Abstract
Objectives:
Cognitive impairment is a leading cause of disability worldwide and cardiometabolic conditions are key contributors to its development. Short sleep is also a potential contributor to brain health, however, its role in predicting mortality remains poorly understood. We investigated whether objective short sleep duration increases the risk of all-cause mortality associated with coexisting cognitive impairment and cardiometabolic conditions, i.e., possible vascular cognitive impairment (VCI).
Design:
Longitudinal
Setting:
Population-based, in-lab study
Participants:
1,524 adults (48.9±13.4 years, 53.4% women) from the Penn State Adult Cohort
Measurements:
All-cause mortality after 19.1±5.1 years of follow-up. Neuropsychological testing to ascertain cognitive impairment. Clinical history and physical examination to ascertain stage 2 hypertension, type 2 diabetes, heart disease and stroke. Possible VCI defined as the presence of any of these cardiometabolic conditions and cognitive impairment. In-lab, 8-hour polysomnography (PSG) to ascertain short sleep duration (i.e., <6 hours).
Results:
Multivariable-adjusted Cox proportional hazard models showed that the risk of all-cause mortality associated with cardiometabolic conditions (n=864) and possible VCI (n=122) was significantly increased in those who slept <6 hours at baseline (HR=1.79, 95%CI=1.28-2.51 and HR=4.01, 95%CI=2.66-6.05, respectively), while it was negligible in those who slept ≥6 hours (HR=1.44, 95%CI=0.99-2.09 and HR=1.41, 95%CI=0.70-2.83, respectively).
Conclusions:
Objective short sleep duration predicts the mortality prognosis of adults with possible VCI. Sleep duration and cognition should be objectively evaluated in patients presenting with a cluster of cardiometabolic conditions and sleep and cognitive complaints. Short sleep is a useful risk factor in the prediction of adverse cardiometabolic and brain health outcomes.
Keywords: Cardiovascular Disease, Cognitive Impairment, Mortality/Survival, Sleep Duration
INTRODUCTION
It is estimated that about 13% of the population has some form of cognitive impairment, while about 6% develop dementia in older adulthood, which make them leading causes of disability worldwide and a priority in healthcare prevention. (1,2) Vascular disease is a cause or contributor in 25% to 50% of cases of dementia, and vascular dementia is the second most common type of major neurocognitive disorder in clinical and population-based samples. (3,4)
The concept of vascular cognitive impairment (VCI) was introduced to capture the entire spectrum of neurocognitive disorders associated with all forms of vascular brain injury that range from mild cognitive impairment to fully-developed dementia. (3) The most recent scientific statement on VCI established that cardiometabolic conditions, including hypertension and diabetes, are key vascular contributors to cognitive impairment (4-6) due to silent cerebrovascular brain injury. (3,7,8) The impact of cardiometabolic conditions on cognition appears to begin in middle-age and additively increases the likelihood of possible cases of VCI progressing into dementia in older adulthood. (3,9) Despite increased preventive efforts and campaigns, hypertension and diabetes remain as the most prevalent cardiometabolic conditions for the development of cardiovascular disease and stroke. (10) Scientific statements on VCI have concluded that detection and control of cardiometabolic conditions may be effective in the prevention of cognitive decline, and pin-pointed the need to identify novel predictors of the prognosis, including survival, of VCI. (3)
Sleep is increasingly recognized as an important contributor to cardiometabolic and brain health. While sleep apnea is an established risk factor (11) and experimental sleep deprivation is known to impact cardiometabolic and neurocognitive outcomes, (12,13) we still lack a complete understanding on whether short sleep duration does play a role in predicting mortality in the general population. (14,15) In order to improve our understanding on how short sleep contributes to adverse cardiometabolic and brain health outcomes, we have contributed to shifting the paradigm and conceptualized objectively-measured sleep duration as an effect modifier of the association between cardiometabolic conditions and cognitive impairment with mortality. In the present study, we hypothesized that objective short sleep duration increases the risk of mortality associated with possible VCI in middle-age adults. To test this novel hypothesis, we examined the effect modification by objective sleep duration on the increased risk of all-cause mortality in a random, general population sample studied in the sleep laboratory via polysomnography (PSG) and objective neuropsychological testing.
PARTICIPANTS AND METHODS
Participants
The data presented here were collected as part of the Penn State Adult Cohort, a population-based study of sleep disorders, which used a two-phase protocol in order to recruit participants from various age groups. (16,17) The second phase of the study randomly recruited 741 men and 1,000 women to be studied in the sleep laboratory (response rates of 67.8% and 65.8%, respectively). Detailed descriptions of the sampling procedure and the cohort composition have been extensively described in previous publications. (16,17) After giving a complete description of the study to the subjects, written informed consent was obtained. The study protocol study complied with the Declaration of Helsinki and was approved by the Institutional Review Board at Penn State University College of Medicine.
Mortality
Death certificates for deceased individuals as of December 31, 2016, were retrieved from the Center of Disease Control and Prevention (CDC). Participants were linked by CDC and to death records from the National Death Index for the years 1992 through 2016 and vital status was determined through a rigorous process of probabilistic matching and death certificate review based on participants’ social security number, full name, date of birth, and gender. (18-20) Of the 1,741 participants, a total of 1,145 subjects were alive and 596 were deceased. Survival time was calculated from the time of the baseline in-lab evaluation to the date of death for those deceased or to December 31, 2016 for those alive.
Cardiometabolic Conditions
We identified the presence of stage 2 hypertension defined as systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg or use of antihypertensive medication. (3,10) Type 2 diabetes was defined as fasting glucose levels ≥126 mg/dL or a report of having been diagnosed with or receiving treatment for type 2 diabetes. (5,10) Cardiovascular disease was defined as a report of having been diagnosed with or receiving treatment for heart disease. Cerebrovascular disease was defined as a report of having been diagnosed with or receiving treatment for stroke. Participants with stage 2 hypertension, type 2 diabetes, heart disease and/or stroke were classified as having cardiometabolic conditions.
Cognitive Impairment
On the evening before the sleep recording, each participant completed a battery of standardized neuropsychological tests (21,22) consisting of measures commonly used in clinical practice to assess a wide range of cognitive domains. All testing sessions occurred between 19:00 and 20:00. During the 1-h testing session five neuropsychological tests were administered, which provided 16 outcomes. The Mini-Mental State Examination (MMSE) assesses global cognitive status and is a sensitive screener for dementia. (23) The lower the score in the MMSE, the worse the global cognitive status. (21,22) A score < 25 is suggestive of probable dementia. (24,25) The Symbol Digit Modalities Test (SDMT) primarily assesses psychomotor processing speed and, secondarily, the visual scanning and tracking aspects of complex attention. (26) The greater the number of correct responses in the SDMT, the better the cognitive function measured. (27) The Trail Making Test (TMT) is a 2-part test that yields four outcomes and assesses different aspects of attention. (28) Part A (TMT-A) assesses visual conceptual tracking, psychomotor processing speed, and attention. (29-33) Part B (TMT-B), in addition, it requires set-switching attention and working memory abilities. The TMT B/A and TMT B–A are derived scores that remove the speed element from the test evaluation by factoring-in the TMT-A and TMT-B scores and assess executive control of attention. The TMT B/A is considered a purer measure of the working memory (maintenance/rehearsal) task required in TMT-B and is obtained by dividing part B by part A. (30-32) The TMT B-A is a purer, reliable and valid, measure of the set-switching attention task required in TMT-B and is obtained by subtracting part A from part B. (30-32) The lower the time (in seconds) to complete any of the parts of the TMT, the better the cognitive function measured. (21,22) The Benton Visual Retention Test (BVRT; administration A, immediate recall) assesses short-term visual memory, visual perception, and visual-constructive abilities. (34) It yields 8 outcomes of number of correct designs (BVRT-Correct) and number of errors (BVRT-Errors) and their type (i.e., omissions, distortions, perseverations, rotations, misplacements, size error). (21,22,34) The greater the number of correct designs in the BVRT, the better the cognitive function measured, while the lower the number of errors, the better the cognitive function measured. The Thurstone Word Fluency Test (TWFT) assesses the spontaneous oral and written production of words under restricted search conditions (i.e., verbal fluency) and the ability to inhibit previous responses. (35). The greater the number of words produced in the TWFT, the better the cognitive function measured. (21,22,35) A total of 217 participants did not have data in all neurocognitive tests and, thus, were excluded from the analyses. Compared to the 1524 participants who completed all neurocognitive tests, there were significantly higher proportions of males (56.3% vs. 46.6), ethnic minorities (20.4% vs. 8.5%), and mental health problems (21.5% vs. 28.5%) among those who did not complete all tests. None of the other demographic, clinical, or sleep-related characteristics were significantly different between the two groups, including cardiometabolic conditions and objective sleep duration.
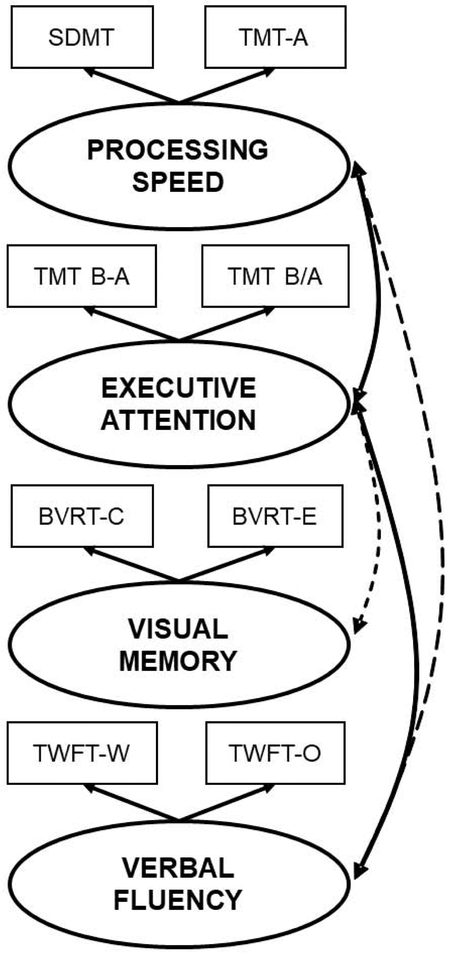
While the availability of these 16 outcomes is clearly an advantage that permits interpretation based on cognitive profiles rather than one single test score, it also imposes statistical (i.e., experiment-wise error rate) and clinical (i.e., overlapping cognitive functions) limitations when testing our hypotheses. We, thus, conducted principal component analysis (PCA) to reduce the 16 outcomes to a smaller number of clinically meaningful and psychometrically reliable cognitive domains consistent with previous scientific statements (3) and established models of cognition. (36-38) Z-scores for each test were computed based on the raw score, with their respective sample mean and standard deviation (SD) and PCA was performed. This factorial analysis yielded a four-factor solution explaining 89% of the total variance. A fifth unspecific factor indexed by the MMSE score was removed from the PCA as it loaded across all domains and represented “global cognitive status”. Figure 1 depicts the four cognitive function domains and the outcome scores loading in each of them. These four cognitive function domains plus global cognitive status were used in our analyses, which 1) reduced the number of outcome variables and multiple comparisons, 2) made individual test scores clinically meaningful, and 3) allowed for replication in future studies using different neuropsychological tests measuring similar cognitive function domains.
Figure 1. Cognitive functioning domains.
Out of the 16 available outcomes from the five neuropsychological tests administered, principal component analysis (PCA) identified a four-factor solution of well-delimited cognitive functioning domains labeled “processing speed”, “executive control of attention”, “short-term visual memory” and “verbal fluency”. Not depicted in the figure was a fifth factor, indexed by the mini-mental state examination (MMSE) score, that was removed from the PCA as it loaded across all domains and represented “global cognitive status” and used to identify those with probable dementia (MMSE<25). Dashed arrows indicate small, statistically significant correlations between factors (r ≤ 0.12), while solid arrows indicate medium, statistically significant correlations between factors (r ≤ 0.32). All other correlations were negligible (r<0.1) and non-statistically significant.
To identify participants with cognitive impairment, a two-fold approach was used. First, individuals with a MMSE score of < 25, regardless of scores from other tests, were identified as cognitively impaired (i.e., probable dementia). (24,25) Second, the sum of Z-scores from all tests (Zsum), except MMSE, was computed. Participants with a Zsum > 90th percentile of the study population were considered as cognitively impaired based on the estimated 13% prevalence of cognitive impairment (1,2). We chose the 90th percentile rather than 85th percentile to rely on a more conservative definition. Since higher Z-scores in SDMT, BVRT-Correct, TWFT-Oral and TWFT-Written represent better cognitive functions, they were multiplied by −1 in the calculation, so that higher value suggests worse cognitive function. The algorithm for calculating Zsum can be written as the following equation.
After applying these criteria, 155 of the 1,524 participants were categorized as cognitively impaired. To evaluate the validity of the categorization of cognitive impairment as well as assess the neurocognitive characteristics of the participants based on their cardiometabolic health, both raw scores and Z-scores of all tests and the factor solution were compared by using two-sample t-tests (Table 1). As expected, participants who were identified as cognitively impaired performed significantly worse across all cognitive function domains. Individuals with cardiometabolic conditions also performed significantly worse across all cognitive function domains (Table 1). A group with “possible VCI” was defined by the coexistence of cardiometabolic conditions and cognitive impairment (n=122). This group was identified as “possible VCI” (vs. “probable VCI”) given the absence of brain imaging or other biomarkers that could establish a definitive vascular etiology. (39)
TABLE 1.
Neurocognitive characteristics of the overall sample as well as stratified by the presence of cognitive impairment and cardiometabolic conditions
| Cognitive Impairment | Cardiometabolic Conditions | ||||||
|---|---|---|---|---|---|---|---|
| Overall (N = 1,524) |
No (n = 1,369) |
Yes (n = 155) |
Pa | No (n = 538) |
Yes (n = 986) |
Pb | |
| Global Cognitive Status | |||||||
| MMSE, score | 29.1 (1.4) | 29.2 (1.2) | 27.4 (2.1) | <0.01 | 29.3 (1.5) | 28.8 (1.3) | <0.01 |
| Probable dementia, % | 1.9 | 0.0 | 17.7 | N/A | 0.6 | 2.3 | 0.01 |
| Processing Speed, z-score | −0.28 (1.68) | −0.48 (1.49) | 2.24 (1.84) | <0.01 | −0.57 (1.86) | 0.09 (1.52) | <0.01 |
| SDMT, number | 47.83 (11.74) | 49.84 (0.97) | 30.08 (11.27) | <0.01 | 51.30 (11.21) | 45.94 (11.59) | <0.01 |
| TMT-A, seconds | 33.31 (14.51) | 31.75 (12.33) | 47.18 (22.77) | <0.01 | 30.57 (12.11) | 34.81 (15.47) | <0.01 |
| Executive Attention, z-score | −0.11 (0.95) | −0.29 (0.52) | 2.17 (1.59) | <0.01 | −0.24 (1.15) | 0.05 (0.81) | <0.01 |
| TMT B-A, seconds | 48.38 (38.68) | 39.81 (21.65) | 124.51 (64.84) | <0.01 | 40.91 (31.43) | 52.46 (41.57) | <0.01 |
| TMT B/A, seconds | 2.53 (1.00) | 2.37 (0.77) | 3.96 (1.54) | <0.01 | 2.41 (0.92) | 2.59 (1.04) | <0.01 |
| Visual Memory, z-score | −0.43 (1.83) | −0.69 (1.60) | 2.80 (1.43) | <0.01 | −0.79 (2.06) | 0.01 (1.61) | <0.01 |
| BVRT Correct, number | 6.46 (1.88) | 6.78 (1.62) | 3.62 (1.54) | <0.01 | 7.00 (1.69) | 6.16 1.91) | <0.01 |
| BVRT Errors, number | 5.54 (3.52) | 4.88 (2.85) | 11.33 (3.64) | <0.01 | 4.48 (3.03) | 6.12 (3.64) | <0.01 |
| Omissions, number | 0.54 (1.29) | 0.38 (0.85) | 1.97 (2.81) | <0.01 | 0.39 (0.94) | 0.63 (1.46) | <0.01 |
| Distortions, number | 2.40 (1.97) | 2.12 (1.67) | 4.86 (2.61) | <0.01 | 2.01 (1.70) | 2.63 (2.08) | <0.01 |
| Perseverations, number | 0.74 (0.99) | 0.68 (0.86) | 1.23 (1.09) | <0.01 | 0.64 (0.84) | 0.80 (0.94) | <0.01 |
| Rotations, number | 0.87 (1.01) | 0.79 (0.95) | 1.58 (1.20) | <0.01 | 0.66 (0.87) | 1.00 (1.06) | <0.01 |
| Misplacements, number | 0.77 (0.99) | 0.71 (0.93) | 1.29 (1.26) | <0.01 | 0.69 (0.92) | 0.82 (1.02) | <0.01 |
| Size error, number | 0.10 (0.39) | 0.09 (0.36) | 0.26 (0.56) | <0.01 | 0.05 (0.27) | 0.14 (0.44) | <0.01 |
| Verbal Fluency, z-score | −0.21 (1.82) | −0.39 (1.76) | 2.00 (1.09) | <0.01 | −0.46 (2.30) | 0.09 (1.45) | <0.01 |
| TWFT Oral, number | 11.59 (4.38) | 12.08 (4.20) | 7.21 (3.46) | <0.01 | 12.20 (4.37) | 11.26 (4.36) | <0.01 |
| TWFT Written, number | 11.49 (4.12) | 12.01 (3.89) | 6.86 (3.20) | <0.01 | 12.35 (4.24) | 11.01 (3.98) | <0.01 |
Data are mean (standard deviation), unless otherwise stated. P a = p value for cognitive impairment. P b = p value for cardiometabolic conditions. MMSE = Mini-mental state examination. SDMT = Symbol digit modalities test. TMT = Trail making test. BVRT = Benton visual retention test. TWFT = Thurstone word fluency test.
Sleep Laboratory
All subjects were evaluated for one night in the sleep laboratory in sound-attenuated, light- and temperature-controlled rooms. During this evaluation, each subject was continuously monitored for eight hours (fixed-time period) using 16-channel polysomnography (PSG) including electroencephalogram, electrooculogram, and electromyogram. Bedtimes were adjusted to conform to subjects’ usual bedtimes, and subjects were recorded between 22:00-23:00 and 06:00-07:00. The sleep recordings were subsequently scored independently, according to standardized criteria. (40) Respiration was monitored throughout the night by use of thermocouples at the nose and mouth and thoracic strain gauges. All-night recordings of hemoglobin oxygen saturation (SpO2) were obtained with an oximeter attached to the finger. The presence of OSA was defined as an apnea/hypopnea index (AHI) ≥ 5 events per hour of sleep based on standard criteria (39). Out of the 8 hours of sleep recording (i.e., time in bed), total sleep time (TST) was defined as the sum of time spent in stages 1, 2, 3, 4 or rapid eye movement (REM) sleep since sleep onset until sleep offset or lights on, whichever occurred first. According to the distribution of PSG-measured TST, we categorized the entire study sample into 2 groups: ≥ 50th percentile (i.e., ≥ 6 hours) and < 50th percentile (i.e., < 6 hours). This cut-off of < 6 hours of sleep has previously shown to be associated with significant morbidity and mortality. (13-15)
Other Assessments
During their in-lab clinical history and physical examination, all subjects completed a standardized questionnaire to assess the presence of physical health conditions, mental health problems, and substance use and their height and weight was measured to calculate body mass index (BMI). Baseline information regarding the subject’s history of mental health conditions included depression, suicidal ideation or attempts, loneliness, marital problems, alcohol abuse, or drug abuse. A composite binary variable was created based on a positive response to any of these mental health conditions. Physical health conditions, other than heart disease and stroke as mentioned above, included allergies/asthma, anemia, birth defects, cancer/tumor, colitis, encephalitis, epilepsy, kidney/bladder disorders, migraine, Parkinson’s disease, rheumatism, thyroid, or ulcer. A composite binary variable was created based on a positive response to any of these physical health conditions. As part of this standardized questionnaire, the presence of sleep difficulty was established as an ordinal variable with three levels: insomnia, defined by a complaint of insomnia with a duration of ≥ 1 year, poor sleep, defined as a moderate-to-severe complaint of difficulty falling asleep, difficulty staying asleep, early final awakening and/or unrefreshing sleep in the absence of insomnia, and normal sleep, defined as the absence of insomnia and poor sleep. Participants’ daily consumption of caffeine (number of cups/day), tobacco (number of cigarettes/day), and alcohol (number of drinks/day) as well as demographic characteristics, including age, sex, race/ethnicity and years of education, were also obtained.
Statistical Analyses
The demographic and clinical characteristics of the study population was evaluated and presented as mean (SD) and proportions for continuous and categorical variables, respectively. To compare these characteristics according to cognitive impairment and cardiometabolic conditions, t-tests or chi-square tests were used, as appropriate.
To evaluate the excess risk of all-cause mortality associated with cognitive impairment and cardiometabolic conditions, a multivariable-adjusted Cox proportional hazards model was used. In this model, age, race, sex, years of education, BMI, AHI, smoking, alcohol, sleep difficulty, objective sleep duration, mental health conditions and physical health conditions (other than hypertension, diabetes, heart disease or stroke) were considered major co-variables and were controlled for. Hazards ratios (HRs) with the corresponding 95% confidence intervals (95%CI) for cognitive impairment and cardiometabolic conditions with respect to their independent relationship with all-cause mortality were estimated.
Based on the presence of cognitive impairment and cardiometabolic conditions, four mutually exclusive groups were established: absence of either cognitive impairment or cardiometabolic conditions (i.e., reference group), cognitive impairment (alone), cardiometabolic conditions (alone) and possible VCI (both cardiometabolic conditions and cognitive impairment, as defined above). The excess risk of all-cause mortality among participants with cognitive impairment, cardiometabolic conditions and possible VCI, compared the reference group, was assessed in a multivariable-adjusted Cox proportional hazards model, while adjusting for age, race, sex, years of education, BMI, AHI, smoking, alcohol, sleep difficulty, objective sleep duration, physical and mental health conditions. In this model, we tested whether objective sleep duration was an effect modifier of the association between cognitive impairment and cardiometabolic conditions with all-cause mortality. We tested the significance of the interaction term between objective sleep duration (<6 hours vs. ≥ 6 hours) and the four mutually exclusive groups as defined above. The HRs for cognitive impairment, cardiometabolic conditions and possible VCI, stratified by objective sleep duration, were thereafter estimated. To graphically present the all-cause mortality risk in each study group, we plotted the multivariable-adjusted survival curves based on the model estimates and the average characteristics of the study population.
Statistical significance was set at a two-sided p<0.05 and all analyses were performed using SAS version 9.4 (SAS Inc, Cary, NC, USA).
RESULTS
The demographic and clinical characteristics of the sample are presented in Table 2. Among the 1,542 participants, 10.1% were cognitively impaired, while 34.9% had cardiometabolic conditions. A total of 32.6% (n=503) of the sample was deceased and the crude mortality in participants with cognitive impairment (60%) or cardiometabolic conditions (41.9%) is presented in Table 2. Multivariable-adjusted Cox proportional hazard models confirmed that cognitive impairment and cardiometabolic conditions were independently associated with an increased risk of all-cause mortality after controlling for major co-variables (HR= 1.75, 95%CI=1.36-2.26 and HR=1.78, 95%CI=1.40-2.25, respectively).
TABLE 2.
Demographic and clinical characteristics of the overall sample as well as stratified by the presence of cognitive impairment and cardiometabolic conditions
| Cognitive Impairment | Cardiometabolic Conditions | ||||||
|---|---|---|---|---|---|---|---|
| Overall (N = 1,524) |
No (n = 1,369) |
Yes (n = 155) |
Pa | No (n = 538) |
Yes (n = 986) |
Pb | |
| Age, years | 48.9 (13.4) | 48.1 (13.3) | 59.3 (11.5) | <0.01 | 44.2 (14.4) | 54.8 (11.0) | <0.01 |
| Male, % | 46.6 | 45.6 | 58.8 | <0.01 | 40.6 | 54.1 | <0.01 |
| White, % | 91.5 | 92.3 | 82.4 | <0.01 | 93.4 | 89.2 | <0.01 |
| Education, years | 13.6 (2.8) | 13.8 (2.8) | 11.2 (2.2) | <0.01 | 14.0 (3.4) | 13.1 (2.4) | <0.01 |
| BMI, kg/m2 | 27.6 (5.6) | 27.5 (5.6) | 28.5 (5.4) | 0.08 | 26.2 (6.0) | 29.4 (5.0) | <0.01 |
| Cardiometabolic conditions | |||||||
| Hypertension, % | 34.9 | 33.9 | 48.6 | <0.01 | 0.0 | 78.7 | |
| Diabetes, % | 13.7 | 12.5 | 29.3 | <0.01 | 0.0 | 31.0 | |
| Heart disease, % | 10.0 | 9.3 | 19.5 | <0.01 | 0.0 | 22.6 | |
| Stroke, % | 1.6 | 1.3 | 4.9 | <0.01 | 0.0 | 3.5 | |
| Smoker, % | 22.0 | 20.6 | 39.2 | <0.01 | 21.6 | 22.5 | 0.68 |
| Alcohol use, drinks/day | 1.1 (5.5) | 1.1 (5.8) | 0.5 (1.2) | <0.01 | 1.3 (8.9) | 0.9 (1.9) | 0.17 |
| Physical health problems, % | 53.6 | 54.3 | 48.8 | 0.05 | 52.2 | 55.4 | 0.21 |
| Mental health problems, % | 21.5 | 21.2 | 24.7 | 0.39 | 19.0 | 24.6 | <0.01 |
| Sleep Difficulty | |||||||
| Normal sleep, % | 69.4 | 69.1 | 73.8 | 0.46 | 72.1 | 66.0 | <0.01 |
| Poor sleep, % | 23.1 | 23.4 | 18.3 | 22.4 | 23.9 | ||
| Insomnia, % | 7.5 | 7.5 | 7.9 | 5.5 | 10.1 | ||
| AHI, events/hour | 2.4 (7.5) | 2.1 (7.0) | 5.3 (10.7) | <0.01 | 1.2 (6.2) | 3.9 (8.0) | <0.01 |
| OSA, % | 10.8 | 10.1 | 19.7 | <0.01 | 6.2 | 16.6 | <0.01 |
| Objective sleep duration, hours | 5.9 (1.2) | 6.0 (1.2) | 5.0 (1.2) | <0.01 | 6.1 (1.4) | 5.6 (1.0) | <0.01 |
| < 6 hours, % | 44.8 | 42.1 | 78.2 | <0.01 | 36.0 | 55.8 | <0.01 |
| Total deaths, % | 33.0 | 30.0 | 60.0 | <0.01 | 16.7 | 41.9 | <0.01 |
| Follow-up Time, years | 19.1 (5.1) | 19.4 (4.8) | 15.0 (6.3) | <0.01 | 20.2 (4.5) | 17.8 (5.2) | <0.01 |
| Alive, years | 21.3 (2.2) | 21.2 (2.3) | 21.8 (1.8) | 0.05 | 21.1 (2.6) | 21.6 (1.8) | <0.01 |
| Deceased, years | 13.1 (5.4) | 13.7 (5.4) | 9.6 (4.4) | <0.01 | 14.2 (6.1) | 12.7 (5.2) | <0.01 |
Data are mean (standard deviation), unless otherwise stated. P a = p value for cognitive impairment. P b = p value for cardiometabolic conditions. AHI = apnea hypopnea index. BMI = body mass index. OSA = obstructive sleep apnea (i.e., AHI ≥ 5 events per hour of sleep).
We further examined our four a priori defined mutually exclusive groups. As shown in Table 3, while cognitive impairment was not associated with a higher risk of all-cause mortality, cardiometabolic conditions and possible VCI were associated with a significant 1.6-fold and 3.1- fold risk, respectively, of all-cause mortality. Importantly, objective sleep duration modified the increased risk of all-cause mortality associated with cardiometabolic conditions and possible VCI (p-value for interaction = 0.03). The association between cardiometabolic conditions and possible VCI with all-cause mortality was significantly stronger among participants who slept objectively < 6 hours (Table 3). Specifically, the risk of all-cause mortality associated with possible VCI was non-significant and 1.4-fold among participants who slept objectively ≥ 6 hours, while significant and 4.0-fold among participants who slept objectively < 6 hours.
TABLE 3.
Risk of all-cause mortality associated with cognitive impairment, cardiometabolic conditions and possible vascular cognitive impairment
| Cardiometabolic Conditions |
Cognitive Impairment |
Group Label | n | Overall | ≥ 6 hours | < 6 hours | P for interaction |
|---|---|---|---|---|---|---|---|
| No | No | Reference | 505 | 1.00 | 1.00 | 1.00 | |
| No | Yes | Cognitive Impairment | 33 | 1.02 (0.51-1.96) | 1.10 (0.33-3.69) | 1.02 (0.46-2.30) | 0.04 |
| Yes | No | Cardiometabolic Conditions | 864 | 1.62 (1.26-2.09) | 1.44 (0.99-2.09) | 1.78 (1.27-2.50) | |
| Yes | Yes | Possible VCI | 122 | 3.14 (2.22-4.45) | 1.40 (0.70-2.82) | 4.09 (2.71-6.18) |
Data are hazard ratios (95% confidence intervals) adjusted for age, race, sex, education, body mass index, apnea/hypopnea index, smoking, alcohol, sleep difficulty, mental health problems, and other physical health problems. VCI = vascular cognitive impairment
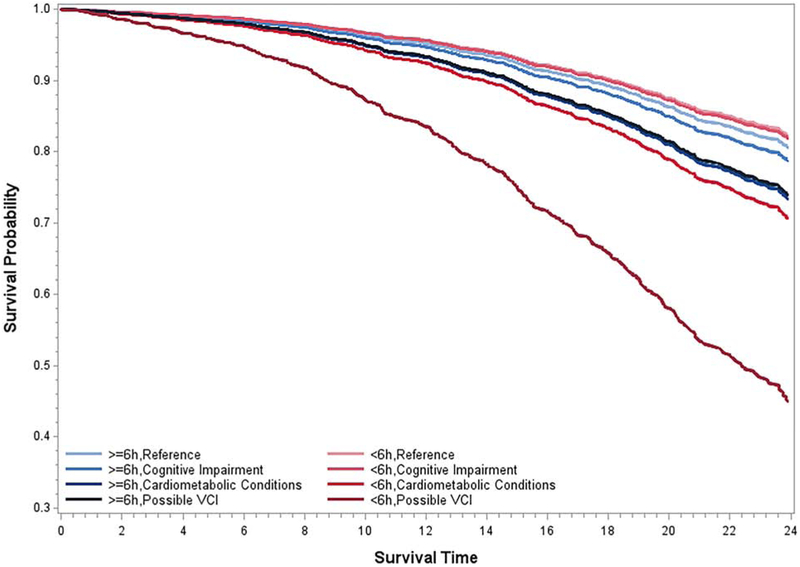
The multivariable-adjusted survival curves presented in Figure 2 further illustrate how possible VCI was associated with a worse survival rate in those with objective short sleep duration. For example, the multivariable-adjusted mortality rates (95%CI) at 15, 20 and 25 years of follow-up in individuals with possible VCI who slept objectively < 6 hours were 23.6% (95%CI=16.5-30.1), 39.8 % (95%CI=29.0-48.9) and 61.3% (95%CI=45.6-72.5), respectively, and these rates were 17.0-40.0% higher than the reference group who also slept objectively < 6 hours [i.e., 6.5% (95%CI=4.3-8.7), 11.9% (95%CI=8.0-15.7) and 21.2% (95%CI=13.8-27.8)]. In contrast, the mortality rates at the same years of follow-up, in individuals with possible VCI who slept objectively ≥ 6 hours were 10.1% (95%CI=3.8-16.0), 18.1% (95%CI=7.1-27.7) and 31.2% (95%CI=12.6-45.8), respectively, and only 3-8% higher than the reference group who also slept more than 6 hours [i.e., 7.3% (95%CI=4.8-9.6), 13.3% (95%CI=9.1-17.2) and 23.3% (95%CI=15.5-30.4), respectively].
Figure 2. Survival curves for individuals with cognitive impairment, cardiometabolic conditions and possible vascular cognitive impairment (VCI) as a function of objective short sleep duration.
All data adjusted for age, race, sex, education, BMI, AHI, smoking, alcohol, sleep difficulty, mental health conditions, and other physical health conditions.
DISCUSSION
This is the first study to demonstrate that objective short sleep duration predicts the mortality prognosis of middle-aged adults with coexisting cardiometabolic conditions and cognitive impairment, i.e., possible VCI. These data further support that objectively-measured short sleep duration is useful in predicting the prognosis of individuals with cognitive impairment, particularly those with a suspicion of VCI. From a public health perspective, randomized clinical trials should test whether lengthening sleep via pharmacological or behavioral therapies improves the cognitive functions and underlying cerebrovascular mechanisms of individuals at risk of vascular dementia and related neurodegenerative disorders.
Given the high prevalence of vascular contributors to dementia, including hypertension and diabetes, identifying cognitive impairment early on in individuals with cardiometabolic conditions has become a priority in the prevention of major neurocognitive disorders such as Alzheimer’s disease. However, this remains a significant challenge for clinicians given the mild nature of cognitive impairment in middle-aged patients with cardiometabolic conditions and the lack of clear-cut biomarkers or observable brain changes at such incipient stage of cognitive decline. A clinical suspicion of possible VCI is, thus, common in these patients who are then closely followed-up. Together with cognitive complaints, sleep is also usually impaired in these individuals. However, it has remained unknown whether sleep does play a role in the progression of possible VCI. In this study, we present evidence that objective sleep, as measured by in-lab PSG sleep duration, can serve as a predictor of the mortality prognosis of these individuals. The strength of association found was large with a 4-fold risk of mortality in individuals with possible VCI who slept less than 6 hours in the lab. Furthermore, it appears that this risk is minimal if the individuals with possible VCI have maintained the ability to sleep more than 6 hours. These findings have direct clinical implications given that many of these individuals with cardiometabolic conditions and/or cognitive impairment do usually undergo a one-night sleep study to rule out sleep apnea, (11) but little attention is paid to their sleep duration as a potential marker of the biological severity of their sleep disturbance.
From a pathophysiological standpoint, it has been posited that dysfunction of the neurovascular unit and mechanisms regulating cerebral blood flow underlie VCI. Cerebral amyloid angiopathy, a biomarker of Alzheimer’s disease risk, is also emerging as an important marker of VCI, including underlying microinfarction, microhemorrhage and macro-hemorrhage of the brain. (3) Cognitive impairment during older adulthood will most commonly result from a mixture of Alzheimer’s disease and microvascular brain damage, such as asymptomatic deep brain infarction, white matter hyper-intensities and accelerated brain atrophy. (3) For this reason, neuroimaging is an important tool in the diagnosis and detection of probable vs. possible VCI. Given the known role of sleep in cognition, memory consolidation and autonomic and immune-system regulation, (12,13) it is likely that individuals with possible VCI who sleep objectively less than 6 hours may indeed be suffering from greater central blood flow dysregulation and systemic inflammation, which may lead to faster cognitive decline and early death.
From a conceptual standpoint, however, there is the possibility that the increased risk of death as a function of objective short sleep duration in those with possible VCI may indicate that cognitive impairment is a “common path” (or mediator) for early death. We tested this alternative model in which cognitive functioning at baseline mediated the increased risk of mortality in those with objective short sleep duration (Supplemental Table 1) and found that this potential mediating effect is minimal (about 10%) and cannot explain the high increased risk when objective short sleep is treated as an effect modifier of cardiometabolic conditions, cognitive impairment and possible VCI. Nevertheless, an important limitation of this study is the lack of a follow-up in-lab study with sleep and neuropsychological testing before death that could examine the decline over time as well as a proximal mechanistic relationship between cognition and early death. Future longitudinal studies with multiple time points and objective sleep and cognitive measures should test these hypotheses.
This study has some limitations that should be considered when interpreting our results. First, the objective sleep duration in this study was based on one night of PSG, which may be affected by the first night effect. There is the possibility that one-night PSG, as routinely conducted in sleep clinics, may reflect physiologic sleep ability rather than habitual sleep duration in the home environment. Nevertheless, the average objective sleep duration in large epidemiologic studies is about 6 hours, which is independent of whether sleep is recorded at home with PSG, i.e., Sleep Heart Health Study, in the lab with PSG, i.e., Penn State Adult Cohort and Wisconsin Sleep Cohort, or at home with actigraphy, i.e., Coronary Artery Risk Development in Young Adults study. The consistency among these large epidemiological studies in terms of objective sleep duration reinforces our belief that the inherent limitations of a 1-night PSG recording in large samples do not compromise the validity of the findings and may provide clinically useful data when participants are classified as short (<6hours) and normal (>6hours) sleepers. Second, the study lacked an in-lab follow-up visit before death to examine the change in sleep duration over time and precluded examining the longitudinal trajectory of objective sleep duration. Third, we did not have available data on circadian preference or phase (e.g., chronotype, sleep midpoint, dim light melatonin onset), which may have influenced some individuals’ objective sleep duration during the 8-hour PSG; however, in this random general sample of central Pennsylvania, the vast majority of participants went to bed between 22:00 and 23:00 and only a small minority went to sleep outside of this time window and none for more than 1 hour, thereby making a 1-hour maximum adjustment. The lack of circadian data also precluded examining the potential interplay of circadian misalignment and objective short sleep in the relationships tested. Fourth, we did not have the ability to confirm the type of diagnoses using medical record data, either for heart disease, stroke or other physical or mental health conditions as reported by the subjects during the clinical history and physical examination. Similarly, the lack of brain imaging precluded the definitive diagnosis of VCI and, thus, the use of the term possible VCI, which should be interpreted cautiously. Finally, the significant differences in some of the demographic characteristics between participants who did and did not complete all neurocognitive tests suggest a potential selection bias. For example, the high proportion of non-Hispanic white population with an average of 13.5 years of education in our study population may not be entirely representative of the racial/ethnic and educational composition of the general U.S. population. Thus, future population-based studies should incorporate multiple night recordings, multiple time points and circadian assessments and test our hypotheses in more ethnically and sex/gender diverse cohorts, thereby extending the generalizability of our findings.
In summary, objective short sleep duration, as measured by in-lab PSG, is a clinically useful predictor of all-cause mortality in middle-aged adults with possible VCI. Sleep and cognitive complaints should be objectively evaluated in individuals with cardiometabolic conditions in a routine manner. There is a need for clinical trials targeting cognition and sleep physiology and behavior in individuals at risk of vascular dementia and related neurodegenerative disorders.
CONCLUSIONS
The risk of all-cause mortality associated with cognitive impairment of possible vascular origin (VCI) in middle-aged adults is four times greater if they demonstrate objective short sleep duration upon a sleep study. Given the high prevalence of cardiometabolic conditions and the need to better predict their impact on cognitive impairment and its prognosis, including its evolution into dementia and mortality, the identification of objective sleep as a modifiable risk factor provides with a specific public health target. Individuals with coexisting cardiometabolic conditions and cognitive impairment who demonstrate objective short sleep duration upon a sleep study may require targeted multidisciplinary interventions. A sleep study in these patients may not only rule in/out the presence of sleep apnea but also estimate in-lab objective sleep duration, which appears to help ascertain the biological severity of their sleep impairment.
Supplementary Material
ACKNOWLEDGEMENTS
This work was performed at the Sleep Research & Treatment Center at Penn State College of Medicine and the staff is especially commended for their efforts.
FUNDING
American Heart Association Award Number 14SDG19830018 (PI: Fernandez-Mendoza) and National Heart Lung and Blood Institute of the National Institutes of Health Awards Number R01HL51931 and R01HL40916 (PI: Bixler).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
None of the authors have any conflicts of interest to disclose.
REFERENCES
- 1.Gillis C, Mirzaei F, Potashman M, Ikram MA, Maserejian N. The incidence of mild cognitive impairment: A systematic review and data synthesis. Alzheimers Dement (Amst). 2019;11:248–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu C, Yu D, Sun X, Zhang M, Wang L, Qin H. The prevalence and progression of mild cognitive impairment among clinic and community populations: a systematic review and meta-analysis. Int Psychogeriatr. 2017;29(10):1595–1608. [DOI] [PubMed] [Google Scholar]
- 3.Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, et al. ; American Heart Association Stroke Council, Council on Epidemiology and Prevention, Council on Cardiovascular Nursing, Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(9):2672–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knopman D, Boland LL, Mosley T, Howard G, Liao D, Szklo M, McGovern P, Folsom AR; Atherosclerosis Risk in Communities (ARIC) Study Investigators. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology. 2001;56:42–48. [DOI] [PubMed] [Google Scholar]
- 5.Biessels GJ, Staekenborg S, Brunner E, et al. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol 2006; 5:64. [DOI] [PubMed] [Google Scholar]
- 6.Luchsinger JA, Reitz C, Honig LS, et al. Aggregation of vascular risk factors and risk of incident Alzheimer disease. Neurology 2005; 65:545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeCarli C Clinically asymptomatic vascular brain injury: a potent cause of cognitive impairment among older individuals. J Alzheimers Dis. 2013;33 Suppl 1:S417–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Debette S, Beiser A, DeCarli C, Au R, Himali JJ, Kelly-Hayes M, Romero JR, Kase CS, Wolf PA, Seshadri S. Association of MRI markers of vascular brain injury with incident stroke, mild cognitive impairment, dementia, and mortality: the Framingham Offspring Study. Stroke. 2010;41(4):600–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeCarli C Cerebrovascular disease: Assessing the brain as an end-organ of vascular disease. Nat Rev Cardiol. 2012; 9(8): 435–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Danaei G, Ding EL, Mozaffarian D, Taylor B, Rehm J, Murray CJ, Ezzati M. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med. 2009;6(4):e1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, Daniels S, Floras JS, Hunt CE, Olson LJ, Pickering TG, Russell R, Woo M, Young T; American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology; American Heart Association Stroke Council; American Heart Association Council on Cardiovascular Nursing; American College of Cardiology Foundation. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). Circulation. 2008;118(10):1080–111. [DOI] [PubMed] [Google Scholar]
- 12.Goel N, Rao H, Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2009;29(4):320–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mullington JM, Haack M, Toth M, Serrador JM, Meier-Ewert HK. Cardiovascular, inflammatory, and metabolic consequences of sleep deprivation. Prog Cardiovasc Dis. 2009; 51(4): 294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurina LM, McClintock MK, Chen JH, Waite LJ, Thisted RA, Lauderdale DS. Sleep duration and all-cause mortality: a critical review of measurement and associations. Ann Epidemiol. 2013; 23(6): 361–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gallicchio L, Kalesan B. Sleep duration and mortality: a systematic review and meta-analysis. J Sleep Res. 2009;18(2):148–58. [DOI] [PubMed] [Google Scholar]
- 16.Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men: I. Prevalence and severity. Am J Respir Crit Care Med. 1998;157:144–148. [DOI] [PubMed] [Google Scholar]
- 17.Bixler EO, Vgontzas AN, Lin HM, Ten Have T, Rein J, Vela-Bueno A, Kales A. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med. 2001;163:608–613. [DOI] [PubMed] [Google Scholar]
- 18.National Center for Health Statistics. 2003 revision of the U.S. Standard Certificate of Death. 2003. Available from: http://www.cdc.gov/nchs/data/dvs/DEATH11-03final-acc.pdf.
- 19.National Center for Health Statistics. Report of the panel to evaluate the U.S. standard certificates. 2000. Available from: http://www.cdc.gov/nchs/data/dvs/panelreport_acc.pdf.
- 20.Vital statistics, instructions for classifying the underlying cause of death NCHS instruction manual, part 2a. Hyattsville, MD: Public Health Service. Published annually. [Google Scholar]
- 21.Lezak MD, Howieson DB, Loring DW, Hannay HJ, Fischer JS. Neuropsychological Assessment, 4th ed. New York: Oxford University Press, 2004. [Google Scholar]
- 22.Strauss E, Sherman EMS, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. 3rd edition. New York: Oxford University Press, 2006. [Google Scholar]
- 23.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189. [DOI] [PubMed] [Google Scholar]
- 24.Crum RM, Anthony JC, Bassett SS, Folstein MF. Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA 1993; 269:2386. [PubMed] [Google Scholar]
- 25.Kukull WA, Larson EB, Teri L, et al. The Mini-Mental State Examination score and the clinical diagnosis of dementia. J Clin Epidemiol 1994; 47:1061. [DOI] [PubMed] [Google Scholar]
- 26.Smith A Symbol Digits Modalities Test. Los Angeles: Western Psychological Services, 1982. [Google Scholar]
- 27.Sheridan LK, Fitzgerald HE, Adams KM, Nigg JT, Martel MM, Puttler LI, Wong MM, Zucker RA. Normative Symbol Digit Modalities Test performance in a community-based sample. Arch Clin Neuropsychol. 2006; 21(1):23–8. [DOI] [PubMed] [Google Scholar]
- 28.Reitan RM. The relation of the trail making test to organic brain damage. J Consult Psychol. 1955; 19(5):393–4. [DOI] [PubMed] [Google Scholar]
- 29.Crowe SF. The differential contribution of mental tracking, cognitive flexibility, visual search, and motor speed to performance on parts A and B of the Trail Making Test. J Clin Psychol. 1998;54(5):585–91. [DOI] [PubMed] [Google Scholar]
- 30.Sánchez-Cubillo I, Periáñez JA, Adrover-Roig D, Rodríguez-Sánchez JM, Ríos-Lago M, Tirapu J, Barceló F. Construct validity of the Trail Making Test: role of task-switching, working memory, inhibition/interference control, and visuomotor abilities. J Int Neuropsychol Soc. 2009;15(3):438–50. [DOI] [PubMed] [Google Scholar]
- 31.Gaudino EA, Geisler MW, Squires NK. Construct validity in the Trail Making Test: what makes Part B harder? J Clin Exp Neuropsychol. 1995. August;17(4):529–35. [DOI] [PubMed] [Google Scholar]
- 32.Kortte KB, Horner MD, Windham WK. The Trail Making Test, part B: cognitive flexibility or ability to maintain set? Applied Neuropsychol 2002;9:106–09. [DOI] [PubMed] [Google Scholar]
- 33.Zakzanis KK, Mraz R, Graham SJ. An fMRI study of the Trail Making Test. Neuropsychologica 2005;43:1878–86. [DOI] [PubMed] [Google Scholar]
- 34.Sivan AB. Benton Visual Retention Test (5th Edition), San Antonio, TX: Psychological Corporation, 1992. [Google Scholar]
- 35.Thurstone LL, Thurstone TG. Primary Mental Abilities. Chicago, University of Chicago Press, 1938. [Google Scholar]
- 36.Mirsky AF, Anthony BJ, Duncan CC, Ahearn MB, Kellam SG. Analysis of the elements of attention: a neuropsychological approach. Neuropsychol Rev. 1991;2(2):109–45. [DOI] [PubMed] [Google Scholar]
- 37.Strauss ME, Thompson P, Adams NL, Redline S, Burant C. Evaluation of a model of attention with confirmatory factor analysis. Neuropsychology. 2000;14(2):201–8. [DOI] [PubMed] [Google Scholar]
- 38.Ríos M, Periáñez JA, Muñoz-Céspedes JM. Attentional control and slowness of information processing after severe traumatic brain injury. Brain Inj. 2004;18(3):257–72. [DOI] [PubMed] [Google Scholar]
- 39.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association, 2013 [Google Scholar]
- 40.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Bethesda, MD: National Institutes of Health, 1968. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.