Abstract
Overactive bladder (OAB) patients suffer from a frequent urge to urinate, which can lead to a poor quality of life. Current neurostimulation therapy uses open-loop electrical stimulation to alleviate symptoms. Continuous stimulation facilitates habituation of neural pathways and consumes battery power. Sensory feedback-based closed-loop stimulation may offer greater clinical benefit by driving bladder relaxation only when bladder contractions are detected, leading to increased bladder capacity. Effective delivery of such sensory feedback, particularly in real-time, is necessary to accomplish this goal. We implemented a Kalman filter-based model to estimate bladder pressure in real-time using unsorted neural recordings from sacral-level dorsal root ganglia, achieving a 0.88 ± 0.16 correlation coefficient fit across thirty-six normal and simulated OAB bladder fills in five experiments. We also demonstrated closed-loop neuromodulation using the estimated pressure to trigger pudendal nerve stimulation, which increased bladder capacity by 40% in two trials. An offline analysis indicated that unsorted neural signals had a similar stability over time as compared to sorted single units, which would require a higher computational load. We believe this study demonstrates the utility of decoding bladder pressure from neural activity for closed-loop control; however, real-time validation during behavioral studies is necessary prior to clinical translation.
Index Terms–: bladder, Kalman filter, dorsal root ganglia, neuromodulation, closed-loop
I. Introduction
Overactive bladder (OAB) is defined as urinary urgency, with or without incontinence, typically accompanied by frequency and nocturia [1]. The cause of overactive bladder can be neurogenic (e.g. spinal cord injury, traumatic brain injury, multiple sclerosis, stroke), non-neurogenic (e.g. aging, multiple pregnancies) or nonspecific. OAB affects millions of people worldwide, leading to a variety of side effects such as poor sleep, anxiety, and depression [2].
Sacral neuromodulation (SNM), in which a stimulation lead is placed near the S3 sacral root, is a standard clinical treatment after conservative approaches fail [3]. SNM is applied constantly to reduce the effects of OAB. However, continuous stimulation can facilitate habituation of neural pathways [4] and consume battery power [5]. Sensory feedback-based, or closed-loop stimulation may offer greater clinical benefit by driving bladder function only when necessary, leading to increased bladder capacity and voiding efficiency [5], [6].
Several studies have demonstrated the possibility of detecting bladder pressure changes, or bladder volume increases by recording at different nerve sites with offline analyses of recorded data [7]–[11]. These offline validation studies applied various parameters combinations to a detecting or decoding algorithm and selected parameters that generated the lowest error. However, final parameters were not tested in new sets of data in real-time to rule out overfitting. A few studies have applied optimized parameters to nerve recordings [5], [12] to detect bladder volume or contractions in real-time, though pressure was not explored in this manner. For OAB, we hypothesize that pressure feedback will be more valuable because it may directly reflect the level of urgency [13]. In this study, we specifically examine real-time estimation of bladder pressure towards closed-loop neuromodulation.
Bladder-related neural signals can be observed at potential neuromodulation sites such as the pudendal nerve [5], pelvic nerve [14] and sacral dorsal root ganglia (DRG) [10], [15], making closed-loop control possible without an additional surgical procedure to implant a bladder pressure sensor. Among these recording sites, sacral-level DRG uniquely contain afferent-only signals from the detrusor muscle and urethra, via proximal pelvic and pudendal nerve fibers, while also containing pudendal sensory pathways that can drive spinal circuits modulating bladder function [16], [17]. Our long-term goal is to take advantage of this sensory nerve convergence at sacral DRG and develop a single-site neural interface for both monitoring and controlling the bladder state.
In this study, we used sacral-level DRG as a recording site to estimate bladder pressure and the onset of bladder contractions in real-time for both normal and simulated OAB conditions, through the infusion of acetic acid irritant. Although acetic acid-induced bladder overactivity does not fully imitate the urgency component of clinical OAB, it is commonly used in preclinical studies to simulate an increase in spontaneous contractions and a decrease in bladder capacity [18]. In two experiments, we used the estimated pressure to initiate pudendal nerve stimulation upon detection of bladder contractions, as a demonstration of closed-loop relaxation of the bladder. This is the first study to test a bladder pressure decoding algorithm from neural signals in real-time, as well as apply it to a demonstration of closed-loop bladder control.
II. Methods
A. Animals
All procedures were approved by the University of Michigan Institutional Animal Care and Use Committee, in accordance with the National Institute of Health’s guidelines for the care and use of laboratory animals. Five spinal-intact adult, domestic, short-hair cats (0.97 ± 0.20 years old, 4.48 ± 0.83kg, 2 female, 3 male, Liberty Research, Inc., Waverly, NY) were used in this study (designated as experiments 1–5). Cats were used due to their high relevance to human physiology and their long history of study in bladder neurophysiology [19]. Prior to use, animals were free-range housed with up to 3 other cats in a 413 ft2 room with controlled temperature (19–21 °C) and relative humidity (35–60%), food and water available ad lib, and a 12-hour light/dark cycle. Animals received enrichment via staff interaction and toys.
B. Surgical Procedure
As in prior studies [20], animals were induced for anesthesia with a mixture of ketamine (6.6 mg/kg), butorphanol (0.66 mg/kg), and dexmedetomidine (0.02–0.03 mg/kg) administered intramuscularly. Animals were intubated and subsequently maintained on isoflurane anesthesia (0.5–4%) during surgical procedures. Respiratory rate, heart rate, end-tidal CO2, O2 perfusion, temperature, and intra-arterial blood pressure were monitored continuously using a Surgivet vitals monitor (Smiths Medical, Dublin, OH). Fluids (1:1 ratio of lactated Ringers solution and 5% dextrose) were infused intravenously via the cephalic vein at a rate of 5–10 mL/kg/hr (increased up to 30 mL/kg/hr during surgery as needed). Catheters were inserted into the bladder at the bladder dome via a laparotomy (experiment 1–2, both female) or via the urethra (experiment 35, all male, catheter diameter 3.5–5 Fr) for intravesical fluid infusion and pressure monitoring. The urethra was not ligated.
A midline dorsal incision was made to expose the L7 to S3 vertebrae and a partial laminectomy was performed to access sacral DRG. Iridium oxide microelectrode arrays with shank length of 1.0 mm and inter-shank spacing of 0.4 mm (4×8 configuration; Blackrock Microsystems, Salt Lake City, UT) were implanted bilaterally into S1 DRG using a pneumatic inserter (Blackrock Microsystems). Although S2 DRG contain more bladder neurons than S1 DRG in cats [21], the larger size of S1 DRG aligned better with the microelectrode array footprint and our prior work obtained sufficient bladder signals for decoding from S1 DRG [20]. Array reference wires were placed near the spinal cord and ground wires were attached to a stainless steel needle inserted below the skin (lateral and caudal to the laminectomy incision site). At the conclusion of surgical procedures (8.3±0.7 hr surgery length, plus 0.1–0.5 hr for array insertion), prior to experimental testing, animals were transitioned to intravenous alpha-chloralose (C0128, Sigma Aldrich; 70 mg/kg induction; 20 mg/kg maintenance). As this transition was at least six hours after induction, we expect that there were no residual effects on bladder function due to the induction dosing of ketamine, butorphanol, or dexmedetomidine. Analgesia was augmented with 0.01 mg/kg buprenorphine every 8–12 hours subcutaneously.
C. Experimental set-up and data collection
Neural data was sampled at 30 kHz and band-passed filtered (250 Hz to 7.5 kHz) using a Grapevine Neural Interface Processer and Trellis recording system (Ripple, Salt Lake City, UT). Threshold crossings were detected by setting a dual threshold at 3–5.5 times the root-mean-squared value of the signal. Bladder pressure was monitored with a pressure transducer (DPT-100, Utah Medical Products, Midvale, UT) and transducer amplifier (TBM4M, World Precision Instruments, Sarasota, FL). The bladder pressure signal was sampled by the Grapevine system at 1 kHz, and all data was streamed to an online decoder developed in MATLAB Graphic User Interface (Mathworks, Natick, MA) through a Trellis MATLAB software interface. During each bladder infusion trial, 0.9% saline or 0.5% acetic acid (R13032, Fisher Scientific, Hampton, NH) in saline was infused into the bladder with a syringe pump (AS50 Infusion Pump, Baxter International, Deerfield, IL and Model NE-1000, New Era Pump Systems, Inc., Farmingdale, NY). Acetic acid is an irritant that increases bladder overactivity and was infused to simulate an OAB condition [18], [22].
Each experiment had two or more bladder fill sequences, in which bladder pressure and neural recordings were collected during infusion at 2 mL/min. Prior to an infusion, the bladder was emptied by withdrawing from the catheter. Fluid infusion was stopped after the bladder was full (leakage observed or a sustained bladder contraction was observed for over 10 seconds). In experiment 3, some trials were stopped early because the heart rate increased at least 40 bpm above baseline. Because of this, summary statistics on the bladder capacity was not included below for experiment 3. The bladder capacity was estimated as the volume withdrawn from the bladder at the end of the trial. The first infusion in each sequence was used to train a decoding model, and the second fill was used to test the model in real-time. Depending on experimental progress, that second fill may have then been used to train a new model as the first fill of a new sequence. At least 3 saline-only fills were conducted prior to acetic acid trials. In general, training and testing fills used the same condition (saline-normal or acetic acid-simulated OAB) but in a few cases saline fills were used to train a model that was tested for a simulated OAB fill.
D. Real-time Decoding Algorithm
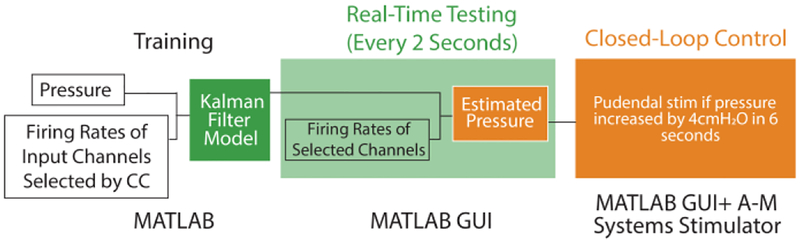
The real-time closed-loop control system was developed as a MATLAB graphic user interface (GUI). A previously described Kalman filter modeling algorithm [10] was trained offline then tested in real-time, using the following parameters. For offline training, after a full bladder fill cycle was completed, the firing rates of threshold crossings on all channels were calculated at a 2-second interval using a boxcar smoothing method, as a balance between computational load and estimation accuracy [10]. The firing rates were then normalized by dividing each by the firing rate range of the first 30 seconds of each trial. The bladder pressure was also averaged every 2 seconds. The correlation coefficient (CC) between each channel firing rate and the bladder pressure was calculated. While 64 total microelectrodes were implanted for each experiment, only channels highly correlated with bladder pressure (CC > 0.7) during a training fill sequence were used. If no channel met this criterion, the threshold was iteratively lowered by 0.1 until sufficient channels were available to make a robust estimate. Following channel selection, the Kalman algorithm was used to model bladder pressure as a weighted average of the previous bladder pressure and firing rates of the input channels. During testing, the threshold crossing times were streamed to the GUI in real-time (1.1 ± 1.4 ms calculation time). Firing rates were calculated every 2 seconds and fed into the model to obtain an estimate of the bladder pressure for comparison to the measured pressure. Fig. 1 shows the experimental workflow.
Fig. 1.
Flow diagram of real-time decode training and testing, and closed-loop control.
E. Real-time Closed-loop Control
In experiments 3 and 4, a bipolar nerve cuff (2.0 mm inner diameter Silastic 508–009 tubing; 0.4 mm stainless steel Cooner wire contacts) was placed on the left pudendal nerve after a postero-lateral gluteal incision and access. During one testing trial in experiment 3, electrical stimulation (Model 2100 Isolated Pulse Stimulator, A-M Systems, Sequim, WA) was manually turned on for 23.7 ± 3.6 seconds after the onset of each of five contractions, visually identified from the real-time estimated pressure. In experiment 4, this process was automated in MATLAB to trigger stimulation when the estimated pressure monotonically increased at least 4 cm H2O in 6 seconds. The six-second interval was selected as three consecutive pressure estimates, based on the 2-second real-time pressure updates. In each stimulation interval, 10 Hz stimulation (balanced biphasic, 200 μs pulse width) was used to drive bladder relaxation, based on a previous study that reported its efficacy over other frequencies in a similar experimental set-up [23]. Neither the closed-loop timing nor stimulation paradigm were optimized for this study, but were selected to demonstrate the potential utility in this application. A cross-channel invalidation and blanking method was used to reduce stimulation artifacts in DRG recordings. In the artifact blanking method, a 2 ms sliding time window was applied in real-time to the input spike times. If a spike was present in the same 2 ms window for over 50% of the channels, then that spike and any occurring in the next 40 ms were removed. This method was empirically determined from offline analysis to remove the effect of stimulation artifacts on decoding performance.
F. Euthanasia
After completion of all testing, animals were euthanized with 2–3 mL of intravenous or intracardiac sodium pentobarbital (390 mg/mL) while under deep isoflurane anesthesia.
G. Statistical Analyses
Two primary metrics were used to evaluate the accuracy of bladder decoding. The correlation coefficient (R, equation 1) and normalized root-mean-squared error (NRMSE, equation 2) between the measured (P) and estimated pressure () were calculated for each real-time testing trial. The correlation plays an important role because it reflects the level of similarity in shape between the estimated pressure and the measured pressure. A high correlation coefficient indicates that the basic features (reflex contraction, sustained contraction, relaxation etc.) in the cystometry curve were captured, and that it is possible to extract events with machine learning algorithms. Although R and CC use the same underlying correlation coefficient calculation, we use different variables to distinguish the different types of data sets being compared (R compares estimated to actual bladder pressure, and CC compares neural firing rates to the actual bladder pressure).
| (1) |
| (2) |
To verify that acetic acid was producing a simulated OAB state, an unpaired t-test was used to determine if bladder capacity was reduced significantly from saline-only fills to acetic-acid fills. An unpaired t-test was also used to determine if acetic acid caused significantly earlier onset of non-voiding contractions. To analyze the stability of threshold crossings within each experiment, a paired t-test was used to quantify any changes between firing rates and bladder pressure from training to testing trials and from saline to acetic acid trials. For all tests, a significance level of 0.05 was used. When appropriate, values are given as mean ± standard deviation.
III. Results
Real-time decoding was performed in all 5 experiments, with 20 saline and 15 acetic acid testing trials in total. We demonstrated closed-loop control in experiments 3 and 4.
A. Normal Bladder and Simulated OAB Models
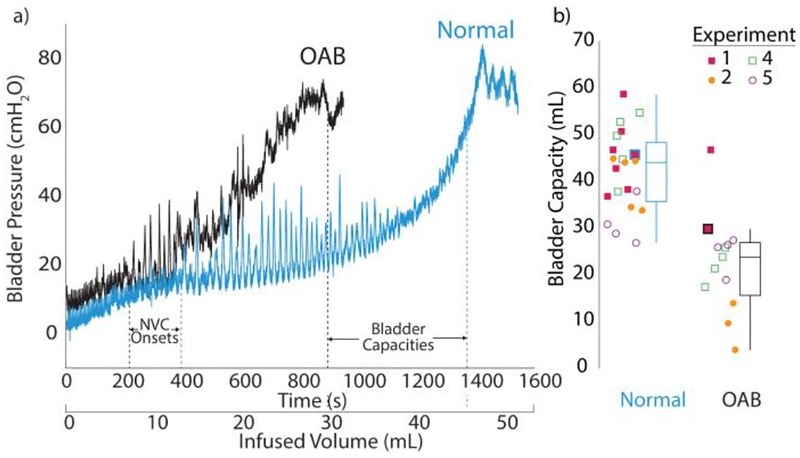
The simulated OAB model produced significantly lower bladder capacities (23.4 ± 12.1 mL, n = 13, p < 0.0001; Fig. 2) than normal saline bladder capacities (44.5 ± 10.1 mL, n = 21). In experiments 1 and 4, an earlier onset of non-voiding contractions during acetic acid infusion was observed, though this finding was not significant. The infused volumes until the first non-voiding contraction were 12.1 ± 2.7 mL (saline, n = 7) and 7.8 ± 1.1 mL (acetic acid, n = 2) for experiment 1 (example in Fig. 2), and 17.2 ± 2.7 mL (saline, n = 5) and 13.7 ± 1.8 mL (acetic acid, n = 4) for experiment 4. No other experiments had consistently identifiable non-voiding contractions across all trials. Experiments with urethra catheters had significantly larger bladder contractions (p < 0.001; 55.6 ± 6.7 cm H2O, n = 9 for Experiment 4; 54.8 ± 8.3 cm H2O, n = 8 for Experiment 5; not measured in Experiment 3) than those with supra-pubic catheters (20.7 ± 3.1 cm H2O, n = 9; 20.1 ± 5.0 cm H2O, n = 8 for Experiments 1 and 2), likely due to the catheter partially occluding the urethra. These later experiments also used larger males (5.7, 5.1 kg) than the females in supra-pubic experiments (3.4, 4.0 kg), which may have been a factor in the larger pressures. Bladder volumes for either saline or acetic acid infusion trials were not different between supra-pubic and urethra catheter experiments (Fig. 2).
Fig. 2.
a) Example bladder fills sequences for saline (in blue) and acetic acid (in black) infusions from experiment 1. b) Summary of bladder capacities in experiment 1, 2, 4 and 5 in box plots. Simulated OAB capacities (n = 13) were significantly lower than normal bladder capacities (n = 21; p < 0.0001). Icons for fills in (a) indicated with thick outline in blue or black.
B. Real-time Decoding
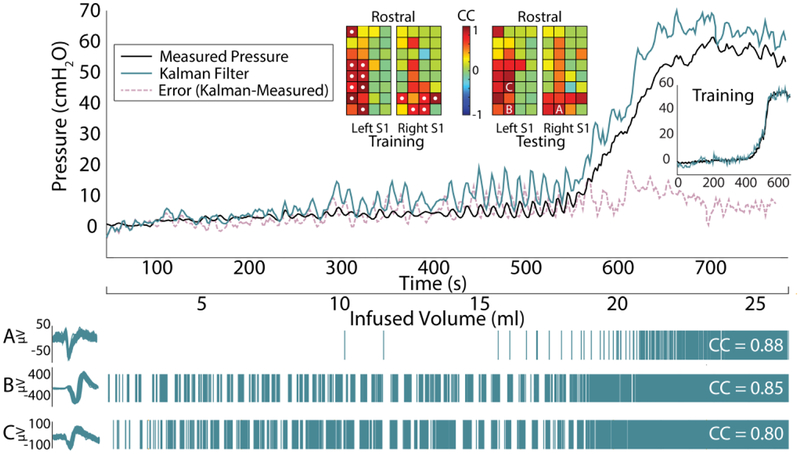
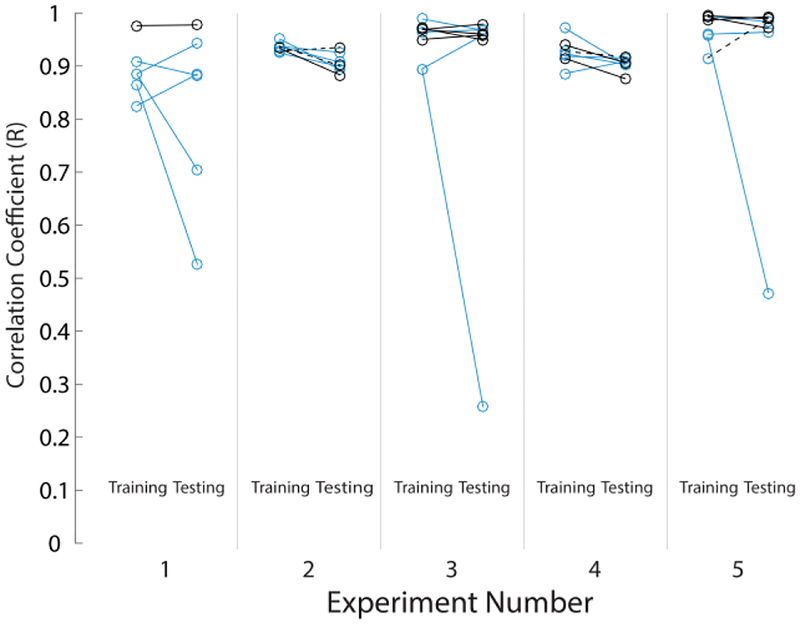
Thirty-five real-time decoding testing trials were performed, with a minimum of six testing trials per cat (Table I). The first bladder fill for an experiment and bladder condition was used to train a model and a following bladder fill was used for real-time decode testing of that model (for both stimulation and nonstimulation trials). That second bladder fill then became a training data set for a new model, in an alternating manner up to the total number of testing bladder fills per experiment and condition given in Table I. On average, 5.6 ± 3.7 DRG channels were used in each testing trial (range 1–14). Table I lists the mean channel counts used in each experiment. Twenty-two of 35 trials (62.9%) used the maximum 0.7 CC threshold for channel selection, with an average CC threshold of 0.58 ± 0.18 (range: 0.2–0.7) used across all trials. Table II summarizes the NRMSE and R metrics for the real-time algorithm performance. On average, simulated OAB trials had a significantly higher correlation (p = 0.026) and non-significantly lower NRMSE (p = 0.445) than normal saline trials. Fig. 3 shows an example real-time decoding trial. Fig. 4 shows the individual R values for all training and testing trials. There was no trend in R or NRMSE across testing trials within each individual experiment. Four simulated OAB testing trials across the experiments used models created from normal saline training fills. The performance metrics for these four trials (R: 0.94 ± 0.04; NRMSE: 0.30 ± 0.26) were not different from the simulated OAB testing trials trained on acetic acid fills (n = 11; R: 0.95 ± 0.04; NRMSE: 0.20 ± 0.14).
Table I.
Trial Summary
| Experiment | Saline | Acetic Acid | Closed-Loop | Channels per Model |
|---|---|---|---|---|
| 1 | 5 | 1 | 0 | 5.8 ± 2.1 |
| 2 | 4 | 3 | 0 | 2.1 ± 0.4 |
| 3 | 4 | 4 | 1 (manual) | 6.8 ± 3.1 |
| 4 | 4 | 3 | 2 | 4.4 ± 3.9 |
| 5 | 3 | 4 | 0 | 8.6 ± 4.6 |
| Total | 20 | 15 | 3 | 5.6 ± 3.7 |
Table II.
Real-Time Decoding Summary Statistics
| R | NRMSE | |
|---|---|---|
| Normal (saline) (n =20) | 0.84 ± 0.19 | 0.28 ± 0.13 |
| OAB (acetic acid) (n = 15) | 0.95 ± 0.04 | 0.23 ± 0.18 |
| Normal & OAB (n = 35) | 0.88 ± 0.16 | 0.26 ± 0.21 |
| Automated Closed-loop (n=2) | 0.79 ± 0.05 | 0.19 ± 0.02 |
Fig. 3.
Example real-time OAB decoding trial from Experiment 5. NRMSE = 0.098, R = 0.9895. Fourteen channels were used for decoding. At middle top, the correlation coefficient mapping of threshold crossings and bladder pressure are shown for all microelectrodes, with channels used for decoding indicated by a white dot. At bottom are raster plots for units from three example channels.
Fig. 4.
Performance summary of correlation coefficients (R) for all trials. Saline trials are denoted by blue icons; acetic acid OAB trials by black icons. Transitional trials (training with saline and testing with acetic acid) are denoted by black dashed lines. Table II contains summary statistics across all trials.
We performed three post-hoc analyses to understand the sources of prediction error and to inform improvements to the algorithm. First we tested the stability of unsorted neural activity between paired training and testing trials. In two experiments, there was a significant change in the correlation coefficients (CC) according to a paired t-test (Table III). We also modeled the relationship between pressure and singlechannel threshold crossing firing rates with a linear regression model and tested whether the slopes and intercepts changed between training and testing trials. In two experiments, there was a statistically significant change in slope, and in four experiments, there was a significant change in intercept (Table III). Each experiment had a significant change in at least one parameter but only one experiment had a significant change in all three. Table III also gives the mean ratio between each parameter in the testing data set to the training data set value.
Table III.
Mean Test:Train ratio and (P-value) summary for tests of unsorted neural activity between training and testing trials
| Exp. | CC | Slope | Intercept |
|---|---|---|---|
| 1 | 0.97 (0.76) | 0.63 (0.01) | 1.06 (0.73) |
| 2 | 0.90 (0.15) | 1.03 (0.81) | 2.55 (<0.001) |
| 3 | 0.92 (0.01) | 1.00 (0.96) | 2.43 (<0.001) |
| 4 | 0.51 (<0.001) | 0.33 (0.002) | 3.13 (0.006) |
| 5 | 0.93 (0.18) | 0.99 (0.86) | 1.23 (<0.001) |
(p < 0.05 highlighted with bold text)
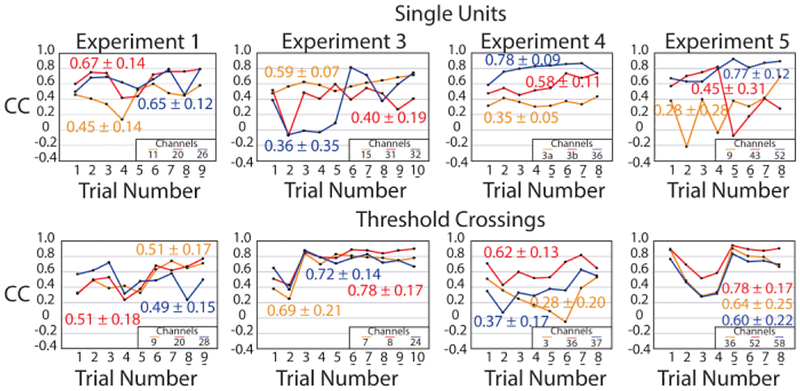
Next, we investigated if changes in channel firing-rate correlation coefficients resulted from changes in single-unit activity. An expert spike sorter manually sorted single units (Offline Sorter, Plexon, Dallas, TX) that responded to bladder filling in each trial. Thirty-six sortable bladder single units were identified across the five experiments (range: 1–15 per experiment), fourteen of which occurred in all trials for their respective experiment (no sorted units were persistent in experiment 2). Fig. 5 shows CC across trials for twelve of these persistent units. Fig. 5 also shows the variation in CC for unsorted channels that have the highest average CC in the same experiment. The averages of the standard deviation in CC for these single units and the unsorted threshold crossings were not different (0.16 and 0.17, respectively; p = 0.65), suggesting that variations in channel firing rate coefficients were related to changes in underlying single unit activity.
Fig. 5.
Sorted single units (top) and highly-correlated unsorted channels (bottom) had similar variability across each experiment. Mean (± standard deviation) CC is given next to each trace. An underlined trial number indicates acetic acid infusion trials.
Because acetic acid trials had both lower NRMSE and higher R (Table II, Fig. 4), our final post-hoc analysis investigated the effect of the simulated OAB model on single-unit firing rate to bladder pressure correlation. We quantified the behavior of single-unit neurons and threshold crossings from saline to acetic acid trials. Out of twelve single-unit neurons that we analyzed, ten neurons had an increased sensitivity to bladder pressure during acetic acid trials (average change in CC: +0.10 ± 0.25; range: −0.51 – +0.58), though the overall mean CC was not different between the saline and acetic acid trials (saline: 0.48 ± 0.22; acetic acid: 0.59 ± 0.20; p = 0.25). Out of twelve unsorted channel multi-units, eleven had an increased sensitivity to bladder pressure during acetic acid trials (average change in CC change per channel: +0.19 ± 0.14; range: −0.17 – +0.30), and the overall mean CC was significantly higher (saline: 0.48 ± 0.13; acetic acid: 0.67 ± 0.19; p = 0.009).
C. Closed-loop Control
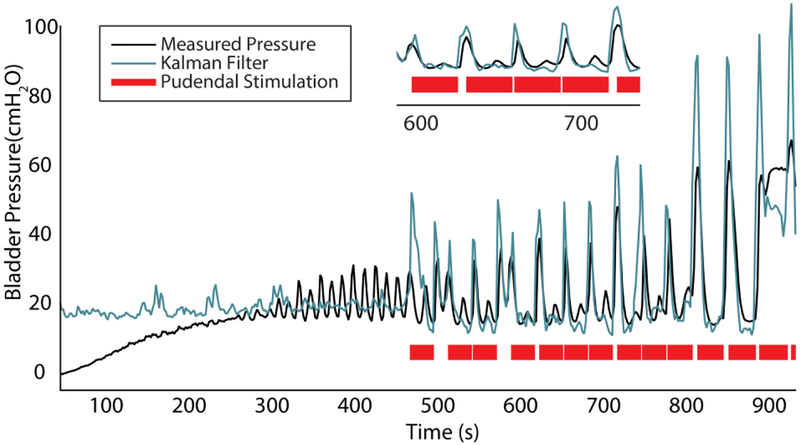
One decoding trial in experiment 3 and two trials in experiment 4 were combined with manual or automated closed-loop pudendal stimulation (Table I). In experiment 3, manualdriven closed-loop stimulation successfully suppressed bladder contractions during an acetic acid trial. However, stimulation produced artifacts in DRG recordings, which led to large decoding errors during stimulation. In experiment 4, the real-time algorithm was updated to reject stimulation artifacts. For two closed-loop trials, the NRMSE (0.17, 0.21) and R (0.84, 0.74) were similar to non-closed-loop trials. We also successfully demonstrated that bladder contractions can be automatically inhibited (Fig. 6). The maximum bladder volume increased from 22.3 ± 3.2 mL (n = 4; range: 17–26 mL) without stimulation to 31.3 ± 1.3 mL (n = 2; range 30–32.5 mL) with closed-loop stimulation, a 40.4% increase in bladder capacity. Across the two automated closed-loop trials, 33 stimulation epochs preceded a drop in actual pressure with an average delay of 0.64 +/− 0.70 s while 11 epochs followed a decrease in actual pressure by an average of 1.17 +/− 0.34 s. After stimulation was terminated, the bladder pressure resumed non-voiding contractions until eventually leakage occurred (Fig. 6). In these closed-loop trials, stimulation was turned on 114.1 ± 14.1 ms after the rising-were not performed in experiment 5 due to a lack of an inhibitory response to pudendal nerve stimulation at 5 Hz and 10 Hz.
Fig. 6.
Real-time closed-loop control of bladder relaxation with pudendal nerve stimulation (R = 0.74). A zoomed-in view of part of the trial is shown at top. Pudendal stimulation was applied for 30 seconds when there was an increase in decoded pressure of 4 cm H2O in 6 seconds. The stimulation effect was most pressure condition was met. Closed-loop trials obvious for large non-voiding contractions.
IV. Discussion
To our knowledge, this is the first study to decode bladder pressure from neural signals in real-time. This development is necessary towards the implementation of an effective closed-loop neuromodulation algorithm. In prior studies decoding bladder pressure from neural signals [7]–[10], only offline validation was performed. In this study, we built upon a previously developed model [10], and showed that it can be applied during active recording from electrodes. Additionally, we demonstrated that the level of accuracy in these new data sets is sufficient to provide closed-loop feedback.
We demonstrated the feasibility of decoding bladder pressure from DRG recordings in both healthy and simulated OAB conditions with high performance (Fig. 3, 4; Table II). As we have shown previously, there is a hysteretic relationship between bladder neuron firing rates and bladder pressure during contraction and relaxation cycles [20]. The real-time Kalman filter successfully modelled this relationship by combining previous estimated bladder states with current firing rate inputs using a weighted average method. During post hoc analyses, we quantified the stability of sensory neurons during the experiments, observing variability across neural signals (Table III) that was similar between unsorted channels and sorted single units (Fig. 5). We also demonstrated how this method can be incorporated into real-time closed-loop control of bladder relaxation (Fig. 6).
The average correlation coefficients for saline trials and acetic acid trials for real-time estimation (Table II) are higher than two previous offline studies where correlation coefficients were reported (0.81 in Ross et al. and ~0.82 in Im et al.) [9], [10]. The higher bladder decoding correlations in our study suggest that our Kalman model provides an improved approach over prior studies, particularly in tracking relative changes in bladder pressure. Other offline decoding studies reported NRMSE (or RMSE, which can be translated to NRMSE) ranging from 0.06 to 0.17 [7], [8], [10], [11]. Our study had a higher NRMSE, but unlike offline studies there was no room for parameter tuning, which can yield more accurate results. Lumbar-level DRG have been used to decode limb state with similar performance offline (NRMSE of 0.04–0.22, R of 0.120.88) [24]. For an ultimate closed-loop application, the high correlation coefficients are a more critical parameter than NRMSE as they demonstrate our ability to detect relative changes in bladder pressure that can be used to trigger stimulation upon the start of a contraction. Also, our results suggest that an irritant like acetic acid is correlated with a general increase in the sensitivity of both threshold crossings and single units, leading to an improved decode. This increased sensitivity could be due to greater activation of bladder neurons by the irritant or a secondary effect of a faster rise in the bladder pressure due to an effectively smaller bladder. Simulated OAB trials were able to use a saline-fill training model successfully, suggesting there was consistency in neural activity across at least those four bladder fill pairs.
For the conditions used in our study, our results support the use of unsorted channel activity to create robust bladder decoding models. The offline analyses of our data indicated that channel threshold crossings are as stable as single units, in terms of correlation coefficient with bladder pressure (Fig. 5). Also, systems that use threshold crossings consume less computational power than those that use single units, which can be challenging to extract with high accuracy [25]. However, this study was performed under anesthesia and only bladder sensory neurons were actively driven during bladder filling, thereby limiting non-relevant signals within the unsorted channel recordings. In an ultimate clinical use, other sensory pathways will also be active and may generate confounding signals. Studies using extraneural cuff or wire electrodes to detect bladder activity[5], [8], [11], [14] may have challenges ignoring non-bladder signals [26]. As we observed in this study, previous work has shown that bladder activity can be differentiated from non-bladder activity with intraneural microelectrodes at DRG [15], [27], [28], which we expect will increase decoding tolerance to non-bladder signals. It may be necessary to sort neural activity to obtain a robust algorithm for differentiating bladder and non-bladder activity.
Among the five experiments, the shift in baseline firing rates was the most common cause of an error in absolute pressure estimation. This is represented by the significant change in the intercept of a linear pressure-firing rate relationship from training to testing trials in four of the five experiments (Table III), some of which had a large increase in the ratio between testing and training intercepts. One example of this effect can be seen in the decoded pressure offset at the start of Fig. 6. In the majority of experiments, the correlation coefficient and slope between threshold crossing firing rates and bladder pressure remained stable (note ratios near 1.0 in Table III), which made it possible to decode relative pressure increases and decreases. The instability could be a result of the trauma from array insertion or a slight settling in of the array during the experiment. With durable recording electrodes, these sensory neuron recordings are expected to stabilize over time during chronic implant experiments [27]. The estimated bladder pressure, even with an initial offset in some cases, still allowed us to reliably detect the onset of bladder contractions. In three closed-loop trials, we successfully suppressed bladder contractions when they were detected and increased the bladder capacity compared to fills without stimulation. Seventy-five percent of the stimulation epochs preceded a decrease in bladder pressure, which generally occurred within a second of stimulation onset. The remaining epochs were initiated about one second after the pressure had already started to decrease, suggesting that shorter time intervals between bladder pressure updates may have led to a quicker initiation of stimulation or no triggering of stimulation for a smaller estimated pressure change. As the closed-loop algorithm was not optimized for this study, we expect that the total duration of applied stimulation can be significantly shortened.
The methods developed in this study are translatable to clinical use. Implementation of closed-loop control such that continuous neuromodulation like SNM is only applied when needed could lead to a larger bladder capacity [5] and longer battery life for implanted pulse generators. For clinical use, the closed-loop algorithm will need to be optimized to have high specificity for bladder contraction onsets and/or an increase in baseline tone, depending on a patient’s symptoms. In order to mimic a potential clinical calibration method, in the last three experiments we transitioned from supra-pubic bladder catheters to less-invasive urethral catheters. Decoding trials had similar accuracy using either catheter approach (Fig. 4), even with larger bladder contractions. While DRG were accessed with a laminectomy in this study, DRG can also be accessed percutaneously at the lumbosacral level [29]. In that approach, a non-penetrating electrode may be more feasible than penetrating arrays, which may need more vertical space for insertion. Recent research has demonstrated that DRG cell bodies are more likely to be located near the surface of the DRG [30], and that a thin-film surface electrode can record bladder pressure single units [28]. New electrodes with a lower profile and minimal immune response may be more feasible to implement clinically and may yield a higher count of bladderrelevant signals.
This study had several limitations. We observed a normal low level of overfitting, which can be alleviated through adding regularization components to the algorithm. Infusion of acetic acid into the bladder can cause damage to the tissue for a prolonged exposure [22]. An increased heart rate and the presence of blood in urine were occasionally observed. An elevated heart rate may also be a result of the anesthetic depth changing over time. Elevated heart rates resulted in some trials being ended prematurely. Although additional resting time between trials was given in order to return the stability of vitals, an alternate simulated OAB model such as the use of Prostaglandin E2 (PGE2) might have better clinical relevance [31]. We made an effort to keep the number of saline and acetic acid trials consistent across different experiments, however we were limited by experimental and anesthetic factors in some cases. This arrangement still allowed us to achieve statistically significant results in key parameters. Prediction errors were often due to shifts in channel and underlying single-unit correlations to bladder pressure. This may have been the effect of electrodes settling during an acute procedure. We anticipate that a stable chronic interface will alleviate this variation. Finally, our anesthetized animal model limited sensory inputs. Testing with varied bladder and non-bladder signals, such as with behavioral studies, are necessary to determine whether unsorted microelectrode recordings provide sufficient specificity.
Further development of real-time estimation and closed-loop control opens the possibility of a more practical bladder neuroprosthetic device. In future studies, we plan to compare on-demand closed-loop stimulation with other paradigms such as a fixed 50% duty cycle. We also plan to optimize bladder pressure estimation, including evaluating algorithm robustness during activation of non-bladder pathways and across repeated test sessions for chronic-implanted animals, and incorporate adaptive stimulation to maximize the efficacy of bladder neuromodulation in both normal and simulated OAB models. As microelectrode development continues, future studies interfacing with bladder-neuron dominant S2 DRG may also yield improvements in algorithm efficiency. Future work will also include pilot clinical trials with electrodes and stimulation parameters that are more suitable for human use.
V. Data Availability
All data and MATLAB code are available online (https://osf.io/h6835/).
Acknowledgment
The authors thank Dr. Cynthia Chestek, Ahmad Jiman, Lauren Zimmerman, Eric Kennedy, Chris Stephan, Indie Rice, Kora Dreffs, Samuel Kim, Kathleen Finn, Alex Mundorf, Manorama Kadwani and Nicholas Peck-Dimit for assisting with algorithm development and/or data collection. The authors also thank the University of Michigan Unit for Laboratory Animal Medicine for assistance with animal care.
This work was supported by the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health under Award Numbers U18EB021760, R21EB020811, OT2OD023873, and OT2OD024907. The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health.
References
- [1].Wein AJ and Rovner ES, “Definition and epidemiology of overactive bladder.,” Urology, vol. 60, no. 5 Suppl 1, p. 7–12; discussion 12, November 2002. [DOI] [PubMed] [Google Scholar]
- [2].Latini JM and Giannantoni A, “Pharmacotherapy of overactive bladder: Epidemiology and pathophysiology of overactive bladder,” Expert Opin. Pharmacother, vol. 12, no. 7, pp. 1017–1027, 2011. [DOI] [PubMed] [Google Scholar]
- [3].Siegel S et al. , “Five-Year Followup Results of a Prospective, Multicenter Study of Patients with Overactive Bladder Treated with Sacral Neuromodulation,” J. Urol, vol. 199, no. 1, pp. 229–236, 2018. [DOI] [PubMed] [Google Scholar]
- [4].Cariga P, Catley M, Mathias CJ, and Ellaway PH, “Characteristics of habituation of the sympathetic skin response to repeated electrical stimuli in man,” Clin. Neurophysiol, vol. 112, no. 10, pp. 1875–1880, 2001. [DOI] [PubMed] [Google Scholar]
- [5].Wenzel BJ, Boggs JW, Gustafson KJ, and Grill WM, “Closed loop electrical control of urinary continence.,” J. Urol, vol. 175, no. 4, pp. 1559–1563, 2006. [DOI] [PubMed] [Google Scholar]
- [6].Horvath EE, Yoo PB, Amundsen CL, Webster GD, and Grill WM, “Conditional and continuous electrical stimulation increase cystometric capacity in persons with spinal cord injury,” Neurourol. Urodyn, vol. 29, no. 3, pp. 401–407, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bruns TM, Gaunt RA, and Weber DJ, “Estimating bladder pressure from sacral dorsal root ganglia recordings,” in Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, 2011, vol. 2011, pp. 4239–4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lubba C, Mitrani E, Hokanson J, Grill WM, and Schultz SR, “Real-time decoding of bladder pressure from pelvic nerve activity,” in International IEEE/EMBS Conference on Neural Engineering, NER, 2017, pp. 617–620. [Google Scholar]
- [9].Im C et al. , “Decoding intravesical pressure from local field potentials in rat lumbosacral spinal cord,” J. Neural Eng, vol. 13, no. 5, p. 056005, October 2016. [DOI] [PubMed] [Google Scholar]
- [10].Ross SE, Ouyang Z, Rajagopalan S, and Bruns TM, “Evaluation of Decoding Algorithms for Estimating Bladder Pressure from Dorsal Root Ganglia Neural Recordings,” Ann. Biomed. Eng, vol. 46, no. 2, pp. 233–246, November 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Geramipour A, Makki S, and Erfanian A, “Neural network based forward prediction of bladder pressure using pudendal nerve electrical activity,” in Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, 2015, vol. 2015, pp. 4745–4748. [DOI] [PubMed] [Google Scholar]
- [12].Mendez A, Sawan M, Minagawa T, and Wyndaele J-J, “Estimation of bladder volume from afferent neural activity,” IEEE Trans. Neural Syst. Rehabil. Eng, vol. 21, no. 5, pp. 704–15, September 2013. [DOI] [PubMed] [Google Scholar]
- [13].Fowler CJ, Griffiths D, and De Groat WC, “The neural control of micturition,” Nat. Rev. Neurosci, vol. 9, no. 6, pp. 453–466, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Jezernik S, Wen JG, Rijkhoff NJM, Djurhuus JC, and Sinkjær T, “Analysis of bladder related nerve cuff electrode recordings from preganglionic pelvic nerve and sacral roots in pigs,” J. Urol, vol. 163, no. 4, pp. 1309–1314, 2000. [PubMed] [Google Scholar]
- [15].Bruns TM, Gaunt RA, and Weber DJ, “Multielectrode array recordings of bladder and perineal primary afferent activity from the sacral dorsal root ganglia,” J. Neural Eng, vol. 8, no. 5, p. 056010, August 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wang Z, Liao L, Deng H, Li X, and Chen G, “The inhibitory effect of sacral dorsal root ganglion stimulation on nociceptive and nonnociceptive bladder reflexes in cats,” World Journal of Urology, vol. 36, no. 5, Springer Berlin Heidelberg, pp. 1–8, 27-May-2018. [DOI] [PubMed] [Google Scholar]
- [17].Bruns TM, Weber DJ, and Gaunt RA, “Microstimulation of afferents in the sacral dorsal root ganglia can evoke reflex bladder activity,” Neurourol. Urodyn, vol. 34, pp. 65–71, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Choudhary M, van Asselt E, van Mastrigt R, and Clavica F, “Neurophysiological modeling of bladder afferent activity in the rat overactive bladder model,” J. Physiol. Sci, vol. 65, no. 4, pp. 329–338, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Fry CH et al. , “Animal models and their use in understanding lower urinary tract dysfunction,” Neurourol. Urodyn, vol. 29, no. 4, pp. 603–608, April 2010. [DOI] [PubMed] [Google Scholar]
- [20].Ross SE, Sperry ZJ, Mahar CM, and Bruns TM, “Hysteretic behavior of bladder afferent neurons in response to changes in bladder pressure,” BMC Neurosci, vol. 17, no. 1, p. 57, December 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Downie JW, Champion JA, and Nance DM, “A quantitative analysis of the afferent and extrinsic efferent innervation of specific regions of the bladder and urethra in the cat,” Brain Res. Bull, vol. 12, no. 6, pp. 735–740, 1984. [DOI] [PubMed] [Google Scholar]
- [22].Kullmann FA, Wells GI, Langdale CL, Zheng J, and Thor KB, “Stability of the Acetic Acid-Induced Bladder Irritation Model in Alpha Chloralose-Anesthetized Female Cats,” PLoS One, vol. 8, no. 9, p. e73771, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Snellings AE and Grill WM, “Effects of stimulation site and stimulation parameters on bladder inhibition by electrical nerve stimulation,” BJU Int, vol. 110, no. 1, pp. 136–143, 2012. [DOI] [PubMed] [Google Scholar]
- [24].Rigosa J, Weber DJ, Prochazka A, Stein RB, and Micera S, “Neuro-fuzzy decoding of sensory information from ensembles of simultaneously recorded dorsal root ganglion neurons for functional electrical stimulation applications,” in Journal of Neural Engineering, 2011, vol. 8, no. 4, p. 046019. [DOI] [PubMed] [Google Scholar]
- [25].Christie BP et al. , “Comparison of spike sorting and thresholding of voltage waveforms for intracortical brain-machine interface performance,” J. Neural Eng, vol. 12, no. 1, p. 016009, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wenzel BJ, Boggs JW, Gustafson KJ, and Grill WM, “Detecting the onset of hyper-reflexive bladder contractions from the electrical activity of the pudendal nerve,” IEEE Trans. Neural Syst. Rehabil. Eng, vol. 13, no. 3, pp. 428–435, 2005. [DOI] [PubMed] [Google Scholar]
- [27].Khurram A et al. , “Chronic monitoring of lower urinary tract activity via a sacral dorsal root ganglia interface,” J. Neural Eng, vol. 14, no. 3, p. 036027, June 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sperry ZJ et al. , “Flexible microelectrode array for interfacing with the surface of neural ganglia,” J. Neural Eng, vol. 15, p. 036027, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Liem L et al. , “One-Year Outcomes of Spinal Cord Stimulation of the Dorsal Root Ganglion in the Treatment of Chronic Neuropathic Pain,” Neuromodulation Technol. Neural Interface, vol. 18, no. 1, pp. 41–49, January 2015. [DOI] [PubMed] [Google Scholar]
- [30].Ostrowski AK, Sperry ZJ, Kulik G, and Bruns TM, “Quantitative models of feline lumbosacral dorsal root ganglia neuronal cell density,” J. Neurosci. Methods, vol. 290, pp. 116–124, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hokanson JA, Langdale CL, Sridhar A, and Grill WM, “OAB without an Overactive Bladder in the Acute Prostaglandin E2 Rat Model,” Am. J. Physiol. - Ren. Physiol, vol. 313, no. 5, pp. F1169–F1177, November 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and MATLAB code are available online (https://osf.io/h6835/).