Abstract
Expression of caveolin-1 (Cav-1) is an important pathophysiological factor in acne. Cav-1 strongly interacts with such well-recognized etiopathogenic factors such as hyperseborrhea, follicular hyperkeratinization, and pathogenicity of C. acnes. Cav-1 is a strong negative regulator of transforming growth factor beta (TGF-β) expression. It acts as a critical determinant of autophagy, which is significantly induced in acne lesions through C. acnes and by absorption of fatty acids. Cav-1 also demonstrates different correlations with the development of innate immunity. We propose that normalization of Cav-1 expression can serve as a target in anti-acne therapy.
Keywords: Acne, pathophysiology, caveolin, hyperseborrhea, follicular hyperkeratinization, C. acnes, autophagy, innate immunity
1. INTRODUCTION
Plasma membranes of eukaryotic cells have spatially heterogeneous structures containing lipid clusters, which can appear in form of flattened areas (lipid rafts) or plasma membrane invaginations (caveolae). Caveolae are involved in rapid adaptation to cellular volume changes, in various signal transduction processes as well as in endo- and exocytosis. Caveolin-1 (Cav-1) is the principal structural component of caveolae, its expression is strongly dependent on the differentiation stage of the cell, and it is often considered as a master regulator of several important signaling cascades. Cav-1 exists not only in plasma membranes or intracellularly but can also be transported in extracellular vesicles, providing a long-range mechanism of communication inside of the tissue or even between adjacent tissues.[1-3] Low levels of Cav-1 expression in tissues are generally connected with the appearance of hyperproliferative and inflammatory conditions and are also substantially involved in psoriasis and scarring.[3,4] Keratinocytes in which lipid rafts are disrupted are also associated with the appearance of atopic dermatitis.[5]
Acne vulgaris is the most common chronic inflammatory skin disease related to pilosebaceous units (PSUs). Its pathophysiology was connected with different etiopathogenic factors, among them hyperseborrhea, follicular hyperkeratinization, and pathogenic behavior of the gram-positive bacterium C. acnes (formerly P. acnes).[6,7] Traditionally, hyperseborrhea was linked with increased sebum excretion from sebaceous gland induced by androgens; follicular hyperkeratinization was believed to be mainly caused by abnormal proliferation, terminal differentiation and desquamation of keratinocytes, and the pathogenic behavior of C. acnes was related to uncontrolled proliferation of these bacteria in PSUs. At the same time, inflammatory events are present in the very early stages of acne development and can be found both in involved and uninvolved acne skin.[8] This allows acne vulgaris to be considered as a primary inflammatory skin disorder.[9]
There is no increase in the proliferation of keratinocytes in acne lesions.[10] The transition from commensalism to pathogenicity of bacteria is often realized without the need for hyperproliferation of pathogens and is normally connected with a modified host-pathogen interaction.[11] This modification is not inherently connected with altered properties of the pathogen itself, but may mainly be caused by internal modifications of the host cells. Further, local overloading of the skin with free fatty acids causing inflammation can be provided not only by the sebaceous gland. In fact, dermal adipocytes can provide significant local levels of the free fatty acids around the distal end of PSU during their physiological de-differentiation into adipocyte-derived preadipocytes (ADPs) [12] in the catagen phase of the hair follicle (HF) cycle.[13]
Transforming growth factor β (TGF-β) is actively produced by human fibroblasts and plays an important role in functioning of sebocytes and keratinocytes. Whereas TGF-β can exist in different isoforms, it appears that only TGF-β1 plays an important role in acne, being strongly upregulated in acne lesions.[14] Cav-1 is involved in the internalization and intracellular degradation of TGF-β receptors, [15] providing an inverse correlation between TGF-β1 and Cav-1 in different hyperproliferative and inflammatory conditions.[3,4]. Correspondingly, the observed overexpression of TGF-β1 points to a local deficiency of Cav-1 in acne lesions. This mechanism can also influence the lipid production in human sebocytes.[16]
Here, we discuss how the main etiopathogenic factors in acne can be connected with the expression of Cav-1 and whether Cav-1 can be a target in the treatment of this skin disease.
2. CAV-1 IN HYPERKERATINIZATION
Follicular obstruction observed in acne lesions may be connected either with uncontrolled hyperproliferation of keratinocytes, with their abnormal terminal differentiation and/or with reduced desquamation of these cells. Since enhanced proliferation of keratinocytes in acne lesions is not observed,[10] the main focus is on the differentiation and desquamation processes.
Both terminal differentiation and desquamation of keratinocytes are significantly regulated by epidermal lamellar bodies.[17,18] Cav-1 deficiency delays the terminal differentiation of these cells. Application of methyl-β-cyclodextrin (MβCD), which is a cholesterol-depleting agent that disrupts caveolae, demonstrates skin-specific anti-Cav-1 activity, and significantly reduces the secretion of lamellar bodies,[18] which must affect the desquamation process.
Thus, Cav-1 expression must be an important factor in terminal differentiation of follicular keratinocytes and their desquamation and thus in the development of hyperkeratinization.
3. CAVEOLIN AND HYPERSEBORRHEA
Hyperseborrhea, traditionally connected with increased sebum excretion from sebaceous gland, is considered as a necessary condition for acne development. Mature sebocytes accumulate lipid droplets and express pro- and anti-inflammatory adipokines, thus linking lipid metabolism and inflammation.[18] This resembles the functioning of adipocytes providing far-reaching analogies with adipocyte biology.
3.1. Cav-1 in sebocyte functioning
The physiological activity of sebocytes demonstrates multiple correlations with Cav-1 expression. Sebocytes express adiponectin and adiponectin receptors (AdipoR1 and AdipoR2) both in vitro and in vivo,[19-21] and it was reported that adiponectin strongly regulates lipid production in sebocytes in a dose-dependent manner.[20] AdipoR1 is co-localized with Cav-1 in the plasma membrane, producing AdipoR1/Cav-1 “signalsomes”, which are critical for adiponectin signaling. From here, enhanced expression of Cav-1 in sebocytes may be needed for hyperseborrhea. Suppression of Cav-1 will lead to a dissociation of AdipoR/Cav-1 signalsomes and thus to a reduction of hyperseborrhea. This can explain the early reports concerning reduction of hyperseborrhea through of β-cyclodextrins, which deplete cholesterol and reduce Cav-1. Interestingly, such a dissociation of the AdipoR1/Cav-1 signalsome is typical in thw diabetic state, which corresponds to the theory of metabolic syndrome of the PSU proposed in [22].
Secretion of anti-inflammatory markers, such as adiponectin, and pro-inflammatory cytokines, such as leptin and interleukin (IL)-6, as well as expression of Cav-1 are much higher in differentiated than in undifferentiated adipocytes.[23] Activation of TGF-β signaling was shown to be necessary and sufficient for maintaining sebocytes in an undifferentiated state, whereas reduced expression of TGF-β receptors (TβR) in these cells caused an increased lipid production.[16] Internalization and degradation of TGF-β receptors can occur through caveolae-dependent and independent pathways; whereas caveolin-independent internalization of TβR enhances TGF-β signaling, caveolin-dependent internalization of these receptors causes its effective suppression.[15] This also points to an increased expression of Cav-1 in hyperseborrhea. It was reported that hyaluronan (HA) increases co-localization of TβR with Cav-1.[24] Whereas originally this effect was reported for human renal proximal tubular cells, it appears to be of general significance. Of note, exogenous HA attenuates lipid production in human sebocytes in a dose-dependent manner both in vitro and in vivo.[25]
Whereas TGF-β1 signaling was shown to be enhanced in acne lesions,[14] this signaling must be suppressed in hyperseborrhea.[16] Concerning the negative correlation between TGF-β1 and Cav-1 observed in different hyperproliferative and inflammatory skin conditions, this points to an inhomogeneous distribution of Cav-1 in acne skin.
3.2. Dermal adipocytes as an additional source for fatty acid loading
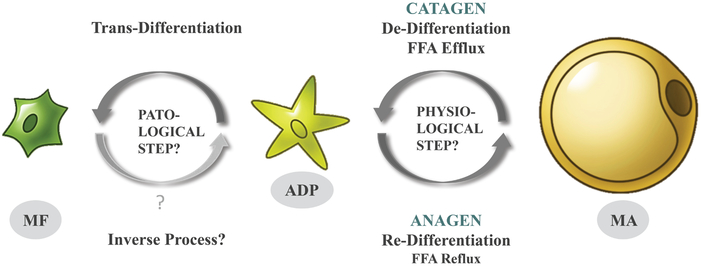
While the hyperseborrhea is mainly linked to the sebaceous glands, there is another source for fatty acids in the skin - dermal white adipose tissue (dWAT). This special fat depot located in the superficial hypodermis and around the distal parts of PSUs demonstrates significant volume oscillations during HF cycle.[12,26,27] As we discovered very recently, this effect is connected with repeated de- and re-differentiation processes, which affect 90-95% of the adipose cells from dWAT (Fig. 1).[13] The de-differentiation of mature dermal adipocytes in adipocyte-derived preadipocytes (ADPs) is connected to a dramatic reduction of the cell volumes.[12] During this process, dWAT provides a local source for fatty acids,[13] which can - upon release - be transported to the reticular and even to the papillar dermis. This release must be spatiotemporally correlated in the case of the synchronized HF cycling, as seen in rodents. However, it has a more local character in the microenvironment during asynchronous HF cycling, as observed in humans. At the same time, as we argued previously,[28] short range synchronization of neighboring HFs in humans is possible and may be connected with high expression of the multi-ligand receptor megalin/LRP2. Substantial portion (up to 40-fold) of cellular megalin/LRP2 is concentrated in caveolae where it can be phosphorylated.[29] Megalin/LRP2 levels temporally vary during HF cycle. They are much higher in early anagen than in catagen or telogen.[30] This means that the degree of the spatial HF synchronization should vary during HF cycle. This effect can increase the area loaded with fatty acids extending it from a single HF to a spatially correlated group of HFs.
Fig.1.
Processes of de-, re-, and trans-differentiation of mature adipocytes are strongly dependent on the phase of the HF cycle. MA – mature adipocyte, ADP – adipocyte-derived preadipocyte, MF – myofibroblast.
On the other hand, the re-differentiation of ADPs into mature adipocytes is taking place during the anagen phase[13] and requires a significant flux of fatty acids back to the adipocytes. This demand must be at least partially covered by the local source of fatty acids in sebum and requires a strong secretory capacity from sebocytes. As a result, the reticular dermis experiences oscillations of fatty acids loading and unloading during the HF cycle. Cav-1 is strongly involved both in uptake and efflux of lipids in adipocytes and is therefore a critical factor in these processes.[31,32] Consequently, we would argue that these oscillations must correlate with the local Cav-1 expression levels in the areas adjacent to PSUs.
As demonstrated in [13], ADPs can be alternatively trans-differentiated in myofibroblasts in vivo. These myofibroblasts typically display significantly lower levels of Cav-1 expression compared to normal fibroblasts. The differentiation process into myofibroblasts is enhanced under conditions of Cav-1 deficiency.[4] This trans-differentiation, as it takes place in acne skin, will lead to localized scarring. The specific signals triggering transition from the cyclic de-/re-differentiation of dermal adipocytes to their trans-differentiation into myofibroblasts remain to be elucidated. We assume that this transformation can be caused by prolonged inflammation accompanied by the development of excessive autophagy. These processes lead to Cav-1 deficiency, which, in turn, can promote the trans-differentiation of ADPs into myofibroblasts. The trans-differentiation of adipocytes into myofibroblasts in vitro requires TGF-β stimulation and an additional mechanical stress, provided by increasing the adhesion area in a stiff matrix.[33] Of note, both TGF-β [15] and intracellular adhesion molecule 1 (ICAM-1) [8] were found to be strongly upregulated in acne lesions.
Since Cav-1 is substantially involved in the re-differentiation and very likely in the de-differentiation processes in dermal adipocytes as well as in the trans-differentiation of ADPs into myofibroblasts, the manipulation of its expression can be an important target in the regulation of the fatty acid content at the distal ends of HFs.
4. CAV-1 IN OPPORTUNISTIC PATHOGENICITY OF C. ACNES
Even though C. acnes is highly abundant in a healthy skin, it can act as an opportunistic pathogen in the development of acne vulgaris. This pathogenic transformation is not simply connected with enhanced proliferation of bacteria, but must be essentially based on a modified interaction host-pathogen.
4.1. Cav-1 in host-pathogen interactions
Different pathogens interact with lipid raft microdomains in the plasma membrane to invade the host cells.[34,35] In the course of this, critical constituents of caveolae are actively recruited to the sites of bacterial entrance [36] demonstrating a significant involvement of Cav-1 in this process. It was reported that internalization of various pathogens can positively or negatively depend on the Cav-1 expression in the host cells. Non-professional phagocytic cells deficient in Cav-1 demonstrate a remarkably increased uptake of S. aureus and it was proposed that Cav-1 overexpression generally reduces the internalization of fibronectin-binding pathogens.[37] This phenomenon was connected with a negative regulation of the membrane microdomain mobility through Cav-1, which anchors these domains to the cytoskeleton. C. acnes are also fibronectin-binding bacteria, and, whereas this topic was not investigated properly, it can be strongly assumed that internalization of these pathogens should demonstrate a similar dependence on Cav-1. Also, Cav-1 KO mice demonstrate significantly elevated bacterial burdens in different types of cells.[38] It was further reported that Cav-1 can protect host cells against bacterial invasion by a caveola-independent mechanism.[39] Additionally, skin infections with S. aureus cause rapid proliferation of dermal preadipocytes and their differentiation into mature adipocytes.[40] Since Cav-1 expression in immature adipocytes is significantly lower than in mature adipocytes, these cells should demonstrate a higher susceptibility to bacterial infection. As a result, the de-differentiation of dermal adipocytes into ADPs can lead to an enhanced level of bacterial infection in these immature cells, which in turn is transmitted to the mature adipocytes during re-differentiation process in the anagen phase. In fact, the inhibition of TGF-β receptors increases resistance of adult mice to S. aureus.[41]
C. acnes interacts with the skin not only through invading the host cells. This pathogen also constitutively releases extracellular vesicles, which can induce innate immunity and acne-like phenotype in human epidermal keratinocytes.[42,43] Similar mechanism based on the S. aureus-derived extracellular vesicles was stated to be an important factor in pathogenesis of atopic dermatitis.[44]
C. acnes produce coproporphyrin III, which arises from heme synthesis. It demonstrates a high correlation with the appearance of comedonal and papulopustular acne lesions.[45] Heme degradation enzyme heme oxygenase (HO-1) is localized to caveolae; moreover, Cav-1 physically interacts with HO-1, modulating its enzymatic activity.[46]
4.2. Possible role of hyaluronan in C. acnes pathogenicity
Additionally, C. acnes of the type IA, which is predominantly associated with acne-affected skin, demonstrates high hyaluronic acid lyase/hyaluronidase activity and can intensively degrade the HA producing oligosaccharides of various sizes.[47] Partitioning of TGF-β receptors into Cav-1 lipid raft-associated pools takes place only in the presence of high molecular weight HA (HMW-HA); in contrast, low molecular weight HA (LMW-HA) was stated to be unable to antagonize the effect of TGF-β1 by Cav-1.[48] This phenomenon must be of importance in inflammatory acne lesions where it should lead to detachment of TGF-β receptors from Cav-1 providing deregulation of their physiological interaction and effectively increasing the TGF-β content.
Binding of HA to the cell surface mainly occurs through CD44 receptor and is strongly regulated by the matrix metalloproteinase MT1-MMP; both CD44 and MT1-MMP are spatially co-localized with caveolae.[49] Furthermore, lipid rafts sufficiently regulate the interaction between CD44 and HA, and this regulation largely depends on the HA size.[50] Completing this picture, C. acnes directly induces the expression of MT1-MMP in human dermal fibroblasts.[51] Additionally, sebocytes express CD44 both in vitro and in vivo, and exogenous HMW-HA downregulates lipid synthesis in these cells in a dose-dependent manner.[52] Whereas these authors did not directly investigate the effect of the LMW-HA on the sebum production, they inferred that it should be different from HMW-HA. Consequently, the regulation of sebum production by HA must be strongly modified in acne skin where C. acnes intensively degrade HA.
These results collectively infer that Cav-1 must be involved both directly and indirectly in the pathogenic behavior of C. acnes in a diverse and multifunctional way.
5. CAV-1, AUTOPHAGY AND ACNE
Healthy epidermis and the normal sebaceous gland demonstrate significant autophagy.[53,54] Distribution of the autophagy markers spatially correlates with expression of Cav-1,[55] as well as with the production of lamellar bodies. Autophagosome-like structures were also found in PSUs.[53,56] It was reported that C. acnes induces autophagy in macrophages and mesenchymal cells obtained from sarcoid lesions,[57] and in epidermal keratinocytes.[58] This catabolic process is essential for removal of dysfunctional mitochondria and influences the survival and differentiation of keratinocytes.[59] Of note, autophagy can be stimulated both by intracellular infection,[57] as well as by extracellular C. acnes,[58] which means that extracellular vesicles released by C. acnes can be involved in this process.[44]
Under physiological conditions, Cav-1 co-localizes with different autophagy markers and is a critical determinant of autophagy.[60] All the while, prolonged or strongly overstimulated autophagy leads to the degradation of Cav-1 prompting a local deficiency.[61,62] Autophagic degradation of Cav-1 can be induced by palmitic acid (PA),[63] which is a typical component of sebum, and also released by enlarged adipocytes.[64]
Autophagy demonstrates interactions with TGF-β signaling at multiple levels. On the one hand, TGF-β can activate autophagy;[65] on the other hand, excessive autophagy promotes degradation of TGF-β.[66] Inhibition of autophagy antagonizes the effects of TGF-β1.[67] The degree of interaction between TGF-β and Cav-1 is obviously critically dependent on the Cav-1 expression. siRNA-mediated Cav-1 reduction triggers a significant activation of autophagy in endothelial cells.[60] Cav-1 deficiency promotes both basal and inducible autophagy in mouse embryonic fibroblasts, enhancing lysosomal function and autophagosome-lysosome interaction.[68]
On the other hand, senescent keratinocytes having high Cav-1 expression demonstrate extremely enhanced autophagic activity, which seems to contradict the results described above.[69] It should be, however, noted that accumulation of autophagy markers may be connected either with induction of autophagy or with impaired clearance of autophagosomes. For example, increased numbers of autophagosomes was also observed in aged dermal fibroblasts, but their appearance was connected with impaired autophagic flux (failure of autophagosomes to fuse with lysosomes) and not with enhanced autophagy.[70]
Both saturated and unsaturated fatty acids are able to induce the autophagy in dose-dependent manner. PA is one of the typical components of the human sebum which enhances the innate immune response of human sebocytes against C. acnes.[71] PA-triggered autophagy observed in [63] was mTOR-independent.[72] This allows the induction of autophagy even in the context of strong upregulation of mTOR, as seen in acne vulgaris.[73] Importantly, Chen et al. demonstrated that PA induces autophagy in a Cav-1-independent manner, that degradation of Cav-1 is responsible for the development of inflammation, and that chronic high-fat diet can induce autophagy and Cav-1 degradation.[63] Moreover, induced overexpression of Cav-1 was shown to attenuate these effects. These results indicate that the Cav-1 expression and the appearance of acne can be at least partly connected with the nutritional status and that Cav-1 can be a causal factor. Of note, Cav-1 is required for the uptake of fatty acids through regulation of the fatty acid translocase (FAT/CD36) at the plasma membrane.[74]
Since fatty acids are released by mature adipocytes during their de-differentiation into ADPs in the catagen phase and are re-absorbed during re-differentiation of these cells into mature adipocytes in anagen,[13] local autophagy in the skin should demonstrate HF cycle specific variations. Indeed, whole-mount staining of HFs and surrounding dWAT reveals an increased expression of the autophagy marker LC3B during catagen.[75] On the other hand, the transition from anagen VI to catagen is characterized by a remarkable reduction of the autophagic flux in hair matrix keratinocytes and such inhibition of autophagy is necessary for catagen development.[76]
These results collectively demonstrate that Cav-1 is substantially involved in the regulation of autophagy. Non-physiological autophagy can strongly reduce the Cav-1 content in the tissue leading to development of spatially limited hyperproliferative and inflammatory conditions in the skin which are typical in acne.
6. CAV-1 IN INNATE IMMUNITY
Traditionally, it is believed that C. acnes can interact with the innate immune system through four primary pathways: matrix-metalloproteinases (MMPs), toll-like receptors (TLRs), inflammasomes, and anti-microbial peptides (AMPs).[7] On the other hand, Cav-1 is a known regulator of innate immunity [77] and thus is strongly involved in these processes.
Silencing of Cav-1 in keratinocytes in vitro provided significantly increased expression of different cytokines/chemokines, such as IL-6, CXCL8, and CXCL9, whereas the application of Cav-1 scaffolding domain peptides in murine skin models of psoriasis significantly reduced the expression of cytokines.[78] Application of MβCD also caused dramatic induction of cytokines in keratinocytes.[5] Cav-1 is substantially involved in the regulation of some MMPs, which are strongly upregulated in acne skin.[79] Cav-1 was reported to be a negative regulator of MMP-1 in human dermal fibroblasts.[80] Further, C. acnes activates TLR2 and TLR4 in the membranes of inflammatory cells,[81,82] and TLR4 is co-localized and physically interacts with Cav-1,[77] whereas the Cav-1 deficiency dampens the TLR4 signaling.[83]
AMPs demonstrate not only bactericidal effects, but can also promote inflammatory reactions. Differential expression of AMPs was found in acne skin, among them ß-defensins, psoriasin and cathelicidin.[7,84] Cathelicidin and ß-defensins are abundantly expressed by sebocytes in response to S. aureus and C. acnes.[85] The main source for cathelicidin in the murine skin is provided by differentiating immature dermal adipocytes.[40] Very recently, we have performed gene expression analysis on highly purified dermal adipocytes and identified Camp gene expression for cathelicidin as a marker for these cells.[13]
Whereas expression of cathelicidin by dermal adipocytes was originally reported for S. aureus,[40] it can be strongly inferred that dWAT also demonstrates an antimicrobial reaction against C. acnes. Indeed, HR-1 hairless mice, which are considered as a murine model for acne, demonstrate a pronounced expandability of the dWAT layer [86] and exhibit strong inflammatory reactions, epithelial proliferation and formation of microcomedone-like cysts after C. acnes injection.[87]
7. CONCLUSION
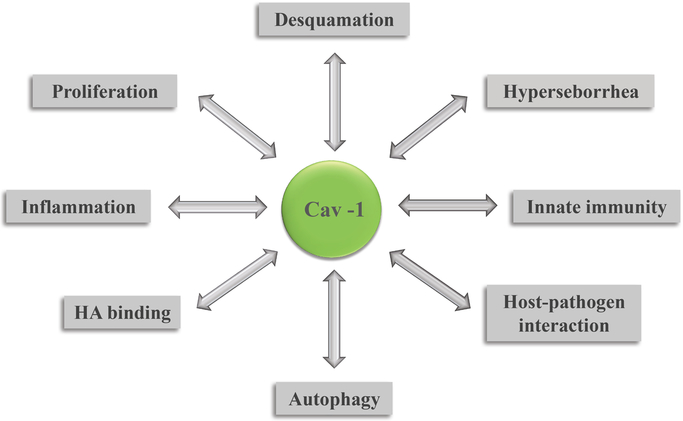
The presence of Cav-1 is essential for different hyperproliferative and inflammatory processes in the skin, acting not only as an important etiopathogenic factor (Fig.2), but also as a master regulator of these conditions. Previous analysis demonstrated its essential role in the pathogenesis of skin diseases such as psoriasis and hypertrophic scarring.[3,4] Here, we propose that Cav-1 is also casually involved in pathogenesis of acne. Etiopathogenic factors in acne such as follicular hyperkeratinization, hyperseborrhea, and pathogenic behavior of C. acnes all demonstrate a clear dependence on Cav-1 expression.
Fig. 2.
Cav-1 as an etiopathogenic factor involved in appearance of acne lesions.
Cav-1 content at the distal end of PSUs undergoes physiological HF cycle-dependent oscillations. These are connected with cyclic processes of de- and re-differentiation of dermal adipocytes located in this area.[13] Such oscillations of the cellular content provide not only the spatially limited and HF cycle-dependent variations of Cav-1 expression, but also lead to corresponding variations in the local susceptibility of the host cells to bacterial infections and to the spatio-temporal variations of fatty acids loading and autophagy in these areas. This suggests a connection of acne lesions with HF cycling. It should be mentioned that the initiation of acne lesions was a long time ago already assigned mainly to the telogen phase of HF cycle,[88] but these effects must now be re-investigated with state-of-the-art experimental techniques.
Altogether, these results collectively demonstrate that Cav-1 expression is not only an important etiopathogenic factor in acne, but can also serve as a target in anti-acne treatment.
ACKNOWLEDGMENTS
PES is supported by NIH grants R01-DK55758, R01-DK099110, RC2-DK118620, P01-DK088761 and P01-AG051459. PES is also supported by an unrestricted grant from the Novo Nordisk Research Foundation.
Footnotes
CONFLICT OF INTREST
ILK is the managing partner of Wellcomet GmbH. Wellcomet GmbH provided support in the form of salaries for ILK, but did not have any additional role in decision to publish or preparation of the manuscript. The commercial affiliation of ILK with Wellcomet GmbH does not alter the adherence to all journal policies on sharing data and materials. PES declares no conflict of interest.
REFERENCES
- [1].Chang CC, Chen CY, Wen HC, Huang CY, Hung MS, Lu HC, Chen WL, Chang CH, Obesity. 2017, 25, 1932. [DOI] [PubMed] [Google Scholar]
- [2].Crewe C, Joffin N, Rutkowski JM, Kim M, Zhang F, Towler DA, Gordillo R, Scherer PE, Cell. 2018, 175, 695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kruglikov IL, Scherer PE, npj. Aging Mech. Dis 2019, 5, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kruglikov IL, Scherer PE, npj Regen. Med 2019, 4, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Mathay C, Pierre M, Pittelkow MR, Depiereux E, Nikkels AF, Colige A, Poumay Y, Invest YJ. Dermatol. 2011, 131, 46. [DOI] [PubMed] [Google Scholar]
- [6].Tuchayi SM, Makrantonaki E, Ganceviciene R, Dessinioti C, Feldman SR, Zouboulis CC, Nat. Rev. Dis. Primers 2015, 1, 15029. [DOI] [PubMed] [Google Scholar]
- [7].Dréno B, Gollnick HPM, Kang S, Thiboutot D, Bettoli V, Torres V, Leiden J, J. Eur. Acad. Dermatol. Venereol 2015, 29, 3. [DOI] [PubMed] [Google Scholar]
- [8].Jeremy AH, Holland DB, Roberts SG, Thomson KF, Cunliffe WJ, J. Invest. Dermatol 2003, 121, 20. [DOI] [PubMed] [Google Scholar]
- [9].Kircik LH, Drug LHJ. Dermatol. 2016, 15(1 Suppl 1), S7. [PubMed] [Google Scholar]
- [10].Persson G, Johansson-Jänkänpää E, Ganceviciene R, Karadag AS, Bilgili SG, Omer H, Alexeyev OA, Exp. Dermatol 2018, 27, 668. [DOI] [PubMed] [Google Scholar]
- [11].Chen YE, Fischbach MA, Belkaid Y, Nature. 2018, 553, 427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kruglikov IL, Zhang Z, Scherer PE, Trends Endocrinol. Metab 2018, 30, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zhang Z, Shao M, Hepler C, Zi Z, Zhao S, An YA, Zhu Y, Ghaben A, Wang MY, Li N, Onodera T, Joffin N, Crewe C, Zhu Q, Kumar A, Xing C, Wang QA, Deng Y, Gordillo R, Kruglikov I, Kusminski CM, Gupta RK, Scherer PE, J Clin Invest. 2019, doi: 10.1172/JCI130239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kelhälä HL, Palatsi R, Fyhrquist N, Lehtimäki S, Väyrynen JP, Kallioinen M, Kubin ME, Greco D, Tasanen K, Alenius H, Bertino B, Carlavan I, Mehul B, Deret S, Reiniche P, Martel P, Marty C, Blume-Peytavi U, Voegel JJ, Lauerma A, PLoS One. 2014, 9, e105238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Del Galdo FD, Sotgia F, de Almeida CJ, Jasmin JF, Musick M, Lisanti MP, Jimenez SA, Arth. Rheum 2008, 58, 2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].McNairn AJ, Doucet Y, Demaude J, Brusadelli M, Gordon CB, Uribe-Rivera A, , Lambert PF, Bouez C, Breton L, Guasch G, BMC Dermatol. 2013, 13, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Roelandt T, Roseeuw D, Giddelo C, Hachem JP, Open Dermatol. J 2009, 3, 146. [Google Scholar]
- [18].Roelandt T, Giddelo C, Heughebaert C, Denecker G, Hupe M, Crumrine D, Kusuma A, Haftek M, Roseeuw D, Declercq W, Feingold KR, Elias PM, Hachem JP, J. Invest. Dermatol 2009, 129, 927. [DOI] [PubMed] [Google Scholar]
- [19].Kovács D, Lovászi M, Póliska S, Oláh A, Bíró T, Veres I, Zouboulis CC, Stahle M, Rühl R. Remenyik E, Törőcsik D, Exp. Dermatol 2016, 25, 194. [DOI] [PubMed] [Google Scholar]
- [20].Jung YR, Lee JH, Sohn KC, Lee Y, Seo YJ, Kim CD, Lee JH, Hong SP, Seo SJ, Kim SJ, Im M, PloS One. 2017, 12, e0185081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Won CH, Yoo HG, Park KY, Shin SH, Park WS, Park PJ, Chung JH, Kwon OS, Kim KH, J. Invest. Dermatol 2012, 132, 2849. [DOI] [PubMed] [Google Scholar]
- [22].Melnik BC, Clin. Dermatol 2018, 36, 29. [DOI] [PubMed] [Google Scholar]
- [23].Palacios-Ortega S, Varela-Guruceaga M, Milagro FI, Martínez JA, de Miguel C, PloS One. 2014, 9, e95100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ito T, Williams JD, Fraser DJ, Phillips AO, J. Biol. Chem 2004, 279, 25326. [DOI] [PubMed] [Google Scholar]
- [25].Jung YR, Hwang C, Ha JM, Choi DK, Sohn KC, Lee Y, Seo YJ1, Lee YH, Kim CD, Lee JH, Im M, J. Invest. Dermatol 2017, 137, 1215. [DOI] [PubMed] [Google Scholar]
- [26].Guerrero-Juarez CF, Plikus MV, Nat. Rev. Endocrinol 2018, 14, 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Foster AR, Nicu C, Schneider MR, Hinde E, Paus R, Arch. Dermatol. Res 2018, 310, 453. [DOI] [PubMed] [Google Scholar]
- [28].Kruglikov IL, Scherer PE, Exp. Dermatol 2016, 25, 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Boucher P, Liu P, Gotthardt M, Hiesberger T, Anderson RG, Herz J, J. Biol. Chem 2002, 277, 15507. [DOI] [PubMed] [Google Scholar]
- [30].Adly MA, Histochem. Cell Biol 2010, 134, 591. [DOI] [PubMed] [Google Scholar]
- [31].Pohl J, Ring A, Ehehalt R, Schulze-Bergkamen H, Schad A, Verkade P, Stremmel W, Biochem. 2004, 43, 4179. [DOI] [PubMed] [Google Scholar]
- [32].Pilch PF, Liu L, Trends Endocrinol. Metab 2011, 22, 318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bielczyk-Maczyńska E, Taylor B, Miller CM, Zhao ML, Shah A, Bahrami-Nejad Z, Dunn AR, Teruel MN, bioRxiv. 2019, 604231. [Google Scholar]
- [34].Zaas DW, Swan Z, Brown BJ, Wright JR, Abraham SN, Commun. Integr. Biol 2009, 2, 535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Vieira FS, Correa G, Einicker-Lamas M, Coutinho-Silva R, Biol. Cell 2010, 102, 391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Duncan MJ, Shin JS, Abraham SN, Cell SN. Microbiol. 2002, 4, 783. [DOI] [PubMed] [Google Scholar]
- [37].Hoffmann C, Berking A, Agerer F, Buntru A, Neske F, Chhatwal GS, Ohlsen K, Hauck CR, J. Cell Sci 2010, 123, 4280. [DOI] [PubMed] [Google Scholar]
- [38].Feng H, Guo L, Song Z, Gao H, Wang D, Fu W, Han J, Li Z, Huang B, Li XA, J. Biol. Chem 2010, 285, 25154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Lim JY, Barnett TC, Bastiani M, McMahon KA, Ferguson C, Webb RI, Parton RG, Walker MJ, Cell. Microbiol 2017, 19, e12772. [DOI] [PubMed] [Google Scholar]
- [40].Zhang LJ, Guerrero-Juarez CF, Hata T, Bapat SP, Ramos R, Plikus MV, Gallo RL, Science. 2015, 347, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zhang LJ, Chen SX, Guerrero-Juarez CF, Li F, Tong Y, Liang Y, Liggins M, Chen X, Chen H, Li M, Hata T, Zheng Y, Plikus MV, Gallo RL, Immunity. 2019, 50, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Choi EJ, Lee HG, Bae IH, Kim W, Park J, Lee TR, Cho EG, J. Invest. Dermatol 2018, 138, 1371. [DOI] [PubMed] [Google Scholar]
- [43].Dagnelie MA, Corvec S, Khammari A, Dréno B, Exp. Dermatol 2019, doi: 10.1111/exd.14050. [DOI] [Google Scholar]
- [44].Kim J, Bin BH, Choi EJ, Lee HG, Lee TR, Cho EG, Clin. Exp. Allergy 2019, 49, 68. [DOI] [PubMed] [Google Scholar]
- [45].Patwardhan SV, Richter C, Vogt A, Blume-Peytavi U, Canfield D, Kottner J, Arch. Dermatol. Res 2017, 309, 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kim HP, Wang X, Galbiati F, Ryter SW, Choi AM, FASEB J. 2004, 18, 1080. [DOI] [PubMed] [Google Scholar]
- [47].Nazipi S, Stødkilde K, Scavenius C, Brüggemann H, Microorganisms. 2017, 5, E57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ito T, Williams JD, Fraser DJ, Phillips AO, Am. J. Pathol 2004, 164, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Annabi B, Thibeault S, Moumdjian R, Béliveau R, J. Biol. Chem 2004, 279, 21888. [DOI] [PubMed] [Google Scholar]
- [50].Murai T, Front. Immunol 2015, 6, 420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Choi JY, Piao MS, Lee JB, Oh JS, Kim IG, Lee SC, J. Invest. Dermatol 2008, 128, 846. [DOI] [PubMed] [Google Scholar]
- [52].Jung YR, Hwang C, Ha JM, Choi DK, Sohn KC, Lee Y, Kim CD, Lee JH, Im M, Invest MJ. Dermatol. 2017, 137, 1215. [DOI] [PubMed] [Google Scholar]
- [53].Sukseree S, Eckhart L, Tschachler E, Watanapokasin R, Front. Biosci 2013, 5, 1000. [DOI] [PubMed] [Google Scholar]
- [54].Rossiter H, Stübiger G, Gröger M, König U, Gruber F, Sukseree S, Mlitz V, Buchberger M, Oskolkova O, Bochkov V, Eckhart L, Tschachler E, Exp. Dermatol 2018, 27, 1142. [DOI] [PubMed] [Google Scholar]
- [55].Sando GN, Zhu H, Weis JM, Richman JT, Madison KC, Wertz PW, J. Invest. Dermatol 2003, 120, 531. [DOI] [PubMed] [Google Scholar]
- [56].Rossiter H, Stübiger G, Gröger M, König U, Gruber F, Sukseree S, Mlitz V, Buchberger M, Oskolkova O, Bochkov V, Eckhart L, Tschachler E, Exp. Dermatol 2018, 27, 1142. [DOI] [PubMed] [Google Scholar]
- [57].Nakamura T, Furukawa A, Uchida K, Ogawa T, Tamura T, Sakonishi D, Wada Y, Suzuki Y, Ishige Y, Minami J, Akashi T, Eishi Y, PloS One. 2016, 11, e0156298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Megyeri K, Orosz L, Bolla S, Erdei L, Rázga Z, Seprényi G, Urban E, Szabo K, Kemény L, Invest LJ. Dermatol. 2018, 138, 750. [DOI] [PubMed] [Google Scholar]
- [59].Li L, Chen X, Gu H, Oncotarget. 2016, 7, 50682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Shiroto T, Romero N, Sugiyama T, Sartoretto JL, Kalwa H, Yan Z, Shimokawa H, Michel T, PloS One. 2014, 9, e87871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Martinez-Outschoorn UE, Trimmer C, Lin Z, Whitaker-Menezes D, Chiavarina B, Zhou J, Wang C, Pavlides S, Martinez-Cantarin MP, Capozza F, Witkiewicz AK, Flomenberg N, Howell A, Pestell RG, Caro J, Lisanti MP, Sotgia F, Cell Cycle. 2010, 9, 3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Martinez-Outschoorn UE, Pavlides S, Whitaker-Menezes D, Daumer KM, Milliman JN, Chiavarina B, Migneco G, Witkiewicz AK, Martinez-Cantarin MP, Flomenberg N, Howell A, Pestell RG, Lisanti MP, Sotgia F, Cell Cycle. 2010, 9, 2423. [DOI] [PubMed] [Google Scholar]
- [63].Chen Z, Nie SD, Qu ML, Zhou D, Wu LY, Shi XJ, Ma LR, Li X, Zhou SL, Wang S, Wu J, Cell Death Dis. 2018, 9, 771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Ezure T, Amano S, J. Invest. Dermatol 2011, 131, 2004. [DOI] [PubMed] [Google Scholar]
- [65].Suzuki HI, Kiyono K, Miyazono K, Autophagy. 2010, 6, 645. [DOI] [PubMed] [Google Scholar]
- [66].Ding Y, II Kim S, Lee SY, Koo JK, Wang Z, Choi ME, J. Am. Soc. Nephrol 2014, 25, 2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Alizadeh J, Glogowska A, Thliveris J, Kalantari F, Shojaei S, Hombach-Klonisch S, Klonisch T, Ghavami S, Biochim. Biophys. Acta Mol, Cell Res 2018, 1865, 749. [DOI] [PubMed] [Google Scholar]
- [68].Shi Y, Tan SH, Ng S, Zhou J, Yang ND, Koo GB, McMahon KA, Parton RG, Hill MM, Del Pozo MA, Kim YS, Shen HM, Autophagy. 2015, 11, 769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Gosselin K, Deruy E, Martien S, Vercamer C, Bouali F, Dujardin T, Slomianny T,C, Houel-Renault L, Chelli F, De Launoit Y, Abbadie C, C. Am. J. Pathol 2009, 174, 423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Tashiro K, Shishido M, Fujimoto K, Hirota Y, Yo K, Gomi T, Tanaka Y, Biochem. Biophys. Res. Commun 2014, 443, 167. [DOI] [PubMed] [Google Scholar]
- [71].Nakatsuji T, Kao MC, Zhang L, Zouboulis CC, Gallo RL, Huang CM, J. Invest. Dermatol 2010, 130, 985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Tan SH, Shui G, Zhou J, Li JJE, Bay BH, Wenk MR, Shen HM, J. Biol. Chem 2014, 289, 9501. [Google Scholar]
- [73].Monfrecola G, Lembo S, Caiazzo G, De Vita V, Di Caprio R, Balato A, Fabbrocini G, Exp. Dermatol 2016, 25, 153. [DOI] [PubMed] [Google Scholar]
- [74].Ring A, Le Lay S, Pohl J, Verkade P, Stremmel W, Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipid 2006, 1761, 416. [DOI] [PubMed] [Google Scholar]
- [75].Nicu C, Hardman J, Pople J, Paus R, Exp. Dermatol 2019, 28, 432. [DOI] [PubMed] [Google Scholar]
- [76].Parodi C, Hardman JA, Allavena G, Marotta R, Catelani T, Bertolini M, Paus R, Grimaldi B, PLoS Biol. 2018, 16, e2002864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Wang XM, Kim HP, Nakahira K, Ryter SW, Choi AM, J. Immunol 2009, 182, 3809. [DOI] [PubMed] [Google Scholar]
- [78].Yamaguchi Y, Watanabe Y, Watanabe T, Komitsu N, Aihara M, J. Invest. Dermatol 2015, 135, 2764. [DOI] [PubMed] [Google Scholar]
- [79].Sato T, Kurihara H, Akimoto N, Noguchi N, Sasatsu M, Ito A, Biol. Pharm. Bull 2011, 34, 295. [DOI] [PubMed] [Google Scholar]
- [80].Haines P, Samuel GH, Cohen H, Trojanowska M, Bujor AM, J. Dermatol. Sci 2011, 64, 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Jugeau S, Tenaud I, Knol AC, Jarrousse V, Quereux G, Khammari A, Dreno B, Br. J. Dermatol 2005, 153, 1105. [DOI] [PubMed] [Google Scholar]
- [82].Shibata M, Katsuyama M, Onodera T, Ehama R, Hosoi J, Tagami H, J. Invest. Dermatol 2009, 129, 375. [DOI] [PubMed] [Google Scholar]
- [83].Mirza MK, Yuan J, Gao XP, Garrean S, Brovkovych V, Malik AB, Tiruppathi C, Zhao YY, Am. J. Pathol 2010, 176, 2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Niyonsaba F, Kiatsurayanon C, Chieosilapatham P, Ogawa H, Exp. Dermatol 2017, 26, 989. [DOI] [PubMed] [Google Scholar]
- [85].Lee DY, Yamasaki K, Rudsil J, Zouboulis CC, Park GT, Yang JM, Gallo RL, J. Invest. Dermatol 2008, 128, 1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Hwang IS, Kim JE, Choi SI, Lee HR, Lee YJ, Jang MJ, Son HJ, Lee HS, Oh CH, Kim BH, Lee SH, Hwang DY, Int. J. Mol. Med 2012, 30, 392. [DOI] [PubMed] [Google Scholar]
- [87].Jang YH, Lee KC, Lee SJ, Kim DW, Lee WJ, Ann. Dermatol 2015, 27, 257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].van Scott EJ, MacCardle RC, J. Invest. Dermatol 1956, 27, 405. [DOI] [PubMed] [Google Scholar]