejection: a review. J Cardiovasc Magn Reson 2009;11:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wisenberg G, Pflugfelder PW, Kostuk WJ, McKenzie FN, Prato FS. Diagnostic applicability of magnetic resonance imaging in assessing human cardiac allograft rejection. Am J Cardiol 1987;60: 130–6. [DOI] [PubMed] [Google Scholar]
- 14.Coelho-Filho OR, Shah R, Lavagnoli CFR, et al. Myocardial tissue remodeling after orthotopic heart transplantation: a pilot cardiac magnetic resonance study. Int J Cardiovasc Imaging 2018; 34:15–24. [DOI] [PubMed] [Google Scholar]
- 15.Miller CA, Naish JH, Shaw SM, et al. Multiparametric cardiovascular magnetic resonance surveillance of acute cardiac allograft rejection and characterization of transplantation-associated myocardial injury: a pilot study. J Cardiovasc Magn Reson 2014;16:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferreira VM, Piechnik SK, Robson MD, Neubauer S, Karamitsos TD. Myocardial tissue characterization by magnetic resonance imaging: novel applications of T1 and T2 mapping. J Thorac Imaging 2014;29:147–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giri S, Chung YC, Merchant A, et al. T2 quantification for improved detection of myocardial edema. J Cardiovasc Magn Reson 2009;11:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Messroghli DR, Radjenovic A, Kozerke S, Higgins DM, Sivananthan MU, Ridgway JP. Modified looklocker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart. Magn Reson Med 2004;52:141–6. [DOI] [PubMed] [Google Scholar]
- 19.Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002;105:539–42. [DOI] [PubMed] [Google Scholar]
- 20.Kellman P, Wilson JR, Xue H, Ugander M, Arai AE. Extracellular volume fraction mapping in the myocardium, part 1: evaluation of an automated method. J Cardiovasc Magn Reson 2012;14: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berry GJ, Burke MM, Andersen C, et al. The 2013 International Society for Heart and Lung Transplantation working formulation for the standardization of nomenclature in the pathologic diagnosis of antibody-mediated rejection in heart transplantation. J Heart Lung Transplant 2013;32: 1147–62. [DOI] [PubMed] [Google Scholar]
- 22.Markl M, Rustogi R, Galizia M, et al. Myocardial T2- mapping and velocity mapping: changes in regional left ventricular structure and function after heart transplantation. Magn Reson Med 2012;70:517–26. [DOI] [PubMed] [Google Scholar]
- 23.Perea RJ, Ortiz-Perez JT, Sole M, et al. T1 mapping: characterisation of myocardial interstitial space. Insights Imaging 2015;6:189–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kidambi A, Motwani M, Uddin A, et al. Myocardial extracellular volume estimation by CMR predicts functional recovery following acute myocardial infarction. J Cardiovasc Magn Reson 2015;17 suppl 1:Q63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pichler M, Rainer PP, Schauer S, Hoefler G. Cardiac fibrosis in human transplanted hearts is mainly driven by cells of intracardiac origin. J Am Coll Cardiol 2012;59:1008–16. [DOI] [PubMed] [Google Scholar]
- 26.Maisch B Ventricular remodeling. Cardiology 1996;87 Suppl 1:2–10. [DOI] [PubMed] [Google Scholar]
- 27.Spinale FG. Myocardial matrix remodeling and the matrix metalloproteinases: Influence on cardiac form and function. Physiol Rev 2007;87: 1285–342. [DOI] [PubMed] [Google Scholar]
- 28.Ramzy D, Rao V, Brahm J, Miriuka S, Delgado D, Ross HJ. Cardiac allograft vasculopathy: a review. Can J Surg 2005;48:319–27. [PMC free article] [PubMed] [Google Scholar]
- 29.Anguita M, Lopez-Rubio F, Arizon JM, et al. Repetitive nontreated episodes of grade 1B or 2 acute rejection impair long-term cardiac graft function. J Heart Lung Transplant 1995;14: 452–60. [PubMed] [Google Scholar]
- 30.Ibrahim EH. Myocardial tagging by cardiovascular magnetic resonance: evolution of techniques–pulse sequences, analysis algorithms, and applications. J Cardiovasc Magn Reson 2011; 13:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Korosoglou G, Osman NF, Dengler TJ, et al. Strain-encoded cardiac magnetic resonance for the evaluation of chronic allograft vasculopathy in transplant recipients. Am J Transplant 2009;9: 2587–96. [DOI] [PubMed] [Google Scholar]
- 32.Foll D, Jung B, Schilli E, et al. Magnetic resonance tissue phase mapping of myocardial motion. Circ Cardiovasc Imaging 2010;3:54–64. [DOI] [PubMed] [Google Scholar]
- 33.Vivekananthan K, Kalapura T, Mehra M, et al. Usefulness of the combined index of systolic and diastolic myocardial performance to identify cardiac allograft rejection. Am J Cardiol 2002;90: 517–20. [DOI] [PubMed] [Google Scholar]
- 34.Mena C, Wencker D, Krumholz HM, McNamara RL. Detection of heart transplant rejection in adults by echocardiographic diastolic indices: a systematic review of the literature. J Am Soc Echocardiography 2006;19:1295–300. [DOI] [PubMed] [Google Scholar]