Abstract
Peak oxygen uptake (V̇O2peak), a primary determinant of prognosis, mortality, and quality of life, is diminished in patients with chronic obstructive pulmonary disease (COPD). Mounting evidence supports an important role of the periphery, particularly skeletal muscle, in the diminished V̇O2peak with COPD. However, the peripheral determinants of V̇O2peak have not been comprehensively assessed in this cohort. Thus, the hypothesis was tested that both muscle convective and diffusive oxygen (O2) transport, and therefore skeletal muscle peak O2 uptake (V̇MO2peak), are diminished in patients with COPD compared to matched healthy controls, even when ventilatory limitations (i.e. attainment of maximal ventilation) are minimized by using small muscle mass exercise. Muscle O2 transport and utilization were assessed at peak exercise from femoral arterial and venous blood samples and leg blood flow (by thermodilution) in 8 patients with severe COPD (FEV1±SE = 0.9±0.1 L, 30% of predicted) and 8 controls during single leg knee-extensor exercise. Both muscle convective O2 delivery (0.44±0.06 vs. 0.69±0.07 l/min, p<0.05) and muscle diffusive O2 conductance (6.6±0.8 vs. 10.4±0.9 ml/min/mmHg, p<0.05) were ≈1/3 lower in patients with COPD than controls, resulting in an attenuated V̇MO2peak in the patients (0.27±0.04 vs. 0.42±0.05 l/min, p<0.05). When cardiopulmonary limitations to exercise are minimized, the convective and diffusive determinants of V̇MO2peak, at the level of the skeletal muscle, are greatly attenuated in patients with COPD. These findings emphasize the importance of factors, beyond the lungs, that may ultimately influence this population’s prognosis, mortality, and quality of life.
Keywords: Lung disease, O2 uptake, blood flow, O2 delivery
INTRODUCTION
Diminished exercise capacity (i.e. peak O2 uptake, V̇O2peak) is a defining symptom of chronic obstructive pulmonary disease (COPD) and a primary determinant of prognosis, mortality, and quality of life (Maltais et al., 1998; Hajiro et al., 1999; Oga et al., 2003; Amann et al., 2010; O’Donnell et al., 2014). The traditional explanation for this diminished V̇O2peak is impaired lung function (O’Donnell & Webb, 2008; O’Donnell et al., 2014), but mounting evidence supports an important role for the periphery. The presence of peripheral dysfunction with COPD is emphasized by the persistent reduction in V̇O2peak even after lung function is improved (Lands et al., 1999; Amann et al., 2010; Bartels et al., 2011) and the weak relationship between lung function and V̇O2peak (Jones et al., 1971; Maltais et al., 1997; Lands et al., 1999). Thus, understanding factors, beyond the lungs, that contribute to the diminished V̇O2peak in COPD may, ultimately, promote improvements in patient prognosis, mortality, and quality of life. It has been difficult, however, to assess peripheral dysfunction in COPD because of the substantial constraint on V̇O2peak resulting from the reduced maximal ventilation due to the mechanical effects of the disease on the lungs and chest wall.
V̇O2peak in COPD is determined, at least in part, by O2 availability, as increasing O2 transport using hyperoxia or small-muscle mass exercise improves V̇O2peak (Richardson et al., 1999; Richardson et al., 2004). In turn, O2 availability is determined by an integrated O2 transport system comprised of ventilation, lung diffusion, muscle circulation, and muscle diffusion (Figure 1) (Wagner, 1992; Wagner, 1996). Evidence supporting impaired central O2 transport as the primary determinant of the reduced V̇O2peak with COPD is predominantly from two-legged cycle exercise. However, the recruitment of such a large muscle mass, in the face of marked lung dysfunction, likely masks any peripheral O2 transport dysfunction due to the reduced ventilatory capacity typical of COPD. Maximal exercise ventilation, as measured during conventional two-legged cycling exercise, is not reached during maximal single-leg knee-extensor exercise (KE). This is because a much smaller muscle mass is involved during two-legged cycling (Andersen et al., 1985; Richardson et al., 1999; Lawrenson et al., 2003). Indeed, maximum ventilation is 20% less during KE than during two-legged cycle exercise even in patients with severe COPD (Richardson et al., 1999). Therefore, maximal KE is a strategy to achieve maximal muscle exercise capacity without being constrained by the limited ability of the patient to ventilate. Importantly, this allows a greater peak O2 delivery and V̇O2 per unit of muscle for KE than cycle exercise (Richardson et al., 2004). Thus, incremental KE, is an ideal modality to appropriately assess how peripheral O2 transport determines the diminished V̇O2peak in patients with COPD.
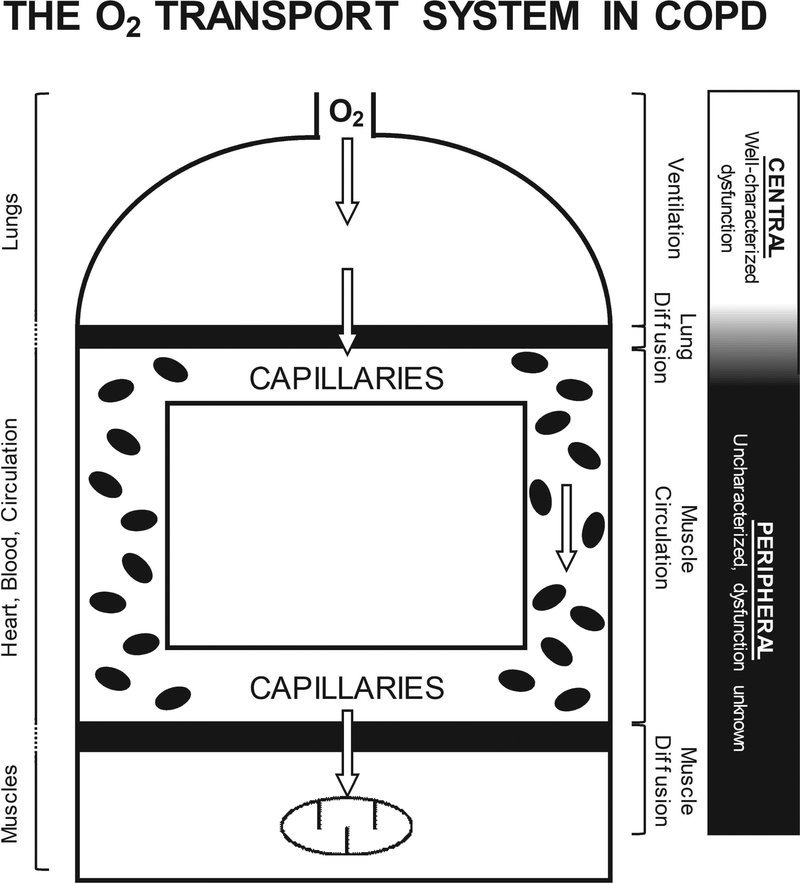
Figure 1. Schematic of the O2 transport system in COPD.
An illustration of the principal structures (lungs, heart, blood, circulation, and muscles) and associated functions (ventilation, lung diffusion, muscle circulation, and muscle diffusion) integrated in the transport of O2 from air to muscle. Patients with COPD demonstrate characterized central O2 transport dysfunction (i.e. ventilation and lung diffusion), which has been considered the primary mechanism responsible for the diminished exercise capacity in these patients. Importantly, previous studies have predominantly utilized exercise modalities that recruit a large muscle mass in the face of the marked lung dysfunction in patients with COPD (e.g. cycle ergometry), which would accentuate the influence of central O2 transport on exercise capacity, while masking peripheral dysfunction. However, peripheral O2 transport (i.e. muscle circulation and muscle diffusion) has yet to be comprehensively assessed in patients with COPD, when the central cardiopulmonary limitations to exercise capacity are minimized. Such an assessment is essential for a better understanding of the functional consequences of peripheral maladaptations with COPD and the identification of novel therapeutic targets, beyond the lungs, ultimately, facilitating the optimization of treatment and improving exercise capacity in patients with COPD.
The muscle metabolic reserve capacity during KE in patients with COPD strongly supports peripheral O2 transport as a primary determinant of V̇O2peak in this patient population (Richardson et al., 1999). The peripheral determinants of V̇O2peak can be quantified by integrating the Fick principle and Fick’s law of diffusion at peak exercise intensities (Wagner, 1992; Wagner, 1996). This establishes that muscle V̇O2peak (V̇MO2peak) is determined by the interaction between muscle convective O2 delivery (Q̇MO2) and muscle diffusive O2 transport (ḊMO2), dictating O2 supply to the capillaries and O2 flux to the muscle, respectively (Figure 1). Importantly, ḊMO2 is strongly related to V̇O2peak across small muscle mass and whole-body exercise modalities in both healthy controls and patients with compromised central O2 transport (Esposito et al., 2011). The skeletal muscle structural alterations that typically accompany COPD (Jakobsson et al., 1990; Satta et al., 1997; Jobin et al., 1998; Whittom et al., 1998; Maltais et al., 1999; Richardson et al., 2004; Eliason et al., 2010) are likely to manifest in the reduction of Q̇MO2, ḊMO2, and, therefore, V̇MO2peak. To date, however, the effects of COPD on muscle blood flow, O2 delivery, and V̇O2 are equivocal. Blood flow and O2 delivery during submaximal KE in patients with COPD have been documented to be both greater (Richardson et al., 2004) and less (Bronstad et al., 2012; Iepsen et al., 2017) than control subjects, with no differences in V̇O2 between patients and control subjects. During maximal KE, blood flow, O2 delivery, and V̇O2peak have been demonstrated to be both not different (Richardson et al., 2004) and less (Bronstad et al., 2012) than control subjects in patients with COPD. In addition to resolving these findings, the specific role played by Q̇MO2 and ḊMO2 in determining V̇MO2peak in patients with COPD remains unknown. Thus, there is a need to quantify the peripheral determinants of V̇MO2peak in patients with COPD, which is imperative for effectively guiding therapies to improve exercise capacity and the accompanying clinical outcomes.
Therefore, this study addressed the importance of peripheral dysfunction in determining exercise capacity in patients with COPD. We tested the hypothesis that, compared to matched healthy controls, both Q̇MO2 and ḊMO2, and, therefore, V̇MO2peak, assessed during KE, would be attenuated in patients with COPD, even when central factors, typically limiting O2 availability to the active muscle, are minimized.
METHODS
Ethical Approval
The experimental protocol was approved by the University of California San Diego, Human Research Protection Program (#990152). The protocol conformed to the standards set by the Declaration of Helsinki, except for registration in a database. Written informed consent was attained after subjects were informed of the experimental protocol and the potential risks of participation.
Subjects
Eight men with severe COPD (FEV1=0.9±0.1 L, 30% predicted) and eight matched healthy men volunteered for this study. Subject characteristics are presented in Table 1. Patients and controls were matched for age, sex, height, weight, quadriceps muscle mass, and, especially, activity level using the Minnesota Leisure Time Physical Activity Questionnaire (Taylor et al., 1978; Folsom et al., 1986; Jacobs et al., 1993). Quadriceps muscle mass was measured using thigh length, circumference, and skin-fold measurements (Andersen et al., 1985; Lawrenson et al., 2003). For further characterization, subjects performed a preliminary incremental cycle ergometer exercise test to exhaustion, with pulmonary gas exchange measurements.
Table 1.
Subject Characteristics
| COPD | Control | ||
|---|---|---|---|
| Age (years) | 66 ± 4 | 67 ± 2 | |
| Height (cm) | 172 ± 20 | 174 ± 20 | |
| Body mass (kg) | 74 ± 3 | 77 ± 5 | |
| Quadriceps muscle mass (kg) | 2.1 ± 0.1 | 2.2 ± 0.1 | |
| Body mass index (kg/m2) | 25.2 ± 1.3 | 25.2 ± 1.2 | |
| Cycle VO2peak (ml/min/kg) | 14.9 ± 1.6 | 20.3 ± 1.8† | |
| FEV1 (L) | 0.9 ± 0.1 | 2.2 ± 0.3† | |
| FEV/FVC (%) | 39 ± 2 | 79 ± 3† |
Values are reported as mean ± standard error.
significantly different from Control (p < 0.05).
Experimental Protocol
Subjects performed KE seated on an adjustable chair with the ankle of 1 leg attached by a rigid bar to a cycle ergometer (Andersen et al., 1985; Richardson et al., 1995a). On a preliminary day, subjects were familiarized with KE to ensure maximal effort was achieved during the catheter study. For the catheter study, femoral arterial and venous lines were placed using the Seldinger technique and a femoral venous thermocouple was advanced through a catheter until 10 cm proximal to the tip of the infusion catheter (Gonzalez-Alonso et al., 2001). KE work rate was increased to the previously determined maximum, with a minimum of three stages. Data were obtained at each stage between 2–4 minutes depending on the exercise intensity, allowing for stead-state when possible (Richardson et al., 2004).
Measurements and Calculations
Iced saline was infused through the femoral venous catheter at flow rates sufficient to decrease blood temperature at the thermocouple by ≈1°C. Infusions were continued for 15–20 s until femoral vein temperature had stabilized at a lower value. Saline injection rate was measured by weight change in a reservoir bag suspended from a force transducer, which was calibrated before and after each experiment. The calculation of quadriceps muscle blood flow (Q̇M) was performed on thermal balance principles (Andersen & Saltin, 1985; Gonzalez-Alonso et al., 2001). Heart rate was measured from a 3-lead electrocardiogram (Lifepak 9A, Lifeline, Santa Barbara, CA). Femoral arterial and venous blood pressures were continuously monitored by pressure transducers at heart level (PX-MK099, Baxter, Irvine, CA). Mean arterial (MAP) and venous (MVP) pressures were calculated by the integration of each pressure curve and leg vascular resistance was calculated as (MAP-MVP)/Q̇M. Simultaneous arterial and venous blood samples (blood gas values corrected to the temperature measured in the femoral vein) were collected at rest and maximal exercise for analysis of: oxygen (PO2) and carbon dioxide (PCO2) partial pressures corrected to femoral venous temperature, pH, hemoglobin concentration ([Hb], g/dl), %saturation (SaO2) (IL-683, Clayton, NC), and lysed whole-blood lactate concentrations (YSI 2300 Stat Plus, Yellow Springs, OH). Oxygen content (ml/dl) was calculated as (1.39 × [Hb] × %saturation/100 + 0.003 × PO2). All blood sample data were pooled for each subject and the standard P50 calculated by a least squares method (Kelman, 1966, 1967). Net venous lactate outflow was calculated as the product of Q̇M and venous-arterial lactate concentration. V̇MO2peak was calculated as: V̇MO2peak = Q̇M × (CaO2-CvO2) and muscle O2 delivery (Q̇MO2) was determined as: Q̇MO2 = Q̇M × CaO2, where Ca and Cv are arterial and venous O2 content, respectively. The technical aspects of the study measurements and calculations have been previously provided in detail (Knight et al., 1993; Agusti et al., 1994). Mean capillary PO2 (PcapO2) and ḊMO2 were calculated as previously described (Roca et al., 1988; Wagner, 1988, 1992; Knight et al., 1993; Agusti et al., 1994). ḊMO2, which, as a lumped parameter, is considered to be constant along the capillary length, encompasses all phenomena that facilitate O2 unloading at the muscle and is calculated using a numerical integration technique which varies ḊMO2 as an input variable until the primary output of the integration algorithm, venous PO2, matches the directly measured PvO2 for the directly measured PaO2 and Q̇M (Wagner, 1988, 1992). The assumptions for this calculation are that mitochondrial PO2 (PmitoO2) is negligibly low at peak exercise (< 3–4 mmHg (Richardson et al., 1995b)) and, therefore, is taken to be zero due its negligible mathematical influence, relative to PcapO2 (~40–50 mmHg), on the PO2 pressure gradient from the capillary to muscle, and that the only explanation for O2 remaining in the femoral venous blood is diffusion limitation of O2 efflux from the muscle microcirculation. Mean capillary PO2 (PcapO2) was determined as the numerical average of all PO2 values computed, equally spaced in time, along the capillary from the arterial to the venous end. These assumption have been specifically validated in multiple experimental studies (Roca et al., 1988; Hogan et al., 1991b; Knight et al., 1993; Agusti et al., 1994; Richardson et al., 1998).
Analysis
Least-squares regression provided the slopes and intercepts for variables against work rate. These intercepts and slopes were then used to test each variable for a statistical difference between patients with COPD and control subjects with independent samples t tests. At maximal exercise, variables were tested for a significant difference between groups using independent samples t tests. Statistical significance was set at p < 0.05 and data are expressed as mean ± SEM.
RESULTS
Subject Characteristics
There were no significant differences in the physical characteristics of the patients and controls, but, as expected, lung function was significantly impaired in the patients with COPD (Table 1). The conventional two-legged incremental cycle exercise test to exhaustion revealed that both peak work rate (48±8 vs. 131±12 W) and pulmonary V̇O2peak (14.9±1.6 vs. 20.3±1.8 ml/kg/min) were significantly lower for patients than controls (Table 1).
Peripheral O2 transport and utilization during knee-extensor exercise
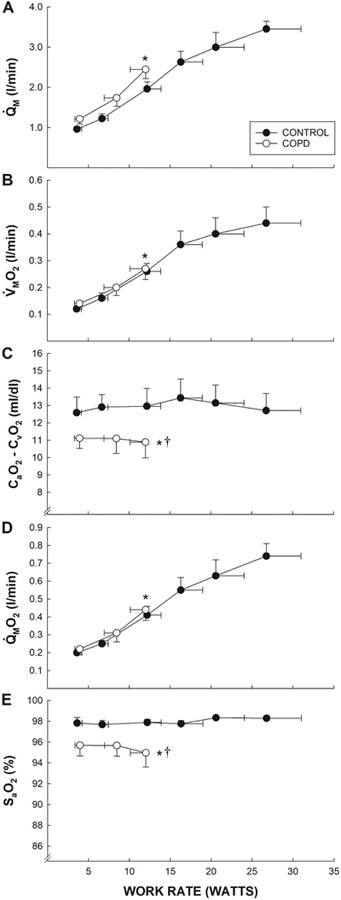
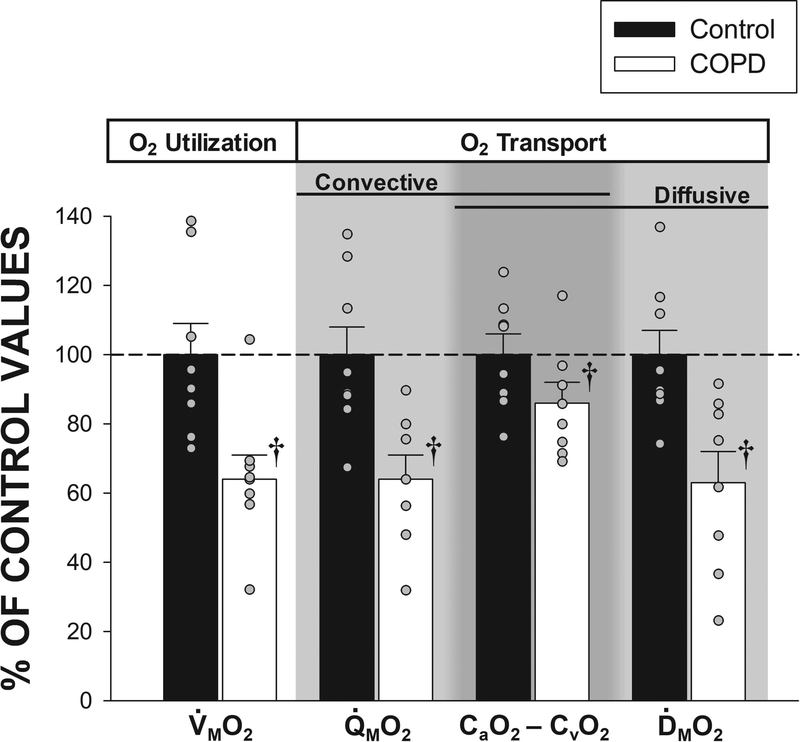
Throughout the submaximal KE, Q̇M, Q̇MO2, and V̇MO2 were not different between the patients with COPD and control subjects (Figure 2). In contrast, SaO2 and the CaO2 - CvO2 difference were lower in the patients throughout the submaximal KE (Figure 2). At peak work rates, peak heart rate and net lactate efflux during KE were not significantly different between patients and controls (Table 2). Peak KE work rate and V̇MO2 were 50% and 36% lower, respectively, in patients compared to controls (Table 2). At maximal KE, Q̇M was 26% lower and CaO2 16% lower in patients compared to controls, and, consequently, Q̇MO2 (convective O2 transport) was 36% lower in the patients (Table 2, Figures 2 and 3). ḊMO2 at maximal KE was 37% lower in patients compared to controls (Table 2, Figures 2and 3). The CaO2-CvO2 difference across the exercising quadriceps muscle at maximal KE, a variable influenced by both convective and diffusive O2 transport, was 14% lower in patients compared to controls (Table 2, Figures 2 and 3).
Figure 2. Peripheral O2 transport and utilization during graded small muscle mass exercise.
A comparison of leg blood flow (Panel A, Q̇M), muscle oxygen uptake (Panel B, V̇MO2), the arterial-venous O2 content difference (Panel C, CaO2 - CvO2), muscle oxygen delivery (Panel D, Q̇MO2), and arterial oxygen saturation (Panel E, SaO2) assessed during graded knee-extensor exercise in patients with chronic obstructive pulmonary disease (COPD) (n = 8) and control subjects (n = 8). Data are presented as mean ± SEM. * significantly different from control subjects at peak work rates. † significantly different y-intercept from control subjects.
Table 2.
Blood flow, hematologic, and metabolic responses to maximal single leg knee-extensor exercise in patients with COPD and matched healthy controls
| COPD | Control | ||
|---|---|---|---|
| Work (Watts) | 12 ± 2 | 24 ± 4† | |
| Q̇M (l/min) | 2.45 ± 0.23 | 3.32 ± 0.32† | |
| Q̇MO2 (l/min) | 0.44 ± 0.06 | 0.69 ± 0.07† | |
| V̇MO2 (l/min) | 0.27 ± 0.04 | 0.42 ± 0.05† | |
| pHa | 7.34 ± 0.02 | 7.38 ± 0.01† | |
| pHv | 7.20 ± 0.02 | 7.23 ± 0.01 | |
| [Hb]total (g/dl) | 13.2 ± 0.4 | 15.1 ± 0.6† | |
| CaO2 (ml/dl) | 17.7 ± 0.8 | 21.0 ± 0.8† | |
| CvO2 (ml/dl) | 6.8 ± 0.9 | 8.3 ± 0.6† | |
| PaO2 (mmHg) | 88 ± 13 | 116 ± 3† | |
| PvO2 (mmHg) | 25 ± 1 | 28 ± 2† | |
| PaCO2 (mmHg) | 43 ± 4 | 31 ± 4† | |
| PvCO2 (mmHg) | 67 ± 5 | 61 ± 3† | |
| SaO2 (%) | 95 ± 1 | 98 ± 0† | |
| SvO2 (%) | 36 ± 4 | 39 ± 3 | |
| MAP-MVP (mmHg) | 109 ± 4 | 121 ± 7† | |
| Heart rate (bpm) | 104 ± 6 | 112 ± 4 | |
| DMO2 (ml/min/mmHg) | 6.6 ± 0.8 | 10.4 ± 0.9† | |
| PcapO2 (mmHg) | 36 ± 1 | 46 ± 2 | |
| Net venous lactate outflow (mmol/min) | 3.1 ± 0.5 | 4.6 ± 1.1 |
Values are mean ± SE. Q̇M, Quadriceps muscle blood flow; Q̇MO2, quadriceps muscle O2 delivery; V̇MO2, quadriceps muscle O2 consumption; pHa, arterial pH; pHv, venous pH; [Hb]total, total hemoglobin; CaO2, arterial O2 content of blood; CvO2, venous O2 content of blood; PaO2, partial pressure of arterial O2; PvO2, partial pressure of venous O2; PaCO2, partial pressure of arterial CO2; PvCO2, partial pressure of venous CO2; SaO2, arterial O2 saturation; SvO2, venous O2 saturation; MAP, mean arterial pressure; MVP, mean venous pressure; DMO2, O2 diffusional conductance; PcapO2, mean capillary partial pressure of O2;
significantly different from Controls (p < 0.05).
Figure 3. Peripheral O2 transport and utilization at peak small muscle mass exercise.
A comparison of O2 transport and utilization parameters assessed at maximal knee-extensor exercise in patients with chronic obstructive pulmonary disease (COPD) (n = 8) and control subjects (n = 8). Control data have been normalized to 100% as a point of reference for data collected in the patients with COPD. CaO2 – CvO2, arterial-venous O2 content difference; ḊMO2, quadriceps muscle O2 diffusional conductance; V̇MO2, quadriceps muscle O2 uptake; Q̇MO2, quadriceps O2 delivery. Data are presented as mean ± SEM. † significantly different from control subjects (p < 0.05).
DISCUSSION
This investigation provides novel insight into the influence of peripheral dysfunction on exercise capacity, in terms of skeletal muscle O2 transport, in patients with COPD. We found that, at maximal KE, both muscle convective and muscle diffusive O2 transport and, therefore, V̇MO2peak, are diminished in patients with COPD compared to activity and anthropometrically matched control subjects, even when ventilatory limitations to exercise are minimized. Thus, peripheral dysfunction, at the level of skeletal muscle, attenuates the peripheral O2 transport determinants of V̇MO2peak in patients with COPD. These findings emphasize the importance of factors, beyond the lungs, that influence exercise capacity in this patient population and may, ultimately, influence the prognosis, mortality, and quality of life for patients with COPD.
Muscle Convective O2 Transport
Despite the reduction in CaO2 throughout the submaximal KE, the tendency for a slightly greater Q̇M in the patients with COPD resulted in a Q̇MO2 that was not different from control subjects (Figure 2). However, at maximal KE the patients with COPD demonstrated a marked attenuation in muscle convective O2 transport (−36%) compared to controls (Figures 2, 3, and 4), which was the result of both a lower muscle blood flow (−26%) and a lower CaO2 (−16%) (Table 2). This is consistent with previous evidence of a reduced convective O2 delivery during submaximal exercise in patients with COPD (Medeiros et al., 2015; Iepsen et al., 2017). The lower convective O2 delivery, in the current patients, was primarily the result of a compromised muscle blood flow, likely resulting from multiple systemic issues related to COPD (e.g. vascular disease, endothelial dysfunction, increased sympathetic activity). The lower CaO2 was primarily the result of a lower hemoglobin concentration in the patients, rather than from hypoxemia. Indeed, >80% of the difference in CaO2 between patients and controls was accounted for by the difference in hemoglobin concentration, with the remainder being due to a lower arterial PO2. Such alterations in hemoglobin can potentially capture a range of prognostic factors, as hemoglobin homeostasis can be impaired by nutrient deficiency, comorbid disease, and medication, and is modified by tissue oxygen supply and systemic inflammation, which is associated with frailty (Toft-Petersen et al., 2016). Historically, polycythemia was considered to be a common consequence of hypoxemia in COPD, however, more recently, due to oxygen and bronchodilator therapies, anemia of chronic disease is more commonly associated with COPD (Kent et al., 2011; Toft-Petersen et al., 2016). The prevalence of anemia in COPD has been reported to vary from 7.5 to 44%, with evidence of a significant impact on quality of life, healthcare utilization, and survival (Toft-Petersen et al., 2016). Of note, some degree of limitation arising from the lung (e.g. VA/Q inequalities, alveolar hypoventilation, etc.) may have been present during peak exercise in the patients of the current study based on the ≈3% lower SaO2, ≈28 mmHg lower PaO2, and ≈12 mmHg higher PaCO2 compared to the control subjects. However, this only accounted for ≈5% of the difference in V̇MO2peak between the patients and the control subjects, supporting the minimal influence of pulmonary and ventilatory limitations when utilizing the KE paradigm. Moreover, a higher PaCO2, per se, is not always indicative of pulmonary ventilatory limitation, but may reflect the respiratory control system responding to mechanical and gas exchange signals stemming from the lungs/chest wall (mechanical) and blood (gas exchange), and does, therefore, not indicate whether or not ventilation was mechanically limited. Indeed, VA/Q inequalities will cause an elevated PaCO2 even with normal, unchanged ventilation. The diminished muscle convective O2 transport in the current patients contrasts with our previous study, in which O2 delivery was not different between patients with COPD and control subjects, despite a substantially lower peak work load (Richardson et al., 2004). Hemoglobin concentration and peak muscle blood flow were not different between patients and controls in the previous study (Richardson et al., 2004). Notably, hemoglobin concentration and peak muscle blood flow were lower in the patients than control subjects in the current study, accounting for ≈20% and ≈60% of the lower convective O2 transport in the patients, respectively. These differences in hemoglobin concentration, peak muscle blood flow, and peak muscle convective O2 transport in the patients with COPD between these two studies are likely a result, and example, of the high degree of heterogeneity in this patient population. Despite this lack of a difference in O2 delivery, the previous study did reveal that V̇MO2peak was dependent on O2 delivery in the patients (Richardson et al., 2004), which is consistent with the findings of the current study regarding muscle convective O2 transport. Thus, the current findings confirm and extend our understanding of muscle convective O2 transport as a critical determinant of the diminished exercise capacity in patients with COPD.
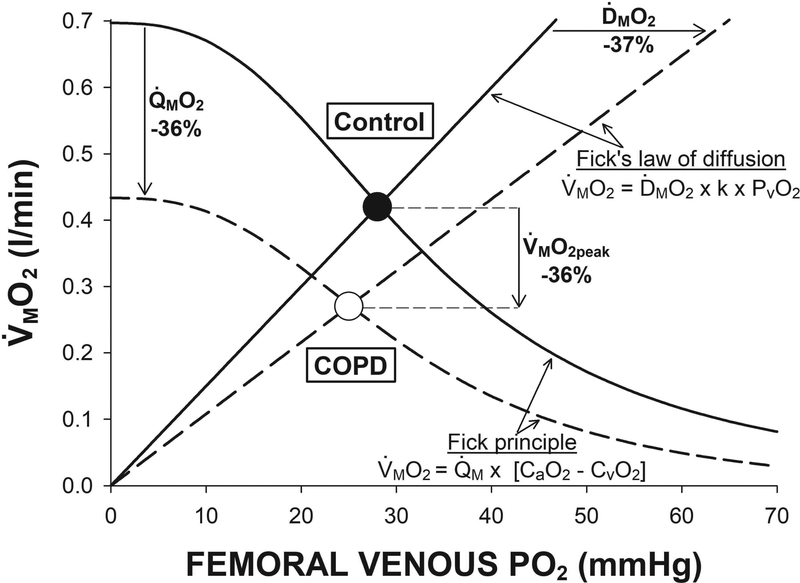
Figure 4. Convective and diffusive components of peripheral O2 transport at peak small muscle mass exercise.
An illustration of the peripheral O2 transport determinants of muscle peak O2 uptake (V̇MO2peak) in both patients with chronic obstructive pulmonary disease (COPD) (n = 8) and control subjects (n = 8) during single leg knee-extension exercise. The Fick principle (convective O2 transport, sigmoidal lines) and Fick’s law of diffusion (diffusive O2 transport, linear lines) are integrated in a model linking V̇MO2peak and venous O2 partial pressure (PvO2). It is the intersection of the convective and diffusive O2 transport components that dictates the V̇MO2peak for each group. This model clearly depicts that the ≈36% lower V̇MO2peak in the patients with COPD compared to the control subjects was the result of both a ≈36% lower muscle O2 delivery (Q̇MO2) and ≈36% diffusional conductance (ḊMO2). Importantly, this model emphasizes the integrated nature of V̇MO2peak, as it is evident that an independent change in either Q̇MO2 or ḊMO2 would have resulted in a substantially smaller alteration (≈25%) in V̇MO2peak in the patients with COPD.
Muscle Diffusive O2 Transport
Muscle diffusive O2 transport at maximal KE was substantially diminished (−37%) in the patients with COPD compared to controls. Recent evidence supports that this was likely not the result of regional heterogeneity in the matching of perfusion to O2 utilization within the quadriceps muscle (Louvaris et al., 2017). The compromised muscle diffusive O2 transport is likely a result of 1) the pernicious effects of COPD on the surface area for gas exchange through muscle structural alterations (increased proportion of type II muscle fibers and decreased muscle-capillary interface (Jakobsson et al., 1990; Satta et al., 1997; Jobin et al., 1998; Whittom et al., 1998; Maltais et al., 1999; Richardson et al., 2004; Eliason et al., 2010)) and 2) the lower hemoglobin concentration (Hogan et al., 1991a). Based on the data from Hogan et al. (Hogan et al., 1991a), the 1.9 g/dl difference in [Hb] between groups in the current study is estimated to account for 0.6 of the 3.8 ml/min/mmHg (≈16%) difference in DMO2. Additionally, microvascular dysfunction (disrupted interaction between red cell and capillary wall, impaired longitudinal recruitment (Poole et al., 2011)) is also likely to contribute to the compromised muscle diffusive O2 transport. Thus, the current findings provide novel insight regarding the critical importance of muscle diffusive O2 transport in determining the diminished exercise capacity in patients with COPD, documenting a compromised ability to move O2 from the capillary to the muscle.
The current findings also highlight the important distinction between muscle diffusive O2 transport and the CaO2-CvO2 difference, which is often, incorrectly, utilized to evaluate O2 diffusion. In the current patients, the CaO2-CvO2 difference was only 14% lower than controls, while muscle diffusive O2 transport was 37% lower (Figure 3). The distinction between the CaO2-CvO2 difference (a consequence of both convective and diffusive O2 transport) and muscle diffusive O2 transport (solely a consequence of diffusive movement) provides important mechanistic insight into the peripheral determinants of exercise capacity in patients with COPD.
Integrated determinants of V̇MO2peak
The principal aim of the current study was to investigate the peripheral convective (bulk delivery of O2) and diffusive (movement of O2 from capillaries to muscle) contributions to determining the diminished V̇MO2peak in patients with COPD. The peripheral O2 transport determinants of V̇MO2peak can be quantified by integrating the Fick principle (V̇MO2peak = Q̇M × [CaO2 – CvO2]) with Fick’s law of diffusion (V̇MO2peak = ḊMO2 × [PcapO2 – PmitoO2]), where PmitoO2 can be taken to be zero at peak exercise (Richardson et al., 1995b). For illustrative purposes (Figure 4), the Fick principle can be integrated with Fick’s law of diffusion using the proportionality constant (k) between PcapO2 and PvO2, in a model linking V̇MO2peak and PvO2 (Roca et al., 1988; Wagner, 1992; Wagner, 1996). To quantify the peripheral O2 transport determinants of V̇MO2peak, it is assumed that O2 utilization is limited by the availability of O2 and not by the metabolic capacity of the muscle itself, which is supported by evidence that the capacity for O2 utilization exceeds peripheral O2 transport during KE in patients with COPD. Quadriceps muscle citrate synthase activity, an index of mitochondrial capacity, was not different between controls and patients with COPD (Richardson et al., 2004). Estimation of peak quadriceps muscle O2 utilization (Gifford et al., 2016), from in vitro mitochondrial respiration data, in patients with COPD is markedly greater (>0.40 l/min) than the V̇MO2peak in the current patients with COPD (Gifford et al., 2015; Gifford et al., 2017). Peak work load was 25% greater during KE in hyperoxia (100% O2) compared to normoxia in patients with COPD (Richardson et al., 1999). Additionally, the current findings of diminished peripheral convective and diffusive O2 transport in patients with COPD are consistent with previous evidence of a metabolic reserve during KE in patients with COPD, where, utilizing hyperoxia, augmented O2 delivery was accompanied by a proportional increase in V̇MO2peak (Richardson et al., 2004). Together, these findings suggest that, in terms of respiratory capacity, the skeletal muscle of patients with COPD is functionally adequate and that it is the availability of O2 (through impairments in both convective and diffusive transport) that results in impaired V̇MO2peak in this patient population.
A primary novel finding of the current study was that, despite minimizing cardiopulmonary limitations with a small muscle mass exercise paradigm, both muscle convective and diffusive components of peripheral O2 transport were attenuated in the patients with COPD, resulting in a markedly diminished V̇MO2peak (Figures 2 and 3). During a sufficiently rapid incremental exercise test, such as the one used this study, primary limitations to peak work rate are O2 transport and utilization (Knight et al., 1993; Richardson et al., 1995a; Wagner, 1996; Gonzalez-Alonso et al., 2001). Thus, the similar Q̇MO2 and V̇MO2 during submaximal exercise and the diminished muscle convective and diffusive O2 transport at maximal exercise in the patients with COPD compared to control subjects supports COPD-specific effects on the peripheral determinants of O2 transport that limited V̇MO2peak, and, consequently, peak work rate. This is consistent with the improvement in KE V̇MO2peak and peak work rate in patients with COPD when supplemented with 100% O2 (Richardson et al., 1999). It is interesting, and likely coincidental, that both the convective and diffusive components were lower in patients with COPD compared to controls by almost the same degree, 38 and 37% respectively (Table 2). The integrated nature of muscle convective and diffusive O2 transport can be brought to light using Figure 4 and considering the influence of each component in isolation. Specifically, either the lower muscle convective O2 transport or diffusive O2 transport, alone, would have diminished V̇MO2peak by ≈25%. Together, one might then have expected these effects to be additive and result in a 50% lower V̇MO2peak. However, interestingly, V̇MO2peak was lower by only 36% due to the integration of both components (Figure 4). In combination, these findings emphasize the importance of peripheral O2 transport, within the active skeletal muscle, in determining the diminished exercise capacity in patients with COPD.
Mechanical Efficiency
It has previously been demonstrated that mechanical efficiency is reduced in patients with COPD compared to controls (Baarends et al., 1997; Sala et al., 1999; Richardson et al., 2004). In the current study, V̇MO2 was not different between patients and control subjects across submaximal work rates (Figure 2). However, when V̇MO2peak is considered relative to peak work rate, the mechanical efficiency was reduced by ≈30% in the patients with COPD (Figure 2). The underlying mechanisms for this reduction in mechanical efficiency, if consistently present in COPD, are likely related to the energetic cost of muscle contraction, as patients with COPD demonstrate a 2.2-fold higher ATP cost of muscle contraction compared to control subjects (Layec et al., 2011; Layec et al., 2012). This is consistent with the Type II fiber shift common in these patients, as the energy cost of contraction for these muscle fibers is three- to four-fold greater than Type I fibers (He et al., 2000). Such a mechanical inefficiency would support the concept that, despite preserved mechanisms of energy production, the lower functional capacity of muscle in patients with COPD is exacerbated by a reduction in mechanical efficiency.
Critical Experimental Design Elements
To properly understand the nature of muscle function in patients with COPD, it is imperative that 1) appropriate control subjects be selected, beyond matching for age, sex, and stature and 2) that the muscles studied are not limited by a compromised central cardiopulmonary capacity. Patients with COPD typically experience long-term locomotor muscle disuse from diminished activity, and matching controls to these very low levels of physical activity is imperative. Previous studies have compared patients with COPD to more physically active control subjects (Maltais et al., 1996; Maltais et al., 1998; Serres et al., 1998), which has confounded the understanding of disease-related versus disuse-related peripheral alterations. The evidence for structural and functional changes in the muscle of patients with COPD (Jakobsson et al., 1990; Satta et al., 1997; Jobin et al., 1998; Whittom et al., 1998; Maltais et al., 1999; Eliason et al., 2010) may be, in part, explained by comparisons made with substantially more active control subjects, as such differences are minimized when appropriate controls are used (Richardson et al., 2004). As with our previous investigation (Richardson et al., 2004), great effort was taken in the current study to match controls to both the physical activity and physical characteristics of the patients with COPD. In light of this, the current findings demonstrate impairments in peripheral convective and diffusive O2 transport associated with COPD, rather than with inactivity alone, that contribute to the diminished V̇MO2peak. Additionally, minimizing ventilatory limitations (i.e. attainment of maximal ventilation) with a small muscle mass exercise paradigm is important because it unbridles the exercising muscle, facilitating much higher levels of O2 utilization than during large muscle mass exercise (Richardson et al., 2004). Despite this, the patients with COPD still attained a peak work rate ≈50% lower than controls. While V̇MO2peak, and its peripheral determinants, being reduced concomitantly with peak work rate is at first intuitive, the mechanistic importance of this can be appreciated by considering the limitations to peak work rate in this context. During a sufficiently rapid incremental exercise test, primary limitations to peak work rate and V̇MO2peak are oxygen transport and utilization (Knight et al., 1993; Richardson et al., 1995a; Wagner, 1996; Gonzalez-Alonso et al., 2001). The lower work rate obtained by the patients, thus, reveals the direct impact of the compromised peripheral determinants of O2 transport in determining the diminished V̇MO2peak in patients with COPD.
Clinical Implications
The insight provided by this investigation highlights the importance of peripheral skeletal muscle dysfunction in patients with COPD. Clinically, this insight can be utilized to better optimize therapies and treatments for improving exercise capacity, and the associated clinical outcomes, in patients with COPD. Activation of group III/IV afferents within the active muscles during whole body exercise in patients with COPD increases the ventilatory response and dyspnea, which compromises V̇O2peak and exercise tolerance (Gagnon et al., 2012). The compromised peripheral O2 transport reported in the current study for patients with COPD would, therefore, not only diminish exercise capacity directly, but also, indirectly, by increasing the ventilatory response and dyspnea. Thus, therapies or treatments that augment muscle convective and diffusive O2 transport would not only increase exercise capacity, but also decrease the ventilatory response and dyspnea during exercise in patients with COPD. The current findings are congruent with the previously documented benefit of O2 supplementation on exercise capacity in patients with COPD (Richardson et al., 1999), and support this as a continued therapy. It is important to recognize, however, that the benefits of acute O2 supplementation alone are likely significantly inhibited by the existing peripheral maladaptations which will continue to compromise peripheral O2 transport. Additionally, chronic use of a high inspired O2 concentration may augment oxidative stress. Thus, therapies and treatments targeting the underlying mechanisms for the compromised convective and diffusive O2 transport are likely to yield greater improvements in exercise capacity in these patients. One such therapy is small muscle mass exercise training (e.g. KE training). Critically, KE training improved whole-body cycling V̇O2peak in patients with heart failure in spite of no improvement in central O2 transport (Esposito et al., 2011). This improvement in whole-body cycling V̇O2peak occurred by augmenting peripheral convective and diffusive O2 transport, with a strong, linear relationship between ḊMO2 and V̇O2peak across exercise modalities (Esposito et al., 2011). This concept is particularly relevant for patients with COPD where cardiopulmonary improvements may be limited and many of the peripheral factors that were enhanced by KE training are diminished (i.e. muscle fiber cross-sectional area, capillary-to-fiber ratio, and number of capillaries around the muscle fiber). Consistent with this premise, single-leg endurance exercise training (i.e. KE or cycling) has been demonstrated to be highly efficacious in terms of improving exercise capacity in patients with COPD (Dolmage & Goldstein, 2008; Bronstad et al., 2012), however, the effects on peripheral O2 transport remain to be assessed. Other therapies, such as dietary nitrates (Zamani et al., 2015), aimed at augmenting peripheral O2 transport may also effectively improve exercise capacity in patients with COPD. As with any study in COPD, it is important to consider that the high degree of heterogeneity in this patient population may limit the complete translation of the study findings to all patients. Hemoglobin concentration is one example of this heterogeneity. Hemoglobin concentration was low in the patients with COPD in the current study, but this does not appear to have been the primary reason for the diminished convective O2 transport in this group. In previous studies, convective O2 transport at maximal small muscle mass or whole body exercise has been both not different and lower than controls for patients with COPD with normal hemoglobin concentrations (Maltais et al., 1998; Simon et al., 2001; Richardson et al., 2004). Future, large-scale, studies are needed to assess the influence of the high degree of heterogeneity in COPD on the determinants of exercise capacity in this population. Overall, the findings of this study provide novel insight into important clinical targets to better optimize therapies and treatments for improving exercise capacity, and the associated clinical outcomes, in patients with COPD.
Conclusions
The current study provides novel mechanistic insight regarding the peripheral O2 transport determinants of the diminished exercise capacity in patients with COPD. These findings document that, at maximal KE, both muscle convective and diffusive O2 transport and, therefore muscle peak O2 uptake, are compromised in patients with COPD compared to activity and anthropometrically matched control subjects, even when ventilatory limitations are minimized by a small muscle mass exercise paradigm. Thus, O2 transport dysfunction, at the level of the active skeletal muscle, greatly attenuates peripheral O2 transport and, therefore, exercise capacity in patients with COPD. These findings emphasize the importance of factors, beyond the lungs, that influence exercise capacity and may, ultimately, influence the prognosis, mortality, and quality of life for patients with COPD.
Supplementary Material
KEY POINTS.
Peak oxygen uptake, a primary determinant of prognosis, mortality, and quality of life, is diminished in patients with COPD, with mounting evidence supporting an important role for peripheral dysfunction, particularly within skeletal muscle.
In patients with severe COPD and activity-matched controls, muscle oxygen transport and utilization were assessed at peak effort during single-leg knee-extensor exercise (KE), where ventilation is assumed to be submaximal. This strategy removes ventilation as the major constraint to exercise capacity in COPD, allowing maximal muscle function to be attained and evaluated.
During maximal KE, both convective arterial oxygen delivery to the skeletal muscle microvasculature and subsequent diffusive oxygen delivery to the mitochondria were diminished in patients with COPD compared to control subjects.
These findings emphasize the importance of factors, beyond the lungs, that influence exercise capacity in this patient population and may, ultimately, influence the prognosis, mortality, and quality of life for patients with COPD.
Acknowledgments
We are indebted to all of the participants for their time and effort expended to complete this study.
Funding: National Heart, Lung, and Blood Institute Grant HL-091830 and Ruth L. Kirschstein National Research Service Award 1T32HL139451 and the Veterans Administration Rehabilitation Research and Development Service (E6910-R, E1697-R, E1433-P, E9275-L and E1572-P).”
Footnotes
Conflict of Interest: The authors have nothing to disclose.
REFERENCES
- Agusti AG, Roca J, Barbera JA, Casademont J, Rodriguez-Roisin R & Wagner PD. (1994). Effect of sampling site on femoral venous blood gas values. J Appl Physiol 77, 2018–2022. [DOI] [PubMed] [Google Scholar]
- Amann M, Regan MS, Kobitary M, Eldridge MW, Boutellier U, Pegelow DF & Dempsey JA. (2010). Impact of pulmonary system limitations on locomotor muscle fatigue in patients with COPD. Am J Physiol Regul Integr Comp Physiol 299, R314–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P, Adams RP, Sjogaard G, Thorboe A & Saltin B. (1985). Dynamic knee extension as model for study of isolated exercising muscle in humans. J Appl Physiol 59, 1647–1653. [DOI] [PubMed] [Google Scholar]
- Andersen P & Saltin B. (1985). Maximal perfusion of skeletal muscle in man. J Physiol 366, 233–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baarends EM, Schols AM, Akkermans MA & Wouters EF. (1997). Decreased mechanical efficiency in clinically stable patients with COPD. Thorax 52, 981–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels MN, Armstrong HF, Gerardo RE, Layton AM, Emmert-Aronson BO, Sonett JR & Arcasoy SM. (2011). Evaluation of pulmonary function and exercise performance by cardiopulmonary exercise testing before and after lung transplantation. Chest 140, 1604–1611. [DOI] [PubMed] [Google Scholar]
- Bronstad E, Rognmo O, Tjonna AE, Dedichen HH, Kirkeby-Garstad I, Haberg AK, Bjork Ingul C, Wisloff U & Steinshamn S. (2012). High-intensity knee extensor training restores skeletal muscle function in COPD patients. Eur Respir J 40, 1130–1136. [DOI] [PubMed] [Google Scholar]
- Dolmage TE & Goldstein RS. (2008). Effects of one-legged exercise training of patients with COPD. Chest 133, 370–376. [DOI] [PubMed] [Google Scholar]
- Eliason G, Abdel-Halim SM, Piehl-Aulin K & Kadi F. (2010). Alterations in the muscle-to-capillary interface in patients with different degrees of chronic obstructive pulmonary disease. Respir Res 11, 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito F, Reese V, Shabetai R, Wagner PD & Richardson RS. (2011). Isolated quadriceps training increases maximal exercise capacity in chronic heart failure: the role of skeletal muscle convective and diffusive oxygen transport. J Am Coll Cardiol 58, 1353–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folsom AR, Jacobs DR Jr., Caspersen CJ, Gomez-Marin O & Knudsen J. (1986). Test-retest reliability of the Minnesota Leisure Time Physical Activity Questionnaire. J Chronic Dis 39, 505–511. [DOI] [PubMed] [Google Scholar]
- Gagnon P, Bussieres JS, Ribeiro F, Gagnon SL, Saey D, Gagne N, Provencher S & Maltais F. (2012). Influences of spinal anesthesia on exercise tolerance in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 186, 606–615. [DOI] [PubMed] [Google Scholar]
- Gifford JR, Garten RS, Nelson AD, Trinity JD, Layec G, Witman MA, Weavil JC, Mangum T, Hart C, Etheredge C, Jessop J, Bledsoe A, Morgan DE, Wray DW, Rossman MJ & Richardson RS. (2016). Symmorphosis and skeletal muscle VO2 max : in vivo and in vitro measures reveal differing constraints in the exercise-trained and untrained human. J Physiol 594, 1741–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford JR, Trinity JD, Kwon OS, Layec G, Garten RS, Park SY, Nelson AD & Richardson RS. (2017). Altered Skeletal Muscle Mitochondrial Phenotype in COPD: Disease versus Disuse. J Appl Physiol 124, 1045–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford JR, Trinity JD, Layec G, Garten RS, Park SY, Rossman MJ, Larsen S, Dela F & Richardson RS. (2015). Quadriceps exercise intolerance in patients with chronic obstructive pulmonary disease: the potential role of altered skeletal muscle mitochondrial respiration. J Appl Physiol 119, 882–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Alonso J, Richardson RS & Saltin B. (2001). Exercising skeletal muscle blood flow in humans responds to reduction in arterial oxyhaemoglobin, but not to altered free oxygen. J Physiol 530, 331–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajiro T, Nishimura K, Jones PW, Tsukino M, Ikeda A, Koyama H & Izumi T. (1999). A novel, short, and simple questionnaire to measure health-related quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 159, 1874–1878. [DOI] [PubMed] [Google Scholar]
- He ZH, Bottinelli R, Pellegrino MA, Ferenczi MA & Reggiani C. (2000). ATP consumption and efficiency of human single muscle fibers with different myosin isoform composition. Biophys J 79, 945–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan MC, Bebout DE & Wagner PD. (1991a). Effect of hemoglobin concentration on maximal O2 uptake in canine gastrocnemius muscle in situ. J Appl Physiol 70, 1105–1112. [DOI] [PubMed] [Google Scholar]
- Hogan MC, Bebout DE & Wagner PD. (1991b). Effect of increased HbO2 affinity on VO2max at a constant O2 delivery in dog muscle in situ. J Appl Physiol 70, 2656–2662. [DOI] [PubMed] [Google Scholar]
- Iepsen UW, Munch GW, Rugbjerg M, Ryrso CK, Secher NH, Hellsten Y, Lange P, Pedersen BK, Thaning P & Mortensen SP. (2017). Leg blood flow is impaired during small muscle mass exercise in patients with COPD. J Appl Physiol 123, 624–631. [DOI] [PubMed] [Google Scholar]
- Jacobs DR, Ainsworth BE, Hartman TJ & Leon AS. (1993). A Simultaneous Evaluation of 10 Commonly Used Physical-Activity Questionnaires. Med Sci Sport Exer 25, 81–91. [DOI] [PubMed] [Google Scholar]
- Jakobsson P, Jorfeldt L & Brundin A. (1990). Skeletal muscle metabolites and fibre types in patients with advanced chronic obstructive pulmonary disease (COPD), with and without chronic respiratory failure. Eur Respir J 3, 192–196. [PubMed] [Google Scholar]
- Jobin J, Maltais F, Doyon JF, LeBlanc P, Simard PM, Simard AA & Simard C. (1998). Chronic obstructive pulmonary disease: capillarity and fiber-type characteristics of skeletal muscle. J Cardiopulm Rehabil 18, 432–437. [DOI] [PubMed] [Google Scholar]
- Jones NL, Jones G & Edwards RH. (1971). Exercise tolerance in chronic airway obstruction. Am Rev Respir Dis 103, 477–491. [DOI] [PubMed] [Google Scholar]
- Kelman GR. (1966). Digital computer subroutine for the conversion of oxygen tension into saturation. J Appl Physiol 21, 1375–1376. [DOI] [PubMed] [Google Scholar]
- Kelman GR. (1967). Digital computer procedure for the conversion of PCO2 into blood CO2 content. Respir Physiol 3, 111–115. [DOI] [PubMed] [Google Scholar]
- Kent BD, Mitchell PD & McNicholas WT. (2011). Hypoxemia in patients with COPD: cause, effects, and disease progression. Int J Chron Obstruct Pulmon Dis 6, 199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight DR, Schaffartzik W, Poole DC, Hogan MC, Bebout DE & Wagner PD. (1993). Effects of hyperoxia on maximal leg O2 supply and utilization in men. J Appl Physiol 75, 2586–2594. [DOI] [PubMed] [Google Scholar]
- Lands LC, Smountas AA, Mesiano G, Brosseau L, Shennib H, Charbonneau M & Gauthier R. (1999). Maximal exercise capacity and peripheral skeletal muscle function following lung transplantation. J Heart Lung Transplant 18, 113–120. [DOI] [PubMed] [Google Scholar]
- Lawrenson L, Poole JG, Kim J, Brown C, Patel P & Richardson RS. (2003). Vascular and metabolic response to isolated small muscle mass exercise: effect of age. Am J Physiol Heart Circ Physiol 285, H1023–1031. [DOI] [PubMed] [Google Scholar]
- Layec G, Haseler LJ, Hoff J & Richardson RS. (2011). Evidence that a higher ATP cost of muscular contraction contributes to the lower mechanical efficiency associated with COPD: preliminary findings. Am J Physiol Regul Integr Comp Physiol 300, R1142–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layec G, Haseler LJ & Richardson RS. (2012). The effect of higher ATP cost of contraction on the metabolic response to graded exercise in patients with chronic obstructive pulmonary disease. J Appl Physiol 112, 1041–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louvaris Z, Habazettl H, Asimakos A, Wagner H, Zakynthinos S, Wagner PD & Vogiatzis I. (2017). Heterogeneity of blood flow and metabolism during exercise in patients with chronic obstructive pulmonary disease. Respir Physiol Neurobiol 237, 42–50. [DOI] [PubMed] [Google Scholar]
- Maltais F, Jobin J, Sullivan MJ, Bernard S, Whittom F, Killian KJ, Desmeules M, Belanger M & LeBlanc P. (1998). Metabolic and hemodynamic responses of lower limb during exercise in patients with COPD. J Appl Physiol 84, 1573–1580. [DOI] [PubMed] [Google Scholar]
- Maltais F, LeBlanc P, Jobin J, Berube C, Bruneau J, Carrier L, Breton MJ, Falardeau G & Belleau R. (1997). Intensity of training and physiologic adaptation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 155, 555–561. [DOI] [PubMed] [Google Scholar]
- Maltais F, LeBlanc P, Simard C, Jobin J, Berube C, Bruneau J, Carrier L & Belleau R. (1996). Skeletal muscle adaptation to endurance training in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 154, 442–447. [DOI] [PubMed] [Google Scholar]
- Maltais F, Sullivan MJ, LeBlanc P, Duscha BD, Schachat FH, Simard C, Blank JM & Jobin J. (1999). Altered expression of myosin heavy chain in the vastus lateralis muscle in patients with COPD. Eur Respir J 13, 850–854. [DOI] [PubMed] [Google Scholar]
- Medeiros WM, Fernandes MC, Azevedo DP, de Freitas FF, Amorim BC, Chiavegato LD, Hirai DM, O’Donnell DE & Neder JA. (2015). Oxygen delivery-utilization mismatch in contracting locomotor muscle in COPD: peripheral factors. Am J Physiol Regul Integr Comp Physiol 308, R105–111. [DOI] [PubMed] [Google Scholar]
- O’Donnell DE, Laveneziana P, Webb K & Neder JA. (2014). Chronic obstructive pulmonary disease: clinical integrative physiology. Clin Chest Med 35, 51–69. [DOI] [PubMed] [Google Scholar]
- O’Donnell DE & Webb KA. (2008). The major limitation to exercise performance in COPD is dynamic hyperinflation. J Appl Physiol 105, 753–755. [DOI] [PubMed] [Google Scholar]
- Oga T, Nishimura K, Tsukino M, Sato S & Hajiro T. (2003). Analysis of the factors related to mortality in chronic obstructive pulmonary disease: role of exercise capacity and health status. Am J Respir Crit Care Med 167, 544–549. [DOI] [PubMed] [Google Scholar]
- Poole DC, Copp SW, Hirai DM & Musch TI. (2011). Dynamics of muscle microcirculatory and blood-myocyte O2 flux during contractions. Enzyme 202, 293–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson RS, Knight DR, Poole DC, Kurdak SS, Hogan MC, Grassi B & Wagner PD. (1995a). Determinants of maximal exercise VO2 during single leg knee-extensor exercise in humans. Am J Physiol 268, H1453–1461. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Leek BT, Gavin TP, Haseler LJ, Mudaliar SR, Henry R, Mathieu-Costello O & Wagner PD. (2004). Reduced mechanical efficiency in chronic obstructive pulmonary disease but normal peak VO2 with small muscle mass exercise. Am J Respir Crit Care Med 169, 89–96. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Noyszewski EA, Kendrick KF, Leigh JS & Wagner PD. (1995b). Myoglobin O2 desaturation during exercise. Evidence of limited O2 transport. J Clin Invest 96, 1916–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson RS, Sheldon J, Poole DC, Hopkins SR, Ries AL & Wagner PD. (1999). Evidence of skeletal muscle metabolic reserve during whole body exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 159, 881–885. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Tagore K, Haseler LJ, Jordan M & Wagner PD. (1998). Increased VO2 max with right-shifted Hb-O2 dissociation curve at a constant O2 delivery in dog muscle in situ. J Appl Physiol 84, 995–1002. [DOI] [PubMed] [Google Scholar]
- Roca J, Ramis L, Rodriguez-Roisin R, Ballester E, Montserrat JM & Wagner PD. (1988). Serial relationships between ventilation-perfusion inequality and spirometry in acute severe asthma requiring hospitalization. Am Rev Respir Dis 137, 1055–1061. [DOI] [PubMed] [Google Scholar]
- Sala E, Roca J, Marrades RM, Alonso J, Gonzalez De Suso JM, Moreno A, Barbera JA, Nadal J, de Jover L, Rodriguez-Roisin R & Wagner PD. (1999). Effects of endurance training on skeletal muscle bioenergetics in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 159, 1726–1734. [DOI] [PubMed] [Google Scholar]
- Satta A, Migliori GB, Spanevello A, Neri M, Bottinelli R, Canepari M, Pellegrino MA & Reggiani C. (1997). Fibre types in skeletal muscles of chronic obstructive pulmonary disease patients related to respiratory function and exercise tolerance. Eur Respir J 10, 2853–2860. [DOI] [PubMed] [Google Scholar]
- Serres I, Gautier V, Varray A & Prefaut C. (1998). Impaired skeletal muscle endurance related to physical inactivity and altered lung function in COPD patients. Chest 113, 900–905. [DOI] [PubMed] [Google Scholar]
- Simon M, LeBlanc P, Jobin J, Desmeules M, Sullivan MJ & Maltais F. (2001). Limitation of lower limb VO(2) during cycling exercise in COPD patients. J Appl Physiol 90, 1013–1019. [DOI] [PubMed] [Google Scholar]
- Taylor HL, Jacobs DR Jr., Schucker B, Knudsen J, Leon AS & Debacker G. (1978). A questionnaire for the assessment of leisure time physical activities. J Chronic Dis 31, 741–755. [DOI] [PubMed] [Google Scholar]
- Toft-Petersen AP, Torp-Pedersen C, Weinreich UM & Rasmussen BS. (2016). Association between hemoglobin and prognosis in patients admitted to hospital for COPD. Int J Chron Obstruct Pulmon Dis 11, 2813–2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner PD. (1988). An integrated view of the determinants of maximum oxygen uptake In Oxygen Transfer from the Atmosphere to Tissues, ed. Gonzalez NC & Fedde MR, pp. 245–256. Plenum Publishing Corporation, New York. [DOI] [PubMed] [Google Scholar]
- Wagner PD. (1992). Gas exchange and peripheral diffusion limitation. Med Sci Sports Exerc 24, 54–58. [PubMed] [Google Scholar]
- Wagner PD. (1996). Determinants of maximal oxygen transport and utilization. Annu Rev Physiol 58, 21–50. [DOI] [PubMed] [Google Scholar]
- Whittom F, Jobin J, Simard PM, Leblanc P, Simard C, Bernard S, Belleau R & Maltais F. (1998). Histochemical and morphological characteristics of the vastus lateralis muscle in patients with chronic obstructive pulmonary disease. Med Sci Sports Exerc 30, 1467–1474. [DOI] [PubMed] [Google Scholar]
- Zamani P, Rawat D, Shiva-Kumar P, Geraci S, Bhuva R, Konda P, Doulias PT, Ischiropoulos H, Townsend RR, Margulies KB, Cappola TP, Poole DC & Chirinos JA. (2015). Effect of inorganic nitrate on exercise capacity in heart failure with preserved ejection fraction. Circulation 131, 371–380; discussion 380. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.