Abstract
Cancer hijacks embryonic development and adult wound repair mechanisms to fuel malignancy. Cancer frequently originates from de-regulated adult stem cells or progenitors, which are otherwise essential units for postnatal tissue remodeling and repair. Cancer genomics studies have revealed convergence of multiple cancers across organ sites, including squamous cell carcinomas (SCCs), a common group of cancers arising from the head and neck, esophagus, lung, cervix and skin. In this review, we summarize our current knowledge on the molecular drivers of SCCs, including these five major organ sites. We especially focus our discussion on lineage dependent driver genes and pathways, in the context of squamous development and stratification. We then use skin as a model to discuss the notion of field cancerization during SCC carcinogenesis, and cancer as a wound that never heals. Finally, we turn to the idea of context dependency widely observed in cancer driver genes, and outline literature support and possible explanations for their lineage specific functions. Through these discussions, we aim to provide an up-to-date summary of molecular mechanisms driving tumor plasticity in squamous cancers. Such basic knowledge will be helpful to inform the clinics for better stratifying cancer patients, revealing novel drug targets and providing effective treatment options.
Keywords: Squamous cell carcinomas, lineage driver, adult stem cells, skin, wounds
1. Introduction
The idea that cancer reactivates embryonic developmental pathways otherwise inaccessible to adult homeostatic tissues in order to sustain malignant behavior has drawn fascinations from cancer and development biologists for many decades. The similarity between cancer and adult wound repair has also gained increasing interests and are of recent intensive research focus (Arwert, Hoste, & Watt, 2012; Beachy, Karhadkar, & Berman, 2004; Dvorak, 1986; Ge & Fuchs, 2018; Martin et al., 2014; Nieto, 2013; Schafer & Werner, 2008). These hypotheses share a similar perspective and draw analogy between a malignant disease to embryonic development, injury repair and tissue regeneration, suggesting cancer hijacks embryogenesis or wound repair mechanisms to sustain malignancy.
Defined by the two golden standards, long-term self-renewal and multi-lineage differentiation (Till & McCulloch, 1961), adult stem cells are the essential units for postnatal tissue remodeling and repair. They come in different flavors: some are constantly cycling, such as those in the hematopoietic system, the gastrointestinal tract, and the skin; and others that are deeply quiescent unless confronted with injury, for example, the skeletal muscle and the brain. There are tissue types whose progenitor or differentiated cells can be adapted to tissue damage repair, such as the lung, the liver and the pancreas. Adult stem cells, or other resident cell types capable of adopting the fate to drive tissue repair and regeneration, are bona fide cells of origin in cancer.
Cancer plasticity mirrors that of adult stem cells bearing tissue repair responsibilities, regardless of whether they function in a constitutive or facultative fashion (Beck & Blanpain, 2013; Blanpain, 2013; Huntly & Gilliland, 2005; Meacham & Morrison, 2013; L. V. Nguyen, Vanner, Dirks, & Eaves, 2012; Pardal, Clarke, & Morrison, 2003; Reya, Morrison, Clarke, & Weissman, 2001; Stingl & Caldas, 2007; Visvader, 2011; Visvader & Lindeman, 2008). While embracing the enthusing marriage of cancer and stem cell biology, it is important not to over-interpret cancer stem cell as a new cell type, nor that they invent their own cellular machinery and molecular pathways. Rather, hallmarks of cancer (Hanahan & Weinberg, 2000, 2011) are manifestations of malignant cells hijacking the existing lineage programs of adult stem cells, and rewiring them from the steady state to a stress-induced network during tumorigenesis (Ge & Fuchs, 2018). Together with the coopted microenvironment (Dotto, 2014; Junttila & de Sauvage, 2013; Kalluri, 2003), they drive malignant initiation and progression.
It has been increasingly demonstrated in various cancer types, that tumor cells exhibit a lineage addiction phenotype and are heavily dependent on their specific lineage survival pathways based on their tissue of origin (Garraway & Lander, 2013; Garraway & Sellers, 2006). Such cancer vulnerability is rooted in lineage programs derived from embryonic development and postnatal injury repair. For example, melanoma is highly addicted to SOX10 and MITF transcription factors, both of which are master regulators of neural crest lineage development and differentiation, from which melanocytes and melanomas originate (Garraway et al., 2005). Basal cell carcinomas (BCCs) of the skin, exquisitely rely upon Sonic hedgehog signaling, mirroring hair follicle morphogenesis programs during embryogenesis (Youssef et al., 2012). Cutaneous squamous cell carcinomas (cSCCs) hijack adult wound repair mechanisms. In both cases, adult stem cells adopt expended fate to cope with stress, a phenomenon referred to as “lineage infidelity” (Ge et al., 2017). It is functionally required to sustain malignancy and to drive re-epithelialization during wound healing (Ge & Fuchs, 2018; Ge et al., 2017).
In this review article, we center our discussions around squamous cell carcinomas (SCCs), a common group of cancers, occurring in many organs. We survey up-to-date large-scale sequencing studies, and summarize genomic and molecular profiles of SCCs from the head and neck, esophagus, lung, cervix and skin. Cutaneous SCCs have been functionally characterized in genetically engineered mouse models (GEMMs), patient-derived tumor graft models, and in vitro cultures. Based on these studies, we review the idea behind cancer plasticity and lineage addiction, and how it relates to adult stem cell plasticity and wound repair. Finally, we discuss the translational significances and clinical implications based on our knowledge of cancer plasticity.
2. Squamous cell carcinomas of the stratified epithelia and squamous lineage convergence
SCCs are one of the most frequent human solid cancers that arise from the skin epithelia, the epithelial lining of respiratory, digestive, and genital tracts including the head and neck, esophagus, lung, cervix, and anogenital region, many exhibiting a poor prognosis (Dotto & Rustgi, 2016; Facompre, Nakagawa, Herlyn, & Basu, 2012). SCCs across different body sites exhibit strong resemblance in their histopathological features. The defining “squamous” characteristics include the presence of keratins, tonofilament bundles, hemidesmosomes and desmosomes. Notably, the relative frequencies of developing SCCs across these tissues differ. At the head and neck and anogenital regions and uterine cervix, the majority of cancers are SCCs. SCCs are also considerably frequent among esophageal and lung carcinomas, although their incidences are relatively lower than adenocarcinomas in these two organ sites. In general, SCCs are the second most common skin cancers next to BCCs; although this ratio is skewed in a subset of patient groups (discussed below).
Cancer genomic studies have revealed striking molecular convergence in mutational landscapes and molecular profiles across SCCs arising in various organs (Campbell et al., 2018; Dotto & Rustgi, 2016; Hoadley et al., 2018; Hoadley et al., 2014; Schwaederle, Elkin, Tomson, Carter, & Kurzrock, 2015). Below we compare the major SCC types and summarize our current understanding of their molecular alterations.
2.1. Head and neck squamous cell carcinomas
The majority of head and neck cancers are SCCs that develop in the upper aerodigestive mucosal epithelium, with an incidence of more than 650,000 cases per year worldwide, and have a 5-year survival rate of ~50% (Argjris, Karamouzis, Raben, & Ferris, 2008; Haddad & Shin, 2008). Strong risk factors include exposure to carcinogens including tobacco and alcohol (Argjris et al., 2008; Brennan et al., 1995; Haddad & Shin, 2008), alpha-type (mucosotropic) human papillomavirus (HPV) infection (D'Souza et al., 2007; Gillison et al., 2000; P. J. Snijders et al., 1992; Syrjanen, 2005), and genetic predisposition (Cloos et al., 1996; Hopkins et al., 2008; Kutler et al., 2003).
Despite being predominantly squamous cancer, HNSCCs are notoriously known for their heterogenicity, including histological subclasses (Woolgar & Triantafyllou, 2009), genomic alterations (Agrawal et al., 2011; Akagi et al., 2014; Beder et al., 2003; Berenson, Yang, & Mickel, 1989; Bhattacharya et al., 2011; Bockmuhl et al., 1997; Cancer Genome Atlas Network, 2015a; C. H. Chung, Ely, et al., 2006; C. H. Chung, Parker, et al., 2006; Fadlullah et al., 2016; Hayes et al., 2016; Hedberg et al., 2016; Hermsen et al., 2001; Jin et al., 2006; Kalu et al., 2017; Lechner et al., 2013; Li et al., 2014; Maitra et al., 2013; Martin et al., 2014; McBride et al., 2014; Morris et al., 2017; Pickering et al., 2013; A. M. Snijders et al., 2005; Stransky et al., 2011; Sun et al., 2014; Walter et al., 2013; Zhao et al., 2011), and transcriptional signatures (Cancer Genome Atlas Network, 2015a; Cao et al., 2013; C. H. Chung et al., 2004; He et al., 2016; Martin et al., 2014; Walter et al., 2013).
HPV infection status is a major contributing factor in segregating HNSCCs’ genomic and transcriptional landscapes. HPV impacts host function via protein interactions (Eckhardt et al., 2018), or via integration into the host genome, which may additionally affect host gene expression (Parfenov et al., 2014). HPV+ HNSCCs exhibit distinct gene expression profiles (Slebos et al., 2006), copy number alterations (Klussmann et al., 2009; Smeets et al., 2006), and mutational preferences (Gaykalova et al., 2014; Gillison et al., 2019; Keck et al., 2015; Seiwert et al., 2015). Not surprisingly, HPV+ HNSCCs strongly cluster with other HPV+ tumors (mainly CxSCC, see below) based on pan-SCCs integrative comparisons, known as “iCluster 27” (Campbell et al., 2018; Hoadley et al., 2018) (Figure 1). In most SCCs HPV infections and TP53 mutations are mutually exclusive (Balz et al., 2003; Braakhuis et al., 2004; Hafkamp et al., 2003; Westra et al., 2008). Unlike HPV− HNSCCs, HPV+ ones preserve wild type alleles of TP53 and CDKN2A, and lack EGFR mutations (Cancer Genome Atlas Network, 2015a; C. H. Chung, Ely, et al., 2006). Instead, they are uniquely mutated in TRAF3 and E2F1 (Cancer Genome Atlas Network, 2015a). Of importance, HPV+ HNSCCs typically exhibit better prognosis compared to the HPV− group (Ang et al., 2010; Ragin & Taioli, 2007), potentially associated with the preponderant wild type status of TP53 in the former group (Poeta et al., 2007; Zhou, Liu, & Myers, 2016).
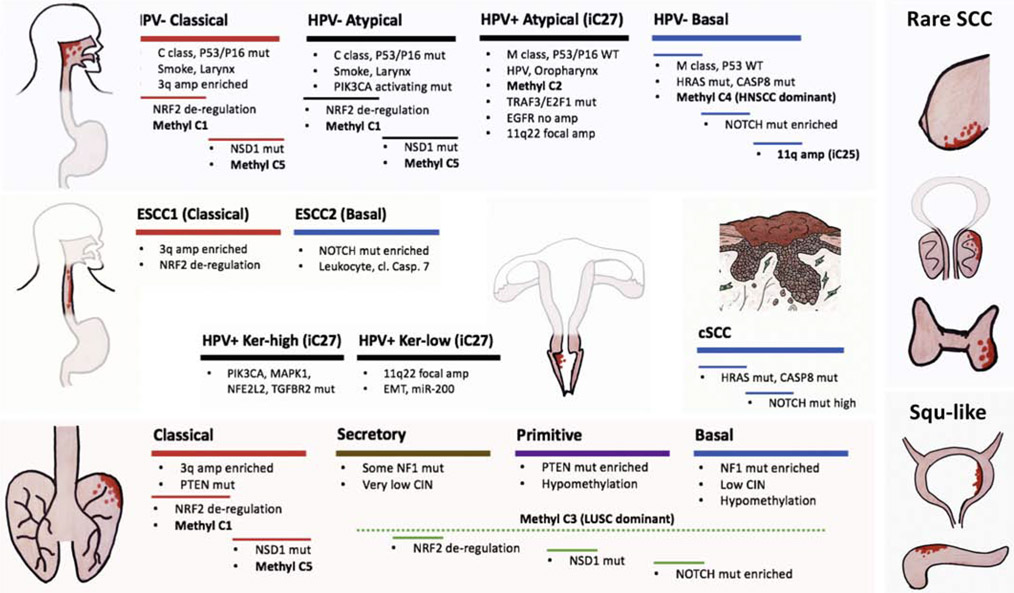
Figure 1. Molecular convergence in squamous cell carcinomas (SCCs) across organ sites.
SCCs occur frequently in head and neck (HNSCC), lung (LSCC), esophagus (ESCC), uterine cervix (CxSCC), and the skin (cSCC). Bladder and pancreas cancers have subgroups deemed squamous like carcinomas. Other organ sites develop rare SCCs including the breast, prostate, and thyroid. Based on integrative molecular analysis, HNSCCs, LSCCs, ESCCs and CxSCCs are divided into subtypes based on transcriptional signatures, each subgroup with distinct genomic alterations associated. Classical and basal subtypes are shared across SCCs. Not depicted here are mesenchymal subgroup of HNSCCs and ESCC3 subgroup of ESCCs.
Among HPV− HNSCCs, it is interesting to note two subgroups that are uniquely distinct from the rest of HPV− tumors, and rather share some common traits with HPV+ tumors. One subgroup has remarkably low copy number alterations just as HPV+ tumors (Cancer Genome Atlas Network, 2015a). Both HPV+ and this subgroup of HPV− HNSCCs belong to the “M” class tumors (Figure 1), known as cancers dominated by mutational alterations, as opposed to the “C” class tumors dominated by copy number changes (Ciriello et al., 2013). In particular, these HPV− HNSCCs include tumors with HRAS-activating-mutations and are mutually exclusive of TP53 mutations (Figure 1). Importantly, this subgroup of TP53 wild type HPV− HNSCCs also tend to exhibit better prognosis similar to HPV+ tumors (Smeets et al., 2009). Meanwhile, most HNSCCs with CASP8-inactivating-mutations also reside within this group, many with co-occurring HRAS mutations. These HRAS-CASP8 mutated tumors, however, differ from their HPV+ counterparts in their methylation patterns, and each belongs to a hypermethylated cluster “C4” and “C2”, respectively, when comparing across Pan-SCCs, resulting in suppressed candidate drivers e.g., TET1, FANCF and PAPRG (Campbell et al., 2018). Transcriptionally, they both fall under the umbrella of so-called “basal” group (Cancer Genome Atlas Network, 2015a; C. H. Chung et al., 2004; Walter et al., 2013). Unlike HPV+ group, “basal” group diverges considerably at the genomic level, encompassing tumors with HRAS/CASP8 mutations, NOTCH1 mutations, and those with 11q amplification (Cancer Genome Atlas Network, 2015a). The latter is a signature shared across SCCs known as “iCluster 25” (Campbell et al., 2018; Hoadley et al., 2018) (Figure 1).
Another subgroup of HPV− HNSCCs intriguingly share transcriptional signature with HPV+ tumors, so called the “atypical” group (Cancer Genome Atlas Network, 2015a; C. H. Chung et al., 2004; Walter et al., 2013) (Figure 1). The molecular basis for such transcriptional convergence is unclear, as their mutational landscape differs quite significantly. The atypical subgroup of HPV− HNSCCs, unlike HPV+ counterparts, have a plethora of common HNSCC mutations including the universal loss of TP53 and CDKN2A. In contrast to HPV+ tumors which occur predominantly in the oropharynx, this subgroup is enriched for laryngeal tumors and is associated with heavy smoking history (Cancer Genome Atlas Network, 2015a). In this regard, it shares similarity with another “classical” group which is also the main smoking group, commonly occurring in the larynx (Figure 1). These two groups contain the most NFE2L2/KEAP1 mutations resulting in aberrant NRF2 signaling, harbor the majority of NSD1 mutations, and maintain NOTCH wild type status. NFE2L2/KEAP1 mutated and NSD1 mutated tumors however exhibit minimal overlap with each other, and each belong to a methylation cluster “C1” and “C5”, respectively. In particular, methylation C5 cluster exhibits a strong association with NSD1 mutations, and presents a unique hypomethylation pattern with activation of putative drivers, e.g., LCK, suggesting (Campbell et al., 2018; Cancer Genome Atlas Network, 2015a), suggesting a potential functional interaction between NSD1 and hypomethylation landscape within this group of HNSCCs. In addition, “classical” HPV− group tends to be enriched with HNSCCs harboring 3q26-3q28 amplification, the genomic region containing SOX2/PIK3CA/TP63 genes, even though it occurs universally in all groups. In contrast, activating mutations in PIK3CA is prevalent in the “atypical” HPV− group (Cancer Genome Atlas Network, 2015a).
The remaining HPV− tumors have been referred to as the “mesenchymal” group, which exhibit intermediate levels of either 3q amplifications or NOTCH mutations, compared to classical and atypical groups, respectively. One caveat here could be that the stromal interference may mask tumor parenchymal alterations, thus, skewing molecular classifications. While the objective is to focus on samples with high tumor purity, and/or bioinformatically deconvolute such complexity, given the intertwined nature of tumor and its microenvironment in solid malignancies, the alternative approaches using single cell sequencing to dissect HNSCC heterogeneity (Puram et al., 2017), or FACS-sorting to enrich for epithelial cells using well-established cell-surface markers while excluding other lineages prior to sequencing are thereby warranted.
2.2. Esophageal squamous cell carcinomas
Esophageal carcinomas consist of two major types. Esophageal squamous cell carcinoma (ESCC) is prevalent worldwide, with an incidence of more than 450,000 cases per year, and is particularly common in certain regions of Asia. On the other hand, esophageal adenocarcinoma (ESAC) has a higher incidence in Western countries. Esophageal cancer patients have a 5-year overall survival rate of ~15%. Smoking and alcohol are common risk factors, which can synergistically couple with genetic variations to enhance esophageal cancer risk (Cui et al., 2009; Rustgi & El-Serag, 2014). Unlike those in HNSCCs where HPV has well-established roles, involvement of HPV has been reported (Ludmir, Stephens, Palta, Willett, & Czito, 2015) but not confirmed for ESCC (Cancer Genome Atlas Network, 2017). Rather, Epstein-Barr virus (EBV) infection, has been strongly associated with ESCC (Shah & Young, 2009), similar to a small group of HNSCC, the non-keratinized nasopharyngeal carcinoma (Shah & Young, 2009).
Genomic and molecular profiling of ESCCs have revealed commonly mutated pathways in cell cycle control, epigenetic regulation, and development (Cheng et al., 2016; Gao et al., 2014; Lin et al., 2014; Mathe et al., 2009; Qin et al., 2016; Sawada et al., 2016; Song et al., 2014; L. Zhang et al., 2015), many of which are also observed in SCCs from other organ sites. Together with ESACs (Bandla et al., 2012; Cancer Genome Atlas Network, 2017; L. Zhang et al., 2015), these studies highlighted common themes behind esophageal cancers and are very well conserved in other organs sites.
First of all, a clear divergence between ESCC and ESAC has been identified: ESACs are much closer to the adenocarcinomas of the stomach and present highly different molecular portraits than ESCCs (Cancer Genome Atlas Network, 2014a; Dulak et al., 2012; K. Wang et al., 2014). ESCCs are strongly enriched for amplifications of CCND1, 3q26-3q28, FGFR1, and mutations of NOTCH1, ZNF750, KDM6A (UTX), whereas EACs are specifically enriched for amplifications of ERBB2, VEGFA, GATA4, GATA6, and mutations in SMAD4 and ARID1A (Cancer Genome Atlas Network, 2017). CDKN2A is inactivated in both, yet it is mainly through deletion in ESCCs and epigenetic silencing in EACs (Cancer Genome Atlas Network, 2017). Divergence within esophageal carcinomas into ESCC and EAC is analogous to those observed in the lung (discussed below), and provides strong rationale for segregation of patient populations in clinical trials and treatments.
Next, ESCCs show a characteristic convergence with the rest of SCCs from other organ sites in terms of molecular subtypes (Figure 1). Based on integrated molecular characterizations (Shen, Olshen, & Ladanyi, 2009), ESCCs are further segregated into two major groups, ESCC1 and ESCC2 (Cancer Genome Atlas Network, 2017). ESCC1 is prevalent in Asian patients, exhibits highly aberrant NRF2 signaling due to NFE2L2/KEAP1/CUL3 mutations, accompanied with characteristic 3q amplification. These genomic alterations are strongly reminiscent to the HPV− “atypical” and “classical” HNSCC groups aforementioned (Cancer Genome Atlas Network, 2015a) (Figure 1).
A second ESCC subgroup, ESCC2 appears more common in East European and South American populations, exhibiting frequent NOTCH1 and ZNF750 mutations, features shared with the HNSCCs “basal” subgroup (Cancer Genome Atlas Network, 2015a) (Figure 1). APOBEC mutational signature is stronger in ESCCs compared to EACs, and appears to be further enriched in this particular ESCC2 group, especially patients from Ukraine and Russia. ESCC2 also has higher leukocyte infiltration (Cancer Genome Atlas Network, 2017), an interesting observation that may be further investigated in similar subgroups from other SCCs.
A third ESCC subgroup, ESCC3 has only very few cases and are found in North America populations, characterized by mutations in ATG7, KMT2D (MLL2), PTEN, PIK3CA (activating), with lower TP53 mutation rates compared to other ESCCs. All four ESCC3 cases under study uniquely harbor SMARCA4 mutations not shared with any other SCCs (Cancer Genome Atlas Network, 2017). In this regard, SMARCA4 is frequently mutated in EAC (Cancer Genome Atlas Network, 2017) and LAC (Cancer Genome Atlas Network, 2014c), suggesting a potential close relationship between ESCC3 subgroup and adenocarcinomas.
2.3. Lung squamous cell carcinomas
Lung carcinomas, occurring at an incidence of 1.8 million cases per year world-wide, are divided into two major types, non-small cell lung cancer (NSCLC), which includes both lung adenocarcinoma (LAC) and lung squamous cell carcinoma (LSCC) and small cell lung cancer (SCLC), referring to small cell neuroendocrine carcinoma (SCNC). The 5-year survival rate for late stage lung cancer patients is only ~5%. Cigarette smoking is prevalent in North American patients, and strongly contributes to all histological types (Kenfeld, Wei, Stampfer, Rosner, & Colditz, 2008).
As lung cancers divergent into LAC, LSCC and SCNC, they also exhibit cross-organ convergence. For example, SCNCs are rare neuroendocrine cancers occurring in several organs, and lung SCNCs highly resemble SCNCs in the prostate at the molecular level, revealing a common neuroendocrine lineage program (Balanis et al., 2019; Park et al., 2018) and remarkable cancer plasticity (Niederst et al., 2015; Sequist et al., 2011) and prostate SCNC (Beltran et al., 2016; Ku et al., 2017; Mu et al., 2017; Zou et al., 2017). An important theme that has emerged through evaluation of SCNCs is that cell cycle regulators such as RB and TP53, are the likely master regulators of lineage reprogramming, and serve as barrier to cancer plasticity (Ku et al., 2017; Mu et al., 2017; Tan et al., 2014; Tschaharganeh et al., 2014; Weissmueller et al., 2014; J. Zhu et al., 2015; Zou et al., 2017).
Likewise, LSCCs converge with other SCCs at the level of copy number alterations (Bass et al., 2009; Beroukhim et al., 2010; Dutt et al., 2011; Hammerman et al., 2012; Ramos et al., 2009; Tonon et al., 2005) and somatic mutations (Hammerman et al., 2012; Hammerman et al., 2011; Kan et al., 2010; Kim et al., 2014; C. Li et al., 2015; Weiss et al., 2010). Based on integrated molecular analysis (Shen et al., 2009), LSCC can be subdivided into four distinct groups, exhibiting different genomic alternations, gene expression signatures, and prognosis (Hammerman et al., 2012; Wilkerson et al., 2010) (Figure 1). The “classical” group strongly resembles those of “classical” HNSCCs and ESCC1 tumors (Figure 1), exhibiting high level of NRF2 signaling deregulation due to NFE2L2/KEAP1/CUL3 mutations. Consistently, classical group also exhibits highest amount of hypermethylation, chromatin instability, and elevated levels of 3q26-3q28 amplification. Largely absent in this group are NOTCH mutations, along with newly discovered ASCL4 and FOXP1 mutations (Hammerman et al., 2012). This is reminiscent of cases observed in HNSCC and ESCCs, where NOTCH mutations are enriched in the “basal” group, but not in the “classical” group (Figure 1). Interestingly, LSCC “basal” group exhibits NF1 mutations that are uncommon in other SCCs. The remaining two subgroups of LSCCs, “primitive”, and “secretory”, do not seem to have clear transcriptional counterparts in the other SCC types. When focusing on methylation patterns, it is clear that a large proportion of LSCCs constitute a major group of methylation cluster “C3” featured with distinct hypomethylation patterns (Campbell et al., 2018)(Figure 1).
Similar to esophagus, LSCCs are clearly distinct from LAC (Campbell et al., 2016). Although many copy number alterations are shared between them, the 3q SOX2/TP63/PIK3CA amplicon is specific to LSCC (Tonon et al., 2005), similar to the “classical” HPV− HNSCCs. In contrast, NKX2-1 amplification, KRAS activation and STK11 inactivation are frequent in LAC (Weir et al., 2007) but are rare in LSCCs. EGFR alterations have been found in both, although their frequencies and types of mutations differ (Cancer Genome Atlas Network, 2014c; Rekhtman et al., 2012). Therapeutically targetable oncogenic fusion events specific for LACs, e.g., ALK, are absent in LSCCs (Cancer Genome Atlas Network, 2014c). In this regard, efforts have been made to seek targetable oncogenic events enriched in LSCCs, e,g., DDR2 and FGFR mutations (Dutt et al., 2011; Hammerman et al., 2011; Hedberg et al., 2016; Liao et al., 2013; Weiss et al., 2010).
2.4. Cervical squamous cell carcinomas
Cervical carcinoma is the most common gynecological tumor, with an incidence of 570,000 cases per year worldwide. While patients presenting with early localized disease can be successfully treated with surgery or radiation, only 56% and 17% of patients remain alive at 5-years after diagnosis, when presenting with regional and distal metastasis, respectively. Ninety-five percent of cervical cancers are caused by persistent infections with carcinogenic alpha-type HPVs (Munoz et al., 2003; Schiffman et al., 2011; Uyar & Rader, 2014; Walboomers et al., 1999; zur Hausen, 2002). Effective vaccines against high-risk HPVs have been developed although the vaccination rate remains unsatisfactory.
Uterine cervical carcinomas are mainly squamous carcinomas (CxSCCs), followed by adenocarcinomas, with occasional cases of adenosquamous carcinomas. Again, genomic landscapes clearly segregate cervix cancers into several groups (Cancer Genome Atlas Research et al., 2017; T. K. Chung et al., 2015; Muller et al., 2015; Ojesina et al., 2014). Cervical adenocarcinomas belong to the “adeno” subgroup based on integrative molecular analysis, and diverge from CxSCCs with frequent amplifications of 17q12 (ERBB2), BCAR4 long non-coding RNA, 13q22 (KLF5), significant activation of hormonal signaling and ERα, FOXA1, FOXA2, FGFR1 pathways, and expression of KRT18 (non-stratified simple epithelial keratin). Overall, the cervical adenocracinomas closely resemble endometrial carcinomas, whereas CxSCCs converge with HNSCCs, particularly the HPV+ HNSCCs (Cancer Genome Atlas Research et al., 2017). APOBEC mutagenesis signature is pronounced in cervical cancers across all groups, but is infrequent in the minority HPV− group, which is more closely related to endometrial carcinomas.
Under the similar integrative molecular analysis, CxSCCs can be further divided into 2 subgroups “keratin-high” and “keratin-low”. The former exhibits stronger squamous characteristics with higher level of TP63, squamous keratins e.g. KRT5, KRT6A, squamous miRNAs miR-205, miR-499. Keratin-high group is also enriched with PIK3CA, MAPK1, NFE2L2 and TGFBR2 mutations. In contrast, keratin-low group presents with relatively higher frequencies of YAP activation (11q22 focal amplification) and epithelial-mesenchymal transition (EMT) features, including high levels of miR200a/b, intact TGFBR2 and suppressed ECAD. Like HPV+ HNSCCs, TP53 and CDKN2A are intact in CxSCCs. Distinct from other SCCs, in CxSCCs TP63 is co-amplified with TERC/MECOM but not SOX2/PIK3CA. NOTCH mutations are notably absent in CxSCCs. They also exhibit higher level of 11q22 focal amplifications compared to HPV− SCCs (Campbell et al., 2018; Cancer Genome Atlas Research et al., 2017). All of these CxSCC-specific characteristics highlight the unique biology behind HPV+ cancers.
2.5. Squamous-like carcinomas of other organs
While SCCs are common in the aforementioned organs, SCCs can also arise rarely in other organs such as the urinary bladder (Botelho, Machado, Brindley, & Correia da Costa, 2011; Odegaard & Hsieh, 2014), breast (Grabowski, Saltzstein, Sadler, & Blair, 2009), prostate (Malik et al., 2011), and thyroid (Tunio, Al Asiri, Fagih, & Akasha, 2012), as a result of chronic inflammation or metaplasia. Moreover, several organs can develop aggressive “squamous-like” carcinomas. A group of bladder cancers has been reported to express ‘basal and squamous’ markers including keratins, forming a tight cluster (Cancer Genome Atlas Network, 2014b; Ho, Kurtova, & Chan, 2012; Sjodahl et al., 2012) (Figure 1). Particularly, bladder SCCs are known to be associated with Schistosoma haemotobium infections, due to either direct carcinogenic effect of their metabolites or more likely secondary to chronic inflammation (Botelho et al., 2011; Odegaard & Hsieh, 2014). In addition, some pancreatic ductal adenocarcinomas (PDACs) also exhibit clear squamous features (Figure 1) and are characterized by worse survival (Bailey et al., 2016; Collisson et al., 2011; Moffitt et al., 2015). Of significance, TP63, a classical basal squamous master regulator, is functionally involved in PDAC invasiveness (Bailey et al., 2016; Weissmueller et al., 2014), suggesting that some of the genes contributing to the squamous phenotype, beyond being biomarkers, could also be bona fide cancer drivers and are, thus putative therapeutic targets.
Another group of cancers that show similarities to SCCs is the basal-like subtype of breast cancer (Figure 1) (including the aggressive triple-negative subgroup) and they are also known to share a molecular profile with LSCC (C. H. Chung, Bernard, & Perou, 2002). This resemblance can also be extrapolated to other gynecological tumors including ovarian cancers (Hoadley et al., 2014). Of note, TP63 signaling is activated in these basal-like breast and ovarian cancers, yet appears to be still stronger in SCC subgroup (Hoadley et al., 2014).
Together, there are multiple implications behind such molecular convergence of squamous cancers (Figure 1). From the view of development biology and cancer cell of origin, it reflects common lineage specification and differentiation programs among these epithelial cells across different organs. From a cancer therapeutic perspective, drugs that are efficient in a particular disease subgroup may be effectively used to treat cancers of different organs exhibiting similar molecular signatures. Therefore, future studies geared toward identifying new and specific driver genes or pathways that lead to squamous lineage acquisition and dependency are likely to aid the discovery of cancer specific vulnerabilities and novel drug targets.
3. Lessons from skin SCC genomics and “cancer are wounds that never heal”
The shear abundance of non-melanoma skin cancers including BCC and SCC, occurring at an incidence of 2 to 3 million cases per year world-wide, exerts a substantial burden on our healthcare and society (Alam & Ratner, 2001; Johnson, Rowe, Nelson, & Swanson, 1992; Madan, Lear, & Szeimies, 2010; Nagarajan et al., 2019). While ultraviolet light (UV) exposure is the principal risk factor for skin cancer, interesting association of certain types of HPV with cutaneous SCCs (cSCCs) have been observed (Nagarajan et al., 2019). Although cSCCs are easily treated in the general population, a subset of patients develops highly aggressive disease with extremely poor prognosis (Lawrence & Cottel, 1994). Surgical, radiation and chemotherapies remain as standard treatments with low efficacy for metastatic disease, until the recent advent of immunotherapy, which has resulted in exceptionally high response rates (Gross et al., 2019; Migden et al., 2018).
To date, numerous studies have been conducted to interrogate mutational landscapes of cSCCs (Brown et al., 2004; Cho et al., 2018; Durinck et al., 2011; Y. Y. Li et al., 2015; Oberholzer et al., 2012; Pickering et al., 2014; Purdie et al., 2009; South et al., 2014; N. J. Wang et al., 2011). Indeed, many mutations and altered pathways are shared with SCCs from other organ sites, including alternations in HRAS and PI3K signaling, squamous differentiation genes, cell cycle regulators, and epigenetic regulators. Interestingly, NOTCH mutation rate appears to be much higher in cSCCs compared to other SCCs, and HRAS/CASP8 mutations are also prevalent in cSCC, similar to the basal groups of other SCCs (Figure 1). On the other hand, it remains unclear whether a group of 3q amplification, NRF2 de-regulation, or NSD1 mutation has an equivalent counterpart in cSCC, given UV damage rather than smoking is top risk factors for skin cancer. Besides discovering recurrent mutations, several important insights into the fundamental properties of adult normal epithelia can be learned from genomic studies of cSCCs.
Mutations in cancer drivers such as TP53 (Brash et al., 1991; Pierceall, Mukhopadhyay, Goldberg, & Ananthaswamy, 1991), NOTCH (Durinck et al., 2011; Pickering et al., 2014; South et al., 2014) and HRAS (Daya-Grosjean, Robert, Drougard, Suarez, & Sarasin, 1993; Pierceall, Goldberg, Tainsky, Mukhopadhyay, & Ananthaswamy, 1991) are all frequent events in cSCC. Interestingly, they occur relatively early rather than late in skin SCCs, and many are found in adjacent ‘normal’ tissues (Durinck et al., 2011; South et al., 2014), consistent with ‘field cancerization’ (Braakhuis, Tabor, Kummer, Leemans, & Brakenhoff, 2003; Leemans, Braakhuis, & Brakenhoff 2011). Field cancerization was initially described in HNSCCs, where it has been frequently observed that local secondary tumours could occur at the site of surgically removed primary tumours (sporadic non-familial cases), suggesting a cancerous ‘field’ of pre-malignant tissues or mutations in the peritumoral epithelia (Slaughter, Southwick, & Smejkal, 1953). The key idea behind this concept is also shared with the model of multi-step carcinogenesis (Fearon, Hamilton, & Vogelstein, 1987; Fearon & Vogelstein, 1990), such that peritumoral tissues are no longer considered “normal”, but often contain several early tumorigenic genetic events (Tomasetti, Vogelstein, & Parmigiani, 2013). Besides HNSCCs (Califano et al., 1996), field cancerization has also been observed in many other carcinomas including lung (Franklin et al., 1997), esophagus (Prevo, Sanchez, Galipeau, & Reid, 1999), urinary tract (T. Takahashi et al., 1998), cervix (Chu, Shen, Lee, & Liu, 1999) and vulva (Rosenthal, Ryan, Hopster, & Jacobs, 2002). TP53 mutations are also known to occur in a patchy fashion (Garcia et al., 1999)(Tabor et al., 2002; Tabor et al., 2001; van Houten et al., 2002), involving the otherwise normal epithelial mucosa of these abovementioned tissues, and most notably in the skin epidermis (Brash & Haseltine, 1982; Brash et al., 1991; Chao, Eck, Brash, Maley, & Luebeck, 2008; Jonason et al., 1996; Klein, Brash, Jones, & Simons, 2010; W. Zhang Remenyik, Zelterman, Brash, & Wikonkal, 2001; Ziegler et al., 1994). Besides epithelial cancers, analogous examples have been found in haematological malignancies, known as pre-leukemic clones in bone marrow (Challen et al., 2011; Corces-Zimmerman, Hong Weissman, Medeiros, & Majeti, 2014; Laurie et al., 2012; Shlush et al., 2014). Of importance, clonal haematopoiesis is directly associated with the risk of therapy-related myeloid neoplasms (K. Takahashi et al., 2017). Recent examples include the ‘normal’ eyelid skin (Martincorena et al., 2015), and esophageal mucosa (Martincorena et al., 2018; Yokoyama et al., 2019). In the case of esophagus, the frequencies of cancer driver mutations could be even higher in normal than cancer (Martincorena et al., 2018), and may occur as early as infancy (Yokoyama et al., 2019). Mechanistically, differentiation imbalance in single esophageal progenitor cells (Alcolea et al., 2014), aberrant signals from the mesenchyme (Hu et al., 2012), clonal selection during regeneration (M. Zhu et al., 2019), or cell competition during embryonic development (Ellis et al., 2019) are all pertinent possibilities that could lead to field change in normal tissues.
While “field cancerization” suggests malignant recurrence originates from peritumoral tissues, the idea “tumors are wounds that never heals” postulates that cancer has its roots in chronic wounds (Balkwill & Mantovani, 2001; Coussens & Werb, 2002; Dvorak, 1986; Ge & Fuchs, 2018; Ge et al., 2017; Schafer & Werner, 2008). Indeed, transcriptional similarities have been widely observed between wounds and tumors in various tissues (Chang et al., 2004; Iyer et al., 1999; Lambert et al., 2014; Pedersen et al., 2003; Riss et al., 2006). Skin is an excellent model to study both wound healing and carcinogenesis. The epidermis harbors highly abundant, well-defined and genetically accessible adult stem cells, responsible for appendage (hair follicles, nails, sebaceous, eccrine and apocrine glands) regeneration, wound repair and tumor development (Balmain & Yuspa, 2014; Benitah, 2012; Blanpain & Fuchs, 2014; Chuong, Cotsarelis, & Stenn, 2007; DeStefano & Christiano, 2014; Ge & Fuchs, 2018; Gurtner, Werner, Barrandon, & Longaker, 2008; Hsu, Li, & Fuchs, 2014; Khavari, 2006; C. Lu & Fuchs, 2014; Millar, 2002; Paus & Cotsarelis, 1999; Rompolas & Greco, 2014; Schepeler, Page, & Jensen, 2014; Takeo, Lee, & Ito, 2015; Wabik & Jones, 2015; Watt, 2014). Historically, organotypical cultures of human skin (proto-type of nowadays organoids) have been the front runner in regenerative and translational medicine due to its effective treatment of burn patients (Gallico, O'Connor, Compton, Kehinde, & Green, 1984; Rheinwald & Green, 1975). In vivo lineage tracing in GEMMs has revealed the cell-of-origin for cSCCs (Lapouge et al., 2011; White et al., 2011). Recent work in the skin has dived deeper into functional significance underlying the observed similarities between wounds and cancer, shedding light on the role of adult stem cells in driving wound repair and cancer progression (Ge et al., 2017).
Many types of skin chronic wounds may predispose patients to cSCCs, and precursor lesions may develop into cSCCs (Alam & Ratner, 2001). Chronically injured skin, including regions affected by non-healing ulcers (Enoch, Miller, Price, & Harding, 2004; Kerr-Valentic, Samimi, Rohlen, Agarwal, & Rockwell, 2009), sinus tracts, osteomyelitis, radiation dermatitis, or vaccination scars, and chronic inflammatory disorders including discoid lupus erythematosus, lichen sclerosus, lichen planus, dystrophic epidermolysis bullosa (Bastin, Steeves, & Richards, 1997), and lupus vulgaris, are all predisposing factors of cSCCs. Actinic keratosis is a classic precursor lesion of cSCC (Callen, Bickers, & Moy, 1997), along with other potential precursor lesions or SCC variants including epidermodysplasia verruciformis, Bowen’s disease and Bowenoid papulosis, erythroplasia of Queyrat, keratoacanthoma, and verrucous carcinoma (Alam & Ratner, 2001). SCCs of other organ sites also have similar precursor lesions, such as oral leukoplakia (Mao et al., 1996) potentially progressing into HNSCC, and Barretts esophagus (Rustgi & El-Serag, 2014; Spechler & Souza, 2014) developing into EAC and/or rarely, ESCC.
Besides chronic wounds, various therapeutic agents may also increase the risk for development of cSCCs, a common secondary malignancy. These disease-predisposed individuals or skin regions may harbor cancerous fields or non-healing wounds, providing a conceptual explanation of increased cancer incidence and severity. cSCCs are known to frequently arise in 15-30% patients receiving the BRAF inhibitors such as vemurafenib, dabrafenib or sorafenib (Arnault et al., 2009; Belum, Fontanilla Patel, Lacouture, & Rodeck, 2013; F. Su et al., 2012). This is likely due to the paradoxical MAPK-pathway activation upon BRAF inhibition (Hall-Jackson et al., 1999; Hatzivassiliou et al., 2010; Heidorn et al., 2010; Poulikakos, Zhang, Bollag, Shokat, & Rosen, 2010). Interestingly, HRAS mutations are relatively infrequent in primary tumors, but become highly enriched in BRAF inhibitor treated tumors (Oberholzer et al., 2012; South et al., 2014), pointing to a predominant role for HRAS in driving secondary cSCCs. Indeed, mouse models of cSCCs are particularly addicted to HRAS activating mutations and high level of pERK signaling (Balmain, Ramsden, Bowden, & Smith, 1984; Burns, Jack, Neilson, Haddow, & Balmain, 1991; Quintanilla, Brown, Ramsden, & Balmain, 1986; Quintanilla et al., 1991).
A group of aggressive cSCCs are known to develop in organ transplantation recipients, heavily associated with immune-suppression (Berg & Otley, 2002; Boukamp, 2005; Euvrard, Kanitakis, & Claudy, 2003; Rangwala & Tsai, 2011). This is particularly prominent in renal (Birkeland et al., 1995; Bouwes Bavinck et al., 1996; Dantal et al., 1998; Hartevelt, Bavinck, Kootte, Vermeer, & Vandenbroucke, 1990; Jensen et al., 1999; Lindelof, Sigurgeirsson, Gabel, & Stern, 2000; Ramsay, Fryer, Hawley, Smith, & Harden, 2002) and heart (Jensen et al., 1999; Veness et al., 1999) transplant recipients, and occur at moderate rates among hematopoietic stem cell transplant recipients (Dargent, Kornreich, Andre, & Lespagnard, 1998; Hasegawa et al., 2005; Leisenring, Friedman, Flowers, Schwartz, & Deeg, 2006; Lishner et al., 1990; Rizzo et al., 2009). Although recipients of solid organ transplants are known to have 3-4-fold increased risk of developing malignancies in general, their risk for cSCCs is particularly noticeable with up to 250-fold (Harwood et al., 2013; Pelisson, Soler, Chardonnet, Euvrard, & Schmitt, 1996; Penn, 2000). cSCCs from these patients are also more aggressive (Euvrard et al., 1995; Lott, Manz, Koch, & Lorenz, 2010; Veness et al., 1999), and exhibit different characteristics than the general population, including altered rates of oncogenic mutations and HPV infections (Pelisson et al., 1996), poor prognosis and increased incidence of metastasis (Carucci et al., 2004; Martinez et al., 2003; Wells & Shirai, 2012). Of note, the fact that incidences of SCCs and BCCs are completely reversed in this subset of patients compared to the general population, and additionally the up-to-100 fold increase of SCCs in the anogenital regions (Boukamp, 2005; Euvrard et al., 2003) strongly implicates these patients’ specific susceptibility to squamous cancers rather than a non-discriminative predisposition of all skin cancer incidences. Changing immune suppressive agent and/or dose (Dantal et al., 1998; Guba, Graeb, Jauch, & Geissler, 2004; Otley, Coldiron, Stasko, & Goldman, 2001; Otley & Maragh, 2005) significantly altered the course of cSCC development in these patients, suggesting a direct mechanistic link between cSCCs and immune modulation. In this regard, the use of a combination of oral methoxsalen, cyclosporine, and UVA radiation for the treatment of psoriasis and other chronic dermatoses is associated with an elevated risk of cSCC (Forman, Roenigk, Caro, & Magid, 1989; Marcil & Stern, 2001; Stern et al., 1984). Interestingly, the calcineurin inhibitor cyclosporine as an immune-suppressant not only inhibits skin resident Langerhans cells (Borghi-Cirri et al., 2001; Dupuy, Bagot, Michel, Descourt, & Dubertret, 1991) and dendritic cells (Abdul, Charron, & Haziot, 2008; Sauma et al., 2003), but also directly acts on tumor epithelial cells in a cell-autonomous manner (Han, Ming, He, & He, 2010; Hojo et al., 1999; Walsh et al., 2011; X. Wu et al., 2010) to promote tumor progression. Many other immune-suppressive drugs include tacrolimus (another calcineurin inhibitor), azathioprine and mycophenolate mofetil (inhibitors of purine synthesis), rapamycin analogs (mTOR inhibitors), monoclonal antibodies against TNFa and IL-12/IL-23, or glucocorticoid have been under intensive studies in their relationship with non-melanoma skin cancer development (Euvrard et al., 2003; Rangwala & Tsai, 2011).
Finally, cSCCs are also among the top secondary tumors observed in chronic lymphocytic leukemia (CLL) patients (Adami, Frisch, Yuen, Glimelius, & Melbye, 1995; Manusow & Weinerman, 1975; Mehrany, Weenig, Pittelkow, Roenigk, & Otley, 2005; Onajin & Brewer, 2012), a low-grade clonal B-cell lymphoproliferative disorder, accounting for 25% of all leukemias in developed countries. In contrast to solid organ transplant recipients where cSCC rate is exceptionally high, in CLL patients, BCCs and HNSCCs are also similarly elevated (Wong et al., 2008). Metastasis and mortality due to cSCCs are found to be increased in these patients (Mehrany, Weenig, Lee, Pittelkow, & Otley, 2005). Immune-dysregulation has been proposed to contribute to development of these second malignancies. Intriguingly, neoplastic B cells may infiltrate cSCCs and compromise anti-tumor T cell responses (Dargent et al., 1998; Smoller & Warnke, 1998). Besides immune dysfunction, the confounding factors of chemo- and radiation therapies as well as common genetic alterations have also been suggested, although a direct causal relationship is yet to be established (Mehrany, Weenig, Lee, et al., 2005).
Future studies of integrated molecular profiling and characterizations of cSCCs, both primary and secondary malignancies as discussed above, will be important to further provide deep mechanistic understanding of squamous carcinogenesis. Of particular biological and therapeutic relevance would be to compare cSCCs to SCCs from other organs, as well as to clinical cases of skin wounds and chronic inflammatory dermatoses, aiming to reveal the functional significance behind squamous lineage convergence in the context of cancer as a non-healing wound.
4. Cancer drivers of the squamous lineage and their context dependency
As cancer genomic studies continue to provide deep insights into cancer drivers, functional studies using GEMMs, patient derived xenograft and organoid models, combined with well-characterized cancer cell line cultures {Behan, 2019 #18069}{Tsherniak, 2017 #18070}{Barretina, 2012 #9639}{Ghandi, 2019 #18071}, together offer comprehensive toolkits to define and refine cancer genes. No models are perfect, but all are useful and complementary to each other. A combination of these mouse models and human samples offers functional validation and drug screening platforms, while providing opportunity for in depth mechanistic dissection of driver programs, a prerequisite knowledge to truly advance cancer therapy.
Many pathways have been repeatedly revealed to be essential for SCCs across various sites, including cell cycle, squamous differentiation and developmental genes, oxidative stress regulators, and epigenetic factors (Dotto & Rustgi, 2016). Here we focus our discussion on frequent alterations that differentiate SCCs from the rest of cancer types (Figure 2). Notably, for driver genes universally altered in many cancers, often the mutation outcomes and mechanisms of action are distinct among cancer types, raising the critical issue of context dependency (Schneider, Schmidt-Supprian, Rad, & Saur, 2017). Studies using various pre-clinical GEMMs and cellular models have revealed pathways known to be important for squamous fate specification and differentiation, and provided unprecedented insights into carcinogenesis mechanisms and as well as putative therapeutic targets.
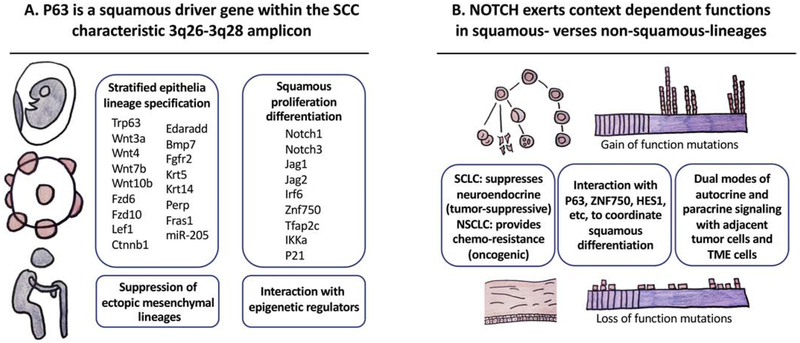
Figure 2. Understanding context dependency of cancer genes with squamous drivers.
(A) The SCC-characteristic amplicon 3q26-3q28 is a prototypical example for understanding context dependency of cancer driver genes. Within this amplicon, TP63 – notably the deltaNp63alpha isoform – is widely involved in the development, homeostasis, and aging of stratified epithelia tissues, and is a canonical driver gene of squamous cancer. Its direct transcriptional targets include regulators of squamous lineage specification, proliferation and differentiation. It also directly suppresses ectopic mesenchymal lineage genes and interacts with epigenetic regulators to orchestrate lineage plasticity. (B) Other key squamous specification, maintenance and stratification genes are frequently altered in SCCs. Meanwhile, many of them have important functions outside squamous lineages. Their functions in these diverse tissues differ drastically dependent on the lineage context. NOTCH is oncogenic in lymphoid organs but tumor-suppressive in myeloid leukemia and squamous tissues, consistent with its function to promote proliferation verses stratification during the development of these two lineages, respectively. Several mechanisms may underlie such context dependency. Notch could provide cell-autonomous dependency of neuroendocrine lineage in SCLC, while non-autonomously drive slow-cycling and chemo-resistance in NSCLC. Notch coordinate with other squamous transcription factors such as P63, ZNF750 and HES1 to drive differentiation program. Notch signaling in both autocrine and paracrine fashion to impact the tumor parenchyma and tumor microenvironment.
4.1. The 3q26-3q28 amplicon in SCCs
As discussed above, SCCs exhibit a characteristic copy number alteration event, namely frequent co-amplification of numerous oncogenes at 3q26-3q28 (Bass et al., 2009; Freier et al., 2010; Hussenet et al., 2010; Long & Hornick, 2009; Maier et al., 2011; Yuan et al., 2010). Below, we review some of the known examples of SCC drivers residing within this region.
One of the defining factors for squamous lineage specification is TP63, residing at 3q28 (Figure 2). TP63 belongs to the same family of transcription factors as TP53, and was initially discovered to bind to TP53 responsive elements (Bian & Sun, 1997; Zeng, Levine, & Lu, 1998). TP63 is highly expressed in the basal layer of stratified squamous but not single-layered epithelia (A. Yang et al., 1998), and is crucial to maintain the proliferative potential of epithelial stem cells and prevent differentiation (Carroll et al., 2006; King et al., 2006; King et al., 2003; Koster, Kim, Mills, DeMayo, & Roop, 2004; Liefer et al., 2000; Okuyama et al., 2007; Parsa, Yang, McKeon, & Green, 1999; Pellegrini et al., 2001; Senoo, Pinto, Crum, & McKeon, 2007; X. Su, Cho, et al., 2009; Truong, Kretz, Ridky, Kimmel, & Khavari, 2006; Yugawa et al., 2010). TP63 mutation is the most common genetic basis for the congenital ectrodactyly, ectodermal dysplasia, and facial clefts (EEC) syndrome (Brunner, Hamel, & Van Bokhoven, 2002). Mice lacking TP63 failed to develop stratified epithelia and epithelial appendages, such as teeth, hair follicles and mammary glands. TP63 along with several other transcription factors are potent enough to reprogram wound fibroblasts into skin epithelial cells (Kurita et al., 2018).
Mechanistically, TP63 directly targets key squamous lineage genes, for example the keratin gene KRT14 (Candi et al., 2006), a tetraspan membrane protein and cell adhesion regulator Perp (Ihrie et al., 2005), and an extracellular matrix gene Fras1 (Koster et al., 2007), all characteristic of stratified epithelia (Figure 2). It also targets master epidermal morphogenesis signals such as JAG1 in the Notch pathway (Sasaki et al., 2002), and determinants of epidermal terminal differentiation IRF6 (Moretti et al., 2010; Richardson et al., 2006) and ZNF750 (Sen et al., 2012). Other major epidermal morphogenesis genes shown as direct P63 targets include AP-2gamma (Koster, Kim, Huang, Williams, & Roop, 2006), IKKa (Koster et al., 2007), microRNA miR-205 {Tucci, 2012 #5371}, ROS regulator REDD1 (Ellisen et al., 2002). Genome-wide search of TP63 targets in human and mouse epithelia further expanded squamous lineage defining target network of TP63, solidifying its active suppression of ectopic mesenchymal lineage, and interaction with other epigenetic regulators (Bao et al., 2015; Fan et al., 2018; Kouwenhoven et al., 2010; Medawar et al., 2008; Romano, Birkaya, & Sinha, 2006; Romano et al., 2012; Shalom-Feuerstein et al., 2011; A. Yang et al., 2006) (Figure 2).
Given its key role in squamous lineage specification and stratification of epithelial tissues (Crum & McKeon, 2010), it is not surprising that TP63 is one of the most well-established oncogenic drivers for squamous cancers and basal subtype carcinomas from stratified epithelia (Hoadley et al., 2018; Westfall & Pietenpol, 2004). TP63 is known to be frequently amplified in various SCCs (Hibi et al., 2000; Maier et al., 2011; Saladi et al., 2017; Schrock et al., 2014; Watanabe et al., 2014; X. Yang et al., 2011). Its molecular mechanisms have been linked to interaction with TP53 (Flores et al., 2005; Flores et al., 2002), P21 (Barbieri, Barton, & Pietenpol, 2003; Barbieri, Tang, Brown, & Pietenpol, 2006; Westfall, Mays, Sniezek, & Pietenpol, 2003) and YAP (Ehsanian et al., 2010; Saladi et al., 2017)
Adding to its complexity, TP63 has multiple isoforms due to both N- and C-terminal alternative splicing (Candi et al., 2006; Dohn, Zhang, & Chen, 2001; Ghioni et al., 2002; Helton, Zhu, & Chen, 2006; Jacobs et al., 2005; King et al., 2003; Laurikkala et al., 2006; X. Su, Paris, et al., 2009; Suzuki, Sahu, Leu, & Senoo, 2015; G. Wu et al., 2003). Two of these isoforms, known as ΔNp63 and TAp63, respectively, have distinct functions. The former is the predominant isoform in the squamous lineage whereas the latter has a broader expression in the early ectoderm prior to stratification commitment, in stromal fibroblast (X. Su, Paris, et al., 2009) and neuronal (Jacobs et al., 2005) lineages. Whereas ΔNp63 is key driver of squamous lineage or those of stratified epithelia, TAp63 appears to be highly expressed in non-squamous cancers including colorectal cancers and lymphomas (Bishop et al., 2012; Di Como et al., 2002; Nylander et al., 2002).
SOX2 is another transcription factor frequently amplified in SCCs, residing at 3q26. SOX2 is a master regulator of multiple lineages during development including embryonic stem cells, lung and neuronal lineages, and its aberrant expression and functional importance has been widely reported in many cancer types. Knocking out or overexpressing SOX2 in cultured SCC cancer cells suggests it is indeed a lineage survival oncogene (Bass et al., 2009; Hussenet et al., 2010). In vivo GEMMs further confirm its crucial function in LSCCs (Ferone et al., 2016; Y. Lu et al., 2010; Mukhopadhyay et al., 2014) and cSCCs (Boumahdi et al., 2014; Siegle et al., 2014). It is worth to note that some SCLCs also exhibit SOX2 amplification and are functionally dependent on its expression (Rudin et al., 2012). However, in SCLCs the SOX2 amplification may reflect the importance of its function in neuronal lineages rather than a squamous lineage.
Interestingly, one of the genes co-amplifying with SOX2 is PRKCI, reported to functionally co-operate with SOX2 in LSCC (Justilien et al., 2014). PKC family members are known to be classical regulators of epidermal differentiation in response to calcium signaling and phorbol ester TPA (12-0-tetradecanoylphorbol- 13-acetate) treatment (Hennings et al., 1980). However, this particular family member, PRKCI encoding PKC iota, is considered calcium insensitive and remains relatively under-characterized. It will be interesting to see whether functional interaction of PKC iota with SOX2 extends to other SCCs and whether there are yet unknown roles of PKC in SCCs.
Another known oncogene co-amplified with SOX2 at 3q26 is PIK3CA (Kozaki et al., 2006; Murugan, Hong, Fukui, Munirajan, & Tsuchida, 2008; Qiu et al., 2006; Redon et al., 2001; Woenckhaus et al., 2002). Even though PIK3CA is frequently mutated across many cancers, its mutational identity in SCCs is unique: PIK3CA E542K and E545K is enriched in CxSCC, HNSCC, and bladder cancer, and is very different from breast cancer and others (Cancer Genome Atlas Research et al., 2017), raising an intriguing possibility that a subset of PI3K pathway genes genetically or biochemically interact with these particular mutant PI3Ks in SCCs compared to other cancer types.
4.2. Regulators of squamous stratification
Several master development regulators important for squamous differentiation have been found to be significantly altered in SCCs; and, hence are bona fide drivers of squamous carcinogenesis.
NOTCH pathway genes are prevalently mutated in human cancers. NOTCH1 mutations in SCCs are loss-of-function type, implicating Notch1 as a tumor suppressor therein (Agrawal et al., 2011; Stransky et al., 2011; N. J. Wang et al., 2011). This is analogous to myeloid leukemia (Klinakis et al., 2011), but is in sharp contrast to lymphoid malignancies, where it is a well-established oncogene (Baldus et al., 2009; Di Ianni et al., 2009; Ellisen et al., 1991; Fabbri et al., 2011; Lee et al., 2009; Puente et al., 2011; Weng et al., 2004).
The tumor suppressive function of NOTCH in cSCCs has been well-corroborated by mouse models and cell culture studies. Skin epidermis-specific deletion of Notch1 leads to accelerated carcinogenesis in mice (Nicolas et al., 2003), possibly due to cell non-autonomous effect from the tumor microenvironment (Demehri, Turkoz, & Kopan, 2009). It is also consistent with NOTCH function in promoting cell cycle exit and squamous differentiation (Devgan, Mammucari, Millar, Brisken, & Dotto, 2005; Lefort et al., 2007; Lowell, Jones, Le Roux, Dunne, & Watt, 2000; Mammucari et al., 2005; B. C. Nguyen et al., 2006; Nickoloff et al., 2002; Rangarajan et al., 2001; Williams, Beronja, Pasolli, & Fuchs, 2011). Of note, clinical observations that patients treated with gamma-secretase inhibitor (therefore blocking Notch ligand processing) frequently developed skin cancers (Extance, 2010), is in line with a tumor suppressive function of Notch in the skin epithelia. Functionally related to NOTCH, the transcription factor ZNF750 is a core component in epidermal stratification program directly regulated by TP63 (Boxer, Barajas, Tao, Zhang, & Khavari, 2014; Rubin et al., 2017; Sen et al., 2012), and appears to be frequently mutated in HNSCCs (Cancer Genome Atlas Network, 2015a; Gillison et al., 2019) and ESCCs (Cancer Genome Atlas Network, 2017; Lin et al., 2014; Sawada et al., 2016; L. Zhang et al., 2015).
NOTCH pathway mutations are also common in NSCLCs (Westhoff et al., 2009) and SCLCs (Cancer Genome Atlas Network, 2015b). Forced NOTCH expression in Notch-mutant SCLCs suppresses neuroendocrine signature, and extend survival of tumor-bearing mice (Cancer Genome Atlas Network, 2015b), supporting the tumor suppressive role of NOTCH in SCLC. Intriguingly, it has been recently reported the non-neuroendocrine NOTCH activated cells are slow-cycling and chemotherapy-resistant, providing trophic support to neuroendocrine tumor cells, where NOTCH inhibition combined with chemotherapy is effective in delaying disease relapse in pre-clinical models (Lim et al., 2017).
Further adding to the complexity of understanding Notch pathway deregulation in cancer, Notch signaling represents one of the classic developmental pathways where ligands and receptors may come from the same (autocrine) or the adjacent cells (paracrine) (Kopan & Ilagan, 2009; Kovall & Blacklow, 2010). On one hand, skin basal epithelial stem cells express the ligand Delta to induce neighboring cells expressing the Notch1 receptor to differentiate (Lowell et al., 2000). On the other, suprabasal cells express both ligand Jagged and receptor Notch to form a positive feedback loop and reinforce differentiation fate (Luo, Aster, Hasserjian, Kuo, & Sklar, 1997; Nickoloff et al., 2002; Rangarajan et al., 2001). Meanwhile, differentiated Notch-high cells actively suppress stem cell fate by inhibiting TP63 expression (B. C. Nguyen et al., 2006). In this regard, it is intriguing to notice that TP63 amplified and NOTCH1 mutated LSCCs exhibit minimal overlap (Hammerman et al., 2012), raising the possibility of direct genetic interaction between these two SCC drivers. Notably, one of the key Notch target and pathway regulator HES1 localizes to 3q29 region adjacent to TP63, is possibly co-amplified and functionally involved in SCCs. It will also be interesting to analyze at single cell level what type of signaling paradigm (autocrine vs paracrine) SCCs exploit to de-regulate NOTCH activity, especially in relationship with neighboring tumor parenchymal cells and tumor-stroma microenvironment.
MYC, a master oncogenic driver across many cancers and the founding member of oncogene amplification (Little, Nau, Carney, Gazdar, & Minna, 1983; Schwab et al., 1983), is amplified in SCCs (Rodrigo, Lazo, Ramos, Alvarez, & Suarez, 1996). Counterintuitively, MYC overexpression alone seems to be capable of both stimulating proliferation and promoting differentiation of the skin epidermis (Arnold & Watt, 2001; Gandarillas & Watt, 1997; Pelengaris, Littlewood, Khan, Elia, & Evan, 1999; Waikel, Kawachi, Waikel, Wang, & Roop, 2001; Waikel, Wang, & Roop, 1999). Notably, the incidence of MYC amplification in SCCs developing in renal transplant recipients is increased to 50% (Pelisson et al., 1996). MYC also appears to be important for SCC resistance to combined miR-21 antagonism and PI3K/mTOR inhibition (Darido et al., 2018). Intriguingly, GRHL2, a well-established squamous stratification gene (Boglev et al., 2011) and functionally synergistic with MYC in reprogramming wound fibroblasts into epidermal keratinocytes (Kurita et al., 2018), is localized at 8q22 close to MYC at 8q24, and is potentially co-amplifed with MYC. These together suggest context-dependent function of MYC across different tumor types, and hence, a foreseeable SCC-specific MYC oncogenic network.
Further speaking of context dependency, another recently reported SCC driver is KLF5 (Ge et al., 2017; X. Zhang et al., 2018). KLF5 promotes lineage infidelity in skin epidermal stem cells, and is functionally required for wound repair and cSCC propagation (Ge et al., 2017). Epidermal-specific KLF5 overexpression induced skin dysplasia (Sur, Rozell, Jaks, Bergstrom, & Toftgard, 2006; Sur, Unden, & Toftgard, 2002). While KLF5 appears to promote proliferation, its close family member KLF4, one of the induced pluripotent stem cell (iPSC) reprogramming factors (K. Takahashi & Yamanaka, 2006), is crucial for epidermal stratification and differentiation (Jaubert, Cheng, & Segre, 2003; Ohnishi et al., 2000; Segre, Bauer, & Fuchs, 1999). Interestingly, KLF5 is also frequently amplified or mutated in other cancers, including HNSCC, ESCC and LSCC (Campbell et al., 2016; X. Zhang et al., 2018; X. Zhang et al., 2016), salivary gland tumors (Giefing et al., 2008), uterine cervical adenocarcinomas (Cancer Genome Atlas Research et al., 2017), gastric cancers (Deng et al., 2012), and bladder cancers (Cancer Genome Atlas Network, 2014b). Indeed, KLF5 has a plethora of functions across epithelial tissues as well as the hematopoietic system (Dong & Chen, 2009; McConnell & Yang, 2010; Turner & Crossley, 1999), consistent with its wide involvement of human cancers. KLF5 function and targets likely differ in various cancer types, whereas its function in SCCs may significantly overlap. It will be crucial to integrate and leverage our current knowledge on squamous regulators to gain deeper understanding of the context dependency mechanisms of cancer drivers.
5. Summary and perspectives
Over the past century, milestones leading to a cure in cancer have been progressively exciting, including the momentous advancement of surgical, radiation- and chemo-therapy, targeted molecular therapy, and most recently immunotherapy in cancer treatment. Pondering over the important lineage addiction phenomenon of cancer, it is also sobering to realize many of the lineage factors discussed above are not readily targetable. That said, signaling events leading to activation of these lineage pathways might serve as putative therapeutic targets.
Meanwhile, with effective treatments, many types of cancers are becoming a chronic disease, and the next challenge in cancer medicine would be to address the pressing issues of treatment toxicities, comorbidities and long-term survival of our cancer patients. One strategy is to exploit cancer specific targets while sparing normal adult tissues, based on our fundamental knowledge of cellular and molecular pathways governing adult tissue homeostasis. By dissecting how pathways of stress and wound repair are hijacked by cancer cells, one could also re-purpose therapeutic agents approved for management of other diseases to cancer patients. Furthermore, one of the most fascinating questions in cancer biology is to understand context dependency of cancer drivers. One ought to take advantage of our knowledge in embryonic development and adult stem cells to dissect cancer vulnerability and lineage addiction across tissues and organs.
Acknowledgments.
Y. Ge. is supported by the NIH K01 Career development award 1K01AR072132, CPRIT first-time recruitment award FP00006955, the Irene Diamond Fund/AFAR Postdoctoral Transition Award, UT Rising Star award, and UT MD Anderson Cancer Center StartUp funds. Illustration is by D. Fails and SciStories.
Abbreviations:
- SCCs
squamous cell carcinomas
- HNSCCs
head and neck squamous cell carcinomas
- ESCCs
esophageal squamous cell carcinomas
- EACs
esophageal adenocarcinomas
- NSCLC
non-small cell lung cancer
- SCLC
small cell lung cancer
- LSCCs
lung squamous cell carcinomas
- LACs
lung adenocarcinomas
- CxSCCs
uterine cervical squamous cell carcinomas
- BCCs
basal cell carcinomas
- cSCCs
cutaneous squamous cell carcinomas
- GEMMs
genetically engineered mouse models
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest. The authors declare that there are no conflicts of interest.
References
- Abdul M, Charron D, & Haziot A (2008). Selective effects of cyclosporine a on Th2-skewed dendritic cells matured with viral-like stimulus by means of toll-like receptors. Transplantation, 86(6), 880–884. doi: 10.1097/TP.0b013e3181861f1d [DOI] [PubMed] [Google Scholar]
- Adami J, Frisch M, Yuen J, Glimelius B, & Melbye M (1995). Evidence of an association between non-Hodgkin's lymphoma and skin cancer. Bmj, 310(6993), 1491–1495. doi: 10.1136/bmj.310.6993.1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal N, Frederick MJ, Pickering CR, Bettegowda C, Chang K, Li RJ, . . . Myers JN (2011). Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science., 333(6046), 1154–1157. doi: 1110.1126/science.1206923. Epub 1202011 Jul 1206928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akagi K, Li J, Broutian TR, Padilla-Nash H, Xiao W, Jiang B, . . . Gillison ML (2014). Genome-wide analysis of HPV integration in human cancers reveals recurrent, focal genomic instability. Genome Res, 24(2), 185–199. doi: 10.1101/gr.164806.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam M, & Ratner D (2001). Cutaneous squamous-cell carcinoma. N Engl J Med., 344(13), 975–983. [DOI] [PubMed] [Google Scholar]
- Alcolea MP, Greulich P, Wabik A, Frede J, Simons BD, & Jones PH (2014). Differentiation imbalance in single oesophageal progenitor cells causes clonal immortalization and field change. Nat Cell Biol., 16(6), 615–622. doi: 610.1038/ncb2963. Epub 2014 May 1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, . . . Gillison ML (2010). Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med, 363(1), 24–35. doi: 10.1056/NEJMoa0912217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argiris A, Karamouzis MV, Raben D, & Ferris RL (2008). Head and neck cancer. Lancet, 371(9625), 1695–1709. doi: 10.1016/s0140-6736(08)60728-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnault JP, Wechsler J, Escudier B, Spatz A, Tomasic G, Sibaud V, . . . Robert C (2009). Keratoacanthomas and squamous cell carcinomas in patients receiving sorafenib. J Clin Oncol, 27(23), e59–61. doi: 10.1200/jco.2009.23.4823 [DOI] [PubMed] [Google Scholar]
- Arnold I, & Watt FM (2001). c-Myc activation in transgenic mouse epidermis results in mobilization of stem cells and differentiation of their progeny. Curr Biol, 11(8), 558–568. [DOI] [PubMed] [Google Scholar]
- Arwert EN, Hoste E, & Watt FM (2012). Epithelial stem cells, wound healing and cancer. Nat Rev Cancer., 12(3), 170–180. doi: 110.1038/nrc3217. [DOI] [PubMed] [Google Scholar]
- Bailey P, Chang DK, Nones K, Johns AL, Patch AM, Gingras MC, . . . Grimmond SM (2016). Genomic analyses identify molecular subtypes of pancreatic cancer. Nature., 531(7592), 47–52. doi: 10.1038/nature16965. Epub 12016 Feb 16924. [DOI] [PubMed] [Google Scholar]
- Balanis NG, Sheu KM, Esedebe FN, Patel SJ, Smith BA, Park JW, . . . Graeber TG (2019). Pan-cancer Convergence to a Small-Cell Neuroendocrine Phenotype that Shares Susceptibilities with Hematological Malignancies. Cancer Cell, 36(1), 17–34.e17. doi: 10.1016/j.ccell.2019.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldus CD, Thibaut J, Goekbuget N, Stroux A, Schlee C, Mossner M, . . . Hofmann WK (2009). Prognostic implications of NOTCH1 and FBXW7 mutations in adult acute T-lymphoblastic leukemia. Haematologica, 94(10), 1383–1390. doi: 10.3324/haematol.2008.005272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkwill F, & Mantovani A (2001). Inflammation and cancer: back to Virchow? Lancet., 357(9255), 539–545. [DOI] [PubMed] [Google Scholar]
- Balmain A, Ramsden M, Bowden GT, & Smith J (1984). Activation of the mouse cellular Harvey-ras gene in chemically induced benign skin papillomas. Nature, 307(5952), 658–660. [DOI] [PubMed] [Google Scholar]
- Balmain A, & Yuspa SH (2014). Milestones in skin carcinogenesis: the biology of multistage carcinogenesis. J Invest Dermatol, 134(e1), E2–7. doi: 10.1038/skinbio.2014.2 [DOI] [PubMed] [Google Scholar]
- Balz V, Scheckenbach K, Gotte K, Bockmuhl U, Petersen I, & Bier H (2003). Is the p53 inactivation frequency in squamous cell carcinomas of the head and neck underestimated? Analysis of p53 exons 2-11 and human papillomavirus 16/18 E6 transcripts in 123 unselected tumor specimens. Cancer Res, 63(6), 1188–1191. [PubMed] [Google Scholar]
- Bandla S, Pennathur A, Luketich JD, Beer DG, Lin L, Bass AJ, . . . Litle VR (2012). Comparative genomics of esophageal adenocarcinoma and squamous cell carcinoma. Ann Thorac Surg, 93(4), 1101–1106. doi: 10.1016/j.athoracsur.2012.01.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao X, Rubin AJ, Qu K, Zhang J, Giresi PG, Chang HY, & Khavari PA (2015). A novel ATAC-seq approach reveals lineage-specific reinforcement of the open chromatin landscape via cooperation between BAF and p63. Genome Biol., 16(1), 284. doi: 210.1186/s13059-13015-10840-13059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbieri CE, Barton CE, & Pietenpol JA (2003). Delta Np63 alpha expression is regulated by the phosphoinositide 3-kinase pathway. J Biol Chem, 278(51), 51408–51414. doi: 10.1074/jbc.M309943200 [DOI] [PubMed] [Google Scholar]
- Barbieri CE, Tang LJ, Brown KA, & Pietenpol JA (2006). Loss of p63 leads to increased cell migration and up-regulation of genes involved in invasion and metastasis. Cancer Res, 66(15), 7589–7597. doi: 10.1158/0008-5472.Can-06-2020 [DOI] [PubMed] [Google Scholar]
- Bass AJ, Watanabe H, Mermel CH, Yu S, Perner S, Verhaak RG, . . . Meyerson M (2009). SOX2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nat Genet, 41(11), 1238–1242. doi: 10.1038/ng.465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastin KT, Steeves RA, & Richards MJ (1997). Radiation therapy for squamous cell carcinoma in dystrophic epidermolysis bullosa: case reports and literature review. Am J Clin Oncol, 20(1), 55–58. [DOI] [PubMed] [Google Scholar]
- Beachy PA, Karhadkar SS, & Berman DM (2004). Tissue repair and stem cell renewal in carcinogenesis. Nature., 432(7015), 324–331. [DOI] [PubMed] [Google Scholar]
- Beck B, & Blanpain C (2013). Unravelling cancer stem cell potential. Nat Rev Cancer, 13(10), 727–738. doi: 10.1038/nrc3597 [DOI] [PubMed] [Google Scholar]
- Beder LB, Gunduz M, Ouchida M, Fukushima K, Gunduz E, Ito S, . . . Shimizu K (2003). Genome-wide analyses on loss of heterozygosity in head and neck squamous cell carcinomas. Lab Invest, 83(1), 99–105. [DOI] [PubMed] [Google Scholar]
- Beltran H, Prandi D, Mosquera JM, Benelli M, Puca L, Cyrta J, . . . Demichelis F (2016). Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat Med, 8(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belum VR, Fontanilla Patel H, Lacouture ME, & Rodeck U (2013). Skin toxicity of targeted cancer agents: mechanisms and intervention. Future Oncol, 9(8), 1161–1170. doi: 10.2217/fon.13.62 [DOI] [PubMed] [Google Scholar]
- Benitah SA (2012). Defining an epidermal stem cell epigenetic network. Nat Cell Biol., 14(7), 652–653. doi: 610.1038/ncb2538. [DOI] [PubMed] [Google Scholar]
- Berenson JR, Yang J, & Mickel RA (1989). Frequent amplification of the bcl-1 locus in head and neck squamous cell carcinomas. Oncogene, 4(9), 1111–1116. [PubMed] [Google Scholar]
- Berg D, & Otley CC (2002). Skin cancer in organ transplant recipients: Epidemiology, pathogenesis, and management. J Am Acad Dermatol, 47(1), 1–17; quiz 18-20. doi: 10.1067/mjd.2002.125579 [DOI] [PubMed] [Google Scholar]
- Beroukhim R, Mermel CH, Porter D, Wei G, Raychaudhuri S, Donovan J, . . . Meyerson M (2010). The landscape of somatic copy-number alteration across human cancers. Nature, 463(7283), 899–905. doi: 10.1038/nature08822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya A, Roy R, Snijders AM, Hamilton G, Paquette J, Tokuyasu T, . . . Albertson DG (2011). Two distinct routes to oral cancer differing in genome instability and risk for cervical node metastasis. Clin Cancer Res, 17(22), 7024–7034. doi: 10.1158/1078-0432.Ccr-11-1944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian J, & Sun Y (1997). p53CP, a putative p53 competing protein that specifically binds to the consensus p53 DNA binding sites: a third member of the p53 family? Proc Natl Acad Sci U S A, 94(26), 14753–14758. doi: 10.1073/pnas.94.26.14753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkeland SA, Storm HH, Lamm LU, Barlow L, Blohme I, Forsberg B, . . . et al. (1995). Cancer risk after renal transplantation in the Nordic countries, 1964-1986. Int J Cancer, 60(2), 183–189. doi: 10.1002/ijc.2910600209 [DOI] [PubMed] [Google Scholar]
- Bishop JA, Teruya-Feldstein J, Westra WH, Pelosi G, Travis WD, & Rekhtman N (2012). p40 (DeltaNp63) is superior to p63 for the diagnosis of pulmonary squamous cell carcinoma. Mod Pathol, 25(3), 405–415. doi: 10.1038/modpathol.2011.173 [DOI] [PubMed] [Google Scholar]
- Blanpain C (2013). Tracing the cellular origin of cancer. Nat Cell Biol, 15(2), 126–134. doi: 10.1038/ncb2657 [DOI] [PubMed] [Google Scholar]
- Blanpain C, & Fuchs E (2014). Stem cell plasticity. Plasticity of epithelial stem cells in tissue regeneration. Science., 344(6189), 1242281. doi: 1242210.1241126/science.1242281. Epub 1242014 Jun 1242212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockmuhl U, Petersen S, Schmidt S, Wolf G, Jahnke V, Dietel M, & Petersen I (1997). Patterns of chromosomal alterations in metastasizing and nonmetastasizing primary head and neck carcinomas. Cancer Res, 57(23), 5213–5216. [PubMed] [Google Scholar]
- Boglev Y, Wilanowski T, Caddy J, Parekh V, Auden A, Darido C, . . . Jane SM (2011). The unique and cooperative roles of the Grainy head-like transcription factors in epidermal development reflect unexpected target gene specificity. Dev Biol, 349(2), 512–522. doi: 10.1016/j.ydbio.2010.11.011 [DOI] [PubMed] [Google Scholar]
- Borghi-Cirri MB, Riccardi-Arbi R, Bacci S, Mori M, Pimpinelli N, Romagnoli P, & Filipponi F (2001). Inhibited differentiation of Langerhans cells in the rat epidermis upon systemic treatment with cyclosporin A. Histol Histopathol, 16(1), 107–112. doi: 10.14670/hh-16.107 [DOI] [PubMed] [Google Scholar]
- Botelho MC, Machado JC, Brindley PJ, & Correia da Costa JM (2011). Targeting molecular signaling pathways of Schistosoma haemotobium infection in bladder cancer. Virulence, 2(4), 267–279. doi: 10.4161/viru.2.4.16734 [DOI] [PubMed] [Google Scholar]
- Boukamp P (2005). Non-melanoma skin cancer: what drives tumor development and progression? Carcinogenesis, 26(10), 1657–1667. doi: 10.1093/carcin/bgi123 [DOI] [PubMed] [Google Scholar]
- Boumahdi S, Driessens G, Lapouge G, Rorive S, Nassar D, Le Mercier M, . . . Blanpain C (2014). SOX2 controls tumour initiation and cancer stem-cell functions in squamous-cell carcinoma. Nature, 511(7508), 246–250. doi: 10.1038/nature13305 [DOI] [PubMed] [Google Scholar]
- Bouwes Bavinck JN, Hardie DR, Green A, Cutmore S, MacNaught A, O'Sullivan B, . . . Hardie IR (1996). The risk of skin cancer in renal transplant recipients in Queensland, Australia. A follow-up study. Transplantation, 61(5), 715–721. doi: 10.1097/00007890-199603150-00008 [DOI] [PubMed] [Google Scholar]
- Boxer LD, Barajas B, Tao S, Zhang J, & Khavari PA (2014). ZNF750 interacts with KLF4 and RCOR1, KDM1A, and CTBP1/2 chromatin regulators to repress epidermal progenitor genes and induce differentiation genes. Genes Dev., 28(18), 2013–2026. doi: 2010.1101/gad.246579.246114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braakhuis BJ, Snijders PJ, Keune WJ, Meijer CJ, Ruijter-Schippers HJ, Leemans CR, & Brakenhoff RH (2004). Genetic patterns in head and neck cancers that contain or lack transcriptionally active human papillomavirus. J Natl Cancer Inst, 96(13), 998–1006. doi: 10.1093/jnci/djh183 [DOI] [PubMed] [Google Scholar]
- Braakhuis BJ, Tabor MP, Kummer JA, Leemans CR, & Brakenhoff RH (2003). A genetic explanation of Slaughter's concept of field cancerization: evidence and clinical implications. Cancer Res, 63(8), 1727–1730. [PubMed] [Google Scholar]
- Brash DE, & Haseltine WA (1982). UV-induced mutation hotspots occur at DNA damage hotspots. Nature, 298(5870), 189–192. [DOI] [PubMed] [Google Scholar]
- Brash DE, Rudolph JA, Simon JA, Lin A, McKenna GJ, Baden HP, . . . Ponten J (1991). A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proc Natl Acad Sci U S A, 88(22), 10124–10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan JA, Boyle JO, Koch WM, Goodman SN, Hruban RH, Eby YJ, . . . Sidransky D (1995). Association between cigarette smoking and mutation of the p53 gene in squamous-cell carcinoma of the head and neck. N Engl J Med, 332(11), 712–717. doi: 10.1056/nejm199503163321104 [DOI] [PubMed] [Google Scholar]
- Brown VL, Harwood CA, Crook T, Cronin JG, Kelsell DP, & Proby CM (2004). p16INK4a and p14ARF tumor suppressor genes are commonly inactivated in cutaneous squamous cell carcinoma. J Invest Dermatol, 122(5), 1284–1292. doi: 10.1111/j.0022-202X.2004.22501.x [DOI] [PubMed] [Google Scholar]
- Brunner HG, Hamel BC, & Van Bokhoven H (2002). The p63 gene in EEC and other syndromes. J Med Genet, 39(6), 377–381. doi: 10.1136/jmg.39.6.377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns PA, Jack A, Neilson F, Haddow S, & Balmain A (1991). Transformation of mouse skin endothelial cells in vivo by direct application of plasmid DNA encoding the human T24 H-ras oncogene. Oncogene, 6(11), 1973–1978. [PubMed] [Google Scholar]
- Califano J, van der Riet P, Westra W, Nawroz H, Clayman G, Piantadosi S, . . . Sidransky D (1996). Genetic progression model for head and neck cancer: implications for field cancerization. Cancer Res, 56(11), 2488–2492. [PubMed] [Google Scholar]
- Callen JP, Bickers DR, & Moy RL (1997). Actinic keratoses. J Am Acad Dermatol, 36(4), 650–653. [DOI] [PubMed] [Google Scholar]
- Campbell JD, Alexandrov A, Kim J, Wala J, Berger AH, Pedamallu CS, . . . Meyerson M (2016). Distinct patterns of somatic genome alterations in lung adenocarcinomas and squamous cell carcinomas. Nat Genet, 48(6), 607–616. doi: 10.1038/ng.3564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JD, Yau C, Bowlby R, Liu Y, Brennan K, Fan H, . . . Van Waes C (2018). Genomic, Pathway Network, and Immunologic Features Distinguishing Squamous Carcinomas. Cell Rep, 23(1), 194–212.e196. doi: 10.1016/j.celrep.2018.03.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Genome Atlas Network. (2014a). Comprehensive molecular characterization of gastric adenocarcinoma. Nature, 513(7517), 202–209. doi: 10.1038/nature13480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Genome Atlas Network. (2014b). Comprehensive molecular characterization of urothelial bladder carcinoma. Nature, 29(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Genome Atlas Network. (2014c). Comprehensive molecular profiling of lung adenocarcinoma. Nature., 511(7511), 543–550. doi: 510.1038/nature13385. Epub 12014 Jul 13389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Genome Atlas Network. (2015a). Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature, 517(7536), 576–582. doi: 10.1038/nature14129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Genome Atlas Network. (2015b). Comprehensive genomic profiles of small cell lung cancer. Nature, 13(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Genome Atlas Network. (2017). Integrated genomic characterization of oesophageal carcinoma. Nature, 541(7636), 169–175. doi: 10.1038/nature20805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research, N., Albert Einstein College of, M., Analytical Biological, S., Barretos Cancer, H., Baylor College of, M., Beckman Research Institute of City of, H., . . . Washington University in St, L. (2017). Integrated genomic and molecular characterization of cervical cancer. Nature, 543(7645), 378–384. doi: 10.1038/nature21386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candi E, Rufini A, Terrinoni A, Dinsdale D, Ranalli M, Paradisi A, . . . Melino G (2006). Differential roles of p63 isoforms in epidermal development: selective genetic complementation in p63 null mice. Cell Death Differ, 13(6), 1037–1047. doi: 10.1038/sj.cdd.4401926 [DOI] [PubMed] [Google Scholar]
- Cao P, Zhou L, Zhang J, Zheng F, Wang H, Ma D, & Tian J (2013). Comprehensive expression profiling of microRNAs in laryngeal squamous cell carcinoma. Head Neck., 35(5), 720–728. doi: 710.1002/hed.23011. Epub 22012 May 23018. [DOI] [PubMed] [Google Scholar]
- Carroll DK, Carroll JS, Leong CO, Cheng F, Brown M, Mills AA, . . . Ellisen LW (2006). p63 regulates an adhesion programme and cell survival in epithelial cells. Nat Cell Biol, 8(6), 551–561. doi: 10.1038/ncb1420 [DOI] [PubMed] [Google Scholar]
- Carucci JA, Martinez JC, Zeitouni NC, Christenson L, Coldiron B, Zweibel S, & Otley CC (2004). In-transit metastasis from primary cutaneous squamous cell carcinoma in organ transplant recipients and nonimmunosuppressed patients: clinical characteristics, management, and outcome in a series of 21 patients. Dermatol Surg, 30(4 Pt 2), 651–655. doi: 10.1111/j.1524-4725.2004.30151.x [DOI] [PubMed] [Google Scholar]
- Challen GA, Sun D, Jeong M, Luo M, Jelinek J, Berg JS, . . . Goodell MA (2011). Dnmt3a is essential for hematopoietic stem cell differentiation. Nat Genet, 44(1), 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HY, Sneddon JB, Alizadeh AA, Sood R, West RB, Montgomery K, . . . Brown PO (2004). Gene expression signature of fibroblast serum response predicts human cancer progression: similarities between tumors and wounds. PLoS Biol., 2(2), E7. Epub 2004 Jan 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao DL, Eck JT, Brash DE, Maley CC, & Luebeck EG (2008). Preneoplastic lesion growth driven by the death of adjacent normal stem cells. Proc Natl Acad Sci U S A, 105(39), 15034–15039. doi: 10.1073/pnas.0802211105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C, Zhou Y, Li H, Xiong T, Li S, Bi Y, . . . Cui, Y. (2016). Whole-Genome Sequencing Reveals Diverse Models of Structural Variations in Esophageal Squamous Cell Carcinoma. Am J Hum Genet, 98(2), 256–274. doi: 10.1016/j.ajhg.2015.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho RJ, Alexandrov LB, den Breems NY, Atanasova VS, Farshchian M, Purdom E, . . . South AP (2018). APOBEC mutation drives early-onset squamous cell carcinomas in recessive dystrophic epidermolysis bullosa. Sci Transl Med, 10(455). doi: 10.1126/scitranslmed.aas9668 [DOI] [PubMed] [Google Scholar]
- Chu TY, Shen CY, Lee HS, & Liu HS (1999). Monoclonality and surface lesion-specific microsatellite alterations in premalignant and malignant neoplasia of uterine cervix: a local field effect of genomic instability and clonal evolution. Genes Chromosomes Cancer, 24(2), 127–134. [DOI] [PubMed] [Google Scholar]
- Chung CH, Bernard PS, & Perou CM (2002). Molecular portraits and the family tree of cancer. Nat Genet, 32 Suppl, 533–540. doi: 10.1038/ng1038 [DOI] [PubMed] [Google Scholar]
- Chung CH, Ely K, McGavran L, Varella-Garcia M, Parker J, Parker N, . . . Hirsch FR (2006). Increased epidermal growth factor receptor gene copy number is associated with poor prognosis in head and neck squamous cell carcinomas. J Clin Oncol, 24(25), 4170–4176. doi: 10.1200/jco.2006.07.2587 [DOI] [PubMed] [Google Scholar]
- Chung CH, Parker JS, Ely K, Carter J, Yi Y, Murphy BA, . . . Yarbrough WG (2006). Gene expression profiles identify epithelial-to-mesenchymal transition and activation of nuclear factor-kappaB signaling as characteristics of a high-risk head and neck squamous cell carcinoma. Cancer Res, 66(16), 8210–8218. doi: 10.1158/0008-5472.can-06-1213 [DOI] [PubMed] [Google Scholar]
- Chung CH, Parker JS, Karaca G, Wu J, Funkhouser WK, Moore D, . . . Perou CM (2004). Molecular classification of head and neck squamous cell carcinomas using patterns of gene expression. Cancer Cell, 5(5), 489–500. [DOI] [PubMed] [Google Scholar]
- Chung TK, Van Hummelen P, Chan PK, Cheung TH, Yim SF, Yu MY, . . . Wong YF (2015). Genomic aberrations in cervical adenocarcinomas in Hong Kong Chinese women. Int J Cancer, 137(4), 776–783. doi: 10.1002/ijc.29456 [DOI] [PubMed] [Google Scholar]
- Chuong CM, Cotsarelis G, & Stenn K (2007). Defining hair follicles in the age of stem cell bioengineering. J Invest Dermatol, 127(9), 2098–2100. doi: 10.1038/sj.jid.5700947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciriello G, Miller ML, Aksoy BA, Senbabaoglu Y, Schultz N, & Sander C (2013). Emerging landscape of oncogenic signatures across human cancers. Nat Genet., 45(10), 1127–1133. doi: 1110.1038/ng.2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloos J, Spitz MR, Schantz SP, Hsu TC, Zhang ZF, Tobi H, . . . Snow GB (1996). Genetic susceptibility to head and neck squamous cell carcinoma. J Natl Cancer Inst, 88(8), 530–535. doi: 10.1093/jnci/88.8.530 [DOI] [PubMed] [Google Scholar]
- Collisson EA, Sadanandam A, Olson P, Gibb WJ, Truitt M, Gu S, . . . Gray JW (2011). Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat Med, 17(4), 500–503. doi: 10.1038/nm.2344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corces-Zimmerman MR, Hong WJ, Weissman IL, Medeiros BC, & Majeti R (2014). Preleukemic mutations in human acute myeloid leukemia affect epigenetic regulators and persist in remission. Proc Natl Acad Sci U S A, 111(7), 2548–2553. doi: 10.1073/pnas.1324297111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens LM, & Werb Z (2002). Inflammation and cancer. Nature., 420(6917), 860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crum CP, & McKeon FD (2010). p63 in epithelial survival, germ cell surveillance, and neoplasia. Annu Rev Pathol, 5, 349–371. doi: 10.1146/annurev-pathol-121808-102117 [DOI] [PubMed] [Google Scholar]
- Cui R, Kamatani Y, Takahashi A, Usami M, Hosono N, Kawaguchi T, . . . Matsuda K (2009). Functional variants in ADH1B and ALDH2 coupled with alcohol and smoking synergistically enhance esophageal cancer risk. Gastroenterology, 137(5), 1768–1775. doi: 10.1053/j.gastro.2009.07.070 [DOI] [PubMed] [Google Scholar]
- D'Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, Koch WM, . . . Gillison ML (2007). Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med, 356(19), 1944–1956. doi: 10.1056/NEJMoa065497 [DOI] [PubMed] [Google Scholar]
- Dantal J, Hourmant M, Cantarovich D, Giral M, Blancho G, Dreno B, & Soulillou JP (1998). Effect of long-term immunosuppression in kidney-graft recipients on cancer incidence: randomised comparison of two cyclosporin regimens. Lancet, 351(9103), 623–628. doi: 10.1016/s0140-6736(97)08496-1 [DOI] [PubMed] [Google Scholar]
- Dargent JL, Kornreich A, Andre L, & Lespagnard L (1998). Cutaneous infiltrate of chronic lymphocytic leukemia surrounding a primary squamous cell carcinoma of the skin. Report of an additional case and reflection on its pathogenesis. J Cutan Pathol, 25(9), 479–480. [DOI] [PubMed] [Google Scholar]
- Darido C, Georgy SR, Cullinane C, Partridge DD, Walker R, Srivastava S, . . . Jane SM (2018). Stage-dependent therapeutic efficacy in PI3K/mTOR-driven squamous cell carcinoma of the skin. Cell Death Differ, 25(6), 1146–1159. doi: 10.1038/s41418-017-0032-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daya-Grosjean L, Robert C, Drougard C, Suarez H, & Sarasin A (1993). High mutation frequency in ras genes of skin tumors isolated from DNA repair deficient xeroderma pigmentosum patients. Cancer Res, 53(7), 1625–1629. [PubMed] [Google Scholar]
- Demehri S, Turkoz A, & Kopan R (2009). Epidermal Notch1 loss promotes skin tumorigenesis by impacting the stromal microenvironment. Cancer Cell., 16(1), 55–66. doi: 10.1016/j.ccr.2009.1005.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng N, Goh LK, Wang H, Das K, Tao J, Tan IB, . . . Tan P (2012). A comprehensive survey of genomic alterations in gastric cancer reveals systematic patterns of molecular exclusivity and co-occurrence among distinct therapeutic targets. Gut, 61(5), 673–684. doi: 10.1136/gutjnl-2011-301839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeStefano GM, & Christiano AM (2014). The genetics of human skin disease. Cold Spring Harb Perspect Med, 4(10). doi: 10.1101/cshperspect.a015172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devgan V, Mammucari C, Millar SE, Brisken C, & Dotto GP (2005). p21WAF1/Cip1 is a negative transcriptional regulator of Wnt4 expression downstream of Notch1 activation. Genes Dev., 19(12), 1485–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Como CJ, Urist MJ, Babayan I, Drobnjak M, Hedvat CV, Teruya-Feldstein J, . . . Cordon-Cardo C (2002). p63 expression profiles in human normal and tumor tissues. Clin Cancer Res, 8(2), 494–501. [PubMed] [Google Scholar]
- Di Ianni M, Baldoni S, Rosati E, Ciurnelli R, Cavalli L, Martelli MF, . . . Falzetti F (2009). A new genetic lesion in B-CLL: a NOTCH1 PEST domain mutation. Br J Haematol, 146(6), 689–691. doi: 10.1111/j.1365-2141.2009.07816.x [DOI] [PubMed] [Google Scholar]
- Dohn M, Zhang S, & Chen X (2001). p63alpha and DeltaNp63alpha can induce cell cycle arrest and apoptosis and differentially regulate p53 target genes. Oncogene, 20(25), 3193–3205. doi: 10.1038/sj.onc.1204427 [DOI] [PubMed] [Google Scholar]
- Dong JT, & Chen C (2009). Essential role of KLF5 transcription factor in cell proliferation and differentiation and its implications for human diseases. Cell Mol Life Sci, 66(16), 2691–2706. doi: 10.1007/s00018-009-0045-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotto GP (2014). Multifocal epithelial tumors and field cancerization: stroma as a primary determinant. J Clin Invest, 124(4), 1446–1453. doi: 10.1172/jci72589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotto GP, & Rustgi AK (2016). Squamous Cell Cancers: A Unified Perspective on Biology and Genetics. Cancer Cell, 29(5), 622–637. doi: 10.1016/j.ccell.2016.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulak AM, Schumacher SE, van Lieshout J, Imamura Y, Fox C, Shim B, . . . Bass AJ (2012). Gastrointestinal adenocarcinomas of the esophagus, stomach, and colon exhibit distinct patterns of genome instability and oncogenesis. Cancer Res, 72(17), 4383–4393. doi: 10.1158/0008-5472.Can-11-3893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuy P, Bagot M, Michel L, Descourt B, & Dubertret L (1991). Cyclosporin A inhibits the antigen-presenting functions of freshly isolated human Langerhans cells in vitro. J Invest Dermatol, 96(4), 408–413. doi: 10.1111/1523-1747.ep12469772 [DOI] [PubMed] [Google Scholar]
- Durinck S, Ho C, Wang NJ, Liao W, Jakkula LR, Collisson EA, . . . Cho RJ (2011). Temporal dissection of tumorigenesis in primary cancers. Cancer Discov, 1(2), 137–143. doi: 10.1158/2159-8290.cd-11-0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutt A, Ramos AH, Hammerman PS, Mermel C, Cho J, Sharifnia T, . . . Meyerson M (2011). Inhibitor-sensitive FGFR1 amplification in human non-small cell lung cancer. PLoS One, 6(6), e20351. doi: 10.1371/journal.pone.0020351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak HF (1986). Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med., 315(26), 1650–1659. [DOI] [PubMed] [Google Scholar]
- Eckhardt M, Zhang W, Gross AM, Von Dollen J, Johnson JR, Franks-Skiba KE, . . . Krogan NJ (2018). Multiple Routes to Oncogenesis Are Promoted by the Human Papillomavirus-Host Protein Network. Cancer Discov, 8(11), 1474–1489. doi: 10.1158/2159-8290.Cd-17-1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehsanian R, Brown M, Lu H, Yang XP, Pattatheyil A, Yan B, . . . Van Waes C (2010). YAP dysregulation by phosphorylation or DeltaNp63-mediated gene repression promotes proliferation, survival and migration in head and neck cancer subsets. Oncogene, 29(46), 6160–6171. doi: 10.1038/onc.2010.339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis SJ, Gomez NC, Levorse J, Mertz AF, Ge Y, & Fuchs E (2019). Distinct modes of cell competition shape mammalian tissue morphogenesis. Nature, 569(7757), 497–502. doi: 10.1038/s41586-019-1199-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellisen LW, Bird J, West DC, Soreng AL, Reynolds TC, Smith SD, & Sklar J (1991). TAN-1, the human homolog of the Drosophila notch gene, is broken by chromosomal translocations in T lymphoblastic neoplasms. Cell, 66(4), 649–661. doi: 10.1016/0092-8674(91)90111-b [DOI] [PubMed] [Google Scholar]
- Ellisen LW, Ramsayer KD, Johannessen CM, Yang A, Beppu H, Minda K, . . . Haber DA (2002). REDD1, a developmentally regulated transcriptional target of p63 and p53, links p63 to regulation of reactive oxygen species. Mol Cell, 10(5), 995–1005. [DOI] [PubMed] [Google Scholar]
- Enoch S, Miller DR, Price PE, & Harding KG (2004). Early diagnosis is vital in the management of squamous cell carcinomas associated with chronic non healing ulcers: a case series and review of the literature. Int Wound J, 1(3), 165–175. doi: 10.1111/j.1742-4801.2004.00056.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euvrard S, Kanitakis J, & Claudy A (2003). Skin cancers after organ transplantation. N Engl J Med, 348(17), 1681–1691. doi: 10.1056/NEJMra022137 [DOI] [PubMed] [Google Scholar]
- Euvrard S, Kanitakis J, Pouteil-Noble C, Disant F, Dureau G, Finaz de Villaine J, . . . Thivolet J (1995). Aggressive squamous cell carcinomas in organ transplant recipients. Transplant Proc, 27(2), 1767–1768. [PubMed] [Google Scholar]
- Extance A (2010). Alzheimer's failure raises questions about disease-modifying strategies. Nat Rev Drug Discov, 9(10), 749–751. doi: 10.1038/nrd3288 [DOI] [PubMed] [Google Scholar]
- Fabbri G, Rasi S, Rossi D, Trifonov V, Khiabanian H, Ma J, . . . Gaidano G (2011). Analysis of the chronic lymphocytic leukemia coding genome: role of NOTCH1 mutational activation. J Exp Med, 208(7), 1389–1401. doi: 10.1084/jem.20110921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facompre N, Nakagawa H, Herlyn M, & Basu D (2012). Stem-like cells and therapy resistance in squamous cell carcinomas. Adv Pharmacol, 65, 235–265. doi: 10.1016/b978-0-12-397927-8.00008-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadlullah MZ, Chiang IK, Dionne KR, Yee PS, Gan CP, Sam KK, . . . Cheong SC (2016). Genetically-defined novel oral squamous cell carcinoma cell lines for the development of molecular therapies. Oncotarget, 7(19), 27802–27818. doi: 10.18632/oncotarget.8533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Wang D, Burgmaier JE, Teng Y, Romano RA, Sinha S, & Yi R (2018). Single Cell and Open Chromatin Analysis Reveals Molecular Origin of Epidermal Cells of the Skin. Dev Cell, 47(1), 21–37.e25. doi: 10.1016/j.devcel.2018.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon ER, Hamilton SR, & Vogelstein B (1987). Clonal analysis of human colorectal tumors. Science, 238(4824), 193–197. [DOI] [PubMed] [Google Scholar]
- Fearon ER, & Vogelstein B (1990). A genetic model for colorectal tumorigenesis. Cell, 61(5), 759–767. [DOI] [PubMed] [Google Scholar]
- Ferone G, Song JY, Sutherland KD, Bhaskaran R, Monkhorst K, Lambooij JP, . . . Berns A (2016). SOX2 Is the Determining Oncogenic Switch in Promoting Lung Squamous Cell Carcinoma from Different Cells of Origin. Cancer Cell, 30(4), 519–532. doi: 10.1016/j.ccell.2016.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores ER, Sengupta S, Miller JB, Newman JJ, Bronson R, Crowley D, . . . Jacks T (2005). Tumor predisposition in mice mutant for p63 and p73: evidence for broader tumor suppressor functions for the p53 family. Cancer Cell., 7(4), 363–373. [DOI] [PubMed] [Google Scholar]
- Flores ER, Tsai KY, Crowley D, Sengupta S, Yang A, McKeon F, & Jacks T (2002). p63 and p73 are required for p53-dependent apoptosis in response to DNA damage. Nature, 416(6880), 560–564. doi: 10.1038/416560a [DOI] [PubMed] [Google Scholar]
- Forman AB, Roenigk HH Jr., Caro WA, & Magid ML (1989). Long-term follow-up of skin cancer in the PUVA-48 cooperative study. Arch Dermatol, 125(4), 515–519. [PubMed] [Google Scholar]
- Franklin WA, Gazdar AF, Haney J, Wistuba II, La Rosa FG, Kennedy T, . . . Miller YE (1997). Widely dispersed p53 mutation in respiratory epithelium. A novel mechanism for field carcinogenesis. J Clin Invest, 100(8), 2133–2137. doi: 10.1172/jci119748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freier K, Knoepfle K, Flechtenmacher C, Pungs S, Devens F, Toedt G, . . . Radlwimmer B (2010). Recurrent copy number gain of transcription factor SOX2 and corresponding high protein expression in oral squamous cell carcinoma. Genes Chromosomes Cancer, 49(1), 9–16. doi: 10.1002/gcc.20714 [DOI] [PubMed] [Google Scholar]
- Gallico GG 3rd, O'Connor NE, Compton CC, Kehinde O, & Green H (1984). Permanent coverage of large burn wounds with autologous cultured human epithelium. N Engl J Med, 311(7), 448–451. doi: 10.1056/nejm198408163110706 [DOI] [PubMed] [Google Scholar]
- Gandarillas A, & Watt FM (1997). c-Myc promotes differentiation of human epidermal stem cells. Genes Dev, 11(21), 2869–2882. doi: 10.1101/gad.11.21.2869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao YB, Chen ZL, Li JG, Hu XD, Shi XJ, Sun ZM, . . . He J (2014). Genetic landscape of esophageal squamous cell carcinoma. Nat Genet, 24(10). [DOI] [PubMed] [Google Scholar]
- Garraway LA, & Lander ES (2013). Lessons from the cancer genome. Cell, 153(1), 17–37. doi: 10.1016/j.cell.2013.03.002 [DOI] [PubMed] [Google Scholar]
- Garraway LA, & Sellers WR (2006). Lineage dependency and lineage-survival oncogenes in human cancer. Nat Rev Cancer, 6(8), 593–602. doi: 10.1038/nrc1947 [DOI] [PubMed] [Google Scholar]
- Garraway LA, Widlund HR, Rubin MA, Getz G, Berger AJ, Ramaswamy S, . . . Sellers WR (2005). Integrative genomic analyses identify MITF as a lineage survival oncogene amplified in malignant melanoma. Nature, 436(7047), 117–122. doi: 10.1038/nature03664 [DOI] [PubMed] [Google Scholar]
- Gaykalova DA, Mambo E, Choudhary A, Houghton J, Buddavarapu K, Sanford T, . . . Sun W (2014). Novel insight into mutational landscape of head and neck squamous cell carcinoma. PLoS One, 9(3), e93102. doi: 10.1371/journal.pone.0093102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Y, & Fuchs E (2018). Stretching the limits: from homeostasis to stem cell plasticity in wound healing and cancer. Nat Rev Genet, 19(5), 311–325. doi: 10.1038/nrg.2018.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Y, Gomez NC, Adam RC, Nikolova M, Yang H, Verma A, . . . Fuchs E (2017). Stem cell Lineage Infidelity Drives Wound Repair and Cancer. Cell, 169(4), 636–650 e614. doi: 10.1016/j.cell.2017.03.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghioni P, Bolognese F, Duijf PH, Van Bokhoven H, Mantovani R, & Guerrini L (2002). Complex transcriptional effects of p63 isoforms: identification of novel activation and repression domains. Mol Cell Biol, 22(24), 8659–8668. doi: 10.1128/mcb.22.24.8659-8668.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giefing M, Wierzbicka M, Rydzanicz M, Cegla R, Kujawski M, & Szyfter K (2008). Chromosomal gains and losses indicate oncogene and tumor suppressor gene candidates in salivary gland tumors. Neoplasma, 55(1), 55–60. [PubMed] [Google Scholar]
- Gillison ML, Akagi K, Xiao W, Jiang B, Pickard RKL, Li J, . . . Symer DE (2019). Human papillomavirus and the landscape of secondary genetic alterations in oral cancers. Genome Res, 29(1), 1–17. doi: 10.1101/gr.241141.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L, . . . Sidransky D (2000). Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst, 92(9), 709–720. doi: 10.1093/jnci/92.9.709 [DOI] [PubMed] [Google Scholar]
- Grabowski J, Saltzstein SL, Sadler G, & Blair S (2009). Squamous cell carcinoma of the breast: a review of 177 cases. Am Surg, 75(10), 914–917. [PubMed] [Google Scholar]
- Gross N, Ferrarotto R, Nagarajan P, Bell D, El-Naggar A, Johnson JM, . . . Myers J (2019). LBA74 Phase II study of neoadjuvant cemiplimab prior to surgery in patients with stage III/IV (M0) cutaneous squamous cell carcinoma of the head and neck (CSCC-HN). Annals of Oncology, 30(Supplement_5). doi: 10.1093/annonc/mdz394.071 [DOI] [Google Scholar]
- Guba M, Graeb C, Jauch KW, & Geissler EK (2004). Pro- and anti-cancer effects of immunosuppressive agents used in organ transplantation. Transplantation, 77(12), 1777–1782. doi: 10.1097/01.tp.0000120181.89206.54 [DOI] [PubMed] [Google Scholar]
- Gurtner GC, Werner S, Barrandon Y, & Longaker MT (2008). Wound repair and regeneration. Nature, 453(7193), 314–321. doi: 10.1038/nature07039 [DOI] [PubMed] [Google Scholar]
- Haddad RI, & Shin DM (2008). Recent advances in head and neck cancer. N Engl J Med, 359(11), 1143–1154. doi: 10.1056/NEJMra0707975 [DOI] [PubMed] [Google Scholar]
- Hafkamp HC, Speel EJ, Haesevoets A, Bot FJ, Dinjens WN, Ramaekers FC, . . . Manni JJ (2003). A subset of head and neck squamous cell carcinomas exhibits integration of HPV 16/18 DNA and overexpression of p16INK4A and p53 in the absence of mutations in p53 exons 5-8. Int J Cancer, 107(3), 394–400. doi: 10.1002/ijc.11389 [DOI] [PubMed] [Google Scholar]
- Hall-Jackson CA, Eyers PA, Cohen P, Goedert M, Boyle FT, Hewitt N, . . . Hedge P (1999). Paradoxical activation of Raf by a novel Raf inhibitor. Chem Biol, 6(8), 559–568. [DOI] [PubMed] [Google Scholar]
- Hammerman PS, Lawrence MS, Voet D, Jing R, Cibulskis K, Sivachenko A, . . . Network, C. G. A. (2012). Comprehensive genomic characterization of squamous cell lung cancers. Nature, 489(7417), 519–525. doi: 10.1038/nature11404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerman PS, Sos ML, Ramos AH, Xu C, Dutt A, Zhou W, . . . Meyerson M (2011). Mutations in the DDR2 kinase gene identify a novel therapeutic target in squamous cell lung cancer. Cancer Discov, 1(1), 78–89. doi: 10.1158/2159-8274.Cd-11-0005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han W, Ming M, He TC, & He YY (2010). Immunosuppressive cyclosporin A activates AKT in keratinocytes through PTEN suppression: implications in skin carcinogenesis. J Biol Chem, 285(15), 11369–11377. doi: 10.1074/jbc.M109.028142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, & Weinberg RA (2000). The hallmarks of cancer. Cell, 100(1), 57–70. [DOI] [PubMed] [Google Scholar]
- Hanahan D, & Weinberg RA (2011). Hallmarks of cancer: the next generation. Cell, 144(5), 646–674. doi: 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- Hartevelt MM, Bavinck JN, Kootte AM, Vermeer BJ, & Vandenbroucke JP (1990). Incidence of skin cancer after renal transplantation in The Netherlands. Transplantation, 49(3), 506–509. doi: 10.1097/00007890-199003000-00006 [DOI] [PubMed] [Google Scholar]
- Harwood CA, Mesher D, McGregor JM, Mitchell L, Leedham-Green M, Raftery M, . . . Proby CM (2013). A surveillance model for skin cancer in organ transplant recipients: a 22-year prospective study in an ethnically diverse population. Am J Transplant, 13(1), 119–129. doi: 10.1111/j.1600-6143.2012.04292.x [DOI] [PubMed] [Google Scholar]
- Hasegawa W, Pond GR, Rifkind JT, Messner HA, Lau A, Daly AS, . . . Lipton JH (2005). Long-term follow-up of secondary malignancies in adults after allogeneic bone marrow transplantation. Bone Marrow Transplant, 35(1), 51–55. doi: 10.1038/sj.bmt.1704706 [DOI] [PubMed] [Google Scholar]
- Hatzivassiliou G, Song K, Yen I, Brandhuber BJ, Anderson DJ, Alvarado R, . . . Malek S (2010). RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature, 464(7287), 431–435. doi: 10.1038/nature08833 [DOI] [PubMed] [Google Scholar]
- Hayes TF, Benaich N, Goldie SJ, Sipila K, Ames-Draycott A, Cai W, . . . Watt FM (2016). Integrative genomic and functional analysis of human oral squamous cell carcinoma cell lines reveals synergistic effects of FAT1 and CASP8 inactivation. Cancer Lett, 383(1), 106–114. doi: 10.1016/j.canlet.2016.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Shao F, Pi W, Shi C, Chen Y, Gong D, . . . Tang K. (2016). Largescale Transcriptomics Analysis Suggests Over-Expression of BGH3, MMP9 and PDIA3 in Oral Squamous Cell Carcinoma. PLoS One, 11(1), e0146530. doi: 10.1371/journal.pone.0146530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedberg ML, Goh G, Chiosea SI, Bauman JE, Freilino ML, Zeng Y, . . . Grandis JR (2016). Genetic landscape of metastatic and recurrent head and neck squamous cell carcinoma. J Clin Invest, 126(1), 169–180. doi: 10.1172/jci82066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidorn SJ, Milagre C, Whittaker S, Nourry A, Niculescu-Duvas I, Dhomen N, . . . Marais R (2010). Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell, 140(2), 209–221. doi: 10.1016/j.cell.2009.12.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helton ES, Zhu J, & Chen X (2006). The unique NH2-terminally deleted (DeltaN) residues, the PXXP motif, and the PPXY motif are required for the transcriptional activity of the DeltaN variant of p63. J Biol Chem, 281(5), 2533–2542. doi: 10.1074/jbc.M507964200 [DOI] [PubMed] [Google Scholar]
- Hennings H, Michael D, Cheng C, Steinert P, Holbrook K, & Yuspa SH (1980). Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell, 19(1), 245–254. [DOI] [PubMed] [Google Scholar]
- Hermsen M, Guervos MA, Meijer G, Baak J, van Diest P, Marcos CA, & Sampedro A (2001). New chromosomal regions with high-level amplifications in squamous cell carcinomas of the larynx and pharynx, identified by comparative genomic hybridization. J Pathol, 194(2), 177–182. doi: 10.1002/path.862 [DOI] [PubMed] [Google Scholar]
- Hibi K, Trink B, Patturajan M, Westra WH, Caballero OL, Hill DE, . . . Sidransky D (2000). AIS is an oncogene amplified in squamous cell carcinoma. Proc Natl Acad Sci U S A, 97(10), 5462–5467. doi: 10.1073/pnas.97.10.5462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho PL, Kurtova A, & Chan KS (2012). Normal and neoplastic urothelial stem cells: getting to the root of the problem. Nat Rev Urol, 9(10), 583–594. doi: 10.1038/nrurol.2012.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoadley KA, Yau C, Hinoue T, Wolf DM, Lazar AJ, Drill E, . . . Laird PW (2018). Cell-of-Origin Patterns Dominate the Molecular Classification of 10,000 Tumors from 33 Types of Cancer. Cell, 173(2), 291–304.e296. doi: 10.1016/j.cell.2018.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoadley KA, Yau C, Wolf DM, Cherniack AD, Tamborero D, Ng S, . . . Stuart JM (2014). Multiplatform Analysis of 12 Cancer Types Reveals Molecular Classification within and across Tissues of Origin. Cell., 158(4), 929–944. doi: 910.1016/j.cell.2014.1006.1049. Epub 2014 Aug 1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojo M, Morimoto T, Maluccio M, Asano T, Morimoto K, Lagman M, . . . Suthanthiran M (1999). Cyclosporine induces cancer progression by a cell-autonomous mechanism. Nature, 397(6719), 530–534. doi: 10.1038/17401 [DOI] [PubMed] [Google Scholar]
- Hopkins J, Cescon DW, Tse D, Bradbury P, Xu W, Ma C, . . . Liu G (2008). Genetic polymorphisms and head and neck cancer outcomes: a review. Cancer Epidemiol Biomarkers Prev, 17(3), 490–499. doi: 10.1158/1055-9965.Epi-07-2714 [DOI] [PubMed] [Google Scholar]
- Hsu YC, Li L, & Fuchs E (2014). Emerging interactions between skin stem cells and their niches. Nat Med., 20(8), 847–856. doi: 810.1038/nm.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Castillo E, Harewood L, Ostano P, Reymond A, Dummer R, . . . Dotto GP (2012). Multifocal epithelial tumors and field cancerization from loss of mesenchymal CSL signaling. Cell., 149(6), 1207–1220. doi: 1210.1016/j.cell.2012.1203.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntly BJ, & Gilliland DG (2005). Leukaemia stem cells and the evolution of cancer-stem-cell research. Nat Rev Cancer, 5(4), 311–321. doi: 10.1038/nrc1592 [DOI] [PubMed] [Google Scholar]
- Hussenet T, Dali S, Exinger J, Monga B, Jost B, Dembele D, . . . du Manoir S (2010). SOX2 is an oncogene activated by recurrent 3q26.3 amplifications in human lung squamous cell carcinomas. PLoS One, 5(1), e8960. doi: 10.1371/journal.pone.0008960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihrie RA, Marques MR, Nguyen BT, Horner JS, Papazoglu C, Bronson RT, . . . Attardi LD (2005). Perp is a p63-regulated gene essential for epithelial integrity. Cell., 120(6), 843–856. [DOI] [PubMed] [Google Scholar]
- Iyer VR, Eisen MB, Ross DT, Schuler G, Moore T, Lee JC, . . . Brown PO (1999). The transcriptional program in the response of human fibroblasts to serum. Science., 283(5398), 83–87. [DOI] [PubMed] [Google Scholar]
- Jacobs WB, Govoni G, Ho D, Atwal JK, Barnabe-Heider F, Keyes WM, . . . Kaplan DR (2005). p63 is an essential proapoptotic protein during neural development. Neuron, 48(5), 743–756. doi: 10.1016/j.neuron.2005.10.027 [DOI] [PubMed] [Google Scholar]
- Jaubert J, Cheng J, & Segre JA (2003). Ectopic expression of kruppel like factor 4 (Klf4) accelerates formation of the epidermal permeability barrier. Development, 130(12), 2767–2777. [DOI] [PubMed] [Google Scholar]
- Jensen P, Hansen S, Moller B, Leivestad T, Pfeffer P, Geiran O, . . . Simonsen S (1999). Skin cancer in kidney and heart transplant recipients and different long-term immunosuppressive therapy regimens. J Am Acad Dermatol, 40(2 Pt 1), 177–186. [DOI] [PubMed] [Google Scholar]
- Jin C, Jin Y, Wennerberg J, Annertz K, Enoksson J, & Mertens F (2006). Cytogenetic abnormalities in 106 oral squamous cell carcinomas. Cancer Genet Cytogenet, 164(1), 44–53. doi: 10.1016/j.cancergencyto.2005.06.008 [DOI] [PubMed] [Google Scholar]
- Johnson TM, Rowe DE, Nelson BR, & Swanson NA (1992). Squamous cell carcinoma of the skin (excluding lip and oral mucosa). J Am Acad Dermatol, 26(3 Pt 2), 467–484. [DOI] [PubMed] [Google Scholar]
- Jonason AS, Kunala S, Price GJ, Restifo RJ, Spinelli HM, Persing JA, . . . Brash DE (1996). Frequent clones of p53-mutated keratinocytes in normal human skin. Proc Natl Acad Sci U S A, 93(24), 14025–14029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junttila MR, & de Sauvage FJ (2013). Influence of tumour micro-environment heterogeneity on therapeutic response. Nature., 501(7467), 346–354. doi: 310.1038/nature12626. [DOI] [PubMed] [Google Scholar]
- Justilien V, Walsh MP, Ali SA, Thompson EA, Murray NR, & Fields AP (2014). The PRKCI and SOX2 Oncogenes Are Coamplified and Cooperate to Activate Hedgehog Signaling in Lung Squamous Cell Carcinoma. Cancer Cell., 25(2), 139–151. doi: 110.1016/j.ccr.2014.1001.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R (2003). Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer, 3(6), 422–433. doi: 10.1038/nrc1094 [DOI] [PubMed] [Google Scholar]
- Kalu NN, Mazumdar T, Peng S, Shen L, Sambandam V, Rao X, . . . Johnson FM (2017). Genomic characterization of human papillomavirus-positive and -negative human squamous cell cancer cell lines. Oncotarget, 8(49), 86369–86383. doi: 10.18632/oncotarget.21174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan Z, Jaiswal BS, Stinson J, Janakiraman V, Bhatt D, Stern HM, . . . Seshagiri S (2010). Diverse somatic mutation patterns and pathway alterations in human cancers. Nature., 466(7308), 869–873. doi: 810.1038/nature09208. Epub 02010 Jul 09228. [DOI] [PubMed] [Google Scholar]
- Keck MK, Zuo Z, Khattri A, Stricker TP, Brown CD, Imanguli M, . . . Seiwert TY(2015). Integrative analysis of head and neck cancer identifies two biologically distinct HPV and three non-HPV subtypes. Clin Cancer Res, 21(4), 870–881. doi: 10.1158/1078-0432.ccr-14-2481 [DOI] [PubMed] [Google Scholar]
- Kenfield SA, Wei EK, Stampfer MJ, Rosner BA, & Colditz GA (2008). Comparison of aspects of smoking among the four histological types of lung cancer. Tob Control, 17(3), 198–204. doi: 10.1136/tc.2007.022582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr-Valentic MA, Samimi K, Rohlen BH, Agarwal JP, & Rockwell WB (2009). Marjolin's ulcer: modern analysis of an ancient problem. Plast Reconstr Surg, 123(1), 184–191. doi: 10.1097/PRS.0b013e3181904d86 [DOI] [PubMed] [Google Scholar]
- Khavari PA (2006). Modelling cancer in human skin tissue. Nat Rev Cancer., 6(4), 270–280. [DOI] [PubMed] [Google Scholar]
- Kim Y, Hammerman PS, Kim J, Yoon JA, Lee Y, Sun JM, . . . Park K (2014). Integrative and comparative genomic analysis of lung squamous cell carcinomas in East Asian patients. J Clin Oncol, 32(2), 121–128. doi: 10.1200/jco.2013.50.8556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King KE, Ponnamperuma RM, Gerdes MJ, Tokino T, Yamashita T, Baker CC, & Weinberg WC (2006). Unique domain functions of p63 isotypes that differentially regulate distinct aspects of epidermal homeostasis. Carcinogenesis, 27(1), 53–63. doi: 10.1093/carcin/bgi200 [DOI] [PubMed] [Google Scholar]
- King KE, Ponnamperuma RM, Yamashita T, Tokino T, Lee LA, Young MF, & Weinberg WC (2003). deltaNp63alpha functions as both a positive and a negative transcriptional regulator and blocks in vitro differentiation of murine keratinocytes. Oncogene, 22(23), 3635–3644. doi: 10.1038/sj.onc.1206536 [DOI] [PubMed] [Google Scholar]
- Klein AM, Brash DE, Jones PH, & Simons BD (2010). Stochastic fate of p53-mutant epidermal progenitor cells is tilted toward proliferation by UV B during preneoplasia. Proc Natl Acad Sci U S A, 107(1), 270–275. doi: 10.1073/pnas.0909738107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinakis A, Lobry C, Abdel-Wahab O, Oh P, Haeno H, Buonamici S, . . . Aifantis I (2011). A novel tumour-suppressor function for the Notch pathway in myeloid leukaemia. Nature, 473(7346), 230–233. doi: 10.1038/nature09999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klussmann JP, Mooren JJ, Lehnen M, Claessen SM, Stenner M, Huebbers CU, . . . Speel EJ (2009). Genetic signatures of HPV-related and unrelated oropharyngeal carcinoma and their prognostic implications. Clin Cancer Res, 15(5), 1779–1786. doi: 10.1158/1078-0432.Ccr-08-1463 [DOI] [PubMed] [Google Scholar]
- Kopan R, & Ilagan MX (2009). The canonical Notch signaling pathway: unfolding the activation mechanism. Cell, 137(2), 216–233. doi: 10.1016/j.cell.2009.03.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster MI, Dai D, Marinari B, Sano Y, Costanzo A, Karin M, & Roop DR (2007). p63 induces key target genes required for epidermal morphogenesis. Proc Natl Acad Sci U S A., 104(9), 3255–3260. Epub 2007 Feb 3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster MI, Kim S, Huang J, Williams T, & Roop DR (2006). TAp63alpha induces AP-2gamma as an early event in epidermal morphogenesis. Dev Biol, 289(1), 253–261. doi: 10.1016/j.ydbio.2005.10.041 [DOI] [PubMed] [Google Scholar]
- Koster MI, Kim S, Mills AA, DeMayo FJ, & Roop DR (2004). p63 is the molecular switch for initiation of an epithelial stratification program. Genes Dev., 18(2), 126–131. Epub 2004 Jan 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouwenhoven EN, van Heeringen SJ, Tena JJ, Oti M, Dutilh BE, Alonso ME, . . . Zhou H (2010). Genome-wide profiling of p63 DNA-binding sites identifies an element that regulates gene expression during limb development in the 7q21 SHFM1 locus. PLoS Genet, 6(8), e1001065. doi: 10.1371/journal.pgen.1001065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovall RA, & Blacklow SC (2010). Mechanistic insights into Notch receptor signaling from structural and biochemical studies. Curr Top Dev Biol, 92, 31–71. doi: 10.1016/s0070-2153(10)92002-4 [DOI] [PubMed] [Google Scholar]
- Kozaki K, Imoto I, Pimkhaokham A, Hasegawa S, Tsuda H, Omura K, & Inazawa J (2006). PIK3CA mutation is an oncogenic aberration at advanced stages of oral squamous cell carcinoma. Cancer Sci, 97(12), 1351–1358. doi: 10.1111/j.1349-7006.2006.00343.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku SY, Rosario S, Wang Y, Mu P, Seshadri M, Goodrich ZW, . . . Goodrich DW (2017). Rb1 and Trp53 cooperate to suppress prostate cancer lineage plasticity, metastasis, and antiandrogen resistance. Science, 355(6320), 78–83. doi: 10.1126/science.aah4199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurita M, Araoka T, Hishida T, O'Keefe DD, Takahashi Y, Sakamoto A, . . . Izpisua Belmonte JC (2018). In vivo reprogramming of wound-resident cells generates skin epithelial tissue. Nature, 561(7722), 243–247. doi: 10.1038/s41586-018-0477-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutler DI, Auerbach AD, Satagopan J, Giampietro PF, Batish SD, Huvos AG, . . . Singh B (2003). High incidence of head and neck squamous cell carcinoma in patients with Fanconi anemia. Arch Otolaryngol Head Neck Surg, 129(1), 106–112. [DOI] [PubMed] [Google Scholar]
- Lambert SR, Mladkova N, Gulati A, Hamoudi R, Purdie K, Cerio R, . . . Harwood CA (2014). Key differences identified between actinic keratosis and cutaneous squamous cell carcinoma by transcriptome profiling. Br J Cancer, 110(2), 520–529. doi: 10.1038/bjc.2013.760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapouge G, Youssef KK, Vokaer B, Achouri Y, Michaux C, Sotiropoulou PA, & Blanpain C (2011). Identifying the cellular origin of squamous skin tumors. Proc Natl Acad Sci U S A., 108(18), 7431–7436. Epub 2011 Apr 7418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurie CC, Laurie CA, Rice K, Doheny KF, Zelnick LR, McHugh CP, . . . Weir BS (2012). Detectable clonal mosaicism from birth to old age and its relationship to cancer. Nat Genet, 44(6), 642–650. doi: 10.1038/ng.2271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurikkala J, Mikkola ML, James M, Tummers M, Mills AA, & Thesleff I (2006). p63 regulates multiple signalling pathways required for ectodermal organogenesis and differentiation. Development, 133(8), 1553–1563. doi: 10.1242/dev.02325 [DOI] [PubMed] [Google Scholar]
- Lawrence N, & Cottel WI (1994). Squamous cell carcinoma of skin with perineural invasion. J Am Acad Dermatol, 31(1), 30–33. [DOI] [PubMed] [Google Scholar]
- Lechner M, Frampton GM, Fenton T, Feber A, Palmer G, Jay A, . . . Boshoff C (2013). Targeted next-generation sequencing of head and neck squamous cell carcinoma identifies novel genetic alterations in HPV+ and HPV− tumors. Genome Med, 5(5), 49. doi: 10.1186/gm453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SY, Kumano K, Nakazaki K, Sanada M, Matsumoto A, Yamamoto G, . . . Chiba S (2009). Gain-of-function mutations and copy number increases of Notch2 in diffuse large B-cell lymphoma. Cancer Sci, 100(5), 920–926. doi: 10.1111/j.1349-7006.2009.01130.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leemans CR, Braakhuis BJ, & Brakenhoff RH (2011). The molecular biology of head and neck cancer. Nat Rev Cancer, 11(1), 9–22. doi: 10.1038/nrc2982 [DOI] [PubMed] [Google Scholar]
- Lefort K, Mandinova A, Ostano P, Kolev V, Calpini V, Kolfschoten I, . . . Dotto GP (2007). Notch1 is a p53 target gene involved in human keratinocyte tumor suppression through negative regulation of ROCK1/2 and MRCKalpha kinases. Genes Dev., 21(5), 562–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leisenring W, Friedman DL, Flowers ME, Schwartz JL, & Deeg HJ (2006). Nonmelanoma skin and mucosal cancers after hematopoietic cell transplantation. J Clin Oncol, 24(7), 1119–1126. doi: 10.1200/jco.2005.02.7052 [DOI] [PubMed] [Google Scholar]
- Li C, Gao Z, Li F, Li X, Sun Y, Wang M, . . . Wei Q (2015). Whole Exome Sequencing Identifies Frequent Somatic Mutations in Cell-Cell Adhesion Genes in Chinese Patients with Lung Squamous Cell Carcinoma. Sci Rep, 5, 14237. doi: 10.1038/srep14237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Wawrose JS, Gooding WE, Garraway LA, Lui VW, Peyser ND, & Grandis JR (2014). Genomic analysis of head and neck squamous cell carcinoma cell lines and human tumors: a rational approach to preclinical model selection. Mol Cancer Res, 12(4), 571–582. doi: 10.1158/1541-7786.mcr-13-0396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YY, Hanna GJ, Laga AC, Haddad RI, Lorch JH, & Hammerman PS (2015). Genomic analysis of metastatic cutaneous squamous cell carcinoma. Clin Cancer Res, 21(6), 1447–1456. doi: 10.1158/1078-0432.Ccr-14-1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao RG, Jung J, Tchaicha J, Wilkerson MD, Sivachenko A, Beauchamp EM, . . . Hammerman PS (2013). Inhibitor-sensitive FGFR2 and FGFR3 mutations in lung squamous cell carcinoma. Cancer Res, 73(16), 5195–5205. doi: 10.1158/0008-5472.Can-12-3950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liefer KM, Koster MI, Wang XJ, Yang A, McKeon F, & Roop DR (2000). Down-regulation of p63 is required for epidermal UV-B-induced apoptosis. Cancer Res, 60(15), 4016–4020. [PubMed] [Google Scholar]
- Lim JS, Ibaseta A, Fischer MM, Cancilla B, O'Young G, Cristea S, . . . Sage J (2017). Intratumoural heterogeneity generated by Notch signalling promotes small-cell lung cancer. Nature. doi: 10.1038/nature22323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin DC, Hao JJ, Nagata Y, Xu L, Shang L, Meng X, . . . Koeffler HP (2014). Genomic and molecular characterization of esophageal squamous cell carcinoma. Nat Genet, 30(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindelof B, Sigurgeirsson B, Gabel H, & Stern RS (2000). Incidence of skin cancer in 5356 patients following organ transplantation. Br J Dermatol, 143(3), 513–519. [PubMed] [Google Scholar]
- Lishner M, Patterson B, Kandel R, Fyles G, Curtis JE, Meharchand J, . . . Messner HA (1990). Cutaneous and mucosal neoplasms in bone marrow transplant recipients. Cancer, 65(3), 473–476. doi: [DOI] [PubMed] [Google Scholar]
- Little CD, Nau MM, Carney DN, Gazdar AF, & Minna JD (1983). Amplification and expression of the c-myc oncogene in human lung cancer cell lines. Nature, 306(5939), 194–196. [DOI] [PubMed] [Google Scholar]
- Long KB, & Hornick JL (2009). SOX2 is highly expressed in squamous cell carcinomas of the gastrointestinal tract. Hum Pathol, 40(12), 1768–1773. doi: 10.1016/j.humpath.2009.06.006 [DOI] [PubMed] [Google Scholar]
- Lott DG, Manz R, Koch C, & Lorenz RR (2010). Aggressive behavior of nonmelanotic skin cancers in solid organ transplant recipients. Transplantation, 90(6), 683–687. doi: 10.1097/TP.0b013e3181ec7228 [DOI] [PubMed] [Google Scholar]
- Lowell S, Jones P, Le Roux I, Dunne J, & Watt FM (2000). Stimulation of human epidermal differentiation by delta-notch signalling at the boundaries of stem-cell clusters. Curr Biol, 10(9), 491–500. [DOI] [PubMed] [Google Scholar]
- Lu C, & Fuchs E (2014). Sweat gland progenitors in development, homeostasis, and wound repair. Cold Spring Harb Perspect Med., 4(2).(pii), a015222. doi: 015210.011101/cshperspect.a015222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Futtner C, Rock JR, Xu X, Whitworth W, Hogan BL, & Onaitis MW (2010). Evidence that SOX2 overexpression is oncogenic in the lung. PLoS One, 5(6), e11022. doi: 10.1371/journal.pone.0011022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludmir EB, Stephens SJ, Palta M, Willett CG, & Czito BG (2015). Human papillomavirus tumor infection in esophageal squamous cell carcinoma. J Gastrointest Oncol, 6(3), 287–295. doi: 10.3978/j.issn.2078-6891.2015.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo B, Aster JC, Hasserjian RP, Kuo F, & Sklar J (1997). Isolation and functional analysis of a cDNA for human Jagged2, a gene encoding a ligand for the Notch1 receptor. Mol Cell Biol, 17(10), 6057–6067. doi: 10.1128/mcb.17.10.6057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madan V, Lear JT, & Szeimies RM (2010). Non-melanoma skin cancer. Lancet, 375(9715), 673–685. doi: 10.1016/s0140-6736(09)61196-x [DOI] [PubMed] [Google Scholar]
- Maier S, Wilbertz T, Braun M, Scheble V, Reischl M, Mikut R, . . . Perner S (2011). SOX2 amplification is a common event in squamous cell carcinomas of different organ sites. Hum Pathol, 42(8), 1078–1088. doi: 10.1016/j.humpath.2010.11.010 [DOI] [PubMed] [Google Scholar]
- Maitra A, Biswas NK, Amin K, Kowtal P, Kumar S, Das S, . . . Sutradhar D (2013). Mutational landscape of gingivo-buccal oral squamous cell carcinoma reveals new recurrently-mutated genes and molecular subgroups. Nat Commun., 4:2873.(doi), 10.1038/ncomms3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik RD, Dakwar G, Hardee ME, Sanfilippo NJ, Rosenkrantz AB, & Taneja SS (2011). Squamous cell carcinoma of the prostate. Rev Urol, 13(1), 56–60. [PMC free article] [PubMed] [Google Scholar]
- Mammucari C, Tommasi di Vignano A, Sharov AA, Neilson J, Havrda MC, Roop DR, . . . Dotto GP (2005). Integration of Notch 1 and calcineurin/NFAT signaling pathways in keratinocyte growth and differentiation control. Dev Cell., 8(5), 665–676. [DOI] [PubMed] [Google Scholar]
- Manusow D, & Weinerman BH (1975). Subsequent neoplasia in chronic lymphocytic leukemia. Jama, 232(3), 267–269. [PubMed] [Google Scholar]
- Mao L, Lee JS, Fan YH, Ro JY, Batsakis JG, Lippman S, . . . Hong WK (1996). Frequent microsatellite alterations at chromosomes 9p21 and 3p14 in oral premalignant lesions and their value in cancer risk assessment. Nat Med, 2(6), 682–685. [DOI] [PubMed] [Google Scholar]
- Marcil I, & Stern RS (2001). Squamous-cell cancer of the skin in patients given PUVA and ciclosporin: nested cohort crossover study. Lancet, 358(9287), 1042–1045. doi: 10.1016/s0140-6736(01)06179-7 [DOI] [PubMed] [Google Scholar]
- Martin D, Abba MC, Molinolo AA, Vitale-Cross L, Wang Z, Zaida M, . . . Gutkind JS (2014). The head and neck cancer cell oncogenome: a platform for the development of precision molecular therapies. Oncotarget, 5(19), 8906–8923. doi: 10.18632/oncotarget.2417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martincorena I, Fowler JC, Wabik A, Lawson ARJ, Abascal F, Hall MWJ, . . . Jones PH (2018). Somatic mutant clones colonize the human esophagus with age. Science, 362(6417), 911–917. doi: 10.1126/science.aau3879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martincorena I, Roshan A, Gerstung M, Ellis P, Van Loo P, McLaren S, . . . Campbell PJ (2015). Tumor evolution. High burden and pervasive positive selection of somatic mutations in normal human skin. Science., 348(6237), 880–886. doi: 810.1126/science.aaa6806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez JC, Otley CC, Stasko T, Euvrard S, Brown C, Schanbacher CF, & Weaver AL (2003). Defining the clinical course of metastatic skin cancer in organ transplant recipients: a multicenter collaborative study. Arch Dermatol, 139(3), 301–306. doi: 10.1001/archderm.139.3.301 [DOI] [PubMed] [Google Scholar]
- Mathe EA, Nguyen GH, Bowman ED, Zhao Y, Budhu A, Schetter AJ, . . . Harris CC (2009). MicroRNA expression in squamous cell carcinoma and adenocarcinoma of the esophagus: associations with survival. Clin Cancer Res., 15(19), 6192–6200. doi: 6110.1158/1078-0432.CCR-6109-1467. Epub 2009 Sep 6129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride SM, Rothenberg SM, Faquin WC, Chan AW, Clark JR, Ellisen LW, & Wirth LJ (2014). Mutation frequency in 15 common cancer genes in high-risk head and neck squamous cell carcinoma. Head Neck, 36(8), 1181–1188. doi: 10.1002/hed.23430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell BB, & Yang VW (2010). Mammalian Kruppel-like factors in health and diseases. Physiol Rev, 90(4), 1337–1381. doi: 10.1152/physrev.00058.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meacham CE, & Morrison SJ (2013). Tumour heterogeneity and cancer cell plasticity. Nature., 501(7467), 328–337. doi: 310.1038/nature12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medawar A, Virolle T, Rostagno P, de la Forest-Divonne S, Gambaro K, Rouleau M, & Aberdam D (2008). DeltaNp63 is essential for epidermal commitment of embryonic stem cells. PLoS One, 3(10), e3441. doi: 10.1371/journal.pone.0003441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrany K, Weenig RH, Lee KK, Pittelkow MR, & Otley CC (2005). Increased metastasis and mortality from cutaneous squamous cell carcinoma in patients with chronic lymphocytic leukemia. J Am Acad Dermatol, 53(6), 1067–1071. doi: 10.1016/j.jaad.2005.08.055 [DOI] [PubMed] [Google Scholar]
- Mehrany K, Weenig RH, Pittelkow MR, Roenigk RK, & Otley CC (2005). High recurrence rates of squamous cell carcinoma after Mohs' surgery in patients with chronic lymphocytic leukemia. Dermatol Surg, 31(1), 38–42; discussion 42. [DOI] [PubMed] [Google Scholar]
- Migden MR, Rischin D, Schmults CD, Guminski A, Hauschild A, Lewis KD, . . . Fury MG (2018). PD-1 Blockade with Cemiplimab in Advanced Cutaneous Squamous-Cell Carcinoma. N Engl J Med, 379(4), 341–351. doi: 10.1056/NEJMoa1805131 [DOI] [PubMed] [Google Scholar]
- Millar SE (2002). Molecular mechanisms regulating hair follicle development. J Invest Dermatol., 118(2), 216–225. [DOI] [PubMed] [Google Scholar]
- Moffitt RA, Marayati R, Flate EL, Volmar KE, Loeza SG, Hoadley KA, . . . Yeh JJ (2015). Virtual microdissection identifies distinct tumor- and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nat Genet, 47(10), 1168–1178. doi: 10.1038/ng.3398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti F, Marinari B, Lo Iacono N, Botti E, Giunta A, Spallone G, . . . Costanzo A (2010). A regulatory feedback loop involving p63 and IRF6 links the pathogenesis of 2 genetically different human ectodermal dysplasias. J Clin Invest, 120(5), 1570–1577. doi: 10.1172/jci40267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris LGT, Chandramohan R, West L, Zehir A, Chakravarty D, Pfister DG, . . . Ho AL (2017). The Molecular Landscape of Recurrent and Metastatic Head and Neck Cancers: Insights From a Precision Oncology Sequencing Platform. JAMA Oncol, 3(2), 244–255. doi: 10.1001/jamaoncol.2016.1790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu P, Zhang Z, Benelli M, Karthaus WR, Hoover E, Chen CC, . . . Sawyers CL (2017). SOX2 promotes lineage plasticity and antiandrogen resistance in TP53- and RB1-deficient prostate cancer. Science, 355(6320), 84–88. doi: 10.1126/science.aah4307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay A, Berrett KC, Kc U, Clair PM, Pop SM, Carr SR, . . . Oliver TG (2014). Sox2 Cooperates with Lkb1 Loss in a Mouse Model of Squamous Cell Lung Cancer. Cell Rep, 17(14), 00430–00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller E, Brault B, Holmes A, Legros A, Jeannot E, Campitelli M, . . . Castera L (2015). Genetic profiles of cervical tumors by high-throughput sequencing for personalized medical care. Cancer Med, 4(10), 1484–1493. doi: 10.1002/cam4.492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz N, Bosch FX, de Sanjose S, Herrero R, Castellsague X, Shah KV, . . . Meijer CJ (2003). Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med, 348(6), 518–527. doi: 10.1056/NEJMoa021641 [DOI] [PubMed] [Google Scholar]
- Murugan AK, Hong NT, Fukui Y, Munirajan AK, & Tsuchida N (2008). Oncogenic mutations of the PIK3CA gene in head and neck squamous cell carcinomas. Int J Oncol, 32(1), 101–111. [PubMed] [Google Scholar]
- Nagarajan P, Asgari MM, Green AC, Guhan SM, Arron ST, Proby CM, . . . Toland AE (2019). Keratinocyte Carcinomas: Current Concepts and Future Research Priorities. Clin Cancer Res, 25(8), 2379–2391. doi: 10.1158/1078-0432.Ccr-18-1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen BC, Lefort K, Mandinova A, Antonini D, Devgan V, Della Gatta G, . . . Dotto GP (2006). Cross-regulation between Notch and p63 in keratinocyte commitment to differentiation. Genes Dev., 20(8), 1028–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen LV, Vanner R, Dirks P, & Eaves CJ (2012). Cancer stem cells: an evolving concept. Nat Rev Cancer, 12(2), 133–143. doi: 10.1038/nrc3184 [DOI] [PubMed] [Google Scholar]
- Nickoloff BJ, Qin JZ, Chaturvedi V, Denning MF, Bonish B, & Miele L (2002). Jagged-1 mediated activation of notch signaling induces complete maturation of human keratinocytes through NF-kappaB and PPARgamma. Cell Death Differ, 9(8), 842–855. doi: 10.1038/sj.cdd.4401036 [DOI] [PubMed] [Google Scholar]
- Nicolas M, Wolfer A, Raj K, Kummer JA, Mill P, van Noort M, . . . Radtke F (2003). Notch1 functions as a tumor suppressor in mouse skin. Nat Genet., 33(3), 416–421. Epub 2003 Feb 2018. [DOI] [PubMed] [Google Scholar]
- Niederst MJ, Sequist LV, Poirier JT, Mermel CH, Lockerman EL, Garcia AR, . . . Engelman JA (2015). RB loss in resistant EGFR mutant lung adenocarcinomas that transform to small-cell lung cancer. Nat Commun, 6, 6377. doi: 10.1038/ncomms7377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto MA (2013). Epithelial plasticity: a common theme in embryonic and cancer cells. Science, 342(6159), 1234850. doi: 10.1126/science.1234850 [DOI] [PubMed] [Google Scholar]
- Nylander K, Vojtesek B, Nenutil R, Lindgren B, Roos G, Zhanxiang W, . . . Coates PJ (2002). Differential expression of p63 isoforms in normal tissues and neoplastic cells. J Pathol, 198(4), 417–427. doi: 10.1002/path.1231 [DOI] [PubMed] [Google Scholar]
- Oberholzer PA, Kee D, Dziunycz P, Sucker A, Kamsukom N, Jones R, . . . Garraway LA (2012). RAS mutations are associated with the development of cutaneous squamous cell tumors in patients treated with RAF inhibitors. J Clin Oncol, 30(3), 316–321. doi: 10.1200/jco.2011.36.7680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegaard JI, & Hsieh MH (2014). Immune responses to Schistosoma haematobium infection. Parasite Immunol, 36(9), 428–438. doi: 10.1111/pim.12084 [DOI] [PubMed] [Google Scholar]
- Ohnishi S, Laub F, Matsumoto N, Asaka M, Ramirez F, Yoshida T, & Terada M (2000). Developmental expression of the mouse gene coding for the Kruppel-like transcription factor KLF5. Dev Dyn, 217(4), 421–429. doi: [DOI] [PubMed] [Google Scholar]
- Ojesina AI, Lichtenstein L, Freeman SS, Pedamallu CS, Imaz-Rosshandler I, Pugh TJ, . . . Meyerson M (2014). Landscape of genomic alterations in cervical carcinomas. Nature., 506(7488), 371–375. doi: 310.1038/nature12881. Epub 12013 Dec 12825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuyama R, Ogawa E, Nagoshi H, Yabuki M, Kurihara A, Terui T, . . . Ikawa S (2007). p53 homologue, p51/p63, maintains the immaturity of keratinocyte stem cells by inhibiting Notch1 activity. Oncogene, 26(31), 4478–4488. doi: 10.1038/sj.onc.1210235 [DOI] [PubMed] [Google Scholar]
- Onajin O, & Brewer JD (2012). Skin cancer in patients with chronic lymphocytic leukemia and non-Hodgkin lymphoma. Clin Adv Hematol Oncol, 10(9), 571–576. [PubMed] [Google Scholar]
- Otley CC, Coldiron BM, Stasko T, & Goldman GD (2001). Decreased skin cancer after cessation of therapy with transplant-associated immunosuppressants. Arch Dermatol, 137(4), 459–463. [PubMed] [Google Scholar]
- Otley CC, & Maragh SL (2005). Reduction of immunosuppression for transplant-associated skin cancer: rationale and evidence of efficacy. Dermatol Surg, 31(2), 163–168. [DOI] [PubMed] [Google Scholar]
- Pardal R, Clarke MF, & Morrison SJ (2003). Applying the principles of stem-cell biology to cancer. Nat Rev Cancer., 3(12), 895–902. [DOI] [PubMed] [Google Scholar]
- Parfenov M, Pedamallu CS, Gehlenborg N, Freeman SS, Danilova L, Bristow CA, . . . Kucherlapati R (2014). Characterization of HPV and host genome interactions in primary head and neck cancers. Proc Natl Acad Sci U S A., 111(43), 15544–15549. doi: 15510.11073/pnas.1416074111. Epub 1416072014 Oct 1416074113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JW, Lee JK, Sheu KM, Wang L, Balanis NG, Nguyen K, . . . Witte ON (2018). Reprogramming normal human epithelial tissues to a common, lethal neuroendocrine cancer lineage. Science, 362(6410), 91–95. doi: 10.1126/science.aat5749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsa R, Yang A, McKeon F, & Green H (1999). Association of p63 with proliferative potential in normal and neoplastic human keratinocytes. J Invest Dermatol, 113(6), 1099–1105. doi: 10.1046/j.1523-1747.1999.00780.x [DOI] [PubMed] [Google Scholar]
- Paus R, & Cotsarelis G (1999). The biology of hair follicles. N Engl J Med., 341(7), 491–497. [DOI] [PubMed] [Google Scholar]
- Pedersen TX, Leethanakul C, Patel V, Mitola D, Lund LR, Dano K, . . . Bugge TH (2003). Laser capture microdissection-based in vivo genomic profiling of wound keratinocytes identifies similarities and differences to squamous cell carcinoma. Oncogene., 22(25), 3964–3976. [DOI] [PubMed] [Google Scholar]
- Pelengaris S, Littlewood T, Khan M, Elia G, & Evan G (1999). Reversible activation of c-Myc in skin: induction of a complex neoplastic phenotype by a single oncogenic lesion. Mol Cell, 3(5), 565–577. [DOI] [PubMed] [Google Scholar]
- Pelisson I, Soler C, Chardonnet Y, Euvrard S, & Schmitt D (1996). A possible role for human papillomaviruses and c-myc, c-Ha-ras, and p53 gene alterations in malignant cutaneous lesions from renal transplant recipients. Cancer Detect Prev, 20(1), 20–30. [PubMed] [Google Scholar]
- Pellegrini G, Dellambra E, Golisano O, Martinelli E, Fantozzi I, Bondanza S, . . . De Luca M (2001). p63 identifies keratinocyte stem cells. Proc Natl Acad Sci U S A, 98(6), 3156–3161. doi: 10.1073/pnas.061032098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn I (2000). Post-transplant malignancy: the role of immunosuppression. Drug Saf, 23(2), 101–113. doi: 10.2165/00002018-200023020-00002 [DOI] [PubMed] [Google Scholar]
- Pickering CR, Zhang J, Yoo SY, Bengtsson L, Moorthy S, Neskey DM, . . . Frederick MJ (2013). Integrative genomic characterization of oral squamous cell carcinoma identifies frequent somatic drivers. Cancer Discov, 3(7), 770–781. doi: 10.1158/2159-8290.cd-12-0537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering CR, Zhou JH, Lee JJ, Drummond JA, Peng SA, Saade RE, . . . Frederick MJ (2014). Mutational landscape of aggressive cutaneous squamous cell carcinoma. Clin Cancer Res, 20(24), 6582–6592. doi: 10.1158/1078-0432.ccr-14-1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierceall WE, Goldberg LH, Tainsky MA, Mukhopadhyay T, & Ananthaswamy HN(1991). Ras gene mutation and amplification in human nonmelanoma skin cancers. Mol Carcinog, 4(3), 196–202. [DOI] [PubMed] [Google Scholar]
- Pierceall WE, Mukhopadhyay T, Goldberg LH, & Ananthaswamy HN (1991). Mutations in the p53 tumor suppressor gene in human cutaneous squamous cell carcinomas. Mol Carcinog, 4(6), 445–449. [DOI] [PubMed] [Google Scholar]
- Poeta ML, Manola J, Goldwasser MA, Forastiere A, Benoit N, Califano JA, . . . Koch WM (2007). TP53 mutations and survival in squamous-cell carcinoma of the head and neck. N Engl J Med, 357(25), 2552–2561. doi: 10.1056/NEJMoa073770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulikakos PI, Zhang C, Bollag G, Shokat KM, & Rosen N (2010). RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature, 464(7287), 427–430. doi: 10.1038/nature08902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevo LJ, Sanchez CA, Galipeau PC, & Reid BJ (1999). p53-mutant clones and field effects in Barrett's esophagus. Cancer Res, 59(19), 4784–4787. [PubMed] [Google Scholar]
- Puente XS, Pinyol M, Quesada V, Conde L, Ordonez GR, Villamor N, . . . Campo E (2011). Whole-genome sequencing identifies recurrent mutations in chronic lymphocytic leukaemia. Nature, 475(7354), 101–105. doi: 10.1038/nature10113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puram SV, Tirosh I, Parikh AS, Patel AP, Yizhak K, Gillespie S, . . . Bernstein BE(2017). Single-Cell Transcriptomic Analysis of Primary and Metastatic Tumor Ecosystems in Head and Neck Cancer. Cell, 171(7), 1611–1624.e1624. doi: 10.1016/j.cell.2017.10.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdie KJ, Harwood CA, Gulati A, Chaplin T, Lambert SR, Cerio R, . . . Proby CM (2009). Single nucleotide polymorphism array analysis defines a specific genetic fingerprint for well-differentiated cutaneous SCCs. J Invest Dermatol, 129(6), 1562–1568. doi: 10.1038/jid.2008.408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin HD, Liao XY, Chen YB, Huang SY, Xue WQ, Li FF, . . . Jia WH (2016). Genomic Characterization of Esophageal Squamous Cell Carcinoma Reveals Critical Genes Underlying Tumorigenesis and Poor Prognosis. Am J Hum Genet, 98(4), 709–727. doi: 10.1016/j.ajhg.2016.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu W, Schonleben F, Li X, Ho DJ, Close LG, Manolidis S, . . . Su GH (2006). PIK3CA mutations in head and neck squamous cell carcinoma. Clin Cancer Res, 12(5), 1441–1446. doi: 10.1158/1078-0432.Ccr-05-2173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintanilla M, Brown K, Ramsden M, & Balmain A (1986). Carcinogen-specific mutation and amplification of Ha-ras during mouse skin carcinogenesis. Nature., 322(6074), 78–80. [DOI] [PubMed] [Google Scholar]
- Quintanilla M, Haddow S, Jonas D, Jaffe D, Bowden GT, & Balmain A (1991). Comparison of ras activation during epidermal carcinogenesis in vitro and in vivo. Carcinogenesis, 12(10), 1875–1881. [DOI] [PubMed] [Google Scholar]
- Ragin CC, & Taioli E (2007). Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: review and meta-analysis. Int J Cancer, 121(8), 1813–1820. doi: 10.1002/ijc.22851 [DOI] [PubMed] [Google Scholar]
- Ramos AH, Dutt A, Mermel C, Perner S, Cho J, Lafargue CJ, . . . Meyerson M (2009). Amplification of chromosomal segment 4q12 in non-small cell lung cancer. Cancer Biol Ther, 8(21), 2042–2050. doi: 10.4161/cbt.8.21.9764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay HM, Fryer AA, Hawley CM, Smith AG, & Harden PN (2002). Non-melanoma skin cancer risk in the Queensland renal transplant population. Br J Dermatol, 147(5), 950–956. doi: 10.1046/j.1365-2133.2002.04976.x [DOI] [PubMed] [Google Scholar]
- Rangarajan A, Talora C, Okuyama R, Nicolas M, Mammucari C, Oh H, . . . Dotto GP (2001). Notch signaling is a direct determinant of keratinocyte growth arrest and entry into differentiation. Embo J., 20(13), 3427–3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangwala S, & Tsai KY (2011). Roles of the immune system in skin cancer. Br J Dermatol, 165(5), 953–965. doi: 10.1111/j.1365-2133.2011.10507.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redon R, Muller D, Caulee K, Wanherdrick K, Abecassis J, & du Manoir S (2001). A simple specific pattern of chromosomal aberrations at early stages of head and neck squamous cell carcinomas: PIK3CA but not p63 gene as a likely target of 3q26-qter gains. Cancer Res, 61(10), 4122–4129. [PubMed] [Google Scholar]
- Rekhtman N, Paik PK, Arcila ME, Tafe LJ, Oxnard GR, Moreira AL, . . . Ladanyi M (2012). Clarifying the spectrum of driver oncogene mutations in biomarker-verified squamous carcinoma of lung: lack of EGFR/KRAS and presence of PIK3CA/AKT1 mutations. Clin Cancer Res, 18(4), 1167–1176. doi: 10.1158/1078-0432.Ccr-11-2109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reya T, Morrison SJ, Clarke MF, & Weissman IL (2001). Stem cells, cancer, and cancer stem cells. Nature., 414(6859), 105–111. [DOI] [PubMed] [Google Scholar]
- Rheinwald JG, & Green H (1975). Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell, 6(3), 331–343. [DOI] [PubMed] [Google Scholar]
- Richardson RJ, Dixon J, Malhotra S, Hardman MJ, Knowles L, Boot-Handford RP, . . . Dixon MJ (2006). Irf6 is a key determinant of the keratinocyte proliferation-differentiation switch. Nat Genet, 38(11), 1329–1334. doi: 10.1038/ng1894 [DOI] [PubMed] [Google Scholar]
- Riss J, Khanna C, Koo S, Chandramouli GV, Yang HH, Hu Y, . . . Barrett JC (2006). Cancers as wounds that do not heal: differences and similarities between renal regeneration/repair and renal cell carcinoma. Cancer Res., 66(14), 7216–7224. [DOI] [PubMed] [Google Scholar]
- Rizzo JD, Curtis RE, Socie G, Sobocinski KA, Gilbert E, Landgren O, . . . Deeg HJ (2009). Solid cancers after allogeneic hematopoietic cell transplantation. Blood, 113(5), 1175–1183. doi: 10.1182/blood-2008-05-158782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigo JP, Lazo PS, Ramos S, Alvarez I, & Suarez C (1996). MYC amplification in squamous cell carcinomas of the head and neck. Arch Otolaryngol Head Neck Surg, 122(5), 504–507. doi: 10.1001/archotol.1996.01890170038008 [DOI] [PubMed] [Google Scholar]
- Romano RA, Birkaya B, & Sinha S (2006). Defining the regulatory elements in the proximal promoter of DeltaNp63 in keratinocytes: Potential roles for Sp1/Sp3, NF-Y, and p63. J Invest Dermatol, 126(7), 1469–1479. doi: 10.1038/sj.jid.5700297 [DOI] [PubMed] [Google Scholar]
- Romano RA, Smalley K, Magraw C, Serna VA, Kurita T, Raghavan S, & Sinha S (2012). DeltaNp63 knockout mice reveal its indispensable role as a master regulator of epithelial development and differentiation. Development, 139(4), 772–782. doi: 10.1242/dev.071191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompolas P, & Greco V (2014). Stem cell dynamics in the hair follicle niche. Semin Cell Dev Biol, 25–26, 34-42. doi: 10.1016/j.semcdb.2013.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal AN, Ryan A, Hopster D, & Jacobs IJ (2002). Molecular evidence of a common clonal origin and subsequent divergent clonal evolution in vulval intraepithelial neoplasia, vulval squamous cell carcinoma and lymph node metastases. Int J Cancer, 99(4), 549–554. doi: 10.1002/ijc.10362 [DOI] [PubMed] [Google Scholar]
- Rubin AJ, Barajas BC, Furlan-Magaril M, Lopez-Pajares V, Mumbach MR, Howard I, . . . Khavari PA (2017). Lineage-specific dynamic and pre-established enhancer-promoter contacts cooperate in terminal differentiation. Nat Genet, 49(10), 1522–1528. doi: 10.1038/ng.3935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudin CM, Durinck S, Stawiski EW, Poirier JT, Modrusan Z, Shames DS, . . . Seshagiri S (2012). Comprehensive genomic analysis identifies SOX2 as a frequently amplified gene in small-cell lung cancer. Nat Genet., 44(10), 1111–1116. doi: 1110.1038/ng.2405. Epub 2012 Sep 1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustgi AK, & El-Serag HB (2014). Esophageal carcinoma. N Engl J Med, 371(26), 2499–2509. doi: 10.1056/NEJMra1314530 [DOI] [PubMed] [Google Scholar]
- Saladi SV, Ross K, Karaayvaz M, Tata PR, Mou H, Rajagopal J, . . . Ellisen LW (2017). ACTL6A Is Co-Amplified with p63 in Squamous Cell Carcinoma to Drive YAP Activation, Regenerative Proliferation, and Poor Prognosis. Cancer Cell, 31(1), 35–49. doi: 10.1016/j.ccell.2016.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Ishida S, Morimoto I, Yamashita T, Kojima T, Kihara C, . . . Tokino T (2002). The p53 family member genes are involved in the Notch signal pathway. J Biol Chem, 277(1), 719–724. doi: 10.1074/jbc.M108080200 [DOI] [PubMed] [Google Scholar]
- Sauma D, Fierro A, Mora JR, Lennon-Dumenil AM, Bono MR, Rosemblatt M, & Morales J (2003). Cyclosporine preconditions dendritic cells during differentiation and reduces IL-2 and IL-12 production following activation: a potential tolerogenic effect. Transplant Proc, 35(7), 2515–2517. doi: 10.1016/j.transproceed.2003.09.020 [DOI] [PubMed] [Google Scholar]
- Sawada G, Niida A, Uchi R, Hirata H, Shimamura T, Suzuki Y, . . . Mimori K (2016). Genomic Landscape of Esophageal Squamous Cell Carcinoma in a Japanese Population. Gastroenterology, 150(5), 1171–1182. doi: 10.1053/j.gastro.2016.01.035 [DOI] [PubMed] [Google Scholar]
- Schafer M, & Werner S (2008). Cancer as an overhealing wound: an old hypothesis revisited. Nat Rev Mol Cell Biol., 9(8), 628–638. doi: 610.1038/nrm2455. Epub 2008 Jul 1016. [DOI] [PubMed] [Google Scholar]
- Schepeler T, Page ME, & Jensen KB (2014). Heterogeneity and plasticity of epidermal stem cells. Development, 141(13), 2559–2567. doi: 10.1242/dev.104588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffman M, Wentzensen N, Wacholder S, Kinney W, Gage JC, & Castle PE (2011). Human papillomavirus testing in the prevention of cervical cancer. J Natl Cancer Inst, 103(5), 368–383. doi: 10.1093/jnci/djq562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider G, Schmidt-Supprian M, Rad R, & Saur D (2017). Tissue-specific tumorigenesis: context matters. Nat Rev Cancer, 17(4), 239–253. doi: 10.1038/nrc.2017.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrock A, Bode M, Goke FJ, Bareiss PM, Schairer R, Wang H, . . . Perner S (2014). Expression and role of the embryonic protein SOX2 in head and neck squamous cell carcinoma. Carcinogenesis, 35(7), 1636–1642. doi: 10.1093/carcin/bgu094 [DOI] [PubMed] [Google Scholar]
- Schwab M, Alitalo K, Klempnauer KH, Varmus HE, Bishop JM, Gilbert F, . . . Trent J (1983). Amplified DNA with limited homology to myc cellular oncogene is shared by human neuroblastoma cell lines and a neuroblastoma tumour. Nature, 305(5931), 245–248. [DOI] [PubMed] [Google Scholar]
- Schwaederle M, Elkin SK, Tomson BN, Carter JL, & Kurzrock R (2015). Squamousness: Next-generation sequencing reveals shared molecular features across squamous tumor types. Cell Cycle, 14(14), 2355–2361. doi: 10.1080/15384101.2015.1053669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segre JA, Bauer C, & Fuchs E (1999). Klf4 is a transcription factor required for establishing the barrier function of the skin. Nat Genet., 22(4), 356–360. [DOI] [PubMed] [Google Scholar]
- Seiwert TY, Zuo Z, Keck MK, Khattri A, Pedamallu CS, Stricker T, . . . Hammerman PS (2015). Integrative and comparative genomic analysis of HPV-positive and HPV-negative head and neck squamous cell carcinomas. Clin Cancer Res, 21(3), 632–641. doi: 10.1158/1078-0432.ccr-13-3310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen GL, Boxer LD, Webster DE, Bussat RT, Qu K, Zarnegar BJ, . . . Khavari PA (2012). ZNF750 Is a p63 Target Gene that Induces KLF4 to Drive Terminal Epidermal Differentiation. Dev Cell, 21, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senoo M, Pinto F, Crum CP, & McKeon F (2007). p63 Is essential for the proliferative potential of stem cells in stratified epithelia. Cell., 129(3), 523–536. [DOI] [PubMed] [Google Scholar]
- Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, . . . Engelman JA (2011). Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med, 3(75), 75ra26. doi: 10.1126/scitranslmed.3002003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah KM, & Young LS (2009). Epstein-Barr virus and carcinogenesis: beyond Burkitt's lymphoma. Clin Microbiol Infect, 15(11), 982–988. doi: 10.1111/j.1469-0691.2009.03033.x [DOI] [PubMed] [Google Scholar]
- Shalom-Feuerstein R, Lena AM, Zhou H, De La Forest Divonne S, Van Bokhoven H, Candi E, . . . Aberdam D (2011). DeltaNp63 is an ectodermal gatekeeper of epidermal morphogenesis. Cell Death Differ., 18(5), 887–896. doi: 810.1038/cdd.2010.1159. Epub 2010 Dec 1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen R, Olshen AB, & Ladanyi M (2009). Integrative clustering of multiple genomic data types using a joint latent variable model with application to breast and lung cancer subtype analysis. Bioinformatics, 25(22), 2906–2912. doi: 10.1093/bioinformatics/btp543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlush LI, Zandi S, Mitchell A, Chen WC, Brandwein JM, Gupta V, . . . Dick JE (2014). Identification of pre-leukaemic haematopoietic stem cells in acute leukaemia. Nature., 506(7488), 328–333. doi: 310.1038/nature13038. Epub 12014 Feb 13012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle JM, Basin A, Sastre-Perona A, Yonekubo Y, Brown J, Sennett R, . . . Schober M (2014). SOX2 is a cancer-specific regulator of tumour initiating potential in cutaneous squamous cell carcinoma. Nat Commun., 5:4511.(doi), 10.1038/ncomms5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjodahl G, Lauss M, Lovgren K, Chebil G, Gudjonsson S, Veerla S, . . . Hoglund M (2012). A molecular taxonomy for urothelial carcinoma. Clin Cancer Res, 18(12), 3377–3386. doi: 10.1158/1078-0432.Ccr-12-0077-t [DOI] [PubMed] [Google Scholar]
- Slaughter DP, Southwick HW, & Smejkal W (1953). Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer, 6(5), 963–968. [DOI] [PubMed] [Google Scholar]
- Slebos RJ, Yi Y, Ely K, Carter J, Evjen A, Zhang X, . . . Chung CH (2006). Gene expression differences associated with human papillomavirus status in head and neck squamous cell carcinoma. Clin Cancer Res, 12(3 Pt 1), 701–709. doi: 10.1158/1078-0432.Ccr-05-2017 [DOI] [PubMed] [Google Scholar]
- Smeets SJ, Braakhuis BJ, Abbas S, Snijders PJ, Ylstra B, van de Wiel MA, . . . Brakenhoff RH (2006). Genome-wide DNA copy number alterations in head and neck squamous cell carcinomas with or without oncogene-expressing human papillomavirus. Oncogene, 25(17), 2558–2564. doi: 10.1038/sj.onc.1209275 [DOI] [PubMed] [Google Scholar]
- Smeets SJ, Brakenhoff RH, Ylstra B, van Wieringen WN, van de Wiel MA, Leemans CR, & Braakhuis BJ (2009). Genetic classification of oral and oropharyngeal carcinomas identifies subgroups with a different prognosis. Cell Oncol, 31(4), 291–300. doi: 10.3233/clo-2009-0471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoller BR, & Warnke RA (1998). Cutaneous infiltrate of chronic lymphocytic leukemia and relationship to primary cutaneous epithelial neoplasms. J Cutan Pathol, 25(3), 160–164. [DOI] [PubMed] [Google Scholar]
- Snijders AM, Schmidt BL, Fridlyand J, Dekker N, Pinkel D, Jordan RC, & Albertson DG (2005). Rare amplicons implicate frequent deregulation of cell fate specification pathways in oral squamous cell carcinoma. Oncogene, 24(26), 4232–4242. doi: 10.1038/sj.onc.1208601 [DOI] [PubMed] [Google Scholar]
- Snijders PJ, Cromme FV, van den Brule AJ, Schrijnemakers HF, Snow GB, Meijer CJ, & Walboomers JM (1992). Prevalence and expression of human papillomavirus in tonsillar carcinomas, indicating a possible viral etiology. Int J Cancer, 51(6), 845–850. doi: 10.1002/ijc.2910510602 [DOI] [PubMed] [Google Scholar]
- Song Y, Li L, Ou Y, Gao Z, Li E, Li X, . . . Zhan Q (2014). Identification of genomic alterations in oesophageal squamous cell cancer. Nature, 16(10). [DOI] [PubMed] [Google Scholar]
- South AP, Purdie KJ, Watt SA, Haldenby S, den Breems N, Dimon M, . . . Leigh IM (2014). NOTCH1 mutations occur early during cutaneous squamous cell carcinogenesis. J Invest Dermatol, 134(10), 2630–2638. doi: 10.1038/jid.2014.154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spechler SJ, & Souza RF (2014). Barrett's esophagus. N Engl J Med, 371(9), 836–845. doi: 10.1056/NEJMra1314704 [DOI] [PubMed] [Google Scholar]
- Stern RS, Laird N, Melski J, Parrish JA, Fitzpatrick TB, & Bleich HL (1984). Cutaneous squamous-cell carcinoma in patients treated with PUVA. N Engl J Med, 310(18), 1156–1161. doi: 10.1056/nejm198405033101805 [DOI] [PubMed] [Google Scholar]
- Stingl J, & Caldas C (2007). Molecular heterogeneity of breast carcinomas and the cancer stem cell hypothesis. Nat Rev Cancer, 7(10), 791–799. doi: 10.1038/nrc2212 [DOI] [PubMed] [Google Scholar]
- Stransky N, Egloff AM, Tward AD, Kostic AD, Cibulskis K, Sivachenko A, . . . Grandis JR (2011). The mutational landscape of head and neck squamous cell carcinoma. Science., 333(6046), 1157–1160. doi: 1110.1126/science.1208130. Epub 1202011 Jul 1208128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su F, Viros A, Milagre C, Trunzer K, Bollag G, Spleiss O, . . . Marais R (2012). RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N Engl J Med, 366(3), 207–215. doi: 10.1056/NEJMoa1105358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X, Cho MS, Gi YJ, Ayanga BA, Sherr CJ, & Flores ER (2009). Rescue of key features of the p63-null epithelial phenotype by inactivation of Ink4a and Arf. Embo J, 28(13), 1904–1915. doi: 10.1038/emboj.2009.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X, Paris M, Gi YJ, Tsai KY, Cho MS, Lin YL, . . . Flores ER (2009). TAp63 prevents premature aging by promoting adult stem cell maintenance. Cell Stem Cell, 5(1), 64–75. doi: 10.1016/j.stem.2009.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Gaykalova DA, Ochs MF, Mambo E, Arnaoutakis D, Liu Y, . . . Califano JA (2014). Activation of the NOTCH pathway in head and neck cancer. Cancer Res., 74(4), 1091–1104. doi: 1010.1158/0008-5472.CAN-1013-1259. Epub 2013 Dec 1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur I, Rozell B, Jaks V, Bergstrom A, & Toftgard R (2006). Epidermal and craniofacial defects in mice overexpressing Klf5 in the basal layer of the epidermis. J Cell Sci., 119(Pt 17), 3593–3601. Epub 2006 Aug 3515. [DOI] [PubMed] [Google Scholar]
- Sur I, Unden AB, & Toftgard R (2002). Human Kruppel-like factor5/KLF5: synergy with NF-kappaB/Rel factors and expression in human skin and hair follicles. Eur J Cell Biol., 81(6), 323–334. [DOI] [PubMed] [Google Scholar]
- Suzuki D, Sahu R, Leu NA, & Senoo M (2015). The carboxy-terminus of p63 links cell cycle control and the proliferative potential of epidermal progenitor cells. Development, 142(2), 282–290. doi: 10.1242/dev.118307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syrjanen S (2005). Human papillomavirus (HPV) in head and neck cancer. J Clin Virol, 32 Suppl 1, S59–66. doi: 10.1016/j.jcv.2004.11.017 [DOI] [PubMed] [Google Scholar]
- Takahashi K, Wang F, Kantarjian H, Doss D, Khanna K, Thompson E, . . . Futreal PA (2017). Preleukaemic clonal haemopoiesis and risk of therapy-related myeloid neoplasms: a case-control study. Lancet Oncol, 18(1), 100–111. doi: 10.1016/s1470-2045(16)30626-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, & Yamanaka S (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell, 126(4), 663–676. doi: 10.1016/j.cell.2006.07.024 [DOI] [PubMed] [Google Scholar]
- Takahashi T, Habuchi T, Kakehi Y, Mitsumori K, Akao T, Terachi T, & Yoshida O (1998). Clonal and chronological genetic analysis of multifocal cancers of the bladder and upper urinary tract. Cancer Res, 58(24), 5835–5841. [PubMed] [Google Scholar]
- Takeo M, Lee W, & Ito M (2015). Wound Healing and Skin Regeneration. Cold Spring Harb Perspect Med., 5(1).(pii), a023267. doi: 023210.021101/cshperspect.a023267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan HL, Sood A, Rahimi HA, Wang W, Gupta N, Hicks J, . . . Lotan TL (2014). Rb loss is characteristic of prostatic small cell neuroendocrine carcinoma. Clin Cancer Res, 20(4), 890–903. doi: 10.1158/1078-0432.Ccr-13-1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Till JE, & McCulloch EA (1961). A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res, 14, 213–222. [PubMed] [Google Scholar]
- Tomasetti C, Vogelstein B, & Parmigiani G (2013). Half or more of the somatic mutations in cancers of self-renewing tissues originate prior to tumor initiation. Proc Natl Acad Sci U S A., 110(6), 1999–2004. doi: 1910.1073/pnas.1221068110. Epub 1221062013 Jan 1221068123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonon G, Wong KK, Maulik G, Brennan C, Feng B, Zhang Y, . . . Depinho RA (2005). High-resolution genomic profiles of human lung cancer. Proc Natl Acad Sci U S A, 102(27), 9625–9630. doi: 10.1073/pnas.0504126102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong AB, Kretz M, Ridky TW, Kimmel R, & Khavari PA (2006). p63 regulates proliferation and differentiation of developmentally mature keratinocytes. Genes Dev., 20(22), 3185–3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschaharganeh DF, Xue W, Calvisi DF, Evert M, Michurina TV, Dow LE, . . . Lowe SW (2014). p53-dependent Nestin regulation links tumor suppression to cellular plasticity in liver cancer. Cell, 158(3), 579–592. doi: 10.1016/j.cell.2014.05.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunio MA, Al Asiri M, Fagih M, & Akasha R (2012). Primary squamous cell carcinoma of thyroid: a case report and review of literature. Head Neck Oncol, 4, 8. doi: 10.1186/1758-3284-4-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J, & Crossley M (1999). Mammalian Kruppel-like transcription factors: more than just a pretty finger. Trends Biochem Sci, 24(6), 236–240. [DOI] [PubMed] [Google Scholar]
- Uyar D, & Rader J (2014). Genomics of cervical cancer and the role of human papillomavirus pathobiology. Clin Chem, 60(1), 144–146. doi: 10.1373/clinchem.2013.212985 [DOI] [PubMed] [Google Scholar]
- Veness MJ, Quinn DI, Ong CS, Keogh AM, Macdonald PS, Cooper SG, & Morgan GW (1999). Aggressive cutaneous malignancies following cardiothoracic transplantation: the Australian experience. Cancer, 85(8), 1758–1764. [PubMed] [Google Scholar]
- Visvader JE (2011). Cells of origin in cancer. Nature, 469(7330), 314–322. doi: 10.1038/nature09781 [DOI] [PubMed] [Google Scholar]
- Visvader JE, & Lindeman GJ (2008). Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer, 8(10), 755–768. doi: 10.1038/nrc2499 [DOI] [PubMed] [Google Scholar]
- Wabik A, & Jones PH (2015). Switching roles: the functional plasticity of adult tissue stem cells. Embo J., 34(9), 1164–1179. doi: 1110.15252/embj.201490386. Epub 201492015 Mar 201490326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waikel RL, Kawachi Y, Waikel PA, Wang XJ, & Roop DR (2001). Deregulated expression of c-Myc depletes epidermal stem cells. Nat Genet., 28(2), 165–168. [DOI] [PubMed] [Google Scholar]
- Waikel RL, Wang XJ, & Roop DR (1999). Targeted expression of c-Myc in the epidermis alters normal proliferation, differentiation and UV-B induced apoptosis. Oncogene, 18(34), 4870–4878. doi: 10.1038/sj.onc.1203040 [DOI] [PubMed] [Google Scholar]
- Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, . . . Munoz N (1999). Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol, 189(1), 12–19. doi: [DOI] [PubMed] [Google Scholar]
- Walsh SB, Xu J, Xu H, Kurundkar AR, Maheshwari A, Grizzle WE, . . . Athar M (2011). Cyclosporine a mediates pathogenesis of aggressive cutaneous squamous cell carcinoma by augmenting epithelial-mesenchymal transition: role of TGFbeta signaling pathway. Mol Carcinog, 50(7), 516–527. doi: 10.1002/mc.20744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter V, Yin X, Wilkerson MD, Cabanski CR, Zhao N, Du Y, . . . Hayes DN (2013). Molecular subtypes in head and neck cancer exhibit distinct patterns of chromosomal gain and loss of canonical cancer genes. PLoS One, 8(2), e56823. doi: 10.1371/journal.pone.0056823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Yuen ST, Xu J, Lee SP, Yan HH, Shi ST, . . . Leung SY (2014). Whole-genome sequencing and comprehensive molecular profiling identify new driver mutations in gastric cancer. Nat Genet, 11(10). [DOI] [PubMed] [Google Scholar]
- Wang NJ, Sanborn Z, Arnett KL, Bayston LJ, Liao W, Proby CM, . . . Cho RJ (2011). Loss-of-function mutations in Notch receptors in cutaneous and lung squamous cell carcinoma. Proc Natl Acad Sci U S A, 108(43), 17761–17766. doi: 10.1073/pnas.1114669108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H, Ma Q, Peng S, Adelmant G, Swain D, Song W, . . . Bass AJ (2014). SOX2 and p63 colocalize at genetic loci in squamous cell carcinomas. J Clin Invest., 124(4), 1636–1645. doi: 1610.1172/JCI71545. Epub 72014 Mar 71543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt FM (2014). Mammalian skin cell biology: at the interface between laboratory and clinic. Science., 346(6212), 937–940. doi: 910.1126/science.1253734. [DOI] [PubMed] [Google Scholar]
- Weir BA, Woo MS, Getz G, Perner S, Ding L, Beroukhim R, . . . Meyerson M (2007). Characterizing the cancer genome in lung adenocarcinoma. Nature, 450(7171), 893–898. doi: 10.1038/nature06358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J, Sos ML, Seidel D, Peifer M, Zander T, Heuckmann JM, . . . Thomas RK (2010). Frequent and focal FGFR1 amplification associates with therapeutically tractable FGFR1 dependency in squamous cell lung cancer. Sci Transl Med, 2(62), 62ra93. doi: 10.1126/scitranslmed.3001451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissmueller S, Manchado E, Saborowski M, Morris J. P. t., Wagenblast E, Davis CA, . . . Lowe SW (2014). Mutant p53 Drives Pancreatic Cancer Metastasis through Cell-Autonomous PDGF Receptor beta Signaling. Cell., 157(2), 382–394. doi: 310.1016/j.cell.2014.1001.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells JL 3rd, & Shirai K (2012). Systemic therapy for squamous cell carcinoma of the skin in organ transplant recipients. Am J Clin Oncol, 35(5), 498–503. doi: 10.1097/COC.0b013e318201a3ef [DOI] [PubMed] [Google Scholar]
- Weng AP, Ferrando AA, Lee W, Morris J. P. t., Silverman LB, Sanchez-Irizarry C, . . . Aster JC (2004). Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science, 306(5694), 269–271. doi: 10.1126/science.1102160 [DOI] [PubMed] [Google Scholar]
- Westfall MD, Mays DJ, Sniezek JC, & Pietenpol JA (2003). The Delta Np63 alpha phosphoprotein binds the p21 and 14-3-3 sigma promoters in vivo and has transcriptional repressor activity that is reduced by Hay-Wells syndrome-derived mutations. Mol Cell Biol, 23(7), 2264–2276. doi: 10.1128/mcb.23.7.2264-2276.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westfall MD, & Pietenpol JA (2004). p63: Molecular complexity in development and cancer. Carcinogenesis, 25(6), 857–864. doi: 10.1093/carcin/bgh148 [DOI] [PubMed] [Google Scholar]
- Westhoff B, Colaluca IN, D'Ario G, Donzelli M, Tosoni D, Volorio S, . . . Di Fiore PP (2009). Alterations of the Notch pathway in lung cancer. Proc Natl Acad Sci U S A, 106(52), 22293–22298. doi: 10.1073/pnas.0907781106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westra WH, Taube JM, Poeta ML, Begum S, Sidransky D, & Koch WM (2008). Inverse relationship between human papillomavirus-16 infection and disruptive p53 gene mutations in squamous cell carcinoma of the head and neck. Clin Cancer Res, 14(2), 366–369. doi: 10.1158/1078-0432.Ccr-07-1402 [DOI] [PubMed] [Google Scholar]
- White AC, Tran K, Khuu J, Dang C, Cui Y, Binder SW, & Lowry WE (2011). Defining the origins of Ras/p53-mediated squamous cell carcinoma. Proc Natl Acad Sci U S A., 108(18), 7425–7430. Epub 2011 Apr 7418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkerson MD, Yin X, Hoadley KA, Liu Y, Hayward MC, Cabanski CR, . . . Hayes DN (2010). Lung squamous cell carcinoma mRNA expression subtypes are reproducible, clinically important, and correspond to normal cell types. Clin Cancer Res, 16(19), 4864–4875. doi: 10.1158/1078-0432.Ccr-10-0199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SE, Beronja S, Pasolli HA, & Fuchs E (2011). Asymmetric cell divisions promote Notch-dependent epidermal differentiation. Nature., 470(7334), 353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woenckhaus J, Steger K, Werner E, Fenic I, Gamerdinger U, Dreyer T, & Stahl U (2002). Genomic gain of PIK3CA and increased expression of p110alpha are associated with progression of dysplasia into invasive squamous cell carcinoma. J Pathol, 198(3), 335–342. doi: 10.1002/path.1207 [DOI] [PubMed] [Google Scholar]
- Wong J, Breen D, Balogh J, Czarnota GJ, Kamra J, & Barnes EA (2008). Treating recurrent cases of squamous cell carcinoma with radiotherapy. Curr Oncol, 15(5), 229–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolgar JA, & Triantafyllou A (2009). Pitfalls and procedures in the histopathological diagnosis of oral and oropharyngeal squamous cell carcinoma and a review of the role of pathology in prognosis. Oral Oncol, 45(4-5), 361–385. doi: 10.1016/j.oraloncology.2008.07.016 [DOI] [PubMed] [Google Scholar]
- Wu G, Nomoto S, Hoque MO, Dracheva T, Osada M, Lee CC, . . . Trink B (2003). DeltaNp63alpha and TAp63alpha regulate transcription of genes with distinct biological functions in cancer and development. Cancer Res, 63(10), 2351–2357. [PubMed] [Google Scholar]
- Wu X, Nguyen BC, Dziunycz P, Chang S, Brooks Y, Lefort K, . . . Dotto GP (2010). Opposing roles for calcineurin and ATF3 in squamous skin cancer. Nature., 465(7296), 368–372. doi: 310.1038/nature08996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang A, Kaghad M, Wang Y, Gillett E, Fleming MD, Dotsch V, . . . McKeon F (1998). p63, a p53 homolog at 3q27-29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol Cell, 2(3), 305–316. [DOI] [PubMed] [Google Scholar]
- Yang A, Zhu Z, Kapranov P, McKeon F, Church GM, Gingeras TR, & Struhl K (2006). Relationships between p63 binding, DNA sequence, transcription activity, and biological function in human cells. Mol Cell, 24(4), 593–602. doi: 10.1016/j.molcel.2006.10.018 [DOI] [PubMed] [Google Scholar]
- Yang X, Lu H, Yan B, Romano RA, Bian Y, Friedman J, . . . Chen Z (2011). DeltaNp63 versatilely regulates a Broad NF-kappaB gene program and promotes squamous epithelial proliferation, migration, and inflammation. Cancer Res, 71(10), 3688–3700. doi: 10.1158/0008-5472.Can-10-3445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama A, Kakiuchi N, Yoshizato T, Nannya Y, Suzuki H, Takeuchi Y, . . . Ogawa S (2019). Age-related remodelling of oesophageal epithelia by mutated cancer drivers. Nature, 565(7739), 312–317. doi: 10.1038/s41586-018-0811-x [DOI] [PubMed] [Google Scholar]
- Youssef KK, Lapouge G, Bouvree K, Rorive S, Brohee S, Appelstein O, . . . Blanpain C (2012). Adult interfollicular tumour-initiating cells are reprogrammed into an embryonic hair follicle progenitor-like fate during basal cell carcinoma initiation. Nat Cell Biol., 14(12), 1282–1294. doi: 1210.1038/ncb2628. Epub 2012 Nov 1225. [DOI] [PubMed] [Google Scholar]
- Yuan P, Kadara H, Behrens C, Tang X, Woods D, Solis LM, . . . Wistuba II. (2010). Sex determining region Y-Box 2 (SOX2) is a potential cell-lineage gene highly expressed in the pathogenesis of squamous cell carcinomas of the lung. PLoS One, 5(2), e9112. doi: 10.1371/journal.pone.0009112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yugawa T, Narisawa-Saito M, Yoshimatsu Y, Haga K, Ohno S, Egawa N, . . . Kiyono T (2010). DeltaNp63alpha repression of the Notch1 gene supports the proliferative capacity of normal human keratinocytes and cervical cancer cells. Cancer Res, 70(10), 4034–4044. doi: 10.1158/0008-5472.Can-09-4063 [DOI] [PubMed] [Google Scholar]
- Zeng X, Levine AJ, & Lu H (1998). Non-p53 p53RE binding protein, a human transcription factor functionally analogous to P53. Proc Natl Acad Sci U S A, 95(12), 6681–6686. doi: 10.1073/pnas.95.12.6681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Zhou Y, Cheng C, Cui H, Cheng L, Kong P, . . . Cui Y (2015). Genomic analyses reveal mutational signatures and frequently altered genes in esophageal squamous cell carcinoma. Am J Hum Genet, 96(4), 597–611. doi: 10.1016/j.ajhg.2015.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Remenyik E, Zelterman D, Brash DE, & Wikonkal NM (2001). Escaping the stem cell compartment: sustained UVB exposure allows p53-mutant keratinocytes to colonize adjacent epidermal proliferating units without incurring additional mutations. Proc Natl Acad Sci U S A, 98(24), 13948–13953. doi: 10.1073/pnas.241353198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Choi PS, Francis JM, Gao GF, Campbell JD, Ramachandran A, . . . Meyerson M (2018). Somatic Superenhancer Duplications and Hotspot Mutations Lead to Oncogenic Activation of the KLF5 Transcription Factor. Cancer Discov, 8(1), 108–125. doi: 10.1158/2159-8290.cd-17-0532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Choi PS, Francis JM, Imielinski M, Watanabe H, Cherniack AD, & Meyerson M(2016). Identification of focally amplified lineage-specific super-enhancers in human epithelial cancers. Nat Genet, 48(2), 176–182. doi: 10.1038/ng.3470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Sano D, Pickering CR, Jasser SA, Henderson YC, Clayman GL, . . . Myers JN (2011). Assembly and initial characterization of a panel of 85 genomically validated cell lines from diverse head and neck tumor sites. Clin Cancer Res, 17(23), 7248–7264. doi: 10.1158/1078-0432.ccr-11-0690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G, Liu Z, & Myers JN (2016). TP53 Mutations in Head and Neck Squamous Cell Carcinoma and Their Impact on Disease Progression and Treatment Response. J Cell Biochem, 117(12), 2682–2692. doi: 10.1002/jcb.25592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Sammons MA, Donahue G, Dou Z, Vedadi M, Getlik M, . . . Berger SL (2015). Gain-of-function p53 mutants co-opt chromatin pathways to drive cancer growth. Nature., 525(7568), 206–211. doi: 210.1038/nature15251. Epub 12015 Sep 15252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M, Lu T, Jia Y, Luo X, Gopal P, Li L, . . . Zhu H (2019). Somatic Mutations Increase Hepatic Clonal Fitness and Regeneration in Chronic Liver Disease. Cell. doi: 10.1016/j.cell.2019.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler A, Jonason AS, Leffell DJ, Simon JA, Sharma HW, Kimmelman J, . . . Brash DE (1994). Sunburn and p53 in the onset of skin cancer. Nature, 372(6508), 773–776. doi: 10.1038/372773a0 [DOI] [PubMed] [Google Scholar]
- Zou M, Toivanen R, Mitrofanova A, Floch N, Hayati S, Sun Y, . . . Abate-Shen C (2017). Transdifferentiation as a Mechanism of Treatment Resistance in a Mouse Model of Castration-Resistant Prostate Cancer. Cancer Discov, 7(7), 736–749. doi: 10.1158/2159-8290.cd-16-1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Hausen H (2002). Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer, 2(5), 342–350. doi: 10.1038/nrc798 [DOI] [PubMed] [Google Scholar]