Abstract
Background Context:
Peripheral differences often do not adequately account for variation in reports of pain intensity in people with musculoskeletal pain.
Purpose:
Here we sought to determine the extent to which structural differences in the brain (grey matter density) of pain free individuals might relate to subsequent pain (or lack thereof) after standardized peripheral muscle injury (i.e. micro trauma from high intensity exercise). Study design: This was an observational laboratory-based study that was a secondary analysis from a larger trial.
Methods:
Participants completed baseline testing (functional MRI and quantitative pain testing) followed by high intensity trunk exercise to induce delayed onset muscle soreness in the erector spinae. Forty-eight hours later, back pain intensity ratings were collected and all participants were re-imaged. Grey matter density was determined using voxel-based morphometry. The ‘asymptomatic’ group (no reports of any pain within 48 hour after induction) to a ‘pain’ group (rating of pain at rest and movement pf >20 on a 101-point numeric rating scale).
Results:
Our results revealed several large clusters where, compared to participants with pain, asymptomatic participants had significant greater grey matter density. These brain regions included left medial frontal gyrus, left middle occipital gyrus, left middle temporal gyrus, left inferior frontal gyrus, and right superior frontal gyrus.
Conclusions:
Lower grey matter density in brain regions previously linked to discriminative, emotional, and cognitive aspects of cortical processing are associated with reporting musculoskeletal pain after a standardized peripheral muscle injury.
Clinical Significance:
Cortical gray matter density of people without any pain may influence response to a standardized high intensity exercise protocol. This finding adds further support to the the relevance of central factors in explaining the tremendous individual variability in pain report following acute musculoskeletal injury.
Keywords: imaging, cortex, grey matter, pain
Introduction
Effectively managing pain is challenging given the high rates of people with asymptomatic changes in peripheral anatomy. For example, roughly 30% of asymptomatic individuals between the ages of 20 and 30 have structural abnormalities in the lumbar spine, including disk degeneration, disk bulge and disk protrusion [1]. The percentage of the pain-free population with structural abnormalities increases each decade of life [1]. Similar limitations exist when considering the soft-tissues of the lumbar spine. In our own work, for example, we have shown that the extent of lumbar soft-tissue injury is not significantly associated with the intensity of low back pain [2]. The significant personal and societal costs associated with pain disorders [3] and the modest improvements associated with treatments that target peripheral tissues, raises the priority of research aimed at understanding why some individuals report pain and seek care and others with the same peripheral input do not.
Pain is a centrally mediated experience modulated by the activity of a well-characterized set of neural structures involved in the contextualization and evaluation of the nociceptive stimulus, including those involved in sensory discrimination, executive control, and limbic processing [4–7]. There is a substantial body of literature examining differences in brain structure and function between people living with musculoskeletal pain and those without [8]. Lower grey matter density (GMD) in a variety of pain-related brain regions is commonly identified in these studies [9–12]. However, persistent pain has also been associated with greater GMD in several brain areas, including basal ganglia, parahippocampal gyrus, prefrontal cortex, and amygdala [10, 11, 13]. Critically, cortical structural differences associated with persistent pain have also been linked to alterations in functional connectivity [14], suggesting complex associations between brain structure, function, and the pain experience. The majority of these studies are cross-sectional in design comparing people who are pain-free to those people with chronic pain. Thus, these studies cannot establish if GMD differences arise in response to pain or exist before the original injury and contribute to the development of pain.
Studies of the brain morphological correlates of pain intensity in response to induced pain provide a useful starting point for better understanding the extent to which GMD differences identified in other studies might account for differences in pain reporting in the presence of similar peripheral tissue changes. For example, higher GMD in insula, primary somatosensory cortex, cingulate cortex, ventrolateral prefrontal cortex, ortibofrontal cortex, amygdala, precuneus, and basal ganglia all predict lower pain sensitivity in laboratory-based experimental pain induction paradigms [15–17]. However, the majority of experimental pain induction paradigms use noxious stimuli that induce temporally limited experience of pain, often lasting seconds to minutes (e.g., cutaneous thermal heat to capsaicin injection protocols). In addition, these pain induction paradigms are not typically associated with significant functional impairment or modifications of activities of daily living. The short duration of pain perception associated with many laboratory-based induction protocols restricts ecological validity and ability to inform our understanding of brain morphology and variability in the pain experience.
To address these limitations, we used an exercise induced muscle injury paradigm to compare brain structure between healthy, asymptomatic individuals who report pain after exercise to individuals who do not report pain using voxel-based morphometry (VBM). Exercise induced muscle injury provides several advantages over other experimental pain inductions because it produces a clinically-relevant, longer-lasting (days compared vs. minutes) musculoskeletal pain associated with functional impairment and disability.[18–20] Therefore there is a need to explore what factors, other than the severity of tissue damage, influence the severity of pain following muscle injury. Based on existing literature summarized above, we hypothesized that individuals who report no pain after exercise induced muscle injury would have higher GMD in brain regions associated with the cognitive, affective, and sensory components of cortical processing than their counterparts.
Methods
Participants
Healthy adults between the ages of 20 and 40 years were recruited for this study. Exclusion criteria included current back pain, persistent muscle conditions, or regular performances of conditioning exercises for back/trunk extensor muscles. All participants gave informed consent and signed a form approved by the University Institutional Review Board prior to data collection. The current report represents a secondary analysis from a larger randomized clinical trial ().
Screening Procedure
During laboratory-based screening sessions, participants completed a standard demographic and health history questionnaire. Responses on this questionnaire were used to determine study eligibility. Specific exclusion criteria were:
Previous participation in a conditioning program specific to trunk extensors in the past 6 months;
Any report of back or leg pain in the past 3 months;
Any chronic medical conditions that may affect pain perception (e.g., diabetes, high blood pressure, fibromyalgia, headaches), kidney dysfunction, muscle damage, or major psychiatric disorder;
History of previous injury including surgery to the lumbar spine, renal malfunction, cardiac condition, high blood pressure, osteoporosis, or liver dysfunction;
Consumption of any drugs (e.g., caffeine, alcohol, theophyline, tranquilizers, antidepressants, anti-inflammatory medication) that may affect pain perception or hydration status from 24 hr. before participation until completion of the investigation;
Performance of any intervention for symptoms induced by exercise and before the termination of their participation or the protocol;
Recent illness;
Any contraindication to MRI e.g.: pacemakers, metal implants which are not MRI compatible (e.g. aneurysm clip), pregnancy and severe claustrophobia.
Participants also completed the pain catastrophizing questionnaire.
Structural MRI Acquisition
MRI data were acquired with a 3T Philips Achieva equipped with a 32-channel head coil. High-resolution structural brain images were collected using a three-dimensional (3D) T1-weighted magnetization-prepared rapid gradient-echo (MP-RAGE) sequence with a field-of-view (FOV) = 240mm (FH) × 240mm (AP) × 170mm (RL), voxel wise resolution = 1mm3, TR = 8.1 ms, TE = 3.7 ms, FA = 8°. Acquisition time was 7 minutes 56 seconds.
Voxel-based Morphometry (VBM) Analysis
VBM analyses were conducted in SPM12 v6225 (Wellcome Trust Centre for Neuroimaging, University College London, London, UK). Raw T1 images were manually re-oriented, and the origin was placed near the anterior commissure. Images were down-sampled from 1×1×1mm resolution to 1.5×1.5×1.5mm, then segmented using the default automated segmentation procedure in SPM12. This segmentation routine creates six image segmentations (tissue probability maps) corresponding to gray matter, white matter, cerebral spinal fluid, skull, soft tissue outside the brain, and air. All image segmentations were conducted in the participant’s native space; diffeomorphic anatomical registration using exponentiated Lie algebra (DARTEL) imported images were also created for gray and white matter segmentations [21]. After segmentation, a DARTEL template was created. DARTEL increases the accuracy of between-subject alignment by simultaneously aligning gray and white matter between images to create a study specific template [21, 22]. Each individual image was then aligned to the group template, followed by normalization of the group template to Montreal Neurological Institute (MNI) space using an affine transformation. These transformation parameters were then applied to each individual image to bring the subjects into MNI space. Finally, images were smoothed with an 8mm full-width-half-maximum (FWHM) Gaussian kernel.
Quantitative Sensory Testing (QST) Procedure
Participants completed a QST battery designed to assess thermal and pressure pain sensitivity.
Thermal pain threshold was determined using a Medoc Thermosensory Analyzer (TSA; Medoc, Inc., Ramat Yishai, Israel). A continuous heat stimulus was delivered to the subjects’ dominant arm. The stimulus started at 35°C and increased at a rate of 0.5°C with subjects indicating when the temperature reached pain threshold (“when the sensation first transitions from heat to pain”). These procedures were repeated three times. The average of each trial was calculated to determine pain threshold.
Pressure pain threshold was assessed using a Fischer Dolorimeter tipped with a rubber foot-plate 1 cm in diameter (Pain Diagnostics, Great Neck, NY). To determine pressure pain threshold, force was slowly increased until the participant indicated the sensation changed from pressure to pain. Pressure pain threshold was measured in the lumbar musculature, in the hand at the 1st dorsal interosseous muscle, and between the first and second toe on the dorsal aspect of the foot. As with heat pain threshold determination, pressure pain thresholds were collected three times at each site and averaged. Pressure pain thresholds at the hand and foot were averaged to determine pressure pain threshold at the distal extremities.
Standardized Exercise Induced Pain Protocol
All subjects completed a 5-minute warm-up consisting of riding a stationary bicycle. Following the warm up, participants then performed an isometric (static) test of total torque of the trunk extensor muscles using a MedX lumbar extension exercise machine (MedX Holdings, Inc., Ocala, FL, USA), following a standardized protocol [23]. The repeatability of isometric torque production is well-established in participants without pain and patients with low back pain [23, 24]. Participants were seated in the MedX machine with the stabilizing straps attached across the pelvis and knees. The participant was moved through the range of motion (ROM) of the machine in lumbar flexion and extension to determine his or her available ROM. The device was locked into place in maximal flexion and the participant was instructed to build up force gradually against a pad in contact with the mid and lower back. The torque generated by the participant was displayed graphically on the data collection computer. Once peak effort was observed by the research assistant, the participant was instructed to relax, the device released and the participant returned to an upright position. Isometric testing was administered seven times in positions ranging from the participant’s maximum available trunk flexion to maximal trunk extension. The isometric torque collected at each test angle was summed to give measure of total torque produced across the entire range of motion.
After baseline total torque was recorded, participants performed bouts of dynamic exercise to the point of volitional fatigue. To perform an exercise bout, the participants were seated and restrained in a MedX lumbar extension exercise machine. Participants performed as many repetitions as possible using a weight load equal to approximately 90% of the average total torque measured during the isometric test. Each repetition was performed through the full available ROM and the participants were encouraged to perform the lifting portion (concentric) in two seconds and the lowering (eccentric) in four seconds. Repetitions continued until the patient reported being unable to move through a full range of motion (volitional fatigue). After each bout, the isometric torque test was performed again. Using a criterion of 50% reduction in torque, subjects completed another bout of exercise if the torque was above 50% of the baseline value. If the re-estimated torque was 50% or less, the induction protocol was complete. Following the exercise, subjects were instructed not to initiate any medication or apply any intervention to the lumbar spine to reduce symptoms. This human model of exercise induced low back pain has been previously described in greater detail elsewhere [18, 19].
Post-Induction Pain Assessment
Two days after pain induction, participants completed a laboratory assessment of their pain over the previous 48 hours using 100mm visual analog scales (VAS) anchored from ‘no pain sensation’ to ‘most intense pain sensation imaginable’. Current pain intensity, worst pain intensity over the previous two days, and pain intensity during trunk movement (i.e., flexion, extension, and right and left lateral bending) were assessed during this session. We used a threshold >20mm on the Pain VAS to indicating that a participant had developed clinically relevant pain. This threshold was determined a priori based on the twice the minimal detectable change for worsening in low back pain intensity[25]. Participants reporting 0 mm on all VAS measures of pain intensity were considered asymptomatic or ‘pain resilient’. Participants with VAS results between 1 and 19 were included in an ‘intermediate pain’ group.
Analysis Strategy
Potential differences between asymptomatic participants, participants with pain > 20mm, and those with intermediate pain reporting on demographic and psychological variables were assessed using 1-way analysis of variance (ANOVA). Then, comparison of gray matter density (GMD) asymptomatic participants and those reporting clinically relevant pain was conducted using whole-brain voxel-wise GLM. Participant age, sex, and total intracranial volume (TIV) were included as covariates. Cluster-size based family-wise error correction was implemented using the AFNI-based tools 3dClustSim and 3dFWHMx [26], assuming an uncorrected voxelwise p-value threshold of p < .001. Smoothness parameters for input to 3dClustSim were calculated by estimating smoothness of each subject’s preprocessed VBM images using 3dFWHMx, then averaging across the sample. Resulting mean autocorrelation factors and FWHM estimates were ACFa =.50, ACFb = 4.95, ACFc = 14.85, FWHM = 13.74. 3dClustSim simulations indicated that, given these parameters, a cluster size threshold of 294 contiguous voxels was sufficient to achieve αFWE< .05. Mean GMD within the detected clusters was extracted for each participant using the REX toolbox (https://www.nitrc.org/projects/rex) and subjected to 1-way ANOVA to characterize GMD in the intermediate pain group relative to the asymptomatic and pain susceptible groups, followed by pairwise t-tests to determine significant group differences.
Finally, exploratory voxel-wise regression analyses were conducted to determine the association between GMD and pain intensity VAS scores collected prior to scanning across the entire sample, including those with intermediate pain reporting. Use of the same 3dClustSim-based approach with autocorrelation factors and FWHM estimates across the entire sample (ACFa =.50, ACFb = 4.91, ACFc = 14.58, FWHM = 13.61) revealed a cluster size threshold of 283 contiguous voxels was sufficient to achieve αFWE< .05 in this analysis.
Effect sizes are reported as Hedges’ g, a variant of Cohen’s d that provides correction for small sample sizes, or η2p, as appropriate.
Results
Participant Characteristics
A total of 61 subjects completed the baseline MRI scan and DOMS protocol. Eight were asymptomatic (i.e. pain resilient), 33 had clinically relevant pain, and 20 reported intermediate pain between 0 and 20mm on at least one pain intensity VAS (current, worst, or any movement pain measure).
One-way ANOVA indicated a significant effect of group on lumbar pressure pain threshold (F2,57=6.38, p=.003) such that asymptomatic participants reported significantly greater thresholds than either the pain susceptible (p=.007, g=1.14) or intermediate pain (p=.001, g=1.31) groups. No other significant differences between groups on demographics, pain-related psychological measures, or other QST measures of pain sensitivity were noted (p>.13; see Table 1).
Table 1.
Participant Characteristics
| Pain Resilient | Clinically Relevant Pain | Intermediate Pain | |
|---|---|---|---|
| n=8 Mean (SD) or % |
n=33 Mean (SD) or % |
N=20 Mean (SD) or % |
|
| Demographics | |||
| Age (years) | 25.9 (6.1) | 23.1 (3.8) | 22.6 (3.2) |
| % Women | 63 | 64 | 65 |
| BMI (kg/m2) | 25.4 (4.1) | 23.8 (5.4) | 24.3 (5.4) |
| Pain-related Psychological Measures | |||
| Pain Catastrophizing Scale (total score) | 10.4 (9.1) | 11.61 (10.1) | 10.7 (9.13) |
| Fear of Pain Questionnaire (total score) | 16.8 (8.0) | 16.4 (7.9) | 14.7 (7.2) |
| Pain Sensitivity | |||
| Lumbar Pressure Pain Threshold (kg/cm3) | 29.7 (11.7) | 20.38 (7.2) | 17.30 (8.6) |
| Foot and Hand Pressure Pain Threshold (kg/cm3) | 18.1 (3.8) | 15.6 (8.0) | 14.4 (7.1) |
| Heat Pain Threshold (°C) | 45.0 (2.8) | 44.1 (2.0) | 43.8 (2.7) |
Comparison of Whole Brain GMD
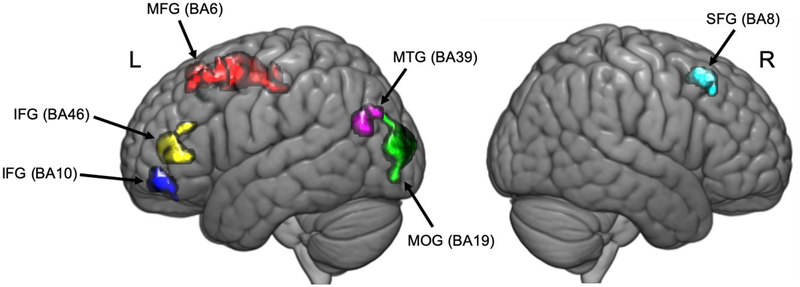
Voxelwise whole-brain comparisons revealed several large clusters where, compared to participants with pain, asymptomatic participants had significant greater GMD. These brain regions included left medial frontal gyrus (MFG), left middle occipital gyrus (MOG), left middle temporal gyrus (MTG), left inferior frontal gyrus (IFG), and right superior frontal gyrus (SFG; Figure 1). Differences in GMD between pain-resilient and clinically relevant pain groups were statistically large (Hedges g > 1.08); see Table 2 for details.
Figure 1.
Clusters where PR participants had significantly higher GMD than PS participants in standard MNI space (pFWE < .05). Red: Left medial frontal gyrus; Green: left middle occipital gyrus; Violet: left middle temporal gyrus; Blue: left inferior frontal gyrus; Yellow: left inferior frontal gyrus; Teal: right superior frontal gyrus.
Table 2.
Regions with Significant Grey Matter Density Differences Between Pain-Resilient Individuals and those with clinically-relevant pain (pFWE < .05)
| MNI Coordinates | Pain-Resilient | Clinically Relevant Pain | ||||||
|---|---|---|---|---|---|---|---|---|
| Brain Region | X | Y | Z | k | Peak t | Mean (SD) | Mean SD) | g |
| Left Medial Frontal Gyrus (BA6) | −9 | 8 | 57 | 2494 | 6.71 | .501 (.034) | .433 (.038) | 1.82 |
| Left Middle Occipital Gyrus (BA19) | −32 | −92 | 10 | 1566 | 6.58 | .470 (.024) | .394 (.058) | 1.42 |
| Left Middle Temporal Gyrus (BA39) | −51 | −69 | 22 | 453 | 5.69 | .537 (.050) | .471 (.063) | 1.08 |
| Left Inferior Frontal Gyrus (BA46) | −40 | 44 | 6 | 886 | 4.94 | .556 (.044) | .478 (.057) | 1.42 |
| Left Inferior Frontal Gyrus (BA10) | −39 | 50 | −9 | 435 | 4.92 | .501 (.048) | .435 (.056) | 1.21 |
| Right Superior Frontal Gyrus (BA8) | 32 | 20 | 51 | 338 | 4.76 | .553 (.056) | .476 (.056) | 1.38 |
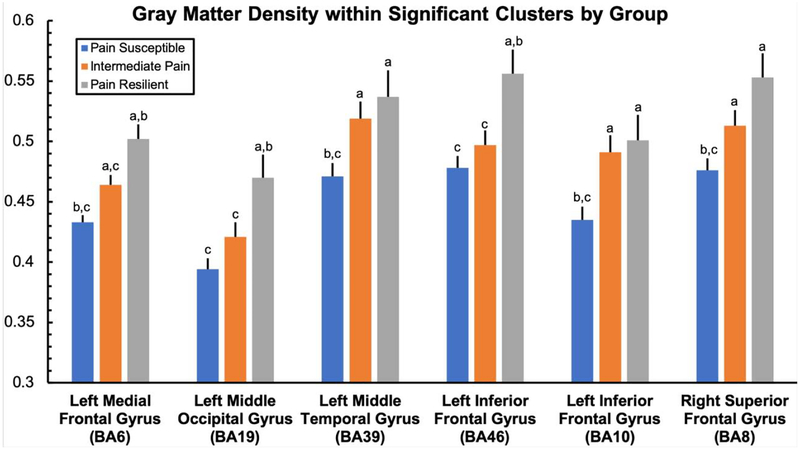
In addition, as expected, 1-way ANOVA revealed significant effects of group on GMD within all detected clusters when participants with intermediate pain were included (F2,60>5.69, p<.006, η2p>.16). Broadly, across regions, participants reporting intermediate pain also showed intermediate GMD volume when compared to asymptomatic and pain-susceptible individuals. Detailed results of each pairwise comparison between groups by cluster are displayed in Figure 2.
Figure 2.
Grey matter density within significant clusters by group. a: significantly difference from the clinically meaningful pain group; b: significantly different from the intermediate pain group; c: significantly difference from the pain resilient group.
Association between GMD and current DOMS pain across the entire sample
We conducted a voxel-by-voxel regression analysis across all subjects to identify regions where higher GMD might predict lower reported pain intensity after the eccentric exercise induction. No clusters survived statistical correction for multiple comparisons (pFWE > .05).
Discussion
Our primary finding in this study is that individual differences in the morphometry of specific brain regions, while an individual is asymptomatic, may underpin subsequent pain development following exercise induced muscle injury in the lower back. Specifically, participants who developed clinically relevant pain in our study had lower GMD in several brain regions, including those demonstrated to be involved with pain processing in prior functional neuroimaging studies: MFG (i.e., supplementary motor area [SMA]), MTG, SFG, and IFG. These regions are highlighted in Figure 1.
Several of the cluster coordinates identified in our analyses are similar to those in previously published studies showing significant activation in response to both experimental and clinical pain or pain-related stimuli. For example, left MFG, MTG, and IFG activity proximal to peak voxels of the clusters where we identified in higher GMD in pain-resilient individuals has been implicated as important for the empathic processing of pain in others [27];[28];[29]. Left MFG activity has also been implicated in the processing of both painful and non-painful mechanical stimulation [30]. We also found that pain resilient individuals had higher GMD in the left IFG than those who experienced clinically relevant pain. Detected clusters within the IFG, which included Brodmann Areas 46 and 10, have been previously demonstrated to be involved in temporal summation of second pain (TSSP) [31]. Perturbation of functional connectivity between left IFG and left mid-insular cortex has also been implicated in development of and recovery from chronic low back pain [32]. Finally, aberrantly high SFG activation during experimental pain induction has been noted in individuals with chronic knee osteoarthritis compared to healthy controls [33].
That the areas identified in our study are consistent with these anatomical regions from other studies that used brief stimuli supporting hypothesis that intensity and duration of a peripheral stimulus is not as important as the cortical structures involved with processing and interpreting the input. Overall, our data suggest that differences in GMD relate more to interpreting sensory input from the periphery as painful or not painful rather than determining the intensity or severity of that of the input.
Study Limitations
Although our findings are novel, the study did have important limitations. Critically, the proportion of the sample in this study who were asymptomatic was small (~13%; n=8). Future studies could avoid this concern by screening larger numbers of participants using the pain induction protocol with the intent to identify sufficient numbers of asymptomatic individuals. Nevertheless, we did identify multiple cortical areas that differed between groups.
Clinical Significance
In our study, asymptomatic individuals did not differ significantly from those who reported intermediate or clinically-relevant pain in their demographics or on pain-related psychological measures, both factors linked to increased intensity and duration of reported pain. There were, however, differences in the grey matter density across several brain regions. Given that the pain experience represents interpretation of sensory information, the differences in GMD identified in our study may help explain variability in pain reporting in the presence of consistent anatomical changes for patients. GMD indicates more cortical substrate and the potential for increased or more efficient processing in these areas. A number of factors contribute to gray matter density, including white matter characteristics (e.g., number of axons), number of neurons and glial cells, the size of these cells, and the degree of dendritic arborization [34]. Histological studies would be needed to determine which exact factors contributed to the gray matter differences we detected in this study between asymptomatic individuals and those that experience clinically-relevant pain. However, studies have indicated that treatment of chronic pain using cognitive behavioral therapy can increase gray matter density in areas related to pain modulation suggesting, plasticity within these regions [35]. Additional cross-sectional studies are needed to compare the extent to which brain morphology differs between symptomatic and asymptomatic individuals with similar peripheral anatomic changes. In addition, longitudinal assessment of brain morphology in people with pain conditions is needed to explore the extent to which an active process of brain remodeling (e.g., an increase in GMD) may underlie clinical improvement.
Conclusions
Results of this study provide preliminary evidence that higher GMD in several brain regions that have been previously linked to discriminative, emotional, and cognitive aspects of pain processing are associated with resilience to musculoskeletal pain after a standardized pain induction in the lower back using exercise. Our findings provide further support for the relevance of brain morphology for explaining individual variability in pain report following acute muscle injury. Our findings also provide preliminary indication that many of the same brain regions involved in maintenance of chronic pain states are already active and show morphological differences during an acute pain episode. This suggests brain morphology during a pain-free state influences not only the subsequent development of acute pain but the longer term pain experience as well.
Acknowledgements
We would like to acknowledge the participants who volunteered for this study and the work of the investigators on the primary trial - Maggie Horn, DPT, PhD, MPH; Meryl Alappattu, DPT, PhD, Joel Bialosky, PT, PhD; Fredy Solis, PT, PhD; Kara Hannibal, DPT; Warren Greenfield, MS; and Cally House, BS. This work was supported by the National Center of Complementary and Integrative Health (grant numbers: R01AT006334, F32AT007729). A portion of this work was performed in the McKnight Brain Institute at the National High Magnetic Field Laboratory’s AMRIS Facility, which is supported by National Science Foundation Cooperative Agreement No. DMR-1157490, and the State of Florida.
Footnotes
Disclosures
Conflict of Interest: The authors declare no conflict of interest.
References
- 1.Brinjikji W, Luetmer PH, Comstock B, et al. Systematic literature review of imaging features of spinal degeneration in asymptomatic populations. AJNR Am J Neuroradiol. 2015;36(4):811–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bishop MD, Horn ME, Lott DJ, Arpan I, George SZ. Magnitude of spinal muscle damage is not statistically associated with exercise-induced low back pain intensity. Spine J. 2011;11(12):1135–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: National Academies Press; 2011. [PubMed] [Google Scholar]
- 4.Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. European journal of pain (London, England). 2005;9(4):463–84. [DOI] [PubMed] [Google Scholar]
- 5.Clarke CF, Lawrence KS. Functional imaging for interpretation of pain pathways: current clinical application/relevance and future initiatives. Current pain and headache reports. 2013;17(2):311. [DOI] [PubMed] [Google Scholar]
- 6.Craggs JG, Price DD, Verne GN, Perlstein WM, Robinson MM. Functional brain interactions that serve cognitive-affective processing during pain and placebo analgesia. NeuroImage. 2007;38(4):720–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Staud R, Craggs JG, Perlstein WM, Robinson ME, Price DD. Brain activity associated with slow temporal summation of C-fiber evoked pain in fibromyalgia patients and healthy controls. European journal of pain (London, England). 2008;12(8):1078–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coppieters I, Meeus M, Kregel J, et al. Relations Between Brain Alterations and Clinical Pain Measures in Chronic Musculoskeletal Pain: A Systematic Review. The journal of pain: official journal of the American Pain Society. 2016;17(9):949–62. [DOI] [PubMed] [Google Scholar]
- 9.Apkarian AV, Sosa Y, Sonty S, et al. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24(46):10410–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seminowicz DA, Labus JS, Bueller JA, et al. Regional gray matter density changes in brains of patients with irritable bowel syndrome. Gastroenterology. 2010;139(1):48–57.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.As-Sanie S, Harris RE, Napadow V, et al. Changes in regional gray matter volume in women with chronic pelvic pain: a voxel-based morphometry study. Pain. 2012;153(5):1006–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niddam DM, Lee SH, Su YT, Chan RC. Brain structural changes in patients with chronic myofascial pain. European journal of pain (London, England). 2017;21(1):148–58. [DOI] [PubMed] [Google Scholar]
- 13.Schweinhardt P, Kuchinad A, Pukall CF, Bushnell MC. Increased gray matter density in young women with chronic vulvar pain. Pain. 2008;140(3):411–9. [DOI] [PubMed] [Google Scholar]
- 14.Yang Q, Wang Z, Yang L, Xu Y, Chen LM. Cortical thickness and functional connectivity abnormality in chronic headache and low back pain patients. Human brain mapping. 2017;38(4):1815–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elsenbruch S, Schmid J, Kullmann JS, et al. Visceral sensitivity correlates with decreased regional gray matter volume in healthy volunteers: a voxel-based morphometry study. Pain. 2014;155(2):244–9. [DOI] [PubMed] [Google Scholar]
- 16.Emerson NM, Zeidan F, Lobanov OV, et al. Pain sensitivity is inversely related to regional grey matter density in the brain. Pain. 2014;155(3):566–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwedt TJ, Chong CD. Correlations between brain cortical thickness and cutaneous pain thresholds are atypical in adults with migraine. PloS one. 2014;9(6):e99791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bishop MD, Horn ME, George SZ. Exercise-induced pain intensity predicted by pre-exercise fear of pain and pain sensitivity. Clin J Pain. 2011;27(5):398–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bishop MD, Horn ME, George SZ, Robinson ME. Self-reported pain and disability outcomes from an endogenous model of muscular back pain. BMC Musculoskelet Disord. 2011;12:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dannecker EA, O’Connor PD, Atchison JW, Robinson ME. Effect of eccentric strength testing on delayed-onset muscle pain. J Strength Cond Res. 2005;19(4):888–92. [DOI] [PubMed] [Google Scholar]
- 21.Ashburner J A fast diffeomorphic image registration algorithm. NeuroImage. 2007;38(1):95–113. [DOI] [PubMed] [Google Scholar]
- 22.Klein A, Andersson J, Ardekani BA, et al. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. NeuroImage. 2009;46(3):786–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graves JE, Pollock ML, Carpenter DM, et al. Quantitative assessment of full range-of-motion isometric lumbar extension strength. Spine (Phila Pa 1976). 1990;15(4):289–94. [DOI] [PubMed] [Google Scholar]
- 24.Robinson ME, Greene AF, O’Connor P, Graves JE, MacMillan M. Reliability of lumbar isometric torque in patients with chronic low back pain. Phys Ther. 1992;72(3):186–90. [DOI] [PubMed] [Google Scholar]
- 25.Hagg O, Fritzell P, Nordwall A, Swedish Lumbar Spine Study G. The clinical importance of changes in outcome scores after treatment for chronic low back pain. Eur Spine J. 2003;12(1):12–20. [DOI] [PubMed] [Google Scholar]
- 26.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–73. [DOI] [PubMed] [Google Scholar]
- 27.Budell L, Jackson P, Rainville P. Brain responses to facial expressions of pain: emotional or motor mirroring? Neuroimage. 2010;53(1):355–63. [DOI] [PubMed] [Google Scholar]
- 28.Decety J, Skelly LR, Kiehl KA. Brain response to empathy-eliciting scenarios involving pain in incarcerated individuals with psychopathy. JAMA Psychiatry. 2013;70(6):638–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage. 2011;54(3):2492–502. [DOI] [PubMed] [Google Scholar]
- 30.Lui F, Duzzi D, Corradini M, Serafini M, Baraldi P, Porro CA. Touch or pain? Spatio-temporal patterns of cortical fMRI activity following brief mechanical stimuli. Pain. 2008;138(2):362–74. [DOI] [PubMed] [Google Scholar]
- 31.Staud R, Craggs JG, Robinson ME, Perlstein WM, Price DD. Brain activity related to temporal summation of C-fiber evoked pain. Pain. 2007;129(1–2):130–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ceko M, Shir Y, Ouellet JA, Ware MA, Stone LS, Seminowicz DA. Partial recovery of abnormal insula and dorsolateral prefrontal connectivity to cognitive networks in chronic low back pain after treatment. Hum Brain Mapp. 2015;36(6):2075–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hiramatsu T, Nakanishi K, Yoshimura S, et al. The dorsolateral prefrontal network is involved in pain perception in knee osteoarthritis patients. Neurosci Lett. 2014;581:109–14. [DOI] [PubMed] [Google Scholar]
- 34.Gennatas ED, Avants BB, Wolf DH, Satterthwaite TD, Ruparel K, Ciric R, … & Gur RC (2017). Age-related effects and sex differences in gray matter density, volume, mass, and cortical thickness from childhood to young adulthood. Journal of Neuroscience, 37(20), 5065–5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seminowicz DA, Shpaner M, Keaser ML, Krauthamer GM, Mantegna J, Dumas JA, … & Naylor MR (2013). Cognitive-behavioral therapy increases prefrontal cortex gray matter in patients with chronic pain. The Journal of Pain, 14(12), 1573–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]