Previous studies have found haemoconcentration during hospitalization for heart failure (HF) to correlate with superior decongestion and improved post-discharge outcomes, despite increased risk of in-hospital worsening renal function (WRF).1–3 These data suggest that haemoconcentration may represent an objective and evidence-based measure of the adequacy of in-hospital decongestion.1,2,4 However, assessment of congestion remains challenging after the in-hospital period.5 This post-discharge ‘vulnerable phase’ represents a high-risk period for death, rehospitalization, and worsening haemodynamics, and despite strong guideline recommendations for early post-discharge follow-up, there are little data to guide titration and assessment of decongestive therapy at these outpatient visits.6 As such, it is possible that post-discharge haemodilution could signal re-accumulation of intravascular fluid and be an early subclinical marker of worsening HF. The EVEREST (Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan) trial offers a novel opportunity (i) to study the relationship between early post-discharge haemodilution, WRF, and markers of congestion among patients recently hospitalized for HF, and (ii) to determine the association between post-discharge haemodilution and subsequent long-term clinical outcomes.
The study design and primary results of the EVEREST trial have been previously reported.7,8 In brief, EVEREST was a prospective, global, randomized clinical trial that studied the effect of tolvaptan vs. placebo on clinical outcomes among patients hospitalized for worsening chronic HF with reduced ejection fraction (HFrEF) [ejection fraction (EF) ≤40%]. Median trial follow-up was 9.9 months. Complete blood counts (including haematocrit), basic chemistries, and body weight were collected at randomization, discharge (or day 7 if occurred first), and 1-month post-discharge. Since tolvaptan has been shown to increase renal excretion of free water, the present post-hoc analysis included only trial participants assigned to placebo. Other inclusion criteria for the present study were complete data for haematocrit at discharge (or day 7) and 1-month post-discharge. The variable of interest in the present study was post-discharge haematocrit absolute change, defined as the difference between discharge and 1-month post-discharge haematocrit. Patients were divided into quartiles by degree of post-discharge haematocrit change, with negative values (i.e. decreasing haematocrit) reflecting haemodilution. Quartile 1 represented patients with the greatest haemodilution in the 1-month post-discharge period and quartile 4 represented patients with the least haemodilution (i.e. most haemoconcentration).
Primary endpoints were (i) all-cause mortality and (ii) the composite of cardiovascular mortality (CVM) or HF hospitalization. Endpoint events were landmarked at 1 month post-discharge, so that only events occurring after the haemodilution assessment interval were counted. All endpoints were assessed as time-to-first event. We also assessed the association between post-discharge haematocrit change and changes in renal function, body weight, and B-type natriuretic peptide (BNP) from discharge to 1 month. Worsening serum creatinine was defined as an increase in the serum creatinine level ≥0.3 mg/dL. Worsening estimated glomerular filtration rate (eGFR) and blood urea nitrogen (BUN) were defined as ≥25%decrease and increase, respectively, in the 2 measures.
Patient characteristics at discharge were compared across quartiles of haematocrit change using χ2 tests, analysis of variance (ANOVA), and Kruskal–Wallis tests, as appropriate. Continuous variables were reported as mean ± standard deviation or median (interquartile range) based on distribution. Multivariable Cox proportional hazards models were used to assess the association between continuous post-discharge change in haematocrit (per 5% absolute decrease in haematocrit) and both all-cause mortality and the composite endpoint (CVM or HF hospitalization). Models were adjusted for 24 pre-specified covariates measured at discharge, including age, sex, geographic region, ischaemic HF aetiology, New York Heart Association class, systolic blood pressure, serum sodium, BUN, eGFR, BNP, QRS duration, EF, past medical history (diabetes mellitus, hypertension, chronic kidney disease, coronary artery disease, chronic obstructive pulmonary disease, atrial fibrillation/flutter, prior HF hospitalization) and concurrent medications (angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, beta-blockers, mineralocorticoid receptor antagonists, digoxin, and intravenous inotropes).
Among 2061 EVEREST patients assigned to placebo, 463 (22%) did not have complete haematocrit data at discharge and/or 1 month. The remaining 1598 (78%) patients alive with available discharge and 1-month post-discharge haematocrit data were included. Mean absolute haematocrit change from discharge to 1 month was −1.5 ± 4.0% (range: −21.0% to +14.0%). Overall, 452 (26%) patients had ≥4% absolute decrease in haematocrit and were considered ‘haemodilutors’ (quartile 1). Patient characteristics were generally well-balanced by quartile of haematocrit change (Table 1). Discharge haematocrit was highest among haemodilutors (quartile 1), and decreased progressively across quartiles of post-discharge haematocrit change (P < 0.01). Patients with the greatest degree of post-discharge haemodilution had lower BNP level and weight at discharge (all P ≤ 0.02).
Table 1.
Characteristics at discharge by 1-month post-discharge haematocrit change
| Quartile 1 (n = 452) Most haemodilution |
Quartile 2 (n = 329) | Quartile 3 (n = 171) | Quartile 4 (n = 346) Least haemodilution |
P-value | |
|---|---|---|---|---|---|
| Hct levels | |||||
| Absolute ΔHct, range (%) | −21, −4 | −3, −2 | −1,2 | 2, 14 | |
| Hct (%), mean ± SD | 46 ± 6 | 43 ± 5 | 42 ± 5 | 40 ± 6 | <0.01 |
| Patient characteristics | |||||
| Age (years), mean ± SD | 65 ± 12 | 65 ± 12 | 65 ± 12 | 66 ± 12 | 0.23 |
| Male sex, n (%) | 349 (77) | 251 (76) | 347 (7.4) | 273 (79) | 0.35 |
| SBP (mmHg), mean ± SD | 114 ± 16 | 116 ± 16 | 116 ± 17 | 115 ± 18 | 0.37 |
| HR (bpm), mean ± SD | 75 ± 12 | 73 ± 11 | 74 ± 13 | 75 ± 12 | 0.31 |
| Weight (kg), mean ± SD | 78 ± 17 | 81 ± 18 | 81 ± 18 | 82 ± 19 | 0.02 |
| LVEF (%), mean ± SD | 27 ± 8 | 28 ± 8 | 28 ± 8 | 28 ± 8 | 0.58 |
| BNP (pg/mL), median (25th-75th) | 372 (131–809) | 429 (177–979) | 404 (176–980) | 632 (271–1193) | <0.01 |
| Creatinine (mg/dL), median (25th-75th) | 1.2(1.0–1.5) | 1.2 (1.0–1.5) | 1.2 (1.0–1. 6) | 1.3 (1.0–1.7) | 0.51 |
| BUN (mg/dL), median (25th-75th) | 27 (21–38) | 27 (21–37) | 27 (20 −38) | 27 (21–41) | 0.66 |
| Diabetes mellitus, n (%) | 137 (30) | 122 (37) | 175 (37) | 138 (40) | 0.03 |
| Chronic kidney disease, n (%) | 85 (19) | 69 (21) | 126 (27) | 104 (30) | <0.01 |
| Prior HF Hospitalization, n (%) | 348 (77) | 260 (80) | 377 (81) | 263 (76) | 0.34 |
| Coronary artery disease, n (%) | 305 (68) | 221 (67) | 349 (74) | 250 (72) | 0.07 |
| Hypertension, n (%) | 296 (66) | 228 (69) | 354 (75) | 250 (72) | 0.01 |
| Hyperlipidaemia, n (%) | 205 (45) | 135 (41) | 223 (4.8) | 173 (50) | 0.10 |
| Signs/symptoms of HF, n (%) | |||||
| Peripheral oedema | 87 (24) | 88 (34) | 133 (37) | 136 (51) | <0.01 |
| Rales | 60 (17) | 43 (17) | 74 (21) | 60 (23) | 0.20 |
| Dyspnoea | 59 (17) | 37(14) | 53 (15) | 54 (20) | 0.24 |
| JVP >10 cm | 19(5) | 9(4) | 14(4) | 16(6) | 0.46 |
| Medical therapy, n (%) | |||||
| ACEI/ARB | 386 (86) | 297 (90) | 403 (86) | 283 (82) | 0.02 |
| Beta-blocker | 327 (73) | 257 (78) | 349 (7.4) | 259 (75) | 0.44 |
| MRA | 310 (69) | 211 (64) | 314 (67) | 208 (60) | 0.05 |
| Diuretic | 431 (96) | 313 (95) | 443 (94) | 331 (96) | 0.39 |
| Event rates, n (%) | |||||
| All-cause mortality | 104 (23) | 60 (18) | 93 (20) | 78 (23) | 0.31 |
| CVM or HF hospitalization | 174 (39) | 116 (35) | 177 (38) | 141 (41) | 0.52 |
| CVM | 85 (19) | 42 (13) | 77 (16 | 58 (17) | 0.16 |
| HF hospitalization | 123 (27) | 83 (25) | 140 (30) | 108 (31) | 0.30 |
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BNP, B-type natriuretic peptide; BUN, blood urea nitrogen; CVM, cardiovascular mortality; Hct, haematocrit; HF, heart failure; HR, heart rate; JVP, jugular venous pressure; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; SBP, systolic blood pressure; SD, standard deviation.
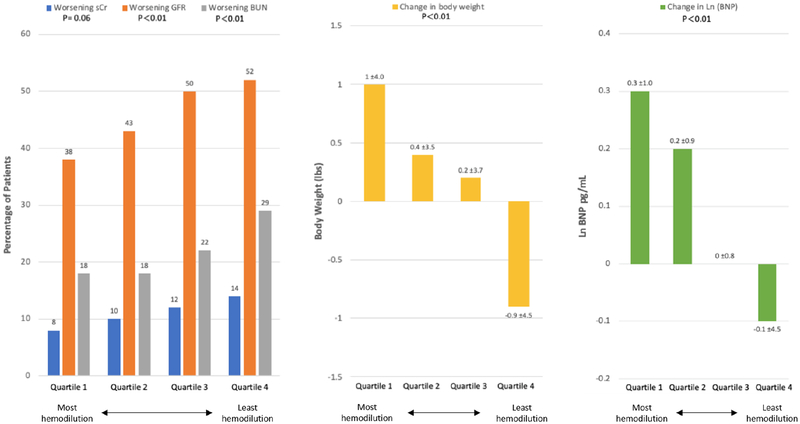
Patients with post-discharge haemodilution had the lowest rates of WRF as defined by eGFR and BUN (all P < 0.01) (Figure 1). Rates of WRF increased in stepwise fashion from quartile 1 to quartile 4. Haemodilutors experienced the greatest post-discharge increases in body weight and BNP, with lesser increases in quartiles 2 and 3, and decreases in these measures in quartile 4. After adjustment for patient characteristics, every 5% decrease in change in haematocrit from discharge to 1 month was significantly associated with an increased risk of all-cause mortality [hazard ratio (HR) 1.03, 95% confidence interval (CI) 1.00–1.06, P = 0.045), but not the composite of CVM or HF hospitalization (HR 1.01, 95% CI 0.99–1.03, P = 0.39).
Figure 1.
Changes in post-discharge renal function and congestion by post-discharge haematocrit change. Patients with the most haemodilution (quartile 1) had lower rates of worsening renal function (left panel), but experienced the greatest increases in weight (middle panel) and B-type natriuretic peptide (BNP). BUN, blood urea nitrogen; GRF, glomerular filtration rate; sCr, serum creatinine.
To our knowledge, we present the first study evaluating the clinical significance of early post-discharge haemodilution after HF hospitalization. Haemodilution at 1-month post-discharge correlated with lower risk of WRF, but worsening measures of congestion, during this interval. After adjustment for clinical factors, early post-discharge haemodilution was significantly associated with subsequent higher risk of mortality over long-term post-discharge follow-up.
Although early post-discharge follow-up is recommended following HF hospitalization, such transitional care strategies have not conclusively improved clinical outcomes.6,9 These inconsistent results may stem from the difficulty and variability in the assessment of congestion by clinicians.5 Haematocrit represents a widely available and inexpensive laboratory test that can be easily ordered in the outpatient clinic. The current data suggest that post-discharge haemodilution may provide a simple and objective marker of the degree of congestion, beyond physical exam and natriuretic peptides, that is independently associated with subsequent mortality. In the context of no guideline-recommended standardized approaches for grading congestion at these early follow-up visits, measurement of early post-discharge haematocrit and comparison with the discharge value may hold promise as an evidence-based marker of congestive status that reflects clinical risk.
Multiple studies have examined the role of haemoconcentration as a surrogate for adequate in-hospital decongestion.1–3 These analyses have generally found greater degrees of in-hospital haemoconcentration to be associated with improved post-discharge clinical outcomes, despite higher rates of WRF.1,3 Our findings build on prior work, and suggest that the concept of haemodilution/haemoconcentration extends beyond the HF hospitalization to the early post-discharge period, where haemodilution is associated with worsening markers of congestion and higher risk of clinical events, despite a lower risk of WRF. Moreover, the current study provides further support for the relative importance of congestive status in interpreting the clinical significance of WRF, and further highlights the assessment of congestion as a critical piece of the early post-discharge clinic visit.
Limitations of this study should be noted. First, this post-hoc observational analysis cannot definitively prove causal relationships. Moreover, data for other surrogates of plasma volume including albumin and uric acid were not available for internal validation of the haemodilution findings based on haematocrit changes. Likewise, these data should be recognized as hypothesis-generating and further data from other cohorts are needed to confirm the relationships seen here. Finally, although simultaneous changes in weight and BNP suggest that decreases in haematocrit were mediated by intravascular volume expansion, alternative reasons for interval changes in haematocrit (e.g. bleeding, anaemia of chronic disease, regression to the mean) cannot be excluded.
In conclusion, among patients recently hospitalized for worsening HFrEF, early post-discharge haemodilution was associated with interval worsening of congestion and increased risk of all-cause mortality, despite a lower risk of WRF. Haemodilution may represent a simple, objective, and evidence-based approach for assessing the level of congestion and clinical risk during early post-discharge follow-up. Further prospective studies are needed to determine whether assessment of post-discharge congestion by haemodilution can inform changes in decongestive therapy and improve clinical outcomes for patients recently hospitalized for HF.
Acknowledgements
This work is dedicated to the mentorship and teaching of Dr. Mihai Gheorghiade, who passed away in August 2017.
Funding
Haris Subacius conducted all final analyses for this report with funding from the Center for Cardiovascular Innovation, Northwestern University Feinberg School of Medicine, Chicago, IL, USA.
Footnotes
Conflict of interest: M.V. is supported by the KL2/Catalyst Medical Research Investigator Training award from Harvard Catalyst (NIH/NCATS Award UL 1TR002541), and has served on advisory boards or received research funding from Amgen, AstraZeneca, Baxter Healthcare, Bayer AG, and Boehringer Ingelheim. R.B.P. is supported by the NHLBI T32 postdoctoral training grant T32HL069771. G.C.F. reports research funding from the National Institutes of Health (NIH) and serving as a consultant for Amgen, Bayer, Medtronic, and Novartis. M.A.K. has received research support and consulting fees from LivaNova, Ironwood, and SC Pharma and consulting fees for data monitoring committee activity from Amgen, Boehringer-Ingelheim, Bristol-Myers Squibb, Array, and Luitpold. F.Z. reports personal fees from Janssen, Bayer, Novartis, Boston Scientific, Resmed, Amgen, CVRx, Quantum Genomics, General Electric, Boehringer, AstraZeneca, Vifor Fresenius, Cardior, outside the submitted work. J.B. has received research support from the NIH, PCORI and the European Union; and serves as a consultant for Amgen, Array, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, CVRx, G3 Pharmaceutical, Innolife, Janssen, Luitpold, Medtronic, Merck, Novartis, Relypsa, StealthPeptide, SC Pharma, Vifor, and ZS Pharma. S.J.G. has received a Heart Failure Society of America/Emergency Medicine Foundation Acute Heart Failure Young Investigator Award funded by Novartis; has received research support from Amgen, Bristol-Myers Squibb and Novartis; has served on an advisory board for Amgen; and has served as a consultant for Amgen. All other authors declare no relevant disclosures.
References
- 1.Greene SJ, Gheorghiade M, Vaduganathan M, Ambrosy AP, Mentz RJ, Subacius H, Maggioni AP, Nodari S, Konstam MA, Butler J, Filippatos G. Haemoconcentration, renal function, and post-discharge outcomes among patients hospitalized for heart failure with reduced ejection fraction: insights from the EVEREST trial. Eur J Heart Fail 2013;15: 1401–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Breidthardt T, Weidmann ZM, Twerenbold R, Gantenbein C, Stallone F, Rentsch K, Rubini Gimenez M, Kozhuharov N, Sabti Z, Breitenbucher D, Wildi K, Puelacher C, Honegger U, Wagener M, Schumacher C, Hillinger P, Osswald S, Mueller C. Impact of haemoconcentration during acute heart failure therapy on mortality and its relationship with worsening renal function. Eur J Heart Fail 2017;19: 226–236. [DOI] [PubMed] [Google Scholar]
- 3.Testani JM, Chen J, McCauley BD, Kimmel SE, Shannon RP. Potential effects of aggressive decongestion during the treatment of decompensated heart failure on renal function and survival. Circulation 2010;122:265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mullens W, Damman K, Harjola VP, Mebazaa A, Brunner-La Rocca HP, Martens P, Testani JM, Tang WH, Orso F, Rossignol P, Metra M, Filippatos G, Seferovic PM, Ruschitzka F, Coats AJ. The use of diuretics in heart failure with congestion – a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2019;21:137–155. [DOI] [PubMed] [Google Scholar]
- 5.Gheorghiade M, Follath F, Ponikowski P, Barsuk JH, Blair JE, Cleland JG, Dickstein K, Drazner MH, Fonarow GC, Jaarsma T, Jondeau G, Sendon JL, Mebazaa A, Metra M, Nieminen M, Pang PS, Seferovic P, Stevenson LW, van Veldhuisen DJ, Zannad F, Anker SD, Rhodes A, McMurray JJ, Filippatos G. Assessing and grading congestion in acute heart failure: a scientific statement from the Acute Heart Failure Committee of the Heart Failure Association of the European Society of Cardiology and endorsed by the European Society of Intensive Care Medicine. Eur J Heart Fail 2010;12:423–433. [DOI] [PubMed] [Google Scholar]
- 6.Greene SJ, Fonarow GC, Vaduganathan M, Khan SS, Butler J, Gheorghiade M. The vulnerable phase after hospitalization for heart failure. Nat Rev Cardiol 2015;12:220–229. [DOI] [PubMed] [Google Scholar]
- 7.Gheorghiade M, Orlandi C, Burnett JC, Demets D, Grinfeld L, Maggioni A, Swedberg K, Udelson JE, Zannad F, Zimmer C, Konstam MA. Rationale and design of the multicenter, randomized, double-blind, placebo-controlled study to evaluate the Efficacy of Vasopressin antagonism in Heart Failure: Outcome Study with Tolvaptan (EVEREST). J Card Fail 2005;11:260–269. [DOI] [PubMed] [Google Scholar]
- 8.Konstam MA, Gheorghiade M, Burnett JC Jr, Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannad JE, Zannad F, Cook T, Ouyang J, Zimmer C, Orlandi C. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST outcome trial. JAMA 2007;297:1319–1331. [DOI] [PubMed] [Google Scholar]
- 9.Van Spall HG, Lee SF, Xie F, Oz UE, Perez R, Mitoff PR, Maingi M, Tjandrawidjaja MC, Heffernan M, Zia MI, Porepa L, Panju M, Thabane L, Graham ID, Haynes RB, Haughton D, Simek KD, Ko DT, Connolly SJ. Effect of patient-centered transitional care services on clinical outcomes in patients hospitalized for heart failure: the PACT-HF randomized clinical trial. JAMA 2019;321:753–761. [DOI] [PMC free article] [PubMed] [Google Scholar]