Abstract
Background:
Short interval intracortical inhibition (SICI) is commonly used to assess inhibition in the motor cortex and is known to be affected by the paired-pulse stimulus parameters (i.e., interstimulus interval [ISI], conditioning stimulus intensity [CSI] and test stimulus intensity [TSI]) used during testing. While the effects of stimulus parameters are well-studied in the upper-extremity, evidence in the lower-extremity is lacking.
Objective:
To comprehensively examine the effects of alterations in paired-pulse stimulus parameters on the two phases of SICI in the quadriceps muscle group.
Methods:
Seventeen adults (8 males, 9 females) volunteered to participate in this study. SICI was examined over a range of CSIs (70-90% active motor threshold [AMT]), TSIs (100-140% AMT), and ISIs (1.0-3.0 ms) using both EMG and torque responses elicited by transcranial magnetic stimulation (TMS).
Results:
The results indicated that SICI at 1.0 ms ISI was best revealed with a CSI of 70% and TSI ≥ 110% AMT, whereas SICI at 2.5 ms ISI was best revealed with a CSI of 80-90% and a TSI of ≥ 130% AMT. Unlike upper-extremity muscles, evaluating SICI with a CSI of 70% AMT and an ISI of 1.0 ms produced the greatest inhibition for all TSIs. In general, inhibitory effects were contaminated by facilitatory effects when using a TSI of 100% AMT.
Conclusions:
The amount of inhibition was dependent on the stimulation parameters used during testing. A CSI of 70% AMT, ISI of 1.0 ms, and TSI of ≥ 110% AMT appear to be optimal for measuring SICI in the quadriceps muscle; however, other parameters can be used if careful consideration is given to the described interaction between the parameters.
Keywords: Cortical excitability, Knee, Paired-pulse stimulation, Two phases, Twitch, Bayesian
INTRODUCTION
Short interval intracortical inhibition (SICI) is a paired-pulse transcranial magnetic stimulation (TMS) measure that is commonly used to assess inhibition in the cerebral motor cortex and monitor changes in corticomotor excitability after an intervention (Cengiz et al., 2013; Di Pino et al., 2014; Hunter et al., 2016; Kujirai et al., 1993). In this paradigm, two TMS pulses, separated by 1-5 ms interstimulus interval (ISI), are delivered via a single stimulating coil over the motor cortex (Di Pino et al., 2014). The first subthreshold pulse, which is commonly referred to as the conditioning stimulus, is known to suppress the second suprathreshold pulse (test stimulus) given 1-5 ms later. SICI has been studied extensively in healthy as well as patient population and is thought to be primarily mediated by the activation of intracortical inhibitory GABAergic interneurons (Hanajima et al., 1998; Ziemann et al., 1996a, b). The neurophysiological features of SICI has been used in a variety of basic science and clinical applications. For example, SICI has been used to study the effects of noninvasive brain stimulation (e.g., transcranial direct current stimulation) on cortical inhibitory circuits (Di Lazzaro et al., 2012), understand the role of dopaminergic treatment on inhibitory circuits (Dubbioso et al., 2017a; Ni et al., 2013), evaluate the integrity of inhibitory circuits underpinned by GABA-A network in individuals with neurological disorders (Dubbioso et al., 2017b), and monitor the long-term effect of therapy on the neurological status of individuals with genetic disorders (Dubbioso et al., 2016).
A number of studies have shown that the extent of intracortical inhibition measured by paired-pulse protocols are affected by the pulse parameters and state of the muscle (active vs rest) during TMS (Brownstein et al., 2018b; Cengiz et al., 2013; Fisher et al., 2002; Ortu et al., 2008; Roshan et al., 2003; Stokic et al., 1997; Vucic et al., 2009). For example, two phases of SICI have been reported with maximum inhibition observed at ISIs of 1.0 and 2.5 ms (Fisher et al., 2002; Roshan et al., 2003). Further, a lower conditioning stimulus intensity (CSI) and higher test stimulus intensity (TSI) are typically known to induce greater inhibition (Ortu et al., 2008; Roshan et al., 2003; Sidhu et al., 2013; Vucic et al., 2009). However, the inhibitory effect of the conditioning stimulus is contaminated by facilitatory effects if both the conditioning and test stimuli are provided at near motor threshold (i.e., ~100% motor threshold) or when SICI is evaluated during voluntary contraction (Hanajima et al., 1998; Ilic et al., 2002; Ortu et al., 2008). Accordingly, most researchers evaluate SICI at rest by providing a subthreshold conditioning stimulus (~80% motor threshold) and suprathreshold test stimulus (>120 % motor threshold) at an ISI of 2.5-3.0 ms.
While the evaluation of SICI at rest has obvious advantages, eliciting motor evoked responses (without much discomfort) when the muscles are relaxing may be challenging in some muscles (e.g., leg muscles such as quadriceps) and patient populations such as stroke. Hence, it is not uncommon to evaluate SICI during a slight contraction of the target muscle, particularly when studying lower-extremity muscles. Such active muscle contraction is also known to stabilize cortical excitability, minimize variability of motor evoked responses, and improve reliability between sessions (Darling et al., 2006; Thomas et al., 2016). However, studies evaluating SICI in lower-extremity muscles typically extrapolate stimulation parameters from upper-extremity studies (Kittelson et al., 2014; Luc-Harkey et al., 2017; Stevens-Lapsley et al., 2013; Ward et al., 2016; Zarzycki et al., 2018), which may not be appropriate considering the differences in recruitment thresholds and somatotopic organization. Further, it is not clear whether the two distinct phases of SICI observed in upper-extremity muscles are also present in the lower-extremity muscles. Therefore, the purpose of this study was to fully characterize the effects of different stimulation parameters on SICI in order to verify the presence of two distinct phases of SICI and to determine the optimal parameters for measuring SICI in the quadriceps muscle group during active contraction.
MATERIALS AND METHODS
Subjects
Seventeen healthy adults (8 males, 9 females; 20.1 ± 2.8 years, 1.7 ± 0.1 meters; 64.7 ± 14.3 kilograms) participated in this study after signing an informed consent document that was approved by the University of Michigan Institutional Review Board. All subjects were right leg dominant based on their preferred leg to kick a ball (Krishnan, 2015; Ranganathan et al., 2016; Washabaugh et al., 2016b). Subjects were included in the study if they did not have a history of significant orthopaedic or neurological conditions, psychiatric illness, or any contraindications to transcranial magnetic stimulation (e.g., recent skull fracture or head injury, metal implants in the head, epilepsy, cardiac pacemakers, pregnant females, recurrent fainting spells or syncope, etc.) (Keel et al., 2001).
Experimental protocol
EMG and Torque Measurements
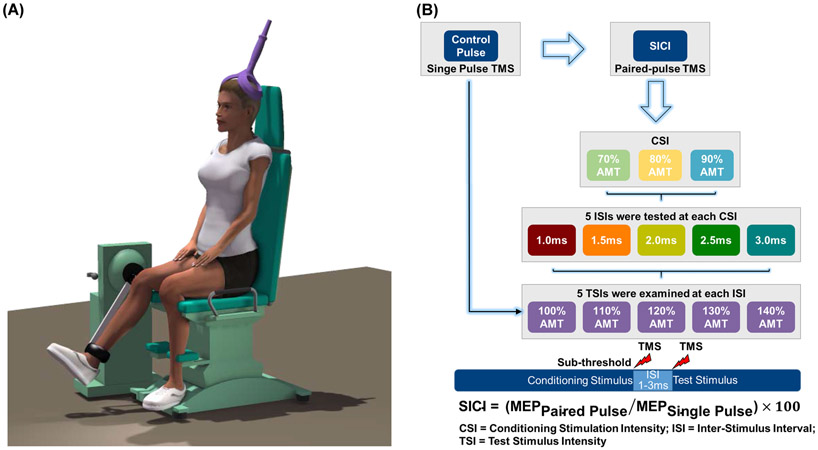
A schematic of the experimental protocol is provided in Fig. 1. After cleaning the skin over the electrode placement sites on the subject’s dominant leg with alcohol swabs, surface electromyography (EMG) electrodes (Trigno, Delsys, Natick, MA) were placed on the muscle bellies of vastus medialis (VM), rectus femoris (RF), and vastus literalis (VL) muscles according to the established guidelines (www.seniam.org). The EMG electrodes were tightly secured to the skin using self-adhesive tapes and cotton elastic bandages. The quality of the EMG signals was visually inspected in real time using a custom written program in LabVIEW 2011 (National Instruments Corp., Austin, TX, USA) (Washabaugh et al., 2016a; Washabaugh and Krishnan, 2018; Washabaugh et al., 2018).
Fig. 1:
A schematic of the (A) experimental set-up and (B) protocol.
The subject was then seated on a Biodex isokinetic dynamometer (System 4 Pro, Biodex Medical Systems, Inc., Shirley, NY, USA) with their hip at 85° and knee at 70° of flexion. The dynamometer’s axis of rotation was aligned to the subject’s knee axis by manually adjusting the dynamometer and chair positioning settings. The subject was then secured to the dynamometer using chest, waist, thigh, and leg straps. The subject was instructed to perform six submaximal quadriceps contractions (2 × 25%, 2 × 50%, and 2 × 75%) as a warm-up, following which two minutes of rest was provided (Krishnan and Theuerkauf, 2015). Next, the subject was asked to perform a 5-second maximal voluntary isometric contraction (MVIC) of the knee extensors by having them kick as hard as possible against the torque-arm pad of the dynamometer. Loud verbal encouragement and visual feedback of the torque curves were provided during the MVICs to facilitate maximal effort.
Short Interval Intracortical Inhibition
After a two-minute rest period, the effect of interstimulus interval (ISI) and conditioning stimulus intensity (CSI) on SICI was evaluated at various test stimulus intensities (TSIs). TMS pulses were delivered using a Magstim Bistim2 stimulator (Magstim, Whitland, UK) via a 110 mm diameter double-cone coil (oriented to induce a posterior-to-anterior current flow in the cortex) while the subject maintained a small background contraction (5% of MVIC) of their quadriceps muscle. First, a linen cap was tied tightly on the subject’s head and the estimated location of the vertex was marked on the cap using an indelible marker. An initial stimulation location was then determined by measuring 1 cm posterior and 2 cm lateral from the vertex (Krishnan and Dhaher, 2012). From this location, the coil was systematically moved to determine the location at which TMS produced the largest and most consistent knee extensor twitch torque at the lowest intensity (i.e., hotspot) (Washabaugh et al., 2016b). The hotspot location was registered digitally using a custom developed frameless stereotaxic camera system (NeuRRoNav) (Rodseth et al., 2017). The coil was then secured to this location using an adjustable coil holding arm and the feedback from NeuRRoNav system was used to maintain the coil location over the hotspot throughout the experiment. After which, the subject’s active motor threshold was established. Active motor threshold was defined as the minimum stimulus intensity required to evoke a clear distinguishable motor evoked response ≥ 50% of the time while the subject maintained an active contraction (5% MVIC) of their quadriceps muscle. Five single-pulse TMS was then provided at 100%, 110%, 120%, 130%, and 140% of active motor threshold to obtain unconditioned motor evoked torque (control MEP torque) and EMG (control MEP EMG) responses (25 trials in total). Following which, paired-pulse TMS was delivered to obtain conditioned MEP torque and EMG responses. Five ISIs (1.0, 1.5, 2.0, 2.5, and 3.0 ms) were studied at three CSIs (70%, 80%, and 90% of active motor threshold) using five TSIs (100%, 110%, 120%, 130%, and 140% of active motor threshold) in a randomized order. Five TMS pulses were delivered at each testing intensity (375 trials in total; 5 trials × 5 TSIs × 5 ISIs × 3 CSIs) and the average of the five trials was used in the analysis. SICI was quantified by expressing the size of conditioned responses obtained using paired-pulse TMS as a percentage of unconditioned control responses obtained using single-pulse TMS [i.e., SICI = (MEPPaired Pulse/MEPSingle Pulse) × 100] (Bashir et al., 2016; Hunter et al., 2016; Kujirai et al., 1993; Ortu et al., 2008; Roshan et al., 2003; Schambra et al., 2016; Stokic et al., 1997).
DATA MANAGEMENT
EMG and torque data were recorded using a custom-written LabVIEW program (version 2011). The EMG and torque signals were low pass filtered at 500 Hz using an 8th order analog Butterworth filter (SCXI 1143, National Instruments) and sampled at 1000 Hz using a Windows desktop computer with an 18-bit high-accuracy M-series multifunction data acquisition module (USB 6281, National Instruments). The raw torque signals were converted to torque values (N·m) using conversion factors obtained from a calibration testing performed prior to the testing. The raw EMG signals were band-pass filtered (20-500Hz) using a 4th order zero lag Butterworth digital filter. The size of the MEP torque and EMG responses was quantified by taking the average peak-to-peak amplitudes at each testing intensity.
STATISTICAL ANALYSES
All statistical analyses were performed using SPSS version 24.0 (SPSS Inc., Chicago, IL, USA.) and JASP version 0.9.2.0. We adopted an inclusive statistical approach where we performed both classical and Bayesian analyses (Wagenmakers et al., 2018a; Wagenmakers et al., 2018b). Data were pooled across the sexes because our initial analysis that included sex as an independent variable indicated no significant differences (p > 0.05). Descriptive statistics were computed for each variable to summarize subject demographics, MEP torque, and MEP EMG data. The relationship between ISI, TSI, and SICI at various CSIs were visualized using three-dimensional (3D) plots with best fit surfaces generated using TableCurve® 3D version 4.0.01 (TableCurve 3D, Systat Software, Inc., Chicago, IL). One sample t-tests were used to test for significant induction of SICI (i.e., if SICI ratio was < 100%) for each of the ISIs (1.0, 1.5, 2.0, 2.5, and 3.0 ms), CSIs (70%, 80%, and 90% active motor threshold), and TSIs (100, 110, 120, 130, and 140% active motor threshold). The effect of CSI, ISI, and TSI on SICI was evaluated using a linear mixed model with CSI, ISI, TSI, CSI × ISI, CSI × TSI, ISI × TSI, and CSI × ISI × TSI as fixed factors and subject as a random factor in the model. A significant main or interaction effect was followed by appropriate post-hoc analyses (i.e., simple main effects for interactions and Benjamini–Hochberg false discovery rate [FDR] corrections for main effects) (Benjamini and Hochberg, 1995; Cohen, 2008; Salkind, 2010). A significance level of α = 0.05 was established for all statistical analyses. We also performed Bayesian analyses (Bayesian Repeated Measures ANOVA) using the default prior in JASP to obtain Bayesian inference. The Vovk-Sellke Maximum p-Ratio (MPR) was also computed to estimate the maximum possible odds in favor of alternative hypothesis (H1) over null hypothesis (H0) based on two-sided p-value using the following equation MPR = 1/(−e × p × ln(p)) for p ≤ 0.37 (Sellke et al., 2001; Vovk, 1993). Note that formal statistical analyses were only performed on the MEP torque data because a priori correlational analyses indicated significant correlations between MEP torque and MEP EMG data for all of the quadriceps muscles (p < 0.001).
RESULTS
Descriptive Data
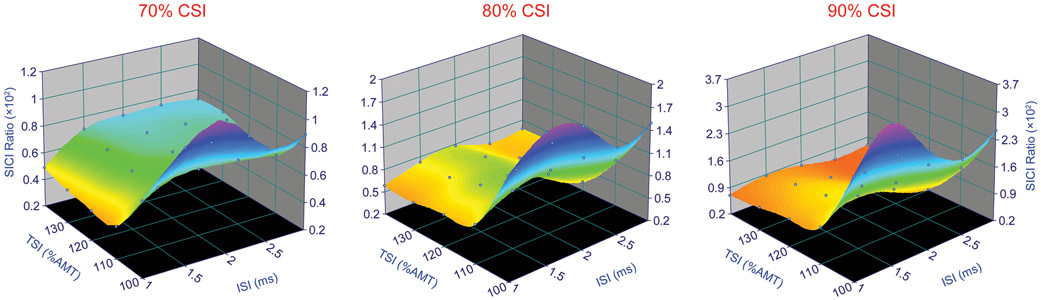
The mean SICI values observed for each of the ISIs, CSIs, and TSIs, and the 3D surface models are shown in Fig. 2 and Fig. 3. In general, paired-pulse TMS delivered with 1.0 ms ISI at 70% CSI produced the greatest inhibition for all TSIs (Table and Appendix 1, Supplementary Tables 1, 2, and 3). There were two phases of SICI, where greater inhibition was generally observed at ISIs of 1.0 and 2.5 ms, and least inhibition was observed at ISIs of 1.5 and 2.0 ms (Fig. 2). The amount of inhibition also increased with the increase in TSI for all CSIs, except for 70% CSI, where the inhibition was greatest when the TSI was 110% and 120% of AMT (Table 1). The amount of inhibition was typically higher at 70% CSI and reduced as the CSI increased. It was also found that as CSI increased, greater TSI was required to achieve inhibition.
Fig. 2:
3D surface plots showing the relationship between interstimulus interval (ISI), test stimulus intensity (TSI), and short interval intracortical inhibition (SICI) for the motor evoked quadriceps torque responses at various conditioning stimulus intensities (CSIs). Note the colors are only for illustration purposes.
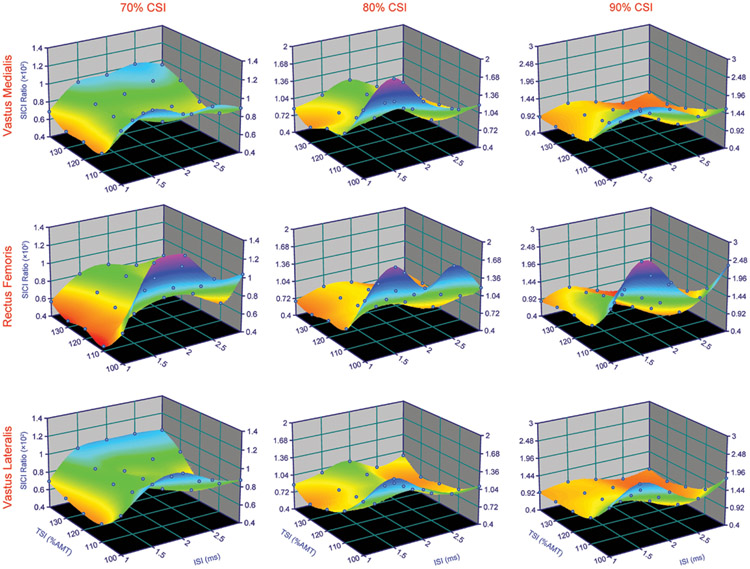
Fig. 3:
3D surface plots showing the relationship between interstimulus interval (ISI), test stimulus intensity (TSI), and short interval intracortical inhibition (SICI) for the EMG-based motor evoked potentials at various conditioning stimulus intensities (CSIs). Note the colors are only for illustration purposes.
Table 1.
Mean (± S.D.) intracortical inhibition observed at various stimulation parameters.
| ISI | TSI | 70% CSI | 80% CSI | 90% CSI |
|---|---|---|---|---|
| 1.0 | 100 | 73.9 ± 42.7 | 152.6 ± 92.0 | 275.8 ± 197.3 |
| 110 | 43.4 ± 24.6 | 71.4 ± 39.9 | 113.0 ± 39.4 | |
| 120 | 42.0 ± 22.5 | 62.6 ± 27.5 | 90.6 ± 28.1 | |
| 130 | 44.3 ± 22.5 | 57.1 ± 27.9 | 78.8 ± 26.0 | |
| 140 | 49.1 ± 16.4 | 58.7 ± 19.6 | 70.3 ± 18.7 | |
| Total | 50.5 ± 29.2 | 80.5 ± 60.2 | 125.7 ± 118.2 | |
| 1.5 | 100 | 101.9 ± 50.6 | 187.6 ± 60.2 | 367.3 ± 269.8 |
| 110 | 62.7 ± 28.0 | 95.0 ± 38.4 | 153.3 ± 50.7 | |
| 120 | 62.8 ± 24.0 | 82.2 ± 26.0 | 114.8 ± 24.3 | |
| 130 | 66.5 ± 19.9 | 72.4 ± 22.9 | 102.4 ± 26.9 | |
| 140 | 70.8 ± 16.1 | 73.1 ± 15.1 | 87.0 ± 16.1 | |
| Total | 72.9 ± 33.1 | 102.0 ± 76.1 | 165.0 ± 159.7 | |
| 2.0 | 100 | 113.7 ± 54.1 | 179.0 ± 90.8 | 270.1 ± 182.0 |
| 110 | 68.0 ± 28.6 | 98.5 ± 40.0 | 133.6 ± 36.2 | |
| 120 | 69.0 ± 25.6 | 84.6 ± 24.9 | 103.0 ± 23.9 | |
| 130 | 71.9 ± 19.7 | 82.2 ± 16.5 | 86.5 ± 23.8 | |
| 140 | 74.1 ± 12.6 | 78.0 ± 11.5 | 79.1 ± 17.7 | |
| Total | 79.4 ± 35.3 | 104.5 ± 59.4 | 134.5 ± 108.8 | |
| 2.5 | 100 | 86.6 ± 46.7 | 135.3 ± 57.0 | 214.1 ± 125.6 |
| 110 | 67.0 ± 28.9 | 70.1 ± 23.4 | 97.6 ± 39.8 | |
| 120 | 68.5 ± 26.5 | 65.4 ± 24.3 | 75.7 ± 27.1 | |
| 130 | 71.1 ± 19.4 | 63.1 ± 18.8 | 64.7 ± 27.0 | |
| 140 | 75.1 ± 14.1 | 63.5 ± 16.4 | 61.2 ± 18.6 | |
| Total | 73.7 ± 29.5 | 79.5 ± 41.8 | 102.6 ± 83.4 | |
| 3.0 | 100 | 89.4 ± 53.8 | 149.8 ± 107.9 | 253.8 ± 174.4 |
| 110 | 63.0 ± 28.7 | 81.0 ± 36.9 | 118.0 ± 47.7 | |
| 120 | 66.0 ± 20.2 | 65.0 ± 22.7 | 88.0 ± 28.5 | |
| 130 | 73.2 ± 16.7 | 66.6 ± 22.2 | 77.2 ± 25.3 | |
| 140 | 72.8 ± 14.0 | 68.0 ± 16.6 | 69.5 ± 21.6 | |
| Total | 72.9 ± 31.0 | 86.1 ± 61.5 | 121.3 ± 106.4 | |
| Total | 100 | 93.1 ± 50.5 | 160.9 ± 98.6 | 276.3 ± 197.4 |
| 110 | 60.8 ± 28.6 | 83.2 ± 37.3 | 123.1 ± 46.2 | |
| 120 | 61.7 ± 25.4 | 72.0 ± 26.3 | 94.4 ± 29.1 | |
| 130 | 65.4 ± 22.1 | 68.3 ± 23.1 | 81.9 ± 28.1 | |
| 140 | 68.4 ± 17.4 | 68.3 ± 17.1 | 73.4 ± 20.2 | |
| Total | 69.9 ± 33.1 | 90.5 ± 61.4 | 129.8 ± 119.2 |
Values that are bold indicate significant SICI at p ≤ 0.05. SICI = short interval intracortical inhibition, CSI = conditioning stimulus intensity, TSI = test stimulus intensity, ISI = interstimulus interval.
Short Interval Intracortical Inhibition
At 70% CSI, SICI was induced at all TSIs except at 100% TSI (p < 0.05), where significant SICI was only induced when a 1.0 ms ISI was used (p < 0.05). At 80% CSI, all TSIs except 100% TSI induced significant SICI when the ISI was 1.0, 2.5, and 3.0 ms (p < 0.05); whereas, when the ISI was 1.5 and 2.0 ms, significant SICI was observed only at 120%, 130%, and 140% TSI (p < 0.05). At 90% CSI, SICI was induced at 130% and 140% TSI when the ISI was 1.0, 2.0, 2.5, and 3.0 ms (p < 0.05); whereas, when the ISI was 1.5 ms, significant SICI was observed only at 140% TSI (p < 0.05). Significant SICI was also observed at 120% TSI when the ISI was 2.5 ms.
Effect of Stimulation Parameters on SICI
There was a significant main effect of CSI [F(2, 1184) = 120.178, p < 0.001, MPR = 1.1×1045)], ISI [F(4, 1184) = 12.232, p < 0.001, MPR = 1.9×107)], and TSI [F(4, 1184) = 159.792, p < 0.001, MPR = 6.8×10105)] on the amount of SICI. There were also significant CSI × ISI [F(8, 1184) = 4.091, p < 0.001, MPR = 4.9×102)] and CSI × TSI [F(8, 1184) = 34.35, p < 0.001, MPR = 6.4×1045)] interaction effects. There was no significant ISI × TSI [F(16, 1184) = 1.466, p = 0.104, MPR = 1.6×100)] or CSI × ISI × TSI [F(32, 1184) = 0.452, p = 0.997, MPR = Not Applicable)] interaction effect. The Bayesian Repeated Measures ANOVA with default prior scales also revealed that the model with CSI × ISI and CSI × TSI interaction terms was preferred to the main effects or any other interaction models by a Bayes factor of > 10 (Appendix 2, Supplementary Materials). Because the interaction effects were primarily mediated by CSI, the effects of ISI and TSI were also examined at each CSI separately using two-way models. These results are presented as supplementary statistics in the Supplementary Materials (Appendix 2).
Modulation of SICI with ISI
Tests of simple main effects of CSI × ISI interaction indicated a significant effect of ISI within each levels of CSI [70% CSI: F(4,1184) = 3.222, p = 0.012, MPR = 6.9×100; 80% CSI: F(4,1184) = 3.686, p = 0.005, MPR = 1.3×101; 90% CSI: F(4,1184) = 13.507, p < 0.001, MPR = 1.8×108]. Post-hoc analysis of the CSI × ISI interaction effect at 70% CSI indicated that 1.0 ms ISI produced greater inhibition than 1.5 (p = 0.026), 2.0 (p = 0.005), 2.5 (p = 0.026), and 3.0 ms ISIs (p = 0.026). However, there were no differences in the amount of SICI between 1.5, 2.0, 2.5, and 3.0 ms ISIs (all p’s > 0.05). Post-hoc analysis of the CSI × ISI interaction effect at 80% CSI indicated that 1.0 and 2.5 ms ISIs produced greater inhibition than 1.5 ms (p = 0.031 and p = 0.026) and 2.0 ms (p = 0.024 and p = 0.02) ISIs. Post-hoc analysis of the CSI × ISI interaction effect at 90% CSI indicated that 1.0, 2.0, 2.5, and 3.0 ms ISIs produced greater inhibition than 1.5 ms ISI (p < 0.001, p = 0.003, p < 0.001, and p < 0.001). Further, 2.5 ms ISI produced greater inhibition than 1.0 and 2.0 ms ISIs (p = 0.026 and p = 0.002).
Modulation of SICI with TSI
Tests of simple main effects of CSI × TSI interaction indicated a significant effect of TSI within each levels of CSI [70% CSI: F(4,1184) = 4.599, p = 0.001, MPR = 4.9 ×101; 80% CSI: F(4,1184) = 41.053, p < 0.001, MPR = 1.7×1029; 90% CSI: F(4,1184) = 182.84, p < 0.001, MPR = 2.6 ×10118]. Post-hoc analysis of the CSI × TSI interaction effect at 70% CSI indicated that 100% TSI produced lower inhibition in comparison with 110% (p = 0.001), 120% (p = 0.001), 130% (p = 0.004), and 140% TSIs (p = 0.01). However, there were no differences in the amount of SICI between 110%, 120%, 130%, and 140% TSIs (all p’s > 0.05). Post-hoc analysis of the CSI × TSI interaction effect at 80% CSI indicated that 100% TSI produced lower inhibition in comparison with 110% (p < 0.001), 120% (p < 0.001), 130% (p < 0.001), and 140% (p < 0.001) TSIs. However, there were no differences in the amount of SICI between 110%, 120%, 130%, and 140% TSIs (all p’s > 0.05). Post-hoc analysis of the CSI × TSI interaction effect at 90% CSI indicated that 100% TSI produced lower inhibition in comparison with 110% (p < 0.001), 120% (p < 0.001), 130% (p < 0.001), and 140% (p < 0.001) TSIs. Further, 110% TSI produced lower inhibition in comparison with 120% (p = 0.003), 130% (p < 0.001) and 140% (p < 0.001) TSIs, and 120% TSI produced lower inhibition in comparison with 140% TSI (p = 0.032).
Modulation of SICI with CSI
Tests of simple main effects of CSI × ISI interaction indicated a significant effect of CSI within each levels of ISI [1.0 ms ISI: F(2,1184) = 37.121, p < 0.001, MPR = 4.6 ×1013; 1.5 ms ISI: F(2,1184) = 57.369, p < 0.001, MPR = 4.1 ×1021; 2.0 ms ISI: F(2,1184) = 19.716, p < 0.001, MPR = 5.0 ×106; 2.5 ms ISI: F(2,1184) = 6.087, p = 0.002, MPR = 2.6 ×101; 3.0 ms ISI: F(2,1184) = 16.249, p < 0.001, MPR = 2.1 ×105]. Post-hoc analysis of the CSI × ISI interaction effect at 1.0, 1.5, and 2.0 ms ISIs indicated that 70% CSI produced greater inhibition in comparison with 80% (p = 0.001, p = 0.001, and p = 0.005) and 90% (p < 0.001, p < 0.001, and p < 0.001) CSIs, and 80% CSI produced greater inhibition in comparison with 90% CSI (p < 0.001, p < 0.001, and p = 0.001). Post-hoc analysis of the CSI × ISI interaction effect at 2.5 and 3.0 ms ISIs indicated that 70% (p = 0.001 and p < 0.001) and 80% (p = 0.01 and p < 0.001) CSIs produced greater inhibition in comparison with 90% CSI.
Tests of simple main effects of CSI × TSI interaction indicated a significant effect of CSI at 100% [F(2,1184) = 222.298, p < 0.001, MPR = 1.8 ×1079], 110% [F(2,1184) = 25.776, p < 0.001, MPR = 1.3 ×109], and 120% TSIs [F(2,1184) = 7.263, p = 0.001, MPR = 7.0 ×101]. However, there were no differences in the amount of SICI between 70%, 80%, and 90% CSIs at 130% [F(2,1184) = 2.014, p = 0.134, MPR = 1.4 ×100] and 140% TSIs [F(2,1184) = 0.226, p = 0.798, MPR = Not Applicable]. Post-hoc analysis of the CSI × TSI interaction effect at 100% and 110% TSIs indicated that 70% CSI produced greater inhibition in comparison with 80% (p < 0.001 and p = 0.021) and 90% (p < 0.001 and p < 0.001) CSIs, and 80% CSI produced greater inhibition in comparison with 90% CSI (p < 0.001 and p < 0.001). Post-hoc analysis of the CSI × TSI interaction effect at 120% TSI indicated that 70% (p = 0.001) and 80% (p = 0.021) CSIs produced greater inhibition in comparison with 90% CSI.
DISCUSSION
This study evaluated the effects of different stimulation parameters on SICI in the quadriceps muscle group during active contraction. The principal finding of this study was that the magnitude of SICI was affected by the choice of stimulation parameters such that 1.0 ms ISI at 70% CSI produced the greatest amount of inhibition. In general, marked SICI was observed when the conditioning stimulus was delivered at 70% active motor threshold with test stimulus intensity ≥ 110% active motor threshold. However, inhibitory effects were minimal (or replaced by facilitatory effects) when the conditioning stimulus was delivered at 90% active motor threshold with test stimulus intensity ≤ 120% active motor threshold. There were also two phases of SICI, where greater inhibition was generally observed at ISIs of 1.0 and 2.5 ms; although, SICI observed at 1.0 ms ISI with 70% CSI was much pronounced than SICI observed at 2.5 ms regardless of CSI or TSI.
Studies evaluating the effect of interstimulus interval on SICI have shown two distinct phases of SICI, with maximum inhibition occurring at ISIs of 1.0 ms and 2.5 ms (Fisher et al., 2002; Roshan et al., 2003). Further, these studies have also shown that the ISI at which SICI peaks vary based on the conditioning and test stimulus intensity (Ortu et al., 2008; Roshan et al., 2003; Vucic et al., 2009). Our results corroborate some of these previous findings from the upper extremity literature. First, the results of this study support the presence of two phases of SICI; however, unlike many of the previous studies, peak SICI was observed at an ISI of 1.0 ms instead of 2.5 ms. This difference could be due to the fact that TMS to the leg motor area predominantly activates early I-waves (I1) (Terao et al., 2000), which are known to be suppressed to a greater extent with an ISI of 1.0 ms than 2.5 ms during paired-pulse TMS (Hanajima et al., 2003). Second, we found that the intensity of the conditioning stimulus affected the ISI at which SICI peaked; though to a lesser extent in comparison with previous studies. At 70% and 80% CSIs, peak SICI occurred at an ISI of 1.0 ms, whereas at 90% CSI, peak SICI occurred at an ISI of 2.5 ms. Finally, the amount of SICI was generally higher at TSIs ≥ 130% AMT, but the inhibitory effects were replaced by facilitatory effects at low TSIs (particularly near AMT). Collectively, the results demonstrated that the amount of inhibition observed at the two phases of SICI was dependent on both CSI and TSI such that SICI at 1.0 ms ISI was best revealed with a CSI of 70% and TSI of 110-130% AMT, and SICI at 2.5 ms ISI was best revealed with a CSI of 80-90% and a TSI of ≥ 130% AMT.
When considering the physiological mechanisms underlying SICI, the SICI peak observed at 2.5 ms has been shown to be of synaptic origin, whereas the SICI peak at 1.0 ms has been attributed to axonal refractoriness, synaptic mechanisms, or both (Fisher et al., 2002; Hanajima et al., 2003; Roshan et al., 2003; Vucic et al., 2009). Hanajima et al. (2003) used different coil orientations to selectively activate different waves (I-waves and D-waves) and showed that SICI at 3 ms suppressed mainly the later I-waves, whereas SICI at 1.0 ms suppressed all waves, including the D-wave (Hanajima et al., 2003). Based on these results, the authors concluded that axonal refractoriness was the most likely reason for the SICI observed at 1.0 ms. However, Di Lazzaro et al. (1998), through epidural recordings of descending corticospinal volleys, found that SICI at 1.0 ms did not affect the early I-wave (I1) and thus argued that inhibition of later I-waves was not likely due to refractory corticospinal axons, but due to synaptic inhibition within the motor cortex (Di Lazzaro et al., 1998). Roshan et al. (2003) examined the effect of TSI on SICI at 1.0 ms and found that the amount of inhibition was greater when higher magnitudes of test stimulus were used (Roshan et al., 2003). Similarly, Vucic et al. (2009) evaluated the effect of CSI on SICI at 1.0 ms and found that the inhibitory effects reversed to facilitatory effects when higher intensities of conditioning stimulus were used (Vucic et al., 2009). The findings from this study corroborate both these findings. Collectively, these results argue against axonal refractoriness as the underlying mechanism for SICI at 1.0 ms because higher TSI should overcome the neuronal refractoriness (thus reducing the amount of inhibition) and higher CSI should increase the neuronal refractoriness (thus increasing the amount of inhibition).
It has been argued that SICI is a complex neurophysiological measure, mediated in part by the interaction of multiple cortical circuits, reflecting a balance between inhibition and facilitation (Ni and Chen, 2008; Peurala et al., 2008). Evidence from upper-limb studies indicate that SICI is reduced during voluntary muscle contraction than when the muscle is at rest (Fisher et al., 2002; Ridding et al., 1995; Roshan et al., 2003). The reduction in SICI during mild voluntary contraction has been attributed to the reduction in excitability of the cortical inhibitory circuits that project onto the corticomotoneuronal cells controlling the active muscle (Ridding et al., 1995). Thus, studies examining cortico-cortical inhibition in the motor cortex often evaluate SICI when the target muscle is at rest. Evaluation of SICI at rest indeed provides a cleaner test model; however, it is not always a feasible approach, particularly for individuals (or muscles) with high resting motor threshold. Accordingly, a number of researchers have tested SICI during a slight voluntary contraction of the target muscle (Brownstein et al., 2018a; Luc-Harkey et al., 2017; Sidhu et al., 2013; Ward et al., 2016). Evaluating SICI during an active contraction has also been shown to be more reliable for leg muscles than SICI measured at rest (Thomas et al., 2016). However, the interactions between cortical inhibitory and excitatory circuits could confound the analysis if optimal parameters were not selected during the investigation. This study addresses this important issue and provides detailed information on the effect of different stimulation parameters on SICI of the quadriceps muscle during active contraction.
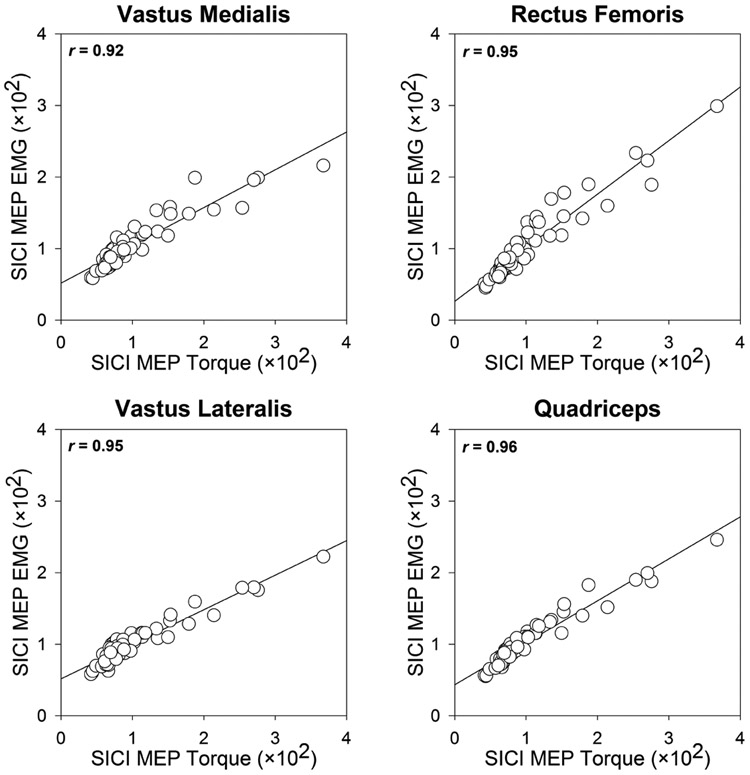
Changes in corticospinal and intracortical excitability are typically quantified by examining the size of motor evoked potentials (MEPs) measured using EMG. While there are advantages to the use of EMG-based MEPs, this may not be ideal when evaluating multi-headed muscles such as the quadriceps during active muscle contraction. This is because of the variability in EMG responses induced by motor unit rotations and alternate muscle activity among synergistic muscles during low-level sustained contractions (Akima et al., 2012; Bawa and Murnaghan, 2009; Fallentin et al., 1993; Kouzaki et al., 2002). Further, evaluating excitability changes from a single head of a multi-headed muscle may not provide a complete picture of the changes happening at the group level. To address these issues, we quantified SICI using motor evoked torque responses (in addition to the EMG responses) of the quadriceps muscle. Our results indicate that evaluating SICI using motor evoked torque response robustly captures intracortical inhibition of the entire muscle group. Further, such torque-based responses could provide additional insight regarding the functional implications of the changes observed at the MEP level (Tan and Dhaher, 2017). Thus, for these reasons and considering that there are good correlations between torque and EMG responses (Fig. 4), motor evoked torque responses may serve as a suitable alternative for EMG-based MEP responses to evaluate corticospinal excitability.
Fig. 4:
Scatterplots demonstrating the relationship between short interval intracortical inhibition (SICI) ratios obtained from motor evoked torque responses (MEP torque) and motor evoked potentials (MEP EMG) of vastus medialis (VM), rectus femoris (RF), vastus lateralis (VL), and quadriceps muscles (average of VM, RF, and VL).
There are some limitations to this study. First, we only evaluated the effect of paired-pulse stimulus parameters on SICI in the lower-extremity muscles. As a result, we are unable to directly compare and contrast the results of this study with those obtained from the upper-extremity. Second, we only provided five TMS pulses at each testing intensity, which may not be sufficient to obtain reliable MEP measurements (Brownstein et al., 2018b; Chang et al., 2016). However, we note that the MEP responses were primarily quantified using torque measurements, which are less variable and more reproducible than EMG responses (Darling et al., 2006; Dutt-Mazumder et al., 2018).
In summary, this study comprehensively examined the effects of conditioning stimulus intensity (CSI), test stimulus intensity (TSI), and interstimulus interval (ISI) on short interval intracortical inhibition (SICI), and tested the presence of two phases of SICI in a lower-extremity muscle group, such as the quadriceps. The results confirm the presence of two phases of SICI and show that the magnitude of SICI during active contraction of the quadriceps muscle depends not only on the strength of the conditioning and test stimulus, but also on the interval between conditioning and test stimulus. In general, 1.0 ms ISI at 70% CSI produced greatest inhibition and also required the least intensity of the test stimulus in comparison to other stimulation parameters. However, other parameters (e.g., ISI of 2.5 ms) can be used if the described interaction between the parameters are properly understood. The findings may have important implications for studies assessing SICI of the quadriceps muscle during an active contraction.
Supplementary Material
Appendix 1. Supplemental Tables for the experiment.
Appendix 2. Supplemental statistics (2-Way Linear Mixed Models and Bayesian Repeated Measures ANOVA) for the experiment.
ACKNOWLEDGMENTS
This work was partly supported by the National Institutes of Health (Grant # R21 HD092614, Grant # R01EB019834, and Pilot Grant [Grant # P2CHD086844] from National Center of Neuromodulation for Rehabilitation [NM4R]). The funders had no role in the collection, analysis and interpretation of data, and in the writing of the manuscript. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author and do not necessarily reflect the views of the funding sources. The author would like to thank Mr. Robert Saner and Ms. Aastha Dharia for their assistance in data collection and processing. The author has no financial relationships that may pose a conflict of interest.
References
- Akima H, Saito A, Watanabe K & Kouzaki M (2012). Alternate muscle activity patterns among synergists of the quadriceps femoris including the vastus intermedius during low-level sustained contraction in men. Muscle and Nerve, 46(1), 86–95. [DOI] [PubMed] [Google Scholar]
- Bashir S, Vernet M, Najib U, Perez J, Alonso-Alonso M, Knobel M, Yoo WK, Edwards D & Pascual-Leone A (2016). Enhanced motor function and its neurophysiological correlates after navigated low-frequency repetitive transcranial magnetic stimulation over the contralesional motor cortex in stroke. Restorative Neurology and Neuroscience, 34(4), 677–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bawa P & Murnaghan C (2009). Motor unit rotation in a variety of human muscles. Journal of Neurophysiology, 102(4), 2265–2272. [DOI] [PubMed] [Google Scholar]
- Benjamini Y & Hochberg Y (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal statistical society: series B (Methodological), 57(1), 289–300. [Google Scholar]
- Brownstein CG, Ansdell P, Skarabot J, Frazer A, Kidgell D, Howatson G, Goodall S & Thomas K (2018a). Motor cortical and corticospinal function differ during an isometric squat compared with isometric knee extension. Experimental Physiology. [DOI] [PubMed] [Google Scholar]
- Brownstein CG, Ansdell P, Skarabot J, Howatson G, Goodall S & Thomas K (2018b). An optimal protocol for measurement of corticospinal excitability, short intracortical inhibition and intracortical facilitation in the rectus femoris. Journal of the Neurological Sciences, 39445–56. [DOI] [PubMed] [Google Scholar]
- Cengiz B, Murase N & Rothwell JC (2013). Opposite effects of weak transcranial direct current stimulation on different phases of short interval intracortical inhibition (SICI). Experimental Brain Research, 225(3), 321–331. [DOI] [PubMed] [Google Scholar]
- Chang WH, Fried PJ, Saxena S, Jannati A, Gomes-Osman J, Kim YH & Pascual-Leone A (2016). Optimal number of pulses as outcome measures of neuronavigated transcranial magnetic stimulation. Clinical Neurophysiology, 127(8), 2892–2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen BH (2008). Three-Way ANOVA, Explaining psychological statistics. John Wiley & Sons. [Google Scholar]
- Darling WG, Wolf SL & Butler AJ (2006). Variability of motor potentials evoked by transcranial magnetic stimulation depends on muscle activation. Experimental Brain Research, 174(2), 376–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Manganelli F, Dileone M, Notturno F, Esposito M, Capasso M, Dubbioso R, Pace M, Ranieri F, Minicuci G, Santoro L & Uncini A (2012). The effects of prolonged cathodal direct current stimulation on the excitatory and inhibitory circuits of the ipsilateral and contralateral motor cortex. J Neural Transm (Vienna), 119(12), 1499–1506. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Restuccia D, Oliviero A, Profice P, Ferrara L, Insola A, Mazzone P, Tonali P & Rothwell JC (1998). Magnetic transcranial stimulation at intensities below active motor threshold activates intracortical inhibitory circuits. Experimental Brain Research, 119(2), 265–268. [DOI] [PubMed] [Google Scholar]
- Di Pino G, Pellegrino G, Assenza G, Capone F, Ferreri F, Formica D, Ranieri F, Tombini M, Ziemann U, Rothwell JC & Di Lazzaro V (2014). Modulation of brain plasticity in stroke: a novel model for neurorehabilitation. Nature Reviews: Neurology, 10(10), 597–608. [DOI] [PubMed] [Google Scholar]
- Dubbioso R, de Rosa A, Esposito M, Peluso S, Iodice R, de Michele G, Santoro L & Manganelli F (2017a). Does motor cortex plasticity depend on the type of mutation in the leucine-rich repeat kinase 2 gene? Movement Disorders, 32(6), 947–948. [DOI] [PubMed] [Google Scholar]
- Dubbioso R, Esposito M, Peluso S, Iodice R, De Michele G, Santoro L & Manganelli F (2017b). Disruption of GABA(A)-mediated intracortical inhibition in patients with chorea-acanthocytosis. Neuroscience Letters, 654107–110. [DOI] [PubMed] [Google Scholar]
- Dubbioso R, Ranucci G, Esposito M, Di Dato F, Topa A, Quarantelli M, Matarazzo M, Santoro L, Manganelli F & Iorio R (2016). Subclinical neurological involvement does not develop if Wilson’s disease is treated early. Parkinsonism & Related Disorders, 2415–19. [DOI] [PubMed] [Google Scholar]
- Dutt-Mazumder A, Brown S, Washabaugh E, Dharia A, Talati R, Vogel A, Gardi A & Krishnan C (2018). Evaluation of Motor Cortical Excitability Using Evoked Torque Responses: A New Tool With High Reliability. Neurorehabilitation and Neural Repair, 32(12), 1069. [Google Scholar]
- Fallentin N, Jorgensen K & Simonsen EB (1993). Motor unit recruitment during prolonged isometric contractions. European Journal of Applied Physiology and Occupational Physiology, 67(4), 335–341. [DOI] [PubMed] [Google Scholar]
- Fisher RJ, Nakamura Y, Bestmann S, Rothwell JC & Bostock H (2002). Two phases of intracortical inhibition revealed by transcranial magnetic threshold tracking. Experimental Brain Research, 143(2), 240–248. [DOI] [PubMed] [Google Scholar]
- Hanajima R, Furubayashi T, Iwata NK, Shiio Y, Okabe S, Kanazawa I & Ugawa Y (2003). Further evidence to support different mechanisms underlying intracortical inhibition of the motor cortex. Experimental Brain Research, 151(4), 427–434. [DOI] [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, Terao Y, Sakai K, Furubayashi T, Machii K & Kanazawa I (1998). Paired-pulse magnetic stimulation of the human motor cortex: differences among I waves. Journal of Physiology, 509 ( Pt 2)607–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter SK, McNeil CJ, Butler JE, Gandevia SC & Taylor JL (2016). Short-interval cortical inhibition and intracortical facilitation during submaximal voluntary contractions changes with fatigue. Experimental Brain Research, 234(9), 2541–2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilic TV, Meintzschel F, Cleff U, Ruge D, Kessler KR & Ziemann U (2002). Short-interval paired-pulse inhibition and facilitation of human motor cortex: the dimension of stimulus intensity. Journal of Physiology, 545(Pt 1), 153–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keel JC, Smith MJ & Wassermann EM (2001). A safety screening questionnaire for transcranial magnetic stimulation. Clinical Neurophysiology, 112(4), 720. [DOI] [PubMed] [Google Scholar]
- Kittelson AJ, Thomas AC, Kluger BM & Stevens-Lapsley JE (2014). Corticospinal and intracortical excitability of the quadriceps in patients with knee osteoarthritis. Experimental Brain Research, 232(12), 3991–3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzaki M, Shinohara M, Masani K, Kanehisa H & Fukunaga T (2002). Alternate muscle activity observed between knee extensor synergists during low-level sustained contractions. J Appl Physiol (1985), 93(2), 675–684. [DOI] [PubMed] [Google Scholar]
- Krishnan C (2015). Are practice trials required for hop tests? Gait and Posture, 41(4), 960–963. [DOI] [PubMed] [Google Scholar]
- Krishnan C & Dhaher Y (2012). Corticospinal responses of quadriceps are abnormally coupled with hip adductors in chronic stroke survivors. Experimental Neurology, 233(1), 400–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan C & Theuerkauf P (2015). Effect of knee angle on quadriceps strength and activation after anterior cruciate ligament reconstruction. J Appl Physiol (1985), 119(3), 223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P & Marsden CD (1993). Corticocortical inhibition in human motor cortex. Journal of Physiology, 471501–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luc-Harkey BA, Harkey MS, Pamukoff DN, Kim RH, Royal TK, Blackburn JT, Spang JT & Pietrosimone B (2017). Greater intracortical inhibition associates with lower quadriceps voluntary activation in individuals with ACL reconstruction. Experimental Brain Research, 235(4), 1129–1137. [DOI] [PubMed] [Google Scholar]
- Ni Z, Bahl N, Gunraj CA, Mazzella F & Chen R (2013). Increased motor cortical facilitation and decreased inhibition in Parkinson disease. Neurology, 80(19), 1746–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Z & Chen R (2008). Short-interval intracortical inhibition: a complex measure. Clinical Neurophysiology, 119(10), 2175–2176. [DOI] [PubMed] [Google Scholar]
- Ortu E, Deriu F, Suppa A, Tolu E & Rothwell JC (2008). Effects of volitional contraction on intracortical inhibition and facilitation in the human motor cortex. Journal of Physiology, 586(21), 5147–5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peurala SH, Muller-Dahlhaus JF, Arai N & Ziemann U (2008). Interference of short-interval intracortical inhibition (SICI) and short-interval intracortical facilitation (SICF). Clinical Neurophysiology, 119(10), 2291–2297. [DOI] [PubMed] [Google Scholar]
- Ranganathan R, Krishnan C, Dhaher YY & Rymer WZ (2016). Learning new gait patterns: Exploratory muscle activity during motor learning is not predicted by motor modules. Journal of Biomechanics, 49(5), 718–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridding MC, Taylor JL & Rothwell JC (1995). The effect of voluntary contraction on cortico-cortical inhibition in human motor cortex. Journal of Physiology, 487 ( Pt 2)541–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodseth J, Washabaugh EP & Krishnan C (2017). A novel low-cost approach for navigated transcranial magnetic stimulation. Restorative Neurology and Neuroscience, 35(6), 601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roshan L, Paradiso GO & Chen R (2003). Two phases of short-interval intracortical inhibition. Experimental Brain Research, 151(3), 330–337. [DOI] [PubMed] [Google Scholar]
- Salkind NJ (2010). Simple Main Effects, Encyclopedia of research design. Sage. [Google Scholar]
- Schambra HM, Martinez-Hernandez IE, Slane KJ, Boehme AK, Marshall RS & Lazar RM (2016). The neurophysiological effects of single-dose theophylline in patients with chronic stroke: A double-blind, placebo-controlled, randomized cross-over study. Restorative Neurology and Neuroscience, 34(5), 799–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellke T, Bayarri M & Berger JO (2001). Calibration of ρ values for testing precise null hypotheses. The American Statistician, 55(1), 62–71. [Google Scholar]
- Sidhu SK, Cresswell AG & Carroll TJ (2013). Short-interval intracortical inhibition in knee extensors during locomotor cycling. Acta Physiologica (Oxford, England), 207(1), 194–201. [DOI] [PubMed] [Google Scholar]
- Stevens-Lapsley JE, Thomas AC, Hedgecock JB & Kluger BM (2013). Corticospinal and intracortical excitability of the quadriceps in active older and younger healthy adults. Archives of Gerontology and Geriatrics, 56(1), 279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokic DS, McKay WB, Scott L, Sherwood AM & Dimitrijevic MR (1997). Intracortical inhibition of lower limb motor-evoked potentials after paired transcranial magnetic stimulation. Experimental Brain Research, 117(3), 437–443. [DOI] [PubMed] [Google Scholar]
- Tan AQ & Dhaher YY (2017). Contralesional Hemisphere Regulation of Transcranial Magnetic Stimulation-Induced Kinetic Coupling in the Poststroke Lower Limb. Frontiers in Neurology, 8373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terao Y, Ugawa Y, Hanajima R, Machii K, Furubayashi T, Mochizuki H, Enomoto H, Shiio Y, Uesugi H, Iwata NK & Kanazawa I (2000). Predominant activation of I1-waves from the leg motor area by transcranial magnetic stimulation. Brain Research, 859(1), 137–146. [DOI] [PubMed] [Google Scholar]
- Thomas AC, Pietrosimone BG & Bayer CJ (2016). Agreement Between Investigators Using Paired-Pulse Transcranial Magnetic Stimulation to Assess Quadriceps Intracortical Excitability. J Sport Rehabil, 25(4). [DOI] [PubMed] [Google Scholar]
- Vovk VG (1993). A logic of probability, with application to the foundations of statistics. Journal of the Royal statistical society: series B (Methodological), 55(2), 317–341. [Google Scholar]
- Vucic S, Cheah BC, Krishnan AV, Burke D & Kiernan MC (2009). The effects of alterations in conditioning stimulus intensity on short interval intracortical inhibition. Brain Research, 127339–47. [DOI] [PubMed] [Google Scholar]
- Wagenmakers EJ, Love J, Marsman M, Jamil T, Ly A, Verhagen J, Selker R, Gronau QF, Dropmann D, Boutin B, Meerhoff F, Knight P, Raj A, van Kesteren EJ, van Doorn J, Smira M, Epskamp S, Etz A, Matzke D, de Jong T, van den Bergh D, Sarafoglou A, Steingroever H, Derks K, Rouder JN & Morey RD (2018a). Bayesian inference for psychology. Part II: Example applications with JASP. Psychon Bull Rev, 25(1), 58–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenmakers EJ, Marsman M, Jamil T, Ly A, Verhagen J, Love J, Selker R, Gronau QF, Smira M, Epskamp S, Matzke D, Rouder JN & Morey RD (2018b). Bayesian inference for psychology. Part I: Theoretical advantages and practical ramifications. Psychon Bull Rev, 25(1), 35–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SH, Pearce A, Bennell KL, Peitrosimone B & Bryant AL (2016). Quadriceps cortical adaptations in individuals with an anterior cruciate ligament injury. Knee, 23(4), 582–587. [DOI] [PubMed] [Google Scholar]
- Washabaugh EP, Claflin ES, Gillespie RB & Krishnan C (2016a). A Novel Application of Eddy Current Braking for Functional Strength Training During Gait. Annals of Biomedical Engineering, 44(9), 2760–2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washabaugh EP & Krishnan C (2018). A wearable resistive robot facilitates locomotor adaptations during gait. Restorative Neurology and Neuroscience, 36(2), 215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washabaugh EP, Santos L, Claflin ES & Krishnan C (2016b). Low-level intermittent quadriceps activity during transcranial direct current stimulation facilitates knee extensor force-generating capacity. Neuroscience, 32993–97. [DOI] [PubMed] [Google Scholar]
- Washabaugh EP, Treadway E, Gillespie RB, Remy CD & Krishnan C (2018). Self-powered robots to reduce motor slacking during upper-extremity rehabilitation: a proof of concept study. Restorative Neurology and Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarzycki R, Morton SM, Charalambous CC, Marmon A & Snyder-Mackler L (2018). Corticospinal and intracortical excitability differ between athletes early after ACLR and matched controls. Journal of Orthopaedic Research. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Lonnecker S, Steinhoff BJ & Paulus W (1996a). The effect of lorazepam on the motor cortical excitability in man. Experimental Brain Research, 109(1), 127–135. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Lonnecker S, Steinhoff BJ & Paulus W (1996b). Effects of antiepileptic drugs on motor cortex excitability in humans: a transcranial magnetic stimulation study. Annals of Neurology, 40(3), 367–378. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1. Supplemental Tables for the experiment.
Appendix 2. Supplemental statistics (2-Way Linear Mixed Models and Bayesian Repeated Measures ANOVA) for the experiment.